Abstract
Hyperphosphorylation and polymerization of microtubule-associated protein tau into paired helical filaments (PHFs) is one of the hallmarks of Alzheimer’s disease (AD). Here we report that neuronal tau hyperphosphorylation under AD conditions is regulated by Snitrosoglutathione (GSNO), an endogenous nitric oxide carrier molecule. In cultured rat cortical primary neurons, we observed that GSNO treatment decreased the β-amyloid (Aβ25–35)-induced pathological tau hyperphosphorylation (Ser396, Ser404, and Ser202/Thr205). The decreased tau hyperphosphorylation correlated with decreased activity of calpain and decreased p35 proteolysis into p25 and Cdk5 activation. GSNO treatment also attenuated the Aβ25–35-induced activation of GSK-3β which is known to play critical role in tau hyperphosphorylation in addition to Cdk5. Consistent with above studies using cultured neurons, we also observed that systemic GSNO treatment of transgenic mouse model of AD (APPSw/PS1dE9) attenuated calpain-mediated p35 proteolysis and Cdk5/GSK-3β activities as well as tau hyperphosphorylation. In addition, GSNO treatment provided neuro- and cognitive protection in APPSw/PS1dE9 mice. This study describing the GSNO-mediated regulation of tau hyperphosphorylation and cognitive function, for the first time, suggests for therapeutic potential of GSNO as neuro- and cognitive-protective agent for AD.
Keywords: Alzheimer’s disease, β-amyloid, calpain, Cdk5, GSK-3β, GSNO, nitric oxide, p25, p35, S-nitrosoglutathione, tau
Introduction
Alzheimer’s disease (AD) is the most common form of dementia among older individuals, characterized by extracellular deposits of Aβ aggregates as senile plaques, and abnormal neuronal tau hyperphosphorylation leading to its aggregation to form intracellular paired helical filaments (PHFs) and neurofibrillary tangles (NFTs) that destroy circuitry-linked activities in cortical and hippocampal regions of brain [1]. Unfortunately, no effective therapy is available due to limited understanding of disease pathologies.
Endogenous nitric oxide (NO) synthesized from NO synthases (NOS), plays a key role in numerous physiological and pathological processes [2]. NO is known to exert its biological activities via at least three distinct mechanisms; classical cGMP/PKG mechanism mediating NO-dependent relaxation of vascular smooth muscle [3], peroxynitrite (ONOO− formed by reaction between NO and superoxide anion) dependent pathological signaling under oxidative stress conditions [4], and S-nitrosoglutathione (GSNO formed by reaction between NO and GSH) dependent redox-based protein modification (S-nitrosylation) [5].
Recently, our laboratory described activities of GSNO in neuro- and cognitive-protection using rats subjected permanent bilateral common carotid artery occlusion (pBCCAO) as a model for chronic cerebral hypoperfusion [6]. GSNO treatment also reduced the Aβ load and ICAM- 1/VCAM-1 expression in the brains of pBCCAO rats and increased Aβ uptake by microglia and endothelial cells and decreased neuronal Aβ synthesis by inhibiting activity of BACE1 in in vitro cell culture models [6]. Taken together with previously reported role of GSNO in antiinflammation [6,7], anti-oxidation [8,9], and cerebrovascular and BBB protections [10,11], our study documented the potential neuro-cognitive protective efficacy of GSNO in AD.
Since the finding of abnormally phosphorylated tau protein in PHF which forms the NFT and induces neuronal cell loss in AD brain [1], tau hyperphosphorylation-mediated pathology is gaining a more prominent role for the development of AD. Numerous studies have identified a number of protein kinases that cause hyperphosphorylation of tau in AD brain [12,13]. Among these, Cdk5 and GSK-3β are now regarded as the major kinases responsible for pathological tau hyperphosphorylation in AD brain [12,13]. Under AD conditions, Cdk5 is activated aberrantly by intracellular calcium influx and calpain activation [14,15]. Cdk5 is a proline-directed serine/threonine kinase that functions differently from traditional Cdks. Cdk5 does not have a cyclin as its activating partner; instead, it is activated by binding with p35 [16,17]. The p35 localize in membrane through myristoylation and recruit Cdk5 for its activation [16]. Upon the binding with p35, Cdk5 is activated and subsequently undergoes degradation via ubiquitinmediated proteolysis [17]. Under the pathological conditions, however, p35 is processed to p25 by calpain [16]. Since p25 is resistant to ubiquitin-mediated proteolysis and lacks the myristoylation site, the p25/Cdk5 complex is dissociated from the membrane and gains access to various substrates including tau [16]. It is interest to note that p25 preferentially binds and activates GSK-3β [18]. The p25 is accumulated in the brains of patients with AD with increased tau hyperphosphorylation and neuronal apoptosis [19], thereby suggesting that modulation of calpain activity and thus inhibition of p35 proteolysis to p25 are critical for regulation of aberrant activation of Cdk5 as well as GSK-3β under AD conditions.
In this study, we report that GSNO inhibits pathological tau hyperphosphorylation via inhibiting calpain-mediated p35 proteolysis generating p25 and aberrant activation of Cdk5 and/or via inhibiting GSK-3β activity in in vitro neuron culture model and APPSw/PS1dE9 AD mouse model.
Materials and Methods
Primary neuronal cell culture
Primary cultures of cortical neurons were prepared from the cerebral cortex of embryos of Sprague Dawley rats at embryonic day 17 (E17) as described in our previous report [6]. The cultured neurons were maintained in Neurobasal media (Invitrogen, Carlsbad, CA) supplemented with 2% B27 supplement (Invitrogen), 0.5 mM glutamine, 25µM glutamate, 50 units/ml penicillin, 50 µg/ml streptomycin under humidified atmosphere of 5% CO2 and 95% O2, at 37°C.
Western Immunoblot analysis
Western immunoblot analysis was performed using antibodies against phospho-tau (p-tau) S396 (Cell Signaling Technology, Danvers, MA), p-tau S404 (Abcam, Cambridge, MA), p-tau S202/T205 (Pierce, Rockford, IL), pan-tau (Cell Signaling Technology), β-actin (Abcam), p35, phospho-GSK-3β (p-GSK-3β) Y216/Y279 (Abcam), p-GSK-3β S9 (Cell Signaling Technology), pan-GSK-3β (Cell Signaling Technology)
Histology and Immuno-fluorescent staining
Paraffin-embedded sections from the formalin-fixed brain tissues were stained by with Nissl stain kit (IHCWORLD, Woodstock, MD) to detect Nissl body according to the manufacturer’s instruction. The sections were also used for immunofluorescent staining for p-tau S396. BX60 Olympus fluorescent/light microscope equipped with DP-70 camera (Olympus, Tokyo, Japan) was used for imaging. The intensities of fluorescence were quantified by Image-Pro Plus (MediaCybernetics, Bethesda, MD, USA).
Calpain activity assay
Analysis of calpain activity was performed using the same assay kit (Abcam, Cambridge, MA). Briefly, equal amounts of brain and neuronal lysate or purified active calpain-1 were incubated with substrate (Ac-LLY-AFC) and reaction buffer and calpain-mediated cleavage of substrate was analyzed by fluorometric analysis.
In vitro kinase assay for Cdk5, and GSK-3β
The brain tissue and neuronal lysates were immunoprecipitated with anti-CDK5 antibody (Abcam) or anti-GSK-3β antibody (BD Transduction Laboratories). The resulted pellets were incubated with biotin-labeled Cdk5 substrate (Biotin-Ahx-PKTPKKAKKL; Enzo life sciences, Farmingdale, NY) or biotin-labeled GSK-3β substrate (Biotin-RRAAEELDSRAGSPQL; AnaSpec, San Jose, CA) in kinase assay buffer (5 mM MOPS, pH 7. 2, 2.5 mM β-glycerol-phosphate, 5 mM MgCl2, 1 mM EGTA, 0.4 mM EDTA, 100µM ATP, and 0.05mM dithiothreitol). The reactions were then stopped by adding 100 mM EDTA and the levels of phosphorylated substrates in supernatants were analyzed by ELISA using streptavidin coated 96 well plate (Pierce/Thermo Scientific, Rockford, IL) and horse-radish-peroxidase-conjugated anti-phospho-Thr antibody (Cell signaling) for analysis of Cdk5 activity or anti-phospho-Ser antibody for analysis of GSK-3β activity.
Animals and GSNO treatment
All animal procedures were in accordance with the animal experiment guidelines of the Medical University of South Carolina and National Institute of Health. Wild-type (C57Bl/6J) and APPSw/PS1dE9 mice (The Jackson Laboratories, Bar Harbor, ME) were housed in cages under controlled temperature (21 ± 1°C) and humidity (55 ± 10%), with a 12-h light/12-h dark cycle. The 5 month old APPSw/PS1dE9 mice were administered with PBS or GSNO (3mg/kg/day; 50µL in PBS) on a daily basis for 5 months through intraperitoneally.
Morris water maze test
Morris water maze was employed to assess spatial learning and memory according to previously published methods with modification [6]. The test was performed in a circular pool (124 cm in diameter/60 cm in depth) filled with water clouded by nontoxic white paint. The circular pool consisted of four equal virtual quadrants. A circular area (radius 20 cm from the center of the platform) was defined as the target zone, equivalent to 4.9% of the total water maze area. All other experimental conditions are identical with our previous report [6].
Results and Discussion
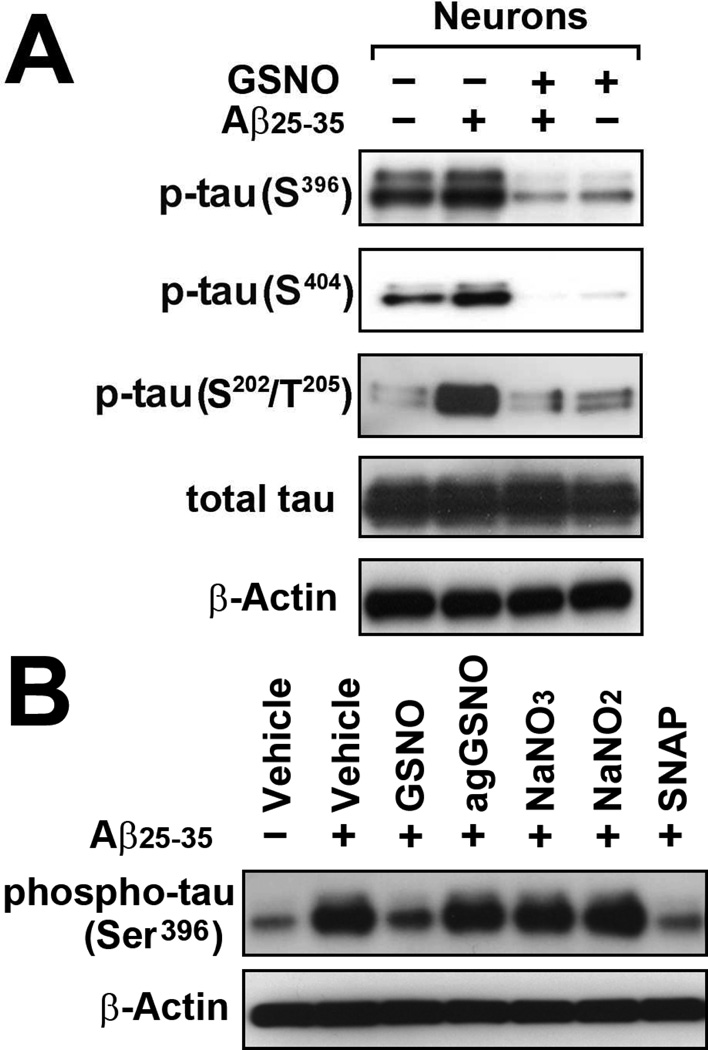
The excessive phosphorylation of tau in the proline-rich region (residues 172–251) and the C-terminal tail region (residues 368–441) have been implicated in the formation of aberrant tau aggregates known as NFTs in AD brain [20]. To understand the role of GSNO on tau phosphorylation, we studied the primary cortical neuron culture model of Aβ-induced tau phosphorylation. Fig. 1A shows that treatment of cortical neurons with Aβ25–35 in culture increased the phosphorylation of tau at Ser202/Thr205 in proline-rich region as well as Ser396/Ser404 in C-terminal tail region. Treatment of cultured neurons with GSNO (100µM) decreased the Aβ25–35-induced tau phosphorylation in both regions. Since GSNO is a thiol based NO donor, we next investigate the role of S-nitrosothiol group of GSNO in Aβ25–35-induced neuronal tau phosphorylation. Similar to GSNO, treatment of Aβ25–35-stimulated neurons with S-nitroso-N-acetylpenicillamine (SNAP), another donor of S-nitrosothiol, also reduced tau phosphorylation. However, aged GSNO (agGSNO), which was decomposed to nitrate and oxidized glutathione under light exposure, or sodium nitrite (NaNO2) and sodium nitrate (NaNO3) had no effect on Aβ-induced tau phosphorylation. These data indicate the specific role of S-nitrosothiol donor in inhibition of Aβ25–35–induced tau phosphorylation.
Fig. 1. Effect of GSNO treatment on pathological tau phosphorylation in primary cultured neurons treated with Aβ25–35.
A. The primary cultured cortical neurons were pretreated with 100µM of GSNO for 4 hrs and treated with Aβ25–35 (40µM) for 12 hrs and neuronal levels of phosphorylated tau (Ser396, Ser404, and Ser202/Thr205), total tau, and β-actin (loading control) were analyzed by Western blot analysis. B. The effects of GSNO metabolites and other S-nitrosothiol donor on Aβ25–35-induced tau phosphorylation (Ser396), cultured neurons were pretreated with GSNO, aged or decomposed GSNO (agGSNO), sodium nitrate (NaNO3), sodium nitrite (NaNO2), or S-nitroso-N-acetylpenicillamine (SNAP) for 4hrs and treated with Aβ25–35 (40µM).
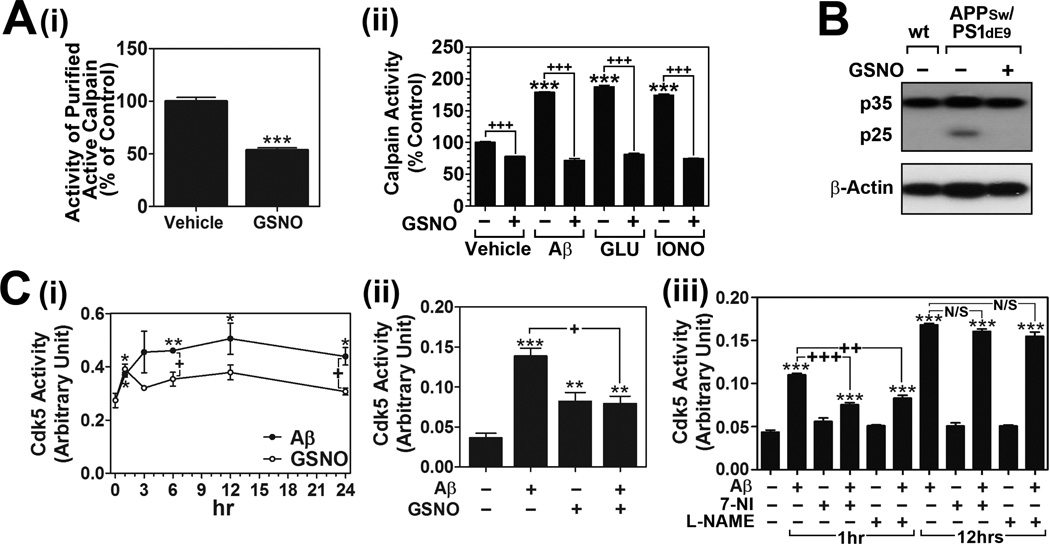
Numerous studies in the last decade have identified a number of protein kinases (e.g. GSK-3β, Cdk5, cAMP-dependent protein kinase/PKA, and stress-activated protein kinases) that cause hyperphosphorylation of tau in AD brain (reviewed in [12,13]). Among these, Cdk5 and GSK-3β are now regarded as the major kinases responsible for pathological tau hyperphosphorylation in AD brain [12,13]. Under AD conditions, aberrant Cdk5 activation by calpain mediated cleavage of p35 to p25 has been implicated in pathological events leading to neurodegeneration and NFTs [14]. Since NO is reported to reversibly regulate calpain activity via S-nitrosylation of calpain [21,22], we next assessed whether GSNO inhibits calpain-mediated p35 proteolytic process to p25. Fig. 2A-i shows that treatment of purified active calpain with GSNO (10µM) in cell free system significantly inhibited its enzyme activity. We next examined the effect of GSNO (100µM) treatment on Aβ25–35-induced calpain activation in cultured neuron cells. GSNO treatment significantly attenuated the Aβ25–35-induced calpain activation (Fig. 2A-ii). Moreover, GSNO treatment also decreased glutamate (100µM) or calcium ionophore (5 µM) induced calpain activations (Fig. 2A-ii). These data indicate a direct role of GSNO in inhibition of calpain activity.
Fig. 2. Effect of GSNO treatment on calpain activation, p35 cleavage to p25, and Cdk5 activation in Aβ25–35 treated neurons.
A. Effect of GSNO on calpain activities was analyzed in cell free system using purified active calpain-1 (i) and in primary cultured cortical neurons (ii). The cortical neurons were pretreated with GSNO (100µM) for 4 hrs and treated with Aβ25–35 (Aβ; 40µM), glutamate (Glu; 50µM), or ionomycine (IONO; A23187; 10µM) for 12 hrs. B. Under the similar experiments, the effect of GSNO on calpain-mediated cleavage of p35 to p25 was examined in cultured neurons treated with Aβ25–35. C. The effect of GSNO (100µM) and Aβ25–35 (40µM) treatment on the activities of Cdk5 was analyzed in a time course manner in primary cultured cortical neurons (i). To examine the effect of GSNO on Aβ25–35-induced Cdk5 activities, the cultured neurons were pretreated with GSNO for 4hrs and treated with Aβ25–35. Following the incubation for 12 hrs, Cdk5 enzyme activities were analyzed by in vitro kinase assay (ii). To examine the role of NOS activation on Aβ25–35-induced Cdk5 activities, the cultured neurons were pretreated with L-Nω-nitroarginine methyl ester (L-NAME; 300µM) or 7-nitroindazole (7-NI; 100µM) for 4hrs and treated with Aβ25–35. Following the incubation for 1 hr or 12hrs, Cdk5 enzyme activities were analyzed (iii). The vertical columns are means of individual data and T-bars are standard error mean. *p<0.01; **p<0.005; and ***p<0.0001 as compared to control group. +p<0.01; ++p<0.005; +++p<0.0001 as compared to Aβ, Glu, IONO treated groups.
Similar to the effect on calpain activity, GSNO treatment also attenuated the proteolysis of p35 detected as p25 levels in cultured neurons treated with Aβ25–35 (Fig. 2B). Based on these data, we next examined the effect of GSNO on the regulation of Cdk5 activity. In a time course study, Cdk5 activity in primary cultured cortical neurons was significantly increased at 1 hr and reached maximum at 12 hrs following the Aβ25–35 treatment (Fig. 2C-i). Interestingly, Cdk5 activity under GSNO (100µM) treatment was significantly higher than untreated neurons, but was significantly lower than that of activities under Aβ25–35 treatment (Fig. 2C-i). In addition, GSNO treatment significantly decreased the Aβ-induced Cdk5 activity (Fig. 2C-ii). Previously, Qu et al. reported that S-nitrosylation of Cdk5 by NO-donors including GSNO increased Cdk5 activity in HEK 293T cells [23]. Therefore, the observed increase in Cdk5 basal activities by GSNO treatment (Fig. 4D-i and ii) may be due to S-nitrosylation of Cdk5. However, under the conditions of Aβ25–35-induced calpain-mediated p35 proteolysis to p25, GSNO inhibited aberrant Cdk5 activation via inhibiting calpain-mediated p25 formation, and thus Cdk5-mediated pathological tau phosphorylation. Consistent with previously described Aβ25–35-induced Cdk5 activation via activation of neuronal NOS (nNOS) and subsequent S-nitrosylation of Cdk5 [23], we also observed that Aβ25–35-induced activation of Cdk5 was dependent on nNOS activity at an early time point (1 hr after Aβ25–35 treatment) as nNOS inhibitors [L-Nω-nitroarginine methyl ester (L-NAME) and 7-nitroindazole (7-NI)] inhibited the Aβ25–35-induced Cdk5 activity (Fig. 2C-iii). However, at a later time point (12 hrs after Aβ25–35 treatment) where Cdk5 activities and p35 proteolysis (Fig. 2B) were aberrantly increased, Cdk5 activities were not affected by L-NAME treatment (Fig. 2C-iii). Along with the data reported by Qu et al. [23], our data suggests that GSNO may have dual roles in regulation of Cdk5 activity; GSNO itself increases the activity of Cdk5 via S-nitrosylation of Cdk5 [23], but under conditions of calpain activation, GSNO inhibits aberrant Cdk5 activation by inhibition of calpain-mediated p35 proteolysis to form p25.
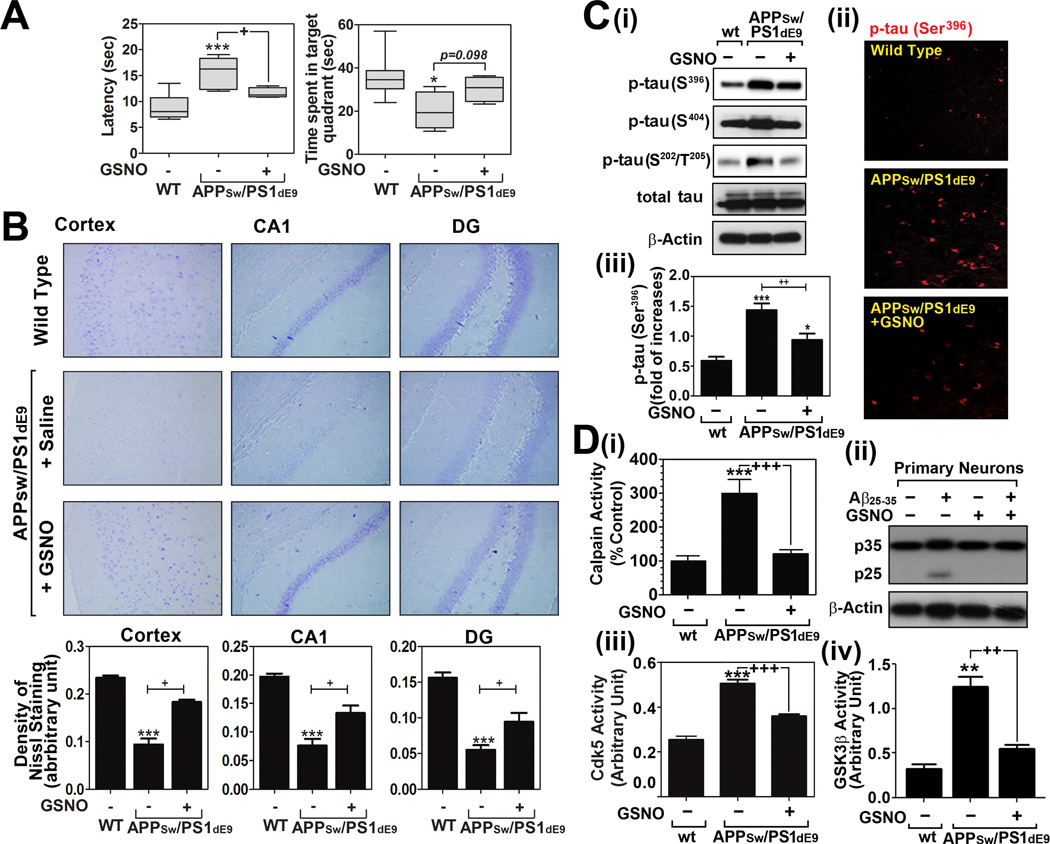
Fig. 4. Effect of GSNO treatment on spatial learning and memory deficits and tau hyperphosphorylation in of APPSw/PS1dE9 mice.
A. Analysis of cognitive function (learning and memory function) of wild type (WT/C57BL6) and APPSw/PS1dE9 mice treated with saline or GSNO (3mg/kg/day). Time (sec) to reach the hidden platform (latency in seconds) for spatial learning performance and time (sec) to spend in target quadrant without platform for spatial memory performance were analyzed by Morris Water maze test (n=8). The columns show 75% of distribution; horizontal bar in each column is median; and vertical T-bars are minimum and maximum values of the data. For statistical analysis and presentation of the data, one way ANOVA test with Turkey’s multiple comparison test was performed. B. Distribution and number of neurons in cortical and hippocampal layers (dentate gyrus; DG and CA1) were analyzed by Nissl staining. C. Brain levels of hyper-phosphorylated tau protein in WT and APPSw/PS1dE9 mice treated with saline or GSNO were analyzed by Western analysis (i) and immunofluorescence staining (ii and iii). D. Calpain activity (i), p35 proteolysis to p25 (ii), Cdk5 activity (iii), and GSK-3β activity (iv) were analyzed in brains of WT and APPSw/PS1dE9 mice treated with saline or GSNO were analyzed as described materials and methods. The vertical columns in panels B~D are means of individual data and T-bars are standard error mean. *p<0.01, **p<0.005, and ***p<0.0001 as compared to the wild type (WT) group. +p<0.01, ++p<0.005, and +++p<0.0001 as compared to untreated APPSw/PS1dE9 mice group.
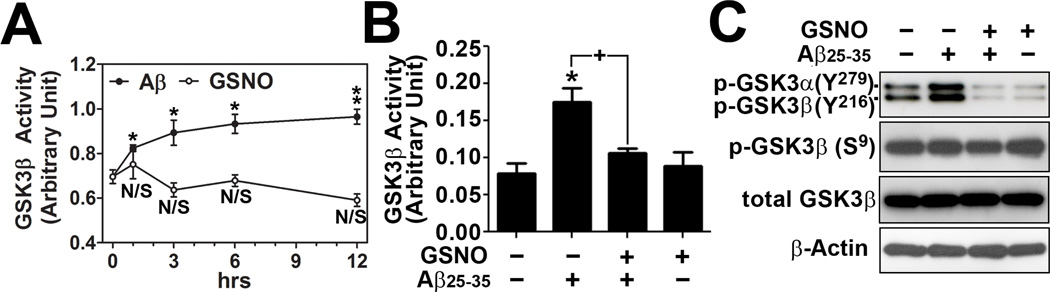
GSK-3β is known to inhibit axonal transport by altering microtubule stability through hyperphosphorylation of tau and PHF/NFT formation [24,25]. GSK-3 is highly active in cells under basal conditions. This is partly due to phosphorylation of a conserved tyrosine residue on the activation loop of the kinase domain (Tyr216 in GSK-3β) that is required for kinase activity of GSK-3β [26]. However, phosphorylation of GSK-3β at an N-terminal serine residue inhibits its kinase activity (Ser9). This phosphorylated Ser9 acts as a pseudo-substrate and binds to the phosphate-binding pocket on GSK-3β, and thus inhibits its activity by preventing interaction with substrates [27]. To evaluate the effects of Aβ25–35 load on neuronal GSK-3β activity, primary cultured neurons were treated with Aβ25–35 peptide and its time course effect was analyzed on GSK-3β enzyme activity. As expected, Aβ25–35 treatment increased GSK-3β activity over 24 hrs, but GSNO treatment had no significant effect on basal GSK-3β activity (Fig. 3A). Next, the neuron cells were treated with Aβ25–35 peptide in the presence or absence of GSNO to examine the effect of GSNO treatment on Aβ25–35-induced activation of GSK-3β. Figs. 3B shows that GSNO treatment had no effect on basal activity but inhibited the Aβ25–35-induced GSK-3β activation. The Aβ25–35-induced increase in GSK-3β activity and its inhibition by GSNO treatment are in coincidence with phosphorylation status of GSK-3β Tyr216 residue (Fig. 3C). However, neither Aβ25–35 nor GSNO had effect on phosphorylation of Ser9 in GSK-3β (Fig. 3C). These data indicate that GSNO modulates mechanisms regulate GSK-3β activity via dephosphorylaltion of its kinase domain loop (Tyr216) without any effect on N-terminal pseudo-substrate domain (Ser9). Taken together, these studies raise interesting question regarding the potential role of GSNO, a natural biological molecule, in relation to protein secondary modification as a regulator of calpain, Cdk5, and GSK-3β signaling mechanisms in AD processes.
Fig. 3. Effect of GSNO treatment on GSK-3β activation in Aβ25–35 treated neurons.
A. Effect of GSNO (100µM) and Aβ25–35 (40µM) treatment on the activities of GSK-3β was analyzed in a time course manner in primary cultured cortical neurons. B and C. To examine the effect of GSNO on Aβ25–35-induced GSK-3β activities, the cultured neurons were pretreated with GSNO for 4hrs and treated with Aβ25–35 for 12 hrs. GSK-3β enzyme activities were analyzed by in vitro kinase assay (B) or Western blot by using antibodies specific to phospho-GSK-3α/β (Tyr279/216; p-GSK-3α-Y279, p-GSK-3β-Y216) and phospho-GSK-3β (Ser9; p-GSK-3β-S9), and total GSK-3β (C). The vertical columns are means of individual data and T-bars are standard error mean. *p<0.01; and **p<0.005 as compared to the control group. +p<0.01 as compared to Aβ treated groups.
Based on the observed inhibitory role of GSNO in tau hyperphosphorylation in cultured neurons, we next evaluated the therapeutic potential of GSNO in mouse model of AD (APPSw/PS1dE9) which express significant Aβ deposition at 6 months of age and senile plaques at 9 months of age [28]. Starting 5 months of age, APPSw/PSEN1dE9 mice received daily GSNO (3mg/kg/day/i.p.) for 5 months. At the end of 5 month of GSNO treatment, the animals were subjected to Morris water maze test for evaluation of spatial learning and memory function. Figure 4A shows that spatial learning and memory functions were significantly compromised in APPSw/PS1dE9 mice. GSNO treatment of APPSw/PSEN1dE9 mice significantly improved the spatial learning performance (escape latency). In addition, GSNO treatment also improved spatial memory performance (percent time in target quadrance), but below the threshold of statistical significance (p=0.098). The improved cognitive functions were well correlated with attenuation of neuronal loss in cortical and hippocampal areas (dentate gyrus/DG and CA1) as observed in Nissl staining (Fig. 4B). Accordingly with the data in in vitro neuron culture, we also observed that the brains of APPSw/PS1dE9 mice had an elevated tau phosphorylation at Ser202/Thr205 and Ser396/Ser404 (Fig. 4C) and increased calpain activity (Fig. 4D-i) and p35 proteolysis to p25 (Fig. 4D-ii) and Cdk5 (Fig. 4D-iii) and GSK-3β (Fig. 4D-iv) activities as compared to wild type control. However, the degree of tau phosphorylation, calpain activity, p35 proteolysis to p25, and Cdk5/GSK-3β activities in APPSw/PS1dE9 mice was markedly reduced by GSNO treatment. Therefore, these data indicate that GSNO-mediated mechanisms also regulate the tau hyperphosphorylation via regulation of calpain/p25/Cdk5 and GSK-3β pathways in brain of AD mice model.
The present study describes the importance of GSNO-mediated mechanisms in neuroprotection and cognitive functions under AD conditions. GSNO is the most abundant S-nitrosothiol in human and animals, formed by redox based reaction between GSH and NO. NO, itself, has been regarded as a cell signaling mediator regulating various physiological processes [2]. However, NO and peroxynitrite are also known to cause nitroso-oxidative damage under inflammatory conditions [29]. The observed opposing roles of NO effectors, such as peroxynitrite in induction of tissue damage of the CNS [29] and AD pathologies (Aβ aggregation and tau modification [30,31]) vs. GSNO in inhibitions of Aβ synthesis, inflammation, cognitive deficit [6,32], and tau hyperphosphorylation suggest GSNO as a neuroprotective effector of NO as compared to degenerating role of peroxynitrite. AD involves chronic inflammation and oxidative stress [33]. Since oxidative stress disease conditions reduced the cellular levels of GSNO, caused by reaction between NO and superoxide anion to form peroxynitrite and by decreased levels of GSH, exogenous supplementation is possibly required to the cellular requirement of GSNO/NO for physiological cellular signaling activities. In this study, we also observed that GSNO supplementation inhibited tau hyperphosphorylation under in vitro cell culture and in vivo AD model. Taken together with the beneficial role of GSNO in anti-inflammation, anti-amyloidogenesis, and cerebrovascular protection [6,32], our data support the possible therapeutic potential of exogenous GSNO supplementation in AD.
Supplementary Material
Highlight.
GSNO inhibits calpain activation and p35 proteolysis to p25 which leads to activation of Cdk5.
GSNO inhibits GSK-3β activation via inhibiting GSK-3β phosphorylation at tyrosine 216 residue.
GSNO inhibits tau hyperphosphorylation in cultured neurons.
GSNO also inhibits tau hyperphosphorylation in brains of mouse model of Alzheimer’s disease.
GSNO attenuates neuronal death and cognitive deficits in mouse model of Alzheimer’s disease.
Acknowledgements
This work was supported by grants from NIH and VA (NS072511, BX001062, NS037766 and BX001072).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Giacobini E, Gold G. Alzheimer disease therapy--moving from amyloid-beta to tau. Nat Rev Neurol. 2013;9:677–686. doi: 10.1038/nrneurol.2013.223. [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Ruiz A, Cadenas S, Lamas S. Nitric oxide signaling: classical, less classical, and nonclassical mechanisms. Free Radic Biol Med. 2011;51:17–29. doi: 10.1016/j.freeradbiomed.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Hammond J, Balligand JL. Nitric oxide synthase and cyclic GMP signaling in cardiac myocytes: from contractility to remodeling. J Mol Cell Cardiol. 2012;52:330–340. doi: 10.1016/j.yjmcc.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 4.McAndrew J, Patel RP, Jo H, Cornwell T, Lincoln T, Moellering D, White CR, Matalon S, Darley-Usmar V. The interplay of nitric oxide and peroxynitrite with signal transduction pathways: implications for disease. Semin Perinatol. 1997;21:351–366. doi: 10.1016/s0146-0005(97)80002-x. [DOI] [PubMed] [Google Scholar]
- 5.Hess DT, Stamler JS. Regulation by S-nitrosylation of protein post-translational modification. J Biol Chem. 2012;287:4411–4418. doi: 10.1074/jbc.R111.285742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Won JS, Kim J, Annamalai B, Shunmugavel A, Singh I, Singh AK. Protective role of S-nitrosoglutathione (GSNO) against cognitive impairment in rat model of chronic cerebral hypoperfusion. J Alzheimers Dis. 2013;34:621–635. doi: 10.3233/JAD-121786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasad R, Giri S, Nath N, Singh I, Singh AK. GSNO attenuates EAE disease by S-nitrosylation-mediated modulation of endothelial-monocyte interactions. Glia. 2007;55:65–77. doi: 10.1002/glia.20436. [DOI] [PubMed] [Google Scholar]
- 8.Chiueh CC. Neuroprotective properties of nitric oxide. Ann N Y Acad Sci. 1999;890:301–311. doi: 10.1111/j.1749-6632.1999.tb08007.x. [DOI] [PubMed] [Google Scholar]
- 9.Qian J, Chen F, Kovalenkov Y, Pandey D, Moseley MA, Foster MW, Black SM, Venema RC, Stepp DW, Fulton DJ. Nitric oxide reduces NADPH oxidase 5 (Nox5) activity by reversible S-nitrosylation. Free Radic Biol Med. 2012;52:1806–1819. doi: 10.1016/j.freeradbiomed.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aggarwal A, Khera A, Singh I, Sandhir R. S-nitrosoglutathione prevents blood-brain barrier disruption associated with increased matrix metalloproteinase-9 activity in experimental diabetes. J Neurochem. 2014 doi: 10.1111/jnc.12939. [DOI] [PubMed] [Google Scholar]
- 11.Khan M, Dhammu TS, Sakakima H, Shunmugavel A, Gilg AG, Singh AK, Singh I. The inhibitory effect of S-nitrosoglutathione on blood-brain barrier disruption and peroxynitrite formation in a rat model of experimental stroke. J Neurochem. 2012;123(Suppl 2):86–97. doi: 10.1111/j.1471-4159.2012.07947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrer I, Gomez-Isla T, Puig B, Freixes M, Ribe E, Dalfo E, Avila J. Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer's disease and tauopathies. Curr Alzheimer Res. 2005;2:3–18. doi: 10.2174/1567205052772713. [DOI] [PubMed] [Google Scholar]
- 13.Mazanetz MP, Fischer PM. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat Rev Drug Discov. 2007;6:464–479. doi: 10.1038/nrd2111. [DOI] [PubMed] [Google Scholar]
- 14.Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40:471–483. doi: 10.1016/s0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- 15.Noble W, Olm V, Takata K, Casey E, Mary O, Meyerson J, Gaynor K, LaFrancois J, Wang L, Kondo T, Davies P, Burns M, Veeranna, Nixon R, Dickson D, Matsuoka Y, Ahlijanian M, Lau LF, Duff K. Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron. 2003;38:555–565. doi: 10.1016/s0896-6273(03)00259-9. [DOI] [PubMed] [Google Scholar]
- 16.Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 17.Patrick GN, Zhou P, Kwon YT, Howley PM, Tsai LH. p35, the neuronal-specific activator of cyclin-dependent kinase 5 (Cdk5) is degraded by the ubiquitin-proteasome pathway. J Biol Chem. 1998;273:24057–24064. doi: 10.1074/jbc.273.37.24057. [DOI] [PubMed] [Google Scholar]
- 18.Chow HM, Guo D, Zhou JC, Zhang GY, Li HF, Herrup K, Zhang J. CDK5 activator protein p25 preferentially binds and activates GSK3beta. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1402627111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- 20.Hanger DP, Anderton BH, Noble W. Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol Med. 2009;15:112–119. doi: 10.1016/j.molmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Michetti M, Salamino F, Melloni E, Pontremoli S. Reversible inactivation of calpain isoforms by nitric oxide. Biochem Biophys Res Commun. 1995;207:1009–1014. doi: 10.1006/bbrc.1995.1285. [DOI] [PubMed] [Google Scholar]
- 22.Samengo G, Avik A, Fedor B, Whittaker D, Myung KH, Wehling-Henricks M, Tidball JG. Age-related loss of nitric oxide synthase in skeletal muscle causes reductions in calpain S-nitrosylation that increase myofibril degradation and sarcopenia. Aging Cell. 2012;11:1036–1045. doi: 10.1111/acel.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qu J, Nakamura T, Cao G, Holland EA, McKercher SR, Lipton SA. S-Nitrosylation activates Cdk5 and contributes to synaptic spine loss induced by beta-amyloid peptide. Proc Natl Acad Sci U S A. 2011;108:14330–14335. doi: 10.1073/pnas.1105172108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rankin CA, Sun Q, Gamblin TC. Tau phosphorylation by GSK-3beta promotes tangle-like filament morphology. Mol Neurodegener. 2007;2:12. doi: 10.1186/1750-1326-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai Z, Zhao Y, Zhao B. Roles of glycogen synthase kinase 3 in Alzheimer's disease. Curr Alzheimer Res. 2012;9:864–879. doi: 10.2174/156720512802455386. [DOI] [PubMed] [Google Scholar]
- 26.Cole A, Frame S, Cohen P. Further evidence that the tyrosine phosphorylation of glycogen synthase kinase-3 (GSK3) in mammalian cells is an autophosphorylation event. Biochem J. 2004;377:249–255. doi: 10.1042/BJ20031259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frame S, Cohen P, Biondi RM. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell. 2001;7:1321–1327. doi: 10.1016/s1097-2765(01)00253-2. [DOI] [PubMed] [Google Scholar]
- 28.Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, Borchelt DR. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet. 2004;13:159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- 29.Torreilles F, Salman-Tabcheh S, Guerin M, Torreilles J. Neurodegenerative disorders: the role of peroxynitrite. Brain Res Brain Res Rev. 1999;30:153–163. doi: 10.1016/s0165-0173(99)00014-4. [DOI] [PubMed] [Google Scholar]
- 30.Reyes JF, Fu Y, Vana L, Kanaan NM, Binder LI. Tyrosine nitration within the proline-rich region of Tau in Alzheimer's disease. Am J Pathol. 2011;178:2275–2285. doi: 10.1016/j.ajpath.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kummer MP, Hermes M, Delekarte A, Hammerschmidt T, Kumar S, Terwel D, Walter J, Pape HC, Konig S, Roeber S, Jessen F, Klockgether T, Korte M, Heneka MT. Nitration of tyrosine 10 critically enhances amyloid beta aggregation and plaque formation. Neuron. 2011;71:833–844. doi: 10.1016/j.neuron.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Kwak YD, Wang R, Li JJ, Zhang YW, Xu H, Liao FF. Differential regulation of BACE1 expression by oxidative and nitrosative signals. Mol Neurodegener. 2011;6:17. doi: 10.1186/1750-1326-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.