Abstract
Purpose of review
Allergic diseases are thought to be driven by aberrant immune responses. Epithelium responds to various environmental factors by releasing key cytokines, such as thymic stromal lymphopoietin (TSLP), IL-33 and IL-25. While there are important differences among these cytokines, there are also similarities which confound a clear understanding of exact roles of these cytokines. The purpose of this review is to analyze advances in biology and functions of these cytokines over recent years, elucidate their differences and similarities, and provide new conceptual understanding as to their roles in allergic diseases.
Recent findings
There are distinct differences in the timing, onset, and kinetics of the responses and perhaps in potency of action of TSLP, IL-33 and IL-25. Newer roles of these cytokines have been described, including airway remodeling and fibrosis-related functions (TSLP, IL-33 and IL-25), fetal-maternal interface (IL-33 and TSLP), T cell biology (TSLP), group 2 innate lymphoid cell (ILC2) biology (TSLP, IL-33 and IL-25), and mast cell-neutrophil axis (IL-33). Novel roles of these cytokines in in pathogenesis of atopic dermatitis and asthma have also been described.
Summary
TSLP, IL-25 and IL-33 are increasingly recognized to play important roles in pathophysiology of allergic diseases. More clear recognition of the differences and similarities of the immunological pathways mediated by these cytokines would help optimize treatment for allergic diseases.
Keywords: TSLP, IL-25, IL-33, airway remodeling, allergic inflammation, asthma, atopic dermatitis
INTRODUCTION
Thymic stromal lymphopoietin (TSLP), IL-25 and IL-33 are produced by epithelial cells as well as other cell types and they show profound downstream effects on various immune cells. There have been important advances in recent years regarding the function of these cytokines, including induction of Th2-type adaptive responses and discovery of the group 2 innate lymphoid cells (ILC2s) [1]. GWAS studies have shown the association of asthma and polymorphisms of several genetic loci, including IL1RL1, TSLP and IL-33 [2]; IL1RL1 encodes ST2, the receptor for IL-33. Increasing evidence suggests associations between these cytokines and allergic diseases but the immunologic mechanisms, by which these cytokines influence the allergic immune responses, have only been recently begun to be revealed. While there are some similarities in the biology and function of these cytokines, better understanding of the differences are also likely to be critical for elucidation of roles of these cytokines in human disease. This review will focus on important recent advances in the fields of TSLP, IL-33 and IL-25 biology and provide high level view with specific focus on overlapping and distinct features.
RECENT ADVANCES IN TSLP BIOLOGY
Regulation of TSLP function
Several biological processes and molecules have been shown to induce or repress TSLP expression by tissue cells. Mechanical injury, proinflammatory milieu and proteases, such as trypsin and papain, cause TSLP production and release from epithelial compartments [3-5]. Newer factors modulating TSLP production have been described and include regulation of TSLP by microRNA and suppression of TSLP by inflammasome in keratinocytes [6, 7]. Furthermore, TSLP expression was reduced in the cells overexpressing caveolin-1, indicating a functional crosstalk between epithelial cell-cell adhesion and induction of airway inflammation [8]. Besides the triggers mentioned above, TSLP stimulation via peptidoglycan matrix of bacterial lactococcus bacteria like particles (BLP) has also been reported [9]. These findings suggest that regulators for TSLP release are more diverse than previously anticipated and that multiple pathways may be involved in pathogenesis of allergic diseases.
Role of TSLP in disease
Effect of TSLP on induction of Th2-type immune responses and generation of allergic inflammation has been known. A novel mechanism for induction of Th2 response by TSLP was reported by Siracusa et. al., who reported that TSLP-mediated extra-medullary hematopoiesis lead to expansion and differentiation of antigen-presenting cells (APCs) [dendritic cells (DCs), macrophages and granulocytic cells]. These APCs then likely promote Th2-type immune responses in lymphocytes [10]. Another new mechanism by which TSLP may regulate allergic inflammation is by promoting Th9-type lymphocyte differentiation. Th9 cells in conjunction with Th2 cells were shown to be important in allergen-induced airway inflammation [11]. The clinical importance for the role of TSLP in allergic airway inflammation has also been demonstrated in a therapeutic context in primates and humans. Blocking of the TSLP pathway with anti-TSLP Receptor antibody or anti-TSLP antibody (AMG157) showed promising efficacy in reducing allergen-induced airway inflammation in cynomolgus monkeys and patients with asthma [12-13]. Interestingly, TSLP was also reported to have additional role in lymphocyte differentiation in non-mucosal compartments. For example, TSLP derived from the trophoblasts induced regulatory T-cells by activating decidual DCs, and TSLP produced from hepatitis C virus-infected hepatocytes promoted a Th17-type response [14, 15]. In addition, TSLP by acting directly on neurons promoted itch sensation, suggesting a pruritogenic role along with an inflammatory role in atopic skin disease [16]. Indeed, abrogation of atopic dermatitis-like responses were seen in TSLPR-deficient mice while mice deficient in IL-33 or IL-25 receptor (IL17RB) demonstrated inflammatory changes, supporting the role of TSLP in evolution of atopic skin diseases [17].
Clinical associations of TSLP
Besides atopic dermatitis and allergic asthma, TSLP is thought to be associated with other diseases as well. In patients with chronic rhinosinusitis (CRSwNP), increased activity of TSLP was reported in nasal polyp (NP) tissue while degradation of TSLP by tissue proteases led to reduced protein amount but augmented cytokine activity [18]. TSLP was also reported to be upregulated in lung fibroblasts in idiopathic pulmonary fibrosis (IPF) and in the pleural fluid of patients with eosinophilic effusion in primary spontaneous pneumothorax [19, 20]. Thus, new role of TSLP in several human diseases has emerged.
RECENT ADVANCES IN IL-25 BIOLOGY
Regulation of IL-25 function
IL-25 and IL-33 appear functionally similar but they have important differences. Unlike IL-33, which is stored in the nucleus, IL-25 is stored in and released from extra-nuclear cellular compartments. Kouzaki et al have reported that IL-25 is constitutively expressed in epithelial cells and is released upon exposure to proteases, such as trypsin, papain or in a more clinically relevant context, allergen proteases present in house dust mite (HDM) extract [21]. Given the immunological effects of IL-25, release of this cytokine by environmental proteases could have important implications in allergic disease. While the cellular mechanisms of IL-25 release and regulation of IL-25 activity is not totally understood, tissue microenvironment likely plays a role. For example,ILC2s activated by IL-25 (and IL-33) showed reduced Th2 cytokine secretion in presence of plate-bound E-cadherin [22], suggesting that cell-cell adhesion may play regulatory role in the biological effects of IL-25. Furthermore, IL-25 inhibited filaggrin gene expression as well as protein level in normal human keratinocytes [23]. Since filaggrin is important in skin barrier function, a defective barrier may promote IL-25 release, which in turn affects FLG expression and thus may initiate a ‘feed-forward’ loop sustaining responses in atopic skin.
Role of IL-25 in disease
Aside from its role in atopic inflammation, IL-25 has been shown to be involved in fibrotic lung disease. IL-25 expression was increased in lungs of idiopathic pulmonary fibrosis patients and correlated with periostin [24]. The authors speculate that IL-25 drives IL-13 production by ILC2s, leading to fibrosis independent of T cells. Additionally, IL-25 may directly induce collagen production human fibroblasts [24]. The pro-fibrotic role for IL-25 was further supported in the HDM-induced airway response model where blockade of IL-25 abolished airway remodeling in mice exposed to HDM. [25]. While results suggest that fibrosis mediated by IL-25 may be a result of direct and indirect mechanisms, there may be a dichotomy in role of IL-25 in relation to airway remodeling versus hyperresponsiveness. IL-25 was shown to be less critical than IL-33 in airway hyperreactivity in mouse airway inflammation model using OVA antigen and ragweed allergens [26].
Clinical associations of IL-25
IL-25 has been known to play a robust role in immunity against helminths in the GI tract [27], but its role in airway is still being characterized. Serum levels of IL-25 were higher in allergic and non-allergic patients with asthma as compared to control individuals [28]. Similarly, increased expression of IL-25 in nasal polyp tissues in patients with CRSwNP correlated with worse CT scores and blood eosinophilia [29]. On the other hand, decreased levels of IL-25 in serum and mucosal tissues were reported in patients with active inflammatory bowel diseases compared to controls [30]. Thus, IL-25 function may depend on anatomic location and tissue microenvironment.
RECENT ADVANCES IN IL-33 BIOLOGY
Regulation of IL-33 function
IL-33 is produced and stored in the nucleus of tissue cells, such as epithelial cells and fibroblasts. The exact pathway for IL-33 production, transport within the cells, and extracellular release is still not well understood. IL-33 activity is likely regulated at several levels, including genetic encoding (single nucleotide polymorphisms, SNPs), factors affecting its biological activity and degradation. Biological activity of IL-33 may be regulated by the presence its decoy receptor, soluble ST2 (sST2). Ho et al [31] describe results from a GWAS study where SNPs in IL1RL1 locus correlate with soluble sST2 concentrations in sera. ST2 molecules may influence activity of IL-33 by functioning as receptor when membrane bound or as a decoy if free in sera. Indeed, over-production of sST2 was shown to prevent airway pathology in a lung injury model by reducing in part the IL-33 levels [32]. Other factors besides ST2 may also affect IL-33 activity. In a HDM model of airway inflammation, IL-33 secretion was dependent on IL-25, suggesting synergistic interaction between these epithelium-derived cytokines [25]. In addition, Roy et al. [33] reported to the ability of mast cell chymase to degrade IL-33, suggesting a complex balance of production and degradation of IL-33 in the tissues that affects its biological function.
Role of IL-33 in disease
Several recent reports add to our knowledge regarding the role of IL-33 in pathophysiology of diseases. The roles for IL-33 and IL-25 may vary depending on the disease studied and organ affected. In atopic dermatitis, skin ILC2s express ST2, respond to IL-33 and home to skin, leading to production of type 2 cytokines in the tissues [22]. Using mice deficient in ST2 (i.e. IL-33 receptor) or IL17RB (i.e. IL-25 receptor), IL-33 played a more important role than IL-25 in induction of IL-13 and airway hyperreactivity [26]. Also, IL-33 has been reported to be crucial for development of HDM-induced allergic airway inflammation and peanut anaphylaxis. IL-33 derived by exposure to peanut and HDM induced OX40L expression by DCs, leading to development of Th2-type CD4+ T cells; TSLP or IL-25 did not show similar roles as IL-33 in this model [34]. A pro-fibrotic role for full length IL-33 independent of Th2-type cytokines was also reported, suggesting that IL-33 may directly act on tissue cells similarly to IL-25. For example, increased levels of IL-33 were seen in lung sections of patients with idiopathic pulmonary fibrosis and those with scleroderma as well as in bleomycin-induced lung injury model in mice [35]. Interestingly, IL-33 showed its inhibitory effect on mast cells. Prolonged exposure of mast cells to IL-33 inhibited mast cell functions, including degranulation [36]. In addition, IL-33 induced neutrophil chemotaxis in cystic fibrosis patients and in mouse peritoneum [37, 38]; this IL-33-mediated neutrophil influx was dependent on mast cells. Thus considering the effect of mast cell chymase on IL-33 proteins and effect of IL-33 on mast cell function, a complex interplay may exist among IL-33, neutrophils, mast cells and epithelial cells. Finally, IL-33 derived from human placental and decidual macrophages induced proliferation of placental trophoblasts, suggesting potential but intriguing role for IL-33 in gestation [39]
Clinical associations of IL-33
Several clinical studies suggest associations of IL-33 with allergic diseases and airway inflammation, including increased levels of IL-33 in patients with asthma, COPD, primary pneumothorax and CRSwNP. For example, not only were soluble ST2 and IL-33 levels reportedly higher in induced sputum and serum from asthmatic children compared to controls, the IL-33 also correlated with severity of asthma [40]. Besides asthma, increased levels of IL-33 were observed in whole lung explants of patients with severe COPD [41]. The mechanistic role for IL-33 in COPD was further supported in a mouse model of COPD where IL-33 appears to be the primary factor driving IL-13-dependent lung pathology, such as prolonged lung inflammation and mucin production [41]. In patients with CRSwNP, IL-33 was reported to drive ILC2-dependent IL-13 production [42]. Finally, higher amounts of IL-33 along with TSLP were reported in pleural fluids of patients with eosinophilic primary spontaneous pneumothorax [18].
NETWORK STUDY OF RECENT CONCEPTS IN IL-25, IL-33 AND TSLP BIOLOGY
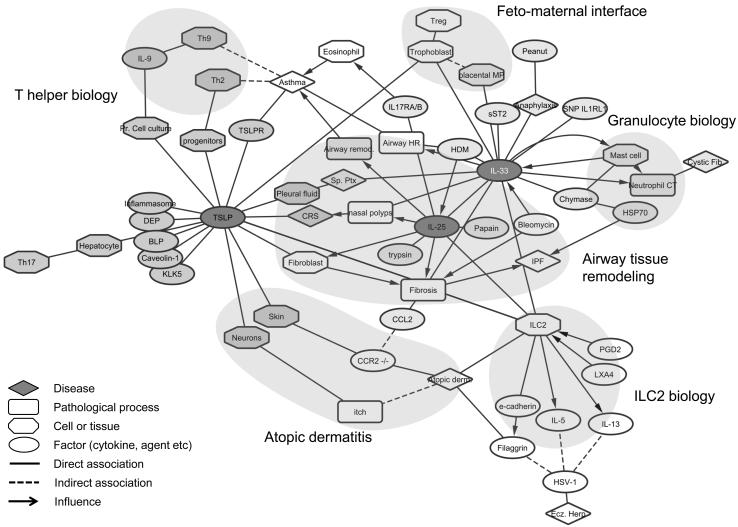
In order to illuminate emerging themes in recently described functions and roles of IL-25, IL-33 and TSLP, we constructed a ‘concept association network’. Each concept (biological process, cell type, mediator etc.) was assigned as a node with a line (edge) connecting the nodes depicting the relationship. Diseases, pathological processes, cells/tissues, and biological factors (cytokines etc.) were assigned distinct shapes (see legend), and networks for each cytokine were generated (see Supplemental Figures 1-3). To understand the commonalities and differences, we then merged the individual networks on common nodes into a single network (Figure 1). After application of a force-directed layout (which pulls connected nodes together and pushes disconnected nodes apart) to the combined network, emergent themes from recent literature related to biology of these cytokines were explored. Some evolving and recognizable sub-groups were identified using domain knowledge as are discussed below:
Figure 1.
Concepts influencing IL-33, TSLP and IL-25 biology are depicted as nodes. Distinct shapes are assigned to a unique biological concept with rhomboid (disease state), rounded squares (biological process), octagon (cell or tissue) and ellipse (factor such as cytokine or allergen). Network was generated using Cytoscape (http://www.cytoscape.org/ v3.1.1). Force directed algorithm was applied which pulls connected nodes together and pushes disconnected nodes apart. Modules are identified based on emergent patterns and domain knowledge.
1. Airway tissue remodeling
Many of the recent advances in biology of all these cytokines suggest their roles in tissue remodeling. The role of IL-33, IL-25 and TSLP in tissue repair and possible secretion of these cytokines mediated by tissue proteases points to a reparative role in health with association with fibrotic disease, polyposis, and airway remodeling in asthma and pulmonary fibrosis in disease.
2. Feto-maternal interface biology (IL-33 and TSLP)
Placental macrophage-derived IL-33 induced trophoblast proliferation while TSLP from trophoblast induced Tregs. Both TSLP and IL-33 seem to have implications in biology of pregnancy.
3. T helper biology (TSLP)
Influence of TSLP in Th9- and Th2-type adaptive immune responses was noted. Also was seen the influence of TSLP on Treg and Th17 pathways. These observations suggest that epithelium-derived cytokines, such as TSLP, have ability to influence downstream adaptive immune responses.
4. Atopic dermatitis related (TSLP, IL-25 and IL-33)
All three cytokines were shown to be involved in atopic dermatitis either working directly on tissues cells (TSLP) or indirectly via regulating functions of intermediaries, such as ILC2s.
5. ILC2 biology related
Many of recent reports pertain to ILC2 biology. TSLP, IL-25 and IL-33 may be involved in regulation of ILC2s directly or indirectly. Effects of these cytokines in ILC2 cytokine production suggests their potential roles in amplification of immune response and inflammation.
6. Mast cell-neutrophil (granulocyte) biology related
The relationship between mast cells, IL-33 and neutrophils is likely complex but noteworthy. IL-33 induced mast cell refractoriness and mast cell-dependent neutrophil chemotaxis, while mast cell chymase degrades IL-33 .
CONCLUSION
Growing body of literature suggests critical roles for epithelium-derived cytokines, TSLP, IL-25 and IL-33, in regulation Th2-type immunity and allergic responses. Several recent advances in biology of these cytokines also point to shared functions as well as distinct characteristics. Discovery of additional information will only add to the growing body of literature that defines the exact role of these important cytokines in allergic diseases. Such knowledge will expedite the clinical application of the modulators of these cytokines to treat allergic diseases and help us to predict potential side effects if these strategies are implemented.
Supplementary Material
Key points.
TSLP, IL-33 and IL-25 may play important roles in tissue remodeling, regulation of innate and adaptive type 2 immune responses, and maintenance of tissue homeostasis.
There are both similarities and distinct differences in biological functions and potency of these cytokines.
These cytokines likely play important roles in initiation, regulation, and persistence of allergic diseases.
ACKNOWLEDGEMENTS
Funding: NIH R56 AI49235 (H.K) and from the Mayo Foundation. There is not conflict of interest.
REFERENCES
- 1.Bartemes KR, Kita Hirohito. Dynamic role of epithelium-derived cytokines in asthma. Clinical Immunology. 2012;143.3:222–235. doi: 10.1016/j.clim.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ober C, Yao TC. The genetics of asthma and allergic disease: a 21st century perspective. Immunol Rev. 2011 Jul;242(1):10–30. doi: 10.1111/j.1600-065X.2011.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oyoshi MK, Larson RP, Ziegler SF, Geha RS. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol. 2010 Nov;126(5):976–84. 984.e1–5. doi: 10.1016/j.jaci.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogiatzi SI, Fernandez I, Bichet JC, et al. Cutting Edge: Proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J Immunol. 2007 Mar 15;178(6):3373–7. doi: 10.4049/jimmunol.178.6.3373. [DOI] [PubMed] [Google Scholar]
- 5.Kouzaki H, O’Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009 Jul 15;183(2):1427–34. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleck B, Grunig G, Chiu A, et al. MicroRNA-375 regulation of thymic stromal lymphopoietin by diesel exhaust particles and ambient particulate matter in human bronchial epithelial cells. J. Immunol. 2013 Apr 1;190(7):3757–63. doi: 10.4049/jimmunol.1201165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuepbach-Mallepell S, Philippe V, Brüggen MC, et al. Antagonistic effect of the inflammasome on thymic stromal lymphopoietin expression in the skin. J Allergy Clin Immunol. 2013 Dec;132(6):1348–57. doi: 10.1016/j.jaci.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 8.Hackett TL, de Bruin HG, Shaheen F, et al. Caveolin-1 controls airway epithelial barrier function. Implications for asthma. Am J Respir Cell Mol Biol. 2013 Oct;49(4):662–71. doi: 10.1165/rcmb.2013-0124OC. [DOI] [PubMed] [Google Scholar]
- 9.Yeh CY, Yeh TH, Jung CJ, et al. Activated human nasal epithelial cells modulate specific antibody response against bacterial or viral antigens. PLoS One. 2013;8(2):e55472. doi: 10.1371/journal.pone.0055472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10 *.Siracusa MC, Saenz SA, Wojno ED, et al. Thymic stromal lymphopoietin-mediated extramedullary hematopoiesis promotes allergic inflammation. Immunity. 2013 Dec 12;39(6):1158–70. doi: 10.1016/j.immuni.2013.09.016. (Role of TSLP in modulating adaptive immune responses)
- 11 *.Yao W, Zhang Y, Jabeen R, et al. Interleukin-9 is required for allergic airway inflammation mediated by the cytokine TSLP. Immunity. 2013 Feb 21;38(2):360–72. doi: 10.1016/j.immuni.2013.01.007. (Role of TSLP in modulating adaptive immune responses)
- 12.Cheng DT, Ma C, Niewoehner J, et al. Thymic stromal lymphopoietin receptor blockade reduces allergic inflammation in a cynomolgus monkey model of asthma. J Allergy Clin Immunol. 2013 Aug;132(2):455–62. doi: 10.1016/j.jaci.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Gauvreau GM, O’Byrne PM, Boulet LP, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014 May 29;370(22):2102–10. doi: 10.1056/NEJMoa1402895. [DOI] [PubMed] [Google Scholar]
- 14.Du MR, Guo PF, Piao HL, et al. Embryonic trophoblasts induce decidual regulatory T cell differentiation and maternal-fetal tolerance through thymic stromal lymphopoietin instructing dendritic cells. J Immunol. 2014 Feb 15;192(4):1502–11. doi: 10.4049/jimmunol.1203425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee HC, Sung SS, Krueger PD, et al. Hepatitis C virus promotes T-helper (Th)17 responses through thymic stromal lymphopoietin production by infected hepatocytes. Hepatology. 2013 Apr;57(4):1314–24. doi: 10.1002/hep.26128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson SR, Thé L, Batia LM, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013 Oct 10;155(2):285–95. doi: 10.1016/j.cell.2013.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim BS, Siracusa MC, Saenz SA, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013 Jan 30;5(170):170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagarkar DR, Poposki JA, Tan BK, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013 Sep;132(3):593–600. e12. doi: 10.1016/j.jaci.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Datta A, Alexander R, Sulikowski MG, et al. Evidence for a functional thymic stromal lymphopoietin signaling axis in fibrotic lung disease. J Immunol. 2013 Nov 1;191(9):4867–79. doi: 10.4049/jimmunol.1300588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon BI, Hong S, Shin K, et al. Innate type 2 immunity is associated with eosinophilic pleural effusion in primary spontaneous pneumothorax. Am J Respir Crit Care Med. 2013 Sep 1;188(5):577–85. doi: 10.1164/rccm.201302-0295OC. [DOI] [PubMed] [Google Scholar]
- 21.Kouzaki H, Tojima I, Kita H, Shimizu T. Transcription of interleukin-25 and extracellular release of the protein is regulated by allergen proteases in airway epithelial cells. Am J Respir Cell Mol Biol. 2013 Nov;49(5):741–50. doi: 10.1165/rcmb.2012-0304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22 **.Salimi M, Barlow JL, Saunders SP, et al. A role for IL-25 and IL-33–driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013 Dec 16;210(13):2939–2950. doi: 10.1084/jem.20130351. (Offers mechanistic basis for function of IL-33 and ILC2 in atopic skin disease)
- 23.Kim BE, Bin L, Ye YM, et al. IL-25 enhances HSV-1 replication by inhibiting filaggrin expression, and acts synergistically with Th2 cytokines to enhance HSV-1 replication. J Invest Dermatol. 2013 Dec;133(12):2678–85. doi: 10.1038/jid.2013.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hams E, Armstrong ME, Barlow JL, et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc Natl Acad Sci U S A. 2014 Jan 7;111(1):367–72. doi: 10.1073/pnas.1315854111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gregory LG, Jones CP, Walker SA, et al. IL-25 drives remodelling in allergic airways disease induced by house dust mite. Thorax. 2013 Jan;68(1):82–90. doi: 10.1136/thoraxjnl-2012-202003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barlow JL, Peel S, Fox J, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013 Oct;132(4):933–41. doi: 10.1016/j.jaci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010 Apr 29;464(7293):1367–70. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang W, Smith SG, Beaudin S, et al. IL-25 and IL-25 receptor expression on eosinophils from subjects with allergic asthma. Int Arch Allergy Immunol. 2014;163(1):5–10. doi: 10.1159/000355331. [DOI] [PubMed] [Google Scholar]
- 29.Lam M, Hull L, McLachlan R, et al. Clinical severity and epithelial endotypes in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013 Feb;3(2):121–8. doi: 10.1002/alr.21082. [DOI] [PubMed] [Google Scholar]
- 30.Su J, Chen T, Ji XY, et al. IL-25 downregulates Th1/Th17 immune response in an IL-10-dependent manner in inflammatory bowel disease. Inflamm Bowel Dis. 2013 Mar-Apr;19(4):720–8. doi: 10.1097/MIB.0b013e3182802a76. [DOI] [PubMed] [Google Scholar]
- 31.Ho JE, Chen WY, Chen MH, et al. Common genetic variation at the IL1RL1 locus regulates IL-33/ST2 signaling. J Clin Invest. 2013 Oct 1;123(10):4208–18. doi: 10.1172/JCI67119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez-González I, Roca O, Masclans JR, et al. Human mesenchymal stem cells overexpressing the IL-33 antagonist soluble IL-1 receptor-like-1 attenuate endotoxin-induced acute lung injury. Am J Respir Cell Mol Biol. 2013 Oct;49(4):552–62. doi: 10.1165/rcmb.2012-0406OC. [DOI] [PubMed] [Google Scholar]
- 33.Roy A, Ganesh G, Sippola H, et al. Mast cell chymase degrades the alarmins heat shock protein 70, biglycan, HMGB1, and interleukin-33 (IL-33) and limits danger-induced inflammation. J Biol Chem. 2014 Jan 3;289(1):237–50. doi: 10.1074/jbc.M112.435156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu DK, Llop-Guevara A, Walker TD, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol. 2013 Jan;131(1):187–200. e1–8. doi: 10.1016/j.jaci.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Luzina IG, Kopach P, Lockatell V, et al. Interleukin-33 potentiates bleomycin-induced lung injury. Am J Respir Cell Mol Biol. 2013 Dec;49(6):999–1008. doi: 10.1165/rcmb.2013-0093OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung MY, Smrz̆ D, Desai A, et al. IL-33 induces a hyporesponsive phenotype in human and mouse mast cells. J Immunol. 2013 Jan 15;190(2):531–8. doi: 10.4049/jimmunol.1201576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roussel L, Farias R, Rousseau S. IL-33 is expressed in epithelia from patients with cystic fibrosis and potentiates neutrophil recruitment. J Allergy Clin Immunol. 2013 Mar;131(3):913–6. doi: 10.1016/j.jaci.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Enoksson M, Möller-Westerberg C, Wicher G, et al. Intraperitoneal influx of neutrophils in response to IL-33 is mast cell-dependent. Blood. 2013 Jan 17;121(3):530–6. doi: 10.1182/blood-2012-05-434209. [DOI] [PubMed] [Google Scholar]
- 39.Fock V, Mairhofer M, Otti GR, et al. Macrophage-derived IL-33 is a critical factor for placental growth. J Immunol. 2013 Oct 1;191(7):3734–43. doi: 10.4049/jimmunol.1300490. [DOI] [PubMed] [Google Scholar]
- 40.Hamzaoui A, Berraies A, Kaabachi W, et al. Induced sputum levels of IL-33 and soluble ST2 in young asthmatic children. J. Asthma. 2013 Oct;50(8):803–9. doi: 10.3109/02770903.2013.816317. [DOI] [PubMed] [Google Scholar]
- 41.Byers DE, Alexander-Brett J, Patel AC, et al. Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest. 2013 Sep 3;123(9):3967–82. doi: 10.1172/JCI65570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaw JL, Fakhri S, Citardi MJ, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013 Aug 15;188(4):432–9. doi: 10.1164/rccm.201212-2227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.