Abstract
Despite successful molecularly targeted, highly specific, therapies for hematologic malignancies, the DNA interstrand crosslinking agents, which are among the oldest and least specific cytotoxic drugs, still have an important role. This is particularly true in stem cell transplantation, where virtually every patient receives conditioning therapy with a DNA-alkylating agent-based program. However, due to concern about serious additive toxicities with combinations of different alkylating drugs, the last several years have seen nucleoside analogs, whose cytotoxic action follows vastly different molecular pathways, introduced in combination with alkylating agents. The mechanistic differences paired with different metabolic pathways for the respective drugs have clinically translated into increased safety without appreciable loss of antileukemic activity. In this report, we review pre-clinical evidence for synergistic antileukemic activity when nucleoside analog(s) and DNA-alkylating agent(s) are combined in the most appropriate manner(s), without a measurable decrease in clinical efficacy compared with the more established alkylating agent combinations. Data from our own laboratory using combinations of fludarabine, clofarabine, and busulfan as prototype representatives for these respective classes of cytotoxic agents are combined with information from other investigators to explain how the observed molecular events will result in greatly enhanced synergistic cytotoxicity. We further present possible mechanistic pathways for such desirable cytotoxic synergism. Finally, we propose how this information-backed hypothesis can be incorporated in the design of the next generation conditioning therapy programs in stem cell transplantation to optimize antileukemic efficacy while still safeguarding patient safety.
Keywords: interstrand crosslink, nucleoside analogs, stem cell transplant, hematologic malignancies, pretransplant conditioning therapy
INTRODUCTION
Hematopoiesis consists of cascades of events from the pluripotent hematopoietic stem cell to various mature and terminally differentiated blood cells. Miscues or inappropriate cell signaling (e.g., DNA mutations, cytokine and growth factor imbalance, hormone imbalance, etc.) to these complex pathways may perturb and inhibit normal cellular differentiation, lead to abnormal accumulation of specific lineage progenitor cells, and eventually cause leukemia, lymphoma or myeloma. Because of the complexity of hematopoiesis such abnormal development may theoretically lead to various disease forms and also partly explains the challenges involved in finding appropriate therapy for these heterogeneous malignancies.
Treatments for hematologic malignancies include cytotoxic chemotherapy, radiation therapy, immunotherapy, use of recombinant proteins or monoclonal antibodies, and stem cell transplantation (SCT); these treatment plans can be used singly or more commonly in various combinations. The development of molecularly targeted therapies heralds a revolution in the application of novel treatment plans for increased efficacy while toxicity is decreasing. However, while such treatment specifically aims at a narrow target, this approach suffers the weakness that it aims at a very specific focus. A prototype example is the tyrosine kinase inhibitor imatinib, which competes at the binding site for tyrosine kinase on myeloid progenitor cells; a point mutation in this binding site may confer three-dimensional alterations that render the target cell resistant to imatinib (Quintás-Cardama et al., 2009). In contrast, and as a back-up strategy to narrowly targeted molecular therapy the more traditional (chemo)-therapeutic agents, like bifunctional DNA-alkylating agents and ionizing radiation (XRT), target a larger fraction of the DNA. By design they have a lower specificity in their cytotoxic action and possess lower risk for development of clinically significant cellular resistance to the particular treatment modality. Unfortunately, there is a risk for clinically significant side effects since normal tissues are also affected to a higher extent. Based partly on a low likelihood of significant cellular resistance development, both alkylating agents and XRT for a long time have been used in pretransplant conditioning therapy in patients undergoing hematopoietic stem cell transplantation (HSCT) to eradicate most of the disease and provide the immunosuppression necessary to support engraftment of allogeneic cells.
The success of HSCT, especially in patients with acute myeloid leukemia (AML), is partly dependent on the dose-intensity of the pretransplant conditioning therapy. This is, in particular, evident in patients transplanted with active leukemia, where it is clear that less intensive conditioning therapy carries with it a higher risk for recurrent disease (de Lima et al., 2004; Shimoni et al., 2006). The benefit of dose-intensity has to be weighed against the risk for serious regimen-related complications and high treatment related mortality (TRM) encountered with the most intensive conditioning programs, especially in older patients. It is a commonly held belief that reducing the intensity of the conditioning program carries the benefit of increased safety, and less treatment-related toxicity, but the risk for recurrent disease post-transplant increases proportionally. In particular, in older and medically infirm patients with low leukemic cell burden (“complete remission” status), it is now being increasingly accepted to favor less intensive conditioning programs to increase the acute safety of the conditioning program (Blaise et al., 2007; Mohty et al., 2009). In view of the increased risk for recurrent disease, however, it is critical to consider factors such as disease stage, patients’ age and health conditions, and under specific conditions a reduced intensity programs may be an acceptable alternative to myeloablative conditioning therapy (Forman, 2009; Giralt, 2009; Sirvent et al., 2010). The serious adverse effects of aggressive myeloablative conditioning regimens underscore the importance of fine-tuning the use of already available agents, for example, using pharmacokinetically directed drug administration to accomplish a uniform systemic drug exposure between patients (Grochow et al., 1993; Slattery et al., 1995; Andersson et al., 2002a), but it also underscores the importance of identifying safe, yet effective new drugs that can safely be added to enhance the cytotoxic efficacy of the treatment program. Historically, the serious toxicities associated with multi-organ damage from total body irradiation (TBI) prompted the development of non-TBI regimens. One of the early replacements for TBI was the use of the bifunctional DNA alkylating agent busulfan (Bu) (Santos et al., 1983; Tutschka et al., 1987). Oral Bu was combined with cyclophosphamide (Cy) to compensate for the limited immunosuppressive activity of Bu, and several randomized studies showed that conditioning with TBI-Cy and Bu-Cy are equivalent (Blaise et al., 1992; Blume et al., 1993; Clift et al., 1994; Ringden et al., 1994; Devergie et al., 1995; Hartman et al., 1998; Socie et al., 2001). It became increasingly apparent, however, that a 25–40% risk for early treatment-related mortality was unacceptable, and this led to two significant developments. First, an intravenous Bu (IV Bu) formulation was developed for high-dose pretransplant conditioning therapy (Bhagwatwar et al., 1996; Andersson et al., 2000; Andersson et al., 2002b). This increased the accuracy, precision, and overall safety of drug delivery because of complete dose assurance and predictable pharmacokinetics as well as avoidance of the hepatic first-pass effect (Benet and Sheiner, 1985; Peters et al., 1987; Kashyap et al., 2002; Thall et al., 2004). Second, it was recognized that both Bu and Cy are detoxified by glutathione (GSH) conjugation when metabolized (Gibbs et al., 1998; McDonald et al., 2003). This latter recognition, together with the realization that the nucleoside analog (NA) fludarabine (Flu) is at least as immunosuppressive as Cy (Terenzi et al., 1996), led to the design of nucleoside analog-DNA alkylating agent combinations for use in conditioning therapy, both reduced-intensity regimens (Giralt et al., 2001; Alyea et al., 2005; Khouri et al., 2008) and myeloablative regimens such as the IV Bu-Flu variant regimens (Russell et al., 2002; de Lima et al., 2004). The potent immunosuppressive activity of Flu combined with its ability to inhibit DNA repair processes initiated by Bu was very effective in conditioning therapy for patients with advanced hematologic malignancies (Slavin et al., 1998; Russell et al., 2002), and disease-specific studies in AML/ MDS and acute lymphoblastic leukemia (ALL) have confirmed these findings (de Lima et al., 2004; Russell et al., 2007; Andersson et al., 2008). Much of the improved outcome after allogeneic SCT for AML/MDS after NA–Bu-based conditioning therapy has been attributed to the dramatically improved safety of these “reduced-toxicity” conditioning programs (de Lima et al., 2004; Russell et al., 2007; Andersson et al., 2008). Although we found that the cytoreductive capability of Bu-Flu and Bu-Cy were equivalent in AML/MDS (Andersson et al., 2008), other investigators disagree with that notion and have expressed concern that replacing Cy with Flu in patients undergoing HSCT for a variety of advanced hematologic malignancies may result in a significant loss of cytoreductive capacity (Shimoni et al., 2006; Bredeson et al., 2008). It is quite possible, that inclusion of anti-thymocyte globulin (ATG) with Flu, and mixing in patients with both myeloid and lymphoid malignancies contribute to this difference of opinion (Shimoni et al., 2006; Bredeson et al., 2008). Overall, the conceptual introduction of NA–DNA alkylating agent combinations has greatly contributed to improved safety and disease control with faster recovery from early regimen- and treatment-related adverse effects. However, patients who are transplanted with active disease still have a similar high risk for recurrent disease as those who are transplanted using conditioning with Bu-Cy or TBI-based regimens (Russell et al., 2002; de Lima et al., 2004; Shimoni et al., 2006). Therefore, although encouraging, the clinical results obtained with NA–Bu-based conditioning therapy still call for ways to fine-tune and optimize the use of already existing NA–DNA alkylating agent regimen(s) and they also mandate a careful search for more efficacious nucleoside analogs and DNA alkylating agents that can be included in the pretransplant conditioning strategy. This review now focuses on DNA interstrand crosslinking drugs and nucleoside analogs that can be incorporated in pretransplant conditioning therapy for (allogeneic) HSCT of hematologic malignancies. We will base our discussion around a more detailed understanding of the cellular events that are triggered by cellular exposure to bifunctional DNA-alkylating agents, using Bu as a prototype agent, and nucleoside analogs and the importance of incorporating such increased knowledge of cellular molecular events to optimize future design of pretransplant conditioning regimens.
DNA ALKYLATING AGENTS USED IN PRETRANSPLANT CONDITIONING THERAPY OF HEMATOLOGIC MALIGNANCIES
DNA alkylating drugs mediate their cytotoxicity by damaging the DNA through formation of covalent linkages between the alkyl groups and mainly the N-7 position of guanine. The N-3 position of cytidine and O-6 of guanine also serve as nucleophiles (Hall and Tilby, 1992). These covalent modifications may lead to inter- or intra-strand DNA crosslink formation, which affects the genomic integrity and causes deleterious consequences during DNA replication. The inability of the cells to accurately repair these DNA adducts may introduce mutations and/or trigger apoptosis.
The classes of DNA alkylating agents used in the treatment of hematologic malignancies include nitrogen mustards [chlorambucil, melphalan (Mel), cyclophosphamide, ifosfamide], alkyl alkane sulfonates (busulfan), and nitro-soureas (1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU), and cyclohexylchlorethylnitrosourea (CCNU). Among these drugs, Bu, Cy, and Mel are the most commonly used agents in conditioning regimens for allogeneic HSCT (for review see Ciurea and Andersson, 2009). Intensive treatment using high dosages of alkylating agents carries a significant risk for serious regimen- and procedure-related toxicities especially when treating older patients (>60 years). Numerous investigators are currently involved in defining an optimal recipe for the conditioning therapy that will safely deliver cytoreduction and immunosuppression while optimizing disease control (Blaise et al., 2007; Mohty et al., 2009).
One way to enhance the efficacy of preparative regimens is to combine slightly reduced doses of different DNA alkylators (e.g., Bu-Cy or Bu-Mel) or to combine a DNA alkylator with one or more nucleoside analog. The latter variant combinations are rapidly gaining popularity in terms of efficacy and reduced-toxicity. For example, a combination of IV Bu and Flu compared favorably with [IV Bu+Cy] as conditioning therapy for patients undergoing allogeneic HSCT for AML or MDS (de Lima et al., 2004; Andersson et al., 2008). Most importantly, these investigators reported a significantly decreased early TRM, likely due to the avoidance of competition for end-organ GSH in the detoxification of Bu and Cy (Gibbs et al., 1998; MacDonald et al., 2003). Further, they found no significant loss of antileukemic activity by replacing Cy with Flu, although the latter finding is still somewhat controversial, other investigators have reported less favorable disease control with Bu-Flu vs that of Bu-Cy2 (Shimoni et al., 2006; Bredeson et al., 2008).
To improve the cytoreductive efficacy without losing engraftment promoting capacity of Bu-Flu, we and others decided to combine the newer NA clofarabine (Clo) with IV Bu. The antileukemic activity of Clo makes this drug suitable for investigation in combination therapy with alkylating agent(s) (Faderl et al., 2005; Steinherz et al., 2007; Jeha et al., 2009; Thomas et al., 2009; Kantarjian et al., 2010). Clo ± Ara-C, plus idarubicin combinations have been clinically shown to be effective salvage therapy for patient’s with primary refractory or first-relapse AML (Faderl et al., 2008). An early multicenter study showed that a combination of Clo with etoposide and Cy was well tolerated and effective in pediatric patients with relapsed/refractory AML or ALL (Hijiya et al., 2009). Current studies are underway to explore the efficacy of Clo combined with DNA alkylating agent(s) in the HSCT setting for patients with advanced hematologic malignancies (Mineishi et al., 2008; Agura et al., 2010; Andersson et al., 2010).
NUCLEOSIDE ANALOGS IN THE TREATMENT OF HEMATOLOGIC MALIGNANCIES
The dependence of leukemia cells on salvage synthesis of nucleosides provides rationale for design of nucleoside analogs as anti-leukemia agents (Fox et al., 1991). In general, these drugs are taken up by the cells via nucleoside transporters, activated by phosphorylation (Arner and Eriksson, 1995), and indiscriminately used by polymerases in the synthesis of DNA and RNA (Parker et al., 1991; Iwasaki et al., 1997; Huang et al., 2000; Chen et al., 2008). The activated analogs may inhibit other enzymes involved in de novo and salvage nucleoside synthesis or induce intrinsic apoptosis (Zhenchuk et al., 2009).
The first nucleoside analogs used to treat hematologic malignancies include cytosine arabinoside (cytarabine, Ara-C), 9-β-D-arabinofuranosyladenine (ara-A), 2’-deoxy-2’,2’-difluorocytidine (gemcitabine), 5-fluorouracil (5FU), fluoroadenine-β-D-arabinoside (fludarabine), and 2-chloro-deoxyadenosine (cladribine). The poor chemical stability and/or low aqueous solubility of these analogs led to the subsequent design and clinical testing of 2-chloro-2’arabino-fluoro-2’ deoxyadenosine (Clo), one of the newest and most efficacious nucleoside analogs in the treatment of leukemia. Clo is cytotoxic to both proliferating and non-proliferating cells. It is resistant to phosphorylitic cleavage and stable under acidic conditions. Clo received regulatory approval by the US Food and Drug Administration for treatment of pediatric leukemia in 2004 (Clolar™, Genzyme, Boston, MA), after extensive clinical phase I and II studies showing high antileukemic efficacy in both ALL and AML (Kantarjian et al., 2003; Jeha et al., 2004; Faderl et al., 2008).
The incorporation of nucleoside analogs into DNA during synthesis may cause termination of DNA-strand elongation and trigger a chain of events including the occurrence of DNA breaks when cells attempt to repair damaged DNA, and subsequent activation of pro-apoptotic pathway(s) (Huang et al., 1990; Iwasaki et al., 1997). Repair of damaged DNA further facilitates the incorporation of nucleoside analogs, propagates DNA breaks, and enhances their cytotoxic effects. The anti-proliferative mechanisms mediated by nucleoside analogs have triggered interest in studying their cellular mechanistic interactions with alkylating agents and XRT, and the resulting data have rationalized their therapeutic use in combination with alkylating agents. These interactions may, somewhat paradoxically, be of special interest in alkylator-resistant leukemia cells that have an inherent increased capacity for excision repair. Such mechanism-based investigations have demonstrated sequence specificity for exposure to the respective drugs (Gandhi and Plunkett, 2002; Valdez et al., 2010a), and show that synergistic cytotoxicity requires cellular pre-exposure to the nucleoside analog(s) (Flu and Clo) with extensive cellular accumulation of the respective nucleoside analog triphophate(s) to optimally inhibit repair of the DNA-damage which is subsequently initiated by the alkylating agents (Yamaguchi et al., 2001; Valdez et al., 2010a).
STUDIES USING COMBINATIONS OF DNA ALKYLATORS AND NUCLEOSIDE ANALOGS
Once taken up by the target cells, nucleoside analogs are phosphorylated by deoxycytidine kinase (DCK) to their respective activated moiety, the nucleoside analog triphosphate. The high enzymatic activity of DCK in lymphoid cells contributes to the more significant efficacy of nucleoside analog substrates in lymphoid malignancies as compared with tumors of myeloid origin, although early data achieved with Clo in AML now promise to renew the interest in using nucleoside analog combinations in myeloid malignancies (Faderl et al., 2008). Although the complete mechanisms for how nucleoside analogs mediate their antineoplastic activity are not entirely known, available data suggest a pronounced synergistic efficacy when they are used in combination with DNA alkylating agents. One in vivo study showed increased anti-leukemia activity of BCNU in mice when coadministered with 2’-deoxyuridine, 2’-deoxycytidine, or thymidine (Lin and Prusoff, 1987). Further, adozelesin (Ado), a DNA alkylating agent, showed enhanced cytotoxicity on DLD-1 human colon carcinoma cells when combined with Ara-C (Cote and Momparler, 1993). Chlorambucil had synergistic cytotoxicity in explanted leukemic cells from CLL patients when combined with [2’-deoxycoformycin + deoxyadenosine] in vitro (Johnston et al., 1994). Several different groups have used combinations of nucleoside analogs and alkylating agents in a conventional dosing range. This is exemplified by Keating et al. who demonstrated high antileukemic activity in patients with CLL that was clinically refractory to Flu alone (Keating et al., 2002; Tam et al., 2008; Tsimberidou et al., 2009). A phase I/II study by the Eastern Cooperative Oncology Group (ECOG) similarly suggests efficacy of pentostatin and chlorambucil in treating patients with B-cell chronic lymphocytic leukemia (B-CLL, Oken et al., 2004). Combinations of pentostatin, a purine nucleoside analog, with the DNA alkylator chlorambucil showed activity in B-CLL patients, but because of the intense immunosuppression experienced with this combination the drugs should be used with anti-bacterial and anti-viral phorphylaxis to reduce the risk for opportunistic infections. The authors obtained an impressive overall objective response rate of 87% with median response duration of 33 months and a median survival of 5 years (Oken et al., 2004).
The immunosuppressive effects of Flu are at least as pronounced as those of Cy (Terenzi et al., 1996). In addition, the lack of effects of GSH in Flu metabolism, its long plasma half-life, and its synergistic cytotoxic interaction with alkylators invited the use of Flu-DNA alkylating agent combinations in pretransplant conditioning therapy for both lymphoid and myeloid malignancies (Slavin et al., 1998; Giralt et al., 2001; Hamaki et al., 2004; Alyea et al., 2005; Blaise et al., 2007; Khouri et al., 2008), showing that this concept can be used not only with different alkylating agents but also at different dosing intensity levels, varying from virtually conventional dosing to fully myeloablative dose levels of the respective alkylating drug (Russell et al., 2002; de Lima et al., 2004; Andersson et al., 2008). In these different programs Flu has typically been limited to an approximately conventional dose range (25–50 mg/m2 daily for 4–5 days), comparing with its use as a single agent. The overall intensity of the conditioning regimen is typically modulated by grading the dose of the alkylator from an almost conventional dose range (Alyea et al., 2005; Khouri et al., 2008) to the supralethal range (Russell et al., 2002, 2007; de Lima et al., 2004; Andersson et al., 2008). Interestingly, the TRM rate, and therefore the acute safety of the conditioning regimen, appears to be related at least as much to the choice of alkylator companion drug as to the overall intensity of the regimen. Thus, the use of the Mel-Flu reduced-intensity regimen in patients with active leukemia yielded a 100-day TRM of almost 40% (Giralt et al., 2001), while the corresponding experience with the fully ablative IV Bu-Flu regimen is in the order of 3% (Russell et al., 2002; de Lima et al., 2004; Russell et al., 2007; Andersson et al., 2008). The latter experience of a very low 100-day TRM rate was mirrored by Khouri et al. (2008), who used a reduced-intensity Flu-Cy combination in patients with follicular lymphoma.
As interference with cellular DNA-repair processes might possibly be conducive to mutagenic activity, one possible long-term risk of NA–DNA alkylator combination therapy is induction of therapy-related AML/MDS. For example, in a clinical study of 142 patients with CLL, 3.5% of patients developed therapy-related MDS or AML after treatment with Flu plus chlorambucil (Morrison et al., 2002). Similarly, in a group of 38 patients with various malignancies (15 CLL, 4 Waldenstro¨m’s macroglobulinemia, 6 mantle cell lymphoma, 10 follicular non-Hodgkin’s lymphoma, and 3 with other low-grade NHL) who received treatment with 2-chloro-2’-deoxyadenosine and Cy, three subjects developed AML/MDS, and two developed lung cancer (Van Den Neste et al., 2004). It is unclear, however, if the risk for secondary AML/MDS is higher than the historical experience with alkylating agents alone, but the reported experience highlights that treating physicians should be aware of this type of complication when treating patients with combinations of NA and DNA-alkylating agents. When used in pretransplant conditioning therapy the NA–DNA alkylating agent regimens have not been used for sufficiently long time to substantiate if the relative risk for secondary malignancies is higher than or only similar to that seen with double DNA alkylating agent combinations, such as Bu-Cy or Bu-Mel, still lower than TBI-Cy (Socie et al., 2000; Baker et al., 2003; Brown et al., 2005). What further complicates this issue is that any alkylating agents that result in mutagenic events may lead to secondary neoplasms. In long-term follow-up studies of patients receiving autologous stem cell transplants for Non-Hodgkin’s lymphomas the risk for secondary AML/MDS appears related to the cumulative amount of previous chemotherapy. In a multivariate analysis previous exposure to Flu and inclusion of TBI in the pretransplant conditioning regimen were independently connected with an increased risk for later development of AML/MDS (Hosing et al., 2002).
USE OF NUCLEOSIDE ANALOGS TO SENSITIZE LEUKEMIA CELLS RESISTANT TO DNA ALKYLATING AGENTS
A major obstacle to successful pretransplant chemotherapy is inherent or drug-induced resistance to DNA alkylating agents when used as part of the treatment program. Cellular drug resistance may be mediated by genetic mutations or epigenetic mechanisms (Nyce, 1997). Gene therapy could be used to correct genetic defects while certain drugs can be used to alter the epigenetic status of cancer cells. Epigenetic changes contribute to drug resistance development through transcriptional suppression of genes involved in pro-drug activation, cell cycle checkpoint control, apoptosis, and other cellular processes (Herman and Baylin, 2003; Teodoridis et al., 2004). Alteration of the enzymatic activities of DNA methyl-transferases, histone deacetylases, and histone methyl-transferases may reverse gene silencing by chromatin remodeling (Teodoridis et al., 2004; Yoo and Jones, 2006). 5-Azacytidine and 5-aza-2’-deoxycitidine are two cytidine analogs currently used to treat leukemia. Both drugs are incorporated into DNA, inhibit DNA methyl-transferase and cause expression of tumor suppression and pro-apoptotic genes (Zhu et al., 2001; Schmelz et al., 2005). Decitabine received regulatory approval for the treatment of MDS including chronic myelomonocytic leukemia in the United States in 2006. 5-Azacytidine similarly received US regulatory approval in 2007.
The combinations of 5-aza-2’-deoxycitidine with DNA intercalating agents result in additive or synergistic biological activities and possible reversal of clinical drug resistance (Plumb et al., 2000; Issa et al., 2004; Gore et al., 2005; Garcia-Manero et al., 2006; Jabbour et al., 2008). Recently, we reported our findings with decitabine as a sensitizing agent when exposing Bu-resistant myeloid leukemia cells in a p53-dependent manner by regulating expression of genes involved in cell cycle checkpoint and apoptosis (Valdez et al., 2010b). These data provide mechanistic support for a clinical study designed to explore the synergistic activity of decitabine and Bu in pretransplant conditioning therapy prior to HSCT for myeloid malignancies.
Purine analogs with documented anti-leukemia activitiy include fluoroadenine-β-D-arabinoside (Flu), 2-chloro-deoxyadenosine (cladribine), and 2-chloro-2’arabino-fluoro-2’deoxyadenosine (Clo) (Carson et al., 1992; Montgomery et al., 1992; Kantarjian et al., 2007; Korycka et al., 2008). Although similar in their chemical structures, these adenosine analogs differ in their mechanisms of action and efficacies in the clinic (Plunkett and Gandhi, 2001). Like other nucleoside analogs, these drugs are taken up by the cells and phosphorylated by deoxynucleo-tide kinases (Arner and Eriksson, 1995). In general, the incorporation of these nucleoside analogs into DNA during synthesis causes termination of DNA-strand elongation and triggers a chain of events including induction of DNA breaks, and subsequent activation of pro-apoptotic pathways (Huang et al., 1990; Iwasaki et al., 1997; Zhenchuk et al., 2009). Initiation of repair of damaged DNA facilitates further incorporation of nucleoside analog triphosphate, propagates DNA-strand breaks and consequently enhances the cytotoxic effects. This activity is also potentiated by the drug-mediated inhibition of ribonucleotide reductase, which leads to decreased deoxynucleotide pools with preferentially enhanced incorporation of the nucleoside analog triphosphates into growing DNA strands (Parker et al., 1991). A previous study demonstrated Clo and Flu to inhibit DNA repair initiated by 4-hydroperoxycyclophosphamide (4-HC) in lymphocytes from CLL patients (Yamaguchi et al., 2001). In a multicenter study it was shown that a combination of Clo with etoposide and Cy was well tolerated and effective in pediatric patients with relapsed/refractory AML or ALL (Hijiya et al., 2009). Taken together, these observations rationalize the use of mechanism-based therapies which combine nucleoside analogs and DNA alkylating agents for more effective cytotoxicity and possible circumvention or reversal of clinical alkylating agent resistance.
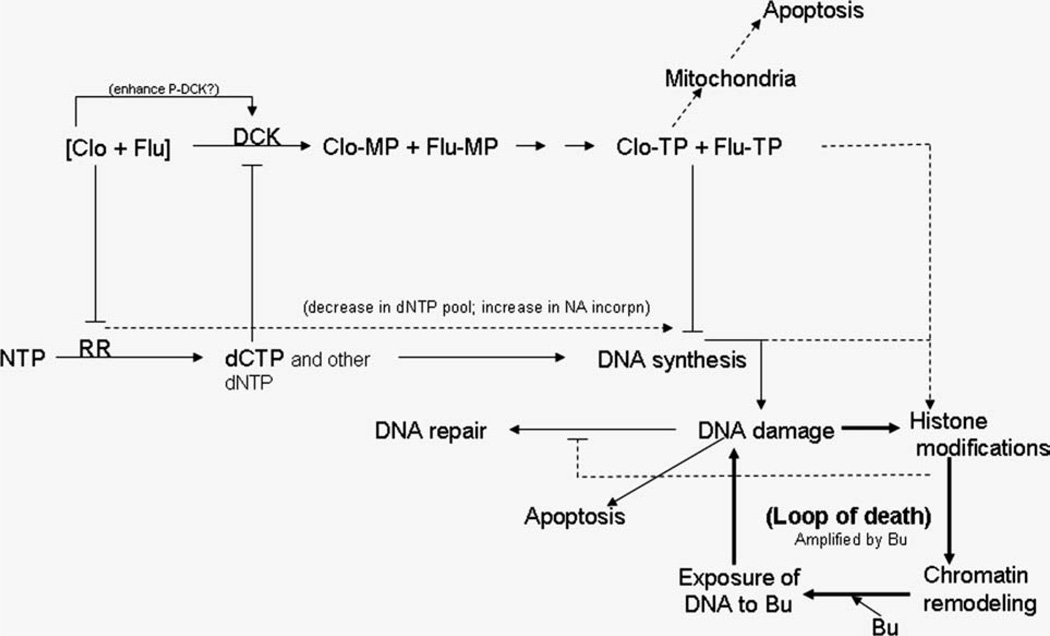
Studies in our laboratory have aimed at investigating a hypothesized synergistic cytotoxicity of [Clo + Flu + Bu] at relatively low concentrations, or the equivalence of the IC20 concentrations of the respective drug, using a Bu-resistant human AML cell line as the model (Valdez et al., 2008; Valdez et al., 2010a). We could show a pronounced cytotoxic synergy when Clo and Flu were combined and this synergy was further enhanced when Bu was subsequently added. Similar effects were observed in freshly explanted leukemia cells from AML patients. We attribute the efficacy and synergism of the three drugs to greater DNA damage response through the ATM pathway. Activation of ATM resulted in the phosphorylation of its substrates CHK2 and SMC1 and subsequent transduction of the signal that resulted in S- and G2-phase arrest and apoptosis. The [Clo + Flu] combination induced methylation of histone 3 and addition of Bu further enhanced this effect. We hypothesize that the [Clo + Flu] combination initially induced histone modifications, caused chromatin remodeling, exposing the genomic DNA, and made it sterically more accessible to Bu alkylation, which resulted in a more efficient Bu-induced DNA-strand crosslinking (Fig. 1). Such processes provide a positive feedback loop mechanism that synergistically potentiates the effects of all three drugs, but with strict sequence specificity, it is mandatory that the nucleoside analog exposure precedes the alkylating agent to achieve this effect, expanding the previous observation for Flu with 4-HC (Yamaguchi et al., 2001). This hypothesis is further supported by an observed susceptibility of DNA to exogenous nuclease in cells pre-exposed to [Clo + Flu] (Valdez et al., 2010a). On the basis of previous reports and our own unpublished results, the observed synergism may be attributed to combined inhibition of DNA synthesis and repair, cell cycle checkpoints, and changes in chromatin structure through histone 3 methylations (Fig. 1). These intriguing results have prompted us to investigate the efficacy of [Clo +- Flu + Bu] in pretransplant conditioning therapy for patients with relapsed and chemotherapy-refractory myeloid leukemia and MDS. Early results suggest improved event-free and overall survival of 41 mostly chemotherapy-refractory myeloid leukemia patients who received [Clo + Flu + Bu] as part of their pre-transplant regimen when compared with historical control patients receiving Flu-Bu alone (de Lima et al., 2004; Andersson et al., 2010).
Fig. 1.
Suggested mechanism of synergistic cytotoxicity of clofarabine (Clo), fludarabine (Flu), and busulfan (Bu). Clo and Flu are taken up by cells and phosphorylated by kinases to their triphosphate forms (Clo-TP and Flu-TP), which are incorporated into growing DNA strands during replication. Clo and Flu inhibit ribonucleotide reductase (RR) and cause a decrease in the dCTP pool. As dCTP is an inhibitor of deoxycytidine kinase (DCK), a decrease in its concentration may result in activation of DCK, preferential phosphorylation of Clo and Flu, and increased inclusion of these analogs into nascent DNA strands. The incorporation of nucleoside analogs into DNA strands triggers DNA damage responses including DNA repair and histone modifications. Modified histones cause chromatin remodeling and better accessibility of genomic DNA to Bu crosslinking, which propagates DNA damage responses and the cycle continues. The inability of cells to repair DNA damage triggers apoptosis.
CONCLUSIONS
Overall, previously available literature reports together with our present studies suggest: (1) nucleoside analogs can be successfully used to alter the epigenetic status of leukemia cells resulting in chromatin remodeling, thereby making genomic DNA more susceptible to DNA alkylating agents; (2) the inherent abilities of nucleoside analogs to inhibit DNA replication and DNA repair contribute to their antileukemic efficacy and cytotoxic synergism when combined with bifunctional DNA alkylating agents; (3) the enhanced synergy when combining two purine analogs with busulfan form the basis for combining not only one but two nucleoside analogs followed by a DNA alkylating agent; and (4) this approach should be expected to be most advantageous where the administered doses of both nucleoside analogs and the DNA alkylating agent can be escalated without concern for any irreversible damage to normal hematopoiesis, i.e., in pretransplant conditioning therapy, where hematopoietic recovery resides with the graft, independently of the resulting damage to the host’s hematopoietic stem cell compartment.
On the basis of the above observations, therefore, the next immediate development of reduced-toxicity conditioning therapy in the setting of allogenic stem cell transplantation for AML/MDS patients at high risk of recurrent leukemia post-transplantation is a proposed triple drug combination of Flu and Clo followed by Bu to maximize the antileukemic efficacy, yet retaining the already documented safety of the Flu-Bu combination. Subsequent translational steps in this ongoing evolution will include further definition of the role(s) of DNA demethylating agents and molecularly targeted drugs that can enhance specific aspects of the cellular pathways involved in triggering apoptosis in the model cell systems.
Acknowledgments
Grant sponsor: The National Institutes of Health; Grant Numbers: P01 CA055164, CCSG Core CA16672; Grant sponsor: The Stephen L and Lavinia Boyd Fund (Leukemia Research).
Abbreviations
- Ado
adozelesin
- ALL
acute lymphoblastic leukemia
- AML
acute myeloid leukemia
- Ara-A
arabinofuranosyladenine
- Ara-C
cytosine arabinoside or cytarabine
- ATG
anti-thymocyte globulin
- B-CLL
B-cell chronic lymphocytic leukemia
- BCNU
1,3-bis(2-chloroethyl)-1-nitrosourea
- Bu
busulfan
- CCNU
cyclohexylchloroethyl nitrosourea
- CLL
chronic lymphocytic leukemia
- Clo
clofarabine
- Cy
cyclophosphamide
- DCK
deoxycytidine kinase
- Flu
fludarabine
- 5FU
5-fluorouracil
- GSH
glutathione
- 4-HC
4-hydroperoxycyclophosphamide
- HSCT
hematopoeitic stem cell transplantation
- IV
intravenous
- MDS
myelodysplastic syndrome
- Mel
melphalan
- NA
nucleoside analog
- RR
ribonucleotide reductase
- TBI
total body irradiation
- TRM
treatment related mortality
- XRT
X-ray or ionizing radiation therapy
REFERENCES
- Agura E, Berryman RB, Luis P, Estil V, Tadic-Ovcina M, Woelfel R, Fay J. Preliminary results of Phase II trial of clofarabine with parenteral busulfan (Clo/Bu) followed by allogeneic related or unrelated donor transplantation for the treatment of hematologic malignancies. Biol Blood Marrow Transplant. 2010;16:S280. [Google Scholar]
- Alyea EP, Kim HT, Ho V, Cutler C, Gribben J, DeAngelo DJ, Lee SJ, Windawi S, Ritz J, Stone RM, Antin JH, Soiffer RJ. Comparative outcome of nonmyeloablative and myeloablative hematopoietic cell transplantation for patients older than 50 years of age. Blood. 2005;105:1810–1814. doi: 10.1182/blood-2004-05-1947. [DOI] [PubMed] [Google Scholar]
- Andersson BS, Madden T, Tran HT, Hu WW, Blume KG, Chow DS, Champlin RE, Vaughan WP. Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplant conditioning therapy: A phase I study. Biol Blood Marrow Transplant. 2000;6:548–554. doi: 10.1016/s1083-8791(00)70064-4. [DOI] [PubMed] [Google Scholar]
- Andersson BS, Thall PF, Madden T, Couriel D, Wang X, Tran HT, Anderlini P, de Lima M, Gajewski J, Champlin RE. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: Defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2002a;8:477–485. doi: 10.1053/bbmt.2002.v8.pm12374452. [DOI] [PubMed] [Google Scholar]
- Andersson BS, Kashyap A, Gian V, Wingard JR, Fernandez H, Cagnoni PJ, Jones RB, Tarantolo S, Hu WW, Blume KG, Forman SJ, Champlin RE. Conditioning therapy with intravenous busulfan and cyclophosphamide (IV BuCy2) for hematologic malignancies prior to allogeneic stem cell transplantation: A phase II study. Biol Blood Marrow Transplant. 2002b;8:145–154. doi: 10.1053/bbmt.2002.v8.pm11939604. [DOI] [PubMed] [Google Scholar]
- Andersson BS, de Lima M, Thall PF, Wang X, Couriel D, Korbling M, Roberson S, Giralt S, Pierre B, Russell JA, Shpall EJ, Jones RB, Champlin RE. Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu) compares favorably with i.v. busulfan and cyclophosphamide (i.v. BuCy2) as pretransplant conditioning therapy in AML/ MDS. Biol Blood Marrow Transplant. 2008;14:672–684. doi: 10.1016/j.bbmt.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson BS, de Lima M, Valdez BC, Thall PF, Worth LL, Popat U, Jones RB, Shpall EJ, Madden T, McAdams PL, Alousi AM, Ron-don G, Kebriaei P, Champlin RE. Clofarabine ± fludarabine with IV busulfan and allogeneic stem cell transplantation for relapsed, refractory myeloid leukemia (ML) and MDS. Biol Blood Marrow Transplant. 2010;16:S271. [Google Scholar]
- Arner ESJ, Eriksson S. Mammalian deoxyribonucleoside kinases. Pharm Ther. 1995;67:155–186. doi: 10.1016/0163-7258(95)00015-9. [DOI] [PubMed] [Google Scholar]
- Baker SK, DeFor TE, Burns LJ, Ramsey NKC, Neglia JP, Robinson LL. New malignancies after blood and marrow stem-cell transplantation in children and adults: Incidence and risk factors. J Clin Oncol. 2003;21:1352–1358. doi: 10.1200/JCO.2003.05.108. [DOI] [PubMed] [Google Scholar]
- Benet LZ, Sheiner LB. Pharmacokinetics: The dynamics of drug absorption, distribution, and elimination. In: Gilman GA, Good-man LS, Rall TW, Murad F, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 7th ed. New York: MacMillan Publishing Co; 1985. p. 8. [Google Scholar]
- Bhagwatwar HP, Phadungpojna S, Chow DS, Andersson BS. Formulation and stability of busulfan for intravenous administration in high-dose chemotherapy. Cancer Chemother Pharmacol. 1996;37:401–408. doi: 10.1007/s002800050404. [DOI] [PubMed] [Google Scholar]
- Blaise D, Maraninchi D, Archimbaud E, Reiffers J, Devergie A, Jouet JP, Milpied N, Attal M, Michallet M, Ifrah N, Kuentz M, Dauriac C, Bordigoni P, Gratecos N, Guilhot F, Guyotat D, Gouvernet J, Gluckman E. Allogeneic bone marrow transplantation for acute myeloid leukemia in first remission: A randomized trial of a busulfan-Cytoxan versus Cytoxan-total body irradiation as preparative regimen: A report from the Group d’Etudes de la Greffe de Moelle Osseuse. Blood. 1992;79:2578–2582. [PubMed] [Google Scholar]
- Blaise D, Vey N, Faucher C, Mohty M. Current status of reduced-intensity-conditioning allogeneic stem cell transplantation for acute myeloid leukemia. Hematologica. 2007;92:533–541. doi: 10.3324/haematol.10867. [DOI] [PubMed] [Google Scholar]
- Blume KG, Kopecky KJ, Henslee-Downey JP, Forman SJ, Stiff PJ, LeMaistre CF, Appelbaum FR. A prospective randomized comparison of total body irradiation-etoposide versus busulfan-cyclophosphamide as preparatory regimens for bone marrow transplantation in patients with leukemia who were not in first remission: A Southwest Oncology Group study. Blood. 1993;81:2187–2193. [PubMed] [Google Scholar]
- Bredeson CN, Zhang MJ, Agovi MA, Bacigalupo A, Bahlis NJ, Ballen K, Brown C, Chaudhry MA, Horowitz MM, Kurian S, Quinlan D, Muehlenbien CE, Russell JA, Savoie L, Rizzo JD, Stewart DA. Outcomes following HSCT using fludarabine, busulfan, and thymoglobulin: A matched comparison to allogeneic transplants conditioned with busulfan and cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:993–1003. doi: 10.1016/j.bbmt.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JR, Yeckes H, Friedberg JW, Neuberg D, Kim H, Nadler LM, Freedman AS. Increasing incidence of late second malignancies after conditioning with cyclophosphamide and total-body irradiation and autologous bone marrow transplantation for non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:2208–2214. doi: 10.1200/JCO.2005.05.158. [DOI] [PubMed] [Google Scholar]
- Carson DA, Wasson DB, Esparza LM, Carrera CJ, Kipps TJ, Cottam HB. Oral antilymphocyte activity and induction of apoptosis by 2-chloro-2’-arabino-fluoro-2’deoxyadenosine. Proc Natl Acad Sci USA. 1992;89:2970–2974. doi: 10.1073/pnas.89.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Plunkett W, Gandhi V. Polyadenylation inhibition by the triphosphates of deoxyadenosine analogs. Leuk Res. 2008;32:1573–1581. doi: 10.1016/j.leukres.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciurea SO, Andersson BS. Busulfan in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:523–536. doi: 10.1016/j.bbmt.2008.12.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clift RA, Buckner CD, Thomas ED, Bryant E, Anasetti C, Bensinger WI, Bowden R, Deeg HJ, Doney KC, Fisher LD, Hansen JA, Martin P, McDonald GB, Sanders JE, Schoch G, Singer J, Storb R, Sullivan KM, Witherspoon RP, Appelbaum FR. Marrow transplantation for chronic myeloid leukemia: A randomized study comparing cyclophosphamide and total body irradiation with busulphan and cyclophosphamide. Blood. 1994;84:2036–2043. [PubMed] [Google Scholar]
- Cote S, Momparler RL. Evaluation of the antineoplastic activity of adozelesin alone and in combination with 5-aza-2’-deoxycytidine and cytosine arabinoside on DLD-1 human colon carcinoma cells. Anticancer Drugs. 1993;4:327–333. doi: 10.1097/00001813-199306000-00006. [DOI] [PubMed] [Google Scholar]
- de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R, Shpall EJ, Shahjahan M, Pierre B, Giralt S, Korbling M, Russell JA, Champlin RE, Andersson BS. Once-daily intravenous busulfan and fludarabine: Clinical and pharmacokinetic results of a myeoloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–864. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- Devergie A, Blaise D, Attal M, Tigaud JD, Jouet JP, Vernant JP, Bordigoni P, Ifrah N, Dauriac C, Cahn JY, Lioure B, Troussard X, Reiffers J, Gratecos N, Milpied N, Belanger C, Guyotat D, Tilly H, Michallet M, Gluckman E. Allogeneic bone marrow transplantation for chronic myeloid leukemia in first chronic phase: A randomized trial of busulfan-cytoxan versus cytoxan-total body irradiation as preparative regimen: A report from the French Society of Bone Marrow Graft (SFGM) Blood. 1995;85:2263–2268. [PubMed] [Google Scholar]
- Faderl S, Gandhi V, Keating MJ, Jeha S, Plunkett W, Kantarjian HM. The role of clofarabine in hematologic and solid malignancies: Development of a next-generation nucleoside analog. Cancer. 2005;103:1985–1995. doi: 10.1002/cncr.21005. [DOI] [PubMed] [Google Scholar]
- Faderl S, Ferrajoli A, Wierda W, Huang X, Verstovsek S, Ravandi F, Estrov Z, Borthakur G, Kwari M, Kantarjian HM. Clofarabine combinations as acute myeloid leukemia salvage therapy. Cancer. 2008;113:2090–2096. doi: 10.1002/cncr.23816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SJ. Role of reduced intensity transplant in adult patients with acute lymphoblastic leukemia: If and when? Best Pract Res Clin Haematol. 2009;2:557–566. doi: 10.1016/j.beha.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M, Boyle JM, Kinsella AR. Nucleoside salvage and resistance to antimetabolite anticancer agents. Br J Cancer. 1991;64:428–436. doi: 10.1038/bjc.1991.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, Yang H, Rosner G, Verstovsek S, Rytting M, Wierda WG, Ravandi F, Koller C, Xiao L, Faderl S, Estrov Z, Cortes J, O’Brien S, Estey E, Bueso-Ramos C, Fiorentino J, Jabbour E, Issa JP. Phase I/II study of the combination of 5-aza-2’-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108:3271–3279. doi: 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghandi V, Plunkett W. Cellular and clinical pharmacology of fludarabine. Clin Pharmacokinet. 2002;41:93–103. doi: 10.2165/00003088-200241020-00002. [DOI] [PubMed] [Google Scholar]
- Gibbs JP, Yang JS, Slattery JT. Comparison of human liver and small intestinal glutathione S-transferase-catalyzed busulfan conjugation in vitro. Drug Metab Dispos. 1998;26:52–55. [PubMed] [Google Scholar]
- Giralt S, Thall PF, Khouri I, Wang X, Braunschweig I, Ippolitti C, Claxton D, Donato M, Bruton J, Cohen A, Davis M, Andersson BS, Anderlini P, Gajewski J, Kornblau S, Andreeff M, Przepiorka D, Ueno NT, Molldrem J, Champlin R. Melphalan and purine analog-containing preparative regimens: Reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001;97:631–637. doi: 10.1182/blood.v97.3.631. [DOI] [PubMed] [Google Scholar]
- Giralt S. Advances in stem cell transplantation: Making it better and safer. Clin Lymphoma Myelomam. 2009;9(Suppl 3):S293–S295. doi: 10.3816/CLM.2009.s.026. [DOI] [PubMed] [Google Scholar]
- Gore SD. Combination therapy with DNA methyltransferase inhibitors in hematologic malignancies. Nat Clin Pract Oncol. 2005;(Suppl):S30–S35. doi: 10.1038/ncponc0346. [DOI] [PubMed] [Google Scholar]
- Grochow LB. Busulfan disposition: The role of therapeutic monitoring in bone marrow transplantation induction regimens. Semin Oncol. 1993;20(Suppl 4):18–25. [PubMed] [Google Scholar]
- Hall AG, Tilby MJ. Mechanisms of action of, and models of resistance to, alkylating agents used in the treatment of haematological malignancies. Blood Rev. 1992;6:163–173. doi: 10.1016/0268-960x(92)90028-o. [DOI] [PubMed] [Google Scholar]
- Hamaki T, Kami M, Kim SW, Onishi Y, Kishi Y, Murashige N, Hori A, Kojima R, Sakiyama M, Imataki O, Heike Y, Tanosaki R, Masuo S, Miyakoshi S, Taniguchi S, Tobinai K, Takaue Y. Reduced-intensity stem cell transplantation from an HLA-identical sibling donor in patients with myeloid malignancies. Bone Marrow Transplant. 2004;33:891–900. doi: 10.1038/sj.bmt.1704477. [DOI] [PubMed] [Google Scholar]
- Hartman AR, Williams S, Dillon JJ. Survival, disease-free survival and adverse effects of conditioning for allogeneic bone marrow transplantation with busulfan/cyclophosphamide vs total body irradiation: A meta-analysis. Bone Marrow Transplant. 1998;22:439–443. doi: 10.1038/sj.bmt.1701334. [DOI] [PubMed] [Google Scholar]
- Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- Hijiya N, Gaynon P, Barry E, Silverman L, Thomson B, Chu R, Cooper T, Kadota R, Rytting M, Steinherz P, Shen V, Jeha S, Abichandani R, Carroll WL. A multicenter phase I study of clofarabine, etoposide and cyclophosphamide in combination in pediatric patients with refractory or relapsed acute leukemia. Leukemia. 2009;23:2259–2264. doi: 10.1038/leu.2009.185. [DOI] [PubMed] [Google Scholar]
- Hosing C, Munsell M, Yazji S, Andersson BS, Couriel D, de Lima M, Donato M, Gajewski J, Giralt S, Körbling M, Martin T, Ueno NT, Champlin RE, Khouri IF. Risk of therapy-related myelodysplastic syndrome/acute leukemia following high-dose therapy and autologous bone marrow transplantation for non-Hodgkin’s lymphoma. Ann Oncol. 2002;13:450–459. doi: 10.1093/annonc/mdf109. [DOI] [PubMed] [Google Scholar]
- Huang P, Chubb S, Plunkett W. Termination of DNA synthesis by 9-beta-D-arabinofuranosyl-2-fluoroadenine. A mechanism for cytotoxicity. J Biol Chem. 1990;265:16617–16625. [PubMed] [Google Scholar]
- Huang P, Sandoval A, Van Den Neste E, Keating MJ, Plunkett W. Inhibition of RNA transcription: A biochemical mechanism of action against chronic lymphocytic leukemia cells by fludarabine. Leukemia. 2000;14:1405–1413. doi: 10.1038/sj.leu.2401845. [DOI] [PubMed] [Google Scholar]
- Issa JP, Garcia-Manero G, Giles FJ, Mannari R, Thomas D, Faderl S, Bayar E, Lyons J, Rosenfeld CS, Cortes J, Kantarjian HM. Phase I study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2’-deoxycytidine (Decitabine) in hematopoietic malignancies. Blood. 2004;103:1635–1640. doi: 10.1182/blood-2003-03-0687. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Huang P, Keating MJ, Plunkett W. Differential incorporation of ara-C, gemcitabine, and fludarabine into replicating and repairing DNA in proliferating human leukemia cells. Blood. 1997;90:270–278. [PubMed] [Google Scholar]
- Jabbour E, Issa J-P, Garcia-Manero G, Kantarjian H. Evolution of decitabine development: Accomplishments, ongoing investigations, and future strategies. Cancer. 2008;112:2341–2351. doi: 10.1002/cncr.23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeha S, Gandhi V, Chan KW, McDonald L, Ramirez I, Madden R, Rytting M, Brandt M, Keating M, Plunkett W, Kantarjian H. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood. 2004;103:784–789. doi: 10.1182/blood-2003-06-2122. [DOI] [PubMed] [Google Scholar]
- Jeha S, Razzouk B, Rytting M, Rheingold S, Albano E, Kadota R, Luchtman-Jones L, Bomgaars L, Gaynon P, Goldman S, Ritchey K, Arceci R, Altman A, Stine K, Steinherz L, Steinherz P. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute myeloid leukemia. J Clin Oncol. 2009;27:4392–4397. doi: 10.1200/JCO.2008.18.8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JB, Verburg L, Shore T, Williams M, Israels LG, Begleiter A. Combination therapy with nucleoside analogs and alkylating agents. Leukemia. 1994;8:S140–S143. [PubMed] [Google Scholar]
- Kantarjian H, Gandhi V, Kozuch P, Faderl S, Giles F, Cortes J, O’Brien S, Ibrahim N, Khuri F, Du M, Rios MB, Jeha S, McLaughlin P, Plunkett W, Keating M. Phase I clinical and pharmacology study of clofarabine in patients with solid and hematologic cancers. J Clin Oncol. 2003;21:1167–1173. doi: 10.1200/JCO.2003.04.031. [DOI] [PubMed] [Google Scholar]
- Kantarjian HM, Jeha S, Gandhi V, Wess M, Faderl S. Clofarabine: Past, present and future. Leuk Lymphoma. 2007;48:1922–1930. doi: 10.1080/10428190701545644. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, Erba HP, Claxton D, Arellano M, Lyons RM, Kovascovics T, Gabrilove J, Craig M, Douer D, Maris M, Petersdorf S, Shami PJ, Yeager AM, Eckert S, Abichandani R, Faderl S. Phase II study of clofarabine monotherapy in previously untreated older adults with acute myeloid leukemia and unfavorable prognostic factors. J Clin Oncol. 2010;28:549–55. doi: 10.1200/JCO.2009.23.3130. [DOI] [PubMed] [Google Scholar]
- Kashyap A, Wingard J, Cagnoni P, Roy J, Tarantolo S, Hu W, Blume K, Niland J, Palmer JM, Vaughan W, Fernandez H, Champlin R, Forman S, Andersson BS. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: Decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant. 2002;8:493–500. doi: 10.1053/bbmt.2002.v8.pm12374454. [DOI] [PubMed] [Google Scholar]
- Keating MJ, O’Brien S, Kontoyiannis D, Plunkett W, Koller C, Beran M, Lerner S, Kantarjian H. Results of first salvage therapy for patients refractory to a fludarabine regimen in chronic lymphocytic leukemia. Leuk Lymphoma. 2002;43:1755–1762. doi: 10.1080/1042819021000006547. [DOI] [PubMed] [Google Scholar]
- Khouri IF, McLaughlin P, Saliba RM, Hosing C, Korbling M, Lee MS, Medeiros LJ, Fayad L, Samaniego F, Alousi A, Anderlini P, Couriel D, de Lima M, Giralt S, Neelapu SS, Ueno NT, Samuels BI, Hagemeister F, Kwak LW, Champlin RE. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111:5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korycka A, Lech-Miranda E, Robak T. Novel purine nucleoside analogues for hematological malignancies. Recent Pat Anticancer Drug Discov. 2008;3:123–136. doi: 10.2174/157489208784638811. [DOI] [PubMed] [Google Scholar]
- Lin TS, Prusoff WH. Enhancement of the anticancer activity of bis(2-chloroethyl)nitrosourea in mice by coadministration of 2’-deoxyuridine, 2’-deoxycytidine, or thymidine. Cancer Res. 1987;47:394–397. [PubMed] [Google Scholar]
- McDonald GB, Slattery JT, Bouvier ME, Ren S, Batchelder AL, Kalhorn TF, Schoch HG, Anasetti C, Gooley T. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101:2043–2048. doi: 10.1182/blood-2002-06-1860. [DOI] [PubMed] [Google Scholar]
- Mineishi S, Magenau J, Pawarode A, Buck T, Jones D, Kato K, Frame D, Kujawski L, Erba HP, Khaled Y, Peres EM, Krijanovski OI, Reddy P, Kitko C, Choi S, Yanik GA, Braun T, Ferrara JLM, Levine JE. Myeloablative conditioning with clofarabine and busulfan X 4 (CloBu4) is well tolerated, facilitates secure engraftment, and exhibits significant anti-tumor activity against non-remission hematologic malignancies including AML. Blood. 2008;112:749. [Google Scholar]
- Mohty M, Rocha V, Chevallier P, Harousseau JL, Nagler A. Reduced-intensity conditioning for allogeneic stem cell transplantation: 10 years later. Curr Opin Oncol. 2009;21(Suppl 1):S1. doi: 10.1097/01.cco.0000357466.38219.0f. [DOI] [PubMed] [Google Scholar]
- Montgomery JA, Shortnacy-Fowler AT, Clayton SD, Riordan JM, Secrist JA., III Synthesis and biologic activity of 2’-fluoro-2-halo derivatives of 9-beta-D-arabinofuranosyladenine. J Med Chem. 1992;35:397–401. doi: 10.1021/jm00080a029. [DOI] [PubMed] [Google Scholar]
- Morrison VA, Rai KR, Peterson BL, Kolitz JE, Elias L, Appelbaum FR, Hines JD, Shepherd L, Larson RA, Schiffer CA. Therapy-related myeloid leukemias are observed in patients with chronic lymphocytic leukemia after treatment with fludarabine and chlorambucil: Results of an intergroup study, cancer and leukemia group B 9011. J Clin Oncol. 2002;20:3878–3884. doi: 10.1200/JCO.2002.08.128. [DOI] [PubMed] [Google Scholar]
- Nyce JW. Drug-induced DNA hypermethylation: A potential mediator of acquired drug resistance during cancer chemotherapy. Mutat Res. 1997;386:153–161. doi: 10.1016/s1383-5742(96)00051-8. [DOI] [PubMed] [Google Scholar]
- Oken MM, Lee S, Kay NE, Knospe W, Cassileth PA. Pentostatin, chlorambucil and prednisone therapy for B-chronic lymphocytic leukemia: A phase I/II study by the Eastern Cooperative Oncology Group study E1488. Leuk Lymphoma. 2004;45:79–84. doi: 10.1080/1042819031000151897. [DOI] [PubMed] [Google Scholar]
- Parker WB, Shaddix SC, Chang CH, White EL, Rose LM, Brockman RW, Shortnacy AT, Montgomery JA, Secrist JA, III, Bennett LL., Jr. Effects of 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabino-furanosyl)adenine on K562 cellular metabolism and the inhibition of human ribonucleotide reductase and DNA polymerases by its 5’-triphosphate. Cancer Res. 1991;51:2386–2394. [PubMed] [Google Scholar]
- Peters WP, Henner WD, Grochow LB, Olsen G, Edwards S, Stanbuck H, Stuart A, Gockerman J, Moore J, Bast RC, Jr, Seigler HF, Colvin OM. Clinical and pharmacologic effects of high dose single agent busulfan with autologous bone marrow support in the treatment of solid tumors. Cancer Res. 1987;47:6402–6406. [PubMed] [Google Scholar]
- Plumb JA, Strathdee G, Sludden J, Kaye SB, Brown R. Reversal of drug resistance in human tumor xenografts by 2’-deoxy-5-aza-cytidine-induced demethylation of the hMLH1 gene promoter. Cancer Res. 2000;60:6039–6044. [PubMed] [Google Scholar]
- Plunkett W, Gandhi V. Purine and pyrimidine nucleoside analogs. Cancer Chemother Biol Response Modif. 2001;19:21–45. [PubMed] [Google Scholar]
- Quintas-Cardama A, Kantarjian HM, Cortes JE. Mechanisms of primary and secondary resistance to imatinib in chronic myeloid leukemia. Cancer Control. 2009;16:122–131. doi: 10.1177/107327480901600204. [DOI] [PubMed] [Google Scholar]
- Ringden O, Ruutu T, Remberger M, Nikoskelainen J, Volin L, Vindeløv L, Parkkali T, Lenhoff S, Sallerfors B, Ljungman P, Mellander L, Jacobsen N. A randomized trial comparing busulfan with total body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia: A report from the Nordic Bone Marrow Transplantation Group. Blood. 1994;83:2723–2730. [PubMed] [Google Scholar]
- Russell JA, Tran HT, Quinlan D, Chaudhry A, Duggan P, Brown C, Stewart D, Ruether JD, Morris D, Glick S, Gyonyor E, Andersson BS. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: Study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002;8:468–477. doi: 10.1053/bbmt.2002.v8.pm12374451. [DOI] [PubMed] [Google Scholar]
- Russell JA, Savoie ML, Balogh A, Turner AR, Larratt L, Chaudhry MA, Storek J, Bahlis NJ, Brown CB, Quinlan D, Geddes M, Stewart DA. Allogeneic transplantation for adult acute leukemia in first and second remission with a novel regimen incorporating daily intravenous busulfan, fludarabine, 400 cGy total-body irradiation, and thymoglobulin. Biol Blood Marrow Transplant. 2007;13:814–821. doi: 10.1016/j.bbmt.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Santos GW, Tutschka PJ, Brookmeyer R, Saral R, Beschorner WE, Bias WB, Braine HG, Burns WH, Elfenbein GJ, Kaizer H. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med. 1983;309:1347–1353. doi: 10.1056/NEJM198312013092202. [DOI] [PubMed] [Google Scholar]
- Schmelz K, Sattler N, Wagner M, Lubbert M, Dorken B, Tamm I. Induction of gene expression by 5-aza-2’-deoxycytidine in acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) but not epithelial cells by DNA-methylation-dependent and -independent mechanisms. Leukemia. 2005;19:103–111. doi: 10.1038/sj.leu.2403552. [DOI] [PubMed] [Google Scholar]
- Shimoni A, Harden I, Shem-Tov N, Yerushalmi R, Nagler A. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: The role of dose intensity. Leukemia. 2006;20:322–328. doi: 10.1038/sj.leu.2404037. [DOI] [PubMed] [Google Scholar]
- Sirvent A, Dhedin N, Michallet M, Mounier N, Faucher C, Yakoub-Agha I, Mohty M, Robin M, Tabrizi R, Clement L, Bilger K, Larosa F, Contentin N, Huyn A, François S, Bulabois CE, Ceballos P, Bourrhis JH, Buzyn A, Cornillon J, Guillerm G, de Revel T, Bay JO, Guilhot F, Milpied N. Low nonrelapse mortality and prolonged long-term survival after reduced-intensity allogeneic stem cell transplantation for relapsed or refractory diffuse large B cell lymphoma: Report of the Société Française de Greffe de Moelle et de Thérapie Cellulaire. Biol Blood Marrow Transplant. 2010;16:78–85. doi: 10.1016/j.bbmt.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Slattery JT, Sanders JE, Buckner CD, Schaffer RL, Lambert KW, Langer FP, Anasetti C, Bensinger WI, Fisher LD, Appelbaum FR. Graft-rejection and toxicity following bone marrow transplantation in relationship to busulfan pharmacokinetics. Bone Marrow Transplant. 1995;16:31–42. [PubMed] [Google Scholar]
- Slavin S, Nagler A, Naperstek E, Kapelushnik Y, Aker M, Cividalli G, Varadi G, Kirschbaum M, Ackerstein A, Samuel S, Amar A, Brautbar C, Ben-Tal O, Eldor A, Or R. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- Socie G, Curtis RE, Deeg JH, Sobocinski KA, Filipovich AH, Travis LB, Sullivan KM, Rowlings PA, Kingma DW, Banks PM, Travis WD, Witherspoon RP, Sanders J, Jaffe ES, Horowitz MM. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. J Clin Oncol. 2000;18:348–357. doi: 10.1200/JCO.2000.18.2.348. [DOI] [PubMed] [Google Scholar]
- Socie G, Clift RA, Blaise D, Devergie A, Ringden O, Martin PJ, Remberger M, Deeg HJ, Ruutu T, Michallet M, Sullivan KM, Chevret S. Busulfan plus cyclophosphamide compared with total-body irradiation plus cyclophosphamide before marrow transplantation for myeloid leukemia: Long-term follow-up of 4 randomized studies. Blood. 2001;98:3569–3574. doi: 10.1182/blood.v98.13.3569. [DOI] [PubMed] [Google Scholar]
- Steinherz PG, Meyers PA, Steinherz LJ, Jeha S. Clofarabine induced durable complete remission in heavily pretreated adolescents with relapsed and refractory leukemia. J Pediatr Hematol Oncol. 2007;29:656–658. doi: 10.1097/MPH.0b013e318142b94b. [DOI] [PubMed] [Google Scholar]
- Tam CS, O’Brien S, Wierda W, Kantarjian H, Wen S, Do KA, Thomas DA, Cortes J, Lerner S, Keating MJ. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodoridis JM, Strathdee G, Brown R. Epigenetic silencing mediated by CpG island methylation: Potential as a therapeutic target and as a biomarker. Drug Resist Update. 2004;7:267–278. doi: 10.1016/j.drup.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Terenzi A, Aristei C, Aversa F, Perruccio K, Chionne F, Raymondi C, Latini P, Martelli MF. Efficacy of fludarabine as an immunosupressor for bone marrow transplantation conditioning: Preliminary results. Transplant Proc. 1996;28:3101. [PubMed] [Google Scholar]
- Thall PF, Champlin RE, Andersson BS. Comparison of 100-day mortality rates associated with i.v. busulfan and cyclophosphamide vs other preparative regimens in allogeneic bone marrow transplantation for chronic myelogenous leukemia: Bayesian sensitivity analyses of confounded treatment and center effects. Bone Marrow Transplant. 2004;33:1191–1199. doi: 10.1038/sj.bmt.1704461. [DOI] [PubMed] [Google Scholar]
- Thomas X, Raffoux E, Elhamri M, Lobe I, Cannas G, Dombret H. Clofarabine for the treatment of adult acute myeloid leukemia. Future Oncol 2009. 2009;5:1197–1210. doi: 10.2217/fon.09.105. [DOI] [PubMed] [Google Scholar]
- Tsimberidou AM, Keating MJ. Treatment of fludarabine-refractory chronic lymphocytic leukemia. Cancer. 115:2824–2836. doi: 10.1002/cncr.24329. [DOI] [PubMed] [Google Scholar]
- Tutschka PJ, Copelan EA, Klein JP. Bone marrow transplantation for acute leukemia following a new busulfan and cyclophosphamide regimen. Blood. 1987;70:1382–1388. [PubMed] [Google Scholar]
- Valdez BC, Murray D, Ramdas L, de Lima M, Jones R, Kornblau S, Betancourt D, Li Y, Champlin RE, Andersson BS. Altered gene expression in busulfan-resistant human myeloid leukemia. Leuk Res. 2008;32:1684–1697. doi: 10.1016/j.leukres.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez BC, Li Y, Murray D, Champlin RE, Andersson BS. Synergistic cytotoxicity of clofarabine, fludarabine and busulfan: Relevance to myeloablative therapy. Biol Blood Marrow Transplant. 2010a;16:S232. [Google Scholar]
- Valdez BC, Li Y, Murray D, Corn P, Champlin RE, Andersson BS. 5-aza-2’-deoxycytidine sensitizes busulfan-resistant myeloid leukemia cells by regulating expression of genes involved in cell cycle checkpoint and apoptosis. Leuk Res. 2010b;34:364–372. doi: 10.1016/j.leukres.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Neste E, Michaux L, Layios N, Costantini S, Francart J, Lambert C, Sonet A, André M, Robert A, Ferrant A. High incidence of complications after 2-chloro-2’-deoxyadenosine combined with cyclophosphamide in patients with advanced lymphoproliferative malignancies. Ann Hematol. 2004;83:356–363. doi: 10.1007/s00277-004-0858-7. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Nowak BJ, Keating MJ, Plunkett W. DNA repair initiated in chronic lymphocytic leukemia lymphocytes by 4-hydroperoxycyclophosphamide is inhibited by fludarabine and clofarabine. Clin Cancer Res. 2001;11:3580–3589. [PubMed] [Google Scholar]
- Yoo CB, Jones PA. Epigenetic therapy of cancer: Past, present and future. Nat Rev Drug Discov. 2006;5:37–50. doi: 10.1038/nrd1930. [DOI] [PubMed] [Google Scholar]
- Zhenchuk A, Lotfi K, Juliusson G, Albertioni F. Mechanisms of anti-cancer action and pharmacology of clofarabine. Biochem Pharm. 2009;78:1351–1359. doi: 10.1016/j.bcp.2009.06.094. [DOI] [PubMed] [Google Scholar]
- Zhu WG, Dai Z, Ding H, Srinivasan K, Hall J, Duan W, Villalona-Calero MA, Plass C, Otterson GA. Increased expression of unmethylated CDKN2D by 5-aza-2’-deoxycytidine in human lung cancer cells. Oncogene. 2001;20:7787–7796. doi: 10.1038/sj.onc.1204970. [DOI] [PubMed] [Google Scholar]