Abstract
Until recently, treatment for metastatic melanoma was characterised by a limited availability of treatment options that offer objective survival benefit. Cytotoxic agents fundamentally lack the ability to achieve disease control and cytokine therapy with interleukin-2 has an unacceptably high – for the use across all patient cohorts – rate of toxicities. The validation of braf as an oncogene driving melanoma tumorigenesis, as well as the discovery of the role of CTLA-4 receptor in the evasion of anticancer immune response by melanoma, has revolutionised our treatment options against a disease with dismal prognosis. Quick implementation of translational discoveries brought about BRAF/MEK inhibition in clinic, while at the same time, wider experience with CTLA-4 blockade enabled clinicians to manage previously fatal immune-related toxicities with greater confidence. The suitability for clinical use of other oncogenic drivers such as NRAS and c-kit is currently being tested whilst the PD-1/PD-L1/PD-L2 axis has emerged as a new immunotherapy target with exciting early phase results. The recent exponential progress in treatment of melanoma has set an example of translational medicine and the current review aims to explain why, as well as suggesting new goals for the future.
Keywords: BRAF/MEK inhibition, ipilimumab, metastatic melanoma, molecularly targeted treatment, PD-1/PD-L1/PD-L2 axis
Introduction
Melanoma is the most lethal form of skin cancer, accounting for the majority of skin cancer deaths. There are approximately 132,000 new cases globally each year, an incidence that has been steadily rising in the western world for the past few decades: this increase relates to both improved detection and an increase in the frequency of exposure to UV radiation [WHO, 2013; De Vries and Coeburgh, 2005]. Factors such as skin colour, hair colour and pre-existence of more than 20 nevi increase the risk of melanoma occurrence; fair-skinned people are the ones mostly suffering from the disease with the White race having a risk ratio of 5–10 versus Black or Asian race [Linette, 2012]. Approximately 20% of melanoma sufferers over the age of 65 years will present with metastases and therefore be incurable at diagnosis; the 5-year survival of patients with metastatic melanoma is 8% for males and 25% for females, with a median survival of 6 months [Cancer Research UK, 2013a]. Metastases to skin, subcutaneous tissue or lymph nodes confer the best prognosis in the metastatic setting whereas lung metastases have an intermediate prognosis. Patients with disease to any other visceral sites (liver, bone, brain) or any site combined with an elevated lactate dehydrogenase (LDH) carry the worst prognosis with a 33% 1-year survival rate [Balch et al. 2009].
Recent successes in the oncological treatment of melanoma have reminded us that sometimes the biggest disappointments can create great opportunities. This review aims to present the poor progress made with conventional cytotoxic therapies for metastatic melanoma, as well as offering some biological and translational insight on why we have over the last few years had a rapid progress with an explosion of potential treatments compared with other cancers.
Melanoma pathophysiology precludes any survival benefit from traditional cytotoxics
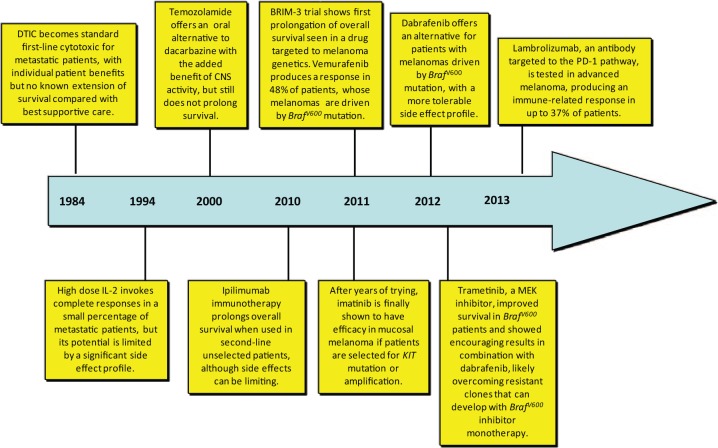
Until recently, no therapy administered to UK patients with metastatic melanoma could extend overall survival. Dacarbazine, a cytotoxic previously considered the standard of care, only offers limited benefit with improvement in symptoms of carefully selected patients (Figure 1) [Tarhini and Agarwala, 2006]. Response rates to dacarbazine (complete and partial response) have most recently been shown to be of approximately 10% and, as a consequence of its historical development, no randomized phase III studies exist to confirm its benefit over best supportive care [Robert et al. 2011]. Temozolamide, an imidazotetrazinone derivative with good brain tissue penetration and the advantage of oral administration, was a more recent hope for cytotoxic development in melanoma, especially in patients with brain metastatic disease [Stevens et al. 1987]. However, in a phase III randomized trial focusing on central nervous system (CNS) involvement, temozolamide affected neither the occurrence of CNS failure as first site of metastases nor the overall survival (OS) in these patients (Figure 1) [Chiarion-Sileni et al. 2011]. Some physicians elect to use the combination of carboplatin/paclitaxel in the second-line setting as it showed modest antitumour activity in a small study of pretreated patients [Rao et al. 2006]. Nevertheless, this treatment offers no survival benefit, similar to a number of other single agents or combination chemotherapeutic regimens that have been assessed [Jilavenau et al. 2009].
Figure 1.
Timeline of key therapeutic developments in metastatic melanoma. Most of these advances have occurred in the last 3–4 years.
CNS, central nervous system; IL-2, interleukin-2; MEK, mitogen-activated protein kinase kinase.
Melanocyte biologists might argue that they are unsurprised by such results since the pathogenesis of melanoma is characterised by two central physiological properties of cells from the melanocyte lineage.
Adult melanocytes (MCs) are resistant to apoptosis. Their major role is lifelong protection of the skin from ultraviolet radiation (UVR) through the production of melanin and the consequent tanning response [Boissy and Nordlund, 1997]. The capacity of MCs to resist apoptosis and survive the highly mutagenic skin environment is reflected in melanoma by its resistance to traditional cytotoxics [Terzian et al. 2010]. Melanoma cells have been shown to display resistance to drug-induced apoptosis in vitro and also show low levels of spontaneous apoptosis in vivo [Soengas and Lowe, 2003]. Thus, melanoma cells can ‘hijack’ the molecular mechanisms of other cells in the MC lineage for their own devices.
MC embryological precursors, melanoblasts (MBs), have a propensity to migrate. The migratory propensity of MCs is indicated by their anatomical localisation in adults, where they classically reside in the skin, but also exist in the eyes, heart, inner ear and brain [Brito and Kos, 2008]. MCs arise from a uniquely motile embryonic precursor population, MBs. This lineage-specific predisposition of MBs to migrate is also present in early stage primary melanomas, where metastases can develop from primary lesions as small as less than 1 mm in size and cause devastating effects through involvement of multiple viscera [Gupta et al. 2005; Corsetti et al. 2000]. Not unusually, we see cases of metastatic melanoma in clinic where identification of the primary is impossible, either because it has regressed or it is too small to detect. Given its biological capacity for aggression, it seems likely that the relatively high 80% survival rate from melanoma accounts only for those early detected primary tumours on an easily visualised organ, the skin. Unlike many other cancers with developed cytotoxic treatment protocols, melanoma therefore offered an attractive niche for the development of innovative targeted treatments that were tailored to its underlying genetic aberrations. There was no ‘standard of care’ to compete with in the treatment of metastatic disease.
The discovery of BrafV600 mutations presents an opportunity
In 2002, a systemic screen of genetic alterations in proteins Ras, Raf, mitogen-activated protein kinase kinase (MEK) and extracellular signal regulated kinase (ERK) was reported in a number of different cancer cell lines [Davies et al. 2002]. Their key discovery was a missense mutation in the serine threonine kinase, BRAF, at codon 600: a single site for mutations which occur with high frequency in cutaneous melanomas. The mutation itself is an oncogenic gain-of-function mutation that renders BRAF constitutively active, thus u-regulating the mitogen-activated protein kinase (MAPK) pathway in the absence of extracellular growth signals. Subsequently, BrafV600 has been functionally validated as an oncogenic driver in both melanoma animal models and cell lines with a prevalence now estimated at 52% across patients with cutaneous melanoma [Gray-Schopfer et al. 2007; Dhomen et al. 2009]. This was the first identification of Braf as an oncogene, and compared with other cancers, its high prevalence in melanoma was unique. Moreover, the amino acid substitutions caused by BrafV600 mutations and their positioning in the BRAF protein residues offered an easy ‘target’ for the subsequent ‘hit-to-lead’ process.
Within 8 years of this discovery, the translational development of BrafV600 inhibition succeeded at near unparalleled levels and set a benchmark for other cancers to follow. A phase I trial of the small molecule BrafV600 inhibitor, vemurafenib, showed remarkable clinical efficacy with a tolerable side effect profile in a large subset of melanoma patients preselected for their BrafV600 status (Figure 1) [Flaherty et al. 2010]. In 2011, a phase III trial confirmed vemurafenib as the first agent targeted to melanoma genetics that could offer an extension in OS of metastatic patients. Median OS was significantly longer in the group treated with vemurafenib than in control group treated with dacarbazine (13.6 months [95% confidence interval (CI) 12.0–15.2] versus 9.7 months [95% CI 7.9–12.8; hazard ratio (HR) 0.70 (95% CI 0.57—0.87); p = 0·0008], as was median progression-free survival (PFS) [6.9 months (95% CI 6.1–7.0) versus 1.6 months (95% CI 1.6–2.1); HR 0.38 (95% CI 0.32–0.46); p < 0.0001] respectively (Table 1) [Chapman et al. 2011]. Patient selection was limited to melanoma patients carrying the BrafV600 mutation and toxicity was generally tolerable.
Table 1.
Key details of recent breakthrough studies in metastatic melanoma.
| BRIM-3 [Chapman et al. 2011] | BREAK-3 [Hauschild et al. 2012] | METRIC [Flaherty et al. 2012b] | COMBI- v [Robert et al. 2014] | MEK162 [Ascierto et al. 2013] | Imatinib [Guo et al. 2011] | Ipilimumab [Hodi et al. 2010] | Ipilimumab/ dacarbazine [Robert et al. 2011] | Nivolumab [Topalian et al. 2014] | |
|---|---|---|---|---|---|---|---|---|---|
| Phase | III | III | III | III | II | II | III | III | I/II |
| Line of treatment | 1st | 1stt | 1st or 2nd | 1st | >2nd | 2ndor 3nd | 2nd | 1nd | > 2nd |
| Number of patients | 675 | 250 | 322 | 704 | 71 | 43 | 676 | 502 | 107 |
| Drug | Vemurafenib | Dabrafenib | Trametinib | Dabrafenib/ trametinib | MEK162 | Imatinib | Ipilimumab | Ipilimumab/ dacarbazine | Nivolumab |
| Mechanism | BrafV600 inhibitor | BrafV600 inhibitor | MEK inhibitor | BrafV600 inhibitor + MEK inhibitor | MEK inhibitor | KIT inhibitor | Anti-CTLA-4 antibody | Anti-CTLA-4 antibody + cytotoxic | Anti-PD-1 antibody |
| Patient selection | BrafV600E or V600K mutated | BrafV600 mutated | BrafV600 mutated | BrafV600 mutated | BrafV600 or NRAS-mutated | cKIT mutated and/or amplified | HLA-A*0201 positive | no | no |
| RR (%) | 57 | 50 | 22 | N/A | 20* | 22 | 10.9 | 15 | 31¥ |
| Median PFS (months) | 6.9* | 6.9* | 4.8* | 11.4 | 4 | 3.5* | 2.9 | <3 | 3.7 |
| Median OS (months) | 13.6 | 18.2 | Not mature | Not mature* | Not mature | Not mature | 10.1* | 11.2* | 16.8 |
All trials were considered to have positive results. Last three columns detail clinical trials with immune checkpoint antibodies.
Primary endpoint
Secondary endpoint
MEK, mitogen-activated protein kinase kinase; OS, overall survival; PFS, progression-free survival; RR, relative risk.
Another BrafV600 inhibitor, dabrafenib, has also emerged into clinical use with striking clinical trial results (Figure 1, Table 1). Following encouraging phase I/II trial results, a phase III comparison of dabrafenib with dacarbazine in patients with BrafV600E expressing melanoma showed a significant improvement in PFS with 6.9 months versus 2.7 months, respectively, and a preliminary median OS favouring dabrafenib (18.2 months versus 15.6 months) [Hauschild et al. 2013]. Dabrafenib has a generally favourable toxicity profile. Taking into consideration that comparisons between trial results are not always precise, a lower incidence of epithelial squamous cell lesions was noticed with dabrafenib when compared with vemurafenib. There are speculations that dabrafenib’s lower specificity for wildtype BRAF and CRAF could prevent undesirable activation of wildtype RAF dimers and therefore lead to less cutaneous adverse events [Hauschild et al. 2012].
The breakthroughs with vemurafenib/dabrafenib were engendered by our predictive selection of patients with melanomas expressing mutated BrafV600, something that can be explored further on two quantifiable levels:
Efficacy. This is reflected by improved response rates and extension of survival with vemurafenib/dabrafenib and may well not have been the case had all patients (i.e. those without BrafV600 mutation) been treated with the BrafV600 inhibitor. In fact there is significant preclinical concern to suggest that patients with melanomas driven by other genetic signatures could respond adversely to drugs such as vemurafenib, with a paradoxical MAPK pathway upregulation and consequent deterioration/progression of their disease [Heidorn et al. 2010].
Toxicity. As vemurafenib is specifically targeted to melanoma cells with BrafV600 mutation, rather than other cells with wildtype Braf, it was tempting to speculate that patients on treatment would be spared side effects due to a lack of effect on cells other than melanoma cells. This has turned out not to be the case and a number of idiosyncratic side-effects have been characterised with BrafV600 inhibition, including an approximately 12% incidence of cutaneous squamous cell cancers [Chapman et al. 2011]. The reasons for this are still not fully elucidated, although it is clear that vemurafenib again invokes a paradoxical upregulation of the MAPK pathway in cells affected by mutated RAS [Su et al. 2012]. Toxicity is therefore not as minor as might have been hypothesised at the outset of clinical trials, but the side effect profile from BrafV600 inhibitors is undoubtedly tolerable in most cases.
To finish, it is important to comment on a level of progress with BrafV600 inhibition that is more difficult to quantify, something that we may not be able to fully assess for a number of years: our knowledge of the tumorigenic process in melanoma now has a preclinical and clinical platform from which we can measure all future advances. The process of development of BrafV600 inhibitors in melanoma, from target identification to clinical implementation, happened with an exemplarily quick pace, proving that the historical 10–15 year duration of drug discovery for small molecule drugs can be achieved or even outreached. It created a paradigm from which cancer researchers can take confidence in their pursuit of successful molecularly targeted treatments for other cancers. One of the first questions oncologists must now ask themselves with all cases of metastatic cancer is: should we still resort to the traditional and molecularly uninformed use of cytotoxics when a trial of an oral drug that specifically targets the main driver mutation of a cancer might suffice?
The historical failure and lack of chemotherapy benefit in metastatic melanoma was in many senses the vehicle for this breakthrough, and the prior success of imatinib in chronic myeloid leukaemia (CML) and gastrointestinal stromal tumours (GIST) followed a similar path [Dematteo et al. 2009; Demetri et al. 2002; Joensuu et al. 2012; Blanke et al. 2008; O’Brien et al. 2003; Verweij et al. 2004]. More recently, the eventual success of erlotinib in nonsmall cell lung cancer (NSCLC) has proven that chemotherapy can be sidelined for less toxic targeted treatments tailored to tumour genetics [Zhou et al. 2011; Rosell et al. 2012; Fukuoka et al. 2011].
With all these advances, a need for innovative clinical trial designs has emerged, whereby patients are carefully selected with the use of validated biomarkers and ‘matched’ to the appropriate drug. These studies, such as enrichment and adaptive-design studies will help patients derive the maximum benefit from targeted treatments and clinical researchers extract a vast amount of translational information [Temple, 2005; Berry, 2011].
MEK inhibition: building on the knowledge gained from BrafV600 inhibition
Shortly after its clinical success, several resistance mechanisms to BrafV600 inhibition were reported in preclinical literature, offering important insights for oncologists to reiteratively dissect treatment of patients resistant to vemurafenib/dabrafenib with further rationally designed second line and combination trials [Heidorn et al. 2010; Poulikakos et al. 2011; Hatzivassiliou et al. 2010; Johannessen et al. 2010; Gopal et al. 2010; Nazarian et al. 2010; Shao and Aplin, 2011; Emery et al. 2009]. One key mechanism of resistance described was the mutation and upregulation of downstream MEK, a protein which has kinase activity in about 90% of untreated human melanomas [Gray-Schopfer et al. 2007; Emery et al. 2009]. A protein susceptible to treatment with kinase inhibitors had once again been uncovered as relevant to melanoma pathogenesis. Trametinib, an allosteric non-adenosine triphosphate (ATP) competitive molecule, is the first of many MEK inhibitors under development for treatment of metastatic melanoma and other malignancies (Figure 1, Table 1). In line with preclinical data which showed efficient inhibition of phosphorylated ERK 1/2, its activity in advanced BrafV600-mutant melanoma was confirmed in phase I trials [Falchook et al. 2012a; Infante et al. 2012]. Clinical efficacy of trametinib was confirmed by the phase III METRIC trial, which showed significant improvements in PFS (4.8 versus 1.5 months) and rate of OS (81% versus 67% 6-month survival) for BrafV600-mutant patients randomized to trametinib rather than chemotherapy (Table 1). Adverse events with trametinib were easily managed and most importantly, skin neoplasms were completely absent. Notably, trametinib-induced rash was papulopustular in nature, as opposed to the hyperkeratotic, maculopapural rash of the BRAF inhibition [Flaherty et al. 2012b]. Severe treatment toxicity was rare, a finding which next led to a feasibility study assessing the combination of trametinib with dabrafenib [Flaherty et al. 2012a]. The biological rationale here was that MEK inhibition could prevent both the resistance and toxicity caused by MAPK upregulation following BrafV600 inhibition. Phase I/II data on 247 patients were reported showing significant improvements in PFS (9.4 versus 5.8 months) and a significant reduction in skin toxicity (5% versus 19% incidence of squamous cell carcinoma) with trametinib/dabrafenib compared with dabrafenib alone [ClinicalTrials.gov identifier: NCT01584648].
These results led to the large-scale phase III trial COMBI-v where the superiority of combination of BRAF/MEK blockade with dabrafenib/tramenitib was tested against monotherapy with vemurafenib in patients with unresectable or metastatic melanoma. Median PFS was 11.4 versus 7.3 months in favour of dabrafenib/trametinib arm (HR 0.56, p < 0.001) whilst median OS has not been reached as yet [Robert et al. 2014]. Even more extended PFS was reported in a phase Ib trial with vemurafenib and a novel MEK inhibitor, cobimetinib; the primary endpoint was safety of the combination treatment but nonetheless a PFS of 9.9 months (hazard ratio for death or disease progression, 0.51; 95% CI 0.39–0.68; p < 0.001) against 6.2 months with vemurafenib monotherapy was demonstrated [Larkin et al. 2014].
One key remaining question for MEK inhibition is whether it may also be efficacious in the 15% of cutaneous melanoma patients with an NRAS (rather than BRAF) mutation. Melanoma activating mutations of BRAF and NRAS are generally mutually exclusive, a finding which suggests they stimulate the same linear pathway involving MAPK deregulation [Davies et al. 2002; Goel et al. 2006; Rajagopalan et al. 2002]. Mutation of NRAS drives the majority of cutaneous melanomas unaccounted for by BrafV600 mutation [Cohen et al. 2002]. Despite their mutual stimulation of the MAPK pathway, there is preclinical evidence to suggest that BrafV600 inhibition will have no effect in patients with melanomas driven by mutation of NRAS, and in fact this may be a counterproductive strategy [Nazarian et al. 2010].
Targeting Ras, a GTPase rather than a kinase, therefore remains an elusive ‘holy grail’ of melanoma, as it is in other cancers. No direct inhibitors of Ras are currently being assessed in clinical trials, although a logical next step would be to consider MEK inhibition in Ras-mutated melanoma given its downstream upregulation of the MAPK pathway. A small phase II study of another MEK inhibitor, MEK-162, suggested that this may well be biologically and clinically plausible as 20% of patients with NRAS mutated advanced melanoma achieved an initial partial response to treatment (Table 1) [Ascierto et al. 2013]. Given the frequent cell cycle checkpoint dysregulation in NRAS-mutant melanoma, MEK-162 was combined with the selective CDK4/6 inhibitor, LEE011, in a phase Ib/II study which saw a 43% rate of partial responses; further results of the phase II part of the study are awaited with great interest for this subtype of melanoma with particularly poor prognostic profile [Sosman et al. 2014].
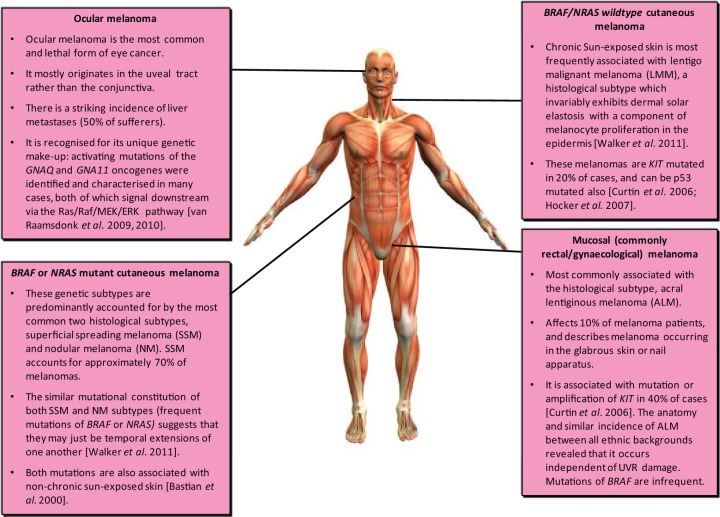
Thus it is quite likely that, in the near future, a vast majority of patients with advanced melanoma will have the option of a molecularly targeted agent, depending on the particular genetic aberrations of their disease. Key driver mutations of nearly all histological subtypes have been identified, and are readily being assessed for targeted therapy (Figure 2). It is clear that we are already beginning to realise the opportunities made available through the knowledge gained with genetic testing both before and after BrafV600 inhibition. The story of developing MEK inhibition strategies after observing drug resistance mechanisms against BRAF inhibition reiterates the importance of understanding tumour biology prior to instigating the drug development of targeted agents.
Figure 2.
The emerging pathological landscape of melanoma: how traditional histological subtypes are molecularly characterised. More than in any other cancers, this genetic constitution has shaped melanoma treatment and may ultimately create pressure for an altered taxonomy by which it can be defined.
Picture of human body obtained from https://washhouseanatomy.wikispaces.com/The+Wonders+Of+The+Human+Body (accessed 26 July 2014).
Perhaps the most disappointing targeted therapeutic applied in melanoma trials is sorafenib, employed on the basis of its anti-VEGFR and anti-Raf activity: poor efficacy was seen in both monotherapy and combination trials [Hauschild et al. 2009; Ott et al. 2010; O’Brien et al. 2003; Eisen et al. 2006]. Initially, the use of imatinib in metastatic melanoma was also an example of poorly considered translational research, with a protracted length of development comparable with that of epidermal growth factor receptor (EGFR) inhibitors in lung cancer [Zhou et al. 2004]. Imatinib is a tyrosine kinase inhibitor which selectively targets BCR-Abl and KIT tyrosine kinases. It is best known for the excellent disease control it offers in treatment of advanced CML, where it targets the BCR-Abl, and GIST, where it targets KIT [Demetri et al. 2002; Blanke et al. 2008; Dematteo et al. 2009]. More recently, its role in the adjuvant treatment of GIST has also been confirmed [Joensuu et al. 2012, Curtin et al. 2006]. KIT is mutated or amplified in 20% of lentigo malignant melanoma and 40% of acral lentiginous melanoma, two subtypes which represent a significant minority of patients (Figure 2) [Wyman et al. 2006]. Three phase II trials of imatinib were initially performed in ungenotyped metastatic melanoma patients, with only 1 of 64 showing a partial treatment response [Ugurel et al. 2005; Kim et al. 2008; Guo et al. 2011]. After a delay of 6 years, a fourth phase II trial assessed its use in metastatic melanoma patients whose cancers were predicatively selected for KIT mutation or amplification, showing a 23% response rate and 30% rate of disease stability (Figure 1, Table 1). The mutations and amplification state of patients resistant to imatinib were also clarified in this trial, offering further opportunities for re-iterative translational research [Wolchok, 2012].
Thus, our biological understanding of simple melanoma genetics was eventually acknowledged in imatinib clinical trial design, leading to its significant success. The portfolio of molecularly targeted therapies available for control of mucosal, as well as cutaneous, melanomas was also further expanded (Figure 2).
What is the treatment niche for immunotherapy?
Immunotherapy is another facet of recent clinical progress in metastatic melanoma, having been responsible for some of the more historical treatment approaches with a degree of early success too. This section is designed to give an overview of the key developments with immunotherapy use in advanced melanoma. There have been a number of trials over the past two decades, some of which we do not expect to comprehensively cover, but more detail is reviewed elsewhere [Rosenberg et al. 1993].
Clinical trials assessing the use of high dose interleukin-2 (IL-2) in advanced melanoma were reported in the 1990s (Figure 1) [Atkins et al. 1999; Hodi et al. 2010]. In general these trials showed that melanoma had a low response rate to treatment (16% objectively), but that this response was durable in a significant percentage of this minority: at follow up of 6 years, 44% of patients with a treatment response were still alive. Unfortunately many clinicians were forced to limit their use of high dose IL-2 due to concern over potentially serious pro-inflammatory side effects, which included problems such as hypotension, arrhythmia, pulmonary oedema and sepsis. Ultimately, with no clear predictive markers for treatment response forthcoming, it was often difficult to justify the risk of these toxicities to patients when they had a less than 1 in 5 chance of benefit.
Approximately 12 years after the approval of IL-2 by the US Food and Drug Administration (US FDA) in 1998, results from the use of ipilimumab in pretreated patients with advanced melanoma were reported [Hodi et al. 2010]. Ipilimumab is a monoclonal antibody which targets cytotoxic T-lymphocyte antigen-4 (CTLA-4), a negative regulator of the activated immune system which would normally prevent a T-cell response against melanoma. In a vaccine-controlled phase III trial of patients with metastatic melanoma, second-line administration of ipilimumab significantly extended median OS from 6.4 months to 10 months. Like IL-2, RR in the ipilimumab monotherapy cohort was relatively low at 10.9%, but 60% of these responders had persisting disease control at 2 years. Grade 3 and 4 immune-related adverse events secondary to ipilimumab (such as dermatitis, colitis) were as high as 10–15% and, more notably, there was a 2.1% rate of drug-related deaths.
First-line administration of ipilimumab with dacarbazine was also reported in a later phase III trial of patients with advanced melanoma (Table 1). The combination treatment led to a statistically significant median survival benefit of 2 months compared with dacarbazine monotherapy, albeit at the cost of significant toxicity with a 56% incidence of grade 3/4 toxicities, commonly in the form of hepatotoxicity. With a better understanding of the immune-related reactions and well-designed algorithms to manage them, drug-related deaths were avoided completely and a 4-year survival of 21.2% was achieved (95% CI 16.1–26.5) versus 12.1% with dacarbazine monotherapy (95% CI 8.1–16.3) [Schadendorf et al. 2013]. An overlap of the PFS curves was observed until week 12 of treatment, confirming once again that the benefit derived by immunotherapy occurs later than the one observed with conventional cytotoxic agents.
More recently, pooled analysis of survival among patients with advanced melanoma treated with ipilimumab monotherapy in either phase II or phase III trials demonstrated a 3-year survival rate of 22% which is further stratified as 20% for pretreated patients and 26% for treatment-naïve patients; OS with ipilimumab seems to reach a plateau at 3 years which extends to ten years [Schadendorf et al. 2013].
The main focus of immunotherapy for melanoma has now shifted to a molecule called programmed cell death 1 (PD-1). PD-1 is an inhibitory cell receptor protein that negatively regulates T lymphocyte activation and their effector mechanisms, consequently inhibiting the immune response against cancer cells [Blank et al. 2004; Freeman et al. 2000]. Its ligands, PD-L1 and PD-L2, are not only expressed on the cell surface of antigen-presenting cells but on the surface of cancer cells too [Latchman et al. 2001; Topalian et al. 2014]. Targeting the PD-1/PD-L1/PD-L2 axis with antibodies against PD-1 such as nivolumab (MDX-1106; BMS 936558; ONO-4538) or lambrolizumab (MK-3475) has offered results that are more promising than anything observed with melanoma immunotherapy in early phase clinical trials before (Figure 1, Table 1). A 31% objective response rate was reported in advanced melanoma patients treated in a phase I/II trial of nivolumab, with an estimated median response duration of 2 years [Topalian et al. 2012]. A 22% incidence of grade 3/4 adverse events occurred amongst 107 patients with melanoma whereas interestingly all the drug-related mortalities (1%) were observed in the nonmelanoma cohorts of the wider trial; two NSCLC patients and one colorectal cancer patient – all attributed to immune-related pneumonitis [ClinicalTrials.gov identifier: NCT01721772]. A phase III study with nivolumab versus dacarbazine as first-line treatment in melanoma is underway [Hamid et al. 2013]. Similarly, early phase results with lambrolizumab with varying dosing schedules in 135 patients with advanced melanoma, including patients pretreated with ipilimumab, showed RR as high as 52% [Brahme et al. 2012]. This striking result has led the US FDA to designate it as a ‘breakthrough therapy’ prioritised for expedited development.
Moreover, PD-L1 blockade with BMS-936559, a PD-L1 specific, immunoglobulin G4 (IgG40 monoclonal antibody achieved up to 29% response rates and disease stability at 24 weeks for 27% of 52 patients with advanced melanoma participating in a phase I trial. At the same time, severe immune-related events frequently noted with CTLA-4 inhibition was relatively infrequent with anti-PD-L1 blockade [Wolchok et al. 2013].
Combination of CTLA-4 and PD-1 blockade was tested in a phase I study where ipilimumab and nivolumab were administered either concurrently or sequentially. Both regimens showed promising clinical activity, but more interestingly, the concurrent treatment achieved deep tumour regressions of more than 80% in 53% of patients who received the highest acceptable dose [Pardoll, 2012].
CTLA-4 and PD-1 are undoubtedly the ‘godfathers’ of immune checkpoints but a plethora of costimulatory (ICOS, CD137, OX-40) and co-inhibitory (BTLA, LAG3, TIM3) molecules have now been identified. Some of them are still in preclinical development, whereas others have already entered early phase clinical trials in cancer immunotherapy [Ribas et al. 2013]. Nevertheless, the activity of PD-1/PD-L1 blockade across a variety of tumour types, previously thought to be nonimmunogenic, will most likely usher in a new paradigm in cancer treatment altogether.
How immunotherapy fits in to the treatment plan for a new patient with metastatic melanoma remains open to question. Currently, second-line ipilimumab represents the main immunotherapy option in Europe available to all patients in the clinic, a context which allows for first-line administration of molecularly targeted treatment (e.g. vemurafenib) in BrafV600 positive tumours, or chemotherapy in BrafV600 negative tumours. The safety of combining immunotherapy with molecularly targeted agents is still being tested in early phase clinical trials, although concerns about high occurrence of grade 3 transaminitis have already been raised. Ribas and colleagues recorded high rates of grade 3 transaminase elevation even in patients who were treated with a ‘lead-in’ period of vemurafenib before the administration of ipilimumab [Ribas et al. 2013]; when the drugs were given concurrently, transaminitis could occur as fast as within 2 weeks of initiation of drugs [Pozanov et al. 2014]. This phenomenon of hepatotoxicity was not observed when vemurafenib was substituted by dabrafenib, even with the addition of tramenitib (ipilimumab + dabrafenib = trametinib), according to early data reported by Puzanov and colleagues, suggesting that a different class of BRAF inhibitors might be better tolerated in combination with ipilimumab [Ascierto et al. 2012].
How clinicians might choose between these two approaches will of course depend on the data produced, although it seems logical to conclude that for patients with mutated BrafV600 melanoma, treatment could be dictated by the disease burden. Patients who are asymptomatic, with low volume or indolent disease and absent poor prognostic factors (such as elevated LDH), could possible derive more benefit from receiving upfront immunotherapy which characteristically offers a late onset and more durable response, with BRAF inhibition reserved in case of clinical/radiological progression. On the other hand, patients with bulky or aggressive disease would be better served by the rapidly induced effects of a BRAF inhibitor as first-line treatment [Leyvraz and Keilholz, 2012].
What does the future hold?
A number of important topics are likely to shape the management of melanoma (and, as a consequence, other cancers) in the years to come. Anticipated advances with immunotherapy and NRAS mutant melanoma have already been described in the sections above.
Uveal melanoma (Figure 2)
Melanoma is the most common type of ocular cancer and is associated with high rates of liver metastases [van Raamsdonk et al. 2009]. This cancer is often driven through upregulation of the MAPK pathway although, unlike cutaneous melanoma, this process is almost never triggered by mutations of BRAF or NRAS. Pioneering work by Bastian and colleagues has now shown that mutations of two G proteins, GNAQ and GNA11, will drive the MAPK pathway in the majority of ocular melanoma cases [van Raamsdonk et al. 2010; McWilliams et al. 2008]. This adds to the detail we already know (described above and in Figure 2) on the various driver mutations implicated in cutaneous and mucosal melanoma. As is the case with NrasQ61-driven tumours, one would anticipate that the new generation of MEK inhibitors may offer a rational, biologically considered, treatment option in ocular melanoma. In the longer term, it would be surprising if a novel generation of ‘orphan’ drugs targeting mutant Gnaq/Gna11 were not developed.
Targeting brain metastases
Brain metastases are common in end-stage melanoma, a problem which is associated with aggressive disease and which confers a life expectancy measured in months. As is the case with chemotherapy, melanoma is mostly resistant to radiotherapy, traditionally the main treatment modality offered for this problem [Falchook et al. 2012b]. Patients with melanoma brain metastases have consequently been almost universally excluded from clinical trial eligibility, or at least been heavily restricted in their access (e.g. stable disease and stable dose of steroids for a period of time). However, an emerging picture of small molecule and immunotherapy efficacy against melanoma brain metastases suggests that this position is becoming untenable [Di Giacomo et al. 2012; Long et al. 2012; Margolin et al. 2012; Cancer Research UK, 2013b]. More brain metastasis specific clinical trials are necessary if the eligibility criteria for these patients are not to be relaxed.
Approaching the new patient
Molecular advances such as the ones described above have altered the nature of new patient consultations in the clinic. Previously a new patient could begin empirical chemotherapy almost immediately, whereas now they are often asked to ‘sit tight’ and wait for their genetic test results to come back. In this circumstance, of course there is no guarantee that the result will be positive, or that they won’t end up on chemotherapy a few weeks later than they might have initially. This can be a difficult and anxious wait for patients who will often want to begin treatment as soon as possible. It could be a justifiable wait given the relative merits of novel small molecules inhibitors compared with chemotherapy. Wherever possible, although there is no positive adjuvant data with small molecule inhibitors in melanoma as yet, it seems sensible to aim for testing of relevant mutations (e.g. BRAF, NRAS, KIT) after primary melanomas have been curatively excised. This will save time later on for the unfortunate few who develop recurrent metastatic disease. An example of such an initiative is the Cancer Research UK Stratified Medicine Programme [Cancer Research UK, 2013b]. Routine postresection computerized tomography (CT) scans in early stage melanoma patients may also become important given the developing portfolio of metastatic treatment options: often there is concern that rapid clinical deterioration of patients, when metastases are left to be diagnosed by clinical presentation alone, may mean that the treatment window is missed.
Lessons for other cancers
Progress with melanoma has created a fresh impetus for other cancers to re-focus their efforts in generating rational molecularly driven trials. A clear example of progress in other cancers is with metastatic NSCLC, where EGFR inhibition has replaced first-line chemotherapy when patients are predictively selected for the presence of an EGFR mutation [Zhou et al. 2011; Rosell et al. 2012; Thatcher et al. 2005]. This treatment was transformed as a consequence, having spent 10 years considered as a second-line treatment with modest benefits in unselected NSCLC patients [Shepherd et al. 2005]. Compared with most other cancers, an unexpected advantage from the outset with molecularly targeted treatments in melanoma was that there was no significant standard of care to replace. The example of NSCLC suggests the inertia that can be involved in replacing historical chemotherapy with successful (and often expensive) biologically targeted drugs.
In most incidences of cancer, opportunities with these novel drugs are still all too rare, but a key and unavoidable challenge for the future will be developing bold clinical trials where new targeted drugs are compared with the traditional option of empirical chemotherapy on carefully selected cohort of patients using validated molecular biomarkers. This is a ‘leap’ that may sometimes be difficult to square when a patient is sitting in front of their oncologist in the clinic, but the progress seen in melanoma so far would suggest that the long-term gains from such an approach could be exponential.
Acknowledgments
‘Blinded by the light’, the title of this review reflects to the first song on the first album recorded by Bruce Springsteen, later made famous when covered by Manfred Mann. Springsteen’s long-term organ player, Danny Federici, passed away with melanoma in 2008. The Danny Federici Melanoma Fund has been set up in his honour, funding research into melanoma progress in the future.
Footnotes
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Contributor Information
Colin R. Lindsay, Beatson West of Scotland Cancer Centre, Glasgow, UK
Pavlina Spiliopoulou, Medical Oncology, Beatson West of Scotland Cancer Centre, 1053 Great Western Road, Glasgow G12 0YN, UK.
Ashita Waterston, Beatson West of Scotland Cancer Centre, Glasgow, UK.
References
- Ascierto P., Schadendorf D., Berking C., Agarwala S., van Herpen C., Queirolo P., et al. (2013) MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol 14: 249–256. [DOI] [PubMed] [Google Scholar]
- Ascierto P., Simeone E., Giannarelli D., Grimaldi A., Romano A., Mozzillo N. (2012) Sequencing of BRAF inhibitors and ipilimumab in patients with metastatic melanoma: a possible algorithm for clinical use. J Transl Med 10: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins M., Lotze M., Dutcher J., Fisher R., Weiss G., Margolin K., et al. (1999) High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 17: 2105–2116. [DOI] [PubMed] [Google Scholar]
- Balch C., Gershenwald J., Soong S., Thompson J., Atkins M., Byrd D., et al. (2009) Final version of 2009 AJCC Melanoma Staging and Classification. J Clin Oncol 27: 6199–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian B.C., Le Boit P.E., Pinkel D. (2000) Mutations and copy number increase of HRAS in spitz nevi with histopathological features. Am JPathol 157: 967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D. (2011) Adaptive clinical trials in oncology. Nature Rev Clin Oncol 9: 199–207. [DOI] [PubMed] [Google Scholar]
- Blank C., Brown I., Peterson A., Spiotto M., Iwai Y., Honjo T., et al. (2004) PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res 64: 1140–1145. [DOI] [PubMed] [Google Scholar]
- Blanke C., Rankin C., Demetri G., Ryan C., von Mehren M., Benjamin R., et al. (2008) Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 26: 626–632. [DOI] [PubMed] [Google Scholar]
- Boissy R., Nordlund J. (1997) Molecular basis of congenital hypopigmentary disorders in humans: a review. Pigment Cell Res 10: 12–24. [DOI] [PubMed] [Google Scholar]
- Brahme J., Tykodi S., Chow L., Hwu W., Topalian S., Hwu P., et al. (2012) Safety and Activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med 366: 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito F., Kos L. (2008) Timeline and distribution of melanocyte precursors in the mouse heart. Pigment Cell Melanoma Res 21: 464–470. [DOI] [PubMed] [Google Scholar]
- Cancer Research UK (2013a) Skin cancer incidence statistics. Available at www.cancerresearchuk.org/cancer-info/cancerstats/types/skin/incidence/(accessed 1 August 2014).
- Cancer Research UK (2013b) Stratified medicine programme. Available at: http://www.cancerresearchuk.org/science/research/how-we-deliver-our-research/others/by-programme/stratified-medicine-programme/). (accessed 1 August 2014).
- Chapman P., Hauschild A., Robert C., Haanen J., Ascierto P., Larkin J., et al. (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364: 2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarion-Sileni V., Guida M., Ridolfi L., Romanini A., Del Bianco P., Pigozzo J., et al. (2011) Central nervous system failure in melanoma patients: results of a randomised, multicentre phase 3 study of temozolomide and dacarbazine- based regimens. Br J Cancer 104: 1816–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C., Zavala-Pompa A., Sequeira J., Shoji M., Sexton D., Cotsonis G., et al. (2002) Mitogen-actived protein kinase activation is an early event in melanoma progression. Clin Cancer Res 8: 3728–3733. [PubMed] [Google Scholar]
- Corsetti R., Allen H., Wanebo H. (2000) Thin < or = 1 mm level III and IV melanomas are higher risk lesions for regional failure and warrant sentinel lymph node biopsy. Ann Surg Oncol 7: 456–460. [DOI] [PubMed] [Google Scholar]
- Curtin J.A., Busam K., Pinkel D., Bastian B.C. (2006) Somatic activation of KIT in distinct subtypes of melanoma. Clin Oncol. 24: 4340–6. [DOI] [PubMed] [Google Scholar]
- Davies H., Bignell G., Cox C., Stephens P., Edkins S., Clegg S., et al. (2002) Mutations of the BRAF gene in human cancer. Nature 417: 949–954. [DOI] [PubMed] [Google Scholar]
- De Vries E., Coebergh J. (2005) Melanoma incidence has risen in Europe. BMJ 331: 698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dematteo R., Ballman K., Antonescu C., Maki R., Pisters P., Demetri G., et al. (2009) Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 373: 1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetri G., von Mehren M., Blanke C., van den Abbeele A., Eisenberg B., Roberts P., et al. (2002) Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347: 472–480. [DOI] [PubMed] [Google Scholar]
- Dhomen N., Reis-Filho J., da Rocha Dias S., Hayward R., Savage K., Delmas V., et al. (2009) Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell 15: 294–303. [DOI] [PubMed] [Google Scholar]
- Di Giacomo A., Ascierto P., Pilla L., Santinami M., Ferrucci P., Giannarelli D., et al. (2012) Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-lab8el, single-arm phase 2 trial. Lancet Oncol 13: 879–886. [DOI] [PubMed] [Google Scholar]
- Eisen T., Ahmad T., Flaherty K., Gore M., Kaye S., Marais R., et al. (2006) Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer 95: 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery C., Vijayendran K., Zipser M., Sawyer A., Niu L., Kim J., et al. (2009) MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci U S A 106: 20411–20416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchook G., Lewis K., Infante J., Gordon M., Vogelzang N., DeMarini D., et al. (2012a) Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol 13: 782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchook G., Long G., Kurzrock R., Kim K., Arkenau T., Brown M., et al. (2012b) Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet 379: 1893–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty K., Infante J., Daud A., Gonzalez R., Kefford R., Sosman J., et al. (2012a) Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 367: 1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty K., Puzanov I., Kim K., Ribas A., McArthur G., Sosman J., et al. (2010) Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 363: 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty K., Robert C., Hersey P., Nathan P., Garbe C., Milhem M., et al. (2012b) Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 367: 107–114. [DOI] [PubMed] [Google Scholar]
- Freeman G., Long A., Iwai Y., Bourque K., Chernova T., Nishimura H., et al. (2000) Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp. Med 192: 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka M., Wu Y., Thongprasert S., Sunpaweravong P., Leong S., Sriuranpong V., et al. (2011) Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 29: 2866–2874. [DOI] [PubMed] [Google Scholar]
- Goel V., Lazar A., Warneke C., Redston M., Haluska F. (2006) Examination of mutations in BRAF, NRAS, and PTEN in primary cutaneous melanoma. J Invest Dermatol 126: 154–160. [DOI] [PubMed] [Google Scholar]
- Gopal Y., Deng W., Woodman S., Komurov K., Ram P., Smith P., et al. (2010) Basal and treatment-induced activation of AKT mediates resistance to cell death by AZD6244 (ARRY-142886) in Braf-mutant human cutaneous melanoma cells. Cancer Res 70: 8736–8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Schopfer V., Wellbrock C., Marais R. (2007) Melanoma biology and new targeted therapy. Nature 445: 851–857. [DOI] [PubMed] [Google Scholar]
- Guo J., Si L., Kong Y., Flaherty K., Xu X., Zhu Y., et al. (2011) Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol 29: 2904–2909. [DOI] [PubMed] [Google Scholar]
- Gupta P., Kuperwasser C., Brunet J., Ramaswamy S., Kuo W., Gray J., et al. (2005) The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nat Genet 37: 1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid O., Robert C., Daud A., Hodi F., Hwu W., Kefford R., et al. (2013) Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 369: 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzivassiliou G., Song K., Yen I., Brandhuber B., Anderson D., Alvarado R., et al. (2010) RAF inhibitors prime wild-type RAF to activate theMAPK pathway and enhance growth. Nature 464: 431–435. [DOI] [PubMed] [Google Scholar]
- Hauschild A., Agarwala S., Trefzer U., Hogg D., Robert C., Hersey P., et al. (2009) Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol 27: 2823–2830. [DOI] [PubMed] [Google Scholar]
- Hauschild A., Grob J., Demidov L., Jouary T., Gutzmer R., Millward M., et al. (2012) Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 380: 358–365. [DOI] [PubMed] [Google Scholar]
- Hauschild A., Grob J., Demidov L., Jouary T., Gutzmer R., Millward M., et al. (2013) An update on BREAK-3, a phase III, randomized trial: Dabrafenib (DAB) versus dacarbazine (DTIC) in patients with BRAF V600E-positive mutation metastatic melanoma (MM). J Clin Oncol 31 (Suppl.): abstract 9013. [Google Scholar]
- Heidorn S., Milagre C., Whittaker S., Nourry A., Niculescu-Duvas I., Dhomen N., et al. (2010) Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 140: 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocker T., Tsao H. (2007) Ultraviolet radiation and melanoma: A systematic review and analysis of reported sequence variants. Hum Mutat 28: 578–588. [DOI] [PubMed] [Google Scholar]
- Hodi F., O’Day S., McDermott D., Weber R., Sosman J., Haanen J., et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante J., Fecher L., Falchook G., Nallapareddy S., Gordon M., Becerra C., et al. (2012) Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol 13: 773–781. [DOI] [PubMed] [Google Scholar]
- Jilaveanu L., Aziz S., Kluger H. (2009) Chemotherapy and biologic therapies for melanoma: do they work? Clin Dermatol 27: 614–625. [DOI] [PubMed] [Google Scholar]
- Joensuu H., Eriksson M., Sundby Hall K., Hartmann J., Pink D., Schutte J., et al. (2012) One versus three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 307: 1265–1272. [DOI] [PubMed] [Google Scholar]
- Johannessen C., Boehm J., Kim S., Thomas S., Wardwell L., Johnson L., et al. (2010) COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 468: 968–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Eton O., Davis D., Frazier M., McConkey D., Diwan A., et al. (2008) Phase II trial of imatinib mesylate in patients with metastatic melanoma. Br J Cancer 99: 734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J., Ascierto P., Dréno B., Atkinson V., Liszkay G., Maio M., et al. (2014) Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 371: 1867–1876. [DOI] [PubMed] [Google Scholar]
- Latchman Y., Wood C., Chernova T., Chaudhary D., Borde M., Chernova I., et al. (2001) PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2: 261–268. [DOI] [PubMed] [Google Scholar]
- Leyvraz S., Keilholz U. (2012) Ocular melanoma: what’s new? Curr Opin Oncol 24:162–169. [DOI] [PubMed] [Google Scholar]
- Linette G. (2012) Cancer of the skin and melanoma. In: Govindan R. (ed.) DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology Review, 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins, pp. 375–382. [Google Scholar]
- Long G., Trefzer U., Davies M., Kefford R., Ascierto P., Chapman P., et al. (2012) Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol 13: 1087–1095. [DOI] [PubMed] [Google Scholar]
- Margolin K., Ernstoff M., Hamid O., Lawrence D., McDermott D., Puzanov I., et al. (2012) Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 13: 459–465. [DOI] [PubMed] [Google Scholar]
- McWilliams R., Rao R., Buckner J., Link M., Markovic S., Brown P. (2008) Melanoma-induced brain metastases. Expert Rev Anticancer Ther 8: 743–755. [DOI] [PubMed] [Google Scholar]
- Nazarian R., Shi H., Wang Q., Kong X., Koya R., Lee H., et al. (2010) Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 468: 973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien S., Guilhot F., Larson R., Gathmann I., Baccarani M., Cervantes F., et al. (2003) Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 348: 994–1004. [DOI] [PubMed] [Google Scholar]
- Ott P., Hamilton A., Min C., Safarzadeh-Amiri S., Goldberg L., Yoon J., et al. (2010) A phase II trial of sorafenib in metastatic melanoma with tissue correlates. PLoS One 5: e15588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D. (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12: 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos P., Persaud Y., Janakiraman M., Kong X., Ng C., Moriceau G., et al. (2011) RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature 480: 387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzanov I., Callahan M., Linette G., Pradyuman Patel S., Luke J., et al. (2014) Phase 1 study of the BRAF inhibitor dabrafenib (D) with or without the MEK inhibitor trametinib (T) in combination with ipilimumab (Ipi) for V600E/K mutation–positive unresectable or metastatic melanoma (MM). J Clin Oncol 32: (Suppl.): abstract 2511. [Google Scholar]
- Rajagopalan H., Bardelli A., Lengauer C., Kinzler K., Vogelstein B., Velculescu V. (2002) Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature 418: 934. [DOI] [PubMed] [Google Scholar]
- Rao R., Holtan S., Ingle J., Croghan G., Kottschade L., Creagan E., et al. (2006) Combination of paclitaxel and carboplatin as second-line therapy for patients with metastatic melanoma. Cancer 106: 375–382. [DOI] [PubMed] [Google Scholar]
- Ribas A., Hodi F., Callahan M., Konto C., Wolchok J. (2013) Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med 368: 1365–1366. [DOI] [PubMed] [Google Scholar]
- Robert C., Karaszewska B., Schachter J., Rutkwoski P., Mackiewicz A., Stroyakovskiy D., et al. Results of the COMBI-v randomised, open-label, phase III study in the first-line treatment of patients with unresectable or metastatic cutaneous melanoma. Available at http://www.esmo.org/Conferences/ESMO-2014-Congress/News-Articles/Combination-Dabrafenib-Trametinib-Improves-OS-in-Comparison-with-Vemurafenib-in-Patients-With-BRAF-Mutation-Positive-Melanoma (accessed 26 October 2014).
- Robert C., Thomas L., Bondarenko I., O’Day S., Weber J., Garbe C., et al. (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. New Engl J Med 364: 2517–2526. [DOI] [PubMed] [Google Scholar]
- Rosell R., Carcereny E., Gervais R., Vergnenegre A., Massuti B., Felip E., et al. (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13: 239–246. [DOI] [PubMed] [Google Scholar]
- Rosenberg S., Lotze M., Yang J., Topalian S., Chang A., Schwartzentruber D., et al. (1993) Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J Natl Cancer Inst 85: 622–632. [DOI] [PubMed] [Google Scholar]
- Schadendorf D., Hodi F., Robert C., Weber J., Margolin K., Hamid O., et al. (2013) Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in metastatic or locally advanced, unresectable melanoma. Presented at European Cancer Congress 2013 Available at http://2013.europeancancercongress.org/Scientific-Programme/Abstract-search.aspx?abstractid=8988 (accessed 1 November 2014). [Google Scholar]
- Shao Y., Aplin A. (2010) Akt3-mediated resistance to apoptosis in B-RAF-targeted melanoma cells. Cancer Res 70: 6670–6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd F., Rodrigues Pereira J., Ciuleanu T., Tan E., Hirsh V., Thongprasert S., et al. (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353: 123–132. [DOI] [PubMed] [Google Scholar]
- Soengas M., Lowe S. (2003) Apoptosisand melanoma chemoresistance. Oncogene 22: 3138–3151. [DOI] [PubMed] [Google Scholar]
- Sosman J., Kittaneh M., Lolkema M., Postow M., Schwartz G., Franklin C., et al. (2014) A phase 1b/2 study of LEE011 in combination with binimetinib (MEK162) in patients with NRAS-mutant melanoma: early encouraging clinical activity. J Clin Oncol 32 (Suppl.): abstract 9009. [Google Scholar]
- Stevens M., Hickman J., Langdon S., Chubb D., Vickers L., Stone R., et al. (1987) Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl- imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M & B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res 47: 5846–5852. [PubMed] [Google Scholar]
- Su F., Viros A., Milagre C., Trunzer K., Bollag G., Spleiss O., et al. (2012) RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med 366: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarhini A., Agarwala S. (2006) Cutaneous melanoma: available therapy for metastatic disease. Dermatol Ther 19: 19–25. [DOI] [PubMed] [Google Scholar]
- Temple R. (2005) Enrichment designs: efficiency in development of cancer treatments. J Clin Oncol 23: 4838–4839. [DOI] [PubMed] [Google Scholar]
- Terzian T., Torchia E., Dai D., Robinson S., Murao K., Stiegmann R., et al. (2010) P53 prevents progression of nevi to melanoma predominantly through cell cycle regulation. Pigment Cell Melanoma Res 23: 781–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher N., Chang A., Parikh P., Rodrigues Pereira J., Ciuleanu T., von Pawel J., et al. (2005) Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 366: 1527–1537. [DOI] [PubMed] [Google Scholar]
- Topalian S., Hodi F., Brahmer J., Gettinger S., Smith D., McDermott D., et al. (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366: 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S., Sznol M., McDermott D., Kluger H., Carvajal R., Sharfman W., et al. (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32: 1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurel S., Hildenbrand R., Zimpfer A., La Rosee P., Paschka P., Sucker A., et al. (2005) Lack of clinical efficacy of imatinib in metastatic melanoma. Br J Cancer 92: 1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk C., Bezrookove V., Green G., Bauer J., Gaugler L., O’Brien J., et al. (2009) Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 457: 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk C., Griewank K., Crosby M., Garrido M., Vemula S., Wiesner T., et al. (2010) Mutations in GNA11 in uveal melanoma. N Engl J Med 363: 2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij J., Casali P., Zalcberg J., LeCesne A., Reichardt P., Blay J., et al. (2004) Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 364: 1127–1134. [DOI] [PubMed] [Google Scholar]
- Walker G.J., Soyer H.P., Handoko H.Y., Ferguson B., Kunisada T., Khosrotehrani K., et al. (2011) Superficial spreading-like melanoma in Arf(-/-)::Tyr-Nras(Q61K)::K14-Kitl Mice: Keratinocyte kit ligand expression sufficient to “translocate” melanomas from dermis to epidermis. J Invest Dermatol 131: 1384–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolchok J. (2012) How recent advances in immunotherapy are changing the standard of care for patients with metastatic melanoma. Ann Oncol 23(Suppl. 8): viii15–viii21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolchok J., Kluger H., Callahan M., Postow M., Rizvi N., Lesokhin A., et al. (2013) Nivolumab plus Ipilimumab in advanced melanoma. N Engl J Med 369: 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2013) Skin cancers. Available at http://www.who.int/uv/faq/skincancer/en/index1.html (accessed 01 August 2014).
- Wyman K., Atkins M., Prieto V., Eton O., McDermott D., Hubbard F., et al. (2006) Multicenter phase II trial of high-dose imatinib mesylate in metastatic melanoma: significant toxicity with no clinical efficacy. Cancer 106: 2005–2011. [DOI] [PubMed] [Google Scholar]
- Zhou C., Wu Y., Chen G., Feng J., Liu X., Wang C., et al. (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12: 735–742. [DOI] [PubMed] [Google Scholar]