Abstract
The Toll and Toll-Like Receptor signaling pathways are evolutionarily conserved pathways that regulate innate immunity in insects and mammals. While efforts have been made to clarify the signal transduction events that occur during infection, much less is known about the components that maintain immune quiescence. Here we show that retromer, an intracellular protein complex known for regulating vesicle trafficking, functions in modulating the Toll pathway in Drosophila melanogaster. In mutant animals lacking retromer function, the Toll pathway but not JAK-STAT or IMD pathway is activated, triggering both cellular and humoral responses. Genetic epistasis and clonal analysis suggest that retromer regulates a component that acts upstream of Toll. Our data further show that in the mutant the Toll ligand Spätzle has a processing pattern similar to that of after infection. Together, the results suggest a novel function of retromer in regulating Toll pathway and innate immunity at a step that modulates ligand processing or activity.
Keywords: Retromer, Vps35, Toll, Spz, Drosophila
INTRODUCTION
The Toll and Toll-Like Receptor (TLR) signaling pathways play a conserved role in innate immunity in insects and vertebrates (Kawai and Akira, 2011). In Drosophila, the Toll pathway is activated through an extracellular protease cascade that detects invading pathogens (Lemaitre and Hoffmann, 2007). The protease cascade culminates in the cleavage and activation of Spätzle Processing Enzyme (SPE), which in turn cleaves and activates Spätzle (Spz) (Jang et al., 2006). As a ligand, active Spz binds and activates the transmembrane receptor Toll (Hu et al., 2004; Weber et al., 2003). The activation of Toll initiates an intracellular signal transduction pathway, which leads to the translocation of Dorsal and Dorsal-related immunity factor (DIF) into the nucleus for the transcriptional activation of downstream targets, including antimicrobial peptide genes (Belvin et al., 1995; Ip et al., 1993; Lemaitre et al., 1996; Meng et al., 1999; Rutschmann et al., 2000; Tanji et al., 2010). Upon infection, the Toll pathway amplifies the signal through enzyme cascades as well as through positive feedback loops (Jang et al., 2006; Lemaitre and Hoffmann, 2007; Lemaitre et al., 1996). In contrast, in the absence of infection, stringent controls are needed to prevent auto-activation of this pathway (Kondo et al., 2012; Levashina et al., 1999). Although the intracellular components of the Toll pathway are well characterized, the factors contributing to negative controls upstream of the Toll receptor are less known.
Retromer is a membrane-associated cytoplasmic protein complex regulating the trafficking of specific transmembrane cargos from endosomes to the trans-Golgi network (Collins, 2008). Retromer is composed of five components: Sorting nexin 1/2 (SNX1/2), SNX5/6, Vacuolar protein sorting 29 (Vps29), Vps26 and Vps35 (Collins, 2008). The complex has been shown to recycle several transmembrane proteins, thereby regulating different biological processes including Wnt secretion, apoptotic cell clearance and apical-basal polarity maintenance (Belenkaya et al., 2008; Chen et al., 2010; Cullen and Korswagen, 2012; Franch-Marro et al., 2008; Pocha et al., 2011; Port et al., 2008; Zhang et al., 2011; Zhou et al., 2011). Here we identify a novel role of retromer in suppressing the auto-activation of the Toll pathway through regulating the processing of the ligand Spz.
MATERIALS AND METHODS
Drosophila strains
dvps351 and UAS-dvps35RNAi were described previously (Belenkaya et al., 2008). w1118, Canton-S, tubGal4, cgGal4, Tlr3, Spz2, Spzrm7 (also known as Spz4), UAS-SpzRNAi-1 (BL28538), UAS-SpzRNAi-2 (BL34699) and UAS-statRNAi (BL33637) were from Bloomington Stock Center. TlRxA was obtained from Dr. K.V. Anderson. YP1-Gal4 was described previously (Hu et al., 2004). UAS-dvps26RNAi (GD18396), UAS-SPERNAi-1 (KK104906) and UAS-SPERNAi-2 (GD30971) were from VDRC (Dietzl et al., 2007). For mosaic clone analysis, y w hsp70-flp;FRTG13 ubiquitin-GFP/FRTG13 dvps351 flies were generated and heat-shocked at 37°C for 2 hours at the beginning of first instar stage. For flip-out clone analysis, y w hsp70-flp/+;AyGal4 UAS-GFP/UAS-Toll10bMyc flies were generated and heat-shocked at 37°C for 30 minutes at the beginning of first instar stage.
Real-Time quantitative PCR
Total RNA was isolated using TRIzol Reagent (Invitrogen). cDNA was synthesized according to a protocol as previously described (Cha et al., 2011). Real-time PCR was performed using SsoFast Evagreen Supermix (BIO-RAD) and CFX96 Real-time System (BIO-RAD). The following gene-specific primers were used:
Drosomycin, 5’-TACTTGTTCGCCCTCTTCG-3’ and 5’-GTATCTTCCGGACAGGCAGT-3’; IM1, 5’- CTCAGTCGTCACCGTTTTTG-3’, 5’-CATTGCACACCCTGCAATC-3’; Diptericin, 5’-CCGCAGTACCCACTCAATCT-3’ and 5’-ACTGCAAAGCCAAAACCATC-3’; CecropinA1, 5’- CATCTTCGTTTTCGTCGCTC-3’ and 5’-CGACATTGGCGGCTTGTTGA-3’; AttacinA, 5’-AGGTTCCTTAACCTCCAATC-3’ and 5’-CATGACCAGCATTGTTGTAG-3’; SPE, 5’-CTTTTCGCTGATCGCATTTT-3’ and 5’-CACCGGATTTGTCCAGTTCT-3’; spz, 5’-GTCCAGTTCGCCATCACTTT-3’ and 5’-GGAAGCTGGTGTACCCAAAA-3’; STAT, 5’-CCGGTTATGTGAAGAGCACA-3’ and 5’-TAGCGACACACGTTCAGGAG-3’; dvps35, 5’-CTTTTCTGGAGTGGAAAGCA-3’ and 5’-TTCAGCAGCTCCACATACAA-3’; ribosomal protein 49 (rp49), 5’-AAGCTAGCCCAACCTGCTTC-3’ and 5’-GTGCGCTTCTTCACGATCT-3’, 5’-ATGCTAAGCTGTCGCACAAA-3’ and 5’-GTTCGATCCGTAACCGATGT-3’ or 5’-CAAGAAGCTAGCCCAACCTG-3’ and 5’-AGCATATCGATCCGACTGGT-3’.
Hemocyte counts and immunostaining
Circulating hemocytes were obtained and counted as previously described (Lanot et al., 2001). Plasmatocytes and lamellocytes were identified by cell appearance. Lymph gland was dissected and stained as previously described (Qiu et al., 1998). Drosophila larval or adult female fat body was fixed and stained as previously described (Scott et al., 2007). Drosophila hemocytes were fixed and stained as previously described (Lanot et al., 2001). The following primary antibodies were used: mouse anti-Dorsal (7A4, 1:5, DSHB), rabbit anti-DIF (Rutschmann et al., 2000) (1:100), rabbit anti-phosphoHistone H3 (Singh et al., 2007) (1:500), mouse anti-Cut (2B10, 1:5, DSHB), rat anti-DE-cad (DCAD2, 1:2.5, DSHB) and mouse anti-Antp (8C11, 1:5, DSHB). Alexa Fluor 488 (Molecular Probes), Cy3 and Cy5 (Jackson Immuno)-conjugated secondary antibodies were used at a dilution of 1:400. Alexa Fluor 488 Phalloidin (1:400, Molecular Probes) was used for F-actin staining. Alexa Fluor 555-conjugated Wheat Germ Agglutinin (WGA, 1:500, Molecular Probes) was used for nuclear membrane staining. DAPI (1 μg/μl, Roche) was used for nuclei staining.
Septic injury and immunoblotting
Septic injuries were performed at room temperature by pricking wandering third instar larvae with a thin tungsten needle previously dipped into a concentrated culture of Micrococcus luteus. Larvae were recovered at 25°C for 1 hour. For immunoblotting, Drosophila wandering 3rd instar larvae were collected and homogenized on ice in lysis buffer (1% Triton X-100, 50mM NaCl, 20mM Tris at pH7.5 and 1mM EDTA) plus Protease Inhibitors (Roche). Lysates were subject to centrifugation at 13,000 rpm. Supernatants were taken out carefully to avoid the lipid layer on the top, followed by a second centrifugation at the same speed. Final supernatants were mixed with Laemmli sample buffer (Bio-Rad) plus 5% 2-Mercaptoethanol (FisherBiotech). Lysates were boiled for 5 minutes and subjected to immunoblot analysis using the following primary antibodies: mouse anti-V5 (1:3000, Invitrogen) and mouse anti-β-Actin (1:5000, Abcam). Horseradish peroxidase (HRP)-conjugated secondary antibodies (Jackson Immuno) were used at a dilution of 1:3000.
Imaging and Statistical analysis
Images of third instar larvae were collected on an Olympus SZ-CTV dissection microscope. Confocal images were collected on a Zeiss LSM 510 confocal microscope with 10x dry, 20x dry, 40x oil and 63x oil objectives. All images were arranged with Adobe Photoshop. Error bars represent standard deviation. Statistical comparisons were made using Student's T-test, with P value listed in figures.
RESULTS
Loss of retromer function triggers an auto-activation of the innate immune response in Drosophila
Drosophila innate immunity involves the cellular immune response and the humoral immune response (Lemaitre and Hoffmann, 2007). Hyper-activation of the cellular immune response can generate melanotic aggregates (Minakhina and Steward, 2006). We noted that homozygous null mutants of Drosophila vps351 (labelled as dvps35 mutants hereafter) generated such melanotic aggregates in the larval body cavity (Figure 1A and 1B). Similarly, when knocking down dvps35 or another retromer component dvps26 by RNAi (RNA interference) using the ubiquitous driver tubulin-Gal4 (tubGal4-dvps35RNAi), we also observed melanotic aggregates (Figure 1C and data not shown for vps26RNAi). These phenotypes were observed in the absence of infections. Thus, loss of retromer activity can cause the formation of melanotic aggregates, implying that retromer may have a role in modulating Drosophila cellular immune response.
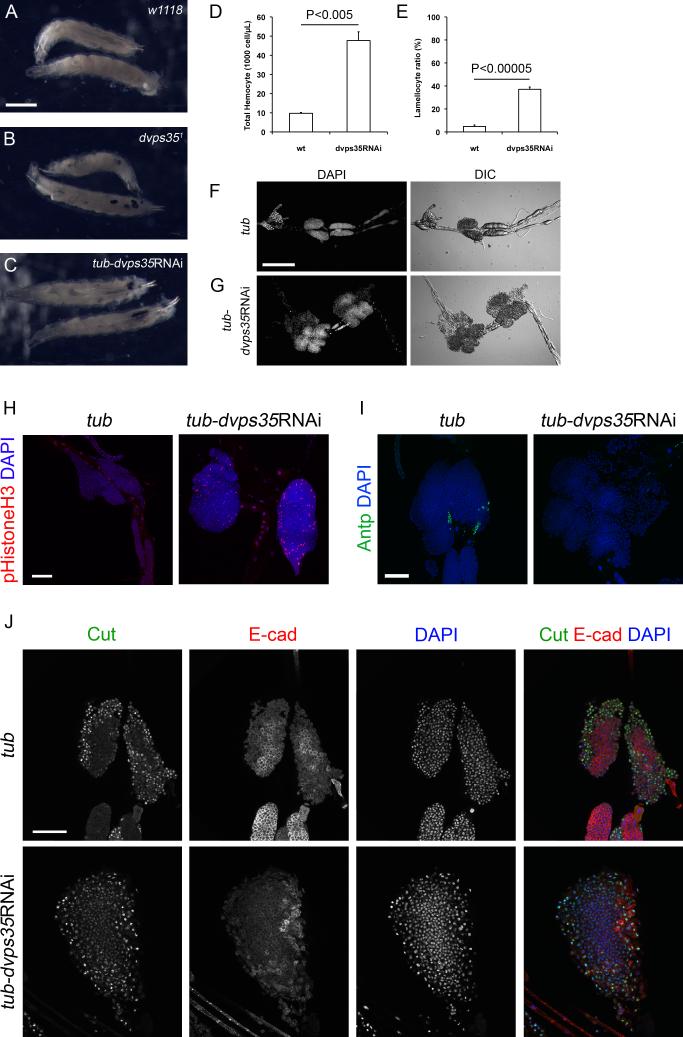
Figure 1. Loss of retromer activity activates the cellular immune response in Drosophila prior to infections.
(A-C) Third instar larvae of (A) wild type, (B) dvps35 mutants and (C) tubGal4-dvps35RNAi. Scale bar, 1 mm. (D) Total hemocyte count of wild type and tubGal4-dvps35RNAi third instar larvae. (E) Lamellocyte ratio in total hemocytes of wild type and tubGal4-dvps35RNAi third instar larvae. (F and G) Lymph glands of (F) tub-Gal4 and (G) tub-Gal4-dvps35RNAi third instar larvae. Anterior, left. Scale bar, 200 μm. (H) phospho-Histone H3 and DAPI staining in lymph glands of tub-Gal4 and tub-dvps35RNAi third instar larvae. (I) Antp and DAPI staining in lymph glands of tub-Gal4 and tubGal4-dvps35RNAi third instar larvae. (J) Cut, DE-cadherin and DAPI staining in lymph glands of tub-Gal4 and tubGal4-dvps35RNAi third instar larvae. Anterior to the top in all images in H-J. Scale bar in H-J, 50 μm.
Upon activation of the cellular immune response, the formation of melanotic aggregates is often associated with excessive proliferation of total hemocytes, over-differentiation into lamellocytes and/or a hypertrophy of lymph gland, which is a hematopoietic organ (Harrison et al., 1995; Lemaitre and Hoffmann, 2007; Luo et al., 1995; Minakhina and Steward, 2006; Qiu et al., 1998). In tubGal4-dvps35RNAi larvae, the total hemocyte count and the ratio of lamellocytes were significantly enhanced compared to the wild type (Figure 1D and 1E). tubGal4-dvps35RNAi larvae also displayed lymph gland hypertrophy when compared to the control tubGal4 larvae (Figure 1F and 1G). Phospho-histone H3 staining suggested a higher proliferation rate in lymph glands in tubGal4-dvps35RNAi larvae than that in control (Figure 1H). We also observed an expansion of the cortical zone, a reduction of the medullary zone and an absence of the posterior signalling center (Figure 1I and 1J), as marked by Cut, DE-cadherin and Antp staining, respectively. This is consistent with the increased proliferation of precursor cells in the absence of control by the signalling center, leading to lymph gland hypertrophy. Taken together, the cellular immune response was highly activated in animals lacking retromer activity.
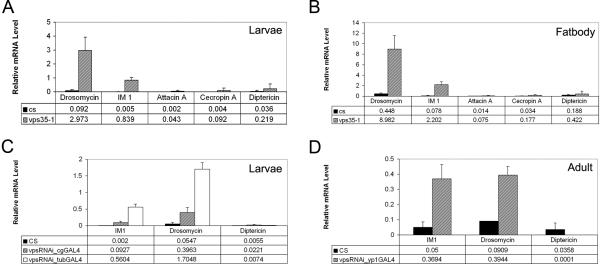
Next, we examined the humoral branch of the innate immune system, which features the production of antimicrobial peptides from an immune organ, the fat body (Lemaitre and Hoffmann, 2007). We monitored the expression of several antimicrobial peptide genes using real-time PCR. A subset of antimicrobial peptide genes including Drosomycin (Drs) and Immune-induced Molecule 1 (IM1) were highly expressed in whole larvae of dvps35 mutants (Figure 2A). Furthermore, Drs and IM1 were greatly induced in the dissected larval fat bodies of dvps35 mutant larvae (Figure 2B). Consistent with null mutants, induction of Drs and IM1 was also observed in whole larvae, knocking down dvps35 by RNAi driven by cgGal4 (fat body and hemocyte expression) or tubGal4 (ubiquitous expression) (Figure 2C). We also used an adult female fat body driver, YP1Gal4 to knock down dvps35 in the adult fat bodies, and observed an induction of Drs and IM1 expression after dvps35RNAi (Figure 2D). These results suggest that the regulation of antimicrobial peptide gene expression by dvps35 is likely similar in both larvae and adults. Taken together, loss of dvps35 function causes an activation of the humoral immune response in addition to the cellular response shown above.
Figure 2. Loss of retromer activity activates the humoral response regulated by Toll.
(A and B) mRNA expression level assayed by q-PCR of Toll or IMD pathway-regulated antimicrobial peptide genes in (A) whole larvae or (B) larval fat body of wild type and dvps35 mutants. CS, Canton-S. Drosomycin and IM1 are Toll-regulated genes. AttacinA, CecropinA and Diptericin are IMD-regulated genes. Ribosomal protein 49 (rp49) served as internal control. (C and D) Expression level of Toll or IMD pathway-regulated antimicrobial peptide genes in (C) larvae or (D) adult of wild type, cgGal4-dvps35RNAi, tubGal4-dvps35RNAi and yp1Gal4-dvps35RNAi.
Loss of retromer function activates specifically the Toll pathway
There are probably more than 50 antimicrobial peptide genes with diverse functions in the Drosophila genome. The expression of different antimicrobial peptide genes is controlled by different signaling pathways. The expression of Drs and IM1 is regulated by the Toll pathway, while the expression of several other antimicrobial peptide genes including AttacinA, CecropinA and Diptericin is regulated by the IMD pathway (Lemaitre and Hoffmann, 2007). Although the expression of Drs and IM1 was highly activated in the absence of dvps35 function, the expression of AttacinA, CecropinA and Diptericin was not significantly changed (Figure 2A-2D). This argues that the Toll pathway but not the IMD pathway is activated in the absence of dvps35 function. Consistent with this view, the activation of the Toll, but not the IMD pathway has been reported to generate many aspects of the cellular immune response observed in tubGal4-dvps35RNAi larvae (Figure 1) (Huang et al., 2005; Qiu et al., 1998).
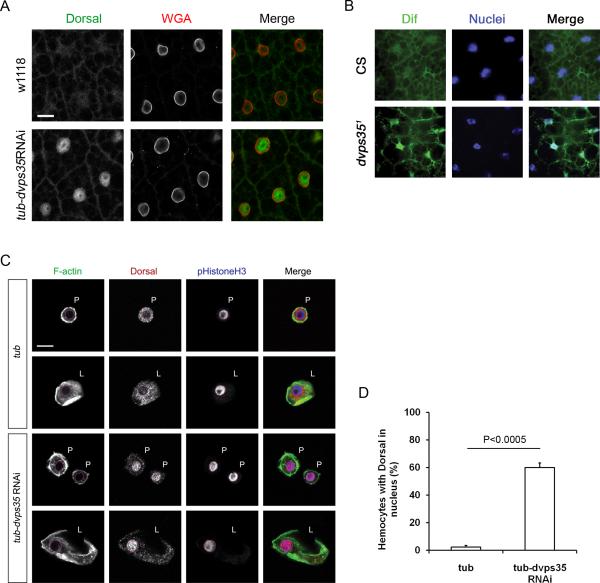
To further confirm the activation of the Toll pathway, we examined the nuclear translocation of the transcription factors Dorsal and DIF. In the fat bodies and hemocytes of tubGal4-dvps35RNAi larvae, Dorsal was localized in the nuclei, in contrast to a cytoplasmic distribution in wild type samples (Figure 3A). Similarly, DIF was also localized in the nuclei of the fat bodies of dvps35 mutants (Figure 3B). These results strongly suggest that abolishing dvps35 activates the Toll pathway. We performed similar staining in the hemocyte collections. The Dorsal protein staining could be observed in the nuclei of many plasmatocytes and lamellocytes (Fig. 3C), consistent with the activation of the Toll pathway. Quantification of the result revealed that Dorsal nuclear localization in hemocytes was highly increased (Fig. 3D).
Figure 3. Loss of retromer activity activates the Toll but not IMD pathway.
(A) Staining of Dorsal and nuclear membrane (WGA) in the fat body of wild type (w1118) and tubGal4-dvps35RNAi third instar larvae. Scale bar, 20 μm. (B) Staining of DIF and DAPI in the adult fat body of wild type (CS) and dvps35 mutant. CS, Canton-S. (C) Staining of F-actin, Dorsal and phospho-Histone H3 in hemocytes of tubGal4 and tubGal4-dvps35RNAi third instar larvae. P, plasmatocytes; L, lamellocytes; red dotted circles, nuclei. Scale bar, 10 μm. (D) Ratio of hemocytes with Dorsal in the nuclei in tubGal4 and tubGal4-dvps35RNAi third instar larvae.
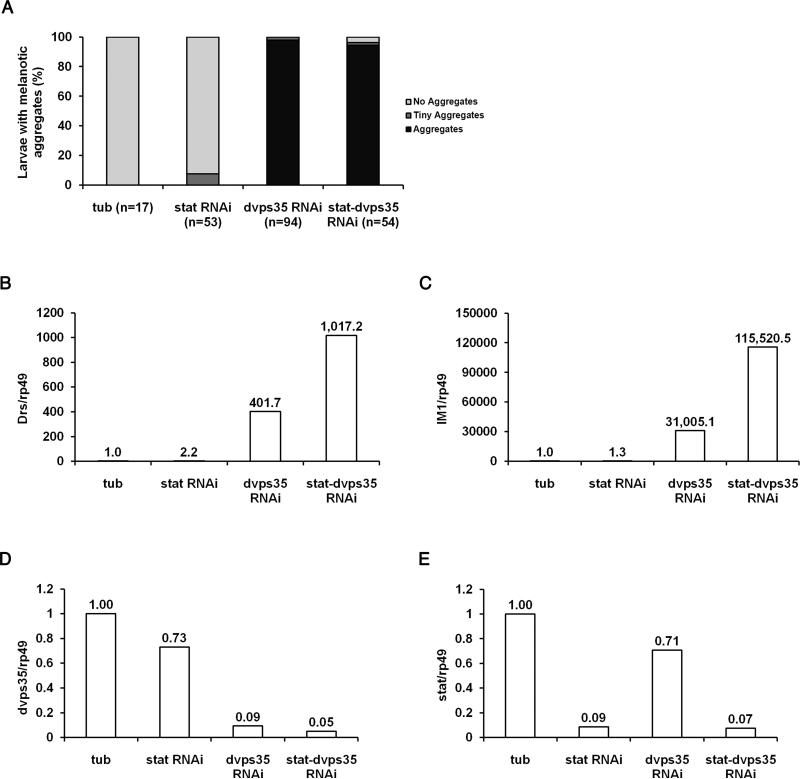
In addition to the Toll pathway, the activation of the JAK/STAT pathway can also generate melanotic aggregates (Harrison et al., 1995; Luo et al., 1995). Furthermore, crosstalk between the Toll pathway and the JAK/STAT pathway has been reported (Sackton et al., 2010). Therefore, we examined whether the generation of melanotic aggregates and the high level expression of antimicrobial peptide genes was a result of the JAK/STAT pathway activation in dvps35 mutants. In tubGal4-dvps35RNAi larvae, knocking down stat (tubGal4-statRNAi-dvps35RNAi), which encodes a transcriptional factor in the JAK/STAT pathway, neither rescued the generation of melanotic aggregates (Figure 4A), nor suppressed the overexpression of Toll-regulated antimicrobial peptide genes (Drs and IM1, Figure 4B-4C). As controls, the knock down of vps35 and stat was highly efficient (Figure 4D-4E). These data argue that the JAK/STAT pathway is not required for the generation of melanotic aggregates or the induction of Toll-regulated antimicrobial peptide genes in dvps35 mutants. Instead, there was some enhancement of the gene expression, suggesting the above mentioned crosstalk between Toll and JAK/STAT pathways.
Figure 4. The JAK-STAT pathway is not essential for the generation of melanotic aggregates or the activation of the Toll pathway in the absence of dVps35 activity.
(A) Ratio of third instar larvae with melanotic aggregates in tubGal4, tubGal4-statRNAi, tubGal4-dvps35RNAi and tubGal4-statRNAi-dvps35RNAi. (B-E) Expression level of (B) Drs, (C) IM1, (D) dvps35 and (E) stat in third instar larvae of tubGal4, tubGal4-statRNAi, tubGal4-dvps35RNAi and tubGal4-statRNAi-dvps35RNAi.
Retromer suppressed the processing of the Toll ligand Spz
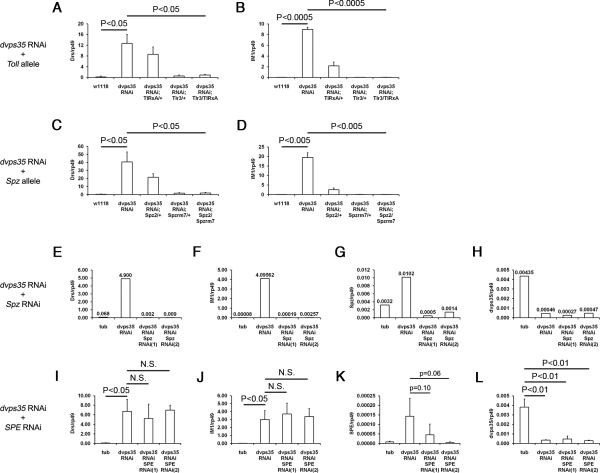
Having established a role of retromer in the Toll pathway, we next conducted epistasis analysis to determine the position of retromer action along the Toll pathway. The initial focus of our epistasis analysis was on the transmembrane receptor Toll and its ligand Spz because previous studies showed that retromer targeted transmembrane proteins (Collins, 2008). We used different alleles of Toll and Spz (Tlr3, TlRxA, Spz2 and Spzrm7) and two independent lines of Spz RNAi to reduce the activity of these two genes. In tubGal4-dvps35RNAi larvae, reducing the activity of Toll (Tlr3/TlRxA) efficiently suppressed the induction of Toll-regulated antimicrobial peptide genes (Figure 5A and 5B). Similarly, reducing the activity of Spz (Spz2/Spzrm7 and Spz RNAi) also gave rise to an efficient suppression in tubGal4-dvps35RNAi larvae (Figure 5C-5H). It is note worthy that the knock down of dvps35 caused a modest increase of Spz transcript (Figure 5G). This is consistent with previous observations that many of the Toll pathway components are up-regulated when the pathway is activated (Ip et al., 1993; Lemaitre et al., 1996). Overall, both the receptor and the ligand of the Toll pathway are required for the activation of downstream events in the absence of dVps35 activity.
Figure 5. Genetic epistasis analysis places the action of retromer at the level of Spz.
(A and B) Expression level of (A) Drs and (B) IM1 in third instar larvae of wild type and tubGal4-dvps35RNAi with different combinations of Toll hypomorphic (Tlr3) or null (TlRxA) alleles. (C and D) Expression level of (C) Drs and (D) IM1 in third instar larvae of wild type and tubGal4-dvps35RNAi with different combinations of Spz null alleles (Spz2 and Spzrm7). (E-H) Expression level of (E) Drs, (F) IM1, (G) Spz and (H) dvps35 in third instar larvae of tubGal4, tubGal4-dvps35RNAi, tubGal4-dvps35RNAi-SpzRNAi(1) and tubGal4-dvps35RNAi-SpzRNAi(2). (I-L) Expression level of (I) Drs, (J) IM1, (K) SPE and (L) dvps35 in third instar larvae of tubGal4, tubGal4-dvps35RNAi, tubGal4-dvps35RNAi-SPERNAi(1) and tubGal4-dvps35RNAi-SPERNAi(2).
Next we investigated components upstream of the ligand. SPE is the immediate upstream protease of Spz in the Toll pathway during the immune response (Jang et al., 2006). We conducted epistasis analysis between dvps35 and SPE. In tubGal4-dvps35RNAi larvae, knocking down SPE by two independent RNAi lines (driven by tub-Gal4) did not suppress the induction of Toll-regulated antimicrobial peptide genes (Figure 5I-5L). We noted that again there was an enhanced expression of SPE in tubGal4-dvps35RNAi (compared to tubGal4, Figure 5K), suggesting a positive feedback regulation of the SPE gene after activation of Toll (Jang et al., 2006; Lemaitre et al., 1996). These data demonstrate that SPE is not required for the activation of the Toll pathway in the absence of dvps35 activity in the absence of infection. Collectively, the above data indicate that retromer regulates Spz independently of the upstream proteolytic cascade.
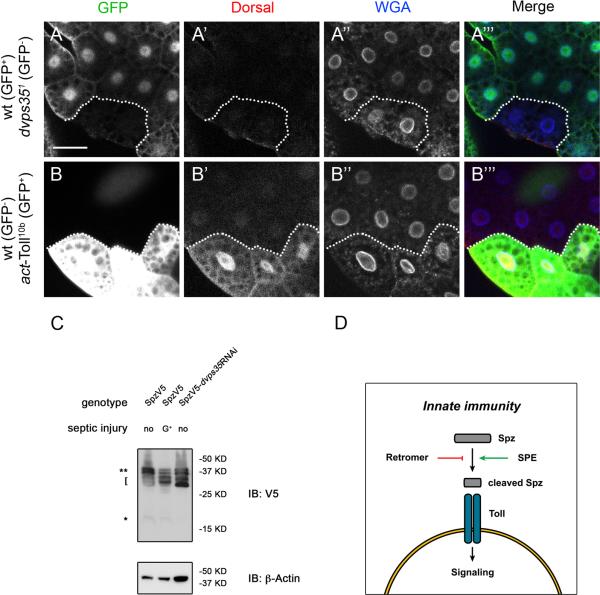
It is somewhat surprising that the epistasis analysis placed retromer function at the ligand level because the most logical target for retromer is the transmembrane protein Toll. To further investigate if retromer acts at the receptor level or above, we tested whether deleting dvps35 could activate the Toll pathway in the same cells. If retromer regulates the receptor, one would predict a cell autonomous activation of the Toll pathway. We generated homozygous mutant cell clones of dvps35 in fat bodies, marked by the absence of GFP. In these mutant cells, we were not able to observe a nuclear localization of Dorsal (Figure 6A). In a parallel experiment where we induced flip-out clones to allow the expression of a gain-of-function Toll (Toll10b, marked by GFP expression), there was a clear nuclear accumulation of Dorsal (Figure 6B). These results suggest that dvps35 is not required cell autonomously to regulate the Toll pathway activity. Instead, dvps35 may regulate a step upstream of the receptor Toll.
Figure 6. Retromer regulates the maturation/processing of the ligand Spz.
(A-A’’’) Staining of GFP, Dorsal and nuclear membrane (WGA) in larval fat bodies containing mosaic clones of dvps35 mutant cells. dvps35 mutant cells are marked by absence of GFP. (B-B’’’) Staining of GFP, Dorsal and nuclear membrane (WGA) in larval fat bodies containing flip-out clones of cells expressing Toll10b protein. Ectopic expressing cells are marked by GFP. Scale bar in A and B, 50μm. (C) Immunoblotting of V5 and β-Actin in third instar larvae of tubGal4-SpzV5 (no infection), tubGal4-SpzV5 (infection with Gram+ bacteria) and tubGal4-SpzV5-dvps35RNAi (no infection). Double asterisks, full length Spz. Bracket, two-band pattern around the 30 kD range of cleaved Spz. Asterisk, 16 kD C-terminal fragment of Spz. (D) A model illustrating retromer suppresses the activation of Spz independently of SPE.
Taken together, the genetic epistasis suggests that retromer regulates a component between Toll and SPE. A logical speculation is that retromer regulates Spz, thereby modulating the Toll pathway activity. To test this idea, we monitored the processing/maturation of Spz, by using a transgenic construct that contains the V5 epitope tag. Spz maturation is probably a multi-step process (Weber et al., 2007), but the final cleaved product is a 16 kD C-terminal fragment of 106 amino acids that binds to Toll (Hu et al., 2004; Lemaitre and Hoffmann, 2007; Weber et al., 2003). Nonetheless, multiple processed forms with sizes smaller than the full-length 37 kD precursor but larger than the 16 kD final product were previously observed during both early embryogenesis and infections correlating with the activation of the Toll pathway (Levashina et al., 1999; Morisato and Anderson, 1994). Here we observed a similar cleavage pattern of Spz during infections and in tubGal4-dvps35RNAi. In larvae with Gram+ bacterial infections, which activate the Toll pathway, Spz was mainly cleaved into two bands migrating around 30 kD (indicated by the bracket in Figure 6C). The putative active form of Spz (16 kD, indicated by an * in Figure 6C) was present at a very low level in the control and was not substantially enhanced after Gram+ bacterial infection. The similar two-band pattern around the 30 kD range was also seen in tubGal4-dvps35RNAi larvae, with no detectable 16 kD form of Spz. The very low or undetectable level of the 16 kD form of Spz observed during infections or in tubGal4-dvps35RNAi is possibly due to a rapid degradation when in complex with Toll. The two bands of Spz at 30 kD might represent more stable forms during Spz processing/maturation. Based on these observations, we propose that in the absence of dvps35 activity, Spz undergoes protein cleavage similar to that occurs during infections. Therefore, retromer may play a role in restricting the processing of Spz into the mature ligand prior to infection.
DISCUSSION
Here we have identified a role of retromer in negatively regulating the Toll pathway to maintain immune quiescence. In the absence of infection, the loss of retromer activity alone is capable of activating the Toll pathway and launching both the cellular and humoral immune responses. Furthermore, genetic epistasis and mosaic analysis suggest that retromer acts upstream of Toll and downstream of SPE, and we uncovered a retromer function in restricting the processing/maturation of Spz. In summary, retromer plays a critical role in suppressing the auto-activation of the innate immune system through Spz in the Toll pathway in Drosophila.
Based on previous knowledge that retromer regulates trafficking of transmembrane proteins, one can envisage that retromer normally may regulate the Toll pathway in one of the four following ways: (1) in Toll-responsive cells to transport the Toll-Spz complex for destruction; (2) in Spz secreting cells to suppress the release of active Spz; (3) in certain cells to assist the clearance of active Spz in the hemolymph; or (4) in certain cells to repress Spz through an indirect effect of other yet to be indentified components. In the dvps35 mutant clonal cells in fat bodies we did not observe increased Dorsal nuclear localization, indicating that retromer is not simply regulating the Toll pathway cell-autonomously. Our epistasis analysis suggests that retromer acts between Toll and SPE. Even though Spz is the only known component in between, there can be many other proteins that regulate the processing, maturation, trafficking or degradation of Spz in normal flies in order to restrict the activity of the Toll signaling pathway prior to infections. The full mechanism of Spz maturation is not yet unveiled and the retromer function in this process requires further investigation. Although we cannot exclude the possibility that retromer has an indirect effect on Spz, we favor a function of retromer in anti-release and/or clearance of active Spz (Figure 6D). Retromer has been shown to target transmembrane proteins. It will be intriguing to identify the transmembrane target of retromer in the context of regulating Spz and explore the mechanism of how this transmembrane target suppresses the release and/or assists the clearance of active Spz. Equally important is to examine whether modulating retromer-dependent Spz maturation is part of the activation mechanism of the Toll pathway during infections.
ACKNOWLEDGEMENTS
We thank Dr. K.V. Anderson, the Bloomington Stock Center and VDRC for Drosophila stocks; Dr. S. Hou and the Iowa Developmental Studies Hybridoma Bank (DSHB) for antibodies; Q. Tao, S. Cha, C. Liu and Y. Feng for technical assistance; Drs. T. Belenkaya and S. Wasserman for comments on the manuscript. This work was supported by a grant from NIH (R01DK83450) to Y.T.I., NIH grants (2R01 GM063891 and 1R01GM087517) to X.L. as well as grants from the National Basic Research Program of China (2011CB943901) and National Natural Science Foundation of China (31101036) to X.L.. Y.T.I. is a member of the UMass DERC (DK32520), a member of the UMass CCTS (UL1TR000161) and a member of the Guangdong Innovative Research Team Program (No. 201001Y0104789252).
Footnotes
AUTHOR CONTRIBUTION
B. Zhou, Y.T. Ip and X. Lin designed the experiments. B. Zhou, E.Y. Yun, L. Ray and J. You performed the experiments. B. Zhou, Y.T. Ip and X. Lin wrote the manuscript.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- Belenkaya TY, Wu Y, Tang X, Zhou B, Cheng L, Sharma YV, Yan D, Selva EM, Lin X. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell. 2008;14(1):120–131. doi: 10.1016/j.devcel.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Belvin MP, Jin Y, Anderson KV. Cactus protein degradation mediates Drosophila dorsal-ventral signaling. Genes Dev. 1995;9(7):783–793. doi: 10.1101/gad.9.7.783. [DOI] [PubMed] [Google Scholar]
- Cha SW, Tadjuidje E, Wylie C, Heasman J. The roles of maternal Vangl2 and aPKC in Xenopus oocyte and embryo patterning. Development. 2011;138(18):3989–4000. doi: 10.1242/dev.068866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Xiao H, Zhang K, Wang B, Gao Z, Jian Y, Qi X, Sun J, Miao L, Yang C. Retromer is required for apoptotic cell clearance by phagocytic receptor recycling. Science. 2010;327(5970):1261–1264. doi: 10.1126/science.1184840. [DOI] [PubMed] [Google Scholar]
- Collins BM. The structure and function of the retromer protein complex. Traffic. 2008;9(11):1811–1822. doi: 10.1111/j.1600-0854.2008.00777.x. [DOI] [PubMed] [Google Scholar]
- Cullen PJ, Korswagen HC. Sorting nexins provide diversity for retromer-dependent trafficking events. Nat Cell Biol. 2012;14(1):29–37. doi: 10.1038/ncb2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448(7150):151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Franch-Marro X, Wendler F, Guidato S, Griffith J, Baena-Lopez A, Itasaki N, Maurice MM, Vincent JP. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol. 2008;10(2):170–177. doi: 10.1038/ncb1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DA, Binari R, Nahreini TS, Gilman M, Perrimon N. Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 1995;14(12):2857–2865. doi: 10.1002/j.1460-2075.1995.tb07285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Yagi Y, Tanji T, Zhou S, Ip YT. Multimerization and interaction of Toll and Spatzle in Drosophila. Proc Natl Acad Sci U S A. 2004;101(25):9369–9374. doi: 10.1073/pnas.0307062101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Ohsako S, Tanda S. The lesswright mutation activates Rel-related proteins, leading to overproduction of larval hemocytes in Drosophila melanogaster. Dev Biol. 2005;280(2):407–420. doi: 10.1016/j.ydbio.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Ip YT, Reach M, Engstrom Y, Kadalayil L, Cai H, Gonzalez-Crespo S, Tatei K, Levine M. Dif, a dorsal-related gene that mediates an immune response in Drosophila. Cell. 1993;75(4):753–763. doi: 10.1016/0092-8674(93)90495-c. [DOI] [PubMed] [Google Scholar]
- Jang IH, Chosa N, Kim SH, Nam HJ, Lemaitre B, Ochiai M, Kambris Z, Brun S, Hashimoto C, Ashida M, Brey PT, Lee WJ. A Spatzle-processing enzyme required for toll signaling activation in Drosophila innate immunity. Dev Cell. 2006;10(1):45–55. doi: 10.1016/j.devcel.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Kondo T, Kawai T, Akira S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 2012;33(9):449–458. doi: 10.1016/j.it.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Lanot R, Zachary D, Holder F, Meister M. Postembryonic hematopoiesis in Drosophila. Dev Biol. 2001;230(2):243–257. doi: 10.1006/dbio.2000.0123. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annual review of immunology. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86(6):973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Levashina EA, Langley E, Green C, Gubb D, Ashburner M, Hoffmann JA, Reichhart JM. Constitutive activation of toll-mediated antifungal defense in serpin-deficient Drosophila. Science. 1999;285(5435):1917–1919. doi: 10.1126/science.285.5435.1917. [DOI] [PubMed] [Google Scholar]
- Luo H, Hanratty WP, Dearolf CR. An amino acid substitution in the Drosophila hopTum-l Jak kinase causes leukemia-like hematopoietic defects. EMBO J. 1995;14(7):1412–1420. doi: 10.1002/j.1460-2075.1995.tb07127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Khanuja BS, Ip YT. Toll receptor-mediated Drosophila immune response requires Dif, an NF-kappaB factor. Genes Dev. 1999;13(7):792–797. doi: 10.1101/gad.13.7.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakhina S, Steward R. Melanotic mutants in Drosophila: pathways and phenotypes. Genetics. 2006;174(1):253–263. doi: 10.1534/genetics.106.061978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisato D, Anderson KV. The spatzle gene encodes a component of the extracellular signaling pathway establishing the dorsal-ventral pattern of the Drosophila embryo. Cell. 1994;76(4):677–688. doi: 10.1016/0092-8674(94)90507-x. [DOI] [PubMed] [Google Scholar]
- Pocha SM, Wassmer T, Niehage C, Hoflack B, Knust E. Retromer controls epithelial cell polarity by trafficking the apical determinant Crumbs. Curr Biol. 2011;21(13):1111–1117. doi: 10.1016/j.cub.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Port F, Kuster M, Herr P, Furger E, Banziger C, Hausmann G, Basler K. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol. 2008;10(2):178–185. doi: 10.1038/ncb1687. [DOI] [PubMed] [Google Scholar]
- Qiu P, Pan PC, Govind S. A role for the Drosophila Toll/Cactus pathway in larval hematopoiesis. Development. 1998;125(10):1909–1920. doi: 10.1242/dev.125.10.1909. [DOI] [PubMed] [Google Scholar]
- Rutschmann S, Jung AC, Hetru C, Reichhart JM, Hoffmann JA, Ferrandon D. The Rel protein DIF mediates the antifungal but not the antibacterial host defense in Drosophila. Immunity. 2000;12(5):569–580. doi: 10.1016/s1074-7613(00)80208-3. [DOI] [PubMed] [Google Scholar]
- Sackton TB, Lazzaro BP, Clark AG. Genotype and gene expression associations with immune function in Drosophila. PLoS Genet. 2010;6(1):e1000797. doi: 10.1371/journal.pgen.1000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RC, Juhasz G, Neufeld TP. Direct induction of autophagy by Atg1 inhibits cell growth and induces apoptotic cell death. Curr Biol. 2007;17(1):1–11. doi: 10.1016/j.cub.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SR, Liu W, Hou SX. The adult Drosophila malpighian tubules are maintained by multipotent stem cells. Cell Stem Cell. 2007;1(2):191–203. doi: 10.1016/j.stem.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji T, Yun EY, Ip YT. Heterodimers of NF-kappaB transcription factors DIF and Relish regulate antimicrobial peptide genes in Drosophila. Proc Natl Acad Sci U S A. 2010;107(33):14715–14720. doi: 10.1073/pnas.1009473107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber AN, Gangloff M, Moncrieffe MC, Hyvert Y, Imler JL, Gay NJ. Role of the Spatzle Pro-domain in the generation of an active toll receptor ligand. J Biol Chem. 2007;282(18):13522–13531. doi: 10.1074/jbc.M700068200. [DOI] [PubMed] [Google Scholar]
- Weber AN, Tauszig-Delamasure S, Hoffmann JA, Lelievre E, Gascan H, Ray KP, Morse MA, Imler JL, Gay NJ. Binding of the Drosophila cytokine Spatzle to Toll is direct and establishes signaling. Nat Immunol. 2003;4(8):794–800. doi: 10.1038/ni955. [DOI] [PubMed] [Google Scholar]
- Zhang P, Wu Y, Belenkaya TY, Lin X. SNX3 controls Wingless/Wnt secretion through regulating retromer-dependent recycling of Wntless. Cell Res. 2011;21(12):1677–1690. doi: 10.1038/cr.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Wu Y, Lin X. Retromer regulates apical-basal polarity through recycling Crumbs. Dev Biol. 2011;360(1):87–95. doi: 10.1016/j.ydbio.2011.09.009. [DOI] [PubMed] [Google Scholar]