Abstract
Objectives
Over the past three decades, twin studies have shown variation in the heritability of obesity. This study examined the difference of body mass index (BMI) heritability (BMI-H) by population characteristics, such as sex, age, time period of observation, and average BMI, as well as by broad social-environmental factors as indicated by country-level gross domestic product (GDP) per capita and GDP growth rate.
Methods
Twin studies that reported BMI-H and were published in English from Jan 1990 to Feb 2011 after excluding those with disease, special occupations or combined heritability estimates for country/ethnic groups were searched in PubMed. 32 studies were identified from Finland (7), the UK (6), the US (3), Denmark (3), China (3), Netherlands (2), South Korea (2), Sweden (2) and four from other countries. Meta regression models with random effect were used to access variation in BMI-H.
Results
Heterogeneity of BMI-H is significantly attributable to variations in age (<20yr, 20–55yr & ≥56yr), time period of observation (i.e., year of data collection), average BMI, and GDP (≤$20,000, $20,001–26,000 & >$26,000). BMI-H was higher in adolescents (<20yr), in studies done in past years, and in populations with higher average BMIs or higher GDP per capita (≥$26,000) than their counterparts. Consistent lowering effects of high GDP growth rate (>median) on BMI-H were shown through stratified analyses by GDP. BMI-H was lower in countries of mid-level GDP, particularly those experiencing rapid economic growth.
Conclusions
BMI-H is sensitive to age, time period of observation, average BMI, GDP, and rapid economic growth.
Keywords: Heritability, body mass index, obesity, GDP, environment, twin study
Introduction
While genetic epidemiologic studies show that genetic factors are an important determinate of obesity in twin and adoption studies, numerous other population-based studies show that social-environmental factors are key drivers of the global obesity epidemic1. However, the relative contributions of genetic and social-environmental factors to obesity remain controversial. Studies on single-nucleotide polymorphisms (SNPs) and their interactions with selective environmental factors shed limited insight on this question. Although 68 SNPs (FTO, MC4R, etc.) have been significantly linked to adult BMI, waist circumference and waist to hip ratio and their additional connection of age at menarche have been recently discovered2, they explain only a small proportion of genetic variance in body mass index (BMI) (e.g., two common variants in FTO and MC4R account for less than 2% of the variance in adult BMI)3. Less physical activity and high fat and carbohydrate intakes have also co- contributed with these genes, indicating accelerated risks of obesity with variant genotype4, 5.
Twin studies provide a unique method for separating the phenotypic impacts of “nurture”-environmental variations from those of “nature”-genetic variations through the comparison of monozygotic (MZ) twins and dizygotic (DZ) twins. Heritability, the proportion of phenotypic variation in a population attributable to genetic variation among individuals, provides estimates of the relative contributions of differences in genetic vs. non-genetic factors. Genetic variance is an aggregate of the effect of all genetic loci6.
Similar degree of heritability may imply differing influences of genetic variance dependent on the within- population heterogeneity of environmental determinants of traits in question. Recent studies have alerted the importance of the relative contribution of environmental factors to obesity since changes in the population gene pool are too slow to explain the increasing obesity epidemic in recent decades. In fact, the risk of assorted marriages between high BMI (>95th BMI percentile) couples increased from 1.39 (1.10–1.81) to 2.39 (1.85–3.09) in three decades, which may lead the offspring’s predisposition to obesity7. Some studies have reported that dietary intake and physical activity might modify the heritability of obesity in children and adults8, 9. This implies that the wide range of BMI-H (e.g., 45–85%) in recent twin studies10 may result from different characteristics in study populations. For example, age distribution of study sample is likely to cause variation in BMI-H due to the activation of certain genes for physical growth, which occurs at younger ages11. Nutrition transitions occurred by economic growth have introduced more obesogenic environments12 and resulting in large variation within a population’s lifestyle behaviors (e.g., regarding diet, transportation, and work practices) 13, 14.
Few studies have assessed whether obesity heritability may vary by population and social-environmental characteristics. Previous research has only partly examined this question (e.g., only studied young age groups) or provided inconclusive results (e.g., given incomparable characteristics within family, adoption, and twin studies)10, 15, 16. This systematic review and meta-analysis aimed to answer whether and how obesity heritability might vary by population characteristics (e.g., sex, age, and average BMI) and environmental conditions (e.g., social-environmental factors such as GDP, GDP growth rate, and time period of observation).
Materials and Methods
We searched PubMed for studies with twin subjects that reported BMI-H. Information on variables of interest (e.g., data collection methods, study population characteristics, social-environmental factors, and BMI-H estimates) was abstracted using a standardized form (Table 1). To reflect the obesogenic conditions of study population at the time of investigation, average BMIs of the study population (reported in the articles) were used since they represent the level of environmental obesogenicity at the time and place of investigation more accurately than the concurrent obesity prevalence of country in general. GDP per capita and annual GDP growth rate were obtained from the Organisation for Economic Cooperation and Development (OECD) website for the corresponding year of data collection of each study so as to examine the effects of country’s economic status on BMI-H. This is because the prosperity of a country has been shown to be significantly associated with the level of BMI in a previous study1. Countries experiencing rapid economic growth (e.g., as reflected by the annual GDP growth rate) are likely to have widely ranging lifestyles due to influences of the burgeoning economy. Accompanying cultural shifts, such as in diet, transportation, and the work place, etc., result and create both mixtures and fusions of the new with the traditional as these countries did not have enough time to adjust their changes13, 14.
Table 1.
Summary of the main characteristics and findings of 32 identified twin studies regarding BMI heritability
| No | Reference | Country/ethnic group |
Data level | Year(s) of data collectio n |
sex | N (pairs) | Key analytic al method s† |
Mean age (yr; range) |
BMI (kg/m2; SD) |
BMI- Heritability (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 47 | Korkeila et al. 1991 | Finland/Finnish | nationwide | 1975 | M, F | MZ: 2372, DZ: 4873 | AE model | 18–54 | M: 24.0 (2.6); F: 22.6 (2.9) | M: 73, F: 68 |
| 50 | Turula et al. 1990 | Finland/Finnish | nationwide | 1975 | M, F | MZ & DZ: 7245 | ACE model | 22 (18–54) | M: 23.8; F: 21.5 | M: 72, F: 66 |
| 39 | Pietilainen et al. 1999 | Finland/Finnish | nationwide | 1993 | M, F | MZ: 692, DZ: 1419 | AE model | 16, 17 | 20.4 (2.4); 20.8 (2.5) | M: 81, F: 87; M: 86 F: 85 |
| 38 | Lehtovirta et al. 2000 | Finland/Finnish | nationwide | 1997 | M, F | MZ: 21, DZ: 20 | AE model | 62 (54–72) | 26.4 (3.0) | 54 |
| 9 | Mustelin et al. 2009 | Finland/Finnish | nationwide | 2001 | M, F | MZ: 696, DZ: 1396 | AE model | 25 (22–27) | 23.1 (3.3) | M: 79, F:78 |
| 33 | Lajunen et al. 2009 | Finland/Finnish | nationwide | 3 times f/u; 1997, 1999, 2001 | M, F | MZ: 2413 | ACE model | 11–12, 14, 17 | 17.7 (2.6); 19.4 (2.8); 21.5 (2.9) | M: 69, F: 58; M: 66, F: 58; M: 83, F: 74 |
| 45 | Alonso et al. 2009 | Finland/Finnish | nationwide | 5 times f/u; 1975, 1981, 1990, 2001, 2004 | F | MZ: 102, DZ: 114 | AE model | 43, 49, 58, 69, 72 (63–76) | 23.9; 24.6; 25.7; 28.0; 28.2 | 54; 58; 61; 68; 72 |
| 48 | Hewitt et al. 1991 | Birmingham, UK/British | community - based | 1988 | M | MZ: 40, DZ: 40 | path analysis of genes, individual environments, age | 19 (3) | 21.5 (3.2) | 87 |
| 37 | Baird et al. 2001 | Birmingham, UK/British | community - based | 1996 | M, F | MZ: 58, DZ: 140 | ADE model | 44 (1) | 25.8 (4.0) | 77 |
| 34 | Wallace et al. 2004 | Scotland, UK/Scottish | community - based | 2000 | F | MZ: 54, DZ: 39 | DE model (χ2- goodness of fit statistics) | 56 (46–65) | 23.6 (21.0–27.5) | 71 |
| 6 | Manek. Et al. 2003 | London, UK/British | community - based | 2000 | F | MZ: 261, DZ: 524 | ACE model | 54 (8) | 24.8 (4.3) | 55 |
| 30 | Beardsall et al. 2009 | Cambridge, UK/British | community - based | 2001 | M, F | MZ: 88, DZ: 98 | ACE model | 9 (7–11) | 16.5 (2.3) | 72 |
| 51 | Haworth et al. 2008 | UK/British | nationwide | 4 times f/u; 1999, 2002, 2005, 2006 | M, F | MZ: 1422, DZ: 2160 | ACE model | 4, 7, 10, 11 | 15.8 (2.0); 15.7 (2.0); 17.2 (3.0); 17.8 (3.1) | 49; 66; 82; 77 |
| 46 | Allison et al. 1994 | Georgia-Kentucky-Indiana, US/Black, white | community - based | 1965 | M, F | MZ: 108, DZ: 130 | AE models | 15 (12–18) | 20.1 | white: 89; black: 90 |
| 43 | Carmichael et al. 1995 | Minnesota, US | community - based | 1994 | M, F | MZ: 586, DZ: 447 | AE model | 32 (18–38), 48 (39–59), 67 (60–81) | 23.4 (3.5); 25.2 (3.8); 25.8 (4.3) | 82; 70; 63 |
| 41 | Austin et al. 1997 | Oakland, US | community - based | 2 times f/u; 1979, 1990 | F | MZ: 185, DZ: 130 | AE model | 41 (18–85), 51 (30–91) | 23.2 (4.6) | 80; 79 |
| 42 | Herskind et al. 1996 | Denmark/Danish | nationwide | 1966 | M, F | MZ: 493, DZ: 740 | AE model | 46–59, 60–76 | 25.1 (3.4); 25.9 (3.6) | M: 46, F: 77; M:61, F: 75 |
| 19 | Poulsen et al. 2001 | Denmark/Danish | nationwide | 1998 | M, F | MZ: 125, DZ: 178 | 2(rMZ-rDZ) | 67 (55–74) | N/A | all: 80; M: 58; F: 90 |
| 32 | Malis et al. 2005 | Denmark/Danish | nationwide | 2003 | M, F | MZ: 108, DZ: 88 | DE for young, AE for old | 28 (25–32), 62 (58–66) | 24.1 (3.1); 26.1 (4.8) | 85; 77 |
| 53 | Ouyang et al. 2010 | China/Chinese | community - based | 2002 | M, F | MZ: 407, DZ: 300 | ACE model | 17 (13–20) | 19.4 (2.0) | M: 86, F: 48 |
| 54 | Lee et al. 2010 | China/Chinese | community - based | 2003 | M, F | MZ: 779, DZ: 481 | ACE model | M: 39 (11); F: 38 (9) | 22.8 (3.2) | 61 |
| 55 | Wu et al. 2010 | China/Chinese | community - based | 2001 | M, F | MZ: 1243, DZ: 833 | ACE model | 38 (19–81) | 23.0 (3.2) | 74 |
| 31 | Burk et al. 2006 | Netherlands/Dutch | nationwide | 1992 | M, F | MZ: 1039, DZ: 1957 | ACE model | 5 | 15.0 (1.5) | M: 34; F: 74 |
| 18 | Silventoinen et al. 2007 | Netherlands/Dutch | nationwide | 1996 | M, F | MZ: 2615, DZ: 5140 at age 3, 6 times f/u | ACE model | 3, 4, 5, 7, 10, 12 | 15.6 (1.3); 15.2 (1.3); 15.9 (1.5); 15.3 (1.8); 16.4 (2.2); 17.2 (2.6) | M: 70, F: 68; M: 73, F−67; M: 31, F−71; M: 60, F: 69; M: 78, F−70; M: 75, F: 82 |
| 28 | Hur. 2007 | South Korea/Korean | nationwide | 2006 | M, F | MZ: 598, DZ: 290 | AE model | 16 (13–19) | 20.1 (2.6) | M:82; F:87 |
| 27 | Song et al. 2010 | South Korea/Korean | nationwide | 2007 | M, F | MZ:468, DZ:120 | SOLAR package by additive genetic effects | 39 (30–74) | 23.3 (3.1) | 63 |
| 49 | Stunkard et al. 1990 | Sweden/Swedish | nationwide | 1984 | M, F | MZ: 247, DZ: 426 | ADE model | 59 (14) | 24.7 (2.8) | M: 74; F: 69 |
| 52 | Silventoinen et al. 2008 | Sweden/Swedish | nationwide | 2005 | M | MZ: 1582, DZ: 1864 | ACE model | 18 (16–25) | 21.2 (2.4) | 84 |
| 44 | Harris et al. 1995 | Norway/Norwegian | nationwide | 1992 | M, F | MZ: 944, DZ: 1626 | ACE model | 18–25 | 22.0 (2.6) | M: 71; F: 79 |
| 40 | Narkiewicz et al. 1999 | Poland/Polish | N/A | 1995 | F | MZ: 19, DZ: 14 | ACE model | 21 (6) | 21.3 | 79 |
| 29 | Souren et al. 2007 | Belgium/Belgian | community - based | 1998 | M, F | MZ: 240, DZ: 138 | AE model | 25 (18–34) | 27.5 (1.2) | M: 85; F:75 |
| 35 | Hanisch et al. 2004 | Germany/German | hospital | 2002 | M, F | MZ:20, DZ: 10 | (VDZ-VMZ)/VDZ | 19–62 | 25.1 (4.7) | 57 |
[NOTES] The studies are sorted first by country, then by the study sample size (n), and then by the year of data collection.
[Abbreviations] N/A: data not available; BMI: body mass index; M: male; F: female; MZ: monozygotic; DZ: dizygotic; V: variation; SOLAR package: Sequential oligogenic linkage analysis routines; r: correlation coefficient.
[Statistical model] Structural equation modeling decomposed the BMI variation into variances of additive genetic (A), dominant genetic (D), common environment (C), and individual environment (E). Final model selection was based on Akaike’s Information Criterion (AIC); the model with the lowest AIC was chosen as the best balance of goodness of fit and parsimony (by default).
Age and BMI of study subjects are presented as mean and standard deviation or range. The median of BMI heritability was 73% across the 32 studies and varied between 31% and 90%. Ages of study populations were ranged from between 4 to 91 years old. Sample sizes varied from 40 to 7755 twin pairs.
Literature search strategy
We searched PubMed for articles published between January 1, 1990, and February 18, 2011, with the following search terms in combination with specific field tags, such as [mh] for MeSH terms and [tiab] for “title and abstract”: “twin”, “twin study”, “heritability”, “body mass index”, “BMI”, “body weight”, “waist”, “hip”, “body fat mass”, “fat”, “obesity”, “overweight”, and “adiposity”.
Study inclusion criteria: Language, sample size, and other conditions
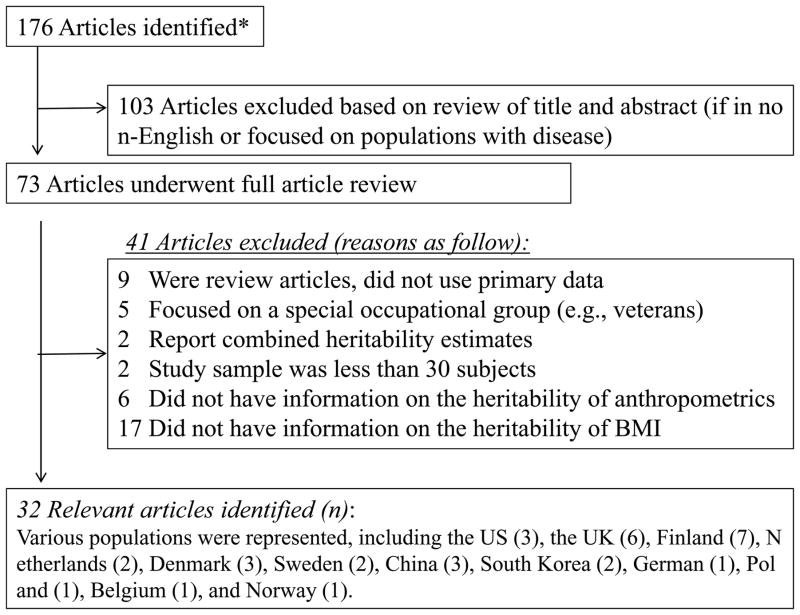
A total of 176 articles were returned with the search above. We then screened the titles and abstracts. Excluding non-English studies and study populations with disease, a total of 73 English language articles involving healthy human subjects remained. During article screening, 41 more entries were excluded for reasons including: not using primary data, focusing only on a specific occupational group (considering the exposure to extraordinary environments in a long period, e.g., soldiers or veterans), reporting combined heritability estimates across multiple ethnic or country groups, having a sample size less than 30, or not including quantitative information about the heritability of obesity (BMI or anthropometrics). To maximize comparability, we chose to focus only on the articles using BMI as their main outcome (instead of overweight, obesity, or another body composition indicator) as the great majority (80%) of remaining articles fit this description. In the end, 32 twin studies were identified as being eligible (see Appendix 1). These studies were from Finland (n=7), the UK (6), the US (3), Denmark (3), China (3), Netherlands (2), South Korea (2), Sweden (2), and four other countries: (German, Poland, Belgium and Norway).
Appendix 1. Literature search flowchart and results.
*PubMed was searched for articles published between January 1, 1990, and February 18, 2011, with following keywords in combination with specific field tags, such as [mh] for MeSH terms and [tiab] for “title and abstract”: “twin”, “twin study”, “heritability”, “body mass index”, “BMI”, “body weight*”, “waist”, “hip”, “body fat mass”, “fat”, “obesity”, “overweight”, and “adiposity”.
Lastly, we checked whether any of the 32 eligible articles were based on data from the same study sample or cohort; we did not find this to be the case. Some of the work in Finland, Netherland and Denmark was based on their shared national twin registry, but concerned different age groups and time periods.
Data abstraction
Information on year of data collection, data collection methods, study design, sample size, study population’s characteristics (e.g., sex, age, country/ethnicity) and average BMI, statistical methods for analysis (e.g., for estimating BMI-H), and BMI-H estimates were abstracted from each article using a standardized form we developed for this review (Table 1). Sex- and age-specific BMI-H values were specifically abstracted unless the articles reported an overall heritability estimate for the whole study population. A total of 74 unique estimates of BMI-H were collected from the 32 studies. We also contacted authors for information that we were abstracting but may not have been published.
Key study variables
The key outcome variable was the BMI-H estimate(s) reported in the articles, and key exposure variables included the study population’s characteristics and average BMI, GDP per capita, and annual GDP growth rate.
1) Population’s characteristics: Sex, age, country
To better attribute BMI-H estimates, they were categorized by sex as ‘male only’, ‘female only’, or ‘both/non-specific’ (if the article gave overall BMI-H values or did not present sex-specific BMI-H values). Ages of subjects were categorized into three age groups: younger than 20 years old, between ages 20–55 years, and 56 years and older for use in regression models. This approach was indicated by previous studies16 as well as by this study’s meta-analyses (see Statistical analysis section). We considered diversity of populations by country of study rather than by regions or racial groups represented.
2) Obesogenicity of the environment: Average BMI
To evaluate the obesogenic nature of a population’s environment, we used the study population’s average BMI (as reported in the articles) as a proxy measure. Compared to using a country’s prevalence of obesity in general, this indicator better reflects the study population’s characteristics by defined age and area, although both of them could be contemporaneous impact of a diverse range of lifestyle factors and behaviors (e.g., consumption of high fat and/or high calorie diets, healthy food preferences, sedentary behaviors, social norms regarding body image, etc.). For example, Haworth et al. 2008, Allison et al. 1994 and Silventoinen et al. 2007 had young children but Lehtovirta et al. 2000 and Alonso et al. 2009 observed old adults instead. Also most of the studies had wide range of age, which prohibits using the age- specific prevalence of obesity. This key variable was modeled as a continuous factor in our study as previous studies have observed a linear relationship between average BMI and BMI-H12.
3) Country-level economic factors: GDP per capita and annual GDP growth rate
Because a country’s economic growth and prosperity can impact a population’s environment and GDP has been shown to be significantly associated with BMI in Egger et al., 2012, in order to examine the effects of a country’s economic status on BMI-H, we obtained information on GDP per capita and annual GDP growth rate1. Data for both GDP and the annual GDP growth rate were downloaded from the website of ‘OECD.Stat’ (stat.oecd.org) for the year of data collection for each relevant country and study17. Data for GDP was calculated using the expenditure approach [per head, in US dollars, using constant prices, constant purchasing power parities (ppps) and OECD base year] and estimated values were provided for China. To examine the effect of GDP on BMI-H, developed countries (26 studies defined as having a GDP per capita over $20,000 in the year 2000; South Korea, Poland and China were excluded) were limitedly used in data analysis along with the year of data collection since the large gap between GDPs of developed countries and developing countries caused a bimodal distribution of GDP [mean (SD) = 23531.1 (585.9) vs. 10758.5 (3434.2), p <0.001)]. GDP level was treated as a categorical variable with GDP under $20,000 per capita set as the low group, over $26,000 as the high group, and between $20,000 to $26,000 the middle group. The cut-off points were the points of slope changes in a locally weighted scatterplot smoothing (LOWESS) curve of GDP and BMI-H and these values also corresponded to the definition of wealthy countries (>$20,000–30,000 GDP) in a previous study that examined the relationship between GDP and BMI1.
The effect of GDP and GDP growth rate may differentially affect environmental conditions since variation of environments largely depends on the rate of economic growth. Once developed countries achieve a certain level of GDP, their GDP growth rates become relatively steady and stable. In contrast, in less developed countries, higher GDP growth rates are expected although their GDP may still be lower than that of more developed countries. The level of GDP in all of the 32 studies was significantly and negatively associated with GDP growth rate (r =−0.50, p <0.001). To estimate the effect of rapid economic growth on BMI-H in different levels of GDP, GDP growth rates were classified as high or low. For this classification, we used the classifications of countries based on GDP level (low, middle and high) described above, and compared each country’s GDP growth rate to the median rate in their respective group. The median rates were as follows: 1.80 in low GDP group, 3.41 in middle GDP group, and 2.44 in high GDP group for the developed countries (26 studies) (See Statistical analysis section for more details).
Statistical analysis
The age effect on BMI-H was examined by a bubble plot using the LOWESS method to produce a nonparametric smoothing curve to show its relationship. Fluctuation of BMI-H with age was observed as in previous studies. The size of the circles depended on the weight of the study (where the weighting factor was equivalent to sample size) (Appendix 1). Age was categorized at the points of slope change into three groups (below 20, between 20–55, and above 56 years old). These age categories were subsequently used in regression models given the non-linear relationship of age with BMI-H.
In conducting a test for heterogeneity in our meta-analysis, we found strong evidence of heterogeneity in BMI-H between studies (using the “meta” command in STATA 11, test for heterogeneity by the Der Simonian and Laird estimate of between studies variance = 167.965, p-value <0.001). As a result, we regarded meta-regression models with random effects as most appropriate for analyzing the associations between BMI-H and the study characteristics of interest. This approach provided restricted maximum likelihood estimates of regression parameters, and random effects allowed for the potential variations in the associations between BMI and study characteristics across the multiple studies. To give more weight for study-estimates with better precision (e.g. smaller standard errors, etc.), sample size was considered relative to weighting factor and used for Meta-regression models with random effects using the “metareg” command in STATA 11.
The basic meta-regression model we used is shown below:
where yi is a statistic from study i; θi is the true effect of study i, θi~N (θ, τ2); and εi is a measure of within- study uncertainty, εi~N (0, σi2). The regression coefficients (βi, ······ βp) are the estimated increase per unit increase of the covariates (xi1,······· xip). The covariates included in the model are the population characteristic variables that we wanted to examine as contributing factors to the heterogeneity of BMI-H (e.g., age, sex).
The basic meta-regression model with random-effects included variables that had recently been suggested to be contributing factors to the heterogeneity of BMI-H, such as sex10, age16, and average BMI12. Again, age and sex were both treated as categorical variables and average BMI as a continuous variable.
To examine the effect of economic status on BMI-H, GDP per capita, annual GDP growth rate, and the year of data collection were added to the model above. Again, due to the bimodal distribution of GDP levels, developed countries’ data (26 studies) were separately entered into the basic model along with the year of data collection and GDP and GDP growth rate were both treated as categorical variables. Additional analysis was done by including the remaining countries of South Korea, Poland, and China with the developed countries’ data (for a total of 32 studies) to examine whether countries overall experienced similar effects of GDP growth rate on BMI-H as developed countries.
Finally, we tested for interaction between GDP and GDP growth rate on BMI-H through analysis of variance (ANOVA). The predicted BMI-H by the previous model (described in Table 2) were compared between countries with high and low GDP growth rates within the different GDP levels.
Table 2.
Random effects meta-regression analyses: Estimates of predictors of heterogeneity in BMI-heritability in developed countries*
| Predictors | BMI-heritability | ||
|---|---|---|---|
| β | Standard error | p-value | |
| Age- | |||
| Below 20 years old | (reference) | ||
| Between 20 and 55 years old | −21.65 | 6.19 | 0.001 |
| Age 56 and above | −26.98 | 7.75 | 0.001 |
| Sex- | |||
| Male | (reference) | ||
| Female | 4.62 | 3.47 | 0.18 |
| Both male & female/non-specific | −0.88 | 3.71 | 0.81 |
| Average BMI (kg/m2) | 2.58 | 0.78 | 0.002 |
| GDP per capita- | |||
| Below $20,000 | (reference) | ||
| Between $20,000 and $25,999 | 8.81 | 6.91 | 0.21 |
| $26,000 and above | 18.36 | 8.43 | 0.03 |
| Annual GDP growth rate (%) | −2.13 | 1.06 | 0.04 |
| Year of data collection (year) | −0.56 | 0.29 | 0.05 |
A total of 26 of 32 studies were from developed countries and from these studies, 66 unique BMI-H estimates were abstracted.
Random effects meta-regression modeling was used to analyze whether study characteristics could be related to the statistical heterogeneity observed between BMI-H estimates across multiple studies. Heterogeneity in BMI-heritability between studies was evident (p =0.01). The regression coefficients can be understood as the estimated changes (increase, decrease, or no change) in BMI-heritability per unit increase in the covariate after adjusting for other covariates and considering statistical significance. We used dummy variables for age, GDP per capita, and sex in the model. Given the non-linear relationship between age and GDP on BMI-H, age and GDP were categorized both into three groups according to the points of change in slope (for age, the three groups were: below 20 years old, between 20–55, and 56 years and older; for GDP per capita, the three groups were: below $20,000, between $20,001–25,999, and $26,000 or above). Beta coefficients for each dummy variable were presented compared to the reference group (as marked).
Results
Characteristics of studies
The median of the reported BMI-H from all 32 studies was 73% and estimates ranged from 31% (Silventioinen et al., 2007)18 to 90% (Poulsen et al., 2001)19. The majority of the studies included both sexes and was cross-sectional. Only four were cohort studies. Most of the studies (84%) were from the US (n=3) and European countries (n=24). Several different statistical models were used to estimate BMI-H such as the ACE model, which was implemented in 86% studies. In this model, structural equation modeling decomposes the total variation in BMI into sources of additive genetic variance (A), common environmental variance (C), or unique environmental variance (E) after model selection based on Akaike’s Information Criteria (where the model with the lowest AIC is chosen as the best balance of goodness of fit and parsimony). Falconer’s formula, path analysis, and other methods were used in other studies.
The studies were widely varied. For example, many studies in Finland, the Netherlands, Denmark, Sweden, Norway, South Korea, and the UK (one article) utilized data from nationwide twin registries or other health record systems (n=18). Other studies in the UK (five articles), the US, Germany, and China used community- or hospital-based data sources (n=13). There were wide ranges in age (from 3 to 91 years old), time period of data collection (from 1965 to 2007), average BMI (from 15.0 to 28.2 kg/m2), GDP per capita (from $2,555 to $31,358), and annual GDP growth rate (from −0.81 to 10.03%).
Random-effects meta-regression analysis
1) Differences of BMI-H with age, sex, and average BMI
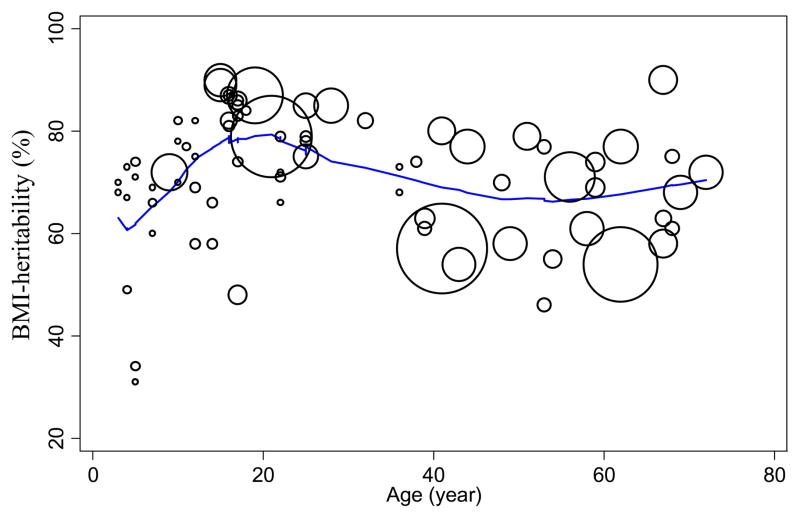
BMI-H varied by age (see Appendix 2). BMI-H increased until around 20 years old [β =1.55, SE =0.39, p <0.001] at which point it reached its peak (BMI-H at age 20=79% on the LOWESS curve). Then, BMI-H decreased steadily until the mid-50’s (β =−0.47, SE =0.16, p <0.01; BMI-H at age 55=66% on the LOWESS curve) and then afterwards gradually increased again (β =0.15, SE =0. 57, p =0.80).
Appendix 2. Bubble plot of BMI-heritability against age using the LOWESS method, based on overall heritability estimates from all 32 twin studies.
The effect of age on BMI-heritability is described by this bubble plot using the locally weighted scatterplot smoothing (LOWESS) method for regression to create a non-parametric smoothing curve. The size of the circles reflects the weight of the study (where the weighting factor was equivalent to study sample size). The curve increased until around age 20 (β =1.55, SE =0.39, p <0.001) and peaked in early adulthood (age 20). BMI-heritability at age 20 was 79%. Then, the heritability curve decreased steadily until the mid-50’s (β =-0.47, SE =0.16, p <0.01; BMI-heritability at age 55 was 66%), and then gradually increased afterwards, though not significantly so (β =0.15, SE =0.57, p =0.80).
After adjustment for sex and average BMI, BMI-H was found to be lower in the two older age groups compared to the youngest age group (β =−15.16 in the age 20–55 group and β =−23.09 in the age ≥56 group; both p <0.01 compared to the age 20 and below group). BMI-H increased with the level of average BMI (β =2.34 per kg/m2, p <0.01). No significant effects were observed in BMI-H with sex (β =2.65 in women compared to men, p =0.45; β= 1.78 in both sexes compared to only men, p =0.60).
2) Differences of BMI-H with GDP per capita level, annual GDP growth rate, and secular trends
To examine the effect of economic factors on BMI-H, analyses limited to the developed countries (n =26 of the 32 total studies) were performed. GDP, GDP growth rate, and the year of data collection were added to the previous model that already included sex, age, and average BMI. BMI-H increased with average BMI (β =2.58 per kg/m2, p <0.01) and it was significantly higher in the high GDP group (≥$26,000) than in the low GDP (<$20,000) (β =18.36, p =0.03). In contrast, BMI-H decreased in the older age groups (β =−21.7 in the age 20–55 year group and β =−27.0 in the age 56 years and above group; both p <0.01 compared to younger than 20 years old age group), with rapid economic growth (β =−2.13, p =0.04), and secularly over time (β =−0.56 per year, p =0.03).
3) The combined effects of GDP per capita and GDP growth rate on BMI-H
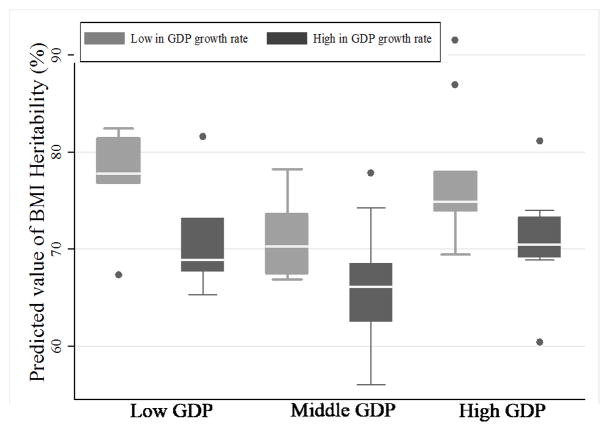
Overall, BMI-H was lower in the group of high GDP growth rate countries than in the group of low GDP growth rate countries [mean (SD) of BMI-H= 68.63 (5.55) for high growth rate countries vs. 75.28 (6.28) for low growth rate countries; p <0.001]. Similarly, in analyzing each strata of GDP individually, those countries with higher GDP growth rates had lower levels of BMI-H than those with lower GDP growth rates. The mean differences of BMI-H between the high and low GDP growth rate groups (high-low) were, by GDP strata, as follows: 6.06% in the high GDP group, 5.07% in the middle GDP group, and 6.34% in the low GDP group. BMI-H was observed to be the lowest in the middle level GDP, high GDP growth rate group at 66.01% (SD =4.96; the 4th group in Figure 1). This group’s heritability was also significantly lower compared to that in the low GDP, low GDP growth rate group, which was 77.16% (SD =6.00; the 1st group in Figure 1), as well as that in the high GDP, low GDP growth rate group, which was 77.27% (SD =6.78; the 5th group in Figure 1]. These analyses were done using Scheffé post-hoc tests.
Figure 1. The combined effects of GDP and GDP growth rates on BMI-heritability: Findings of pooled analysis based results from 32 twin studies.
Predicted values of BMI-heritability were modeled by several predictors in Table 2. As described in the Methods section, studies were classified into one of three groups according to their level of GDP per capita: countries with GDP levels below $20,000 were referred to as the low GDP group (n=16 studies), those between $20,000 and $25,999 were referred to as the middle GDP group (n=28), and those above $26,000 were referred to as the high GDP group (n=22). Studies were further characterized as having either a low or high GDP growth rate within their GDP strata. Within each strata, studies with growth rates higher than the median growth rate were considered as high GDP growth rate countries and those below the median value were considered as low GDP growth rate countries. The median values were 1.80 in the low GDP group, 3.41 in middle GDP group, and 2.44 in the high GDP group.
Finally, the number of studies for each combination of GDP and GDP growth rate in our study were as follows: low GDP-low GDP growth rate (n =5), low-high (11), middle-low (7), middle-high (21), high-low (10), high-high (12). The mean level of BMI-heritability was significantly different (p <0.001) across the six groups according to ANOVA tests. This was evident between the Low-Low and the Middle-High groups as well as the Middle-High and the High-Low groups given Scheffé post-hoc tests.
Additional analysis to examine whether countries overall had similar effects of GDP growth rates on BMI-H as developed countries was done by including the remaining countries of South Korea, Poland, and China with the developed countries’ data. Similar results were observed as above. The BMI-H was lowest in countries in the middle GDP, high GDP growth rate group at 67.80% (SD =4.06). This value was significantly lower than that in the low GDP, low growth rate group (74.60%, SD =5.37%, p <0.001) and the high GDP, low growth rate group (75.66%, SD =4.74%, p <0.001).
Discussion
The relative contributions of genetic and social-environmental factors to body weight status have been controversial. Due to the polygenic nature of obesity, previous studies have been limited in their ability to examine the genetic contribution to obesity development beyond a few single nucleotide polymorphisms (SNPs) or genetic interactions with a few environmental factors. In contrast, as an aggregated index of all genetic loci, BMI-H shows the relative contributions of differences in genetic and environmental factors to total phenotypic variance in a population. Most twin studies present a relatively high level of BMI-H, but reported BMI-H estimates show great variation, from 31% to 90%. Also, few studies have assessed whether obesity heritability may vary by population demographic characteristics. Such questions have only been partially explored (e.g., in younger age groups only) or remain unresolved (e.g., due to incomparable characteristics across family, adoption, and twin studies)10, 15, 16. Also, in terms of social-environmental influences, few studies have suggested a non-linear relationship and time lag between GDP and BMI to date1, 20. Our systematic review found that heterogeneity of BMI-H is significantly attributable to variations in age, average BMI, the time period of data collection (i.e., secular effects), GDP, and GDP growth rates. Children and adolescents, populations with higher average BMIs and higher levels of GDP are likely to have greater BMI-H. In contrast, lowering effects of high GDP growth rate and recent years are shown on BMI-H. The activation of obesity-related genes during periods of heightened growth activity in adolescence (i.e., growth spurts) and/or in obesity-promoting environments (e.g., as reflected by high average BMIs and high GDP levels) are likely to increase contributions to BMI-H from genetic variations compared to environmental influences. Conversely, the mixing of traditional lifestyle behaviors with newer, more sedentary ones that tends to accompany rapid economic growth is likely to lead to large variations in the environment within countries during such transitions. Under these conditions, social-environmental influences on BMI-H are likely to be stronger. With the interplay of population characteristics and the influence of the study environment, BMI-H may stabilize.
Effects of age on BMI-H
The relationship between BMI-H and age is dynamic. In our meta-analysis, BMI-H increased until around age 20 when it reached its peak. BMI-H then decreased steadily until age 55, after which it gradually increased again, but not to its earlier heights. After adjusting for sex and average BMI, BMI-H was lower in older age groups compared to the youngest age group (β =−15.16 in the age 20–55 group and β =−23.09 in age ≥56 year old group both p <0.01 compared to the <20 years old group).
One review of childhood obesity studies up to age 18 (which included nine twin and five adoption studies) observed moderate to high levels of BMI-H (mean =75%) and a nonlinear relationship between age and BMI-H. The study found BMI-H during adolescence higher than in early childhood. In contrast, common environmental effects from shared family background (such as parents’ BMI, food preference, eating behavior, etc.) on BMI-H became relatively small around adolescence compared to early childhood in the twin and adoption studies16. Silventoinen et al. examined cases of strong BMI tracking from birth to late-middle age in adulthood and suggested that continuity of BMI from early childhood to the onset of adulthood was related to genes that started to affect BMI during periods of physical growth15. Thus, various events in gene expression and gene activation for physical growth during childhood and adolescents that occur in sequential pathways (e.g., for weight and height gain11) could increase genetic variation and genetic contribution to BMI-H in young ages. To distinguish the shared environments and non-additive genetic variance (e.g., food availability at home vs. appetite gene effect, etc.), Dr. Segal and colleagues tried ACDE models of virtual twins (composed by adoptee and same-age unrelated siblings)21. However, as most of heritability studies to date do not use advanced ACDE modeling and because of the limited comparability of family adoption and twin studies, we were only able to include twins in our study population.
Conversely, unique environments become relatively more diverse in adulthood compared to childhood. As people age, they tend to encounter more physical exposures (e.g., smoking, alcohol, chemical toxins, diet, and sedentary-promoting lifestyles, etc.), as well as more social factors (e.g., peer pressure, social norms for body image, cultural influences on body shape, etc.) that can impact body weight. Non-genetic factors may interact with and influence one’s genetic potential of influence. For example, in one study, physical activity modified genetic variations in BMI [−0.18 (95% CI: −0.31 to −0.05)] and resulted in lower BMI-H in the active group compared to the sedentary group. High dietary protein have also been found to reduce genetic variances in BMI and waist circumference8, 9. Epigenetic studies have supported the role of the environment in activating and inactivating genes as well since starvation, folic acid, and various chemical exposures have all been linked to epigenetic changes22.
Positive relationships of average BMI and GDP with BMI-H
We found BMI-H increased with average BMI of the study population (β =+2.58 per kg/m2, p <0.01). BMI-H was also significantly higher in countries of high GDP (annual GDP >$26,000) than in those of lower GDP (annual GDP <$20,000).
The associations between population level of BMI and obesity prevalence with BMI-H were recently examined in Swedish and Danish populations12, 23. Additive genetic variance (ranging from 4.3 to 7.9) and unique environmental variance (ranging from 1.4 to 2.0) of BMI variance was observed to linearly increase with the population mean of BMI over three decades. BMI-H increased from 75% to 78.8% over the same period. The authors hypothesized that a relationship of this nature was more likely due to environmental influences modifying the genetic influences on adiposity since the population gene pool changes at a much slower rate than could explain the fast-rising obesity patterns observed over the last 30–50 years. They also surmised it was possible for obesogenic environments to inspire new gene activation, gene expression, and/or epigenetic changes related to obesity.
Egger and Swinburn (1997)24 proposed an ecological model for obesity comprised of two different layers of the environment that could together explain the epidemic rise of obesity over the past years. First, a proximal layer influences changes on a more individual level (for example, in energy intake or physical activity levels) and also includes the food environment and the built environment. Second, a more distal layer includes factors (for example, economic growth) that have a more macro-level reach and can affect proximal level entities. GDP is the most commonly used indicator for national prosperity and it can therefore impact an in-country level of obesogenicity since economic growth is often achieved through increasing consumption. Therefore, populations living in developed countries are more likely to be exposed to obesogenic environments as well as have a greater prevalence of obesity and/or higher population means of BMI. Indeed, as Egger et al. examined, average BMI was more likely to be higher in developed countries, although GDP was not observed to have a linear relationship with average BMI beyond a GDP per capita of $5,0001. Interestingly, in a study of 103 countries, a dose-response relationship has been observed between the level of GDP and the level of caloric sweetener intakes25.
Negative influences of GDP growth rate and secular trends on BMI-H
BMI-H was consistently lower in countries with high GDP growth rates in both overall analysis (β =−2.13, p =0.04) and stratified analyses by GDP level. In our study, the lowering effects were seen across all three levels of GDP groups. The mean differences in BMI-H between the high and low GDP growth rate groups were, by GDP strata: 6.06% in the high GDP group, 5.07% in the middle GDP group, and 6.34% in the low GDP group. The secular trend in BMI-H was observed to be significantly negative (β =−0.56 per year, p =0.03) as well.
There is potential for public health and, specifically, obesity rates in mid-level GDP countries to be adversely affected by rapid economic growth. For example, the pattern of rapid increases in diabetes prevalence over short periods of time in Asian populations seems to be attributed mostly to lifestyle changes resulting from rapid socio-economic growth26. China’s urbanization between 1991 and 2006 saw a 32% reduction in physical activity across the population14. As we have discussed, the introduction of new social and cultural influences on lifestyle behaviors (i.e., dietary habits, nutrition, transportation, work habits, etc.) due to economic growth and technology have led to large variation in people’s environments14, 25 through mixing with traditional lifestyle behaviors; this may result in a reduction of BMI-H. Secular trends are also important to note as these results suggest a gradual push towards more environmental variation over time and, with it, partly decreased BMI-H.
Additionally, the combined or interactive effects of GDP and GDP growth rates could influence the BMI-H. Developed countries have relatively high levels of GDP but lower and steadier GDP growth rates. This may happen after a country achieves a certain level of GDP. It follows that developed countries with low GDP growth rates are more likely to have higher levels of BMI-H than those with high GDP growth rates. This is because obesogenic environments may activate the expression of adiposity-related genes while more stable lifestyles and cultures may weaken the power of environmental variation.
Along this pattern, countries with middle-level GDP and high GDP growth rates, in contrast, would be expected to have the lowest BMI-H; this is what we observed in our meta-analysis. Countries of mid-level GDP and high growth rates are subject to relatively greater environmental heterogeneity because traditional and newer lifestyles coexist in the same population to a greater extent than in countries of high- or low-level GDP. Less obesogenic environments compared to what is found in countries with high GDP drive less activation of the adiposity related genes. For example, while we observed a high level of BMI-H in the low GDP group, there was also a higher level of environmental homogeneity maintained in this group compared to the middle and high GDP groups.
The main strengths of this study include: a) the use of stringent study inclusion criteria (e.g., study had to involve healthy populations, observe twins, have sample sizes greater than 30, not focus on a non-generalizable and specific occupational group, etc.), which minimized possibilities of biased interpretation of results and allowed for analyses of various population characteristics and environmental factors of interest, b) a wide representation of countries, years of data collection, GDP, and GDP growth rates across the 32 included studies, and c) the use of meta-regression models with random effects, which allowed for the possibilities of both i) variation in the associations between study characteristics and BMI-H across multiple studies and ii) identification of significant study characteristics for BMI-H heterogeneity. This study also had its limitations, including: a) BMI-heritability between developing versus developed countries could not be compared due to the small number of twin studies from developing countries, and b) the effects of ethnic differences on BMI-H could not be assessed since most of populations we reviewed were Caucasians and/or from the US or European countries.
In summary, we found that BMI-H varied by population characteristics and social-environmental factors, especially with age, average BMI of the study population, time period of observation, and economic development. Monitoring various aspects of environments is needed to help evaluate genetic effects on total phenotypic variations in a population. Continued research on modifiers of BMI-H will help public health scientists better understand the genetic, socio-environmental, and other potential drivers of the obesity pandemic.
Acknowledgments
This study was supported in part by the National Institute of Health (NIH) grant (U54HD070725) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) and Ewha Womans University (RP-Grant 2012). The U54 project is co-funded by the NICHD and the Office of Behavioral and Social Sciences Research (OBSSR). The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by the NIH. All authors were involved in writing the paper and had final approval of the submitted and published versions. We thank Hsin-jen Chen, PhD who provided important data analysis guidance and support.
Abbreviations
- BMI
Body mass index
- BMI-H
BMI heritability
- GDP
Gross domestic product
Footnotes
Conflict of interest: The authors report no conflict of interest.
References
- 1.Egger G, Swinburn B, Islam FM. Economic growth and obesity: an interesting relationship with world-wide implications. Economics and human biology. 2012;10(2):147–53. doi: 10.1016/j.ehb.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Rhodes L, Demerath EW, Cousminer DL, et al. Association of Adiposity Genetic Variants With Menarche Timing in 92,105 Women of European Descent. American journal of epidemiology. 2013 doi: 10.1093/aje/kws473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogardus C. Missing heritability and GWAS utility. Obesity (Silver Spring) 2009;17(2):209–10. doi: 10.1038/oby.2008.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rampersaud E, Mitchell BD, Pollin TI, et al. Physical activity and the association of common FTO gene variants with body mass index and obesity. Archives of internal medicine. 2008;168(16):1791–7. doi: 10.1001/archinte.168.16.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corella D, Arnett DK, Tucker KL, et al. A high intake of saturated fatty acids strengthens the association between the fat mass and obesity-associated gene and BMI. The Journal of nutrition. 2011;141(12):2219–25. doi: 10.3945/jn.111.143826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rende R. Beyond Heritability: Biological process in social context. Nature and Nurture. 2004;Chap 6:107–26. [Google Scholar]
- 7.Ajslev TA, Angquist L, Silventoinen K, et al. Assortative marriages by body mass index have increased simultaneously with the obesity epidemic. Frontiers in genetics. 2012;3:125. doi: 10.3389/fgene.2012.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silventoinen K, Hasselbalch AL, Lallukka T, et al. Modification effects of physical activity and protein intake on heritability of body size and composition. Am J Clin Nutr. 2009;90(4):1096–103. doi: 10.3945/ajcn.2009.27689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mustelin L, Silventoinen K, Pietilainen K, Rissanen A, Kaprio J. Physical activity reduces the influence of genetic effects on BMI and waist circumference: a study in young adult twins. Int J Obes (Lond) 2009;33(1):29–36. doi: 10.1038/ijo.2008.258. [DOI] [PubMed] [Google Scholar]
- 10.Schousboe K, Willemsen G, Kyvik KO, et al. Sex differences in heritability of BMI: a comparative study of results from twin studies in eight countries. Twin research : the official journal of the International Society for Twin Studies. 2003;6(5):409–21. doi: 10.1375/136905203770326411. [DOI] [PubMed] [Google Scholar]
- 11.Meyer J. Genetic studies of obesity across the life span. New York: Plenum Press; 1995. [Google Scholar]
- 12.Rokholm B, Silventoinen K, Tynelius P, Gamborg M, Sorensen TI, Rasmussen F. Increasing genetic variance of body mass index during the Swedish obesity epidemic. PloS one. 2011;6(11):e27135. doi: 10.1371/journal.pone.0027135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drewnowski A, Popkin BM. The nutrition transition: new trends in the global diet. Nutrition reviews. 1997;55(2):31–43. doi: 10.1111/j.1753-4887.1997.tb01593.x. [DOI] [PubMed] [Google Scholar]
- 14.Ng SW, Norton EC, Popkin BM. Why have physical activity levels declined among Chinese adults? Findings from the 1991–2006 China Health and Nutrition Surveys. Soc Sci Med. 2009;68(7):1305–14. doi: 10.1016/j.socscimed.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silventoinen K, Kaprio J. Genetics of tracking of body mass index from birth to late middle age: evidence from twin and family studies. Obes Facts. 2009;2(3):196–202. doi: 10.1159/000219675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silventoinen K, Rokholm B, Kaprio J, Sorensen TI. The genetic and environmental influences on childhood obesity: a systematic review of twin and adoption studies. Int J Obes (Lond) 2010;34(1):29–40. doi: 10.1038/ijo.2009.177. [DOI] [PubMed] [Google Scholar]
- 17.GDP, volume – annual growth rates in percentage, data extracted on 05 Jul 2012 08 31 U TC (GMT) from OECD.Stat http://stats.oecd.org/index.aspx?queryid=19801
- 18.Silventoinen K, Bartels M, Posthuma D, et al. Genetic regulation of growth in height and weight from 3 to 12 years of age: a longitudinal study of Dutch twin children. Twin Res Hum Genet. 2007;10(2):354–63. doi: 10.1375/twin.10.2.354. [DOI] [PubMed] [Google Scholar]
- 19.Poulsen P, Vaag A, Kyvik K, Beck-Nielsen H. Genetic versus environmental aetiology of the metabolic syndrome among male and female twins. Diabetologia. 2001;44(5):537–43. doi: 10.1007/s001250051659. [DOI] [PubMed] [Google Scholar]
- 20.Bua J, Olsen LW, Sorensen TI. Secular trends in childhood obesity in Denmark during 50 years in relation to economic growth. Obesity (Silver Spring) 2007;15(4):977–85. doi: 10.1038/oby.2007.603. [DOI] [PubMed] [Google Scholar]
- 21.Segal NL, Feng R, McGuire SA, Allison DB, Miller S. Genetic and environmental contributions to body mass index: comparative analysis of monozygotic twins, dizygotic twins and same-age unrelated siblings. Int J Obes (Lond) 2009;33(1):37–41. doi: 10.1038/ijo.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pozharny Y, Lambertini L, Clunie G, Ferrara L, Lee MJ. Epigenetics in women’s health care. Mt Sinai J Med. 2010;77(2):225–35. doi: 10.1002/msj.20176. [DOI] [PubMed] [Google Scholar]
- 23.Rokholm B, Silventoinen K, Angquist L, Skytthe A, Kyvik KO, Sorensen TI. Increased genetic variance of BMI with a higher prevalence of obesity. PloS one. 2011;6(6):e20816. doi: 10.1371/journal.pone.0020816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egger G, Swinburn B. An “ecological” approach to the obesity pandemic. BMJ. 1997;315(7106):477–80. doi: 10.1136/bmj.315.7106.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popkin BM, Nielsen SJ. The sweetening of the world’s diet. Obesity research. 2003;11(11):1325–32. doi: 10.1038/oby.2003.179. [DOI] [PubMed] [Google Scholar]
- 26.Ramachandran A, Snehalatha C, Shetty AS, Nanditha A. Trends in prevalence of diabetes in Asian countries. World journal of diabetes. 2012;3(6):110–7. doi: 10.4239/wjd.v3.i6.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song Y, Lee K, Sung J, Lee D, Lee MK, Lee JY. Genetic and environmental relationships between Framingham Risk Score and adiposity measures in Koreans: the Healthy Twin study. Nutr Metab Cardiovasc Dis. 2012 Jun;22(6):503–9. doi: 10.1016/j.numecd.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Hur YM. Sex difference in heritability of BMI in South Korean adolescent twins. Obesity (Silver Spring) 2007 Dec;15(12):2908–11. doi: 10.1038/oby.2007.346. [DOI] [PubMed] [Google Scholar]
- 29.Souren NY, Paulussen AD, Loos RJ, et al. Anthropometry, carbohydrate and lipid metabolism in the East Flanders Prospective Twin Survey: heritabilities. Diabetologia. 2007 Oct;50(10):2107–16. doi: 10.1007/s00125-007-0784-z. Epub 2007 Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beardsall K, Ong KK, Murphy N, et al. Heritability of childhood weight gain from birth and risk markers for adult metabolic disease in prepubertal twins. J Clin Endocrinol Metab. 2009 Oct;94(10):3708–13. doi: 10.1210/jc.2009-0757. Epub 2009 Sep 1. [DOI] [PubMed] [Google Scholar]
- 31.Estourgie-van Burk GF, Bartels M, van Beijsterveldt TC, Delemarre-van de Waal HA, Boomsma DI. Body size in five-year-old twins: heritability and comparison to singleton standards. Twin Res Hum Genet. 2006 Oct;9(5):646–55. doi: 10.1375/183242706778553417. [DOI] [PubMed] [Google Scholar]
- 32.Malis C, Rasmussen EL, Poulsen P, et al. Total and regional fat distribution is strongly influenced by genetic factors in young and elderly twins. Obes Res. 2005 Dec;13(12):2139–45. doi: 10.1038/oby.2005.265. [DOI] [PubMed] [Google Scholar]
- 33.Lajunen HR, Kaprio J, Keski-Rahkonen A, et al. Genetic and environmental effects on body mass index during adolescence: a prospective study among Finnish twins. Int J Obes (Lond) 2009 May;33(5):559–67. doi: 10.1038/ijo.2009.51. Epub 2009 Mar 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallace AM, Banfield E, Ingram M, et al. Glucocorticoids contribute to the heritability of leptin in Scottish adult female twins. Clin Endocrinol (Oxf) 2004 Jul;61(1):149–54. doi: 10.1111/j.1365-2265.2004.02068.x. [DOI] [PubMed] [Google Scholar]
- 35.Hanisch D, Dittmar M, Höhler T, Alt KW. Contribution of genetic and environmental factors to variation in body compartments--a twin study in adults. Anthropol Anz. 2004 Mar;62(1):51–60. [PubMed] [Google Scholar]
- 36.Manek NJ, Hart D, Spector TD, MacGregor AJ. The association of body mass index and osteoarthritis of the knee joint: an examination of genetic andenvironmental influences. Arthritis Rheum. 2003 Apr;48(4):1024–9. doi: 10.1002/art.10884. [DOI] [PubMed] [Google Scholar]
- 37.Baird J, Osmond C, MacGregor A, Snieder H, Hales CN, Phillips DI. Testing the fetal origins hypothesis in twins: the Birmingham twin study. Diabetologia. 2001 Jan;44(1):33–9. doi: 10.1007/s001250051577. [DOI] [PubMed] [Google Scholar]
- 38.Lehtovirta M, Kaprio J, Forsblom C, Eriksson J, Tuomilehto J, Groop L. Insulin sensitivity and insulin secretion in monozygotic and dizygotic twins. Diabetologia. 2000 Mar;43(3):285–93. doi: 10.1007/s001250050046. [DOI] [PubMed] [Google Scholar]
- 39.Pietiläinen KH, Kaprio J, Rissanen A, et al. Distribution and heritability of BMI in Finnish adolescents aged 16y and 17y: a study of 4884 twins and 2509 singletons. Int J Obes Relat Metab Disord. 1999 Feb;23(2):107–15. doi: 10.1038/sj.ijo.0800767. [DOI] [PubMed] [Google Scholar]
- 40.Narkiewicz K, Szczech R, Winnicki M, et al. Heritability of plasma leptin levels: a twin study. J Hypertens. 1999 Jan;17(1):27–31. doi: 10.1097/00004872-199917010-00005. [DOI] [PubMed] [Google Scholar]
- 41.Austin MA, Friedlander Y, Newman B, Edwards K, Mayer-Davis EJ, King MC. Genetic influences on changes in body mass index: a longitudinal analysis ofwomen twins. Obes Res. 1997 Jul;5(4):326–31. doi: 10.1002/j.1550-8528.1997.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 42.Herskind AM, McGue M, Sørensen TI, Harvald B. Sex and age specific assessment of genetic and environmental influences on body mass index in twins. Int J ObesRelat Metab Disord. 1996 Feb;20(2):106–13. [PubMed] [Google Scholar]
- 43.Carmichael CM, McGue M. A cross-sectional examination of height, weight, and body mass index in adult twins. J Gerontol A Biol Sci Med Sci. 1995 Jul;50(4):B237–44. doi: 10.1093/gerona/50a.4.b237. [DOI] [PubMed] [Google Scholar]
- 44.Harris JR, Tambs K, Magnus P. Sex-specific effects for body mass index in the new Norwegian twin panel. Genet Epidemiol. 1995;12(3):251–65. doi: 10.1002/gepi.1370120303. [DOI] [PubMed] [Google Scholar]
- 45.Ortega-Alonso A, Sipilä S, Kujala UM, Kaprio J, Rantanen T. Genetic influences on change in BMI from middle to old age: a 29-year follow-up study of twin sisters. Behav Genet. 2009 Mar;39(2):154–64. doi: 10.1007/s10519-008-9245-9. Epub 2008 Dec 10. [DOI] [PubMed] [Google Scholar]
- 46.Allison DB, Heshka S, Neale MC, Heymsfield SB. Race effects in the genetics of adolescents’ body mass index. Int J Obes Relat Metab Disord. 1994 Jun;18(6):363–8. [PubMed] [Google Scholar]
- 47.Korkeila M, Kaprio J, Rissanen A, Koskenvuo M. Effects of gender and age on the heritability of body mass index. Int J Obes. 1991 Oct;15(10):647–54. [PubMed] [Google Scholar]
- 48.Hewitt JK, Stunkard AJ, Carroll D, Sims J, Turner JR. A twin study approach towards understanding genetic contributions to body size and metabolic rate. ActaGenet Med Gemellol (Roma) 1991;40(2):133–46. doi: 10.1017/s0001566000002567. Erratum in: Acta Genet Med Gemellol(Roma) 1991;40(3–4):following 309. [DOI] [PubMed] [Google Scholar]
- 49.Stunkard AJ, Harris JR, Pedersen NL, McClearn GE. The body-mass index oftwins who have been reared apart. N Engl J Med. 1990 May 24;322(21):1483–7. doi: 10.1056/NEJM199005243222102. [DOI] [PubMed] [Google Scholar]
- 50.Turula M, Kaprio J, Rissanen A, Koskenvuo M. Body weight in the Finnish Twin Cohort. Diabetes Res Clin Pract. 1990;10(Suppl 1):S33–6. doi: 10.1016/0168-8227(90)90137-i. [DOI] [PubMed] [Google Scholar]
- 51.Haworth CM, Carnell S, Meaburn EL, Davis OS, Plomin R, Wardle J. Increasing heritability of BMI and stronger associations with the FTO gene over childhood. Obesity (Silver Spring) 2008 Dec;16(12):2663–8. doi: 10.1038/oby.2008.434. Epub 2008 Oct 9. [DOI] [PubMed] [Google Scholar]
- 52.Silventoinen K, Magnusson PK, Tynelius P, Kaprio J, Rasmussen F. Heritability of body size and muscle strength in young adulthood: a study of one million Swedish men. Genet Epidemiol. 2008 May;32(4):341–9. doi: 10.1002/gepi.20308. [DOI] [PubMed] [Google Scholar]
- 53.Ouyang F, Christoffel KK, Brickman WJ, et al. Adiposity in inversely related to insulin sensitivity in relatively lean Chinese adolescents: a population based twin study. Am J Clin Nutr. 2010;91:662–71. doi: 10.3945/ajcn.2009.28750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee J, Chen L, Snieder H, et al. Heritability of obesity-related phenotypes and association with adiponectin gene polymorphisms in the Chinese national twin registry. Ann Hum Genet. 2010 Mar;74(2):146–54. doi: 10.1111/j.1469-1809.2010.00565.x. Epub 2010 Feb 18. [DOI] [PubMed] [Google Scholar]
- 55.Wu T, Snieder H, Li L, et al. Genetic and environmental influences on blood pressure and body mass index in Han Chinese: a twin study. Hypertens Res. 2011 Feb;34(2):173–9. doi: 10.1038/hr.2010.194. Epub 2010 Nov 4. [DOI] [PubMed] [Google Scholar]