Abstract
Insulin sensitivity (IS) is measured by the euglycemic-hyperinsulinemic clamp under a non-physiological condition. Daily C-peptide urinary excretion may be a physiological index of IS, since C-peptide is co-secreted with insulin as a function of nutrient intake and IS. The amount of 2H2O released from glycolytic glucose metabolism after [6,6-2H2]-glucose ingestion was recently proposed as a physiological measure of IS. We compared these IS surrogates to the gold standard (euglycemic-hyperinsulinemic clamp). Thirty (15M/15F) sedentary, non-diabetic participants (27.2±4.0 [SD] kg/m2, 35±12 y) were admitted for 3 days to our in-patient unit. After a 10-h fast, they received 60 g of glucose and 15 g of [6,6-2H2]-glucose. Before glucose ingestion and hourly thereafter for 4 h, plasma glucose and insulin concentrations, and plasma deuterium enrichment were determined. Plasma 2H2O production divided by insulin response was used as the glycolytic index. On Day 2, subjects spent 23 h in a metabolic chamber (eucaloric diet, 50% CHO, 30% fat). Urinary C-peptide excretion was divided by energy intake yielding the C-peptide to energy intake ratio (CPEP/EI). After leaving the chamber (Day 3, 10-h fast), IS was measured by a 2-h clamp (120 mU/m2/min). Average IS (clamp) was 7.3±2.6 mg glucose/kg estimated metabolic body size/min (range: 3.6–13.2). These values were inversely correlated with CPEP/EI (r=−0.62; p < 0.01) and positively with the glycolytic rate (r=0.60; p < 0.01). In non-diabetic subjects, two novel estimates of IS –daily urinary C-peptide urinary excretion and glycolytic rate during an OGTT– were related to IS by a clamp.
Keywords: Insulin Resistance, Insulin Secretion, Glucose Metabolism, Glucose Tolerance
Introduction
Insulin resistance (IR) is defined as a state in which higher than “normal” insulinemia is necessary to mediate the action of insulin. It is widely acknowledged that insulin resistance, independent of obesity, predicts the development of type 2 diabetes mellitus and cardiovascular disease 1. Appropriate methods to assess insulin action are essential to better understand the pathogenesis of insulin resistance. The euglycemic-hyperinsulinemic clamp is considered to be the gold standard to quantify insulin sensitivity 2. Numerous methods to assess insulin sensitivity have been developed 3–5 and compared against the clamp.
Recently, a new method to measure insulin sensitivity was proposed by Beysen et al. 6. The method is based on the assessment of glycolytic glucose metabolism using [6,6-2H2]-glucose after a 75-g glucose load containing 15 g of [6,6-2H2]-glucose. The 2H label is lost as water by carboxylation-decarboxylation between pyruvate and dicarboxylic acids (i.e., oxaloacetate, malate and fumarate) and also in liver by action of glutamate-pyruvate transaminase 7. This metabolic water is then incorporated to the body water pool. Thus, release of deuterated water (2H2O) after a standard glucose dose is an estimate of the ability of insulin to facilitate glucose transport, phosphorylation and glycolytic pathway.
Most methods to measure insulin sensitivity including the clamp and OGTT (with or without tracers) require blood sampling, making them unsuitable in persons with difficult access to veins, anemic individuals or in children. For that reason, we here propose a novel non-invasive method to assess insulin sensitivity. C-peptide is secreted from the pancreas in equimolar quantities with insulin and unlike insulin is not cleared by the liver. Thus, insulin secretion can be estimated from urinary C-peptide excretion. Kruszynska et al. 8 observed in healthy humans that urinary C-peptide excretion was directly associated with insulin secretion and plasma insulin concentration. In addition, insulin and C-peptide secretion depend on nutrient intake e.g. carbohydrate and protein. We propose to assess insulin sensitivity by the ratio of 24-hour urinary C-peptide excretion to 24-hour energy intake under standardized feeding. We anticipate that insulin-sensitive individuals will secrete lower amounts of insulin in response to the same amount of nutrients ingested as compared with insulin-resistant individuals.
The objective of this study was to compare two physiological measures of insulin sensitivity (24-h urinary C-peptide excretion to energy intake ratio and deuterated-glucose disposal test) against insulin sensitivity measured by a euglycemic-hyperinsulinemic clamp.
Methods and Procedures
Subjects
Thirty healthy (as defined by physical examination and routine medical laboratory tests), non-smoking and non-diabetic participants (15 males and 15 females) were recruited by advertising (Table 1). None of them were engaged in regular physical activity (> 60 min/week), under/over feeding or taking any medication. They had stable body weight (change < 2 kg for the past 3 months). The protocol was approved by the Pennington Biomedical Research Center Institutional Review Board and all subjects provided written informed consent prior to study participation. Body fat mass was measured on a Hologic Dual Energy X-ray Absorptiometer (DXA) in the fan beam mode (QDR 4500; Hologic, Waltham, MA). Fat-free mass was calculated as the difference between body mass and fat mass. Whole-body water content was calculated as fat-free mass times 0.73 9.
Table 1.
Subjects characteristics
| Mean ± SD | Range | |
|---|---|---|
| Male / Female | 15 / 15 | |
| Age (y) | 35.4 ± 11.5 | 18.7 – 53.6 |
| Body mass (kg) | 78.8 ± 15.2 | 57.1 – 107.8 |
| Height (cm) | 169.9 ± 9.5 | 151.1 – 188.9 |
| Body fat (%) | 28.8 ± 8.7 | 10 – 48 |
| Total body water (kg) | 41.0 ± 9.2 | 26.5 – 59.6 |
| Body mass index (kg/m2) | 27.2 ± 4.0 | 21.6 – 34.2 |
| Fasting glucose (mmol/l) | 5.1 ± 0.4 | 4.6 – 5.9 |
| 2-h OGTT glucose (mmol/l) | 7.3 ± 1.7 | 4.3 – 11.2 |
| Fasting insulin (pmol/l) | 63 ± 49 | 11 – 223 |
Experimental design
Participants were instructed to avoid intense physical activity for the 2 days preceding metabolic testing. On Day −1, subjects came to the Pennington Biomedical Research Center to eat a standardized diet (50% carbohydrate, 20% protein and 30% fat). After their evening meal, subjects were admitted to our in-patient unit. Following a 12-hour overnight fast, the deuteratedglucose disposal test was performed (Day 1). In the afternoon, body composition was determined by DXA. The next morning (Day 2) subjects entered the respiratory chamber at 8:00 AM for assessment of 24-hour energy expenditure. Urine was collected during the whole period for measurements of nitrogen, creatinine, and C-peptide excretion. Upon leaving the metabolic chamber at 7:15 AM the next morning (Day 4), subjects were prepared for the euglycemic-hyperinsulinemic clamp.
Twenty-four hour urine collection for C-peptide excretion
Twenty-four hour energy expenditure and respiratory quotient (RQ) were determined in a whole-room respiration calorimeter as previously described 10 while urine was collected for determination of nitrogen, creatinine, and C-peptide content. Total daily energy expenditure and fuel oxidation were measured at energy balance using an algorithm to balance intake and expenditure within the day 11. No exercise was allowed in the chamber. The diet (50% carbohydrates, 30% fat and 20% protein) was standardized for energy supply, type of food, meal preparation and served according to a fixed schedule. Breakfast, lunch and supper provided 25%, 35% and 40% of the total energy, respectively. Participants were instructed to eat all foods provided.
Deuterated glucose disposal test (2H-GDT)
The 2H-GDT consists of a 75-g glucose load containing 15 g of [6,6-2H2]-glucose dissolved in 300 ml of water. Complete glycolytic disposal of 15 g of [6,6-2H2]-glucose results in the release of 0.0824 mol of 2H2O. Dilution in the body water pool (~2,200 mol in a 70-kg human) results in body deuterium enrichment of about 0.0037% (250 δ units). The limit for deuterium detection by isotope ratio-mass spectrometry is about 1 δ. Blood samples were taken at −30, −20 and −10 min before glucose ingestion, and every 30 minutes for 4 hours thereafter to determine plasma glucose and insulin concentrations. For blood 2H2O enrichment determination the same schedule was followed, except after the first hour blood samples were taken every hour.
From plasma samples, 100-µl aliquots in the cap of an inverted vial were placed in a 70°C glass bead–filled heating block overnight. Water distillate inside the vial was then collected and analysis was run in triplicate. Deuterium content of the plasma samples was determined using a Thermo Finnigan High Temperature Conversion/Elemental Analyzer coupled with a Thermo Finnigan MAT 253 isotope ratio-mass spectrometer via a Conflo-III Interface. Deuterium isotope abundance was first calculated in 2H values relative to the International Vienna Standard Mean Ocean Water (SMOW) and then transformed to atom percent excess. The net plasma 2H2O enrichment was calculated as the difference between 2H2O at a given time minus baseline 2H2O. Then, this value was converted to millimoles by multiplying 2H2O enrichment by whole-body water content divided by 20 (molecular weight 2H2O). Insulin sensitivity was then calculated as 2H2O production after 4 hours divided by the 4-h insulin area under the curve (AUC) alone or by insulin and glucose AUCs together to account for differences in glucose effectiveness (glucose-dependent tissue glucose uptake). Plasma insulin and glucose AUCs were calculated using the trapezoidal method. Additionally, the HOMA-IR ([fasting glycemia (mmol/l) × insulinemia (pmol/l)]/22.5) 3 and Matsuda insulin sensitivity index 4 were calculated.
Euglycemic-hyperinsulinemic clamp
Insulin sensitivity was measured by a euglycemic-hyperinsulinemic clamp 2. After an overnight fast, insulin (120 mU/m2 per min) was infused for 2 hours while a 20% glucose solution was infused to maintain glycemia at 5 mmol/l. Plasma concentration of insulin was measured at 10 minute intervals at baseline and during steady-state from 90 to 120 min. A high insulin dose was selected in order to fully suppress hepatic glucose production and better represent skeletal muscle insulin sensitivity 12. The glucose disposal rate, a measure of insulin sensitivity, was adjusted for estimated metabolic body size (EMBS = FFM [kg] + 17.7) 13. Indirect calorimetry was performed 30 min before initiating insulin infusion and during steady-state from 90 to 120 min using a Deltatrac II metabolic cart (Deltatrac II, Datex-Ohmeda, Helsinki, Finland). Oxygen consumption, CO2 production and energy expenditure standardized for temperature, pressure, and moisture were calculated at one minute intervals. Energy substrate oxidation was calculated taking into account urinary nitrogen excretion rate 14.
Blood and urine analysis
Plasma glucose concentration was measured by the glucose-oxidase method on a Beckman Coulter DXC 600 Pro instrument. Plasma insulin and C-peptide were analyzed using immunoassays on a Siemen's 2000 instrument.
Statistical analysis
Data are presented as means ± SDs, except in figures in which SEs are shown. Analyses were performed using SAS version 9.1.3 (SAS Institute, Cary, NC). Spearman correlation analysis was used to evaluate the association between variables. The mixed model with repeated measures was used to assess differences as a function of time. When the mixed model provided a significant effect, the data was further tested by a Tukey-Kramer post-hoc test. The rejection level for statistical tests was 5%.
Results
Euglycemic-hyperinsulinemic clamp
All individuals completed the euglycemic-hyperinsulinemic clamp. During the last 30 minutes of the 2-h clamp, steady-state glucose and insulin concentrations were 5.0 ± 0.1 mmol/l and 1158 ± 202 pmol/l, respectively, while insulin-stimulated glucose disposal rate was on average 7.3 ± 2.6 mg/kg EMBS/min (range: 3.6 – 13.2).
Urinary C-peptide excretion over 24 hours in a respiratory chamber
Twenty four-hour energy expenditure, energy balance, and RQ were 1905 ± 400 kcal/d, − 89 ± 270 kcal/d and 0.90 ± 0.03, respectively. On average, energy balance was not different from zero (t-test p=0.09). However, the measured 24-h RQ was slightly higher than the expected value based on the food quotient (FQ=0.88). This resulted in small negative carbohydrate (−35 ± 52 g) and positive fat (20 ± 26 g) balances (both different from zero; p < 0.01) whereas protein balance was in equilibrium (−3 ± 33 g, p=0.62).
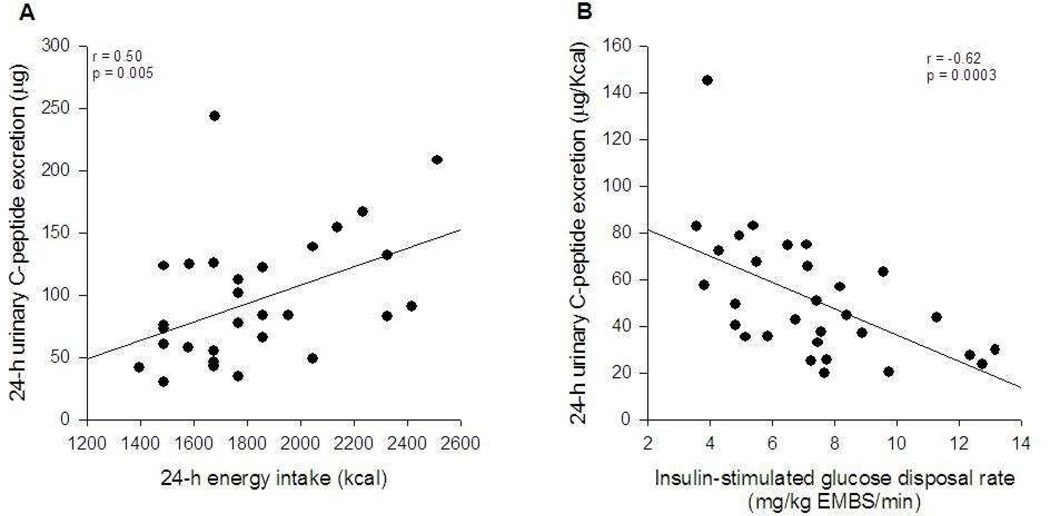
The 24-h urinary C-peptide excretion was directly related to total energy intake in the respiratory chamber (r=0.50; p=0.005) with an intercept not different from zero (−40 kcal/d; p=0.46). The relationship between energy intake and urinary C-peptide excretion is shown in Figure 1A. Since the macronutrient composition of the diet was fixed, identical relationships between nutrient intake and C-peptide excretion were found. The ratio between 24-h urinary Cpeptide excretion and total energy intake (UCPEP/EI), our proposed index of insulin sensitivity, was inversely associated to insulin-stimulated glucose disposal rate during the clamp (r=−0.62; p=0.0003; Figure 1B and Table 2). Similar results were obtained when insulin-stimulated glucose disposal rate was divided by steady-state plasma insulin concentration (data not shown). Thus, insulin-resistant individuals had higher urinary excretion of C-peptide per unit of energy ingested when compared to insulin-sensitive subjects.
Figure 1.
Relationship between 24-h urinary C-peptide excretion and 24-h energy intake (A) and 24-h urinary C-peptide excretion to energy intake ratio against insulin sensitivity measured by a euglycemic-hyperinsulinemic clamp (B).
Table 2.
Spearman correlation analysis among insulin sensitivity estimates.
| Insulin sensitivity measured by… | Clamp | 4-h 2H- GDT |
24-h urinary C-peptide | HOMA-IR | Matsuda index |
|---|---|---|---|---|---|
| Clampa | - | ||||
| 4-h 2H-GDTb | 0.60† | - | |||
| 24-h urinary C-peptidec | −0.62 * | −0.68 * | - | ||
| HOMA-IR | −0.45‡ | −0.78 * | 0.71 * | - | |
| Matsuda index | 0.51† | 0.88 * | −0.69 * | −0.97 * | - |
| Insulin AUC | −0.55† | −0.96* | 0.68* | 0.85* | −0.93* |
Correlations assessed by Spearman analysis.
p<0.0001;
p<0.01;
p<0.05. HOMA-IR (n=30), Matsuda index (n=30), clamp (n=30), glycolytic rate (n=28) and C-peptide excretion (n=30).
Insulin-stimulated glucose disposal rate measured by a 2-h euglycemic-hyperinsulinemic clamp (mg glucose/kg EMBS/min).
2H2O production in millimoles after 4 hours of ingested a 75-g glucose dose containing 15 g of [6,6-2H2] glucose divided by the 4-h insulin area under the curve.
24-h urinary C-peptide excretion divided by 24-h energy intake.
Glycolytic glucose disposal rate during an oral glucose tolerance test
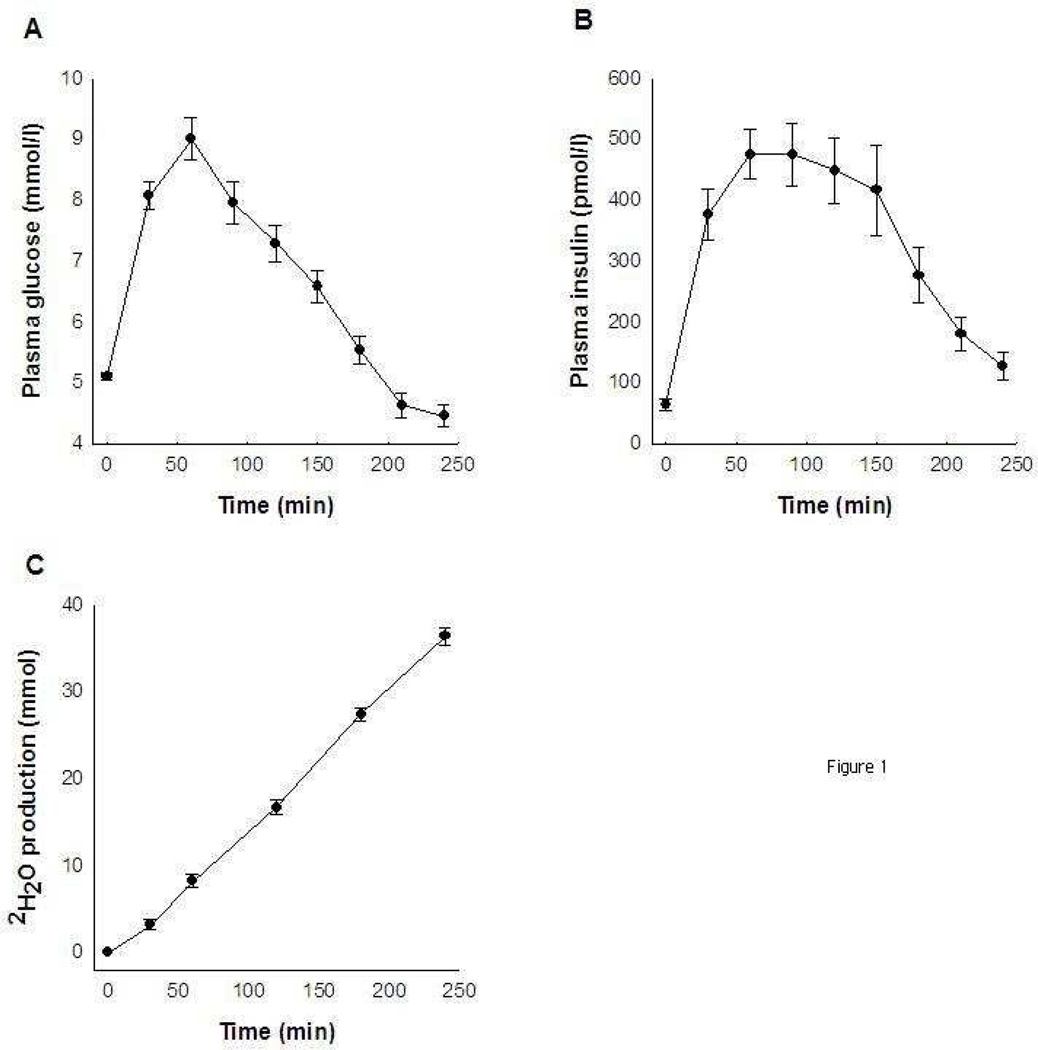
In 2 out of 30 individuals, plasma deuterium enrichment could not be determined (technical difficulties), while in one other volunteer the 240-min sample was not available. Sixty minutes after glucose ingestion, plasma glucose and insulin concentrations reached maximal values. Plasma glucose concentrations were similar to baseline values between 180 to 210 min, and at the end of the procedure, plasma glucose concentrations were even lower than at baseline. However, plasma insulin concentrations did not return to fasting levels for the entire 240-min period (Figure 2A and 2B).
Figure 2.
Plasma glucose (A) and insulin (B) concentration and deuterated water production (C) in response to a 75-g oral glucose tolerance test.
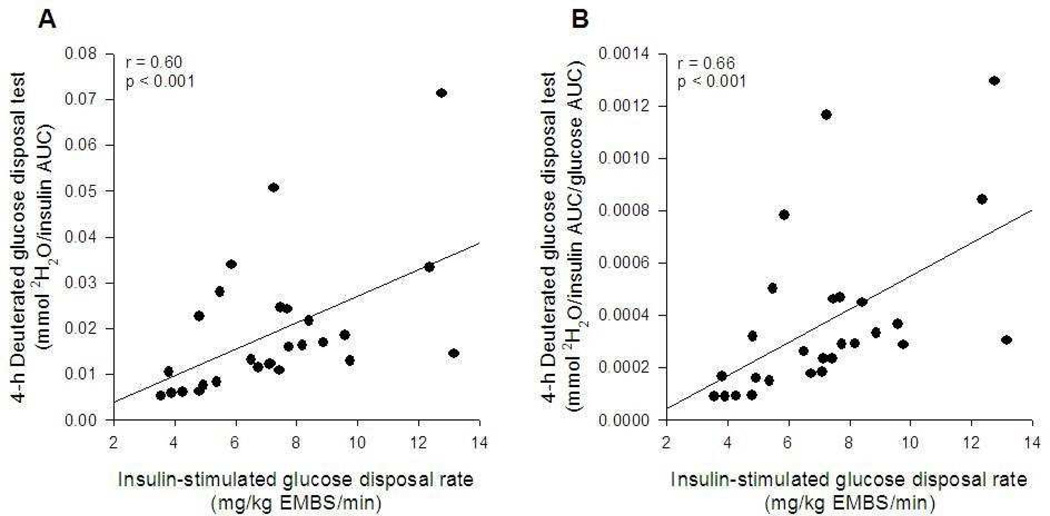
The net 2H2O production increased during the whole period and at the end of the 4-h period was 36.3 ± 5.2 mmol (Figure 2C). The ratio between 2H2O production and plasma insulin AUC after 4 hours was 0.019 ± 0.015 mmol of 2H2O per unit of insulin (AUC). This index of insulin sensitivity correlated positively with insulin-stimulated glucose disposal rate (r=0.60; p < 0.001; Figure 3A and Table 2) and non-oxidative glucose disposal rate (r=0.58; p=0.001) during the clamp. To account for differences in plasma glucose –which influences glucose-dependent tissue glucose uptake– the index was further divided by the 4-h plasma glucose AUC. In this instance, a slightly better association was detected with insulin-stimulated glucose disposal rate (r=0.66, p < 0.001; Figure 3B). Similar results were found when the analysis was repeated using the 3-h 2H2O production and 3-h insulin and glucose AUCs (data not shown). All results were similar when insulin-stimulated glucose disposal rate (clamp) was divided by steady-state plasma insulin concentration (data not shown).
Figure 3.
Relationship between insulin sensitivity measured by a euglycemic-hyperinsulinemic clamp and the deuterated glucose disposal test adjusted for plasma insulin area under the curve (A) or insulin and glucose areas under the curve (B).
Insulin sensitivity surrogates
The relationships of the three estimates of insulin sensitivity measured in this study against other surrogates of insulin sensitivity (HOMA, the insulin sensitivity index by Matsuda and 4-h insulin AUC) were also tested (Table 2). On average, HOMA-IR, Matsuda index and insulin AUC were 2.4 ± 1.9, 5.6 ± 4.2 and 2738 ± 1663 pmol × min/l, respectively. HOMA-IR, Matsuda index and insulin AUC showed similar associations when compared against the clamp (r: 0.45 – 0.55; p < 0.05). However, these IS surrogates were better related to UCPEP/EI and glycolytic glucose disposal rate (r: 0.68 – 0.96; p < 0.0001).
Discussion
The present study showed that our novel non-invasive measure of insulin sensitivity in physiological conditions using daily amount of C-peptide excreted was fairly well associated (r=− 0.62) with insulin sensitivity measured by the gold standard, the hyperinsulinemic-euglycemic clamp. Similarly, the more invasive method of glucose undergoing glycolysis per unit of plasma insulin was also associated with insulin sensitivity measured by the clamp. However, the latter association was weaker than previously published (r= 0.60 vs. r=0.956). For both methods, several arguments may explain the lack of better associations.
Urinary C-peptide excretion
We found a direct association between urinary C-peptide excretion and energy intake with an intercept that was not different from zero. Additionally, we detected an inverse but moderate correlation between urinary C-peptide excretion per calorie ingested and insulin sensitivity measured by a euglycemic-hyperinsulinemic clamp (r=−0.62). Several factors might explain the lack of a better association with insulin sensitivity. First, insulin is secreted in response to multiple nutritional factors 15. This led us to use a standardized diet in terms of macronutrient composition, preparation and cooking procedures to decrease the variability in nutrient digestion and absorption 16. Second, urinary C-peptide excretion is also influenced by the renal C-peptide uptake and clearance 8, 17. Approximately 25% of arterial C-peptide is filtered by the kidneys, with most of that filtered being taken up and degraded to amino acids (~85%), and the remaining 15% being excreted into the urine 17. This process has an inter-individual variability ranging from 6 to 24% 17. Therefore, these factors can affect the relationship between insulin secretion and C-peptide excretion, and further the extent to which insulin sensitivity relates to the C-peptide to energy intake ratio. We here present a novel totally non-invasive method to assess insulin sensitivity in physiological conditions which can be particularly useful in metabolic studies including 24-h assessment in response to diet, drugs or acute exercise. It should be borne in mind that the urinary C-peptide method cannot be used in individuals with diabetes or kidney diseases.
Glycolytic glucose disposal rate
We confirmed that glycolytic glucose disposal rate after an oral glucose tolerance test correlates with the insulin-stimulated glucose disposal rate assessed during a clamp. In the present study we found a weaker relationship between these two variables than reported in the initial description of the method 6. The reason may be related to the fact that we calculated total body water as 73% of fat-free mass 9 instead of evaluated by bio-impedance and the equation of Hume et al. 18. An additional factor may be the use of a different index to express the insulin-stimulated glucose disposal rate during the clamp. Beysen et al. 6 divided glucose disposal rate by kilogram of body weight, while in the present study we used what we consider a better normalization using the estimated metabolic body size 13. The latter adjustment takes into account differences in body composition (i.e., metabolically active tissue), which is overlooked when glucose disposal rate is just divided by body weight. However, even after calculating total body water by using the Hume’s equation and insulin-stimulated glucose disposal rate divided by body weight, we only observed a slightly better association between both variables (r=0.70), but still far from that previously reported (r=0.95) 6. Another aspect that might have played a role between the two studies is the insulin infusion rate, which in our study was 3-fold higher than used by Beysen et al. 6. Since we recruited volunteers with a large range of glucose homeostasis status (from normal to impaired glucose tolerance), we choose this insulin dose to fully suppress hepatic glucose production in all participants 12 and avoid an underestimation of insulin-stimulated glucose disposal rate in those individuals with impaired hepatic insulin sensitivity. Whether lower insulin concentrations would have yielded to a better correlation between the two methods cannot be answered here. However, it is known that insulin-stimulated glucose disposal rate determined at different insulin doses correlates well within individuals 19.
A critical aspect in the interpretation of the 4-h 2H-GDT index is the normalization of 2H2O production by plasma insulin response. It is predicted that insulin-resistant subjects would require a greater amount of insulin per unit of glucose metabolized in the glycolysis (i.e., 2H2O released). However, we found no relationship between 2H2O production and plasma insulin AUC after 3 h (r=0.10) or 4 h (r=−0.07) making the ratio between 2H2O production and insulin AUC questionable. Similarly, we found no relationship between 2H2O production and body weight, percent body fat, glucose AUC, insulin sensitivity and age. In fact, the association between 4-h 2H-GDT index and insulin-stimulated glucose disposal rate (clamp) was mostly driven by the plasma insulin AUC.
Other insulin sensitivity surrogates
The 4-h 2H-GDT index and UCPEP/EI ratio were also compared to often used surrogates of insulin sensitivity such as HOMA-IR 3, the Matsuda index 4 and insulin AUC. In general, these surrogates were well correlated to UCPEP/EI and 4-h 2H-GDT indexes (Table 2). Because insulin AUC is included in the Matsuda index and 4-h 2H-GDT calculation, it is not surprising to find a high association level (r: 0.93 – 0.95). Interestingly, insulin AUC is itself as good as the Matsuda index or 4-h 2H-GDT in predicting insulin sensitivity by clamp.
Conclusion
We here provide evidence that a new method to determine insulin sensitivity noninvasively and in physiological conditions (based on urinary C-peptide excretion) provides a reasonable marker of insulin sensitivity. This method which does not require blood sampling can be easily included in 24-h (or longer) metabolic studies assessing the role of diet, drugs or acute exercise on insulin resistance. Future studies should also assess the reproducibility of this index and its eventual usefulness in field conditions. Furthermore, we confirmed that the glycolytic glucose disposal index proposed by Hellerstein’s group also provides an estimate of insulin sensitivity; however, its performance is not better than plasma insulin concentration itself.
Acknowledgements
This work was funded by a NORC P30 grant DK072476. J.E.G. was supported by a fellowship from The International Nutrition Foundation/Ellison Medical Foundation.
Abbreviations
- IR
insulin resistance
- 2H2O
deuterated water
- DXA
Dual Energy X-ray Absorptiometer
- RQ
respiratory quotient
- 2H-GDT
deuterated glucose disposal test
- SMOW
International Vienna Standard Mean Ocean Water
- EMBS
estimated metabolic body size
- FQ
food quotient
- UCPEP/EI
24-h urinary C-peptide excretion and total energy intake
Footnotes
Duality of interest: None
References
- 1.Reaven GM. Insulin resistance/compensatory hyperinsulinemia, essential hypertension, and cardiovascular disease. J Clin Endocrinol Metab. 2003;88:2399–2403. doi: 10.1210/jc.2003-030087. [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 3.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 4.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 5.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E126. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 6.Beysen C, Murphy EJ, McLaughlin T, et al. Whole-body glycolysis measured by the deuterated-glucose disposal test correlates highly with insulin resistance in vivo. Diabetes Care. 2007;30:1143–1149. doi: 10.2337/dc06-1809. [DOI] [PubMed] [Google Scholar]
- 7.Katz J, Rognstad R. The metabolism of tritiated glucose by rat adipose tissue. J Biol Chem. 1966;241:3600–3610. [PubMed] [Google Scholar]
- 8.Kruszynska YT, Home PD, Hanning I, Alberti KG. Basal and 24-h C-peptide and insulin secretion rate in normal man. Diabetologia. 1987;30:16–21. doi: 10.1007/BF01788901. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Deurenberg P, Wang W, Pietrobelli A, Baumgartner RN, Heymsfield SB. Hydration of fat-free body mass: new physiological modeling approach. Am J Physiol. 1999;276:E995–E1003. doi: 10.1152/ajpendo.1999.276.6.E995. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen T, de Jonge L, Smith SR, Bray GA. Chamber for indirect calorimetry with accurate measurement and time discrimination of metabolic plateaus of over 20 min. Med Biol Eng Comput. 2003;41:572–578. doi: 10.1007/BF02345320. [DOI] [PubMed] [Google Scholar]
- 11.de Jonge L, Nguyen T, Smith SR, Zachwieja JJ, Roy HJ, Bray GA. Prediction of energy expenditure in a whole body indirect calorimeter at both low and high levels of physical activity. Int J Obes Relat Metab Disord. 2001;25:929–934. doi: 10.1038/sj.ijo.0801656. [DOI] [PubMed] [Google Scholar]
- 12.Campbell PJ, Mandarino LJ, Gerich JE. Quantification of the relative impairment in actions of insulin on hepatic glucose production and peripheral glucose uptake in non-insulin-dependent diabetes mellitus. Metabolism. 1988;37:15–21. doi: 10.1016/0026-0495(88)90023-6. [DOI] [PubMed] [Google Scholar]
- 13.Lillioja S, Bogardus C. Obesity and insulin resistance: lessons learned from the Pima Indians. Diabetes Metab Rev. 1988;4:517–540. doi: 10.1002/dmr.5610040508. [DOI] [PubMed] [Google Scholar]
- 14.Jequier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Annu Rev Nutr. 1987:187–208. doi: 10.1146/annurev.nu.07.070187.001155. [DOI] [PubMed] [Google Scholar]
- 15.Wolever TM, Bolognesi C. Prediction of glucose and insulin responses of normal subjects after consuming mixed meals varying in energy, protein, fat, carbohydrate and glycemic index. J Nutr. 1996;126:2807–2812. doi: 10.1093/jn/126.11.2807. [DOI] [PubMed] [Google Scholar]
- 16.Pi-Sunyer FX. Glycemic index and disease. Am J Clin Nutr. 2002;76:290S–298S. doi: 10.1093/ajcn/76.1.264S. [DOI] [PubMed] [Google Scholar]
- 17.Zavaroni I, Deferrari G, Lugari R, et al. Renal metabolism of C-peptide in man. J Clin Endocrinol Metab. 1987;65:494–498. doi: 10.1210/jcem-65-3-494. [DOI] [PubMed] [Google Scholar]
- 18.Hume R, Weyers E. Relationship between total body water and surface area in normal and obese subjects. J Clin Pathol. 1971;24:234–238. doi: 10.1136/jcp.24.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olefsky JM. LIlly lecture 1980. Insulin resistance and insulin action. An in vitro and in vivo perspective. Diabetes. 1981;30:148–162. doi: 10.2337/diab.30.2.148. [DOI] [PubMed] [Google Scholar]