Abstract
There has been only modest change in cancer incidence and mortality during the past several decades, but the number of cancer survivors has almost tripled during the same period. With an increasing cohort of cancer survivors, efforts to prevent, diagnose, and manage side effects of cancer therapy in general and, specifically those of radiation therapy have intensified. Many cancer survivors have undergone radiation therapy of tumors in the pelvis or abdomen, thus rendering the bowel at risk for injury. In fact, the current prevalence of patients with long term radiation-induced intestinal side effects exceeds that of ulcerative colitis and Crohn’s disease combined. Significant progress toward reducing toxicity of radiation therapy has been made by the introduction of so-called dose-sculpting treatment techniques, which allow more precise delivery of the radiation beam. Moreover, new insight into the underlying pathophysiology have resulted in an improved understanding of mechanisms of radiation-induced bowel toxicity and in development of new diagnostic strategies and management opportunities. This article discusses the pathogenesis of early and delayed radiation-induced bowel toxicity, reviews current management options, and outlines priorities for future research. The gastroenterologist by adding insight into molecular and cellular mechanisms of related bowel disorders can substantially strengthen these efforts.
Introduction
Radiation therapy is used in at least 50% of cancer patients and plays a critical role in 25% of cancer cures. Despite recent advances in treatment delivery techniques, normal tissue radiation toxicity remains the overwhelmingly most important obstacle to cancer cure in patients with localized disease. During radiation therapy of tumors in the abdomen or pelvis, the intestine is an important normal tissue at risk. Early radiation enteropathy occurs within 3 months of radiation therapy and affects the quality of life at the time of treatment. Moreover, treatment interruption or changes in the original treatment plan may be required, thereby compromising the likelihood of tumor control. Delayed intestinal radiation toxicity is a highly important issue for long term cancer survivors. It is a progressive condition with few therapeutic options and substantial long-term morbidity and mortality.
This review article discusses radiation enteropathy as a clinical problem, pathological features of radiation enteropathy, cellular and molecular mechanisms of radiation enteropathy, and contemporary approaches for prevention and management of radiation enteropathy. The aim of the review is to provide an introduction of the subject tailored to the needs of the gastroenterologist.
Radiation Enteropathy – Magnitude of the Clinical Problem
Radiation therapy plays a definitive role in a quarter of all cancer cures and more than half of all cancer patients undergo radiation therapy during the course of their disease. Developments in treatment techniques have made it possible to deliver radiation to tumors with much greater precision than before. Nevertheless, normal tissue toxicity remains the single-most important radiation dose-limiting factor and obstacle to cancer cure. Moreover, some authors have expressed concern about the newer treatment techniques and what they mean for the spectrum of toxicities 1. During radiation therapy of tumors in the abdominal cavity or pelvis, parts of the small bowel, colon, or rectum are inevitably included in the treatment field and represent important normal tissues at risk.
The incidence and severity of intestinal radiation toxicity depend on a number of therapy-related and patient-related factors. Therapy-related factors include radiation dose, volume (length) of bowel irradiated, time-dose-fractionation parameters, and the use of concomitant chemotherapy or biotherapy. Patient-related factors include body mass index – obesity protects, while reduced body mass index predisposes to radiation toxicity 2,3; previous abdominal surgery, which increases the risk of radiation-induced bowel injury because peritoneal adhesions lead to fixation of small bowel loops in the radiation field; and certain co-morbidities, e.g., inflammatory bowel disease 4, diabetes 5, vascular disorders 6, and collagen vascular disease 7,8. Tobacco smoking is a strong independent predictor of intestinal complications after radiation therapy of tumors in the pelvis or abdomen. Genetic predisposition also plays a significant role and may explain why certain patients go through therapy without side effects, while others who get exactly the same treatment experience severe toxicities 9.
Intestinal radiation toxicity (radiation enteropathy) is generally classified as early (acute) when it occurs within 3 months of radiation therapy, or delayed (chronic) when it occurs more than 3 months after radiation therapy. Annually, an estimated 300,000+ patients receive pelvic or abdominal radiation therapy with an 60-80% incidence of symptoms of acute bowel toxicity. There are more than 13 million cancer survivors in the US today and the number will likely increase to 18 million by 2022 10. More than half of these patients are survivors of abdominal or pelvic cancers (Figure 1). While the incidence of severe (grade 3-4) delayed intestinal radiation toxicity has diminished over time, largely thanks to developments in radiation treatment planning and radiation delivery techniques, series with careful follow-up show that at least half of the patients will have some form of chronic GI dysfunction. Most clinical studies greatly underestimate the true prevalence of delayed bowel toxicity 11. However, some authors claim that some degree of GI dysfunction is an almost inevitable consequence of pelvic/abdominal radiation therapy 12,13. A conservative estimate of the number of patients with post-radiation intestinal dysfunction living in the US most certainly exceeds 1.6 million (Box 1). This is compared to a prevalence of 396/100,000 for inflammatory bowel disease (IBD) 14, or about 1.4 million suffering from IBD in the US 15.
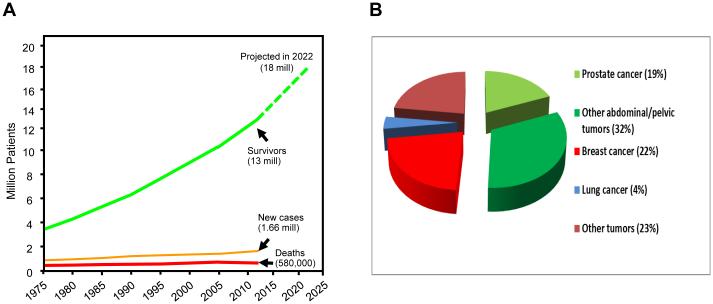
Figure 1. Cancer survivors and cancer prevalence rates in the US.
A) Cancer incidence and death rates have been fairly flat during the past 4 decades, while the cohort of cancer survivors increases by 3% per year, exceeds 13 million in 2013, and is expected to reach 18 million in 2022 10.
B) Approximately half of all cancer survivors are survivors after abdominal or pelvic tumors 93, many of whom have had or will have radiation therapy. This points to radiation enteropathy as a major obstacle to uncomplicated cancer cures.
Box 1. Incidence and prevalence of radiation enteropathy.
60-80% of patients experience temporary symptoms of bowel toxicity during radiation therapy.
50% of patients who have undergone abdominal or pelvic radiation therapy suffer from some degree of chronic intestinal dysfunction, and radiation enteropathy is one of the most common side effects among long term cancer survivors.
The risk of radiation enteropathy limits the uncomplicated cancer cure rate and adversely impacts the quality of life of cancer survivors.
The prevalence of radiation enteropathy exceeds that of inflammatory bowel disease.
Clinical and Pathologic Characteristics of Radiation Enteropathy
Early intestinal injury becomes manifest within days of beginning a course of radiation therapy and is primarily a result of cell death in the rapidly proliferating crypt epithelium and a protracted acute inflammatory reaction in the lamina propria. Crypt cell death results in insufficient replacement of the villus epithelium, breakdown of the mucosal barrier, and mucosal inflammation. Figure 2 shows an example of experimental radiation mucositis using a clinically relevant model for localized irradiation of rat small bowel 16, while Figure 3 shows an example of clinical radiation mucositis in the rectum from a patient undergoing radiation therapy for prostate cancer. Symptoms of early bowel toxicity (nausea, abdominal pain, diarrhea, and fatigue) develop in 60-80% of patients during radiation therapy of tumors in the abdomen or pelvis. Nausea typically occurs relatively early, while diarrhea and abdominal pain usually become problematic 2-3 weeks into the course of radiation therapy. In most patients, acute symptoms of bowel toxicity resolve within 1-3 months of completing treatment (Box 2).
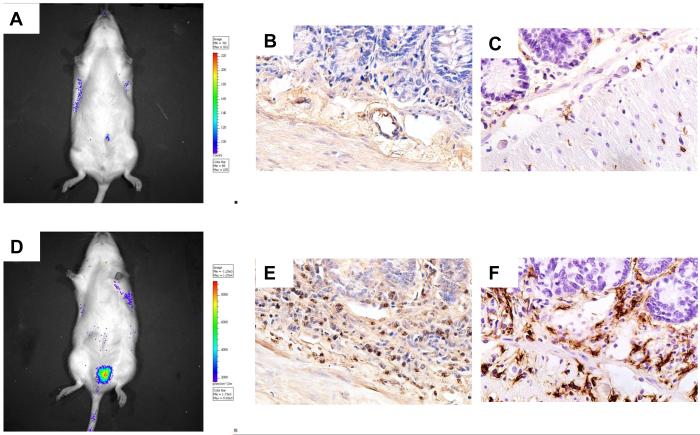
Figure 2. Radiation mucositis (rat intestine, all images original magnification 40X).
A) Bioluminescence image (luminol) of an unirradiated (control) rat with intestine transposed to the left scrotum for fractionated irradiation. No increase in bioluminescence indicating little or no myeloperoxidase activity.
B) Unirradiated (control) rat small intestine stained with anti-transferrin antibody. Few granulocytes are seen in the mucosa/submucosa.
C) Unirradiated (control) rat small intestine stained with anti-ED2 (CD163) antibody. There are few macrophages in the mucosa/submucosa.
D) Bioluminescence image (luminol) 5 days after localized irradiation of a segment of small bowel transposed to the left scrotum. Significant increase in bioluminescence indicating substantial myeloperoxidase activity.
E) Intestine procured 2 weeks after irradiation showing accumulation of granulocytes in mucosa/submucosa.
F) Intestine procured 2 weeks after irradition showing accumulation of macrophages in mucosa/submucosa.
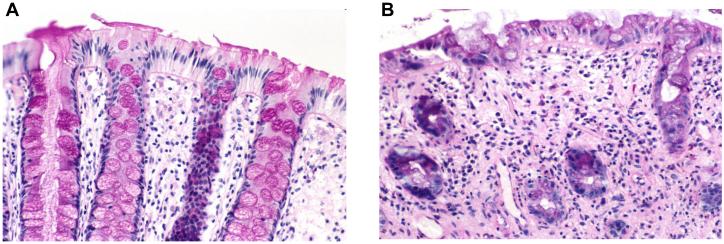
Figure 3. Human endoscopic biopsies of rectal mucosa obtained from patient before and during ongoing radiation therapy of prostate cancer.
A) Periodic acid Schiff (PAS)-staining of normal rectal mucosa before start of radiation therapy. Note intact surface epithelium, straight glands, and the many PAS-positive goblet cells (original magnification 20X).
B) Glandular atrophy, mucosal inflammation, and loss of PAS-positive goblet cells 2 weeks into the course of radiation therapy (original magnification 20X).
Box 2. Symptomatology and pathophysiology of radiation enteropathy.
Early radiation enteropathy occurs because of epithelial barrier dysfunction and mucosal inflammation. The main symptoms (nausea/vomiting, diarrhea, abdominal pain) become manifest during radiation therapy, but usually subside once the course of radiation therapy is over.
Delayed radiation enteropathy injury occurs 3 months or more after radiation therapy and is characterized by mucosal atrophy, vascular sclerosis, and progressive intestinal wall fibrosis. The symptoms are chronic and progressive and mainly characterized by malabsorption of nutrients and abnormal propulsion of intestinal contents.
Symptoms of delayed bowel toxicity can develop before symptoms attributable to early toxicity subside, but typically present after a latency period of 6 months to 3 years. However, latency periods of 20-30 years after radiation therapy are not uncommon. The pathogenesis of delayed radiation enteropathy is complex and involves changes in most compartments of the intestinal wall. Atrophy of the mucosa, fibrosis of the intestinal wall, and microvascular sclerosis are prominent, and currently irreversible features (Figure 4). The main clinical features of delayed radiation enteropathy are altered intestinal transit, malabsorption, and dysmotility 17. Delayed radiation enteropathy is a chronic, often progressive disorder and associated with substantial long-term morbidity. Severe (grade 3-4) late effects have become less common than they were in the past. Nevertheless, for example, the incidence of severe toxicity after chemo-radiation therapy of cervical cancer remains around 10% 18. Severe delayed radiation enteropathy may progress to intestinal obstruction, fistula formation, or frank intestinal perforation. Corrective surgery is associated with high postoperative morbidity and mortality. Long term, the majority of patients have persistent or recurrent symptoms, and about 10% die as a direct result of radiation enteropathy 19,20. Patients with isolated colonic injury are not commonly metabolically deranged, and their long-term prognosis is better.
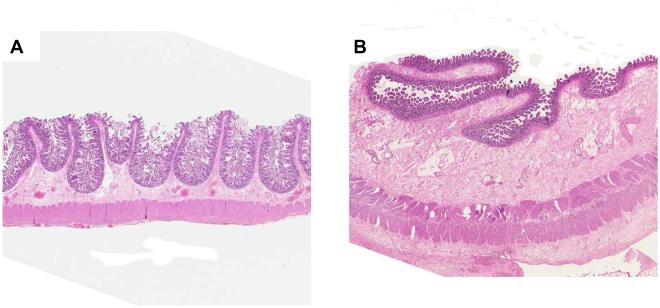
Figure 4. Resection specimens of normal human small bowel and delayed radiation enteropathy.
A) Normal intestine (original magnification 0.5X).
B) Resected small intestine from a woman with severe delayed radiation enteropathy. Note atrophic mucosa and severe fibrosis in submucosa and subserosa (original magnification 0.5X, same as panel A).
For a comprehensive description of clinical and pathological features of radiation enteropathy, the reader is referred to the chapter on the alimentary tract in Radiation Pathology by Fajardo, Berthrong, and Anderson 21, to the very comprehensive review by Carr 22, and to the chapters on the small bowel, colon, rectum, and anus in Human Radiation Injury, edited by Shrieve and Loeffler 23.
Understanding Radiation-Induced Gastrointestinal Toxicity
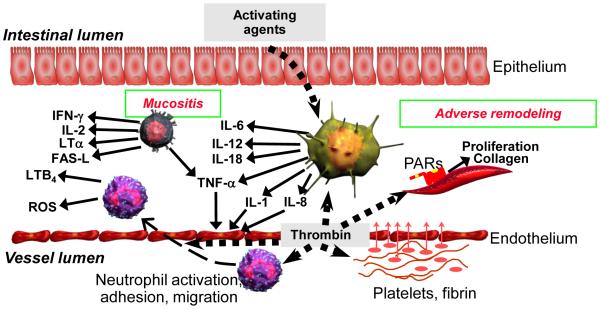
The classical understanding of intestinal radiation toxicity was based entirely on the target cell theory. According to this concept, the intestine was considered a more or less inert tube, covered on the inside by a rapidly proliferating epithelium and the rest of the bowel tissues were considered more or less irrelevant. The severity of epithelial injury was the only determinant of early pathology, while a different, more slowly proliferating target cell (fibroblast, endothelial cell) was used to explain delayed effects. While the sequence of structural and functional manifestations of radiation enteropathy has not changed, our understanding of the underlying pathobiology has improved over the years. Hence, the contemporary view is that many tissues and cell types in the gut participate and contribute to injury. For example, the intestinal immune system is the largest in the human body and influences the development of secondary changes after radiation profoundly. The enteric nervous system is the second largest in the body with a greater number of neurons than the spinal cord and strongly regulates radiation enteropathy development. The intestinal microvasculature is recognized as an important contributor to radiation toxicity of the bowel. There are 100 trillion bacteria in the gut lumen, 10-fold the number of cells in the human body, and the intestinal microbiome profoundly influences radiation enteropathy development. In other words, we have progressed beyond the single target cell concept and now recognize that, in addition to epithelial injury, the intestinal microvasculature, immune mechanisms, neuro-immune interactions, the intestinal microbiome, the composition of the intraluminal contents, and a host of other factors play important roles (Figure 5).
Figure 5. Involvement of the intestinal immune system and microvascular endothelium in the regulation of acute radiation mucositis and subsequent adverse tissue remodeling (intestinal fibrosis).
When the mucosal barrier becomes disrupted, as after radiation exposure, bacterial products and other activating agents gain access to the intestinal tissues and stimulate a variety of immune cells to produce cytokines and other pro- and anti-inflammatory mediators.
Moreover, radiation-induced endothelial dysfunction leads to endothelial dysfunction with loss of thromboresistance, resulting in thrombin formation, neutrophil recruitment and activation, and stimulation of mesenchymal cells.
In addition to the mechanisms depicted here, a host of other mechanisms, for example, those related to mast cells, the enteric nervous system, and the gut microbiome plays important roles in the pathogenesis of radiation enteropathy (see the section Important Unanswered Questions in Radiation Enteropathy).
Consequential Late Effects
The recognition that delayed radiation injury may develop in the wake of severe acute injury was recognized clinically by Bourne and colleagues 24 and subsequently coined “consequential late effects” by Peters 25. Consequential late effects served an important purpose by helping eradicate the old dogma of independence between early and delayed radiation effects, for improving our understanding of the pathophysiology and pathogenesis of delayed normal tissue injury, and for interpreting and modeling radiation responses in vivo.
However, it became increasingly clear that the terminology failed to recognize A) the complexity of radiation effects in multicellular tissues and organs; B) the co-existence of consequential and primary injury (although the response may be skewed in one direction or the other); and C) evidence that early effects unrelated to cell death may give rise to subsequent chronic injury. Many pharmacological and genetic models also showed that the relationship cannot be explained simply by consequential late effects, for example, mast cell deficient rats, sensory nerve-ablated rats, and TGF-beta heterozygous rats all show dissociations between early and delayed injury 26–29, i.e., all have exacerbated epithelial injury, but reduced levels of intestinal fibrosis.
As there was a fundamental conflict between these observations and the traditional notion of “consequential late tissue injury”, a new terminology for classifying normal tissue radiation responses was proposed in 2001 30. According to this classification, there are 1) cytocidal effects, where radiation causes cell death (clonogenic cell death, mitotic catastrophe, apoptosis, etc.); 2) functional effects where radiation causes changes in the intracellular environment, plasma membrane, and extracellular space (transcription factor activation, protein modification, etc.); and 3) secondary effects in response to the initial radiation insult (such as, cellular inflammation, release of cytokines and other mediators, etc.). It is important to remember that all 3 types of effects (cytocidal, functional, and secondary) interact and contribute to organ dysfunction.
Fractionated Radiation Therapy and Radiation Enteropathy
Most radiation therapy regimens are delivered as fractions of 1.8-2 Gy on weekdays for a number of weeks. The radiotherapy response differs from other types of tissue damage in that a burst of free radicals is produced, which not only causes immediate DNA damage, but also alters proteins, lipids, carbohydrates, and other complex molecules. When considering normal tissue radiation responses, it is important to also consider that they occurs as results of multiple repetitive injuries (fractions) rather than as a response to a single insult 31. Hence, each fraction contributes to inflammatory cell recruitment as well as to the accumulation of direct tissue injury. Furthermore, each fraction affects tissue that already exhibits a dynamic spectrum of cellular injury, ongoing repair, inflammation, and other pathophysiologic responses. Therefore, with repetitive radiation exposure, many cellular and molecular responses will be substantially exacerbated, suppressed, or substantially altered compared to the situation after a single exposure to radiation or traumatic injury. Nowhere is this more evident than in the intestine. Hence, the number of patients with symptoms of toxicity increases steadily during a 6-week course of fractionated radiation as does the “toxicity score”. However, mucosal pathology and functional bowel injury (intestinal permeability assessed with differential urinary excretion of di-/mono-saccharides) is actually substantially worse after just 2 weeks irradiation than toward the end of the treatment course 32,33. The fact that the patient has received a 3-fold higher radiation dose at the end of the radiation therapy course than at 2 weeks, points to the remarkable regenerative capacity of the intestine and raises interesting questions of what is the real cause of the symptoms.
Radiation Enteropathy as a Model of Inflammatory Bowel Disease (IBD)
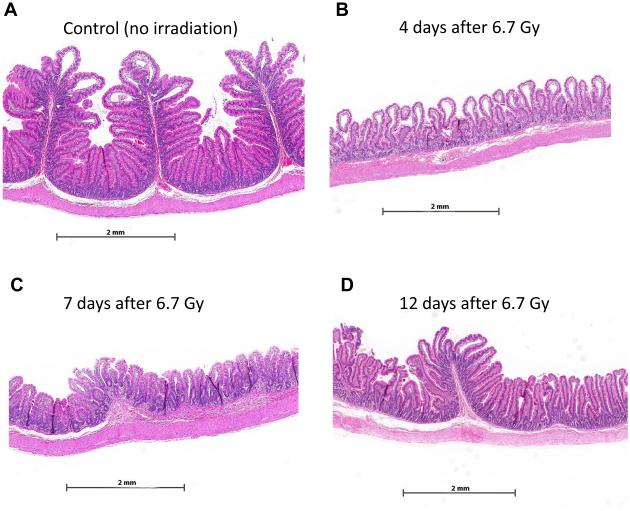
Radiation enteropathy may be considered an ideal model of gastrointestinal inflammation. First, radiation enteropathy is highly clinically relevant – as pointed out previously, the prevalence of radiation-induced GI dysfunction is higher than that of IBD. Second, animal models and patients have an identical pathology and pathophysiology, so the translational value of the observations made in the animal model is obvious (Figure 6). Third, with radiation enteropathy animal models, it is possible to easily and precisely adjust the dose of the “toxic” agent (radiation), thus making it possible to investigate dose-response relationships in greater detail than is true for other IBD models. Finally, by utilizing the identical causative agent (radiation), radiation enteropathy studies may be directly translated to the human disease situation. Methodological and mechanistic insight from other types of gastrointestinal injury or disease processes, applied to radiation enteropathy, will likely help advance this important area of research. Nevertheless, as all animal models, radiation enteropathy models have limitations, for example, in terms of the applicability of radiosensitivity, repair capacity, and differential responses to certain treatments.
Figure 6. Radiation-induced changes in the proximal jejunum from the non-human primate.
Proximal jejunum from unirradiated Rhesus macaque (A) and Rhesus macaque 4-12 days after exposure to single-dose irradiation (B-D). Note conspicuous disappearance of plicae circulares, crypt irregularity, and pronounced villus atrophy after irradiation. Partial recovery of post-irradiation changes is seen at 12 days, with near complete recovery of the epithelium and beginning re-appearance of plicae circulares. Original magnification of all images 1.4X.
Important Unanswered Questions in Radiation Enteropathy
Much discussion has evolved around whether the predominant mode of radiation-induced cell death in the epithelium is by mitotic cell death, apoptosis, or some other mechanism. The role of enterocyte apoptosis has been particularly hotly debated. While crypt cell apoptosis has been well described and, to some extent, taken as a measure of intestinal radiation toxicity 34, p53 deficient mice, while exhibiting greatly diminished crypt cell apoptosis, are actually sensitized to intestinal radiation injury 35. Moreover, using conditional Bax-deficient mice on a Bak1-deficient background, Kirsch and coworkers showed that deletion of pro-apoptotic genes from the intestinal epithelium did not protect from the intestinal radiation syndrome 36. It is possible that the explanation is to be found in the effect of p53/p21 on apoptotic versus non-apoptotic cell death 37.
Radiation kills predominantly rapidly proliferating cells, such as the progenitor cells in the intestinal crypts. This leads to insufficient replacement of the villus epithelium. Much attention has therefore been devoted to the intestinal stem cell population over the years 38,39. It is currently believed that there are at least two types of intestinal stem cells 40. Lgr5+ cells are normally mitotically active and are considered radioresistant 41, whereas Bmi1+cells are quiescent and considered more radiosensitive. While both types of stem cells appear to contribute to regeneration intestinal crypts after irradiation 42, recent evidence suggest a requirement for Lgr5+ cells 43.The intense activity in the field of regenerative medicine, as well as the recognition of the unmet need for medical countermeasures for use in radiological/nuclear emergencies highlights the area of intestinal stem cells as particularly promising.
It is well known that the microvasculature plays a central role in the regulation of radiation responses in many normal tissues, including the intestine 44,45. Radiation induces many changes in endothelial cells, such as, apoptosis, detachment from the basement membrane, increased endothelial permeability, interstitial fibrin deposition, and shifting of the thrombo-hemorrhagic balance toward coagulation. While it is clear that microvascular injury plays at least some role in explaining the self-perpetuating nature of chronic radiation fibrosis 46–48, its role in early intestinal radiation toxicity, particularly in the so-called acute gastrointestinal radiation syndrome, is more controversial. Paris and coworkers, in 2001, published the first in a series of articles showing that endothelial apoptosis was the primary lesion responsible for the gastrointestinal radiation syndrome 49. While subsequent publications are supportive 50, there has been serious challenge to this notion 36,51,52. Moreover, because radiation threshold for apoptosis in the endothelium is high, endothelial cell apoptosis is unlikely to play a significant role during conventionally fractionated radiation therapy. Despite this (still ongoing) controversy, it is known from other fields of biology that preserving the intestinal microcirculation after an insult exert a protective effect on the gut epithelium and the intestinal mucosa. Hence, it is conceivable that radiation-induced endothelial cell apoptosis may be the bellwether, or “tip of the iceberg”, indicating a broader state of dysfunction in the intestinal microvasculature, and that endothelial dysfunction indirectly affects the radiation tolerance and/or repair capacity of the crypt epithelium 53,54.
Under normal conditions, the enteric nervous system regulates intestinal motility, blood flow, and enterocyte function and plays a central role in maintaining the physiological state of the intestinal mucosa, as well as in coordinating inflammatory and fibroproliferative processes. Interactions between afferent nerves, mast cells, and other cells of the resident mucosal immune system maintain mucosal homeostasis and ensure an appropriate response to injury, including radiation enteropathy development 55. These interactions are mediated by substance P, calcitonin gene-related peptide, and other neuropeptides secreted by the sensory nerves, while resident immune cells signal to enteric nerves by the release of cytokines, growth factor, and other mediators.
The parallel between radiation-induced inflammation and primary IBD is noteworthy: colonization with microbiota is necessary for the development of colitis in mouse models, while germ-free mice are resistant to both inflammatory colitis and radiation enteropathy development 56. A similar parallel is apparent for non-steroidal anti-inflammatory drug (NSAID)-induced enteropathy, which is exacerbated by dysbiosis and greatly attenuated in germ-free animals 57. Because of the critical role of gut-associated sepsis in lethality after exposure to total body irradiation, there is a substantial literature dating back more than 5 decades on the importance of intestinal bacteria in the radiation response 58–60. Most intestinal bacteria are considered detrimental in the context of radiation and antibiotic therapy or “gut-decontamination” generally improves outcome in experimental animals after exposure to total body irradiation. Early studies also suggest that microbiota are critical in humans. On the other hand, it is clear that A) it is impossible to sterilize the bowel in the clinical situation; B) antibiotic use tends to select resistant organisms; and C) certain bacteria are more harmful than others. As with IBD, the potential of probiotics in mitigating gastrointestinal inflammation during radiation therapy has generated substantial interest 61–64. New techniques for assessment of bacterial flora in the normal state and after exposure to radiation; the development of powerful new gnotobiotic animal models; and improved understanding of the critical importance of the gut microbiome in health and disease are some of the reasons why this promising area of research is constantly evolving.
Therapy and Prevention of Radiation Enteropathy
Many natural products, peptides, and small molecules have been tested preclinically for the purpose of preventing, mitigating (strategies that are applied after irradiation, but before symptoms occur), and treating radiation enteropathy. However, there is a substantial difference between what has proven to be effective in animal models and what has proven to be effective clinically, especially when proper evidence-based criteria are used 65.
Clinical Strategies
The management of acute radiation enteropathy remains largely symptomatic and follows guidelines for treating similar symptoms in other situations. Patients with severe diarrhea that do not respond to first-line antidiarrheal medication can be treated with octreotide or other somatostatin analogs. The free radical scavenger, amifostine, is the only drug currently approved for reduction of radiation therapy side effects. While amifostine has shown impressive effects in some animal studies, and has also show some effect in preventing clinical gastrointestinal radiation toxicity, serious side effects from the drug and a narrow therapeutic time window severely hampers its use.
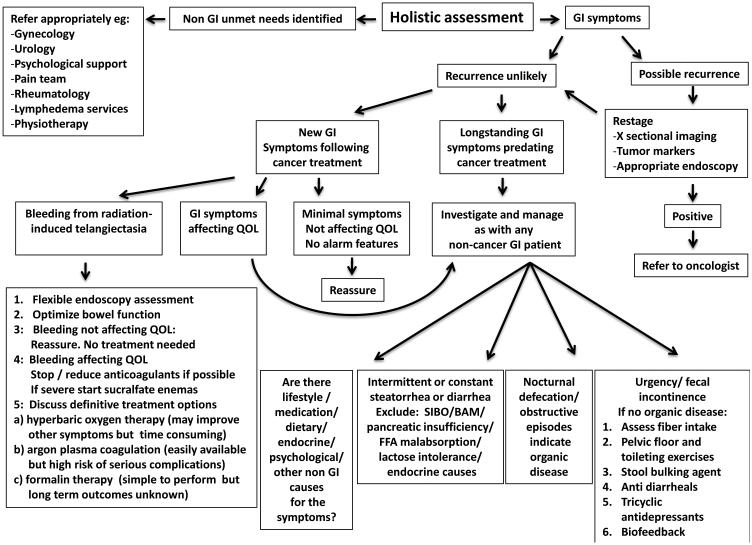
Medical management of patients with delayed radiation enteropathy should be individualized and directed at the specific underlying abnormalities. A comprehensive discussion of specific diagnosis and management principles and algorithms in delayed radiation enteropathy is beyond the scope of this review. However, it is evident that many patients can be significantly helped by a systematic approach 66. An algorithm depicting the approach to patients with radiation induced bowel problems and common treatment options is provided in Figure 7.
Figure 7.
Algorithm depicting simplified principles of work-up and common approaches for managing patients with delayed gastrointestinal symptoms after radiation therapy
Rectal radiation injury (radiation proctopathy) is usually considered separately from injury to the small bowel and colon. It is a common complication of radiation therapy for prostate cancer. First-line therapy for radiation proctopathy with bleeding is sucralfate enemas, which often produce rapid and dramatic effect 67,68. In patients with hemorrhagic proctopathy refractory to sucralfate enemas, bleeding may be controlled with local (endoscopic) interventions or hyperbaric oxygen (HBO) therapy 69.
Evidence-based reviews of strategies to minimize early and/or delayed radiation enteropathy have been published, for example, by the Multinational Association of Supportive Care in Cancer (MASCC) 70.
Preclinical research to prevent radiation enteropathy
Drugs to protect normal tissues from radiation represent a striking unmet need, both in cancer treatment and in the context of radiological emergencies. Therefore, there is intense interest in finding safe, non-toxic radioprotective compounds that do not confer tumor protection.
Interventions aimed at protecting normal tissue against radiation injury fall in two conceptually different categories. The first involves strategies that interfere directly with radiation-specific mechanisms of injury. Because most radiation injury is initiated by reactive oxygen species, antioxidants, free radical scavengers, and various cytoprotectors have been subjects of active study for more than half a century. For example, amifostine is a potent scavenger of free radicals 71. Superoxide dismutase (SOD) and various SOD mimetics are also being pursued as potential radioprotective strategies 72. The vitamin E analog, gamma-tocotrienol is the most potent non-toxic natural radioprotective compound discovered to date 73. The problems with most of these compounds in the cancer therapy situation, however, is that it is often unclear to what extent they also protect tumor cells. Toxicity and a narrow therapeutic time window are also obstacles related to some of the compounds in this category.
The second, fundamentally different approach consists of agents that modulate various pathophysiological, cellular, or molecular responses that occur downstream from radiation. These interventions seek to increase radiation tolerance, ameliorate secondary normal tissue injury, or enhance repair capacity. Such approaches include, for example, various immune-modulating drugs 74,75, enterotrophic agents 76–83, compounds that modulate intraluminal contents 84,85, and a variety of other strategies 86–91. Interventions that target downstream radiation effects may be generally more appealing in the cancer treatment situation because they do not interfere directly with the mechanism of radiation. Therefore, tumor protection is often, albeit by no means always, less of a concern than it is with free radical scavengers and antioxidants. A comprehensive, up-to-date discussion of the various strategies that have been and are under investigation is beyond the scope of this review. For this, the reader is referred to, for example, the discussion in chapter 38 (Small Bowel and Colon) in the book Human Radiation Injury 23.
Screening for New Radiation Protectors or Mitigators
Many compounds have demonstrated fairly robust radioprotective effects in animal studies. However, few have advanced to clinical testing and most of those that do fail, either because of clinical toxicities, a lackluster protective effect, or concerns about tumor protection. Moreover, there are substantial barriers to overcome in the drug development process. First, there is a false perception that the prevalence of radiation enteropathy is lower than it really is (again, the prevalence of radiation enteropathy is actually higher than that of IBD). Second, there is a lack of general public appeal for radiation enteropathy and also a lack of interest from clinicians and institutions (sometimes motivated by financial or medico-legal considerations). Third, radiation enteropathy is a complex disorder that requires multidisciplinary expertise often not readily available in the cancer treatment environment. Fourth, many clinicians feel that any treatment of delayed radiation enteropathy is unlikely to be successful. Finally, there has been little attention devoted to radiation enteropathy research from the pharmaceutical and biotechnology industry.
The recent interest in finding so-called “medical countermeasures against radiation” (drugs for use in the radiological and nuclear emergency situation, where radiation injury to the bone marrow and intestine is the main determinant of survival) has spawned a resurgence in activities to find compounds to protect the intestine against radiation. Such drugs can potentially also benefit the cancer patient who undergoes radiation therapy, i.e., so called “dual benefit” drugs. The recommended steps in the development process of such drugs have been reviewed by Movsas and coworkers 92. First, for a candidate drug to be selected there needs to be evidence of general or organ-specific normal tissue protection and lack of tumor protection. Drug candidates that fulfill these criteria undergo testing to determine maximal tolerated dose, pharmacokinetics, pharmacodynamics, and toxicity. The next stage is to verify evidence of normal tissue protection in vitro and in vivo, absence of tumor protection in vivo in pertinent xenograft models, and further testing of biological mechanisms. Finally, before proceeding to clinical screening, comprehensive drug evaluation and formulation studies should be performed.
The Role of the Gastroenterologist
Few gastroenterologists fully appreciate how much can be achieved for the symptomatic patient after pelvic irradiation. Moreover, they also do not recognize the value of a preclinical model, which has so many parallels with IBD and where the initiating insult (radiation) can be so finely adjusted. Indeed, while fibrosis in the liver has deservedly received substantial attention, the mechanisms of intestinal fibrosis has barely been investigated, even though it is a critical pathophysiological process in a large numbers of patients following radiotherapy. Progressive fibrosis not only contributes to significant morbidity, but is also important in many other gastrointestinal diseases, such as, pouchitis, Crohn’s disease, ischemic colitis, and scleroderma. It is easy to study in a model where the onset of fibrosis can be predicted. Conversely, the radiation biology community has until now been largely deprived of the insights which gastroenterologists could bring from their knowledge of gastrointestinal pathophysiology in other disease processes. Cross-fertilization between the fields of gastroenterology and radiation biology may thus generate substantial methodological and mechanistic insight into gastrointestinal disease processes initiated by radiation and how these processes are influenced by human genetic profiles, immunological responses, and microbial make-up.
Conclusions
Despite technological advances in radiation therapy, radiation enteropathy remains an important obstacle to uncomplicated cancer cures.
Radiation enteropathy is a much more important clinical problem than previously recognized – the prevalence of radiation enteropathy exceeds that of IBD.
The pathogenesis of radiation enteropathy is multifactorial and far more complex than previously assumed – the traditional “target cell concept” is largely obsolete.
While there is some correlation, histopathological and endoscopic changes do not parallel subjective symptoms.
The complexity of radiation enteropathy requires re-thinking of some of the old “dogmas”, but opens up the field for development of exciting new therapeutic strategies.
As an “IBD model”, radiation enteropathy offers substantial advantages in terms of methods and clinical relevance.
A paradigm shift is needed to make it possible to adequately deal with the ever-increasing cohort of cancer survivors and their side effects (Box 3).
Box 3. Priorities for future research.
Obtain an improved understanding of physiological versus pathological responses of the intestine to radiation injury.
Perform clinical, epidemiological, and outcomes studies in well-defined cohorts of cancer survivors to define true prevalence of late effects of radiation.
Determine the medical, quality of life-related, social, and financial consequences of radiation-induced bowel injury.
Develop predictive assays to identify patients who are more prone than others to develop delayed normal tissue toxicity after radiation therapy.
Strengthen molecular epidemiology research aimed at identifying genetic or epigenetic characteristics that correlate with susceptibility to delayed radiation toxicity.
Testing of radiation response modifiers in clinical trials.
Engage the pharmaceutical and biotechnology industries in developing strategies to modulate intestinal radiation toxicity.
Table of contents summary.
Intestinal radiation toxicity (radiation enteropathy) remains an important obstacle to uncomplicated cancer cures after radiation therapy of pelvic and abdominal malignancies. Moreover, the prevalence of radiation enteropathy in the population exceeds that of inflammatory bowel disease. This review introduces the clinical problem of radiation enteropathy, discusses contemporary concepts in pathogenesis, current therapeutic options, as well as strategies for development of new radioprotective agents.
Key Points.
Radiation therapy planning and delivery methods have improved substantially, but the risk of intestinal radiation injury remains the single-most important dose-limiting factor in radiation therapy for abdominal and pelvic tumors.
Early (acute) radiation enteropathy generally occurs during the course of radiation therapy, while delayed (chronic) radiation enteropathy develops after a latency period of variable length.
Delayed radiation enteropathy is among the most common radiation therapy-related side effects – the prevalence of radiation enteropathy exceeds that of inflammatory bowel diseases.
Because the number of cancer survivors steadily increases, radiation enteropathy represents a significant challenge for future research.
Finding safe and effective pharmacological methods to reduce the incidence and severity of radiation enteropathy is an unmet need.
Review Criteria.
Comprehensive searches in Ovid and/or PubMed were performed by combining the following search statements with “AND”: Statement #1: exp abnormalities, radiation-induced/ or exp dose-response relationship, radiation/ or exp radiation/ or exp radiation dosage/ or exp radiation effects/ or exp radiation injuries/ or exp radiation injuries, experimental/ or exp radiation oncology/ or exp radiation pneumonitis/ or exp radiation tolerance/ or exp radiation, ionizing/ or radiation.mp.. Statement #2: exp fibrosis/ or exp inflammation/ or exp inflammation mediators/. Statement #3: exp intestines/ or intestine.mp. or exp intestinal diseases/ or intestinal diseases.mp.. Unpublished clinical and laboratory data from our own research program were also used.
Competing Interests
Support to MH-J for efforts related to the submitted work was provided by the US National Institutes of Health (grants R37 CA71382 and U19 AI67798), the US Biomedical Advanced Research and Development Authority (BARDA, contract HHSO100201100045C), and by the US Veterans Administration. All authors declare that there is no support from any other organization. There are no financial relationships with any organizations that might have an interest in the submitted work; and no other relationships or activities that could appear to have influenced the submitted work.
Biographies
Biographies
Martin Hauer-Jensen, MD, PhD
Dr. Hauer-Jensen is Professor of Pharmaceutical Sciences, Surgery, and Pathology at University of Arkansas for Medical Sciences (UAMS), Associate Dean for Research in the UAMS College of Pharmacy, Director of the UAMS Division of Radiation Health, and Staff Surgeon at Central Arkansas Veterans Healthcare System in Little Rock, Arkansas. Dr. Hauer-Jensen is internationally recognized as an authority on radiation effects in normal tissues, particularly on the gastrointestinal system. Dr. Hauer-Jensen has published more than 200 scientific articles and book chapters, and he serves on numerous national and international advisory boards, review panels, and editorial boards.
James W. Denham, MD
Dr. Denham is Professor of Radiation Oncology at University of Newcastle, New South Wales, Australia. He has long standing interest in clinical aspects regarding short- and long term side effects of radiation therapy. He was co-founder of the multi-center Australia and New Zealand cancer trials group, TROG, in 1987. Later he led the 96.01 and RADAR prostate cancer trials which are Australia and New Zealand’s largest cancer trials, and has furthered his interests in normal tissue radiation injury. Dr. Denham has published more than 160 articles in international journals and had invitations to speak at numerous international conferences.
H. Jervoise N. Andreyev, MD, PhD
Dr. Andreyev is Consultant Gastroenterologist in Pelvic Radiation Disease at the Royal Marsden Hospital, London UK. He originally graduated with a degree in Arabic Studies from Cambridge University, UK and then switched to Medicine. He obtained his training at the Royal London Hospital. His PhD examined the function of K-Ras genes in colorectal cancer and targeting K-Ras mutations with antisense approaches. He was Senior Lecturer at Imperial College, London 2000-2005. In 2006, Dr. Andreyev was appointed to his present position. His research focuses on etiology and clinical management of gastrointestinal consequences of cancer treatments.
References
- 1.Goitein M. How best to dispose of extra-tumoral dose: a cautionary note for intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2009;75:1–3. doi: 10.1016/j.ijrobp.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 2.Eifel PJ, Jhingran A, Bodurka DC, Levenback C, Thames H. Correlation of smoking history and other patient characteristics with major complications of pelvic radiation therapy for cervical cancer. J Clin Oncol. 2002;20:3651–3657. doi: 10.1200/JCO.2002.10.128. [DOI] [PubMed] [Google Scholar]
- 3.Wedlake LJ, et al. Predicting late effects of pelvic radiotherapy: is there a better approach? Int J Radiat Oncol Biol Phys. 2010;78:1163–1170. doi: 10.1016/j.ijrobp.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Willett CG, et al. Acute and late toxicity of patients with inflammatory bowel disease undergoing irradiation for abdominal and pelvic neoplasms. Int J Radiat Oncol Biol Phys. 2000;46:995–998. doi: 10.1016/s0360-3016(99)00374-0. [DOI] [PubMed] [Google Scholar]
- 5.Herold DM, Hanlon AL, Hanks GE. Diabetes mellitus: a predictor for late radiation morbidity. Int J Radiat Oncol Biol Phys. 1999;43:475–479. doi: 10.1016/s0360-3016(98)00460-x. [DOI] [PubMed] [Google Scholar]
- 6.Potish RA, Twiggs LB, Adcock LL, Prem KA. Logistic models for prediction of enteric morbidity in the treatment of ovarian and cervical cancers. Am J Obstet Gynecol. 1983;147:65–72. doi: 10.1016/0002-9378(83)90086-8. [DOI] [PubMed] [Google Scholar]
- 7.Ross JG, Hussey DH, Mayr NA, Davis CS. Acute and late reactions to radiation therapy in patients with collagen vascular diseases. Cancer. 1993;71:3744–3752. doi: 10.1002/1097-0142(19930601)71:11<3744::aid-cncr2820711144>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Lin A, Abu-Isa E, Griffith KA, Ben-Josef E. Toxicity of radiotherapy in patients with collagen vascular disease. Cancer. 2008;113:648–653. doi: 10.1002/cncr.23591. [DOI] [PubMed] [Google Scholar]
- 9.West CM, Barnett GC. Genetics and genomics of radiotherapy toxicity: towards prediction. Genome Med. 2011;3:52. doi: 10.1186/gm268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegel R, et al. Cancer treatment and survivorship statistics, 2012. CA: A Cancer Journal for Clinicians. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 11.Bentzen SM, et al. Normal tissue effects: reporting and analysis. Semin Radiat Oncol. 2003;13:189–202. doi: 10.1016/S1053-4296(03)00036-5. [DOI] [PubMed] [Google Scholar]
- 12.Fransson P, Widmark A. 15-year prospective follow-up of patient-reported outcomes of late bowel toxicity after external beam radiotherapy for localized prostate cancer. A comparison with age-matched controls. Acta Oncol. 2007;46:517–524. doi: 10.1080/02841860601113596. [DOI] [PubMed] [Google Scholar]
- 13.Yeoh E, et al. Effect of pelvic irradiation on gastrointestinal function: a prospective longitudinal study. Am J Med. 1993;95:397–406. doi: 10.1016/0002-9343(93)90309-d. [DOI] [PubMed] [Google Scholar]
- 14.Lakatos P-L. Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World J Gastroenterol. 2006;12:6102–6108. doi: 10.3748/wjg.v12.i38.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CDC - Home Page - Inflammatory Bowel Disease. 2013 Sep 12; http://www.cdc.gov/ibd/
- 16.Hauer-Jensen M, Poulakos L, Osborne JW. Effects of accelerated fractionation on radiation injury of the small intestine: a new rat model. Int J Radiat Oncol Biol Phys. 1988;14:1205–1212. doi: 10.1016/0360-3016(88)90399-9. [DOI] [PubMed] [Google Scholar]
- 17.Husebye E, Hauer-Jensen M, Kjorstad K, Skar V. Severe late radiation enteropathy is characterized by impaired motility of proximal small intestine. Dig Dis Sci. 1994;39:2341–2349. doi: 10.1007/BF02087648. [DOI] [PubMed] [Google Scholar]
- 18.Vale CL, Tierney JF, Davidson SE, Drinkwater KJ, Symonds P. Substantial improvement in UK cervical cancer survival with chemoradiotherapy: results of a Royal College of Radiologists' audit. Clin Oncol (R Coll Radiol) 2010;22:590–601. doi: 10.1016/j.clon.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regimbeau JM, Panis Y, Gouzi JL, Fagniez PL. Operative and long term results after surgery for chronic radiation enteritis. Am J Surg. 2001;182:237–242. doi: 10.1016/s0002-9610(01)00705-x. [DOI] [PubMed] [Google Scholar]
- 20.Larsen A, Reitan JB, Aase ST, Hauer-Jensen M. Long-term prognosis in patients with severe late radiation enteropathy: a prospective cohort study. World J Gastroenterol. 2007;13:3610–3613. doi: 10.3748/wjg.v13.i26.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fajardo LF, Berthrong M, Anderson RE. Radiation pathology. Oxford University Press; Oxford: 2001. [Google Scholar]
- 22.Carr KE. Effects of radiation damage on intestinal morphology. Int Rev Cytol. 2001;208:1–119. doi: 10.1016/s0074-7696(01)08002-0. [DOI] [PubMed] [Google Scholar]
- 23.Shrieve DC, Loeffler JS. Human radiation injury. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2011. [Google Scholar]
- 24.Bourne RG, Kearsley JH, Grove WD, Roberts SJ. The relationship between early and late gastrointestinal complications of radiation therapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 1983;9:1445–1450. doi: 10.1016/0360-3016(83)90316-4. [DOI] [PubMed] [Google Scholar]
- 25.Peters LJ, Ang KK, Thames HD., Jr Accelerated fractionation in the radiation treatment of head and neck cancer. A critical comparison of different strategies. Acta Oncol. 1988;27:185–194. doi: 10.3109/02841868809090339. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Qiu X, Kulkarni A, Hauer-Jensen M. Calcitonin gene-related peptide and substance P regulate the intestinal radiation response. Clin Cancer Res. 2006;12:4112–4118. doi: 10.1158/1078-0432.CCR-06-0592. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Zheng H, Hauer-Jensen M. Influence of Short-Term Octreotide Administration on Chronic Tissue Injury, Transforming Growth Factor beta (TGF-beta) Overexpression, and Collagen Accumulation in Irradiated Rat Intestine. J Pharmacol Exp Ther. 2001;297:35–42. [PubMed] [Google Scholar]
- 28.Wang J, Zheng H, Kulkarni A, Ou X, Hauer-Jensen M. Regulation of early and delayed radiation responses in rat small intestine by capsaicin-sensitive nerves. Int J Radiat Oncol Biol Phys. 2006;64:1528–1536. doi: 10.1016/j.ijrobp.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 29.Zheng H, Wang J, Hauer-Jensen M. Role of mast cells in early and delayed radiation injury in rat intestine. Radiat Res. 2000;153:533–539. doi: 10.1667/0033-7587(2000)153[0533:romcie]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 30.Denham JW, Hauer-Jensen M, Peters LJ. Is it time for a new formalism to categorize normal tissue radiation injury? Int J Radiat Oncol Biol Phys. 2001;50:1105–1106. doi: 10.1016/s0360-3016(01)01556-5. [DOI] [PubMed] [Google Scholar]
- 31.Denham JW, Hauer-Jensen M. The radiotherapeutic injury--a complex 'wound'. Radiother Oncol. 2002;63:129–145. doi: 10.1016/s0167-8140(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 32.Carratu R, et al. Assessment of small intestinal damage in patients treated with pelvic radiotherapy. Oncol Rep. 1998;5:635–639. doi: 10.3892/or.5.3.635. [DOI] [PubMed] [Google Scholar]
- 33.Hovdenak N, Fajardo LF, Hauer-Jensen M. Acute radiation proctitis: a sequential clinicopathologic study during pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48:1111–1117. doi: 10.1016/s0360-3016(00)00744-6. [DOI] [PubMed] [Google Scholar]
- 34.Potten CS, Merritt A, Hickman J, Hall P, Faranda A. Characterization of radiation-induced apoptosis in the small intestine and its biological implications. Int J Radiat Biol. 1994;65:71–78. doi: 10.1080/09553009414550101. [DOI] [PubMed] [Google Scholar]
- 35.Komarova EA, et al. Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastro-intestinal syndrome in mice. Oncogene. 2004;23:3265–3271. doi: 10.1038/sj.onc.1207494. [DOI] [PubMed] [Google Scholar]
- 36.Kirsch DG, et al. p53 Controls Radiation-Induced Gastrointestinal Syndrome in Mice Independent of Apoptosis. Science. 2010;327:593–596. doi: 10.1126/science.1166202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leibowitz BJ, et al. Uncoupling p53 Functions in Radiation-Induced Intestinal Damage via PUMA and p21. Molecular Cancer Research. 2011;9:616–625. doi: 10.1158/1541-7786.MCR-11-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 39.Potten CS. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977;269:518–521. doi: 10.1038/269518a0. [DOI] [PubMed] [Google Scholar]
- 40.Yan KS, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A. 2012;109:466–471. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hua G, et al. Crypt Base Columnar Stem Cells in Small Intestines of Mice Are Radioresistant. Gastroenterology. 2012;143:1266–1276. doi: 10.1053/j.gastro.2012.07.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Landeghem L, et al. Activation of two distinct Sox9-EGFP-expressing intestinal stem cell populations during crypt regeneration after irradiation. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1111–32. doi: 10.1152/ajpgi.00519.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metcalfe C, Kljavin NM, Ybarra R, de Sauvage Frederic J. Lgr5+ Stem Cells Are Indispensable for Radiation-Induced Intestinal Regeneration. Cell Stem Cell. 2014;14:149–159. doi: 10.1016/j.stem.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Hopewell JW, et al. Microvasculature and radiation damage. Recent Results Cancer Res. 1993;130:1–16. doi: 10.1007/978-3-642-84892-6_1. [DOI] [PubMed] [Google Scholar]
- 45.Baker DG, Krochak RJ. The response of the microvascular system to radiation: a review. Cancer Invest. 1989;7:287–294. doi: 10.3109/07357908909039849. [DOI] [PubMed] [Google Scholar]
- 46.Rezvani M, Hopewell JW, Robbins ME. Initiation of non-neoplastic late effects: the role of endothelium and connective tissue. Stem Cells. 1995;131(Suppl):248–256. doi: 10.1002/stem.5530130730. [DOI] [PubMed] [Google Scholar]
- 47.Wang J, Zheng H, Ou X, Fink LM, Hauer-Jensen M. Deficiency of microvascular thrombomodulin and up-regulation of protease-activated receptor-1 in irradiated rat intestine: possible link between endothelial dysfunction and chronic radiation fibrosis. Am J Pathol. 2002;160:2063–2072. doi: 10.1016/S0002-9440(10)61156-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richter KK, Fink LM, Hughes BM, Sung CC, Hauer-Jensen M. Is the loss of endothelial thrombomodulin involved in the mechanism of chronicity in late radiation enteropathy? Radiother Oncol. 1997;44:65–71. doi: 10.1016/s0167-8140(97)00063-7. [DOI] [PubMed] [Google Scholar]
- 49.Paris F, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 50.Rotolo J, et al. Anti-ceramide antibody prevents the radiation gastrointestinal syndrome in mice. J. Clin. Invest. 2012;122:1786–1790. doi: 10.1172/JCI59920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuller BW, et al. Selective irradiation of the vascular endothelium has no effect on the survival of murine intestinal crypt stem cells. Proc Natl Acad Sci U S A. 2006;103:3787–3792. doi: 10.1073/pnas.0600133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schuller BW, et al. No significant endothelial apoptosis in the radiation-induced gastrointestinal syndrome. Int J Radiat Oncol Biol Phys. 2007;68:205–210. doi: 10.1016/j.ijrobp.2006.12.069. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Boerma M, Fu Q, Hauer-Jensen M. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J Gastroenterol. 2007;13:3047–3055. doi: 10.3748/wjg.v13.i22.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geiger H, et al. Pharmacological targeting of the thrombomodulin-activated protein C pathway mitigates radiation toxicity. Nat Med. 2012;18:1123–1129. doi: 10.1038/nm.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, Hauer-Jensen M. Neuroimmune interactions: potential target for mitigating or treating intestinal radiation injury. Br J Radiol. 2007;80:S41–8. doi: 10.1259/bjr/33057885. Spec No 1. [DOI] [PubMed] [Google Scholar]
- 56.Shanahan F. The host-microbe interface within the gut. Best Pract Res Clin Gastroenterol. 2002;16:915–931. doi: 10.1053/bega.2002.0342. [DOI] [PubMed] [Google Scholar]
- 57.Robert A, Asano T. Resistance of germfree rats to indomethacin-induced intestinal lesions. Prostaglandins. 1977;14:333–341. doi: 10.1016/0090-6980(77)90178-2. [DOI] [PubMed] [Google Scholar]
- 58.Osborne JW, BRYAN HS, QUASTLER H, RHOADES HE. X-irradiation and bacteremia; studies on roentgen death in mice. IV. Am J Physiol. 1952;170:414–417. doi: 10.1152/ajplegacy.1952.170.2.414. [DOI] [PubMed] [Google Scholar]
- 59.WILSON BR. SURVIVAL STUDIES OF WHOLE-BODY X-IRRADIATED GERMFREE (AXENIC) MICE. Radiat Res. 1963;20:477–483. [PubMed] [Google Scholar]
- 60.Crawford PA, Gordon JI. Microbial regulation of intestinal radiosensitivity. Proc Natl Acad Sci U S A. 2005;102:13254–13259. doi: 10.1073/pnas.0504830102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delia P, et al. Prevention of radiation-induced diarrhea with the use of VSL#3, a new high-potency probiotic preparation. Am J Gastroenterol. 2002;97:2150–2152. doi: 10.1111/j.1572-0241.2002.05946.x. [DOI] [PubMed] [Google Scholar]
- 62.Ciorba MA, et al. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut. 2012;61:829–838. doi: 10.1136/gutjnl-2011-300367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Urbancsek H, Kazar T, Mezes I, Neumann K. Results of a double-blind, randomized study to evaluate the efficacy and safety of Antibiophilus in patients with radiation-induced diarrhoea. Eur J Gastroenterol Hepatol. 2001;13:391–396. doi: 10.1097/00042737-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 64.Osterlund P, et al. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study. Br J Cancer. 2007;97:1028–1034. doi: 10.1038/sj.bjc.6603990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gibson RJ, et al. Systematic review of agents for the management of gastrointestinal mucositis in cancer patients. Support Care Cancer. 2013;21:313–326. doi: 10.1007/s00520-012-1644-z. [DOI] [PubMed] [Google Scholar]
- 66.Andreyev HJN, Davidson SE, Gillespie C, Allum WH, Swarbrick E. Practice guidance on the management of acute and chronic gastrointestinal problems arising as a result of treatment for cancer. Gut. 2011;61:179–192. doi: 10.1136/gutjnl-2011-300563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kochhar R, et al. Radiation-induced proctosigmoiditis. Prospective, randomized, double-blind controlled trial of oral sulfasalazine plus rectal steroids versus rectal sucralfate. Dig Dis Sci. 1991;36:103–107. doi: 10.1007/BF01300096. [DOI] [PubMed] [Google Scholar]
- 68.Sanguineti G, Franzone P, Marcenaro M, Foppiano F, Vitale V. Sucralfate versus mesalazine versus hydrocortisone in the prevention of acute radiation proctitis during conformal radiotherapy for prostate carcinoma. A randomized study. Strahlenther Onkol. 2003;179:464–470. doi: 10.1007/s00066-003-1082-4. [DOI] [PubMed] [Google Scholar]
- 69.Clarke RE, et al. Hyperbaric oxygen treatment of chronic refractory radiation proctitis: a randomized and controlled double-blind crossover trial with long-term follow-up. Int J Radiat Oncol Biol Phys. 2008;72:134–143. doi: 10.1016/j.ijrobp.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 70.Gibson RJ, et al. Systematic review of agents for the management of gastrointestinal mucositis in cancer patients. Support Care Cancer. 2013;21:313–326. doi: 10.1007/s00520-012-1644-z. [DOI] [PubMed] [Google Scholar]
- 71.Ben-Josef E, et al. Intrarectal application of amifostine for the prevention of radiation-induced rectal injury. Semin Radiat Oncol. 2002;12:81–85. doi: 10.1053/srao.2002.31379. [DOI] [PubMed] [Google Scholar]
- 72.Salvemini D, Riley DP, Cuzzocrea S. SOD mimetics are coming of age. Nat Rev Drug Discov. 2002;1:367–374. doi: 10.1038/nrd796. [DOI] [PubMed] [Google Scholar]
- 73.Berbee M, et al. gamma-Tocotrienol ameliorates intestinal radiation injury and reduces vascular oxidative stress after total-body irradiation by an HMG-CoA reductase-dependent mechanism. Radiat Res. 2009;171:596–605. doi: 10.1667/RR1632.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boerma M, et al. Local administration of interleukin-11 ameliorates intestinal radiation injury in rats. Cancer Res. 2007;67:9501–9506. doi: 10.1158/0008-5472.CAN-07-0810. [DOI] [PubMed] [Google Scholar]
- 75.Boerma M, Wang J, Richter KK, Hauer-Jensen M. Orazipone, a locally acting immunomodulator, ameliorates intestinal radiation injury: a preclinical study in a novel rat model. Int J Radiat Oncol Biol Phys. 2006;66:552–559. doi: 10.1016/j.ijrobp.2006.05.067. [DOI] [PubMed] [Google Scholar]
- 76.Booth C, Booth D, Williamson S, Demchyshyn LL, Potten CS. Teduglutide (Gly2GLP-2) protects small intestinal stem cells from radiation damage. Cell Prolif. 2004;37:385–400. doi: 10.1111/j.1365-2184.2004.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cai Y, et al. Keratinocyte growth factor pretreatment prevents radiation-induced intestinal damage in a mouse model. Scand J Gastroenterol. 2013;48:419–426. doi: 10.3109/00365521.2013.772227. [DOI] [PubMed] [Google Scholar]
- 78.Bhanja P, et al. Protective role of R-spondin1, an intestinal stem cell growth factor, against radiation-induced gastrointestinal syndrome in mice. PLoS One. 2009;4:e8014. doi: 10.1371/journal.pone.0008014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Lau, Wim BM, Snel B, Clevers HC. The R-spondin protein family. Genome Biol. 2012;13:242. doi: 10.1186/gb-2012-13-3-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khan WB, Shui C, Ning S, Knox SJ. Enhancement of murine intestinal stem cell survival after irradiation by keratinocyte growth factor. Radiat Res. 1997;148:248–253. [PubMed] [Google Scholar]
- 81.Matthews MA, Watkins D, Darbyshire A, Carson WE, Besner GE. Heparin-binding EGF-like growth factor (HB-EGF) protects the intestines from radiation therapy-induced intestinal injury. Journal of Pediatric Surgery. 2013;48:1316–1322. doi: 10.1016/j.jpedsurg.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Torres S, et al. Glucagon-like peptide-2 improves both acute and late experimental radiation enteritis in the rat. Int J Radiat Oncol Biol Phys. 2007;69:1563–1571. doi: 10.1016/j.ijrobp.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 83.Zhou W-J, Geng ZH, Spence JR, Geng J-G. Induction of intestinal stem cells by R-spondin 1 and Slit2 augments chemoradioprotection. Nature. 2013;501:107–111. doi: 10.1038/nature12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fu Q, et al. The somatostatin analog SOM230 (pasireotide) ameliorates injury of the intestinal mucosa and increases survival after total-body irradiation by inhibiting exocrine pancreatic secretion. Radiat Res. 2009;171:698–707. doi: 10.1667/RR1685.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fu Q, et al. Preclinical evaluation of Som230 as a radiation mitigator in a mouse model: postexposure time window and mechanisms of action. Radiat Res. 2011;175:728–735. doi: 10.1667/rr2507.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Burdelya LG, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deng W, et al. Lysophosphatidic acid protects and rescues intestinal epithelial cells from radiation- and chemotherapy-induced apoptosis. Gastroenterology. 2002;123:206–216. doi: 10.1053/gast.2002.34209. [DOI] [PubMed] [Google Scholar]
- 88.Deng W, et al. The lysophosphatidic acid type 2 receptor is required for protection against radiation-induced intestinal injury. Gastroenterology. 2007;132:1834–1851. doi: 10.1053/j.gastro.2007.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saha S, et al. TLR9 agonist protects mice from radiation-induced gastrointestinal syndrome. PLoS One. 2012;7:e29357. doi: 10.1371/journal.pone.0029357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shakhov AN, et al. Prevention and mitigation of acute radiation syndrome in mice by synthetic lipopeptide agonists of Toll-like receptor 2 (TLR2) PLoS One. 2012;7:e33044. doi: 10.1371/journal.pone.0033044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singh VK, et al. CBLB613: a TLR 2/6 agonist, natural lipopeptide of Mycoplasma arginini , as a novel radiation countermeasure. Radiat Res. 2012;177:628–642. doi: 10.1667/rr2657.1. [DOI] [PubMed] [Google Scholar]
- 92.Movsas B, et al. Decreasing the adverse effects of cancer therapy: National Cancer Institute guidance for the clinical development of radiation injury mitigators. Clin Cancer Res. 2011;17:222–228. doi: 10.1158/1078-0432.CCR-10-1402. [DOI] [PubMed] [Google Scholar]
- 93.Hewitt ME, Greenfield S, Stovall E. Lost in transition. National Academies Press; Washington, D.C: 2006. From cancer patient to cancer survivor. [Google Scholar]