Abstract
Background
Dyspnea on exertion is a common and debilitating complaint, yet evidence for the relative value of cardiac and pulmonary tests for the evaluation of chronic dyspnea among adults without known cardiac or pulmonary disease is limited.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) enrolled participants ages 45-84 years who were free of clinical cardiovascular disease from six communities; participants with clinical pulmonary disease were excluded from this report. Dyspnea on exertion was assessed via structured interview. Tests included electrocardiograms, cardiac computed tomography (CT) for coronary artery calcium, cardiac magnetic resonance imaging, spirometry, percent emphysema (percent of lung regions < -950 Hounsfield Units) on CT, inflammatory biomarkers and N-terminal pro-Brain Natriuretic Peptide (NT-proBNP). Logistic regression was used to identify independent correlates of dyspnea after adjustment for age, sex, body mass index, physical activity, anxiety, and leg pain.
Results
Among 1,969 participants without known cardiopulmonary disease, 9% had dyspnea. The forced expiratory volume in one second (FEV1) (p <0.001), NT-proBNP (p=0.004), and percent emphysema on CT (p=0.004) provided independent information on the probability of self-reported dyspnea. Associations with the FEV1 were stronger among smokers and participants with other recent respiratory symptoms or seasonal allergies; associations with NT-proBNP were present only among participants with coexisting symptoms of lower extremity edema. Only the FEV1 provided a significant improvement in the receiver operating curve.
Conclusions
Among adults without known cardiac or pulmonary disease reporting dyspnea on exertion, spirometry, NT-proBNP, and CT imaging for pulmonary parenchymal disease were the most informative tests.
Keywords: dyspnea, spirometry, COPD, emphysema, heart failure, atherosclerosis, diagnostic tests
Introduction
Shortness of breath upon physical exertion affects almost one half of Americans.1 Dyspnea represents an important patient-oriented outcome for cardiopulmonary diseases including chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, and heart failure. Indeed, dyspnea may be interpreted as an integrated measure of pulmonary, cardiac, and vascular function, as well as conditioning.2 Since dyspnea worsens with exertion, many patients will become sedentary, causing a downward spiral towards frailty and reduced quality of life.3,4
The optimal diagnostic work-up for adults with chronic dyspnea and no known cardiopulmonary disease remains unclear. Several algorithms have been proposed based upon the diagnostic yield of tests performed on dyspneic patients referred to specialty clinics,5-11 yet no representative United States (US) population-based studies have been performed. Based on limited data, clinical guidelines recommend that a new complaint of dyspnea in adults warrants, in addition to a thorough history and physical, basic laboratory evaluation and preliminary diagnostic tests such as electrocardiogram (ECG), chest radiography, and possibly spirometry.12
We systematically assessed which diagnostic tests were associated with dyspnea among participants without diagnosed cardiac or pulmonary disease from a large panel of tests that were performed in a multiethnic, population-based cohort.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multi-center prospective cohort study designed to investigate the prevalence, correlates, and progression of sub-clinical cardiovascular disease.13 MESA recruited 6814 adults ages 45-84 years of four race/ethnicities from six communities in the US in 2000-2002. Exclusion criteria were clinical cardiovascular disease defined as a physician diagnosis of myocardial infarction, stroke, transient ischemic attack, heart failure, angina, any cardiovascular procedure or current atrial fibrillation; weight greater than 300 pounds; pregnancy; or impediment to long-term participation.
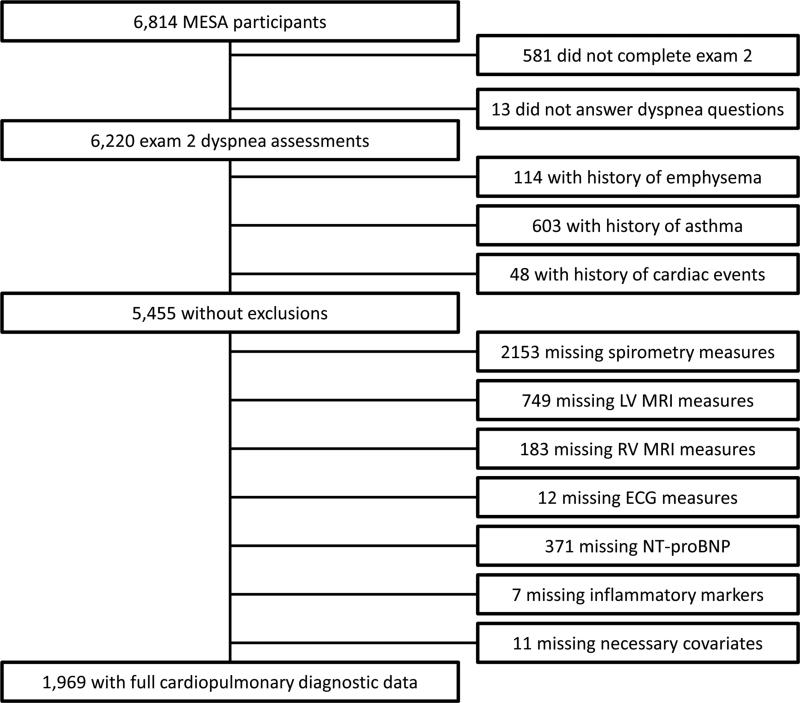
Additional exclusion criteria for the current report included cardiovascular event prior to dyspnea assessment; clinical pulmonary disease (physician diagnosis of asthma or emphysema); or not having performed diagnostic tests or missing relevant questionnaire items (Figure 1).
Figure 1.
Inclusion and exclusion criteria.
MESA=Multi-Ethnic Study of Atherosclerosis; MRI=magnetic resonance imaging; LV=left ventricle;
RV=right ventricle; ECG=electrocardiogram; NT-proBNP=N-terminal pro Brain Natriuretic Peptide.
Necessary covariates=age, sex, body mass index, exercise, leg pain, and anxiety.
All tests were performed at the baseline examination, except as noted below. Protocols were approved by the Institutional Review Boards of all collaborating institutions and the National Heart Lung and Blood Institute.
Dyspnea
Dyspnea was assessed by trained interviewers in 2002-2004 and was defined as a positive response to either of the following: “When walking on level ground, do you get more breathless than people your own age?” and/or “Do you ever have to stop walking due to breathlessness?” This definition correspond to the modified Medical Research Council (mMRC) dyspnea scale grade 2.14
Coronary atherosclerosis
All participants underwent 12-lead ECGs on GE MAC 1200 electrocardiographs (GE Healthcare, Milwaukie, WI) with central quality control via visual inspection and automated classification according to the Minnesota ECG Code.15
Cardiac computed tomography (CT) was performed using standardized protocols on either electron-beam or multidetector CT scanners16 for the assessment of phantom-adjusted Agatston score for coronary artery calcium, as previously described.17,18
Cardiac structure and function
All participants without MRI exclusions were asked to undergo cardiac MRI using a standard protocol.19 Indices of left ventricular structure and function were determined by volumetric imaging.19,20 Left ventricular ejection fraction was calculated as stroke volume divided by end-diastolic volume. Right ventricular parameters were measured similarly.21 Measures of cardiac structure were indexed by body surface area.22
Lung function
Spirometry was performed in 2004-06 for 3965 participants in the MESA Lung Study out of 4483 randomly selected from those who consented to genetic analyses (99%), underwent baseline measures of endothelial function (89%), and attended an examination at that time (91%). Asian-Americans were oversampled. Spirometry was conducted using an automated dry rolling-seal spirometer according to American Thoracic Society guidelines.23 Predicted values were calculated using equations from the National Health and Nutrition Examination Survey III24 with a 0.88 correction factor for Asians, as previously validated in MESA.25
Lung structure
Percentage of emphysema-like lung (hereafter referred to as percent emphysema) was measured as percentage of lung voxels with attenuation less than -950 Hounsfield units (HU), which has been validated on autopsy,26 on the cardiac CT scans.27,28
Percentage of lung with features suggestive of interstitial lung abnormalities (hereafter referred to as percent high attenuation areas [HAA]) was defined as percent of lung voxels with attenuation between -600 and -250 HU.29
Both measures were previously validated against those obtained from full-lung scans (e.g., r=0.96 for percent emphysema on cardiac and full-lung scans on the same MESA scanners).27,29
Biomarkers of inflammation, renal function and fluid overload
Creatinine, fibrinogen, C-reactive protein (CRP), and N-Terminal pro-Brain Natriuretic Peptide (NT-proBNP) were measured using standard techniques.29,30
Other covariates
Body mass index (BMI) was defined as the weight in kilograms (kg) divided by the height in meters (m), squared, which were both measured via standard techniques. Race/ethnicity, educational attainment, leg pain, total intentional exercise per week, and tobacco use were self-reported. Never smoking was defined as a lifetime smoking history of less than 100 cigarettes. Current smoking was defined as cigarette use within the past 30 days. Pack-years were calculated as (cigarettes per day / 20) × years smoked. Hypertension and diabetes were defined by Joint National Committee VI and American Diabetes Association 2003 criteria, respectively. Symptoms of lower extremity swelling, orthopnea, respiratory infections, and seasonal allergies were elicited via structured interview.
Covariates were measured at the time of dyspnea assessment, with the exception of time-invariant covariates (e.g., race/ethnicity) and anxiety, which was assessed at baseline using the Spielberger State-Trait Anxiety Inventory.31
Statistical analysis
Differences in characteristics of participants with and without dyspnea were compared by chi-square, Wilcoxon, and Student's t-tests. Pearson partial correlations were computed for results of diagnostic tests, and variables with a p-value less than 0.50 were incorporated into a backwards-selection multivariable logistic regression model that also included age, BMI, gender, exercise, anxiety, and leg pain, since these were factors that showed strong univariate associations with dyspnea and have been associated with dyspnea in prior studies.1,32-34
The validity of the final model obtained via backwards selection was assessed in two ways. First, backwards selection was repeated using 100 bootstrap datasets constructed via random sampling with replacement from the original set. Inclusion frequencies, defined as the number of times that a predictor was retained in backwards selection,35 were evaluated. Second, model calibration was tested by comparing deciles of actual versus predicted probabilities of dyspnea.36
For each of the retained predictors, adjusting for the pre-specified covariates, receiver operating characteristic (ROC) curves were plotted and c-statistics were compared. Interactions between the retained predictors and age, gender, smoking status, diabetes, hypertension, and other clinical symptoms were tested via multiplicative interaction terms and stratified analyses. Analyses were repeated using clinically-relevant thresholds for diagnostic test results. The prevalence of test results exceeding these thresholds was compared among participants with and without dyspnea.
All analyses were performed in SAS version 9.3 (Cary, North Carolina).
Results
Of 6814 MESA participants, 6220 (91%) answered dyspnea items, of whom 5455 (88%) had no prevalent clinical cardiopulmonary diagnoses. Of these participants, 1969 (36%) completed all relevant tests; the largest decrements in sample size were due to participants not selected for spirometry and those unwilling or unable to undergo MRI (Figure 1). Included participants were somewhat younger and were less likely to be obese, smoke and report dyspnea than excluded participants (Supplementary Table 1).
The mean age of included participants was 62 +/− 10 years, 49% were women, and the race/ethnic distribution was 35% White, 22% African-American, 23% Hispanic and 20% Asian-American. Fifty percent had ever smoked cigarettes, 13% had diabetes, and 42% had hypertension.
Overall, 173 (9%) reported dyspnea. The group reporting dyspnea had significantly more women, higher BMI, greater anxiety, less intentional exercise, more leg pain, and greater smoking history, as well as more major ECG abnormalities, lower lung function, higher inflammatory markers, and higher NT-proBNP (Table 1).
Table 1.
Characteristics of participants in the Multi-Ethnic Study of Atherosclerosis without cardiopulmonary diagnoses, stratified by the self-report of dyspnea.a
| Characteristics | No dyspnea (N=1796) | Dyspnea (N=173) | p-value |
|---|---|---|---|
| Age, years | 62.3 (9.8) | 63.4 (10.0) | 0.162 |
| Male gender | 940 (52.3%) | 65 (37.6%) | <0.001 |
| Race | |||
| White | 619 (34.5%) | 72 (41.6%) | 0.118 |
| Chinese | 369 (20.6%) | 24 (13.9%) | |
| Black | 398 (22.2%) | 37 (21.4%) | |
| Hispanic | 410 (22.8%) | 40 (23.1%) | |
| Education | 0.098 | ||
| No High School | 179 (10.0%) | 21 (12.1%) | |
| High School | 423 (23.6%) | 50 (28.9%) | |
| Some college or associate's degree | 483 (26.9%) | 52 (30.1%) | |
| Bachelor's Degree | 347 (19.3%) | 25 (14.5%) | |
| Graduate or Professional Degree | 363 (20.2%) | 25 (14.5%) | |
| Body Mass Index, kg/m2 | 27.1 (4.7) | 29.7 (5.5) | <0.001 |
| Smoking status | 0.150 | ||
| Never | 900 (50.1%) | 78 (45.1%) | |
| Former | 733 (40.8%) | 72 (41.6%) | |
| Current | 163 (9.1%) | 23 (13.3%) | |
| Pack-years of smoking | 0 (0, 14) | 0.2 (0, 25) | 0.023 |
| Spielberger trait anxiety scale | 15 (12, 18) | 17 (14, 20) | <0.001 |
| Total intentional exercise, MET-hr/wk | 893 (160, 1890) | 315 (0, 1238) | <0.001 |
| Leg pain | 212 (11.8%) | 53 (30.6%) | <0.001 |
| Arthritis | 145 (8.1%) | 24 (13.9%) | 0.009 |
| Cardiac MRI | |||
| LV End-Diastolic Volume / BSA, ml/m2 | 49 (43, 55) | 47 (42, 52) | 0.054 |
| LV Ejection Fraction, percent | 70 (65, 74) | 69 (65, 75) | 0.478 |
| LV End-Diastolic Mass / BSA, g/m2 | 55 (48, 62) | 56 (49, 64) | 0.312 |
| RV Ejection Fraction, percent | 71 (67, 75) | 72 (67, 75) | 0.350 |
| RV End-Diastolic Mass / BSA, g/m2 | 8 (7, 9) | 8 (7, 9) | 0.057 |
| Coronary artery calcium, Agatston score | 0 (0, 53) | 3 (0, 109) | 0.101 |
| Electrocardiographic abnormalities | |||
| Any major abnormalities | 191 (10.6%) | 27 (15.6%) | 0.047 |
| Any minor abnormalities | 636 (35.4%) | 59 (34.1%) | 0.731 |
| Spirometry | |||
| Percent predicted FEV1 | 97.2 (16.1) | 88.5 (19.7) | <0.001 |
| FEV1/FVC ratio | 0.76 (0.07) | 0.74 (0.10) | 0.008 |
| Chest CT | |||
| Percent emphysema | 3.08 (1.33, 5.90) | 3.41 (1.05, 5.94) | 0.570 |
| Percent high attenuation areas | 4.20 (3.55, 5.24) | 4.62 (3.67, 5.58) | 0.163 |
| Inflammatory markers | |||
| CRP | 1.52 (0.70, 3.46) | 2.70 (1.09, 5.06) | 0.002 |
| Fibrinogen | 329 (290, 377) | 354 (309, 391) | 0.002 |
| Creatinine, mg/dl | 0.95 (0.22) | 0.93 (0.21) | 0.308 |
| NT-proBNP, pg/ml | 47 (20, 92) | 66 (34, 135) | 0.001 |
Normally-distributed variables expressed as mean (standard deviation), and p-values pertain to two-sample T-tests. Non-normal variables expressed as median (interquartile range), and p-value derive from two-tailed Wilcoxon Two-Sample Tests with normal approximation. Categorical variables described as frequency (percent), and p-values pertain to chi-square tests.
Kg = kilogram. M = meter. MET = metabolic equivalent. Hr = hour. Wk = week. MRI = Magnetic Resonance Imaging. BSA = body-surface area. m = meters. LV = left ventricular. RV = right ventricular. ml = milliliter. g = grams. L = liter. Min = minute. FEV1 = forced expiratory volume in one second. FVC = forced vital capacity. CT = computed tomography. CRP = C-reactive protein. NT-proBNP = N-Terminal pro-Brain Natriuretic Peptide. Pg = picogram.
After adjusting for age, sex, BMI, exercise, anxiety, and leg pain, the cardiopulmonary tests significantly associated with dyspnea were spirometry, NT-proBNP, percent emphysema, left ventricular ejection fraction and mass, and coronary artery calcium (Table 2). These variables were no more than modestly correlated with each other (Supplementary Table 2).
Table 2.
Multivariate associations of results of diagnostic tests and demographic factors with self-reported dyspnea.a
| Potential predictor | Wald Chi-Square | p-value |
|---|---|---|
| Percent predicted FEV1 | 37.33 | <.0001 |
| FEV1/FVC ratio | 23.38 | <.0001 |
| NT-proBNP | 10.41 | 0.001 |
| Percent emphysema | 7.07 | 0.008 |
| LV End-Diastolic Mass | 5.13 | 0.024 |
| LV Ejection Fraction | 5.00 | 0.025 |
| Smoking status | 4.80 | 0.029 |
| Pack years | 4.35 | 0.037 |
| Coronary artery calcium (Agatston score) | 4.28 | 0.039 |
| Race/ethnicity | 4.21 | 0.040 |
| Any Major ECG Abnormality | 3.01 | 0.083 |
| CRP | 2.95 | 0.086 |
| Fibrinogen | 1.51 | 0.22 |
| Arthritis | 0.82 | 0.37 |
| LV End-Diastolic Volume | 0.74 | 0.39 |
| Any Minor ECG Abnormality | 0.32 | 0.57 |
| Creatinine | 0.21 | 0.65 |
| RV Ejection Fraction | 0.184 | 0.67 |
| Percent high attenuation areas | 0.019 | 0.89 |
| RV End-Diastolic Mass | 0.012 | 0.91 |
| Educational Attainment | 0.54 | 0.97 |
Models adjusted for age, body mass index, sex, exercise, anxiety, and leg pain.
LV = left ventricular. RV = right ventricular. FEV1 = forced expiratory volume in one second. FVC = forced vital capacity. CRP = C-reactive protein. NT-proBNP = N-Terminal pro-Brain Natriuretic Peptide. ECG = electrocardiogram.
Only three of these diagnostic tests, however, provided independent information (Table 3). FEV1 demonstrated the largest and strongest association with dyspnea: for each standard deviation (SD) reduction in FEV1 (17%), there was a 38% increased odds of self-reported dyspnea (95% confidence interval [CI] 28-47%, p <0.001). NT-proBNP was associated with a 20% increase in the odds of dyspnea per SD (123 pg/mL) (95% CI 6-36%, p=0.004) and percent emphysema on CT was associated with a 27% increase per SD (4%) (95% CI 8-51%, p=0.004).
Table 3.
Results of diagnostic tests independently associated with self-reported dyspnea.a
| Predictor | Odds ratiob (95% confidence interval) | Wald Chi-square | p-value |
|---|---|---|---|
| Percent predicted FEV1 | 0.616 (0.525, 0.723) | 35.22 | <0.0001 |
| NT-proBNP | 1.202 (1.062, 1.361) | 8.50 | 0.004 |
| Percent emphysema | 1.274 (1.079, 1.506) | 8.14 | 0.004 |
Models adjusted for age, body mass index, gender, exercise, anxiety, and leg pain.
Odds ratios reported per standard deviation of the predictor. Standard deviations were as follows: percent predicted FEV1 = 17%; NT-proBNP = 123 pg/ml; percent emphysema = 4%.
FEV1 = forced expiratory volume in one second. NT-proBNP = N-Terminal pro-Brain Natriuretic Peptide.
These three test results had the greatest inclusion frequencies in bootstrapped iterations of backward selection. FEV1 was included in 95% of bootstrapped backward selection models, compared to 54% for NT-proBNP, and 35% for percent emphysema; the next most relevant predictor, race, was included in only 20% (Supplementary Figure 1). A calibration plot for the final model with the pre-specified covariates plus the three predictors demonstrated that predicted probabilities were similar to the observed probabilities (Supplementary Figure 2).
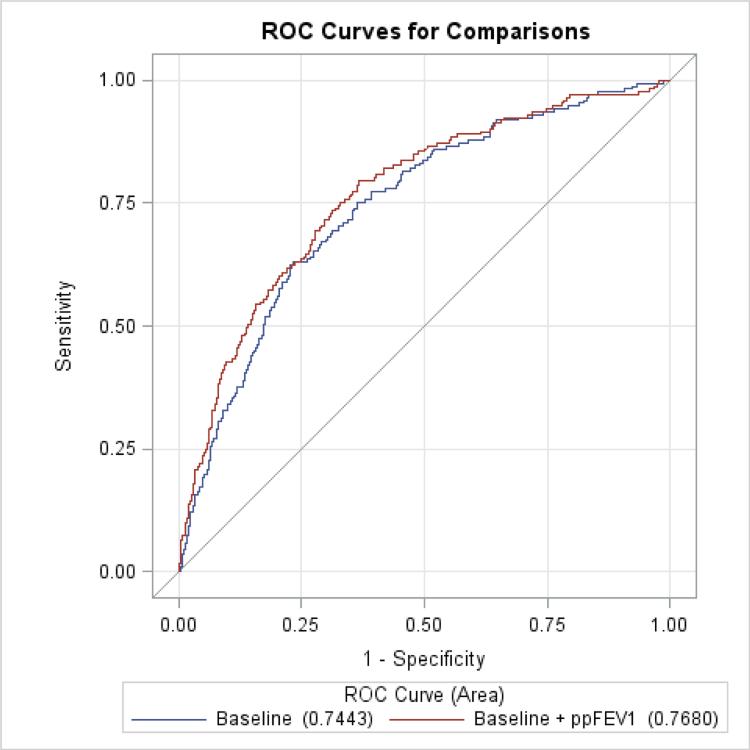
Compared to clinical factors alone, which were strongly predictive of dyspnea (c=0.744), only the FEV1 provided a modest but statistically significant incremental improvement in prediction (c=0.768, p=0.047; Figure 2). Adjustment for NT-proBNP and percent emphysema yielded smaller and non-significant increases in the c-statistic (Table 4).
Figure 2.
Receiver Operating Curves (ROC) for baseline model versus baseline model plus the percent predicted forced expiratory volume in one second (FEV1).
The baseline model includes age, body mass index, gender, exercise, anxiety, and leg pain.
Table 4.
Comparison of Receiver Operating Curves (ROC) for baseline covariates plus selected cardiopulmonary diagnostic tests in prediction of self-reported dyspnea.
| Model | Area Under Curve (95% CI) | Improvement over baseline model (95% CI) | Chi-Square | P-value |
|---|---|---|---|---|
| Baseline modela | 0.744 (0.707, 0.782) | - | - | - |
| Baseline + percent predicted FEV1 | 0.768 (0.731, 0.806) | 0.024 (0.0003, 0.047) | 3.95 | 0.047 |
| Baseline + percent emphysema | 0.752 (0.715, 0.789) | 0.008 (−0.006, 0.021) | 1.289 | 0.256 |
| Baseline + NT-proBNP | 0.752 (0.714, 0.789) | 0.007 (−0.005, 0.019) | 1.434 | 0.231 |
| Baseline + all three tests | 0.774 (0.737, 0.812) | 0.030 (0.003, 0.057) | 4.665 | 0.031 |
Baseline model includes age, body mass index, gender, exercise, anxiety, and leg pain.
FEV1 = forced expiratory volume in one second. NT-proBNP = N-Terminal pro-Brain Natriuretic Peptide.
There were several instances of effect measure modification (Supplementary Table 3). FEV1 was significantly associated with dyspnea among ever-smokers, and especially among current smokers, but not among never-smokers. The FEV1 was also more strongly associated with dyspnea among participants who reported recent bronchitis, sinusitis, cold and flu symptoms, or seasonal allergies, however it remained significant among those without these symptoms. NT-proBNP was only significantly associated with dyspnea among those with self-reported lower extremity edema, however orthopnea did not significantly modify the associations for any of the retained diagnostic tests, and differences in effect estimates between participants with and without these symptoms were inconsistent. The association of percent emphysema with self-reported dyspnea was of greater magnitude in participants greater than 65 years old, but did not differ by smoking history. NT-proBNP was only significantly associated with dyspnea among non-hypertensive individuals, who had lower mean NT-proBNP levels compared to hypertensives (61 vs 110 pg/ml, p<0.001).
Analyses using thresholded test results found that the same three diagnostic tests provided independent information: FEV1 below the lower limit of normal (OR 3.22, 95% CI 2.06-5.02); percent emphysema above the upper limit of normal (OR 1.75, 95% CI 1.05-2.92), and NT-proBNP above 300 pg/ml (OR 2.10, 95% CI 1.05-4.23).
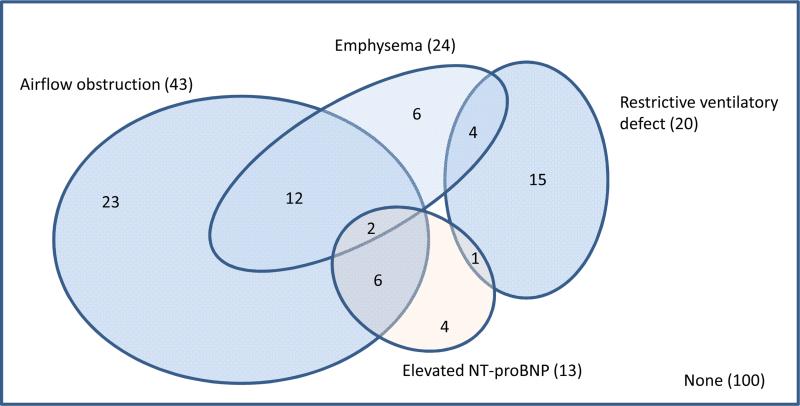
Using clinically relevant thresholds, 36% of dyspneic participants demonstrated abnormal lung function, of which roughly-two thirds was consistent with airflow obstruction, defined as a ratio of the FEV1 to the forced vital capacity (FVC) of less than 0.70 (Figure 3).37 Using a more specific threshold of FEV1/FVC ratio below the lower limit of normal,24 27% had abnormal lung function, of whom approximately one half met criteria for airflow obstruction. Abnormal levels of CT emphysema and NT-proBNP were present for an additional 6% of participants reporting dyspnea.
Figure 3.
Venn diagram for cardiopulmonary diagnoses, according to study test results, among participants with self-reported dyspnea (N=173).
Abnormal values were defined as follows: airflow obstruction as the ratio of forced expiratory volume in one second (FEV1) to forced vital capacity (FVC) < 0.7; restrictive ventilatory defect as percent predicted FVC less than 80% with a normal FEV1/FVC ratio; emphysema as percent emphysema > upper limit of normal; and elevated NT-proBNP as > 300 pg/ml.
Findings were similar when airflow obstruction was defined as FEV1/FVC < lower limit of normal, except the number of participants with airflow obstruction was smaller (N=26).
Among the 100 (57.8%) dyspneic participants for whom spirometry, percent emphysema, and NT-proBNP were within normal limits, 49% had abnormal results on another test. However, in most cases, the abnormal test result was equally common among patients with and without dyspnea (Supplementary Table 4). Thirty six percent had minor ECG abnormalities, 16% had major abnormalities, and 3% had Q waves, which was similar to, non-significantly higher, and significantly higher than nondyspneic participants (Supplementary Table 4). Of the remaining participants with normal ECGs, 6% had elevated coronary artery calcium, similar to the non-dyspneic sample, and none had decreased left ventricular ejection fraction or increased left ventricular mass.
Discussion
In this multiethnic, population-based study, non-invasive diagnostic tests that provided independent diagnostic information for chronic dyspnea among patients without known cardiopulmonary disease included spirometry, NT-proBNP, and CT scanning for pulmonary emphysema. In contrast, measures of atherosclerosis and cardiac function, including ECGs and cardiac MRI, were not independently associated with dyspnea. These findings suggest that these tests of lung function and structure, in addition to NT-proBNP, may be suitable initial tests among outpatients reporting chronic dyspnea.
This is the first population-based study of which we are aware to evaluate the utility of a battery of cardiac and pulmonary tests for self-reported dyspnea in a community setting. Prior literature has examined the diagnostic yield of various tests among patients referred to specialty clinics for evaluation of shortness of breath.6-11 These studies have shown spirometry to have the highest diagnostic yield, consistent with our results, as well as substantial benefit in chest imaging via X-ray or CT. For patients with normal spirometry and lung imaging, other tests such as diffusing capacity of the lung for carbon monoxide (DLCO), and exercise testing provided only incremental improvements in diagnostic accuracy.6,7,11 No consistent approach to screening for cardiovascular causes has been presented in these prior studies, which were predominantly carried out in pulmonary clinics, and due to such problems of selection bias, the generalizability of their results remains unclear.
In our population-based sample, the prevalence of mMRC grade 2 dyspnea (9%) was similar to that observed in the US population-based Cardiovascular Health Study (CHS) and the multinational Burden of Obstructive Lung Diseases (BOLD) study (10% and 7%, respectively).38,39 Consistent with these studies, self-reported dyspnea was associated with female sex, weight, smoking, anxiety, and physical exertion.38-44
Spirometry was the only test that significantly improved prediction of dyspnea after controlling for clinical factors. Among spirometric measurements, a reduction in FEV1 was the strongest predictor of dyspnea, especially among smokers and those with recent bronchitis, sinusitis, “cold” or seasonal allergy symptoms. FEV1/FVC ratio showed a strong bivariate association, however it was not retained in the final model, most likely due to its strong correlation with FEV1. The FEV1 was associated with a relatively modest (3%) incremental improvement in prediction of dyspnea compared to clinical factors alone; nonetheless, up to one in three participants with dyspnea had abnormal spirometry using standard cutoffs, consistent with a high diagnostic yield. These findings underscore the importance of spirometry in the evaluation of chronic dyspnea, a test that is a relatively simple and inexpensive test but which remains underutilized in clinical practice.45,46
Percent emphysema on CT was also significantly associated with dyspnea, particularly among participants over age 65 years, and this association was independent of spirometric airflow measures. These findings build upon previous studies showing associations of percent emphysema with lung cancer, cardiac function, hospitalizations, and all-cause mortality, all independent of lung function.47-52 Furthermore, they highlight that emphysema, which is characterized by destruction of lung parenchyma,53,54 is not infrequent in the absence of COPD.48,55,56 Of note, the prognostic value of percent emphysema was not modified by smoking status, which may suggest detection of panlobular emphysema, which is not related to smoking57 and has broader genetic predispositions than just alpha-1 antitrypsin deficiency.58
NT-proBNP, a biomarker of cardiac myocyte stress that is regularly used to screen for heart failure in the context of acute dyspnea,59-62 also provided independent information regarding chronic dyspnea in this community-based setting. This result supports outpatient screening for heart failure via NT-proBNP, as also suggested by a recent systematic review.63 The evidence of effect modification by hypertensive status, with stronger associations shown among non-hypertensives, indicates that NT-proBNP may be particularly useful in diagnosing dyspnea among patients who lack other known causes for elevations in this highly sensitive but relatively nonspecific test.
NT-proBNP was the only measure of cardiovascular disease which provided independent information despite only modest correlations between the various cardiac measures. Major ECG abnormalities were significantly more common among dyspneic participants, and in bivariate analyses adjusting for clinical factors, left ventricular ejection fraction and mass and coronary artery calcium, a measurement of subclinical atherosclerosis, were significantly associated with self-reported dyspnea. Nonetheless, the associations of these tests of cardiovascular health with prediction of dyspnea were not independent of lung measures and NT-proBNP. Although right ventricular measures have been shown to predict self-reported dyspnea in the MESA sample,64 there were no significant associations observed in these cross-sectional data.
Strengths of this study include a well-characterized, population-based sample with gold standard measurements of subclinical heart and lung disease. Limitations include exclusion of a substantial number of participants who were missing either MRI or spirometry. This may have introduced selection bias, although presumably far less than prior pulmonary clinic-based studies, and refusals and exclusions are common in clinical practice. While most tests were performed at one exam, dyspnea was assessed 18 months later and spirometry about 4 years later. However, results from these tests are not expected to vary substantially over this period, except in the setting of acute decompensations, and therefore should remain relevant to chronic dyspnea, which was the outcome of interest. Duration of dyspnea was not specifically assessed in MESA, yet questionnaire items used to classify dyspnea were consistent with the mMRC scale, which has been interpreted as a measure of chronic dyspnea.
We lacked information on echocardiographic criteria for diastolic dysfunction, which is highly prevalent and predictive of heart failure symptoms, including dyspnea,65 notwithstanding evidence to suggest it is an uncommon cause of dyspnea in the elderly.66 Nonetheless, NT-proBNP has shown to be strongly associated with severity of diastolic dysfunction.67 Our findings pertain to automated quantitative emphysema measurements, yet radiologist-defined emphysema on CT has also been associated with dyspnea and reduced exercise tolerance in the presence and absence of COPD.68 We were also unable to compare the performance of chest CT versus chest X-ray for the evaluation of pulmonary parenchymal diseases; X-ray is more commonly performed as an initial screening test, even though studies indicate that it is relatively low yield in the workup of dyspnea,9,10 likely due to its lower sensitivity for emphysema and other parenchymal lung diseases.69 Regardless, given that lung cancer screening is now recommended in smokers,70 emphysema on CT may be increasingly available for clinical decision-making. We did not evaluate DLCO, which associates with dyspnea71 and correlates modestly with emphysema on CT.72 Bronchoprovocation, exercise testing and plethysmography were not performed. A measure of anemia was not available, however due to physiologic adaptations, anemia must be severe in order to cause dyspnea in otherwise healthy adults, such as those enrolled in MESA. Lastly, data on accompanying clinical symptoms was limited. Coexisting respiratory symptoms and lower extremity swelling modified the strength of the associations, however the FEV1 remained the most significant correlate of dyspnea in all symptom strata.
In conclusion, among adults without pre-existing clinical cardiopulmonary disease, dyspnea was most strongly associated with FEV1, NT-proBNP, and percent emphysema on CT, and only FEV1 significantly improved prediction of dyspnea versus clinical factors alone. Measures of cardiac structure and function on MRI were associated with dyspnea but did not provide information independent of these tests, and measures of atherosclerosis were less strongly associated. Thus, evaluation of dyspnea in the outpatient setting may benefit from prioritization of spirometry, followed by NT-proBNP assay and chest CT imaging for pulmonary parenchymal disease.
Supplementary Material
Clinical Significance.
This study evaluates associations between non-invasive diagnostic tests and self-reported dyspnea on exertion among community-dwelling adults without known cardiopulmonary disease.
Spirometry, N-terminal Brain Natriuretic Peptide, and emphysema on computed tomography provided independent information regarding the likelihood of dyspnea, whereas measures of atherosclerosis and cardiac structure and function did not.
These findings help fill an evidence gap on the prioritization of diagnostic tests for the outpatient work-up of dyspnea.
Acknowledgements
MESA is supported by the National Heart, Lung, and Blood Institute (NHLBI) and was designed and conducted by the MESA investigators in collaboration with NHLBI staff.
Support for MESA is provided by contracts N01-HC-95159 through N01-HC-95169 and UL1-RR-024156 and UL1-RR-025005. The MESA Lung Study is funded by R01-HL077612, RC1-100543 and R01-093081 from the NHLBI.
NHLBI staff routinely monitored study performance and participated in the internal review of this manuscript prior to submission.
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding:
R01-HL077612, RC1-HL100543, R01HL086719, N01-HC95159-165, N01-HC95169.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Dr Oelsner was primarily responsible for data analysis and drafting of the manuscript, and vouches for the integrity of the data and analyses presented. All authors were involved in manuscript preparation, and all have approved the final manuscript.
References
- 1.Gronseth R, Vollmer WM, Hardie JA, et al. Predictors of dyspnoea prevalence: results from the BOLD study. Eur Respir J. 2014;43:1610–1620. doi: 10.1183/09031936.00036813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185:435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho SF, O'Mahony MS, Steward JA, et al. Dyspnoea and quality of life in older people at home. Age Ageing. 2001;30:155–159. doi: 10.1093/ageing/30.2.155. [DOI] [PubMed] [Google Scholar]
- 4.Voll-Aanerud M, Eagan TM, Plana E, et al. Respiratory symptoms in adults are related to impaired quality of life, regardless of asthma and COPD: results from the European community respiratory health survey. Health Qual Life Outcomes. 2010;8:107. doi: 10.1186/1477-7525-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pratter MR, Curley FJ, Dubois J, et al. Cause and evaluation of chronic dyspnea in a pulmonary disease clinic. Arch Intern Med. 1989;149:2277–2282. [PubMed] [Google Scholar]
- 6.DePaso WJ, Winterbauer RH, Lusk JA, et al. Chronic dyspnea unexplained by history, physical examination, chest roentgenogram, and spirometry. Analysis of a seven-year experience. Chest. 1991;100:1293–1299. doi: 10.1378/chest.100.5.1293. [DOI] [PubMed] [Google Scholar]
- 7.Martinez FJ, Stanopoulos I, Acero R, et al. Graded comprehensive cardiopulmonary exercise testing in the evaluation of dyspnea unexplained by routine evaluation. Chest. 1994;105:168–174. doi: 10.1378/chest.105.1.168. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen LS, Svanegaard J, Wiggers P, et al. The yield of a diagnostic hospital dyspnoea clinic for the primary health care section. J Intern Med. 2001;250:422–428. doi: 10.1046/j.1365-2796.2001.00901.x. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen F, Mehlsen J, Raymond I, et al. Evaluation of dyspnoea in a sample of elderly subjects recruited from general practice. Int J Clin Pract. 2007;61:1481–1491. doi: 10.1111/j.1742-1241.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- 10.Pratter MR, Abouzgheib W, Akers S, et al. An algorithmic approach to chronic dyspnea. Respir Med. 2011;105:1014–1021. doi: 10.1016/j.rmed.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Decramer M, Janssens W, Derom E, et al. Contribution of four common pulmonary function tests to diagnosis of patients with respiratory symptoms: a prospective cohort study. The Lancet Respiratory Medicine. 2013;1:705–713. doi: 10.1016/S2213-2600(13)70184-X. [DOI] [PubMed] [Google Scholar]
- 12.Wahls SA. Causes and evaluation of chronic dyspnea. Am Fam Physician. 2012;86:173–182. [PubMed] [Google Scholar]
- 13.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 14.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Dawood FZ, Chen H, et al. Minor isolated Q waves and cardiovascular events in the MESA study. Am J Med. 2013;126:450 e459–450 e416. doi: 10.1016/j.amjmed.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 17.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 18.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 19.Natori S, Lai S, Finn JP, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 20.Heckbert SR, Post W, Pearson GD, et al. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–2292. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tandri H, Daya SK, Nasir K, et al. Normal reference values for the adult right ventricle by magnetic resonance imaging. Am J Cardiol. 2006;98:1660–1664. doi: 10.1016/j.amjcard.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 22.Brumback LC, Kronmal R, Heckbert SR, et al. Body size adjustments for left ventricular mass by cardiovascular magnetic resonance and their impact on left ventricular hypertrophy classification. Int J Cardiovasc Imaging. 2010;26:459–468. doi: 10.1007/s10554-010-9584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 24.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 25.Hankinson JL, Kawut SM, Shahar E, et al. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the multi-ethnic study of atherosclerosis (MESA) lung study. Chest. 2010;137:138–145. doi: 10.1378/chest.09-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gevenois PA, de Maertelaer V, De Vuyst P, et al. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152:653–657. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman EA, Jiang R, Baumhauer H, et al. Reproducibility and validity of lung density measures from cardiac CT Scans--The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16:689–699. doi: 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lederer DJ, Enright PL, Kawut SM, et al. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med. 2009;180:407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patton KK, Heckbert SR, Alonso A, et al. N-terminal pro-B-type natriuretic peptide as a predictor of incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis: the effects of age, sex and ethnicity. Heart. 2013;99:1832–1836. doi: 10.1136/heartjnl-2013-304724. [DOI] [PubMed] [Google Scholar]
- 31.Spielberger C, Gorssuch R, Lushene P, et al. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press, Inc.; 1983. [Google Scholar]
- 32.Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. 2014;9:315–330. doi: 10.2147/COPD.S53255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harms CA, Babcock MA, McClaran SR, et al. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol (1985) 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- 34.Vogiatzis I, Zakynthinos G, Andrianopoulos V. Mechanisms of physical activity limitation in chronic lung diseases. Pulm Med. 2012;2012:634761. doi: 10.1155/2012/634761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heymans MW, van Buuren S, Knol DL, et al. Variable selection under multiple imputation using the bootstrap in a prognostic study. BMC Med Res Methodol. 2007;7:33. doi: 10.1186/1471-2288-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang TJ. Assessing the role of circulating, genetic, and imaging biomarkers in cardiovascular risk prediction. Circulation. 2011;123:551–565. doi: 10.1161/CIRCULATIONAHA.109.912568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 38.Enright PL, Kronmal RA, Higgins MW, et al. Prevalence and correlates of respiratory symptoms and disease in the elderly. Cardiovascular Health Study. Chest. 1994;106:827–834. doi: 10.1378/chest.106.3.827. [DOI] [PubMed] [Google Scholar]
- 39.Nielsen R, Vollmer WM, Hardie JA, et al. Predictors of dyspnoea prevalence: results from the BOLD study. Eur Respir J. 2013 doi: 10.1183/09031936.00036813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ekman I, Boman K, Olofsson M, et al. Gender makes a difference in the description of dyspnoea in patients with chronic heart failure. Eur J Cardiovasc Nurs. 2005;4:117–121. doi: 10.1016/j.ejcnurse.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Katsura H, Yamada K, Wakabayashi R, et al. Gender-associated differences in dyspnoea and health-related quality of life in patients with chronic obstructive pulmonary disease. Respirology. 2007;12:427–432. doi: 10.1111/j.1440-1843.2007.01075.x. [DOI] [PubMed] [Google Scholar]
- 42.Dales RE, Spitzer WO, Schechter MT, et al. The influence of psychological status on respiratory symptom reporting. Am Rev Respir Dis. 1989;139:1459–1463. doi: 10.1164/ajrccm/139.6.1459. [DOI] [PubMed] [Google Scholar]
- 43.Martinez-Moragon E, Perpina M, Belloch A, et al. Determinants of dyspnea in patients with different grades of stable asthma. J Asthma. 2003;40:375–382. doi: 10.1081/jas-120018637. [DOI] [PubMed] [Google Scholar]
- 44.Rector TS, Anand IS, Cohn JN. Relationships between clinical assessments and patients’ perceptions of the effects of heart failure on their quality of life. J Card Fail. 2006;12:87–92. doi: 10.1016/j.cardfail.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Mannino DM, Gagnon RC, Petty TL, et al. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000;160:1683–1689. doi: 10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- 46.Han MK, Kim MG, Mardon R, et al. Spirometry utilization for COPD: how do we measure up? Chest. 2007;132:403–409. doi: 10.1378/chest.06-2846. [DOI] [PubMed] [Google Scholar]
- 47.Haruna A, Muro S, Nakano Y, et al. CT scan findings of emphysema predict mortality in COPD. Chest. 2010;138:635–640. doi: 10.1378/chest.09-2836. [DOI] [PubMed] [Google Scholar]
- 48.Johannessen A, Skorge TD, Bottai M, et al. Mortality by Level of Emphysema and Airway Wall Thickness. Am J Respir Crit Care Med. 2013 doi: 10.1164/rccm.201209-1722OC. [DOI] [PubMed] [Google Scholar]
- 49.Zulueta JJ, Wisnivesky JP, Henschke CI, et al. Emphysema scores predict death from COPD and lung cancer. Chest. 2012;141:1216–1223. doi: 10.1378/chest.11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martinez FJ, Foster G, Curtis JL, et al. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med. 2006;173:1326–1334. doi: 10.1164/rccm.200510-1677OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sverzellati N, Cademartiri F, Bravi F, et al. Relationship and prognostic value of modified coronary artery calcium score, FEV1, and emphysema in lung cancer screening population: the MILD trial. Radiology. 2012;262:460–467. doi: 10.1148/radiol.11110364. [DOI] [PubMed] [Google Scholar]
- 52.McAllister DA, Ahmed FS, Austin JH, et al. Emphysema predicts hospitalisation and incident airflow obstruction among older smokers: a prospective cohort study. PLoS One. 2014;9:e93221. doi: 10.1371/journal.pone.0093221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379:1341–1351. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yokohori N, Aoshiba K, Nagai A. Increased levels of cell death and proliferation in alveolar wall cells in patients with pulmonary emphysema. Chest. 2004;125:626–632. doi: 10.1378/chest.125.2.626. [DOI] [PubMed] [Google Scholar]
- 55.Smith BM, Austin JHM, Newell JD, et al. Pulmonary Emphysema Subtypes on Computed Tomography in Smokers. Am J Med. epublished ahead of print October 9 2013. [Google Scholar]
- 56.Tsushima K, Sone S, Fujimoto K, et al. Identification of occult parechymal disease such as emphysema or airway disease using screening computed tomography. COPD. 2010;7:117–125. doi: 10.3109/15412551003631717. [DOI] [PubMed] [Google Scholar]
- 57.Anderson AE, Jr., Hernandez JA, Eckert P, et al. Emphysema in Lung Macrosections Correlated with Smoking Habits. Science. 1964;144:1025–1026. doi: 10.1126/science.144.3621.1025. [DOI] [PubMed] [Google Scholar]
- 58.Manichaikul A, Hoffman EA, Smolonska J, et al. Genome-wide study of percent emphysema on computed tomography in the general population. The Multi-Ethnic Study of Atherosclerosis Lung/SNP Health Association Resource Study. Am J Respir Crit Care Med. 2014;189:408–418. doi: 10.1164/rccm.201306-1061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Januzzi JL, Jr., Camargo CA, Anwaruddin S, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95:948–954. doi: 10.1016/j.amjcard.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 60.Lainchbury JG, Campbell E, Frampton CM, et al. Brain natriuretic peptide and n-terminal brain natriuretic peptide in the diagnosis of heart failure in patients with acute shortness of breath. J Am Coll Cardiol. 2003;42:728–735. doi: 10.1016/s0735-1097(03)00787-3. [DOI] [PubMed] [Google Scholar]
- 61.Anwaruddin S, Lloyd-Jones DM, Baggish A, et al. Renal function, congestive heart failure, and amino-terminal pro-brain natriuretic peptide measurement: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) Study. J Am Coll Cardiol. 2006;47:91–97. doi: 10.1016/j.jacc.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 62.Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J. 2006;27:330–337. doi: 10.1093/eurheartj/ehi631. [DOI] [PubMed] [Google Scholar]
- 63.Booth RA, Hill SA, Don-Wauchope A, et al. Performance of BNP and NT-proBNP for diagnosis of heart failure in primary care patients: a systematic review. Heart Fail Rev. 2014 doi: 10.1007/s10741-014-9445-8. [DOI] [PubMed] [Google Scholar]
- 64.Kaufmann MR, Barr RG, Lima JA, et al. Right ventricular morphology and the onset of dyspnea: the MESA-right ventricle study. PLoS One. 2013;8:e56826. doi: 10.1371/journal.pone.0056826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wan SH, Vogel MW, Chen HH. Pre-clinical diastolic dysfunction. J Am Coll Cardiol. 2014;63:407–416. doi: 10.1016/j.jacc.2013.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pedersen F, Raymond I, Mehlsen J, et al. Prevalence of diastolic dysfunction as a possible cause of dyspnea in the elderly. Am J Med. 2005;118:25–31. doi: 10.1016/j.amjmed.2004.07.048. [DOI] [PubMed] [Google Scholar]
- 67.McGrady M, Reid CM, Shiel L, et al. N-terminal B-type natriuretic peptide and the association with left ventricular diastolic function in a population at high risk of incident heart failure: results of the SCReening Evaluationof the Evolution of New-Heart Failure Study (SCREEN-HF). Eur J Heart Fail. 2013;15:573–580. doi: 10.1093/eurjhf/hft001. [DOI] [PubMed] [Google Scholar]
- 68.Smith BM, Barr RG. Establishing normal reference values in quantitative computed tomography of emphysema. J Thorac Imaging. 2013;28:280–283. doi: 10.1097/RTI.0b013e3182a0d805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miniati M, Monti S, Stolk J, et al. Value of chest radiography in phenotyping chronic obstructive pulmonary disease. Eur Respir J. 2008;31:509–515. doi: 10.1183/09031936.00095607. [DOI] [PubMed] [Google Scholar]
- 70.Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive services task force recommendation. Ann Intern Med. 2013;159:411–420. doi: 10.7326/0003-4819-159-6-201309170-00690. [DOI] [PubMed] [Google Scholar]
- 71.Spruit MA, Pennings HJ, Janssen PP, et al. Extra-pulmonary features in COPD patients entering rehabilitation after stratification for MRC dyspnea grade. Respir Med. 2007;101:2454–2463. doi: 10.1016/j.rmed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 72.D'Anna SE, Asnaghi R, Caramori G, et al. High-resolution computed tomography quantitation of emphysema is correlated with selected lung function values in stable COPD. Respiration. 2012;83:383–390. doi: 10.1159/000329871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.