Abstract
Objective
HIV-infection is an established risk for diarrheal severity, less is known about specific enteric pathogens associated with HIV status. We determined associations of selected enteric pathogens with HIV-infection and HIV-exposure among Kenyan children.
Design
Cross-sectional study among 6 months to 15 year olds presenting to two Western Kenya District hospitals with acute diarrhea between 2011–2013.
Methods
Stool was tested using standard bacterial culture and microscopy for ova and parasites. HIV testing was obtained on children and mothers. Enteric pathogen prevalence was compared between HIV-infected and HIV-uninfected children and between HIV-exposed uninfected (HEU) and HIV-unexposed. Unadjusted and adjusted prevalence ratios (PR) for selected pathogens by HIV-status were estimated using relative risk (RR) regression and P-values. Age, site, income, household crowding, water source/treatment, anthropometrics, cotrimoxazole use, and breastfeeding history were accounted for in multivariable models.
Results
Among 1,076 children, median age was 22 months (interquartile range: 11–42), 56 (5.2%) were HIV-infected, and 10.3%(105/1020) of HIV-uninfected children were HIV-exposed. The following organisms were most frequently isolated from stool: enteroaggregative Escherichia coli (13.3%), Giardia spp. (11.1%) Campylobacter (6.3%), enteropathogenic Escherichia coli (EPEC) (6.1%) and Cryptosporidium spp. (3.7%). Accounting for age, HIV-infection was associated with EPEC infection (PR: 3.70, P=0.002) while HIV-exposure was associated with Cryptosporidium among HIV-uninfected children (PR: 2.81, P=0.005).
Conclusion
EPEC and Cryptosporidium infections were more common in HIV-infected and HIV-exposed children, respectively. This could explain the increased mortality attributed to these pathogens in other studies. Interventions targeting EPEC and Cryptosporidium may reduce morbidity and mortality in high HIV-prevalence settings.
INTRODUCTION
Diarrheal disease remains a leading cause of death in children under 5 years of age and in resource-limited settings, most diarrhea is attributed to enteric pathogens [1, 2]. In addition to the acute morbidity and mortality attributable to diarrhea, the enteric mucosal damage that occurs in diarrhea leads to decreased nutrient absorptive capacity, growth failure, and cognitive delay [3–7]. HIV-infected children experience more frequent and severe diarrhea episodes and are at higher risk of malnutrition and cognitive impairment than their uninfected counterparts [8–12]. As prevention of mother-to-child HIV transmission (PMTCT) programs expand, pediatric HIV infections are declining, however, there is a growing population of HIV-exposed uninfected (HEU) children [13]. HEU children experience greater risk of death, hospitalization and neurodevelopmental delays compared to HIV-unexposed children [14–17]. The increased morbidity and mortality observed among HEU children may be due to more frequent enteric infections, earlier weaning, reduced breast milk exposure, decreased immunologic development during infancy, poor socioeconomic status, or diminished parental caretaking capacity [12, 18, 19].
Guidelines for syndromic management of diarrheal illness in low-resource settings do not differentiate management strategies by HIV-status [20, 21]. If HIV-infected or HEU children are more likely to be infected with pathogens independently associated with poor growth and death, targeted diagnostic stool testing and/or empiric antibiotic/anti-protozoal therapy for these high risk groups may be helpful in diminishing mortality, morbidity, and transmission. We determined the prevalence of enteric pathogens among HIV-infected, HEU, and HIV-unexposed children presenting with acute diarrhea.
METHODS
Population
Between November 2011 through October 2013 children aged 6 months to 15 years presenting to Kisii Provincial or Homa Bay District Hospital with acute diarrhea (defined as ≥3 loose stools within 24 hours lasting less than 14 continuous days[22]) were enrolled in an ongoing diarrhea surveillance study. Children were excluded if they were unaccompanied by a biological parent or legal guardian, unable to provide a stool sample or rectal swab, or if the primary caregiver elected not to receive HIV counseling on behalf of the child. Study participants were recruited from both outpatient and inpatient settings. Written informed consent was obtained from primary caregivers of enrolled children and assent was obtained from children over 12 years. The University of Washington Institutional Review Board and the Kenya Medical Research Institute Ethical Review Committee approved the current study.
Data collection
Stool was collected, examined for consistency and appearance, and separated into two containers for shipment. When children could not produce stool, 3 rectal swabs were collected. Sociodemographic characteristics, possible exposures (recent antibiotic use [including cotrimoxazole (CTX)], travel history, water source and filtration, sanitation), breastfeeding and vaccination history were obtained from the primary caregiver. Study physicians measured height and weight, and assessed danger and dehydration signs according to the WHO Integrated Management of Childhood Illness (IMCI) algorithm Height for age and weight for height z-scores (HAZ & WHZ) were calculated using the 2006 and 2007 WHO reference populations for children under 5 and 5 or over, respectively and stunting and wasting defined as HAZ <−2 and WHZ <−2, respectively [23, 24]. A child was classified as having a severe illness if one or more IMCI danger signs (unable to drink or breastfeed, convulsions, continuous vomiting, and/or lethargy/unconsciousness) were identified[20]. Children were classified as having MSD if they had sunken eyes, loss of skin turgor, visible blood in stool, or required intravenous hydration or hospital admission based on diarrhea or dysentery[2].
Children were tested for HIV using antibody testing (Abbott Determine™ rapid test kit and confirmed using Uni-Gold™) or HIV DNA polymerase chain reaction (PCR) assays if <18 months. Malaria parasitemia was assessed by both rapid testing (Paracheck Pf® Orchid Biomedical Services, India) and microscopy. Maternal HIV-status was ascertained by antibody testing or by self-report if the mother reported being HIV-infected.
Stool Specimen Processing
All stool/rectal swab specimens were shipped to the United States Army Research Unit Microbiology Hub in Kericho, Kenya, within 24–48 hours of collection. Selective media was used for bacterial identification-BAP (blood agar plate) for hemolysis and oxidase test, Sorbitol-MacConkey agar to select for non-sorbitol fermenting (NSF) Escherichia coli (E. coli), MacConkey agar for E. coli colonies, Hektoen or xylose lysine deoxycholate agar for Salmonella and Shigella spp., cefsulodin irgasan novobiocin agar for Yersinia spp., andcefoperazone vancomycin amphotericin (CVA) agar for Campylobacter spp. Pure colonies exhibiting the proper characteristics on the various media above were further processed using MicroScan WalkAway 40 Plus automated platform.
NSF E. coli isolates were batch tested using multiplex PCR to identify virulent forms of E. coli: enterotoxigenic E. coli (ETEC)- heat labile enterotoxin (elt) and/or heat stable enterotoxin (est), enteroaggregative E. coli (EAEC)- aatA; enteroinvasive E. coli (EIEC)- invasion plasmid antigen H (ipaH); or enterohemmorhagic E. coli (EHEC)- Shiga toxin 1, 2 and variants (stx); and enteropathogenic E. coli (EPEC)- bundle forming pilus (bfpA) [25]. Starting in March 2013, additional gene targets for EPEC- intimin (eae) and for EAEC- aaiC were incorporated. A classification of EAEC was therefore the identification of aatA and/or aaiC. EPEC was disaggregated into two categories: typical EPEC (bfpA with or without eae) and atypical EPEC (eae without either bfpA or stx).
For parasitic identification, the stool was concentrated using the Mini Parasep® Solvent Free concentration kit (DiaSys, Berkshire, England) and then microscopy used to identify parasitic forms. Parasite testing was not performed on rectal swabs.
Statistical Analysis
Two sets of analyses were performed; the first compared risk factors and enteric pathogens between HIV-infected and HIV-uninfected children, and the second compared risk factors and pathogens between HEU and HIV-unexposed children. Children who were missing HIV-status information were excluded and children without maternal HIV status available were excluded from the second analysis.
Sociodemographic and clinical characteristics of included children were compared using Chi-square or Fisher’s exact tests for categorical variables and t-tests for continuous variables. Enteric pathogens were compared using prevalence ratios (PR) and associated P-values estimated using relative risk (RR) regression and associated chi-square or Fisher’s exact tests. For the comparison of enteric pathogens, we adjusted for multiple comparisons using the Benjamini and Hochberg method using a false discovery proportion of 0.05 [26]. For all pathogens associated with HIV-infection and/or HIV-exposure in the univariate analyses, we constructed multivariable models to account for confounding and mediating variables. Because of strong evidence of an age association with specific enteric infections, age was a priori retained as a confounding variable in all multivariable models [2]. Potential confounders, including site, year of enrollment (2011, 2012, 2013), household income, number persons/room, and drinking water source and treatment were included stepwise in multivariable models and maintained in the final model if they changed the PR for the main exposure variable of interest (HIV or HEU) by more than 10%. Variables that we considered to plausibly be on the causal pathway between HIV and enteric pathogens included breastfeeding history (exclusive breastfeeding duration, current breastfeeding), nutritional status indicators (HAZ and WHZ) and recent CTX use [27–30]. Potential mediators were individually added to age-adjusted models. Subgroup analyses were performed among children <24 months and among children with MSD. Confounder, mediator, and subgroup analyses were considered exploratory analyses and therefore an alpha of 0.05 was used to determine statistical significance.
RESULTS
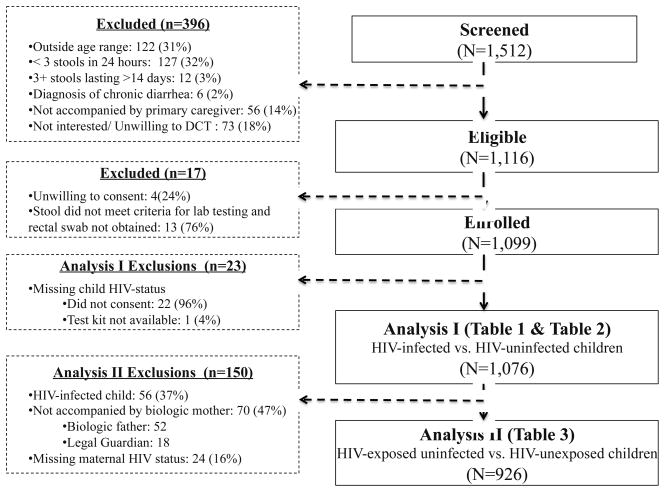
Among 1,512 children presenting with diarrhea, 1,116 met inclusion criteria, 1,099 were enrolled and 1,076 had known HIV-status and therefore were included in analysis I (HIV-infected 5.2% [N=56] and HIV-uninfected 94.8% [N=1,020]) (Figure 1 & Table 1). Among the 56 HIV-infected children, almost half (44.6%) were newly diagnosed as part of this study and among those already diagnosed, 27 (87.1%) were reportedly enrolled in HIV care and 7 (22.6%) reported current ART use. Among the 53 HIV-infected children for whom CD4% or CD4 count was available, 19 (35.9%) were immunosuppressed based on WHO age-specific CD4 cut-offs [31]. Maternal HIV status was ascertained for 926 (90.1%) of the 1,020 HIV-uninfected children, of which 105 (11.3%) and 821 (88.7%) were HEU and HIV-unexposed, respectively. These 926 children were included in analysis II (Figure 1). Only 36 (34.3%) HIV-infected mothers self-reported their last CD4 count (median was 483 cells per cells/mm3 (IQR: 301–834)).
Figure 1.
Participant Inclusion Flow for Analyses I and II
Table 1.
Characteristics of children in Analysis I
| Characteristic | Enrolled N=1,076 |
|---|---|
| n (%a) Median (IQR) | |
| Sociodemographic | |
| Site | |
| Kisii | 513 (47.7%) |
| Homa Bay | 563 (52.3%) |
| ≥1 hour to get to hospital | 177(16.5%) |
| Child accompanied by | |
| Biological mother | 998 (92.8%) |
| Biological father | 53 (4.9%) |
| Legal guardian | 25 (2.3%) |
| Monthly household income <5,000 Kenyan Shillings | 429(40.0%) |
| Household owns ≥ cow | 475(44.3%) |
| # persons/room | 2(1–3) |
| Water source | |
| Piped in house or yard or public tap | 391 (36.5%) |
| Protected well/spring | 456 (42.5%) |
| Unprotected well/spring/surface water | 149 (13.9%) |
| Other b | 76 (7.1%) |
| Household treats drinking water | 795(73.9%) |
| Flush toilet | 75(7.0%) |
| Male | 576(53.5%) |
| Age | |
| 6m–2yr | 585 (54.4%) |
| >2yr–5yr | 367 (34.1%) |
| >5yr | 124(11.5%) |
| Clinical Presentation | |
| 1 or more IMCI General Danger Sign c | 335(31.3%) |
| Moderate to severe diarrhea d | 315(29.3%) |
| Blood observed in stool | 14(1.3%) |
| Mucous in stool | 509(47.3%) |
| Stunted (HAZ<−2) | 171(16.9%) |
| Wastede (WHZ<−2) | 202(21.4%) |
| MUAC <12.5 | 86(8.0%) |
| Malariaf | 106(9.9%) |
| Current fever (≥37.5°C) | 362(33.6%) |
| Clinical History | |
| Child ever breastfed | 1086(98.9%) |
| Child currently breast-feedingamong children less than 2 years | 452(77.4%) |
| # months exclusively breastfed | 6.0(4–6) |
| HIV positive | 56(5.2%) |
| New diagnosis | 25 |
| Known status | 31 |
| Immunosuppressed g | 19(35.9%) |
| HIV-exposed uninfected h | 105(11.3%) |
% among those with non-missing data
Tube well or borehole (n=18), rainwater (n=55), cart with small tank (n=1), bottled water (n=2)
Defined as not able to drink or breastfeed, convulsions, vomits everything, lethargic or unconscious [22]
One or more of the following; sunken eyes, loss of skin turgor, intravenous hydration administered or prescribed, visible blood in stool, or hospital admission based on diarrhea or dysentery [2]
Only calculated for children 5 years and younger
Positive result on microscopy alone (n=8), on RDT alone (n=5) or both RDT and microscopy (n=93)
Defined in terms of CD4% (age ≤11 months: <25%, 12 months–35 months: <20%, 36+ months: <15%) or, in absence of CD4 % data, in terms of CD4 count (age ≤11 months: <1500 cells/mm3, 12 months–35 months: <750 cells/mm3, 36+ months <350 cells/mm3)
Among 926 HIV-uninfected children who were accompanied by the biological mother who was HIV-infected
Bacteria and Parasite Frequency
At least one potential pathogen (bacterial or parasitic) was identified in 45.8% (493/1,076) of the children. Nearly 10% (105/1,076) had 2 organisms identified, 1.4% (15/1076) with 3, <0.5% (2/1076) with 4, and 1 had 5 distinct isolates (Supplementary Figure 1). There were no differences in frequency of bacteria isolation between the 981 children who provided whole stool samples and the 95 who provided rectal swab only (35.1% vs. 31.6%, P=0.50).
One or more diarrheagenic E. colis were identified in the stools of 262/1,076 (24.4%) children. EAEC was identified in 143 (13.3%) of the 1,076 children, EPEC in 66 (6.1%) (4.0% were typical and 2.1% atypical), ETEC in 47 (4.4%), EIEC in 32 (3.0%), and EHEC in 4 (0.4%) stools. Among the 262 children with any identified diarrheagenic E. coli, infection with multiple E. coli serotypes was common (11.1%) (Supplementary Figure 1). Other commonly isolated bacteria included Campylobacter spp. (68 [6.3%]) and Shigella spp. (49 [4.6%]).
Almost a quarter (24.2%) of the 981 children that provided a whole stool sample had at least one parasite identified. The most frequently identified parasites were Giardia spp. (109 children [11.1%]) and Cryptosporidium spp. (36 [3.7%]). Other likely non-pathogenic parasites were also identified, including Blastocystis hominis (73 [7.4%]), Chilomastix spp. (4 [0.40%]) and Endolimax spp. (1 [0.10%]).
HIV-infected vs. HIV-uninfected Children
Compared to HIV-uninfected children, HIV-infected children were older (mean age 50 vs. 31 months, p<0.001), more likely to be enrolled at the Homa Bay site (67.9% vs. 51.5%, P=0.017), more likely to come from low-income households (52.7% vs. 39.3%, P=0.048), and less likely to be accompanied by their biologic mother (85.7% vs. 93.1%, P=0.037) (Table 2a). The mean reported number of months of exclusive breastfeeding was similar between HIV-infected and. HIV-uninfected children (4.8 vs. 5.0 months, P=0.33). However, among the children under 24 months old, HIV-infected children were less likely to be breastfeeding at the time of enrollment (50% vs. 80.5%, P=0.002) even after accounting for age (P=0.018), more likely to have taken CTX within the preceding week (23.2% vs. 5.4%, P <0.001), more likely to be stunted (34.6% vs. 15.8%, P=0.001), and more likely to have MSD (41.1% vs. 28.6%, P=0.046).
Table 2a.
Demographic and clinical differences between HIV-infected and -uninfected children
| HIV-Infected N=56 |
HIV-Uninfected N=1020 |
||
|---|---|---|---|
|
|
|||
| Selected Factors | N/mean (%/SD) | N/mean (%/SD) | P-value |
| Homa Bay Site | 38 (67.9%) | 525 (51.5%) | 0.017 |
| Age in months | 49.7 (40.4) | 31.3 (31.1) | <0.001 |
| Income <5,000 KSH | 29 (52.7%) | 400 (39.3%) | 0.048 |
| # persons/room in house | 2.4 (1.2) | 2.4 (1.4) | 0.73 |
| Unprotected water source a | 16 (29.1%) | 133 (13.1%) | 0.001 |
| Household treats drinking water | 46 (82.1%) | 749 (73.4%) | 0.15 |
| Accompanied by biological mother | 48 (85.7%) | 950 (93.1%) | 0.037 |
| Child <24 months and currently breast-feeding b | 9 (50%) | 437 (80.5%) | 0.002 |
| # months exclusively breastfed c | 4.8 (1.9) | 5.0 (1.8) | 0.329 |
| Child took cotrimoxazole in last 7 days | 13 (23.2%) | 55 (5.4%) | <0.001 |
| Stunted (HAZ<−2) | 18 (34.6%) | 153 (15.9%) | <0.001 |
| Wasted (WHZ<−2) | 12 (29.3%) | 190 (21.1%) | 0.212 |
| Moderate to Severe Diarrhea | 23 (41.1%) | 292 (28.6%) | 0.046 |
| Rectal swab taken | 8 (14.3%) | 87 (8.5%) | 0.140 |
| Blood in stool | 1 (1.8%) | 13 (1.3%) | 0.529 |
Unprotected well, unprotected spring, or surface water
Among 18 HIV-infected children and 544 HIV-uninfected children
When considering only the subset of children < 24 months of age: mean # of months exclusively breastfed were 5.2 (SD: 1.8) vs. 5.1 (SD: 1.8), p=0.861.
The prevalence of enteric bacteria and parasites by HIV-infection status are reported in Table 2b. In univariate analysis, HIV-infected children were nearly 3 times more likely to have typical EPEC identified in their stools compared to HIV-uninfected children (PR=2.95; P=0.008); this association persisted after adjusting for age (adjusted [a]PR: 3.70 [95%CI: 1.6–8.4, P=0.002]) and no additional measured confounders were identified. HIV-status remained associated with typical EPEC in analyses further adjusted for duration (months) of exclusive breastfeeding (aPR: 3.81 [1.68–8.66, P=0.001]), current breastfeeding (aPR: 3.46 [1.51–7.90, P=0.003]), WHZ (aPR: 2.9 [95%CI: 1.1–7.7, P=0.036]), HAZ (aPR: 3.9 [95%CI; 1.7–8.9, P=0.001]), and CTX use (aPR: 3.5 [95%CI: 1.5–8.0, P=0.004]). Among HIV-infected children in whom CD4 data were available (n=53), typical EPEC was identified more commonly among the immunosuppressed than non-immunosuppressed children (15.8% vs. 8.8%) but the difference was not significant (P=0.655).
Table 2b.
Enteric pathogen differences between HIV-infected and -uninfected children
| HIV-Infected N=56 |
HIV-Uninfected N=1020 |
Prevalence Ratio | P-value | |
|---|---|---|---|---|
|
|
||||
| Organism Identified | N % | N % | ||
| Bacteria | ||||
| Campylobacter speciesa | 6 (10.7%) | 62 (6.1%) | 1.76 | 0.165 |
| >1 E. coli serotypeb | 2 (11.1%) | 27 (11.1%) | 1.00 | 1.00 |
| EAEC | 6 (10.7%) | 137 (13.2%) | 0.81 | 0.560 |
| EIEC | 1 (1.8%) | 31 (3.0%) | 0.59 | 1.00 |
| EHEC | 0 (0%) | 4 (0.4%) | -- | 1.00 |
| EPEC-atypicalc | 2 (3.6%) | 21 (2.1%) | 2.74 | 0.178 |
| EPEC-typical | 6 (10.7%) | 37 (3.6%) | 2.95 | 0.008 |
| ETEC | 5 (8.9%) | 42 (4.1%) | 2.17 | 0.092 |
| Salmonella speciesd | 1 (1.8%) | 13 (1.3%) | 1.40 | 0.529 |
| Shigella speciese | 1 (1.8%) | 46 (4.5%) | 0.40 | 0.508 |
| Other bacteriaf | 1 (1.8%) | 22 (2.2%) | 0.83 | 1.00 |
| Parasitesg | ||||
| Giardia species | 5 (10.4%) | 104 (11.2%) | 0.93 | 0.875 |
| Cryptosporidium species | 1 (2.1%) | 35 (3.8%) | 0.56 | 1.00 |
| Entaeombea species | 0 (0%) | 4 (2.6%) | -- | 1.00 |
| Ascaris lumbricoides | 1 (2.1%) | 23 (2.5%) | 0.85 | 1.00 |
| Blastocystis hominis | 4 (8.3%) | 69 (7.4%) | 1.13 | 0.809 |
| Other parasiteh | 1 (2.1%) | 5 (0.5%) | 3.89 | 0.261 |
| No organism identifiedi | 19 (39.6%) | 456 (47.4%) | 0.84 | 0.209 |
HIV-infected: Campylobacter jejuni (n=6), HIV-uninfected: Campylobacter jejuni (n=39), Campylobacter spp. other than jejuni (n=23)
Among children with at least one diarrheagenic E. coli serovar (18 HIV-infected and 244 HIV-uninfected)
Among 417 patients enrolled since March 2013 who had the gene eae tested
HIV-infected: non-typhoidal species (n=1), HIV-uninfected: Salmonella typhi (n=3), Salmonella paratyphi (n=1), Salmonella non-typhoidal species (n=9)
HIV-infected: species not determined (n=1); HIV-uninfected: Shigella flexneri (n=15), Shigella sonnei (n=18), Shigella dysentariae (n=2), species not determined (n=11)
HIV-infected: Plesiomonas shigelloides (n=1); HIV-uninfected: Providencia alcalifaciens(n=6), Providencia stuartii (n=1), Providencia rettgeri (n=1) Citrobacter freundii (n=3), Citrobacter amalonaticus (n=1), Enterobacter agglomerans (n=2), Enterobacter cloacae (n=1), Kluyvera ascorbata (n=2), Escherichia vulneris (n=1), Yersinia enterocolitica (n=1), Aeromonas hydrophila (n=1), Pseudomonas aeruginosa (n=1), Edwardsiella tarda (n=1)
Among 981 children in whom whole stool was collected (HIV-infected: n=48, HIV-uninfected: n=933)
HIV-infected: Chilomastix mesnili (n=1); HIV-uninfected: Chilomastix mesnili (n=3), Cystoisospora belli (n=1), Endolimax nana (n=1)
Among the 981 children who had both bacteria and parasite testing performed (HIV-infected: n=48, HIV-uninfected: n=933)
Subgroup analyses performed among the 562 children aged <24 months (18 (3.2%) HIV-infected and 544 (96.8%) HIV-uninfected) demonstrated no significant differences in the prevalence of typical EPEC between HIV-infected and HIV-uninfected children (11.1% vs. 5.0%, PR: 2.2 [95%CI: 0.6–8.7, P=0.25], age-adjusted PR: 2.6 [95%CI: 0.7–10.2, P=0.17]). In subgroup analyses among 315 children with MSD (23 [7.3%] HIV-infected and 292 [92.7%] HIV-uninfected) HIV-infected children were much more likely to have typical EPEC identified compared to HIV-uninfected children (21.7% vs. 4.1%, PR: 5.3 [95%CI: 2.0–13.7, P<0.001], age-adjusted PR: 6.1 [95%CI: 2.28–16.1, P<0.001]). No other differences in prevalence of other pathogens were observed in these two subgroups.
HEU vs. HIV-Unexposed Children
Compared to HIV-unexposed children (n=821), HEU children (n=105) were more likely to present in Homa Bay than Kisii (87.6% vs. 48.5%, P <0.001) (Table 3a), to live in households with lower incomes (64.8% vs. 36.8%, P<0.001) and were more likely to report having an unprotected water source (28.6% vs. 11.1%, P<0.001) but more likely to treat their drinking water (87.6% vs. 71.8%, P<0.001). HEU and HIV-unexposed children had similar mean number of exclusive breastfeeding months (4.8 vs. 5.0, p=0.329) but among the children under 24 months, HEU children were less likely to be currently breastfeeding (50% vs. 80.5%, p=0.002) despite no difference in mean age among those under 24 months (mean ages in HEU vs HIV-unexposed: 12.0 months vs. 11.1 months, P=0.14). Finally, HEU children were more likely to report having taken CTX in the preceding week (18.3% vs. 4.0%, P<0.001) and were more likely to be stunted (24.5% vs. 15.2%, P=0.016).
Table 3a.
Demographic and clinical differences between HEU and HIV-unexposed children
| HEU N=105 |
HIV-unexposed N=821 |
||
|---|---|---|---|
|
|
|||
| Characteristic | N/Mean (%/SD) | N/mean (%/SD) | P-value |
| Homa Bay Site | 92 (87.6%) | 398 (48.5%) | <0.001 |
| Age in months | 25.2 (20.1) | 30.3 (29.6) | 0.092 |
| Income <5,000 KSH | 68 (64.8%) | 301 (36.8%) | <0.001 |
| # persons/room in house | 4.9 (1.6) | 4.8 (1.7) | 0.6305 |
| Unprotected water source | 30 (28.6%) | 91 (11.1%) | <0.001 |
| Household treats drinking water | 92 (87.6%) | 589 (71.8%) | <0.001 |
| Child <24 months and currently breast-feeding a | 32 (53.3%) | 380 (85.8%) | <0.001 |
| # months exclusively breastfed b | 5.4 (1.6) | 5.0 (1.9) | 0.096 |
| Child took cotrimoxazole in last 7 days | 19 (18.1%) | 33 (4.0%) | <0.001 |
| Stunted (HAZ<−2) | 25 (24.5%) | 117 (15.2%) | 0.016 |
| Wasted (WHZ<−2) | 26 (26.5%) | 148 (20.2%) | 0.145 |
| Moderate to Severe Diarrhea | 35 (33.3%) | 224 (27.3%) | 0.193 |
| Rectal swab taken | 14 (13.3%) | 67 (8.2%) | 0.077 |
| Blood in stool | 0 (0%) | 10 (1.2%) | 0.614 |
Among 60 HEU and 443 HIV-unexposed children
When considering only the subset of children < 24 months of age the mean # of months exclusively breastfed were 5.3 (SD: 1.5) vs. 5.1 (SD: 1.9), p=0.235
The prevalence of bacterial and parasitic infections by HIV-exposure category are presented in Table 3b. Cryptosporidium spp. was more common in HEU children (PR: 3.0, P=0.003). The association between HIV-exposure and Cryptosporidium spp. was independent of age-alone (aPR: 2.81 [95%CI: 1.36–5.80, P=0.005]) and age and site, the only identified confounder (aPR: 2.09 [95%CI: 1.01–4.50, P=0.046]). When we accounted for duration (months) of exclusive breastfeeding and current breastfeeding in age-adjusted models, the association remained significant (aPR: 2.70 [95%CI: 1.31–5.58, P=0.007]; aPR: 2.80 [95%CI: 1.32–5.92, P=0.007]). Similarly, the association between maternal HIV-status and Cryptosporidium spp. infection persisted after including WHZ, HAZ, and CTX use in age-adjusted models (WHZ-aPR: 2.8 [95%CI: 1.4–5.7, P=0.005]; HAZ-aPR: 2.7 [95%CI 1.3–5.6, P=0.008]; CTX-aPR 2.5 [95%CI: 1.2–5.4, P =0.020], respectively). Only 36 of the 105 HIV-infected mothers of HIV-uninfected children knew their most recent CD4 count. Although, the mean CD4 count among the mothers of HEU children with Cryptosporidium spp. appeared to be lower (378.5 cells/mm3) than the maternal CD4 count among those without Cryptosporidium spp. identified (647.9 cells/mm3), this difference was not statistically significant (P=0.300).
Table 3b.
Enteric pathogen differences between HEU and HIV-unexposed children
| HEU N=105 |
HIV-unexposed N=821 |
Prevalence Ratio | P-value | |
|---|---|---|---|---|
|
|
||||
| Organism Identified | N (%) | N (%) | ||
| Bacteria | ||||
| Campylobacter species | 8 (7.6%) | 47 (5.7%) | 1.33 | 0.439 |
| >1 E. coli serotypea | 2 (8.0%) | 21 (10.7%) | 0.75 | 1.00 |
| EAEC | 14 (13.3%) | 111 (13.5%) | 0.99 | 0.958 |
| EIEC | 4 (3.8%) | 24 (2.9%) | 1.3 | 0.548 |
| EHEC | 0 (0%) | 2 (0.24%) | -- | 1.00 |
| EPEC-atypicalb | 4 (3.8%) | 15 (1.8%) | 1.96 | 0.264 |
| EPEC-typical | 3 (2.9%) | 29 (3.5%) | 0.81 | 1.00 |
| ETEC | 2 (1.9%) | 37 (4.5%) | 0.42 | 0.302 |
| Salmonella species | 0 (0%) | 12 (1.5%) | -- | 0.380 |
| Shigella species | 2 (1.9%) | 36 (4.4%) | 0.43 | 0.302 |
| Other bacteria | 4 (3.8%) | 14 (1.7%) | 2.23 | 0.137 |
| Parasitesc | ||||
| Giardia species | 13 (14.3%) | 80 (10.6%) | 1.35 | 0.290 |
| Cryptosporidium species | 9 (9.9%) | 25 (3.3%) | 2.98 | 0.003 |
| Entaeombea species | 0 (0%) | 4 (0.5%) | -- | 1.00 |
| Ascaris lumbricoides | 0 (0%) | 21 (2.8%) | -- | 0.154 |
| Blastocystis species | 8 (8.8%) | 55 (7.3%) | 1.21 | 0.608 |
| Other parasite | 2 (2.2%) | 3 (0.40%) | 5.52 | 0.092 |
| No organism identifiedd | 41 (45.1%) | 377 (50.0%) | 0.90 | 0.597 |
Among children with at least one diarrheagenic E. coli serovar (25 HEU and 196 HIV-unexposed)
Among 367 patients enrolled since March 2013 who had the gene eae tested
Among 845 children in whom whole stool was collected (HEU: n=91, HIV-unexposed: n=754)
Among the 845 children who had both bacteria and parasite testing performed (HEU: n=91, HIV-unexposed: n=754)
Cryptosporidium spp. was also more commonly identified in the stool of the 60 HEU children <24 months of age who were tested for parasites (12.2%) as compared to the 444 HIV-unexposed children <24 months who were tested for parasites (4.7%) (PR 2.59 [95%CI: 1.1–6.2 P=0.033], age-adjusted PR: 2.68 [95%CI: 1.1–6.4, P=0.03]). Among children with MSD (35 HEU & 224 HIV-unexposed), the prevalence of Cryptosporidium spp. infection was also significantly higher in HEU children compared to HIV-unexposed children (PR 4.1 [95%CI: 1.4–11.7, P=0.008], age-adjusted PR: 4.1 [95%CI: 1.5–11.7, P=0.008]). No other differences in pathogen distribution were observed between HEU and HIV-unexposed children in these subgroups.
DISCUSSION
In this cross-sectional study of Kenyan children with acute diarrhea, HIV-infection and HIV-exposure status were both associated with specific enteric pathogens. Typical EPEC was over three times more common in HIV-infected compared to HIV-uninfected children and Cryptosporidium spp. three times more common in HEU compared to HIV-unexposed children. These associations were independent of measured potential confounding and mediating factors. Additionally, we found magnitude of associations to be particularly strong in the subgroup of children with MSD. This finding further supports data from the multi-site GEMS study which found typical EPEC and Cryptosporidium spp. infection during diarrhea episodes to be independently associated with higher case-fatality, particularly in sub-Sahahan African countries where HIV prevalence was highest [2]. If typical EPEC and Cryptosporidium spp. are disproportionally affecting HIV-infected and HEU children, and if HIV-testing rates in health care settings continue to increase, then health workers seeing children with acute diarrhea might consider child and maternal HIV-infection to be an indicator for more intense follow-up or empiric antibiotic/antiprotozoal therapy.
The association between typical EPEC and HIV-infection in children with acute diarrhea suggests a possible immune-modulated mechanism of action; it could be that the attaching and effacing (A/E) lesions characteristic of EPEC exploit deficiencies in the intestinal immune system or epithelial barrier, both common pathologies in HIV-associated intestinal dysfunction [32, 33]. Current Kenyan guidelines for management of diarrhea do not specify antimicrobial treatment of EPEC [34]. In other settings, the recommended treatment for EPEC is either CTX or a floroquinolone [35]. We did not observe differences in the prevalence of other pathogens between HIV-infected and HIV-uninfected children, a finding consistent with reports from other studies [8, 9]. However, among a subset of the GEMS study population in Western Kenya, higher prevalences of ETEC, Cryptosporidium, EPEC and astrovirus in HIV-infected children were reported [36].
We also observed a strong association between HIV-exposure and Cryptosporidium spp. infection among HIV-uninfected children. HIV-infected persons are at greater risk for Cryptosporidium spp. infection and likely shed Cryptosporidium oocysts in greater quantities and for longer periods of time than their HIV-uninfected counterparts [37, 38]. As a result, children of HIV-infected mothers may be exposed to the parasite more frequently than HIV-unexposed children. HEU children may also be less likely to acquire Cryptosporidium-specific antibodies from breast milk [39]. Although we did not observe differences in exclusive breastfeeding duration, we did find that among the younger children (<24 months), HIV-infected and HEU children were less likely to be currently breastfed even after accounting for age differences within the younger age group. Finally HEU children were more likely to have recently taken CTX than HIV-unexposed children. Recent exposure to antibiotics may result in treatment or suppression of bacterial pathogens, resulting in the preferential identification of parasitic infections such as Cryptosporidium spp.
Cryptosporidium spp. infections are associated with nutrient malabsorption, growth faltering, and cognitive disabilities, even in the absence of diarrhea, and these outcomes are common among HEU children [40–42]. It is plausible that some of the failure to thrive observed among HEU children may be the result of increased risk of Cryptosporidium spp. infection in this group. Nitazoxanide has shown efficacy, including among HIV-infected individuals, and could be considered in HEU children with Cryptosporidium spp. infection [43, 44]. Antiretroviral treatment (ART) has also been shown to decrease susceptibility to Cryptosporidium spp. and earlier and/or better coverage of ART among HIV-infected mothers may reduce exposure to Cryptosporidium spp. infection in HEU children [45].
Our study had several strengths and limitations. Strengths include the large cohort with detailed characterization of bacterial and parasitic pathogens associated with diarrhea and consideration of the type 1 error rate from testing for associations among multiple pathogens. Limitations include the lack of non-diarrhea controls which limited our ability to estimate prevalence of asymptomatic carriage of each organism and subsequently estimate the proportion of diarrheal cases attributed to a given organism. However data from a large multi-country case-control study, the GEMS study, has addressed this issue among children with MSD [2]. While our study could not conclusively attribute the cause of diarrhea to the organisms identified, many of the organisms isolated are known to be associated with poor weight gain and linear growth, even in the absence of diarrhea [46–49]. We were also not able to isolate enteric viruses in this study and rotavirus is the leading cause of diarrheal illness in children [2, 36]. However, the introduction of rotavirus vaccination in high burden countries is expected to substantially reduce diarrheal illness due to rotavirus and thus bacterial and parasitic causes of diarrhea may become increasingly important [50, 51].
Although we measured exclusive breastfeeding duration and current breastfeeding status, we did not ascertain the age of weaning and thus could not evaluate how this variable might impact our findings. We also did not collect previous diarrhea morbidity information from the child or mother, and doing so might have strengthened a proposed causal relationship between maternal HIV, maternal Cryptosporidium infection, and Cryptosporidium in HEU children. Future studies that include surveillance stool sampling, ART-status of HIV-infected mothers, detailed weaning and feeding data, and extensive clinical information from the mothers will help us understand why typical EPEC and Cryptosporidium spp. were independently associated with HIV-infection and exposure, respectively, in this study population.
Intriguingly, although we detected an association between HEU and Cryptosporidium spp., we did not find an association with HIV-infection. The lack of association may be due to lack of power-- we had only 56 children with HIV, 8 of whom were not tested for parasites because they could not produce fresh stool. The cohort prevalence of Cryptosporidium spp. was 3.7%, a prevalence similar to studies which utilized microscopy, but lower than studies using PCR-based methods (prevalences as high as 31.3%) [52–54]. In addition, Cryptosporidium spp. infection is more common in children under 2 years old and the average age of HIV-infected children in our cohort was higher (4 years) [2, 55].
Despite these limitations, this study found important and novel relationships between HIV status and two enteropathogens that are significant causes of morbidity and mortality in sub-Saharan Africa. In current management guidelines for acute diarrhea, EPEC and Cryptosporidium are not specifically considered. In the absence of laboratory-based stool testing, ascertaining HIV-infection status of the child and the mother may help clinicians determine optimal empiric treatment for acute diarrhea. If HIV-infected mothers or other HIV-infected household members are indeed exposing children to Cryptosporidium spp. more frequently, then improving HIV care and treatment of mothers and other HIV-infected household members may have indirect benefits such as reducing childhood diarrhea incidence, growth failure, and cognitive delay. Finally, this study suggests that efforts to increase coverage of water, sanitation, and hygiene (WASH) programs are particularly important in high HIV-prevalence settings.
Supplementary Material
Acknowledgments
SOURCES OF SUPPORT
Funding was provided by the National Institute of Health [grant number U19-A2090882]. The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript. The study was designed and implemented by the study investigators and the investigators conducted the analysis and prepared the manuscript. PP is supported by the University of Washington STD/AIDS Research Training Program [grant number T32-AI007140] from the National Institutes of Health. Also GJS is supported by a National Institute of Health mentoring award (grant number K24-HD054314).
We would like to thank all of the participants and clinics who participated in this study. We would also like to acknowledge the staff of the University of Washington/Kenya Medical Research Institute collaboration and at the Walter Reed Project, United States Army Medical Research Unit-Kenya. This research and publication were made possible with support from the University of Washington Center for AIDS Research (CFAR), an NIH funded program (P30 AI027757), which is supported by the following NIH Institutes and Centers (NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA). Additionally we would like to thank Kizazi working group (UW Global Center for Integrated Health of Women, Adolescents and Children [Global WACh]) and the Kenya Research Program for their support during the preparation of this article. The authors would also like to thank Dr. Solomon Mpoke, the director of the Kenya Medical Research Institute, for his support. The findings and conclusions in this paper are those of the authors and do not necessarily reflect the views of their supporting institutions.
Footnotes
CONFLICTS OF INTEREST
None of the authors have a commercial or other association that might pose a conflict of interest.
PBP, GCJ, DMD, BAR, and JLW conceived of the study. Analyses were performed by PBP with the help of FMO, BAR, DMD, and GCJ. EAO and JMN prepared and ran samples for analysis. PBP wrote the final article with the help of all authors. PBPP, BOS, JMN, FMO, EAO, and JLW participated in the larger surveillance study.
References
- 1.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 2.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 3.Moore SR, Lima NL, Soares AM, Oria RB, Pinkerton RC, Barrett LJ, et al. Prolonged episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gastroenterology. 2010;139:1156–1164. doi: 10.1053/j.gastro.2010.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorntz B, Soares AM, Moore SR, Pinkerton R, Gansneder B, Bovbjerg VE, et al. Early childhood diarrhea predicts impaired school performance. The Pediatric infectious disease journal. 2006;25:513–520. doi: 10.1097/01.inf.0000219524.64448.90. [DOI] [PubMed] [Google Scholar]
- 5.Lunn PG, Northrop-Clewes CA, Downes RM. Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet. 1991;338:907–910. doi: 10.1016/0140-6736(91)91772-m. [DOI] [PubMed] [Google Scholar]
- 6.Prendergast A, Kelly P. Enteropathies in the developing world: neglected effects on global health. The American journal of tropical medicine and hygiene. 2012;86:756–763. doi: 10.4269/ajtmh.2012.11-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keusch GT, Rosenberg IH, Denno DM, Duggan C, Guerrant RL, Lavery JV, et al. Implications of acquired environmental enteric dysfunction for growth and stunting in infants and children living in low- and middle-income countries. Food and nutrition bulletin. 2013;34:357–364. doi: 10.1177/156482651303400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Eijk AM, Brooks JT, Adcock PM, Garrett V, Eberhard M, Rosen DH, et al. Diarrhea in children less than two years of age with known HIV status in Kisumu, Kenya. Int J Infect Dis. 2010;14:e220–225. doi: 10.1016/j.ijid.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Thea DM, St Louis ME, Atido U, Kanjinga K, Kembo B, Matondo M, et al. A prospective study of diarrhea and HIV-1 infection among 429 Zairian infants. The New England journal of medicine. 1993;329:1696–1702. doi: 10.1056/NEJM199312023292304. [DOI] [PubMed] [Google Scholar]
- 10.Laughton B, Cornell M, Boivin M, Van Rie A. Neurodevelopment in perinatally HIV-infected children: a concern for adolescence. J Int AIDS Soc. 2013;16:18603. doi: 10.7448/IAS.16.1.18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith R, Chernoff M, Williams PL, Malee KM, Sirois PA, Kammerer B, et al. Impact of HIV severity on cognitive and adaptive functioning during childhood and adolescence. The Pediatric infectious disease journal. 2012;31:592–598. doi: 10.1097/INF.0b013e318253844b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rollins NC, Ndirangu J, Bland RM, Coutsoudis A, Coovadia HM, Newell ML. Exclusive breastfeeding, diarrhoeal morbidity and all-cause mortality in infants of HIV-infected and HIV uninfected mothers: an intervention cohort study in KwaZulu Natal, South Africa. PLoS One. 2013;8:e81307. doi: 10.1371/journal.pone.0081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Global Report: UNAIDS report on the global AIDS epidemic 2013. Geneva, Switzerland: WHO; 2013. [Google Scholar]
- 14.Koyanagi A, Humphrey JH, Ntozini R, Nathoo K, Moulton LH, Iliff P, et al. Morbidity among human immunodeficiency virus-exposed but uninfected, human immunodeficiency virus-infected, and human immunodeficiency virus-unexposed infants in Zimbabwe before availability of highly active antiretroviral therapy. The Pediatric infectious disease journal. 2011;30:45–51. doi: 10.1097/INF.0b013e3181ecbf7e. [DOI] [PubMed] [Google Scholar]
- 15.Le Doare K, Bland R, Newell ML. Neurodevelopment in children born to HIV-infected mothers by infection and treatment status. Pediatrics. 2012;130:e1326–1344. doi: 10.1542/peds.2012-0405. [DOI] [PubMed] [Google Scholar]
- 16.Slogrove A, Reikie B, Naidoo S, De Beer C, Ho K, Cotton M, et al. HIV-exposed uninfected infants are at increased risk for severe infections in the first year of life. Journal of Tropical Pediatrics. 2012;58:505–508. doi: 10.1093/tropej/fms019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filteau S. The HIV-exposed, uninfected African child. Trop Med Int Health. 2009;14:276–287. doi: 10.1111/j.1365-3156.2009.02220.x. [DOI] [PubMed] [Google Scholar]
- 18.Clerici M, Saresella M, Colombo F, Fossati S, Sala N, Bricalli D, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood. 2000;96:3866–3871. [PubMed] [Google Scholar]
- 19.Kasahara TM, Hygino J, Blanco B, Xavier L, Araujo-Lima CF, Guillermo LV, et al. The impact of maternal anti-retroviral therapy on cytokine profile in the uninfected neonates. Hum Immunol. 2013;74:1051–1056. doi: 10.1016/j.humimm.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Handbook: Integrated Management of Childhood Illness for High HIV Settings. Geneva, Switzerland: WHO; 2008. [PubMed] [Google Scholar]
- 21.World Health Organization. WHO recommendations on the management of diarrhoea and pneumonia in HIV-infected infants and children. Geneva, Switzerland: WHO; 2010. [PubMed] [Google Scholar]
- 22.World Health Organization. The Treatment of Diarrhoea. A manual for physicians and other senior health workers. Geneva, Switzerland: WHO; 2007. [Google Scholar]
- 23.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization; 2006. p. 312. (available on the web site: http://www.who.int/childgrowth/publications/en/) [Google Scholar]
- 24.de Onis M, Onyango A, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bulletin of the World Health Organization. 2007;85:661–668. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aranda KR, Fabbricotti SH, Fagundes-Neto U, Scaletsky IC. Single multiplex assay to identify simultaneously enteropathogenic, enteroaggregative, enterotoxigenic, enteroinvasive and Shiga toxin-producing Escherichia coli strains in Brazilian children. FEMS microbiology letters. 2007;267:145–150. doi: 10.1111/j.1574-6968.2006.00580.x. [DOI] [PubMed] [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B-Methodological. 1995;57:289–300. [Google Scholar]
- 27.Goga AE, Doherty T, Jackson DJ, Sanders D, Colvin M, Chopra M, et al. Infant feeding practices at routine PMTCT sites, South Africa: results of a prospective observational study amongst HIV exposed and unexposed infants - birth to 9 months. International breastfeeding journal. 2012;7:4. doi: 10.1186/1746-4358-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isanaka S, Duggan C, Fawzi WW. Patterns of postnatal growth in HIV-infected and HIV-exposed children. Nutrition reviews. 2009;67:343–359. doi: 10.1111/j.1753-4887.2009.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization (WHO) Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents and adults: recommendations for a public health approach. Vol. 2006. Geneva: WHO Department of HIV/AIDS; 2006. [accessed 26 July 2013]. Available from: http://www.who.int/entity/hiv/pub/guidelines/ctxguidelines.pdf. [Google Scholar]
- 30.Sherry B, Embree JE, Mei Z, Ndinya-Achola JO, Njenga S, Muchunga ER, et al. Sociodemographic characteristics, care, feeding practices, and growth of cohorts of children born to HIV-1 seropositive and seronegative mothers in Nairobi, Kenya. Tropical medicine & international health: TM & IH. 2000;5:678–686. doi: 10.1046/j.1365-3156.2000.00631.x. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. Recommendations for a publich health approach. Geneva, Switzerland: WHO; 2006. Antiretroviral therapy of HIV infection in infants and children: Towards universal access. [PubMed] [Google Scholar]
- 32.Stockmann M, Schmitz H, Fromm M, Schmidt W, Pauli G, Scholz P, et al. Mechanisms of epithelial barrier impairment in HIV infection. Annals of the New York Academy of Sciences. 2000;915:293–303. doi: 10.1111/j.1749-6632.2000.tb05257.x. [DOI] [PubMed] [Google Scholar]
- 33.Trabulsi LR, Keller R, Tardelli Gomes TA. Typical and atypical enteropathogenic Escherichia coli. Emerging infectious diseases. 2002;8:508–513. doi: 10.3201/eid0805.010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kenya Ministry of Public Health Sanitation. Policy guidelines on control and management of diarrhoeal diseases in children below five years in Kenya. Nairobi, Kenya: Division of child and adolescent health; 2010. [Google Scholar]
- 35.O’Ryan GM, Ashkenazi-Hoffnung L, O’Ryan-Soriano MA, Ashkenazi S. Management of acute infectious diarrhea for children living in resource-limited settings. Expert review of anti-infective therapy. 2014;12:621–632. doi: 10.1586/14787210.2014.901168. [DOI] [PubMed] [Google Scholar]
- 36.O’Reilly Ciara ERO, Moke Fenny, Ondeng Alex, Hukumu Emmanuel, Ibworo Vincent, Rajasingham Anangu, Ochieng Benjamin, Farag Tamer H, Nasrin Dilruba, Panchalingam Sandra, Nataro James P, Kotloff Karen L, Levine Myron M, Oundo Joseph, Parsons Michele B, Bopp Cheryl, Vulule John, Laserson Kayla, Mintz Eric D, Breiman Robert F. Moderate-to-severe diarrhea among children less than five years old with HIV infected mothers in rural western Kenya. The American Society of Tropical Medicine and Hygiene Annual Meeting; Philadelphia, PA USA. 2011. [Google Scholar]
- 37.Hunter PR, Nichols G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clinical microbiology reviews. 2002;15:145–154. doi: 10.1128/CMR.15.1.145-154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ajjampur SS, Sankaran P, Kang G. Cryptosporidium species in HIV-infected individuals in India: an overview. Natl Med J India. 2008;21:178–184. [PubMed] [Google Scholar]
- 39.Korpe PS, Liu Y, Siddique A, Kabir M, Ralston K, Ma JZ, et al. Breast milk parasite-specific antibodies and protection from amebiasis and cryptosporidiosis in Bangladeshi infants: a prospective cohort study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;56:988–992. doi: 10.1093/cid/cis1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guerrant DI, Moore SR, Lima AA, Patrick PD, Schorling JB, Guerrant RL. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four-seven years later in a poor urban community in northeast Brazil. The American journal of tropical medicine and hygiene. 1999;61:707–713. doi: 10.4269/ajtmh.1999.61.707. [DOI] [PubMed] [Google Scholar]
- 41.Mor SM, Tzipori S. Cryptosporidiosis in children in Sub-Saharan Africa: a lingering challenge. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2008;47:915–921. doi: 10.1086/591539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Checkley W, Epstein LD, Gilman RH, Black RE, Cabrera L, Sterling CR. Effects of Cryptosporidium parvum infection in Peruvian children: growth faltering and subsequent catch-up growth. Am J Epidemiol. 1998;148:497–506. doi: 10.1093/oxfordjournals.aje.a009675. [DOI] [PubMed] [Google Scholar]
- 43.Cabada MM, White AC., Jr Treatment of cryptosporidiosis: do we know what we think we know? Curr Opin Infect Dis. 2010;23:494–499. doi: 10.1097/QCO.0b013e32833de052. [DOI] [PubMed] [Google Scholar]
- 44.Bailey JM, Erramouspe J. Nitazoxanide treatment for giardiasis and cryptosporidiosis in children. The Annals of pharmacotherapy. 2004;38:634–640. doi: 10.1345/aph.1D451. [DOI] [PubMed] [Google Scholar]
- 45.Zardi EM, Picardi A, Afeltra A. Treatment of cryptosporidiosis in immunocompromised hosts. Chemotherapy. 2005;51:193–196. doi: 10.1159/000086920. [DOI] [PubMed] [Google Scholar]
- 46.Lee G, Pan W, Penataro Yori P, Paredes Olortegui M, Tilley D, Gregory M, et al. Symptomatic and asymptomatic Campylobacter infections associated with reduced growth in Peruvian children. PLoS Negl Trop Dis. 2013;7:e2036. doi: 10.1371/journal.pntd.0002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Platts-Mills JA, Gratz J, Mduma E, Svensen E, Amour C, Liu J, et al. Association Between Stool Enteropathogen Quantity and Disease in Tanzanian Children Using TaqMan Array Cards: A Nested Case-Control Study. The American journal of tropical medicine and hygiene. 2014;90:133–138. doi: 10.4269/ajtmh.13-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muhsen K, Levine MM. A systematic review and meta-analysis of the association between Giardia lamblia and endemic pediatric diarrhea in developing countries. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;55(Suppl 4):S271–293. doi: 10.1093/cid/cis762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Mekhlafi HM, Al-Maktari MT, Jani R, Ahmed A, Anuar TS, Moktar N, et al. Burden of Giardia duodenalis infection and its adverse effects on growth of schoolchildren in rural Malaysia. PLoS neglected tropical diseases. 2013;7:e2516. doi: 10.1371/journal.pntd.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atherly DE, Lewis KD, Tate J, Parashar UD, Rheingans RD. Projected health and economic impact of rotavirus vaccination in GAVI-eligible countries: 2011–2030. Vaccine. 2012;30(Suppl 1):A7–14. doi: 10.1016/j.vaccine.2011.12.096. [DOI] [PubMed] [Google Scholar]
- 51.Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–141. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- 52.Cegielski JP, Ortega YR, McKee S, Madden JF, Gaido L, Schwartz DA, et al. Cryptosporidium, enterocytozoon, and cyclospora infections in pediatric and adult patients with diarrhea in Tanzania. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 1999;28:314–321. doi: 10.1086/515131. [DOI] [PubMed] [Google Scholar]
- 53.Tumwine JK, Kekitiinwa A, Bakeera-Kitaka S, Ndeezi G, Downing R, Feng X, et al. Cryptosporidiosis and microsporidiosis in Ugandan children with persistent diarrhea with and without concurrent infection with the Human Immunodeficiency Virus. The American journal of tropical medicine and hygiene. 2005;73:921–925. [PubMed] [Google Scholar]
- 54.Binka E, Vermund SH, Armah GE. Rotavirus diarrhea among children less than 5 years of age in urban Ghana. The Pediatric infectious disease journal. 2011;30:716–718. doi: 10.1097/INF.0b013e318223bd85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molbak K, Hojlyng N, Gottschau A, Sa JC, Ingholt L, da Silva AP, et al. Cryptosporidiosis in infancy and childhood mortality in Guinea Bissau, west Africa. BMJ. 1993;307:417–420. doi: 10.1136/bmj.307.6901.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.