SUMMARY
γ-Secretase is composed of four proteins that are obligatory for protease activity: Presenilin, Nicastrin, Aph1 and Pen-2. Despite the progress towards understanding the function of these individual subunits, there is no information available pertaining to the modulation of γ-secretase in response to environmental changes in cells. Here we show that hypoxia upregulates γ-secretase activity through a direct interaction with Hif-1α, revealing an unconventional function for Hif-1α as an enzyme subunit, which is distinct from its canonical role as a transcription factor. Moreover, hypoxia-induced cell invasion and metastasis are alleviated by either γ-secretase inhibitors or a dominant negative Notch coactivator, indicating that γ-secretase/Notch signaling plays an essential role in controlling these cellular processes. The present study reveals an unprecedented mechanism in which γ-secretase can achieve temporal control through conditional interactions with regulatory proteins, such as Hif-1α, under select physiological and pathological conditions.
INTRODUCTION
γ-Secretase, an intramembranous aspartyl protease composed of Presenilin (PS), Nicastrin (Nct), Aph1 and Pen-2, cleaves multiple type I membrane proteins, including the amyloid precursor protein (APP) and Notch receptor proteins. Mutations of γ-secretase subunits have been associated with Alzheimer’s disease (Levy Lahad et al., 1995; Sherrington et al., 1995), acne inversa (Wang et al., 2010) and acute myeloid leukemia (Klinakis et al., 2011). Although cellular reconstitution studies suggest that all four integral membrane proteins are required for γ-secretase activity (Edbauer et al., 2003), multiple reports indicate that only a fraction of the steady state complexes existing in mammalian cells are catalytically active and that γ-secretase activity is not always correlated with the levels of presenilin (Beher et al., 2003; Gu et al., 2004; Lai et al., 2003; Placanica et al., 2009), the catalytic subunit of γ-secretase (Ahn et al., 2010). Thus, the function of the inactive complexes remains poorly understood. Several γ-secretase interacting proteins, including CD147 (Zhou et al., 2005), p23/TMP21 (Chen et al., 2006) and γ-secretase activating protein (GSAP) (He et al., 2010), have been described to modulate γ-secretase activity and specificity. However, it is unknown whether γ-secretase can be conditionally modulated as an adaptive response to environmental stressors.
Notch and hypoxia-inducible factor-1α (Hif-1α) signaling pathways control many essential biological processes, from stem cell development to organogenesis, and the cross-talk between both pathways has been investigated in several biological systems (Gustafsson et al., 2005; Mukherjee et al., 2011; Wang et al., 2011). Hif-1α, a master regulator of cellular response to hypoxia, regulates the expression of numerous Hif responsive genes, including vascular endothelial growth factor (VEGF), which is essential for angiogenesis, and glucose transporter 1 (Glut-1), which is essential for increased glucose utilization (Denko, 2008). Multiple studies also have shown that Hif-1α can potentiate Notch signaling. For example, Hif-1α was shown to bind and stabilize the Notch intracellular domain (NICD) (Gustafsson et al., 2005), which is generated by γ-secretase cleavage and translocates to the nucleus to activate Notch target genes. Furthermore, the cross-talk between Notch and Hif-1α is required to maintain an undifferentiated cell state under hypoxia (Gustafsson et al., 2005). Alternatively, it has been shown that Hif-1α can bind to the N-boxes in the promoter region of Hes1, a Notch target gene, to alleviate the negative feedback of Notch signaling in cancer stem cells (Wang et al., 2011). In addition, the Aph1a gene promoter contains a Hif-1α response element (HRE) binding site and chemical hypoxia has been shown to lead to the upregulation of γ-secretase activity through an increase in Aph1a gene expression (Wang et al., 2006). Sima, a Drosophila ortholog of Hif-1α, has also been reported to activate Notch signaling in a ligand-independent manner to promote the survival of Drosophila blood cells (Mukherjee et al., 2011). Collectively, these studies indicate that multiple mechanisms can be involved in the regulation of Notch signaling by Hif-1α.
Hypoxia is a common phenomenon shared among many solid tumors and is correlated with poor prognosis and tumor invasiveness (Denko, 2008). The cross-talk between Hif-1α and Notch leads to enhanced activation of Notch signaling, which drives epithelial-to-mesenchymal transition (EMT) events, such as cell motility and invasiveness (Sahlgren et al., 2008). Therefore, investigation of the interplay between Hif-1α and Notch/γ-secretase is critical to understand their role in pathophysiology of cancer and other human disorders. In the present study, we show that hypoxia leads to augmentation of γ-secretase activity by direct binding of Hif-1α to the γ-secretase complex rather than by Hif-1α’s conventional transcriptional activity. Moreover, an increase in γ-secretase activity and Notch signaling is critical for hypoxia-induced cell migration, invasion and metastasis of breast cancer. This work reveals an unconventional role for Hif-1α as a regulatory subunit of the γ-secretase complex under low oxygen environments and a mechanism for temporally controlling γ-secretase activity in response to oxygen deprivation.
RESULTS
Hypoxia increases γ-secretase activity
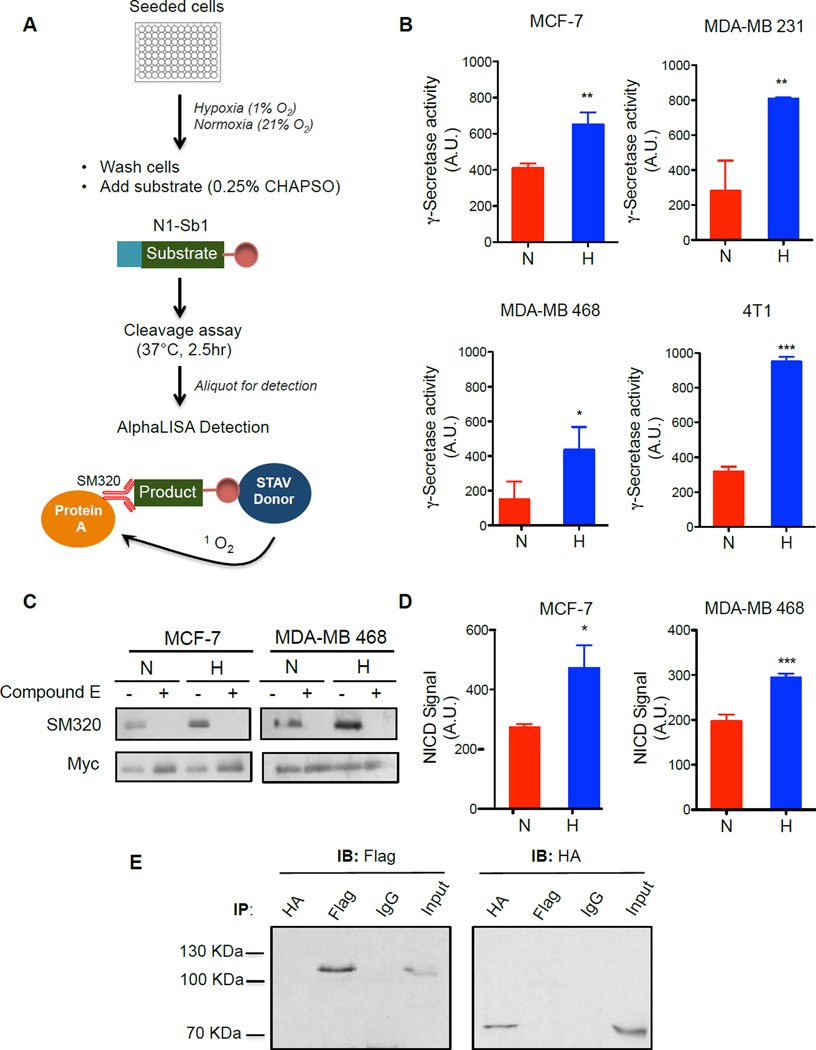
Increasing evidence suggests that the upregulation of Notch signaling promotes breast cancer development (Al-Hussaini et al., 2011; Stylianou et al., 2006). Therefore, we set out to examine if hypoxia potentiates γ-secretase activity and the production of Notch1 intracellular domain (NICD1) in breast cancer cells. To directly measure the effect of hypoxia on γ-secretase, we applied an exo-cell assay (Shelton et al., 2009a) using a recombinant Notch substrate, N1-Sb1 (Chau et al., 2012), which allows for the immediate and real-time analysis of γ-secretase activity without the requirement of cell transfections or membrane preparation (Figure 1A). Three human breast cancer cell lines (MCF-7, MDA-MB-231, MDA-MB-468) and one mouse mammary cancer cell line (4T1) were seeded in a 96-well format and were cultured under normoxic or hypoxic conditions (1% O2) for 24 hours. The recombinant substrate, N1-Sb1 was then added to the cells in the presence of the detergent, CHAPSO, and incubated for an additional 2.5 hours to allow for γ-secretase cleavage. Finally, the N1-Sb1 derived product was detected with an AlphaLISA assay (Chau et al., 2012) (Figure 1A). The specific activity of γ-secretase was determined by normalizing to protein concentration. Hypoxia led to a significant increase in γ-secretase activity in all four cell lines examined (Figure 1B). Compared with normoxia, hypoxia increased γ-secretase activity by 1.6, 2.9, 2.9 and 3.0-fold for MCF-7, MDA-MB 231, MDA-MB 468 and 4T1 cells, respectively (Figure 1B). We next examined the effect of hypoxia on γ-secretase mediated NICD1 production in a cellular setting by transfecting a myc-tagged version of N1-ΔE (N1-ΔE-myc), a truncated form of Notch1 that is cleaved by γ-secretase in a ligand-independent manner (Schroeter et al., 1998). MCF-7 and MDA-MB468 were transfected with N1-ΔE-myc for 24 hours, then cultured under normoxia or hypoxia for another 24 hours. The steady-state levels of NICD1 were then assayed by Western blot analysis. We observed a clear increase in NICD1 generation in hypoxia-treated cells compared with cells cultured in normoxic conditions (Figure 1C). The specificity of protease activity was confirmed by the use of γ-secretase inhibitor, Compound E. Further quantitative analysis with AlphaLISA assay to specially detect NICD1 showed that hypoxia led to an increase in the accumulation of NICD1 by 170% and 150% in MCF-7 and MDA-MB-468, respectively (Figure 1D). We then determined whether Hif-1α binds to NICD1 in breast cancer cells under hypoxia, as was previously reported (Gustafsson et al, 2005). Flag-NICD1 and HA-Hif-1α-ΔODD (a variant of Hif-1α that lacks the oxygen-sensing domain (ODD) and thus is stable in normoxia (Tanimoto et al., 2000)) were co-expressed in MCF-7 cells. However, reciprocal co-immunoprecipitation studies using anti-HA and anti-Flag antibodies failed to reveal any interaction between Flag-NICD1 and HA-Hif-1α-ΔODD, while the same antibodies were able to immunoprecipitate their respective targets, Hif-1α-ΔODD and NICD1, respectively (Fig 1E). Taken together, these findings strongly indicate that increase in NICD1 production under hypoxia is mediated through the upregulation of γ-secretase activity, rather than the direct interaction of Hif-1α and NICD1 (Gustafsson et al., 2005).
Figure 1. Hypoxia potentiates γ-secretase activity.
(A) Schematic of exo-cell γ-secretase activity assay. Breast cancer cells are cultured in normoxia or hypoxia for 24 hours. The level of enzyme activity was determined by the amount of cleaved N1-Sb1 substrate, as detected using NICD-specific SM320 antibody and AlphaLISA donor and acceptor beads. (B) Hypoxia increases γ-secretase activity in breast cancer cells. γ-Secretase activity is expressed as arbitrary units (A.U.). (C) Western blot analysis of NICD1 generated from γ-secretase cleavage of N1-ΔE-myc in breast cancer cells cultured in normoxia or hypoxia. The generation of NICD1 via γ-secretase cleavage was detected by SM320 antibody. Anti-Myc antibody detects expression of uncleaved N1-ΔE-myc. Compound E, a GSI abrogates the production of NICD1. (D) Quantitative measure of NICD1 production from γ-secretase cleavage using AlphaLISA detection shows increase in NICD production under hypoxia. Similar to measurements in (B), NICD was detected using AlphaLISA technology with a pair of antibodies (biotinylated anti-Myc and SM320). Data are expressed as NICD signal in arbitrary units (A.U.). (E) Reciprocal immunoprecipitation of HA-Hif-1α-ΔODD and Flag-NICD1 using HA and Flag antibodies in cells co-expressing Hif-1α and NICD1. Left, immunoblotting with Flag antibody detects only Flag-NICD1, and not HA-Hif-1α-ΔODD. Right, immunoblotting with HA antibody detected only HA-Hif-1α-ΔODD, and not Flag-NICD1. All data are presented as mean ± SD. (n ≥ 3) and two-tailed un-paired t-test was used for statistical analysis, *P<0.05, **P<0.01.
Hypoxia does not increase the level of γ-secretase subunits
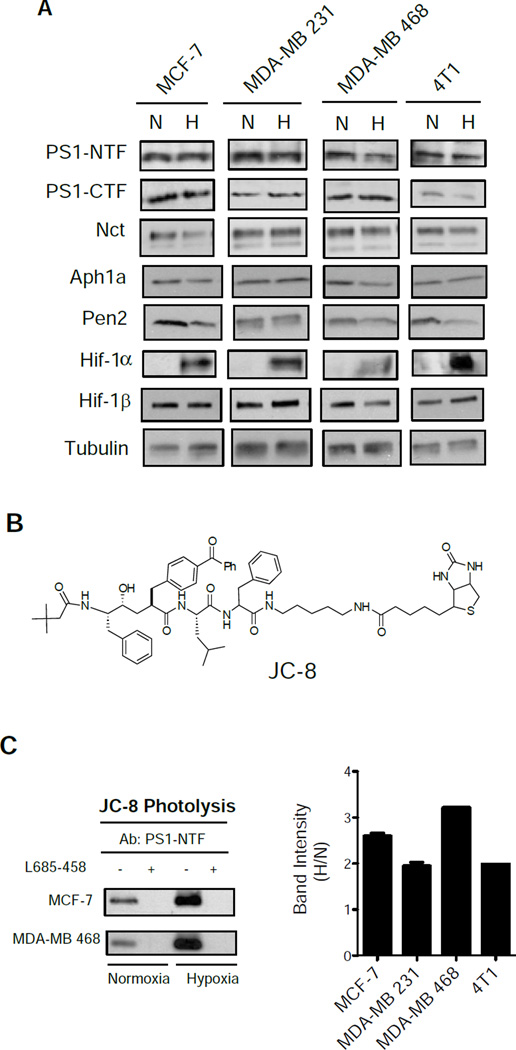
To elucidate the mechanism of the upregulation of γ-secretase activity by hypoxia, we performed Western blot analyses to examine the steady-state protein levels of each γ-secretase subunit under normoxic and hypoxic conditions. The accumulation of Hif-1α confirmed that these cells were cultured in hypoxic conditions while Hif-1β, a Hif-1α binding partner, was present under both conditions (Figure 2A). More importantly, the levels of PS1-NTF, PS1-CTF, Nct, Aph1a and Pen2 were unchanged in all cell lines cultured in normoxic or hypoxic conditions (Figure 2A). Notably, hypoxia did not increase the level of Aph1a (Figure 2A, Figure S1A), a finding inconsistent with an earlier report (Wang et al., 2006), which showed that chemical hypoxia increased the expression of Aph1a. Whether this discrepancy is the result of different treatment paradigms (1% O2 versus NiCl2) is not known. Nevertheless, our findings clearly reveal that hypoxia-induced elevation in γ-secretase activity is not the result of an increase in the overall steady-state levels of each subunit.
Figure 2. Hypoxia increases the formation of active γ-secretase complex, but not the amount of subunits.
(A) Western blot analysis of total γ-secretase subunits PS1-NTF, PS1-CTF, Nct, Aph1a, and Pen2 under hypoxia of breast cancer cell lines MCF-7, MDA-MB 231, MDA-MB 468, and 4T1. Hif-1α is only expressed under hypoxia, and Hif-1β is express in both conditions. (B) Structure of photoprobe JC-8. (C) Western blot analysis of the level of PS1-NTF in active γ-secretase formation using PS1-NTF antibody from JC-8 photolysis of MCF-7 and MDA-MB 468 cells in normoxia or hypoxia. Specificity of JC-8 labeling of the active γ-secretase is represented by addition of L-685,458 as a control to block the binding of JC-8. Quantitation of labeling intensity by JC-8 was performed in all cell lines (right).
It is known that only a small fraction of γ-secretase complexes are catalytically active (Beher et al., 2003; Crump et al., 2013; Gu et al., 2004; Lai et al., 2003; Placanica et al., 2009). Indeed, γ-secretase activity is better correlated with the levels of PS that can be crosslinked with an activity-based photoprobe, rather than the total levels of PS (Lai et al., 2003; Placanica et al., 2009). Therefore, we assessed the levels of active γ-secretase using an activity-based photo-probe, JC-8 (Chun et al., 2004) (Figure 2B), a transition-state inhibitor that only interacts with the active enzyme. Although the levels of PS1-NTF are identical in lysates of cells cultured under both conditions (see Figure 2A), we found a significantly higher level of JC-8 labeled PS1-NTF in the hypoxia-treated cells than in the normoxic cells in all four cell lines (Figure 2C). The labeling is highly specific, as addition of an excess of the parental L685-458 compound inhibited JC-8 photoinsertion into PS1-NTF (Fig 2C, left panel). These results establish that hypoxia does not potentiate γ-secretase activity by increasing the amount of individual subunits of γ-secretase.
Hif-1α is required for upregulation of γ-secretase activity
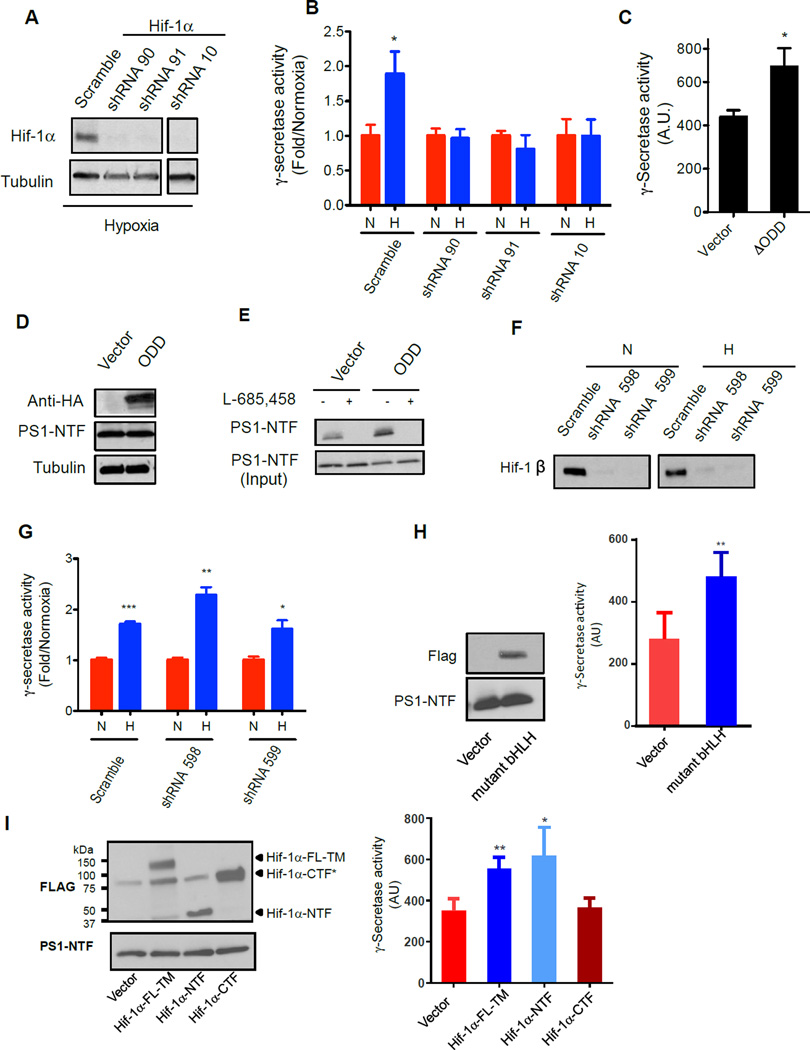
To explore the mechanism(s) by which hypoxia upregulates γ-secretase activity, we investigated Hif-1α, a transcription factor that is essential for orchestrating cellular responses in low O2 concentrations. We first asked whether shRNA-mediated suppression of Hif-1α expression affects the observed hypoxia-mediated enhancement of γ-secretase activity. Three MCF-7 cell lines (lines #90, #91 and #10) that stably express specific shRNA for Hif-1α knockdown were generated, in addition to a scramble control shRNA stable cell line. Under hypoxic conditions, Hif-1α protein was virtually undetectable in the 90, 91 and 10 cell lines, but continued to be expressed in the control line (Figure 3A). Importantly, depletion of Hif-1α in the MCF-7 knockdown cell lines eliminated hypoxia-induced augmentation of γ-secretase activity (Figure 3B), while the control cells retained the ability to potentiate γ-secretase activity upon exposure to hypoxic conditions. These findings indicate that Hif-1α is necessary to upregulate γ-secretase activity under hypoxic conditions. In support of this, expression of HA-Hif-1α-ΔODD in MCF-7 cells resulted in a 1.5-fold increase in γ-secretase activity, compared with control vector transfected cells under normoxic conditions (Figure 3C). Furthermore, the expression of HA-Hif-1α-ΔODD did not alter the overall level of PS1 (Figure 3D). However, JC-8 labeled increased the amounts of PS1-NTF (Figure 3E).
Figure 3. Hif-1α is necessary for upregulation of γ-secretase activity, but independent of its transcriptional activity.
(A) Validation of Hif-1α knockdown by western blot analysis. shRNA targeting Hif-1α were retrovirally introduced in MCF-7 cells and grown under hypoxia. Controls are non-targeting scramble sequence shRNA. (B) Effect of Hif-1α knockdown on the activity of γ-secretase in MCF-7 cells in normoxia and hypoxia. Data are expressed as fold γ-secretase activity (normoxia/hypoxia). (C) γ-Secretase activity assay of MCF-7 expressing HA-Hif-1α-ΔODD construct compared to vector control. Data are represented as arbitrary units (A.U.). (D) Expression level of HA-Hif-1α-ΔODD and PS1-NTF compared to vector only control in MCF-7 cells in normoxia. (E) Active-site photolysis of HA-Hif-1α-ΔODD overexpressing MCF-7 cells in normoxia compared to vector only control as detected using PS1-NTF antibody using western analysis. (F) Validation of Hif-1β knockdown in MCF-7 cells using retrovirally transduced shRNA in normoxia and hypoxia. (G) Effect of Hif-1β knockdown on the activity of γ-secretase in MCF-7 cells. Hif-1β knockdown cells in hypoxia exhibited greater γ-secretase activity than cells in normoxia. Data are expressed as fold γ-secretase activity (normoxia/hypoxia). (H) Expression of Flag-tagged mutant bHLH is verified by anti-Flag antibody (left panel) and led to increase in γ-secretase activity (Right panel). Data are represented as arbitrary units (A.U.). (I) Expression of Hif-1α-TM, Hif-1α-NTF and Hif-1α-CTF were confirmed by tagged Flag antibodies and had no effect on the level of PS1-NTF (Left panel). Expression of Hif-1α-TM, Hif-1α-NTF, but not Hif-1α-CTF potentiates the γ-secretase activity (right panel). All data are presented as mean ± SD (n ≥ 3) and Statistical significance is evaluated by two-tailed un-paired t-test, where *P<0.05, **P<0.01, and ***P<0.001.
We next downregulated the expression of prolyl-hydroxylase 2 (PHD2), a key enzyme that promotes the degradation of Hif-1α under normoxia (Aprelikova et al., 2004; D'Angelo et al., 2003), and examined its effect on γ-secretase. Knockdown of PHD2 by shRNA14 and 17 was confirmed by western blotting for PHD2 protein in MCF-7 cells (Figure S1B, upper panel). The knockdown of PHD2 led to an accumulation of Hif-1α levels (Figure S1B, middle panel), and an increase (2–4 fold) in γ-secretase activity in comparison to scramble control (Figure S1C). Additionally, it appears that the degree of γ-secretase activity increase could be correlated with the degree of PHD2 knockdown and Hif-1α expression.
Taken together, both the knockdown and enhancement of cellular Hif-1α levels demonstrate that Hif-1α is necessary and sufficient for the upregulation of γ-secretase activity in hypoxic conditions.
Hif-1α-mediated γ-secretase potentiation is independent of its transcriptional activity
Findings showing that γ-secretase activity is elevated under hypoxic conditions without changing the individual subunit levels suggest that Hif-1α may regulate γ-secretase through a unique mechanism. We first asked whether Hif-1α plays a non-transcriptional role in modulating γ-secretase activity. Echinomycin has been reported to be a Hif-1α inhibitor that specifically intercalates into the HRE to prevent the binding of the Hif-1α/Hif-1β complex, which is necessary to activate transcription (Wang et al., 2011) (Figure S1D, Top). After the echinomycin treatment of 4T1 cells at 0.1 and 0.3 nM under both normoxic and hypoxic conditions, we examined for the transcription of VEGF-A, a Hif-1α target gene, and found that the drug treatment reduced Hif-1α transcriptional activity in a concentration dependent manner (Figure S1D, bottom). The treatment, however, did not exert an effect on the protein expression of Hif-1α or PS1-NTF (Figure S1E). Moreover, in hypoxic breast cancer cells, chemical inhibition of Hif-1α by echinomycin did not abrogate the stimulation in γ-secretase activity (Figure S1F). These studies suggest that hypoxia augments γ-secretase activity through an unknown mechanism that is independent of the transcriptional activity of Hif-1α.
The Hif-1α and Hif-1β (ARNT) heterodimer is necessary for the transactivation and DNA binding properties of Hif-1α (Chilov et al., 1999). In order to further examine potential transcriptional roles of Hif-1α on γ-secretase activity, we suppressed Hif-1β levels using shRNA knockdown and measured γ-secretase activity in hypoxic and normoxic conditions. Hif-1β is virtually undetectable in two MCF-7 stable cell lines (#598 and # 599) that express shRNA under both conditions (Figure 3F). Notably, the stimulation of activity was still observed under hypoxia in the absence of Hif-1β expression (Figure 3G), providing another line of evidence that the transcriptional activity of Hif-1α is not required for hypoxia-induced γ-secretase potentiation.
To further test that Hif-1α can indeed non-transcriptionally regulate γ-secretase activity, we utilized a Hif-1α-mutant bHLH (Hu et al., 2006). This mutant has four conserved basic residues within the Hif-1α DNA binding domain mutated to alanines so the bHLH region can no longer bind the HRE and activate HIF inducible genes (Hu et al., 2006). We expressed the Hif-1α-mutant bHLH tagged with Flag epitope in MCF-7 cells and showed that while the level of PS1-NTF was not altered (Figure 3H, left panel), expression of this mutant was still able to increase γ-secretase activity (Figure 3H, right panel). This indicates that a transcription-deficient Hif-1α mutant is still capable of stimulating γ-secretase activity.
Lastly, to determine if full length Hif-1α is required for γ-secretase stimulation, we utilized three Flag tagged Hif constructs that include: a full length Hif-1α with triple mutations (Hif-1α-FL-TM contains P420A, P557A, and N813A mutations), a truncated Hif-1α N-terminal fragment (Hif-1α-NTF, amino acids 1–364), and Hif-1α C-terminal fragment (Hif-1α CTF, Δ10-370). Hif-1α-FL-TM is a stable construct under normoxia, Hif-1α-NTF lacks both the N-TAD and C-TAD domains that are important for transcriptional activity, and Hif-1α-CTF does not contain bHLH and PAS domains (Hu et al., 2007). All three constructs were expressed as detected by anti-Flag antibody (Figure 3I, upper panel) and had no effect on the level of PS1 (Figure 3I, low panel). The expression of Hif-1α-FL-TM or Hif-1α-NTF alone in MCF-7 breast cancer cells was able to potentiate γ-secretase activity under normoxia. Hif-1α-CTF expression, however, was unable to stimulate γ-secretase activity (Figure 3I, right panel). These observations indicate that the transcriptional activity of Hif-1α is not required for the augmentation of γ-secretase proteolytic activity, and that the point of interaction between these proteins may reside within the N-terminal domain of Hif-1α. Collectively, our data demonstrate that Hif-1α regulates γ-secretase activity independently from its transcriptional function
Hif-1α directly interacts with the γ-secretase complex
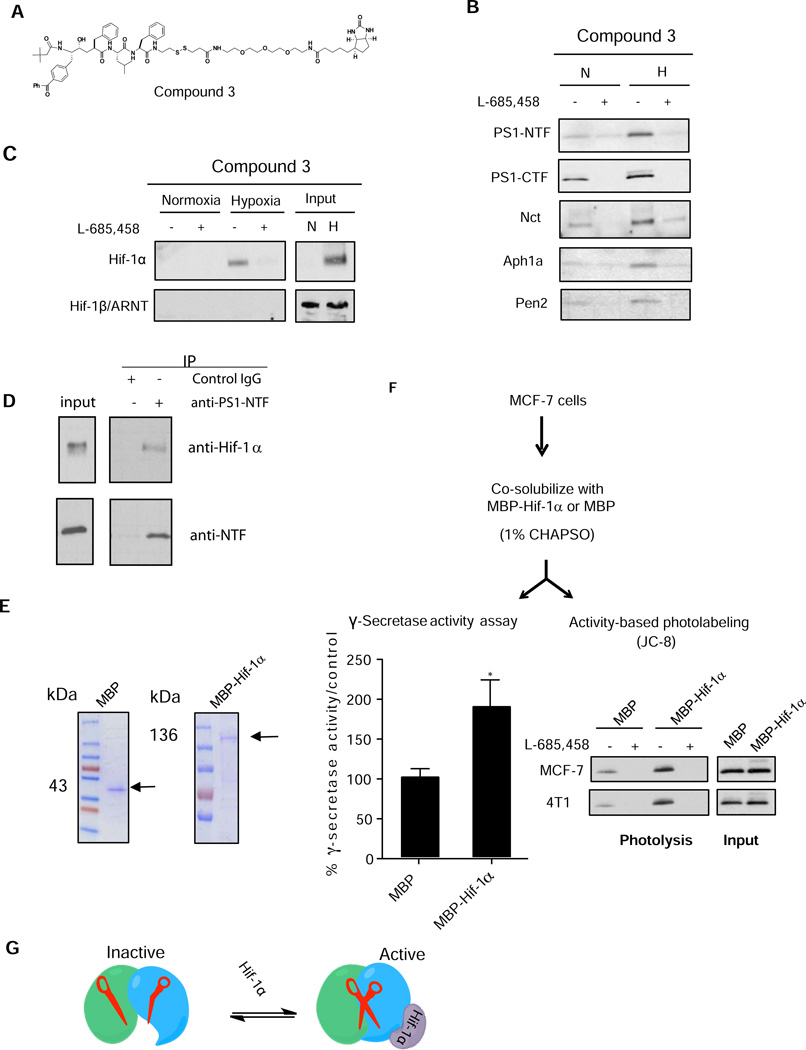
Next, we tested the hypothesis that Hif-1α modulates the activity of γ-secretase through a direct association with the enzyme complex. The γ-secretase complex was isolated using an activity-based probe, Compound 3 (Figure 4A) (Placanica et al., 2009) in order to assess if Hif-1α associates with the complex. Both Compound 3 and a non-biotinylated version of this compound exhibit the same inhibitory profiles (Figure S2A), suggesting that both are equally capable of binding to the complex and capturing γ-secretase and associated proteins. The 4T1 cells were cultured under hypoxic conditions, lysed, and solubilized with CHAPSO. Solubilized γ-secretase complexes from cell lysates and components of the isolated active complex by Compound 3 were assessed by Western blot analysis. The obligatory γ-secretase components (PS1-NTF, PS1-CTF, Aph1a, Nct, and Pen2) were captured under both normoxic and hypoxic conditions (Figure 4B). Compound 3 also captured Hif-1α from CHAPSO-solubilized fraction in hypoxia-treated cells, and not in normoxia (Figure 4C). On the other hand, Hif-1β, which exists in both normoxia and hypoxia samples, was not captured by the activity based probe, indicating that Hif-1α itself was selectively engaged with the active γ-secretase complex, and not with the Hif-1α/Hif-1β transcriptional complex. These activity-based capture studies fully support the earlier Hif-1α and Hif-1β knockdown experiments (see Figure 3 and 4). Furthermore, we found that, in hypoxia, Compound 3 captured elevated levels of all four subunits of γ-secretase specifically. This suggests that Hif-1α binding to the complex increases the overall level of active γ-secretase complexes (Figure 4B). The isolation of γ-secretase subunits and Hif-1α by Compound 3 was specific, as an excess concentration of the parent compound, L-685,458, blocked the capture of both the γ-secretase complex and Hif-1α. Further analyses of PS1 in the active complex showed that the level of captured PS1-NTF and PS1-CTF in hypoxia is approximately 3–5 fold higher than in normoxia (Figure S2B, Western blot in the top panel, quantitation of the blots in the bottom panel). We also captured Hif-1α together with the γ-secretase complex from the CHAPSO-solubilized hypoxic lysates from MDA-MB 231 and MDA-MB 468 cells as well (Figure S2C).
Figure 4. Hif-1α directly interacts with γ-secretase.
(A) Structure of γ-secretase capture probe Compound 3. (B) Affinity capture of all γ-secretase complex components by Compound 3 with normoxic and hypoxic 4T1 cell lysate and analyzed by western blot analysis. (C) Hif-1α is pulled-down with Compound 3 in 4T1 under hypoxia, while Hif-1β is not captured under either condition. Specificity of capture is demonstrated by L-685,458 (D) Hif-1α is co-immunoprecipitated with PS1-NTF by anti-PS1 antibodies whereas control rabbit IgG did not pull-down PS1 or Hif-1α. (E) Recombinant expression and purification of MBP (left) and MBP-Hif-1α fusion protein (right) in E. coli. Purified proteins were visualized on SDS-PAGE by Coomassie staining. (F) Reconstitution of Hif-1α mediated γ-secretase activity in MCF-7 cells with recombinant MBP-Hif-α or MBP. MCF-7 cells are first co-solubilized with MBP-Hif-1α or MBP in 1% CHAPSO. Lysates are then used for γ-secretase activity assay or JC-8 photolysis in 0.25% CHAPSO (Bottom panels). Stimulation of activity is expressed as % γ-secretase activity/control, where MBP-treated cell lysate was used as a control (Bottomleft). JC8 photolysis showed increased labeling of active PS1 as compared to the MBP control in both MCF-7 and 4T1 cell lines (Bottom right) All data are presented as mean ± SD. (n ≥ 3) and Statistical significance is evaluated by two-tailed un-paired t-test, where *P<0.05 and **P<0.01,. (G) Model of γ-secretase activity activation by Hif-1α. Inactive γ-secretase is incapable of catalysis and is represented by uncoordinated catalytic Asp residues, shown by broken scissors. Hif-1α converts inactive γ-secretase to the active enzyme by repositioning the catalytic dyad, as shown by functional scissors to initiate catalysis.
In normoxic conditions, Compound 3 was able to specifically capture flag-tagged Hif-1α-FL-TM in MCF-7 cells that expressed this protein, but not in control cells (Figure. S2D). Additionally, overexpression of Hif-1α-FL-TM increased the level of PS1-NTF capture as compared to control cells that did not express the construct, again to further support that the expression of Hif-1α enhances the formation of active γ-secretase complex.
To further determine that the interaction between the γ-secretase complexes and Hif-1α, we utilized immunoprecipitation to assess their direct association. Immunoprecipitation of CHAPSO-solubilized MCF-7 cells cultured under hypoxia using an anti-PS1 antibody was able to pull-down both PS1-NTF (Figure 4D, lower panel) and Hif-1α (Figure 4D, upper panel). Equivalent amounts of control non-specific rabbit IgG did not precipitate PS1 or HIF-1α (Figure 4D). These findings strongly support our hypothesis that Hif-1α directly interacts with the γ-secretase complex.
In addition, we fractionated CHAPSO-solubilized MCF-7 lysate using a 10–40% glycerol density gradient to analyze the distribution of γ-secretase subunits and Hif-1α (Figure S2E). Western blot analysis of the γ-secretase complex components showed similar distribution of overall protein subunit in both normoxic and hypoxic samples (Figure S2E). Hif-1α was mainly detected in fractions #3–8 and #11 of the hypoxic lysate. To further determine the level of association of Hif-1α with the active γ-secretase complex, each fraction was subjected to Compound-3 capture. Using anti-Hif-1α and anti-PS1 antibodies, western blot analysis identified that fraction #11 contains the most highly active fraction, as indicated by the presence of PS-NTF labeled under both conditions (Figure S2F). This data is consistent with a previous study, which reported that a γ-secretase complex with a molecular mass of greater than 660 kDa is competent for protease activity (Gu et al., 2004). Our study shows that although higher levels of Hif-1α were present in fractions #3–8 as compared to #11 in the hypoxic sample, only Hif-1α from fraction #11 was captured. This clearly demonstrates that the capture of Hif-1α with Compound 3 is dependent on the presence of active γ-secretase complex and that Compound 3 does not bind to Hif-1α alone. These findings indicate that Hif-1α acts as a regulatory subunit of γ-secretase and enhances the level of active complex by recruiting and activating otherwise inactive complexes, leading to an increase in γ-secretase activity.
To directly examine the role of Hif-1α in stimulating γ-secretase activity, we developed an in vitro system using recombinant Hif-1α. We expressed full-length Hif-1α fused to the maltose-binding protein (MBP) at the N-terminus and MBP itself in E. coli. Both MBP-Hif-1α and MBP were purified to homogeneity with Amylose affinity chromatography (Figure 4E). MBP-Hif-1α or MBP was co-solubilized with MCF-7 cell lysates for one hour at 4°C in the presence of 1% CHAPSO detergent. The solubilized fraction was then diluted to 0.25% CHAPSO and assayed for γ-secretase activity using the in vitro activity assay or the activity-based photoprobe, JC-8 (Figure 4F). Addition of MBP-Hif-1α to MCF-7 cell lysates significantly enhanced γ-secretase activity by ~2-fold compared to the control. (Figure 5F, left bottom panel). Similarly, the activity-based labeling detected higher levels of PS1-NTF in MCF-7 cell lysates reconstituted with MBP-Hif-1α than with lysates containing MBP (Figure 4F, right bottom panel). Thus, we have demonstrated that recombinant Hif-1α, alone, was sufficient to potentiate γ-secretase activity and activity-based labeling of PS1-NTF. These biochemical reconstitution studies, along with our demonstration of the co-capture of endogenous Hif-1α with the native γ-secretase complexes in hypoxic cultured cells and co-immunoprecipitation of PS1 and Hif-1α (Figure 4B–4D), establish that Hif-1α operates as a regulatory γ-secretase subunit under hypoxic conditions. Mechanistically, photoaffinity labeling and the capture studies using the activity-based probes suggest that Hif-1α converts a population of inactive complexes to active complexes and thus upregulates γ-secretase activity through a mechanism that is independent of its canonical accepted transcriptional function (Figure 4G).
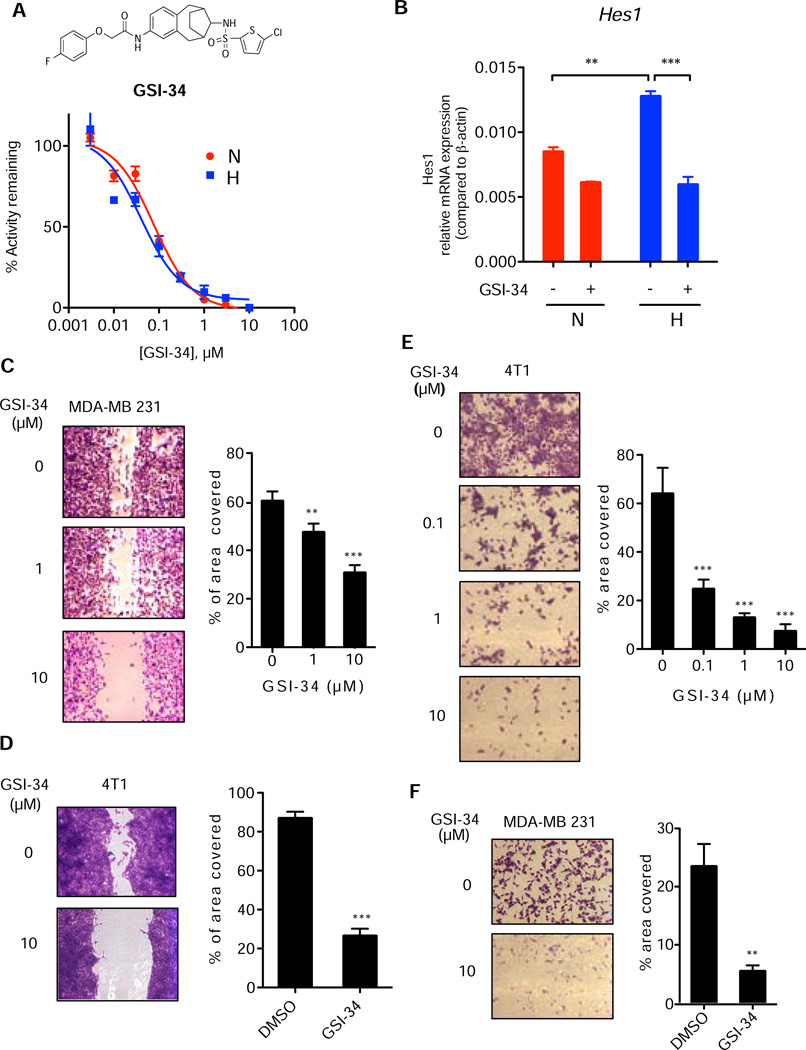
Figure 5. γ-Secretase inhibitor blocks hypoxia promoted cell invasion.
(A) Structure of GSI-34, a sulfonamide GSI, and IC50 using 8X-CBF-fireflyluciferase Notch reporter assay under normoxic and hypoxic conditions. (B) Quantitative RT-PCR analysis of Hes1 expression in breast cells in hypoxia or normoxia in the presence and absence of GSI-34. Data are expressed as relative mRNA expression compared to β-actin. (C) Sensitivity of MDA-MB 231 cell migration to GSI-34 in wound-healing assay. Quantitation by Image J analysis of % of area covered by cells is represented in the right panel. Data are represented as mean (±s.d.) and expressed as % of area covered. (D) Sensitivity of 4T1 cell migration to GSI-34 in wound-healing assay. (E) Matrigel invasion assay of 4T1 cells with 0.1, 1 and 10 µM GSI-34; Statistical significance was analyzed using one-way ANOVA, ***P<0.001. (F) Matrigel invasion assay of MDA-MB 231 cells with 10 µM GSI-34. All data are presented as mean ± SD. (n ≥ 3) and statistical significance is evaluated by two-tailed un-paired t-test otherwise stated, where *P<0.05, **P<0.01, and ***P<0.001.
γ-Secretase inhibitor blocks hypoxia-induced cell invasion and metastasis in cells and in vivo
Hypoxia drives cancer cells to become more malignant, a phenotype that is marked by an increase in cellular migration and invasion (Denko, 2008). To examine the role of hypoxia-induced γ-secretase activity in breast cancer, we determined the migratory propensity of 4T1 and MDA-MB 231, two invasive breast cancer cell lines, in the absence and the presence of GSI-34, a potent γ-secretase inhibitor (Figure 5A top) (Placanica et al., 2010; Shelton et al., 2009a). GSI-34 has an IC50 of approximately 40–70 nM in a cell-based Notch1 reporter assay under both normoxic and hypoxic conditions (Figure 5A bottom). Moreover, inhibition of hypoxia-activated Notch signaling was demonstrated by the suppression of Hes1 mRNA levels in 4T1 cells using GSI-34 (Figure 5B). We then determined the effect of γ-secretase inhibition on cellular migration using a wound-healing assay under hypoxia. GSI-34 attenuated wound closure of MDA-MB 231 cells by 21% and 50% at 1 and 10 µM, respectively (Figure 5C). Similarly, GSI-34 also significantly blocked cell migration of 4T1 cells (Figure 5D).
To assess the effect of γ-secretase inhibition on cell invasiveness, 4T1 cells or MDA-MB 231 cells were seeded in non-serum media onto Matrigel coated transwell inserts and allowed to invade overnight under hypoxia through wells with serum containing media. GSI-34 treatment effectively prevented the invasion of 4T1 cells through the Matrigel platform in a concentration dependent manner; concentrations of 0.1, 1, and 10 µM led to a reduction in cell invasion by 62%, 78%, and 89%, respectively (Figure 5E). Similarly, the compound reduced the invasiveness of MDA-MB 231 cells under hypoxia. GSI-34 at 10 µM was able to reduce MDA-MB 231 cell invasion by 77% (Figure 5F). In addition, while there appears to be some cellular toxicity at 10µM, GSI-34 at lower concentrations did not affect cell viability (Figure S3). These results demonstrate that inhibition of γ-secretase significantly blocks hypoxia-induced invasion of MDA-MB 231 and 4T1 cells.
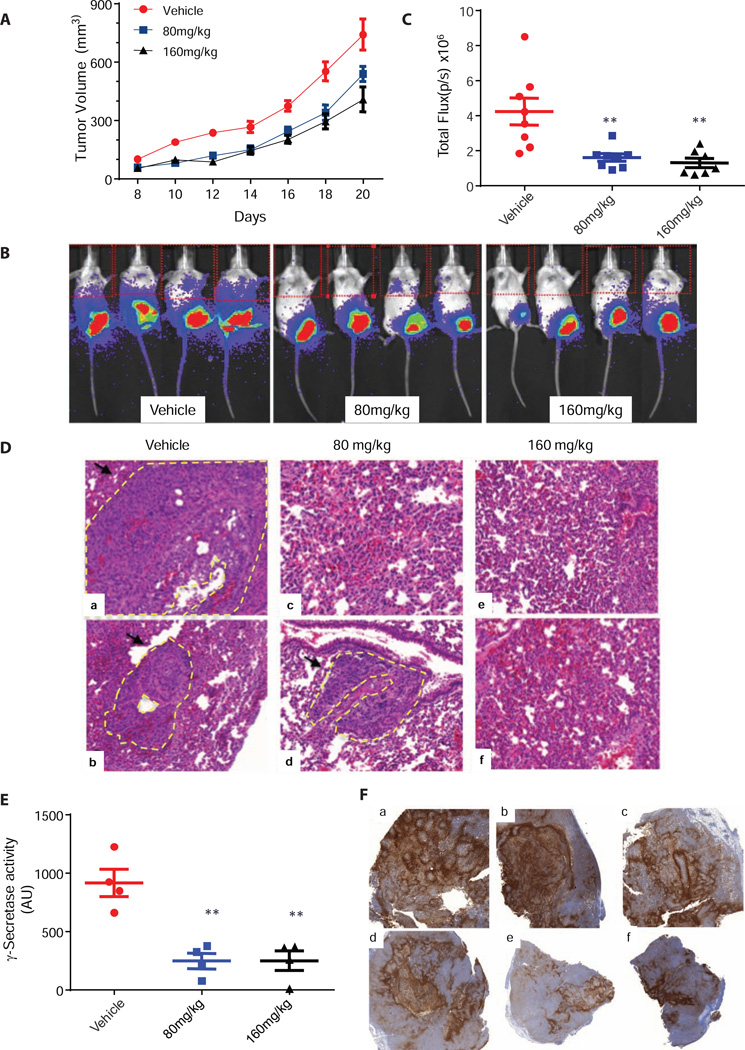
To determine the effect of γ-secretase inhibition on tumor migration and metastasis in vivo, we utilized a luciferase expressing breast cancer cell line 4T1/luc, frequently used to model stage IV breast cancer (Tao et al., 2008). The 4T1/luc mouse mammary cancer cell line can be orthotopically injected into the mammary fatpad. Stable expression of firefly luciferase and in vivo luminescence imaging allows for longitudinal monitoring of tumor growth and metastasis to distant organ sites, such as the lung, a common first site of metastasis (Tao et al., 2008). The 4T1/luc cells (1 million) were implanted at the inguinal mammary fat-pad of female BALB/c mice on day 0. At day 5 post-implantation, animals were imaged for luciferase activity and all mice with similar tumor loads were randomized and separated into 3 treatment groups (vehicle, 80 and 160 mg/kg of GSI-34). Animals received treatment with GSI-34 by intraperitoneal (IP) injection a total of 7 times. No toxicities including weight loss and diarrhea were observed in these mice. From day 5 onward, primary tumor volume was measured every other day with calipers. GSI-34 moderately reduced primary tumor size by 27% and 40% at 80 mg/kg and 160 mg/kg, respectively (Figure 6A).
Figure 6. γ-Secretase inhibition by GSI-34 decreased metastatic progression of 4T1/luc in vivo.
(A) GSI-34 treatment led to a reduction of tumor volume. GSI-34 at 80mg/kg and 160mg/kg reduced primary tumor volume by 27% and 40% respectively by day 20 compared with the vehicle (Average ± SEM, n=8). (B) In vivo luciferase images of tumors. The representative luciferase signal emitted upon luciferin injection of GSI-34 treated and untreated animals. Signal at the lower part of the animal signifies primary tumor, upper thoracic shows signal emitted from 4T1/luc metastasis in the lung region. (C) The effect of GIS-34 treatment on 4T1 lung metastasis. Luciferase signal detected as total flux (p/s) quantitated using based on the upper quadrant of the animal by LivingImage software (Average ± SEM, n=8). (D) H&E Staining. Excised lung were stained analyzed using H&E staining method to detect 4T1/luc colonization of the lung. Lungs from vehicle treated animals (A–B) contain large metastatic lesions while GSI-34 (C–F) treated animals exhibited smaller and less frequent metastatic sites. Metastatic lesions are marked with black arrows and yellow dotted lines. (E) Ex vivo γ-Secretase activity of primary tumor samples. Primary tumor samples were homogenized assayed for ex vivo γ-secretase activity. GSI-34 treatment decreased level of γ-secretase activity compared to tumors from DMSO treated animals (Average ± SEM, n=4). (F) Immunohistochemical staining of Glut-1. Glut-1 marker was used to stain for the prevalence of tumor hypoxia in the primary tumors. In all samples (A–F) there was a strong indication of tumor hypoxia (brown staining) in multiple regions of each tumor sample
To monitor the effect of GSI-34 on the distant metastasis of 4T1/luc cells, mice were injected with D-luciferin and imaged for luminescence. Progression of metastasis was monitored by quantitation of bioluminescence signal in the thoracic region of the animal. We were able detect lung metastases in control mice on day 18. Animals treated at both doses of GSI-34 exhibited reduction in distant metastasis compared to the control group, as determined by the quantification of total flux emitted by the 4T1/luc cells (Figure 6B and 6C) on day 20. Immediately following whole animal imaging, the lungs from four random representative animals of each treatment group were harvested and imaged ex vivo. Three out of four excised lungs from the control group were positive for 4T1/luc, while in the 80 mg/kg GSI-34 treatment group, only one lung was luciferase positive. We did not detect any luminescence signal in the animals treated with GSI-34 at 160mg/kg. Next, we assessed the effect of GSI-34 treatment on γ-secretase activity in primary 4T1/luc tumors ex vivo. Tumor lysate of the primary tumors were assayed for γ-secretase activity using the N1-Sb1 substrate as previously described. GSI-34 treatment demonstrated dose dependent inhibition of γ-secretase activity in tumor tissue membrane (Figure 6E). Overall, GSI-34 treatment resulted in reduction of 4T1/luc tumor burden, distant metastases, and γ-secretase activity in tumor lysates.
Lung metastases were examined using histological techniques on day 22, Hematoxylin and eosin (H&E) staining of formalin fixed lung samples showed differential degree of cancer cell colonization in lungs of control and treated mice. Lungs from control animals exhibited a higher incidence of metastatic lesions and larger metastatic foci compared to lungs from GSI-34 treated animals (Figure 6D). Tumor hypoxia within primary tumors was examined by immunohistochemical staining for glucose transporter-1 (Glut-1), a marker shown to correlate with hypoxia (Airley et al., 2003; Vleugel et al., 2005). Glut-1 staining was observed in all samples at a similar level, indicating hypoxic regions within the tumors were present in both treated and untreated samples (Figure 6F).
Downregulation of Notch signaling by DnMAML1 reduced lung metastasis in vivo
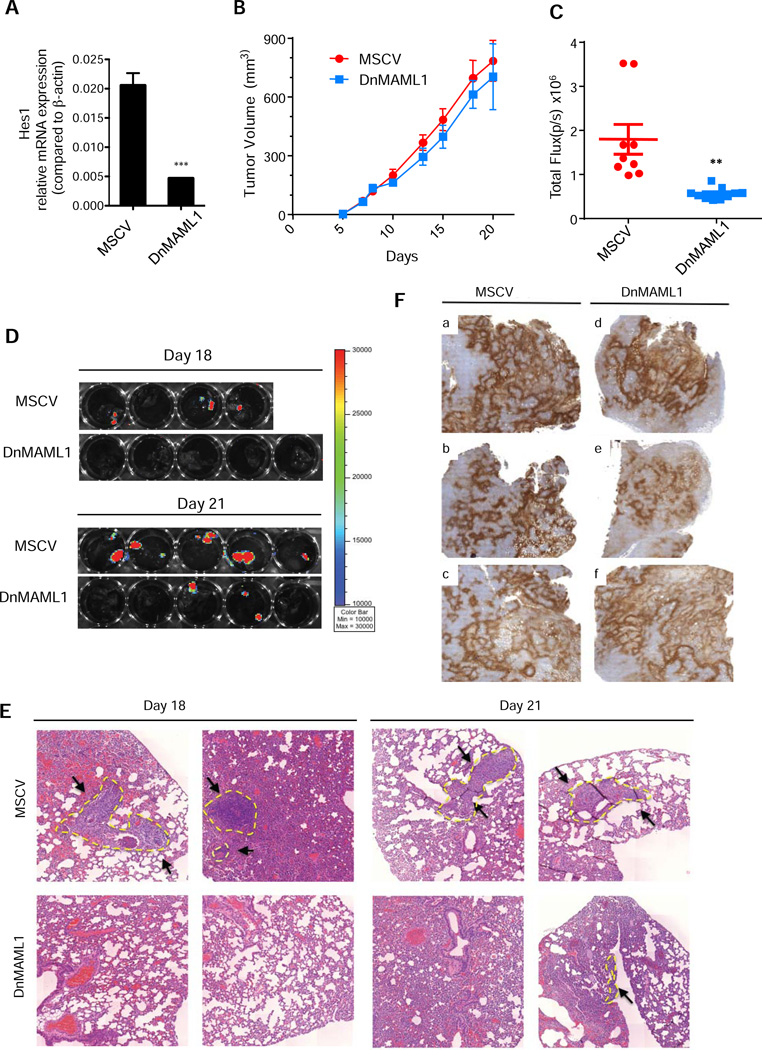
We next investigated whether the effect of GSI-34 treatment on breast cancer cell invasion and metastasis was mediated through the Notch signaling pathway. We thus created a dominant negative Mastermind-like1 (DnMAML1) version of the 4T1/luc cell line that can effectively block Notch signaling (Nam et al., 2003). The stable expression of DnMAML1 in 4T1 cell line showed reduced expression of Hes1 mRNA, indicating that Notch signaling is significantly impaired (Figure 7A). Moreover, DnMAML1 expression did not affect 4T1 cell proliferation or γ-secretase activity in normoxic or hypoxic conditions (Figure S4A–4B). Cell invasion under hypoxia was greatly enhanced in empty vector expressing control, whereas DnMAML1 cells failed to invade through the Matrigel (Figure S4C–4D). Notably, the degree of cell invasiveness under hypoxia for DnMAML1 cells was similar to control cells treated with GSI-34. In both cases, there was a reduction in cell invasion by approximately 50% compared with the control, untreated cells (Figure S4C–4D). These experiments strongly suggest that γ-secretase mediated Notch signaling is the driver of cellular invasion processes and does not play a significant role in the proliferation of breast cancer in hypoxic conditions.
Figure 7. Effect of Down regulation of Notch signaling by DnMAML on tumor growth and metastasis in vivo.
(A) DnMAML1 cells express reduced levels of Notch downstream gene Hes1 compared to control cells as determined by qRT-PCR. (B) Effect on tumor volume. Animals bearing control 4T1 cells and DnMAML1 4T1 cells exhibited similar tumor latency and growth rate throughout the progression of the course of the experiment (Average ± SEM, n=10). (C) Metastatic progression of DnMAML1 and control animals in vivo. The same procedure in Figure 7B was used for the analysis of lung metastasis Luciferase signal detected expressed as total flux (p/s) quantitated by LivingImage software (Average ± SEM, control (n= 9), DnMAML1 (n=10)). (D) Ex vivo biohphotonic imaging of DnMAML1 and control animals Day 18 and Day 21. Excised lung samples were imaged using the Xenogen IVIS imaging system and analyzed with LivingImage software for luciferase signal from control and DnMAML1 4T1 bearing animals sacrificed on Day 18 and Day 21. Control animals exhibited greater incidence and larger metastatic foci of lung metastasis than DnMAML1 animals on both days. (E) H&E staining for metastasis of DnMAML1 and control lung. Histological analysis revealed large metastatic legions in control animals on both days, whereas DnMAML1 animals exhibited smaller micrometastatic sites. Metastatic sites are marked with yellow dotted lines and pointed with black arrows. (F) Glut-1 staining for tumor hypoxia of DnMAML1 and control 4T1 tumors. Glut-1 marker is an indicator for the prevalence of tumor hypoxia in the primary tumors of control and DnMAML1 animals. In all samples (A–F) there was a strong indication of tumor hypoxia (brown staining) at multiple regions of the tumor. Images were taken at 2× magnification.
To further understand whether Notch signaling mediates breast cancer metastases in vivo, we orthotopically implanted 1 million DnMAML-1 4T1/luc cells or control 4T1/luc cells into the mammary fatpad of Balb/C mice. Primary tumor volume was measured over the course of the 21-day period and metastatic spread was monitored by luminescence imaging analysis. The progression of primary tumor growth in DnMAML1 bearing mice and control mice were very similar (Figure 7B), supporting that the downregulation of Notch signaling did not have major anti-proliferative effects. On day 18, luminescence signals from the thoracic region the animals injected with MSCV control cells could be detected, indicating metastatic spread, compared to little or no signal from the DnMAML1 animals (Figure 7C).
On day 18, we randomly chose half of the mice from each group to monitor metastatic progression to the lung. Three of the four mice from the control group had lung metastases, while no mice (0/5) from the DnMAML1 group displayed lung metastases (Figure 7D, upper panel). By day 21, all control animals (5/5) were positive for lung metastases with large metastatic lesions, only 2 of the 5 DnMAML1 mice had lung metastasis (Figure 7D, lower panel).
We did not include a control mouse for ex vivo imaging on day 18 since it had died just prior to our analysis. However, H&E lung staining from this mouse did reveal large portions of the lung invaded with 4T1/luc cells (Figure 7E Day 18 top left). H&E analysis indicated that 9 out of the 10 of the animals in the control group were presented with lung metastases, compared with 2 out of 10 of the DnMAML1 animals. Similar to animals treated with GSI-34, we harvested primary tumors and stained for hypoxia marker Glut-1. We observed obvious hypoxic regions that were prevalent in both control and DnMAML1 tumors (Figure 7F). Furthermore, we did not detect marked differences in the γ-secretase activity of these tumors on day 18 (Figure S4E). Consistent with our previously mentioned in vitro data, DnMAML1 tumors also displayed decreased expression of Hes1 mRNA (Figure S4F). Together, these data demonstrate that Notch signaling has a profound influence in driving cancer metastasis in hypoxic tumors. γ-Secretase inhibition by GSI-34 and the downregulation of notch signaling by DnMAML1 were able to reduce the tendency for metastasis to the lung in a highly metastatic 4T1 breast cancer metastasis model. The inhibition of γ-secretase potently suppresses both migration and invasion of breast cancer cells, and therefore could be a potential therapeutic candidate for the treatment and/or delay the onset of metastatic breast cancer.
DISCUSSION
The present study reveals a novel mechanism of γ-secretase regulation and an unprecedented role for Hif-1α that is independent of its function as a transcriptional regulator during hypoxia. First, we discovered a new role for Hif-1α that is entirely distinct from both the canonical and non-canonical Hif-1α pathways previously reported. The essential and non-transcriptional role of Hif-1α in the modulation of γ-secretase is supported by the following observations: 1) knockdown of Hif-1α abolishes the potentiation of γ-secretase activity under hypoxia and upregulation of Hif-1α level stimulates γ-secretase activity in normoxia (Figure 3), and 2) chemical and genetic inhibition of the transcription function of Hif-1α do not affect the stimulation of γ-secretase activity (Figure 3 & Figure S1). Secondly, we demonstrated that γ-secretase displays enzymatic plasticity in response to hypoxia through a mechanism that involves a direct interaction with Hif-1α. The binding of Hif-1α to the γ-secretase complex is demonstrated by multiple lines of evidence: 1) Hif-1α was co-captured by activity-based affinity probe (Figure 4); 2) Hif-1α was co-immunoprecipitated with PS1 using an anti-PS1 antibody (Figure 4). Hif-1α is co-migrated and co-captured with the more active, high molecular weight γ-secretase complex (Figure S2). Finally, the Hif-1α-containing γ-secretase complex directly contributes to the migratory and invasive phenotype of breast cancer cells through the enhancement of Notch cleavage in cells and in vivo (Figure 6 and 7). Inhibition of γ-secretase activity or downregulation of Notch signaling reduced the invasion and metastasis of breast cancer cells. In this regard, our studies have uncovered a pathway to modulate Notch signaling and offer a potential drug target for hypoxia-driven metastatic breast cancer.
The enhancement of γ-secretase activity under hypoxic conditions relies on two critical factors: inactive γ-secretase complexes and Hif-1α. While the existence of the inactive γ-secretase complex has been established (Beher et al., 2003; Gu et al., 2004; Lai et al., 2003; Placanica et al., 2009), the function of these complexes has been elusive. Our biochemical and cellular studies demonstrate that Hif-1α converts inactive complexes into active complexes, suggesting that the inactive complex serves as a zymogen for activation when enzyme activity is required. γ-Secretase seizes the major hypoxia regulator, Hif-1α, for its activation and to achieve temporal regulation. It will be of interest to further investigate whether this particular mechanism for regulation of γ-secretase also holds true for other forms of cellular stress and environmental cues.
How does Hif-1α upregulate γ-secretase activity? Results from studies using active site-directed inhibitors offer a critical understanding of the mechanism underlying the activation of the inactive γ-secretase complex. We suggest that the orientation and/or coordination of the two catalytic Asp residues within the inactive complex is unsuitable for catalysis, and that the binding of Hif-1α to the inactive complex leads to the repositioning of the catalytic dyad to generate an active form of the enzyme (Figure 4G). However, further insight into the precise structural changes in γ-secretase that are induced by Hif-1α will no doubt require high resolution structures of γ-secretase, which are currently unavailable. It will be important to identify peptides or small molecules that can block or disrupt the interaction of Hif-1α and γ-secretase to precisely investigate the role of Hif-1α in modulating γ-secretase/Notch signaling in cancer and other human disorders.
In the canonical Hif-1α pathway, Hif-1α functions as a master transcriptional regulator of the hypoxic response pathway and has been widely investigated in various physiological and pathological conditions (Buchler et al., 2003; Semenza, 2002). In addition, Hif-1α has been shown to interact with other proteins, such as pyruvate kinase M2 (Luo et al., 2011) and presenilin (De Gasperi et al., 2010) to enhance its stability and promote Hif-1α signaling. Presenilin can also regulate the Hif-1α pathways to enhance the expression of Hif-1α through the cleavages of amyloid precursor protein (APP) to generate APP intracellular domain (Kaufmann et al., 2013). Despite previous report that Hif-1α can bind to NICD1 to promote Notch signaling, our present study clearly demonstrates that Hif-1α binds to γ-secretase and directly modulates its enzymatic activity. This finding is distinct from both canonical and previously reported non-canonical roles of Hif-1α. We also determine that the Hif-1α protein is indispensable for the upregulation of γ-secretase activity under hypoxia, a process that is independent of its transcriptional activity. Moreover, we demonstrate that recombinant Hif-1α binds to and activates the γ-secretase complex. For the first time, we identify Hif-1α as the non-essential subunit of γ-secretase that can shift and modulate enzyme equilibrium from the inactive complex to the active form.
γ-Secretase inhibitors (GSIs) are on trial for the treatment for a variety neoplasms and some clinical benefit has been observed (Gounder and Schwartz, 2012; Krop et al., 2012; Schott et al., 2013; Tolcher et al., 2012; Wei et al., 2010). Notch signaling plays an important role in cancer metastasis (Sethi and Kang, 2011) and GSI treatment reduced Notch-mediated bone metastasis of breast cancer. (Sethi et al., 2011). Our studies further indicate that GSIs can potentially be used as anti-invasive and anti-metastatic agents and more specifically in combination with chemotherapy and radiotherapy as effective adjuvants to prevent or delay the onset of metastatic disease.
The impact of hypoxia-mediated responses is not limited to cancer. Hypoxia may influence neurological disease pathology, such as Alzheimer’s disease and other age-related disorders. Hypoxia has been shown to increase β-amyloid production (Li et al., 2009), and accelerates the deposition of amyloid plaques in AD transgenic mouse models (Sun et al., 2006). Oxygen availability also regulates Notch signaling to influence stem cell differentiation, developmental programming (Simon and Keith, 2008), as well as the outcome of ischemic stroke (Arumugam et al., 2006). Understanding the regulation γ-secretase under physiological and pathological conditions will facilitate the development of selective therapies to combat and control the molecular pathogenesis and progression of both cancer and Alzheimer’s disease.
EXPERIMENTAL PROCEDURES
Compounds
JC-8, Compound 3, Compound E, GSI-34, and L-685,458 are all synthesized in our laboratory (Chun et al., 2004; Placanica et al., 2009; Shelton et al., 2009a; Yang et al., 2009) and dissolved in DMSO.
Exo-cell γ-secretase activity assay
Breast cancer cell lines were seeded in 96-well culture plates and incubated either under normoxic or hypoxic setting for 24 hours. Exo-cell γ-secretase activity was assayed as described (Shelton et al., 2009a) using N1-Sb1 substrate (Chau et al., 2012) (See supplemental information for detail)
Activity-based photoaffinity labeling and capture
Activity-based photoaffinity labeling was performed with breast cancer cell lines in 12-well tissue culture dish with 10 nM of JC-8 (Li et al., 2000; Shelton et al., 2009b). Capture of the active γ-secretase complex was first performed under non-denaturing conditions using compound 3 (Placanica et al., 2009). (See supplemental information for detail)
Reconstitution studies
Breast cancer cells (MCF-7 and 4T1) were co-solubilized with MBP or MBP-Hif-1α in PIPES buffer containing 1% CHAPSO for 1 hour at 4°C. Solubilized fractions were used for assaying γ-secretase activity and activity-based photolabeling (See supplemental information for detail).
Mouse mammary metastasis model and bioluminescence imaging
Animal studies were performed according to protocols approved by the Memorial Sloan Kettering Cancer Center animal facility (See supplemental information for detail).
Immunohistochemistry
Primary tumor and lung samples were immediately fixed upon dissection in 4% paraformaldehyde (v/v) in PBS overnight at 4°C 0See supplemental information for detail).
Supplementary Material
HIGHLIGHTS.
Hypoxia increases the activity of γ-secretase through the non-canonical role of Hif-1α.
Hif-1α directly interacts with γ-secretase to temporally upregulate enzyme activity.
γ-Secretase inhibition reduces hypoxia-induced cancer cell invasion and metastasis.
ACKNOWLEDGMENTS
We thank the Molecular Cytology Facility and the Comparative Pathology Facility in Memorial Sloan-Kettering Cancer Center (MSKCC) for tissue processing, pathology and H&E analyses. We would like to thank Drs. Xuejun Jiang and Pengbo Zhou for advice and comments on the manuscript; Drs. Christina Crump, Deming Chau and Aneesh Sheth for discussions and suggestions regarding this manuscript. Dr. Warren Pear (University of Pennsylvania) for the MigR1-GFP or MigR1-DnMAML1-GFP constructs; and Dr. Hakim Djaballah for the Hif-1α shRNA constructs (MSKCC). This work is supported by NIH RO1 grant AG026660-6A1 (YML), CA055349 (DAS), training grant NIH T32 GM073546 (JCV and DC), the Geoffrey Beene Cancer Research Center of Memorial Sloan-Kettering Cancer Center, Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research, the Experimental Therapeutics Center of MSKCC, and the William Randolph Hearst Fund in Experimental Therapeutics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Extended Experimental Procedures and five figures.
Author Contributions
JCV, DC, AHB carried out biochemical and cellular studies. JCV, FEE, CHV, WFM and EDS conducted in vivo studies. CJH and MCS provided HIF constructs and experimental design. JCV, DC, AHB, SSS, DAS and YML designed experiments and prepared manuscript. DC and AHB contributed equally to this manuscript.
REFERENCES
- Ahn K, Shelton CC, Tian Y, Zhang X, Gilchrist ML, Sisodia SS, Li Y-M. Activation and intrinsic γ-secretase activity of presenilin 1. Proc Natl Acad Sci U S A. 2010;107:21435–21440. doi: 10.1073/pnas.1013246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airley RE, Loncaster J, Raleigh JA, Harris AL, Davidson SE, Hunter RD, West CM, Stratford IJ. GLUT-1 and CAIX as intrinsic markers of hypoxia in carcinoma of the cervix: relationship to pimonidazole binding. International journal of cancer Journal international du cancer. 2003;104:85–91. doi: 10.1002/ijc.10904. [DOI] [PubMed] [Google Scholar]
- Al-Hussaini H, Subramanyam D, Reedijk M, Sridhar SS. Notch signaling pathway as a therapeutic target in breast cancer. Mol Cancer Ther. 2011;10:9–15. doi: 10.1158/1535-7163.MCT-10-0677. [DOI] [PubMed] [Google Scholar]
- Aprelikova O, Chandramouli GV, Wood M, Vasselli JR, Riss J, Maranchie JK, Linehan WM, Barrett JC. Regulation of HIF prolyl hydroxylases by hypoxia-inducible factors. J Cell Biochem. 2004;92:491–501. doi: 10.1002/jcb.20067. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Chan SL, Jo DG, Yilmaz G, Tang SC, Cheng A, Gleichmann M, Okun E, Dixit VD, Chigurupati S, et al. Gamma secretase-mediated Notch signaling worsens brain damage and functional outcome in ischemic stroke. Nat Med. 2006;12:621–623. doi: 10.1038/nm1403. [DOI] [PubMed] [Google Scholar]
- Beher D, Fricker M, Nadin A, Clarke EE, Wrigley JD, Li YM, Culvenor JG, Masters CL, Harrison T, Shearman MS. In vitro characterization of the presenilin-dependent gamma-secretase complex using a novel affinity ligand. Biochemistry. 2003;42:8133–8142. doi: 10.1021/bi034045z. [DOI] [PubMed] [Google Scholar]
- Buchler P, Reber HA, Buchler M, Shrinkante S, Buchler MW, Friess H, Semenza GL, Hines OJ. Hypoxia-inducible factor 1 regulates vascular endothelial growth factor expression in human pancreatic cancer. Pancreas. 2003;26:56–64. doi: 10.1097/00006676-200301000-00010. [DOI] [PubMed] [Google Scholar]
- Chau DM, Crump CJ, Villa JC, Scheinberg DA, Li YM. Familial Alzheimer Disease Presenilin-1 Mutations Alter the Active Site Conformation of gamma-secretase. The Journal of biological chemistry. 2012;287:17288–17296. doi: 10.1074/jbc.M111.300483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Hasegawa H, Schmitt-Ulms G, Kawarai T, Bohm C, Katayama T, Gu Y, Sanjo N, Glista M, Rogaeva E, et al. TMP21 is a presenilin complex component that modulates gamma-secretase but not epsilon-secretase activity. Nature. 2006;440:1208–1212. doi: 10.1038/nature04667. [DOI] [PubMed] [Google Scholar]
- Chilov D, Camenisch G, Kvietikova I, Ziegler U, Gassmann M, Wenger RH. Induction and nuclear translocation of hypoxia-inducible factor-1 (HIF-1): heterodimerization with ARNT is not necessary for nuclear accumulation of HIF-1alpha. J Cell Sci. 1999;112(Pt 8):1203–1212. doi: 10.1242/jcs.112.8.1203. [DOI] [PubMed] [Google Scholar]
- Chun J, Yin YI, Yang G, Tarassishin L, Li YM. Stereoselective Synthesis of Photoreactive Peptidomimetic gamma-Secretase Inhibitors. J Org Chem. 2004;69:7344–7347. doi: 10.1021/jo0486948. [DOI] [PubMed] [Google Scholar]
- Crump CJ, Johnson DS, Li Y-M. Development and Mechanism of γ-Secretase Modulators for Alzheimer’s Disease. Biochemistry. 2013;52:3197–3216. doi: 10.1021/bi400377p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo G, Duplan E, Boyer N, Vigne P, Frelin C. Hypoxia up-regulates prolyl hydroxylase activity: a feedback mechanism that limits HIF-1 responses during reoxygenation. J Biol Chem. 2003;278:38183–38187. doi: 10.1074/jbc.M302244200. [DOI] [PubMed] [Google Scholar]
- De Gasperi R, Gama Sosa MA, Dracheva S, Elder GA. Presenilin-1 regulates induction of hypoxia inducible factor-1alpha: altered activation by a mutation associated with familial Alzheimer's disease. Mol Neurodegener. 2010;5:38. doi: 10.1186/1750-1326-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705–713. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003;5:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- Gounder MM, Schwartz GK. Moving forward one Notch at a time. J Clin Oncol. 2012;30:2291–2293. doi: 10.1200/JCO.2012.42.3277. [DOI] [PubMed] [Google Scholar]
- Gu Y, Sanjo N, Chen F, Hasegawa H, Petit A, Ruan X, Li W, Shier C, Kawarai T, Schmitt-Ulms G, et al. The presenilin proteins are components of multiple membrane-bound complexes that have different biological activities. The Journal of biological chemistry. 2004;279:31329–31336. doi: 10.1074/jbc.M401548200. [DOI] [PubMed] [Google Scholar]
- Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- He G, Luo W, Li P, Remmers C, Netzer WJ, Hendrick J, Bettayeb K, Flajolet M, Gorelick F, Wennogle LP, et al. Gamma-secretase activating protein is a therapeutic target for Alzheimer's disease. Nature. 2010;467:95–98. doi: 10.1038/nature09325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CJ, Iyer S, Sataur A, Covello KL, Chodosh LA, Simon MC. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells. Mol Cell Biol. 2006;26:3514–3526. doi: 10.1128/MCB.26.9.3514-3526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CJ, Sataur A, Wang L, Chen H, Simon MC. The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1alpha and HIF-2alpha. Mol Biol Cell. 2007;18:4528–4542. doi: 10.1091/mbc.E06-05-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann MR, Barth S, Konietzko U, Wu B, Egger S, Kunze R, Marti HH, Hick M, Muller U, Camenisch G, et al. Dysregulation of hypoxia-inducible factor by presenilin/gamma-secretase loss-of-function mutations. J Neurosci. 2013;33:1915–1926. doi: 10.1523/JNEUROSCI.3402-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinakis A, Lobry C, Abdel-Wahab O, Oh P, Haeno H, Buonamici S, van De Walle I, Cathelin S, Trimarchi T, Araldi E, et al. A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature. 2011;473:230–233. doi: 10.1038/nature09999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krop I, Demuth T, Guthrie T, Wen PY, Mason WP, Chinnaiyan P, Butowski N, Groves MD, Kesari S, Freedman SJ, et al. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J Clin Oncol. 2012;30:2307–2313. doi: 10.1200/JCO.2011.39.1540. [DOI] [PubMed] [Google Scholar]
- Lai MT, Chen E, Crouthamel MC, DiMuzio-Mower J, Xu M, Huang Q, Price E, Register RB, Shi XP, Donoviel DB, et al. Presenilin-1 and Presenilin-2 Exhibit Distinct yet Overlapping {gamma}-Secretase Activities. The Journal of biological chemistry. 2003;278:22475–22481. doi: 10.1074/jbc.M300974200. [DOI] [PubMed] [Google Scholar]
- Levy Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, et al. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- Li L, Zhang X, Yang D, Luo G, Chen S, Le W. Hypoxia increases Abeta generation by altering beta- and gamma-cleavage of APP. Neurobiol Aging. 2009;30:1091–1098. doi: 10.1016/j.neurobiolaging.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Li YM, Xu M, Lai MT, Huang Q, Castro JL, DiMuzio-Mower J, Harrison T, Lellis C, Nadin A, Neduvelil JG, et al. Photoactivated gamma-secretase inhibitors directed to the active site covalently label presenilin 1. Nature. 2000;405:689–694. doi: 10.1038/35015085. [DOI] [PubMed] [Google Scholar]
- Luo W, Hu H, Chang R, Zhong J, Knabel M, O'Meally R, Cole RN, Pandey A, Semenza GL. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee T, Kim WS, Mandal L, Banerjee U. Interaction between Notch and Hif-alpha in development and survival of Drosophila blood cells. Science. 2011;332:1210–1213. doi: 10.1126/science.1199643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam Y, Weng AP, Aster JC, Blacklow SC. Structural requirements for assembly of the CSL.intracellular Notch1.Mastermind-like 1 transcriptional activation complex. The Journal of biological chemistry. 2003;278:21232–21239. doi: 10.1074/jbc.M301567200. [DOI] [PubMed] [Google Scholar]
- Placanica L, Chien JW, Li YM. Characterization of an atypical gamma-secretase complex from hematopoietic origin. Biochemistry. 2010;49:2796–2804. doi: 10.1021/bi901388t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placanica L, Tarassishin L, Yang G, Peethumnongsin E, Kim SH, Zheng H, Sisodia SS, Li YM. Pen2 and Presenilin-1 Modulate the Dynamic Equilibrium of Presenilin-1 and Presenilin-2 {gamma}-Secretase Complexes. The Journal of biological chemistry. 2009;284:2967–2977. doi: 10.1074/jbc.M807269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci U S A. 2008;105:6392–6397. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott AF, Landis MD, Dontu G, Griffith KA, Layman RM, Krop I, Paskett LA, Wong H, Dobrolecki LE, Lewis MT, et al. Preclinical and clinical studies of gamma secretase inhibitors with docetaxel on human breast tumors. Clin Cancer Res. 2013;19:1512–1524. doi: 10.1158/1078-0432.CCR-11-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Involvement of hypoxia-inducible factor 1 in human cancer. Internal medicine. 2002;41:79–83. doi: 10.2169/internalmedicine.41.79. [DOI] [PubMed] [Google Scholar]
- Sethi N, Dai X, Winter CG, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell. 2011;19:192–205. doi: 10.1016/j.ccr.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi N, Kang Y. Notch signalling in cancer progression and bone metastasis. Br J Cancer. 2011;105:1805–1810. doi: 10.1038/bjc.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton CC, Tian Y, Frattini MG, Li YM. An exo-cell assay for examining real-time gamma-secretase activity and inhibition. Mol Neurodegener. 2009a;4:22. doi: 10.1186/1750-1326-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton CC, Zhu L, Chau D, Yang L, Wang R, Djaballah H, Zheng H, Li YM. Modulation of gamma-secretase specificity using small molecule allosteric inhibitors. Proc Natl Acad Sci U S A. 2009b;106:20228–20233. doi: 10.1073/pnas.0910757106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stylianou S, Clarke RB, Brennan K. Aberrant activation of notch signaling in human breast cancer. Cancer Res. 2006;66:1517–1525. doi: 10.1158/0008-5472.CAN-05-3054. [DOI] [PubMed] [Google Scholar]
- Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, Staufenbiel M, Huang LE, Song W. Hypoxia facilitates Alzheimer's disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci U S A. 2006;103:18727–18732. doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. Embo J. 2000;19:4298–4309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao K, Fang M, Alroy J, Sahagian GG. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer. 2008;8:228. doi: 10.1186/1471-2407-8-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolcher AW, Messersmith WA, Mikulski SM, Papadopoulos KP, Kwak EL, Gibbon DG, Patnaik A, Falchook GS, Dasari A, Shapiro GI, et al. Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J Clin Oncol. 2012;30:2348–2353. doi: 10.1200/JCO.2011.36.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleugel MM, Greijer AE, Shvarts A, van der Groep P, van Berkel M, Aarbodem Y, van Tinteren H, Harris AL, van Diest PJ, van der Wall E. Differential prognostic impact of hypoxia induced and diffuse HIF-1alpha expression in invasive breast cancer. Journal of clinical pathology. 2005;58:172–177. doi: 10.1136/jcp.2004.019885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Yang W, Wen W, Sun J, Su B, Liu B, Ma D, Lv D, Wen Y, Qu T, et al. Gamma-secretase gene mutations in familial acne inversa. Science. 2010;330(1065) doi: 10.1126/science.1196284. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhang YW, Zhang X, Liu R, Hong S, Xia K, Xia J, Zhang Z, Xu H. Transcriptional regulation of APH-1A and increased gamma-secretase cleavage of APP and Notch by HIF-1 and hypoxia. FASEB J. 2006;20:1275–1277. doi: 10.1096/fj.06-5839fje. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu Y, Malek SN, Zheng P. Targeting HIF1alpha eliminates cancer stem cells in hematological malignancies. Cell Stem Cell. 2011;8:399–411. doi: 10.1016/j.stem.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P, Walls M, Qiu M, Ding R, Denlinger RH, Wong A, Tsaparikos K, Jani JP, Hosea N, Sands M, et al. Evaluation of selective gamma-secretase inhibitor PF-03084014 for its antitumor efficacy and gastrointestinal safety to guide optimal clinical trial design. Mol Cancer Ther. 2010;9:1618–1628. doi: 10.1158/1535-7163.MCT-10-0034. [DOI] [PubMed] [Google Scholar]
- Yang G, Yin YI, Chun J, Shelton CC, Ouerfelli O, Li YM. Stereo-controlled synthesis of novel photoreactive gamma-secretase inhibitors. Bioorganic & medicinal chemistry letters. 2009;19:922–925. doi: 10.1016/j.bmcl.2008.11.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Zhou H, Walian PJ, Jap BK. CD147 is a regulatory subunit of the gamma-secretase complex in Alzheimer's disease amyloid beta-peptide production. Proc Natl Acad Sci U S A. 2005;102:7499–7504. doi: 10.1073/pnas.0502768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.