Abstract
In models of diabetic retinopathy, insulin-like growth factor binding protein-3 (IGFBP-3) protects against tumor necrosis factors-alpha (TNF-α)-mediated apoptosis of retinal microvascular endothelial cells (REC), but the underlying mechanisms are unclear. Our current findings suggest that at least two discrete but complimentary pathways contribute to the protective effects of IGFBP-3; 1) IGFBP-3 directly activates the c-Jun kinase/tissue inhibitor of metalloproteinase-3/TNF-α converting enzyme (c-Jun/TIMP-3/TACE), pathway, which in turn inhibits TNF-α production; 2) IGFBP-3 acts through the IGFBP-3 receptor, low-density lipoprotein receptor-related protein 1 (LRP1), to inhibit signaling of TNF-α receptor 2 (TNFR2). Combined, these two IGFBP-3 pathways substantially reduce REC apoptosis and offer potential targets for the treatment of diabetic retinopathy.
Keywords: Diabetic retinopathy, IGFBP-3, Apoptosis, TNFalpha, c-Jun
Introduction
Diabetic retinopathy is the leading cause of blindness in the world (Cheung et al., 2010; Leon et al., 2013). Retinal microvascular endothelial cell (REC) apoptosis is a key step in the initiation of diabetic-related pathologies (Jiang et al., 2012; Steinle, 2012). Progressive microvascular alterations, including leukocyte adhesion, endothelial cell death and formation of degenerated capillaries, are hallmarks of the disease (Ishida et al., 2003; Joussen et al., 2001). Various mediators, such as tumor necrosis factor alpha (TNF-α), the insulin-like growth factor 1(IGF-1)/insulin like growth factor binding protein (IGFBP) system and vascular endothelial growth factor (VEGF) are known to regulate cell apoptotic pathways in various cell types including REC (Ben-Mahmud et al., 2004; Kielczewski et al., 2011; Titchenell and Antonetti, 2013); however, the specific pathways involved are unclear. We have previously reported that IGFBP-3 inhibits TNF-α production, leading to an inhibition of REC apoptosis (Zhang et al., 2013a,b). Building on this important finding, our goal in the current study is to uncover the mechanistic pathways that underlie IGFBP-3 regulation of TNF-α and REC apoptosis, and specifically to determine if binding of IGFBP-3 to its receptor, LRP1, is involved in its protective effects.
IGFBP-3 is one of seven proteins that constitute the IGFBP family. In addition to being the most abundant circulating IGF-1 binding protein, IGFBP-3 binds to IGF-1 with high affinity and specificity (Baxter, 2000; Mohan and Baylink, 2002), and appears to have multiple functions (Ning et al., 2006). In many cases, the high affinity binding of IGFBP-3 to free IGF-1 acts to control IGF-1 distribution in interstitial fluids (Clemmons, 2001; Lovett-Racke et al., 1998). For example, following tissue injury, IGFBP-3 helps concentrate IGF-1 at wound sites and reduces its rate of clearance. IGFBP-3 binds directly to fibrinogen/fibrin clots and in turn IGF-1 binds the immobilized IGFBP-3/fibrinogen and IGFBP-3/fibrin complexes. The lower IGF-1 affinity for fibrin-bound IGFBP-3 allows IGF-1 release to higher affinity type I IGF receptors of stromal cells migrating into the fibrin clot. Of particular relevance to our studies, IGFBP-3 also has separate, IGF-1 independent actions that have been shown to support cell survival (Jarajapu et al., 2012; Lofqvist et al., 2007), and as such IGFBP-3 had been shown to reduce angiogenesis and mediate protective effects on blood retinal barrier integrity in diabetic retinopathy, independent of its actions on IGF-1. By using a mutant form of IGFBP-3 that cannot bind IGF-1, it is possible to observe IGF-1 independent actions of IGFBP-3. Under these conditions (in our case, transfection of REC with mutant non-binding, IGFBP-3 NB plasmid), we found that overexpression of IGFBP-3 NB led to a reduction in TNF-α levels and a subsequent reduction in REC apoptosis (Zhang et al., 2013a,b). The studies described here also use IGFBP-3 NB plasmid and thus, all the findings contained in this report are limited to actions of IGFBP-3 that are independent of IGF-1 binding.
Since c-Jun N-terminal kinases act as mediators of angiogenesis in other systems, we hypothesize that c-Jun might play a role in vascular changes associated with diabetic retinopathy and be one of the targets of IGFBP-3. It has been established in other tissues that c-Jun can control TNF-α activation by direct transcriptional activation of tissue inhibitor of metalloproteinase-3 (TIMP-3), an inhibitor of the TNF-α-converting enzyme (TACE) (Guinea-Viniegra et al., 2009). Thus, we wished to determine if c-Jun acts as an essential physiological regulator of TNF-α by controlling the TIMP3/TACE pathway in retinal vascular cells and whether IGFBP-3 regulates c-Jun.
TNF-α was first identified as an inducer of cell death (Takahashi et al., 1998; Tracey and Cerami, 1993). It has also been shown to be an inflammatory biomarker associated with the development of vascular leakage and apoptosis in diabetic retinopathy (Zhang et al., 2011). TNF-α interacts with two membrane-bound receptors, tumor necrosis factor receptor 1 (TNFR1; CD120a; p55) and tumor necrosis factor receptor 2 (TNFR2; CD120b; p75) (Riches et al., 1998). While TNFR1 is expressed ubiquitously, basal TNFR2 expression has only been detected in vascular endothelial cells, cardiac myocytes and some neuronal cells (Carpentier et al., 2004; Vandenabeele et al., 1995). TNFR2 expression can also be induced by ischemia in vascular endothelium (Luo et al., 2006). On binding of TNF-α, TNFR recruits the adaptor protein, TNFR associated death domain (TRADD), directly signaling to the Caspase 8 cytoplasmic death complex (Cabal-Hierro and Lazo, 2012).
Changes in retinal microvascular structure, due in part to REC cell death, represent hallmark features of early diabetic retinopathy (Cheung et al., 2010). Because of the potential role of REC in the initiation of this disorder, we have utilized cultured primary human REC grown in normal versus high glucose conditions as a model in which to examine functional relationships between TNF-α and IGFBP-3. Results confirm that cultured REC respond to high glucose, IGFBP-3, and TNF-α in a manner similar to that observed in whole retina. Furthermore, culture conditions permit highly controlled genetic and chemical manipulation of key elements so that mechanistic pathways within REC can be clearly identified. Our findings suggest that functional pathways linking IGFBP-3 to c-Jun N-terminal kinase and to the TNF-α receptor, TNFR2, are responsible for the observed decrease in REC apoptosis.
Methods
Reagents
Caspase 8, TRADD and TIMP3 antibodies were purchased from Cell Signaling (Danvers, MA). LRP1 and actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Human IGFBP-3 immunoassay ELISA kit was purchased from R & D (Minneapolis, MN). InnoZyme™ TACE activity kit was bought from Millipore (San Diego, CA). Human LRP1 siRNA and Non-Targeting siRNA #1 were purchased from Dharmacon RNAi Technologies (Chicago, IL). RNAimax was purchased from Invitrogen (Carlsbad, CA). SuperFect transfection reagent was bought from Qiagen (Valencia, CA). Horseradish peroxidase (HRP) conjugated secondary anti-mouse and anti-rabbit antibodies were purchased from Promega (Madison, WI). Enhanced chemiluminescence for immunoblot development and signal detection was purchased from Amersham Biosciences (Piscataway, NJ). C-Jun peptide was purchased from Tocris Bioscience (Bristol, UK). IGFBP-3 NB plasmid DNA was a gift from Dr. Maria B. Grant (University of Florida).
Cell culture
Primary human REC were acquired from Cell System Corporation (CSC, Kirkland, Washington). Cells were grown in M131 medium containing microvascular growth supplements (Invitrogen) (MVGS), 10 μg/μl gentamycin and 0.25 μg/μl amphotericin B. In the high glucose condition, cells were transferred to high glucose (25 μM) (Cell Systems) medium, supplemented with MVGS and antibiotics for 3 days. Only primary cells within passages 6 were used. Cells were quiesced by incubating in high or normal glucose medium without MVGS for 24 h and used to perform the experiments. For experiments to test inhibition of c-Jun, we treated REC with 400 μM c-Jun peptide to block c-Jun activity as previously reported (Holzberg et al., 2003), followed by transfection with IGFBP-3 NB.
Transfection of siRNA and plasmid
DNA-ON-TARGETplus SMARTpool, human LRP1 siRNA (LRP1 siRNA) were purchased from Dharmacon, Inc. We used 4 sets of siRNA, with target sequences of GCGAAGGCAUUGUGUGUUC, GCACCAUUCUCAAGAGUAU, GCGCAUCGAUCUUCACAAA and GAACAAACACACUGGCUAA. siCONTROL Non-targeting siRNA #1 (Dharmacon) was used as a non-specific control. REC were transfected with siRNA at a final concentration of 20 nM using RNAiMAX transfection reagent according to the manufacturer’s instructions. The cells were used for experiments 24 h after transfection. For the cells in high glucose condition, cells were transfected on Day 2 in high glucose medium and were also kept in high glucose medium during transfection. The cells were also transfected with IGFBP-3 NB plasmid DNA at 1 μg/ml using SuperFect transfect reagent, according to the manufacturer’s instructions. The cells were used for experiment 24 h after transfection.
ELISA analysis
An ELISA for IGFBP-3 and TNF-α levels was performed using the ELISA assay kits according to the manufacturer’s instructions to evaluate the IGFBP-3 and TNF-α levels after the treatment. Equal protein was loaded into all wells for both assays.
TACE analysis
TACE activity was measured using a solution-based assay containing a fluorescently labeled TACE substrate. Equal proteins were added to the TACE-coated 96-well plate. The activity of the captured TACE was measured using an internally quenched fluorescent substrate, MAC-KPLGL-Dpa-AR-NH2. Fluorescence of the cleaved product, MAC-KPLG, was measured at an excitation wavelength of 320 nm and emission wavelength of 405 nm. The specific enzymatic activity of TACE was calculated as relative fluorescence units (RFU).
Western blot analysis
After appropriate treatments and rinsing with cold phosphate-buffered saline, REC were scraped into lysis buffer containing protease and phosphatase inhibitors. Equal amounts of protein from the cell extracts were separated on the pre-cast tris-glycine gel and blotted onto a nitrocellulose membrane. After blocking in TBST (10 mM Tris-HCl buffer, pH 8.0, 150 mM NaCl, 0.1% Tween 20) containing 5% (w/v) BSA, the membrane was treated with anti-phospho-c-Jun, c-Jun, TIMP3, TRADD and caspase 8 antibodies followed by incubation with HRP conjugated secondary antibodies. The antigen–antibody complexes were detected using chemiluminescence reagent kit (Thermo Scientific).
Statistics
All the experiments were repeated at least three times, and the data are presented as mean ± SEM. Data was analyzed by Kruskal–Wallis test, followed by Dunn’s testing with p values < 0.05 were considered statistically significant. In the case of Western blotting, one representative blot is shown. Normal glucose was normalized to 1, with all treatment compared to normal glucose followed by normalization to actin levels.
Results
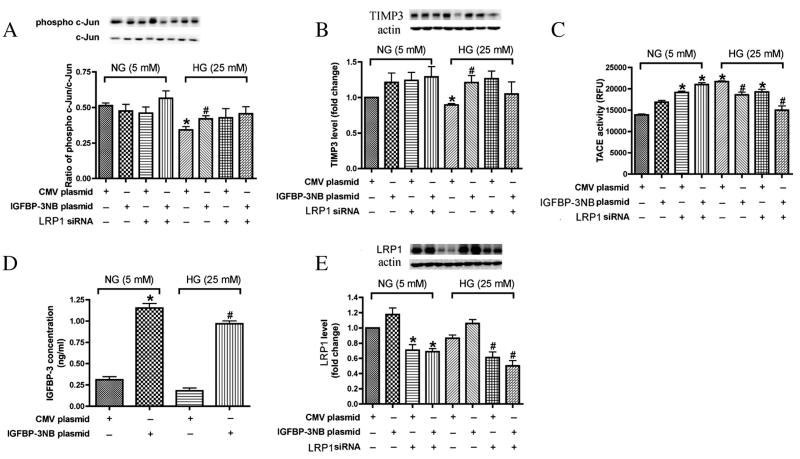
When grown in the presence of 25 mM glucose (diabetic-like conditions) and transfected with IGFBP-3NB plasmid, REC expressed increased levels of IGFBP-3, concomitant with increased levels of phosphorylated c-Jun and TIMP3, as well as decreased levels of TACE. Given our previous observation that IGFBP-3 decreases TNF-α levels and thus protects against REC apoptosis, we wished to determine if the c-Jun pathway mediates this protective effect. For our experiments, we transfected REC with IGFBP-3 NB plasmid DNA at 1.0 μg/ml for 24 h in either normal or high glucose (Fig. 1). We confirmed that the transfection resulted in a large increase (approximately 4-fold) in IGFBP-3 levels in both normal and high glucose samples (Fig. 1A). We then compared changes in the c-Jun/TIMP3/TACE pathways in cells receiving control plasmid versus cells transfected with IGFBP-3 plasmid. Since the phosphorylated form of c-Jun is regarded as the activated form, we used Western blots to monitor changes in the ratio of phospho c-Jun/c-Jun, as shown in Fig. 1B. We found that in normal glucose, the ratio was approximately 50% and it was unaltered by IGFBP-3 transfection (left panel, Fig. 1B). Compared to normal glucose, high glucose conditions caused a significant decrease (approximately 40%) in the phospho c-Jun ratio, while IGFBP-3 transfection returned the ratio to near control levels (right panel, Fig. 1B).
Fig. 1.
IGFBP-3 overexpression inhibited pro-inflammation markers in REC in high ambient glucose. In all experiments, REC cells were treated with IGFBP-3 plasmid and LRP1 siRNA in medium containing normal glucose (NG-5 mM) or high glucose (HG-25 mM) medium. A. Levels of IGFBP-3; B. Western Blot result of ratio of phosphor-cJun/cJun; C. Western blot result of TIMP-3 expression; D. TACE activity; E. Expression of LRP1. *P < 0.05 vs. NG control plasmid DNA transfection. #P < 0.05 vs. HG control plasmid DNA transfection. N = 3.
To determine if IGFBP-3 stimulation of c-Jun expression leads to expected downstream effects, we also monitored changes in TIMP3 and TACE. Since c-Jun is known to stimulate TIMP3, which leads to inhibition of TACE in other cells, we predicted similar changes in REC cells after IGFBP-3 stimulation of c-Jun. As shown in Fig. 1C, TIMP3 levels were significantly lower in high glucose samples compared to normal glucose. IGFBP-3 transfection restored TIMP3 levels to normal. As shown in Fig. 1D, TACE activity was increased in response to high glucose and was significantly decreased after IGFBP-3 transfection. These results support our hypothesis that high glucose inhibits the c-Jun pathway and that IGFBP-3 can restore activity in the pathway to near normal levels. When comparing the IGFBP-3 plasmid group and the IGFBP-3 plasmid + LRP1 siRNA treatment group, whether in NG or HG, there is no significant change suggesting that the LRP1 did not play a role in IGFBP-3 actions on c-Jun/TIMP3/TACE.
In order to determine whether the IGFBP-3 receptor, LR1, was required for IGFBP-3 actions on the c-Jun pathway, we treated control and IGFBP-3 transfected cells with LRP1 siRNA. Compared with control cells transfected with a nonspecific siRNA, LRP1-silenced REC expressed significantly reduced levels of LRP1 (Fig. 1E) in both normal and high glucose samples, including those transfected with IGFBP-3. This reduction in LRP1 levels had little significant effect on IGFBP-3 stimulation of phosphorylated-c-Jun and TIMP3 or on the suppression of TACE activity (Fig. 1B-D).
Taken together, these results suggest that IGFBP-3 may protect against high glucose-induced TNF-α-dependent REC apoptosis by activation of the c-Jun/TIMP3/TACE pathway. Furthermore, IGFBP-3 may directly activate c-Jun, since the effects we observed were independent of the IGFBP-3 receptor, LRP1.
Inhibition of c-Jun by Jun peptide blocked IGFBP-3NB-dependent changes in TIMP-3 expression, TACE activity and TNF-α levels in REC grown under high glucose conditions
In order to verify that c-Jun is required for IGFBP-3 actions, we treated cells with Jun peptide, a cell-permeable peptide containing the JNK-binding site of human c-Jun (Holzberg et al., 2003). This peptide was specifically designed to disrupt c-Jun-JNK and inhibit c-Jun activity. We found that treatment of REC with Jun peptide lowered phosphorylation of c-Jun by approximately 25% (Fig. 2A). IGFBP-3 transfection was unable to stimulate phosphorylation of c-Jun. Similarly, inhibition by Jun peptide treatment led to expected downstream effects, including blockade of the IGFBP-3-dependent increase in TIMP-3 expression (Fig. 2B) and the concomitant decrease in TACE activity (Fig. 2C). Importantly, Jun peptide also blocked the IGFBP-3-dependent decrease in TNF-α levels (Fig. 2D).
Fig. 2.
IGFBP-3 regulating TACE and TIMP3 levels through c-Jun protein. REC were treated with IGFBP-3 plasmid and c-Jun peptide in REC in NG and HG medium. A. Western blot data of phosphorylated and total c-Jun levels; B. Western blot results of TIMP-3 level; C. TACE activity; D. TNF-α level. *P < 0.05 vs. NG control plasmid DNA transfection. #P < 0.05 vs. HG control plasmid DNA transfection. N = 3.
The data show that the addition of c-Jun peptide efficiently disrupted the IGFBP-3 regulation of the c-Jun/TIMP3/TACE pathway and that c-Jun protein is a critical component of IGFBP-3-TNF-α pathway in REC observed in high ambient glucose.
siRNA inhibition of LRP1 blocked the IGFBP-3-mediated decrease in TNF-α receptor, TNFR2
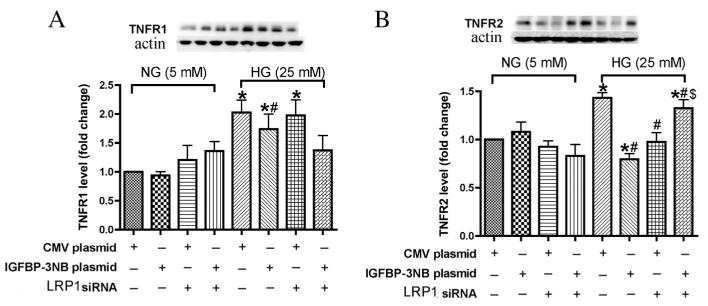
Previous studies have reported that TNF receptors (TNFR) mediate apoptosis/necrosis signal in kidney cells (Al-Lamki et al., 2005), and are a necessary component of the TNF-α signaling pathway (Luo et al., 2006). To investigate the contribution of TNFR in IGFBP-3 regulation of TNF-α signaling, we transfected REC with IGFBP-3 NB plasmid and LRP1 siRNA, followed by Western blotting of the two subtypes of TNFR, 1 and 2. Data show that in REC grown in normal glucose, both TNFR1 and 2 levels remained at control levels regardless of treatment (Fig. 3A and B). When grown under high ambient glucose, REC cells expressed significantly higher levels of both TNFR1 and 2. Cells treated with IGFBP-3 were protected against the high glucose stimulation of both TNFR1 and TNFR2. However, only the IGFBP-3-mediated decrease in TNFR2 was dependent on the LRP1, since LRP1 siRNA negated the effect. Comparison of the BP3 plasmid group and the BP3 plasmid + LRP1 siRNA group demonstrated that the TNFR2 level had a significant change with no changes noted in TNFR1. We conclude that the protective effect of IGFBP-3 on TNF-α-mediated cell death involved TNFR2 rather than TNFR1 and that the TNFR2 effect appeared to be dependent on the LRP1.
Fig. 3.
IGFBP-3 overexpression inhibited TNFR1 and TNFR2 expression in high glucose medium and the TNFR2 inhibition was dependent on LRP1. A. Western Blot of TNFR1 levels in REC transfected with IGFBP-3 NB plasmid DNA or LRP1 siRNA for 24 h in medium containing normal glucose (NG-5 mM) or high glucose (HG-25 mM) medium. B. Western Blot of TNFR2 levels in REC transfected with IGFBP-3 NB plasmid DNA or LRP1 siRNA for 24 h in medium containing normal glucose (NG-5 mM) or high glucose (HG-25 mM) medium.*P < 0.05 vs. NG control plasmid DNA transfection. #P < 0.05 vs. HG control plasmid DNA transfection. $P < 0.05 vs. HG IGFBP-3 NB plasmid. N = 3.
LRP1 siRNA blocked IGFBP-3 regulation of TNF-α receptor signaling partners, TRADD and caspase 8
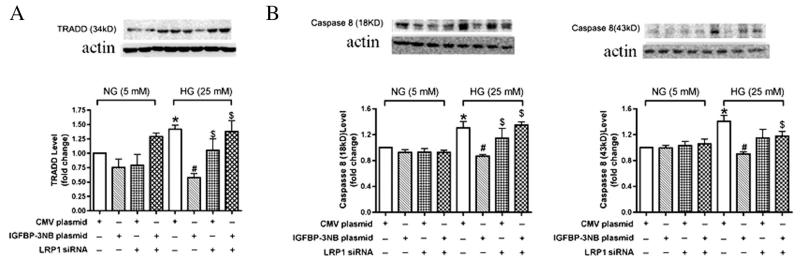
TRADD and caspase 8 are key downstream molecules in the TNF-α receptor signaling cascade that triggers apoptosis (Hsu et al., 1996). Caspase 8 full length is 57 kD and the active fragments of caspase 8 are the cleaved intermediate p43/p41 and fragment p18 (Hsu et al., 1996). Here we studied cleaved caspase 8 to find whether it is involved in the TNFR-induced cell apoptosis. In order to ascertain the effects of IGFBP-3 on these signaling molecules, we examined their protein levels in REC after IGFBP-3 transfection and after treatment with LRP1 siRNA. As expected, high glucose causes a significant increase in all three molecules. Likewise as expected, IGFBP-3 transfection reduced expression to control levels (Fig. 4A-C). Treatment with LRP1 siRNA effectively blocked the protective actions of IGFBP-3 transfection, suggesting that activation of the IGFBP-3 receptor plays a major role in regulation of TNF-α and its signaling partners, TRADD and caspase 8.
Fig. 4.
IGFBP-3 decreased TRADD and caspase 8 in REC in high ambient glucose is LRP1 dependent. A–B. Bar graph of TRADD (A) and caspase 8 (B) of REC in normal or high glucose transfected with LRP1 siRNA and IGFBP-3NB plasmid. *P < 0.05 vs. NG control plasmid, #P < 0.05 vs. HG control plasmid, $P < 0.05 vs. HG IGFBP-3 NB plasmid. N = 4.
Discussion
Based on our results, we propose a novel pathway to describe the IGF-1-independent actions of IGFBP-3 in the regulation of c-Jun and TNF-α cascades under conditions of high ambient glucose (Fig. 5). Our data suggests that IGFBP-3 has two separate interactions; the first (Fig. 5, right) is a direct action on c-Jun that does not involve the IGFBP-3 receptor, LRP1. Using IGFBP-3NB transfection to increase intracellular IGFBP-3NB led to an increase in c-Jun and a concomitant increase in its transcriptional target, TIMP-3. Activation of TIMP-3 was associated with an inhibition of its target, TACE. Since TACE stimulates TNF-α production, the end result of activation of this IGFBP-3 pathway is to decrease TNF-α levels. The central role of c-Jun in this cascade was confirmed by demonstrating that the effect was dependent on an IGFBP-3-stimulated increase in the ratio of activated phosphorylated c-Jun versus non-activated c-Jun, and by demonstrating that the effects downstream to c-Jun activation (i.e., IGFBP-3 stimulation of TIMP3 and inhibition of TACE) were blocked by the inhibitory c-Jun peptide. Our results are in agreement with previous reports that alterations in the cJun/TIMP3/TACE cascade contribute to the progression of diabetic retinopathy (Guinea-Viniegra et al., 2009). In addition, we now identify IGFBP-3 as a likely regulator of the cJun/TIMP3/TACE cascade under conditions of high glucose.
Fig. 5.
Schematic model illustrating how IGFBP-3 proteins regulate the TNF-α and apoptosis pathway in REC: Right. IGFBP-3 proteins transcriptionally activate c-Jun/TIMP-3 expression, which is responsible for TACE inhibition. The absence of JunB and c-Jun leads to an increase in TACE activity, followed by increased levels of TNF-α; Left. IGFBP-3 decreases TNFR-2 levels, which signals through LRP1, inhibiting TRADD and caspase 8 and REC apoptosis.
Our findings also support a second action of IGFBP-3, namely a decrease in the levels of TNF-α receptor, TNFR2 (Fig. 5, left). The decrease in TNFR2 was subsequently reflected in a decrease in levels of its established downstream targets, TRADD and Caspase 8 (Auer et al., 1998; Cesaro et al., 2009; Mahmoodi et al., 2005; Mohammed et al., 2004). These inhibitory effects on the TNFR2 cascade were blocked by treatment with LRP1 siRNA, suggesting that the LRP1 mediates inhibition of the TNF-α receptor-mediated cell death cascade. TNF-α can activate TNFR1 and TNFR2 receptor subtypes, both of which trigger cell death through similar signaling cascades (Leon et al., 2013; Trebicka et al., 2013; Yi et al., 2013). TNFR1 is most commonly associated with cell death in various different cell types, while TNFR2 has been more prominently linked to cell apoptosis in neural tissues (Chen and Palmer, 2013). Although it has been reported that LRP1 function could be linked to JNK and TNFR1 (Gaultier et al., 2008; Lutz et al., 2002), others have also reported that IGFBP-3 have intrinsic role in cell growth independent of its receptor or nucleus transportation (Perks et al., 2011), which could involve components of cholesterol-stabilized integrin receptor signaling, PKA, Rho, and ceramide. Our results presented demonstrate that IGFBP-3 inhibition REC apoptosis in high glucose condition is LRP1-dependent. The mechanisms of IGFBP-3 in pro- or anti-apoptosis in different cell types need to be further investigated. Although both TNFR1 and TNFR2 levels were increased in response to high glucose, with IGFBP-3 inhibiting this increase in both TNFR1 and TNFR2 levels, no significant change of TNFR1 after IGFBP-3 transfection and LRP1 siRNA was observed when compared to cells with only IGFBP-3 transfection. In contrast, TNFR2 levels were significantly reduced after IGFBP-3 transfection and the effect was LRP1 dependent. We conclude that both subtypes of TNF-α receptors may be up-regulated under conditions that mimic diabetic retinopathy; however, IGFBP-3 mediated protection is primarily associated with inhibition of TNFR2-triggered apoptosis. Our results show that the high levels of cell death mediators, TRADD and caspase 8, seen under conditions of high ambient glucose was essentially returned to normal in response to IGFBP-3 transfection.
The combined IGF-1-independent effects of IGFBP-3 to I) decrease TNF-α levels (through LRP1-independent stimulation of the c-Jun cascade) and II) decrease TNF-α receptor (through LRP1-dependent interactions) are complimentary in that both lead to a decrease in cell death. Effects on TNF-α levels are independent of the LRP1, whereas effects on TNF-α receptor signaling require activation of the LRP1. Thus we conclude that IGFBP-3 works through two separate mechanisms, both of which contribute to the substantial protective effects of IGFBP-3.
Our experiments utilized a primary human REC cell line cultured under conditions of high (25 mM) ambient glucose. Previous studies have demonstrated that this model system demonstrates many features of retinal endothelial cells in vivo from diabetic animals (Zhang et al., 2012, 2013a,b). These common features, including high levels of TNF-α as well as vascular cell death, are causative in the development of diabetic retinopathy. Retinal endothelial cell dysfunction is known to play a pivotal role in overall pathophysiological complications of diabetic retinopathy that include subsequent loss of other retinal cell types including neurons. Given the importance of REC in this major blinding disorder, discovery of cellular mechanisms that offer protection to REC against the effects of high glucose may offer new strategies for understanding the overall disease process and for developing potential treatment options. Our current results, along with our previous publications using primary human REC, demonstrate the usefulness of this line of approach. Experiments with these cells have allowed us to uncover novel mechanisms by which IGFBP-3 I) decreases levels of TNF-α through activation of the c-Jun cascade and II) decreases levels of TNF-α receptor, TNFR2 through activation of the LRP1. Both mechanisms serve to inhibit REC apoptosis. Future studies further assessing the regulation of IGFBP-3 systems will be important for the long term goal of creating effective treatments for diabetic retinopathy.
Acknowledgments
We are thankful to Dr. Dianna A Johnson for helping to revise the manuscript.
Funding
Supported by National Eye Institute Vision Grant R01EY022045 (JJS); Juvenile Diabetes Research Foundation Grant (2-2011-597 to JJS); Oxnard Foundation (JJS); Research to Prevent Blindness Award (PI: James C. Fleming); and NEI Vision Core Grant: PHS 3P30 EY013080 (PI: Dianna Johnson).
Footnotes
Author contributions
Qiuhua Zhang performed the experiments, participated in project discussions and wrote the manuscript; and Jena Steinle conceived the overall project, designed the experiments, interpreted the data, and revised the manuscript.
Competing interests
The authors declare that they have no competing interests.
References
- Al-Lamki RS, Wang J, Vandenabeele P, et al. TNFR1- and TNFR2-mediated signaling pathways in human kidney are cell type-specific and differentially contribute to renal injury. FASEB J. 2005;19:1637–1645. doi: 10.1096/fj.05-3841com. [DOI] [PubMed] [Google Scholar]
- Auer KL, Contessa J, Brenz-Verca S, et al. The Ras/Rac1/Cdc42/SEK/JNK/c-Jun cascade is a key pathway by which agonists stimulate DNA synthesis in primary cultures of rat hepatocytes. Mol. Biol. Cell. 1998;9:561–573. doi: 10.1091/mbc.9.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am. J. Physiol. Endocrinol. Metab. 2000;278:E967–E976. doi: 10.1152/ajpendo.2000.278.6.E967. [DOI] [PubMed] [Google Scholar]
- Ben-Mahmud BM, Mann GE, Datti A, et al. Tumor necrosis factor-alpha in diabetic plasma increases the activity of core 2 GlcNAc-T and adherence of human leukocytes to retinal endothelial cells: significance of core 2 GlcNAc-T in diabetic retinopathy. Diabetes. 2004;53:2968–2976. doi: 10.2337/diabetes.53.11.2968. [DOI] [PubMed] [Google Scholar]
- Cabal-Hierro L, Lazo PS. Signal transduction by tumor necrosis factor receptors. Cell. Signal. 2012;24:1297–1305. doi: 10.1016/j.cellsig.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Carpentier I, Coornaert B, Beyaert R. Function and regulation of tumor necrosis factor receptor type 2. Curr. Med. Chem. 2004;11:2205–2212. doi: 10.2174/0929867043364694. [DOI] [PubMed] [Google Scholar]
- Cesaro A, Abakar-Mahamat A, Brest P, et al. Differential expression and regulation of ADAM17 and TIMP3 in acute inflamed intestinal epithelia. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G1332–G1343. doi: 10.1152/ajpgi.90641.2008. [DOI] [PubMed] [Google Scholar]
- Chen Z, Palmer TD. Differential roles of TNFR1 and TNFR2 signaling in adult hippocampal neurogenesis. Brain Behav. Immun. 2013;30:45–53. doi: 10.1016/j.bbi.2013.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- Clemmons DR. Use of mutagenesis to probe IGF-binding protein structure/function relationships. Endocr. Rev. 2001;22:800–817. doi: 10.1210/edrv.22.6.0449. [DOI] [PubMed] [Google Scholar]
- Gaultier A, Arandjelovic S, Li X, et al. A shed form of LDL receptor-related protein-1 regulates peripheral nerve injury and neuropathic pain in rodents. J. Clin. Invest. 2008;118:161–172. doi: 10.1172/JCI32371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinea-Viniegra J, Zenz R, Scheuch H, et al. TNFalpha shedding and epidermal inflammation are controlled by Jun proteins. Genes Dev. 2009;23:2663–2674. doi: 10.1101/gad.543109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzberg D, Knight CG, Dittrich-Breiholz O, et al. Disruption of the c-JUN-JNK complex by a cell-permeable peptide containing the c-JUN delta domain induces apoptosis and affects a distinct set of interleukin-1-induced inflammatory genes. J. Biol. Chem. 2003;278:40213–40223. doi: 10.1074/jbc.M304058200. [DOI] [PubMed] [Google Scholar]
- Hsu H, Shu HB, Pan MG, et al. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- Ishida S, Yamashiro K, Usui T, et al. Leukocytes mediate retinal vascular remodeling during development and vaso-obliteration in disease. Nat. Med. 2003;9:781–788. doi: 10.1038/nm877. [DOI] [PubMed] [Google Scholar]
- Jarajapu YP, Cai J, Yan Y, et al. Protection of blood retinal barrier and systemic vasculature by insulin-like growth factor binding protein-3. PLoS ONE. 2012;7:e39398. doi: 10.1371/journal.pone.0039398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Zhang Q, Soderland C, et al. TNFalpha and SOCS3 regulate IRS-1 to increase retinal endothelial cell apoptosis. Cell. Signal. 2012;24:1086–1092. doi: 10.1016/j.cellsig.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussen AM, Murata T, Tsujikawa A, et al. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am. J. Pathol. 2001;158:147–152. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielczewski JL, Hu P, Shaw LC, et al. Novel protective properties of IGFBP-3 result in enhanced pericyte ensheathment, reduced microglial activation, increased microglial apoptosis, and neuronal protection after ischemic retinal injury. Am. J. Pathol. 2011;178:1517–1528. doi: 10.1016/j.ajpath.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon LR, Dineen S, Blaha MD, et al. Attenuated thermoregulatory, metabolic, and liver acute phase protein response to heat stroke in TNF receptor knockout mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305:R1421–R1432. doi: 10.1152/ajpregu.00127.2013. [DOI] [PubMed] [Google Scholar]
- Lofqvist C, Chen J, Connor KM, et al. IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth. Proc. Natl. Acad. Sci. U. S. A. 2007;104:10589–10594. doi: 10.1073/pnas.0702031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett-Racke AE, Bittner P, Cross AH, et al. Regulation of experimental autoimmune encephalomyelitis with insulin-like growth factor (IGF-1) and IGF-1/IGF-binding protein-3 complex (IGF-1/IGFBP3) J. Clin. Invest. 1998;101:1797–1804. doi: 10.1172/JCI1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Luo Y, He Y, et al. Differential functions of tumor necrosis factor receptor 1 and 2 signaling in ischemia-mediated arteriogenesis and angiogenesis. Am. J. Pathol. 2006;169:1886–1898. doi: 10.2353/ajpath.2006.060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz C, Nimpf J, Jenny M, et al. Evidence of functional modulation of the MEKK/JNK/cJun signaling cascade by the low density lipoprotein receptor-related protein (LRP1) J. Biol. Chem. 2002;277:43143–43151. doi: 10.1074/jbc.M204426200. [DOI] [PubMed] [Google Scholar]
- Mahmoodi M, Sahebjam S, Smookler D, et al. Lack of tissue inhibitor of metalloproteinases-3 results in an enhanced inflammatory response in antigen-induced arthritis. Am. J. Pathol. 2005;166:1733–1740. doi: 10.1016/S0002-9440(10)62483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed FF, Smookler DS, Taylor SE, et al. Abnormal TNF activity in Timp3−/− mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat. Genet. 2004;36:969–977. doi: 10.1038/ng1413. [DOI] [PubMed] [Google Scholar]
- Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J. Endocrinol. 2002;175:19–31. doi: 10.1677/joe.0.1750019. [DOI] [PubMed] [Google Scholar]
- Ning Y, Schuller AG, Bradshaw S, et al. Diminished growth and enhanced glucose metabolism in triple knockout mice containing mutations of insulin-like growth factor binding protein-3, -4, and -5. Mol. Endocrinol. 2006;20:2173–2186. doi: 10.1210/me.2005-0196. [DOI] [PubMed] [Google Scholar]
- Perks CM, Burrows C, Holly JM. Intrinsic, pre-apoptotic effects of IGFBP-3 on breast cancer cells are reversible: involvement of PKA, Rho, and ceramide. Front Endocrinol. (Lausanne) 2011 doi: 10.3389/fendo.2011.00013. http://dx.doi.org/10.3389/fendo.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riches DW, Chan ED, Zahradka EA, et al. Cooperative signaling by tumor necrosis factor receptors CD120a (p55) and CD120b (p75) in the expression of nitric oxide and inducible nitric oxide synthase by mouse macrophages. J. Biol. Chem. 1998;273:22800–22806. doi: 10.1074/jbc.273.35.22800. [DOI] [PubMed] [Google Scholar]
- Steinle JJ. Retinal endothelial cell apoptosis. Apoptosis. 2012;17:1258–1260. doi: 10.1007/s10495-012-0777-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Honeyman MC, Harrison LC. Impaired yield, phenotype, and function of monocyte-derived dendritic cells in humans at risk for insulin-dependent diabetes. J. Immunol. 1998;161:2629–2635. [PubMed] [Google Scholar]
- Titchenell PM, Antonetti DA. Using the past to inform the future: anti-VEGF therapy as a road map to develop novel therapies for diabetic retinopathy. Diabetes. 2013;62:1808–1815. doi: 10.2337/db12-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ, Cerami A. Tumor necrosis factor, other cytokines and disease. Annu. Rev. Cell Biol. 1993;9:317–343. doi: 10.1146/annurev.cb.09.110193.001533. [DOI] [PubMed] [Google Scholar]
- Trebicka J, Krag A, Gansweid S, et al. Soluble TNF-alpha-receptors I are prognostic markers in TIPS-treated patients with cirrhosis and portal hypertension. PLoS ONE. 2013;8:e83341. doi: 10.1371/journal.pone.0083341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenabeele P, Declercq W, Beyaert R, et al. Two tumour necrosis factor receptors: structure and function. Trends Cell Biol. 1995;5:392–399. doi: 10.1016/s0962-8924(00)89088-1. [DOI] [PubMed] [Google Scholar]
- Yi Z, Lin WW, Stunz LL, et al. Roles for TNF-receptor associated factor 3 (TRAF3) in lymphocyte functions. Cytokine Growth Factor Rev. 2013;25(2):147–156. doi: 10.1016/j.cytogfr.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Liu H, Rojas M, et al. Anti-inflammatory therapy for diabetic retinopathy. Immunotherapy. 2011;3:609–628. doi: 10.2217/imt.11.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Guy K, Pagadala J, et al. Compound 49b prevents diabetes-induced apoptosis through increased IGFBP-3 levels. Invest. Ophthalmol. Vis. Sci. 2012;53:3004–3013. doi: 10.1167/iovs.11-8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Jiang Y, Miller MJ, et al. IGFBP-3 and TNF-alpha regulate retinal endothelial cell apoptosis. Invest. Ophthalmol. Vis. Sci. 2013a;54:5376–5384. doi: 10.1167/iovs.13-12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Soderland C, Steinle JJ. Regulation of retinal endothelial cell apoptosis through activation of the IGFBP-3 receptor. Apoptosis. 2013b;18:361–368. doi: 10.1007/s10495-012-0793-3. [DOI] [PMC free article] [PubMed] [Google Scholar]