Abstract
Cholinergic neurotransmission plays a role in regulation of respiratory pattern. Nicotine from cigarette smoke affects respiration and is a risk factor for sudden infant death syndrome (SIDS) and sleep-disordered breathing. The cellular and synaptic mechanisms underlying this regulation are not understood. Using a medullary slice preparation from neonatal rat that contains the preBötzinger Complex (preBötC), the hypothesized site for respiratory rhythm generation, and generates respiratory-related rhythm in vitro, we examined the effects of nicotine on excitatory neurotransmission affecting inspiratory neurons in preBötC and on the respiratory-related motor activity from hypoglossal nerve (XIIn). Microinjection of nicotine into preBötC increased respiratory frequency and decreased the amplitude of inspiratory bursts, whereas when injected into XII nucleus induced a tonic activity and an increase in amplitude but not in frequency of inspiratory bursts from XIIn. Bath application of nicotine (0.2–0.5 μM, approximately the arterial blood nicotine concentration immediately after smoking a cigarette) increased respiratory frequency up to 280% of control in a concentration-dependent manner. Nicotine decreased the amplitude to 82% and increased the duration to 124% of XIIn inspiratory bursts. In voltage-clamped preBötC inspiratory neurons (including neurons with pacemaker properties), nicotine induced a tonic inward current of −19.4 ± 13.4 pA associated with an increase in baseline noise. Spontaneous excitatory postsynaptic currents (sEPSCs) present during the expiratory period increased in frequency to 176% and in amplitude to 117% of control values; the phasic inspiratory drive inward currents decreased in amplitude to 66% and in duration to 89% of control values. The effects of nicotine were blocked by mecamylamine (Meca). The inspiratory drive current and sEPSCs were completely eliminated by 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) in the presence or absence of nicotine. In the presence of tetrodotoxin (TTX), low concentrations of nicotine did not induce any tonic current or any increase in baseline noise, nor affect the input resistance in inspiratory neurons. In this study, we demonstrated that nicotine increased respiratory frequency and regulated respiratory pattern by modulating the excitatory neurotransmission in preBötC. Activation of nicotinic acetylcholine receptors (nAChRs) enhanced the tonic excitatory synaptic input to inspiratory neurons including pacemaker neurons and at the same time, inhibited the phasic excitatory coupling between these neurons. These mechanisms may account for the cholinergic regulation of respiratory frequency and pattern.
INTRODUCTION
Acetylcholine (ACh) plays a role in central respiratory control (Burton et al. 1995; Gesell et al. 1943; Gillis et al. 1988; Metz 1958; Murakoshi et al. 1985; Nattie and Li 1990; Shao and Feldman 2000; Weinstock et al. 1981), which may have important implications in some common disorders of breathing. Impairment in cardiorespiratory control and the responsiveness to hypoxia are hypothesized to be important mechanisms in sudden infant death syndrome (SIDS). The incidence of SIDS is correlated to maternal smoking, smoking by the primary care giver, and nicotine exposure through breast milk (Haglund and Cnattingius 1990; Klonoff-Cohen et al. 1995; Milerad and Sundell 1993; Stepans and Wilkerson 1998), all of which produce elevated circulating levels of nicotine in infants. ACh in brain stem regions associated with sleep is involved in state-dependent respiratory depression (Lydic and Baghdoyan 1993), and smoking is a risk factor for sleep-disordered breathing characterized by repeated episodes of breath cessation (apnea) and reduced ventilation (hypopnea) during sleep (Wetter et al. 1994). Cholinergic mechanisms also underlie central respiratory failure during organophosphate, e.g., nerve gas, poisoning (Lotti 1991). On the beneficial side, nicotine is being investigated as a therapeutic agent for diseases such as Parkinson’s disease, Alzheimer’s disease, and sleep apnea (Benowitz 1996; Gothe et al. 1985).
ACh enhances respiratory motor activity and consequent minute ventilation following intra-arterial injection or application to the fourth ventricle of anesthetized dog in vivo; these effects are potentiated by the cholinesterase inhibitor physostigmine (Gesell et al. 1943). In an en bloc brain stem–spinal cord preparation from neonatal rat generating a rhythmic respiratory motor output, bath application of ACh increases respiratory frequency; this effect is diminished, but not completely abolished, by atropine. Further addition of the nicotinic antagonist dihydro-β-erythroidine (DH-β-E), can completely abolish the effect of ACh (Murakoshi et al. 1985). These effects may be mediated by nAChR. nAChR subunits α4, β2, and α7 are present in the ventrolateral medulla (Dominguez del Toro et al. 1994; Wada et al. 1989). However, the mechanisms underlying the central effects of nicotine on breathing are poorly understood. Basic physiological questions include the following. Where does nicotine act to affect breathing? Is part of this action via direct effects on the neurons postulated to generate respiratory rhythm? How does nicotine affect respiratory neurons and their interactions resulting in modulation of respiratory pattern?
Insight into the cellular mechanisms for cholinergic actions on breathing would provide a physiological understanding of the central effects of smoking, would help to delineate the possible side effects of therapeutic application of nicotine, and could lead to better strategies for treatment of SIDS, sleep apnea, and central respiratory failure during organophosphate poisoning.
The preBötC is hypothesized to be the site for respiratory rhythm generation (Gray et al. 1999; Rekling and Feldman 1998; Smith et al. 1991). A subpopulation of inspiratory neurons in preBötC that have intrinsic pacemaker properties coupled by excitatory synaptic connections are proposed to be the kernel for respiratory rhythm generation (Butera et al. 1999; Rekling and Feldman 1998). The voltage-dependent bursting properties of pacemaker neurons provide a means for controlling frequency by tonic depolarizing or hyperpolarizing input. There are M3-like acetylcholine receptors on preBötC inspiratory neurons, including pacemaker neurons (Shao and Feldman 2000). Activation of these receptors depolarizes inspiratory neurons, which may underlie the cholinergic-induced increase of respiratory frequency. The purpose of this study is to understand the mechanisms underlying the cholinergic modulation of respiratory pattern mediated by nicotinic receptors.
METHODS
Slice preparation
Experiments were performed on medullary slice preparations that retain functional respiratory networks and generate respiratory rhythm (Smith et al. 1991). Sprague-Dawley neonatal rats (0–3 days old) were anesthetized by hypothermia by placing them on crushed ice for 3–4 min and then promptly decerebrated. The cerebellum was removed, and the brain stem–spinal cord was isolated. The brain stem–spinal cord was mounted in the specimen vise of a Vibratome (Technical Products International, VT 100) oriented vertically with rostral end upward. The brain stem was sectioned serially in the transverse plane under a dissection microscope until the landmarks at rostral boundary of preBötC were visible. One transverse slice (500–650 μm thick) was cut. The slice was transferred to a recording chamber of 3-ml volume and stabilized with a threaded frame. The dissection and slicing were performed in an artificial cerebrospinal fluid (ACSF) bubbled with 95% O2-5% CO2 at room temperature. The ACSF contained (in mM) 128 NaCl, 3.0 KCl, 1.5 CaCl2, 1.0 MgSO4, 23.5 NaHCO3, 0.5 NaH2PO4, and 30 glucose. During electrophysiological recording, the slice was continuously superfused (~2.5–3.5 ml/min) with ACSF with increased KCl (9 mM) that was recycled into a reservoir equilibrated with 95% O2-5% CO2. The ACSF in the recording chamber was maintained at 27 ± 1°C during experiments. All slices studied had rhythmic activities from XIIn that were similar in frequency and in temporal pattern to the respiratory activities recorded from the en bloc brain stem–spinal cord preparations (Smith et al. 1991).
Electrophysiological recording
Neurons within 100 μm of the slice surface were visualized with an infrared-differential interference contrast (IR-DIC) microscope (×400, Axioskop, Zeiss). The respiratory neurons we recorded in this study fired in phase with the inspiratory bursts from XIIn and were located ventral to the nucleus ambiguus. Patch electrodes were pulled from thick wall (0.32 mm) borosilicate glass with tip size 1–1.5 μm (resistance: 4–6.5 MΩ). The electrode filling solution contained (in mM) 140 potassium gluconate, 5.0 NaCl, 0.1 CaCl2, 1.1 EGTA, and 2.0 ATP (Mg2+ salt) with the pH adjusted to 7.3 with KOH. Intracellular signals were amplified with a patch-clamp amplifier (AXOPATCH 200A, Axon Instruments). A −10-mV junction potential was determined experimentally; reported values of potential are corrected values.
The respiratory-related nerve activity was recorded from the cut ends of XIIn roots with a suction electrode, amplified 10,000–20,000 times and band-pass filtered (3–3,000 Hz). Both signals from intracellular recording and from XIIn roots were recorded on videocassettes via pulse code modulation (Vetter Instruments).
Data analysis
Selected segments of intracellular signal were low-pass filtered at 1 kHz (except those for average analysis of phasic inspiratory drive current, indicated in figure legends) with an 8-pole Bessel filter (Frequency Devices), and XIIn nerve activity was rectified and integrated (Paynter filter, τ = 15 ms); then both were digitized at sampling frequency 2.5 kHz with DIGIDATA 1200 and software CLAMPEX 8 (Axon Instruments).
Spontaneous excitatory postsynaptic current (sEPSC) data were analyzed with a program written in AXOBASIC (Axon Instruments). This program read the Axon Binary Files (ABF) containing two channels of digitized data: the whole cell patch-clamp signal and the integrated XIIn activity. The program detected sEPSCs during expiratory periods by setting a threshold for the derivative of the membrane current signal and then measured the time as well as the peak amplitude of sEPSCs. The program also detected peaks of the integrated XIIn signal that mark inspirations and muted these periods. The conventional statistical methods for miniature EPSCs, e.g., using the Kolmogorov-Smirnov test to analyze the inter-event intervals of two groups of EPSCs, are not valid, because the expiratory periods are interrupted by variable inspiratory periods. We are not able to obtain continuous inter-event intervals for sEPSCs. To compare the rates between sEPSC series, we assumed that each series was a Poisson process with mean rate λ. If m1 and m2 sEPSCs are observed in time periods t1 and t2, the estimate . If m1 and m2 are large, the estimate tends to be normally distributed with mean λ1 − λ2. The variance of can be estimated with
Then
has a standard normal distribution (Cox and Lewis 1966). Statistical significance for difference in rates can be analyzed by testing the null hypothesis, i.e., λ1 − λ2 = 0. Since the amplitude of sEPSC is not normally distributed, the difference in sEPSC amplitude between two series of sEPSCs was analyzed with Kolmogorov-Smirnov test. The rates and the amplitudes of sEPSCs were tested during application of cholinergic agents versus the control conditions for each neuron. Since the respiratory rhythm is regular in the slice preparation, the distribution of respiratory period can be assumed normal. Thus respiratory periods were measured routinely. Respiratory frequency was determined as the reciprocal of the average of 6–10 consecutive periods in the control or drug application condition for each preparation. Then it was averaged across preparations and presented as means ± SD. While a statistical test for difference in frequency is necessary, a t-test was done on the raw data (the periods). The inspiratory amplitude and duration of XIIn activity, the phasic inward current amplitude, and duration of inspiratory neurons were measured from an averaged envelope of five consecutive inspiratory periods triggered by the up-stroke of the integrated inspiratory bursts from XIIn. Then they were averaged across neurons or preparations and presented as means ± SD. Paired t-tests were used taking the measurements during pharmacological manipulation versus those in the control conditions in the same cell or same preparation as pairs. P ≤ 0.05 was taken as criterion for statistical significance.
Drug application
Nicotine was applied either by adding it to the perfusate or by local pressure injection into preBötC or XII nucleus. For bath application, the effects were measured immediately prior to adding nicotine for control and 3.5–5 min after adding it for the tests. For pressure injection, the effects were measured immediately prior to the injection for control and ~1–2 s after the injection for tests. Five-microliter calibrated glass pipettes (1 μl/division, Fisher Scientific) were pulled and the tips were broken to a diameter of 6–9 μm. The injection pipette was mounted on a micromanipulator and advanced into XII nucleus or 50–150 μm ventral to nucleus ambiguus (XII nucleus and nucleus ambiguus can be identified easily by their distinct anatomical location and the morphology of the neurons under IR-DIC microscopy), 100–200 μm below the slice surface. The injection volume was monitored by the displacement of the fluid meniscus with a microscope containing a calibrated eyepiece reticule. For pressure injection, nicotine was dissolved in a pipette solution containing (in mM) 142 NaCl, 9.0 KCl, 1.5 CaCl2, 1.0 MgSO4, 10 HEPES, and 30 glucose (pH was adjusted to 7.4). The antagonists were applied by adding them to the perfusate. The effects of the antagonists were measured 4.5–6 min after adding them. (−)-Nicotine (hydrogen tartrate salt), mecamylamine hydrochloride, TTX, and 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX) were obtained from SIGMA/RBI.
RESULTS
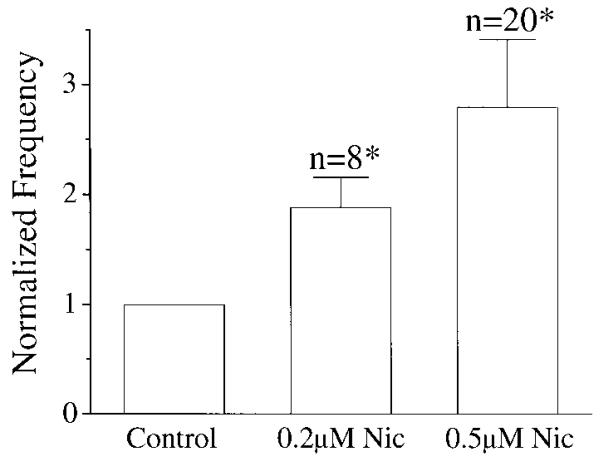
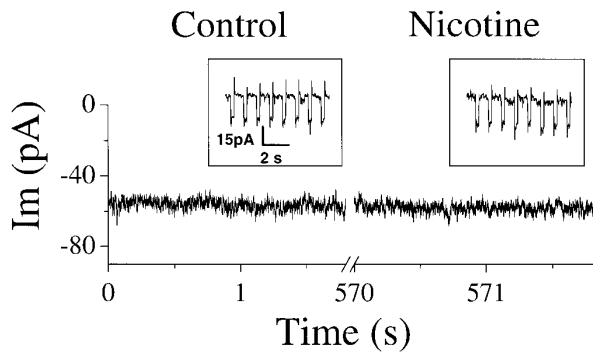
Bath application of 0.2 or 0.5 μM nicotine (equivalent to the arterial blood nicotine concentration shortly after a cigarette has been smoked) (Henningfield et al. 1993) increased respiratory frequency. To avoid possible confounding effects of desensitization, only one concentration was used for each preparation. Under control conditions, the frequency was 5.9 ± 1.7/min (mean ± SD, n = 28). The frequency increased to 9.7 ± 3.3/min (188 ± 28% of control) with 0.2 μM (n = 8) and to 16.6 ± 4.1/min (280 ± 61% of control) with 0.5 μM nicotine (n = 20; Figs. 1 and 3A). The amount of 0.5 μM nicotine also increased the duration of XIIn inspiratory bursts from 640 ± 185 ms to 800 ± 300 ms (124 ± 30% of control, n = 20) and decreased the amplitude from 135 ± 82 μV to 110 ± 69 μV (82 ± 12% of control; Table 1). These effects were maximal within 3–5 min after nicotine was added to the perfusate.
FIG. 1.
Concentration dependence of respiratory frequency for bath-applied nicotine (Nic). Frequency was the reciprocal of the average of 6–10 consecutive periods and was normalized by dividing the number of inspiratory bursts per minute during nicotine application by that in control for each preparation. To avoid possible confounding effects of desensitization, only 1 concentration was used for each preparation. * Statistically significant (P < 0.05) with paired t-test on respiratory periods taking the value during nicotine application and the control value before nicotine application as a pair.
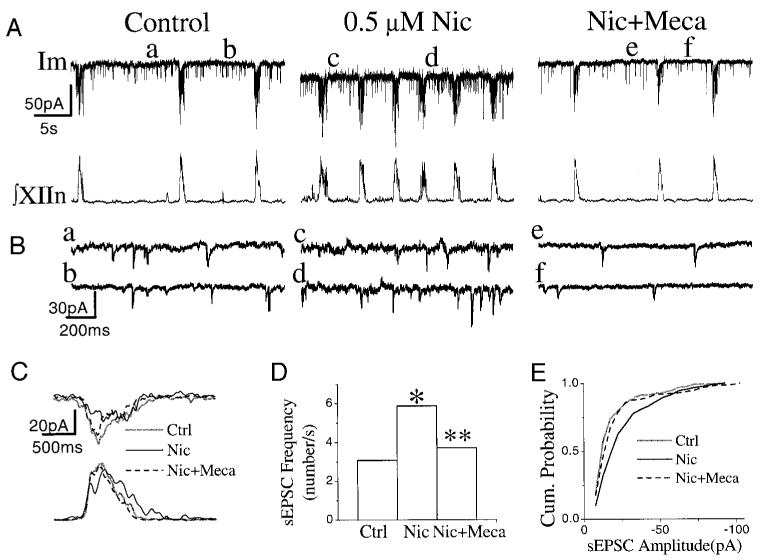
FIG. 3.
Effects of bath application of nicotine (Nic) and mecamylamine (Meca) on respiratory pattern and on inspiratory neurons. A: Im, membrane current of inspiratory neuron, voltage-clamped at −60 mV. ∫XIIn: integrated hypoglossal nerve signal. Meca (1 μM) was bath-applied 5.5 min after the application of 0.5 μM Nic. B: spontaneous excitatory postsynaptic currents (sEPSCs) on an extended time scale. Each trace was taken 3.5 s prior to the start of an inspiratory burst. a–f correspond to a–f, respectively, in A. C: the phasic inspiratory drive current of an inspiratory neuron voltage-clamped at −60 mV in control, nicotine, and Meca conditions. Each trace was low-pass filtered at 10 Hz and averaged for 5 consecutive inspiratory periods triggered by the up-stroke of the integrated inspiratory bursts from XIIn. The effects of Nic on the frequency (D), and on amplitude (E) of sEPSCs in an inspiratory neuron, as well as their recovery under 1 μM Meca. * Statistical significance during nicotine application vs. control. ** Statistical significance during Meca application vs. nicotine application.
TABLE 1. Effects of low concentrations of nicotine on respiratory pattern and preBötC inspiratory neurons.
| Control | 0.5 μM Nicotine | Percent Control, % | n | P Value | |
|---|---|---|---|---|---|
| XIIn motor activity | |||||
| Respiratory frequency, min−1 | 5.9 ± 1.7* | 16.6 ± 4.0 | 279.7 ± 61.4 | 20 | 5.5 E-10 |
| Inspiratory amplitude (integrated), μV | 135.1 ± 82.3 | 110.3 ± 69.4 | 81.5 ± 12.2 | 20 | 0.00013 |
| Inspiratory duration, ms | 640 ± 185 | 800 ± 300 | 124.0 ± 30.0 | 20 | 0.00077 |
| PreBötC neurons | |||||
| Frequency of sEPSCs, s−1 | 2.68 ± 1.17 | 4.28 ± 1.8 | 176.2 ± 85.4 | 15 | |
| Amplitude of sEPSCs, pA | −19.56 ± 4.98 | −22.24 ± 5.68 | 117.0 ± 30.3 | 15 | |
| Phasic inward current amplitude, pA | −57.7 ± 38.3 | −38.20 ± 26.8 | 65.5 ± 11.7 | 17 | 1.6 E-5 |
| Phasic inward current duration, ms | 934.7 ± 190.5 | 824.1 ± 196.5 | 89.0 ± 15.9 | 17 | 0.013 |
Values are means ± SD; n is sample size. Respiratory frequency is the reciprocal of the average of 6–10 consecutive respiratory periods. The data for nicotine application were measured 3.5–5 min after bath application of nicotine. Inspiratory amplitude, inspiratory duration, phasic inward current amplitude, and phasic inward current duration were measured from the averaged envelope of 5 consecutive inspiratory periods triggered by the up-stroke of the integrated inspiratory bursts from XIIn for each condition. Durations were measured at 20% of peak amplitude. sEPSC indicates spontaneous excitatory postsynaptic current during the expiratory period. The frequency of sEPSCs is the number of sEPSCs divided by total expiration time during ;1 min recording time. Please refer to METHODS and RESULTS for the statistical tests for frequency and amplitude of sEPSCs.
n = 28 for this value.
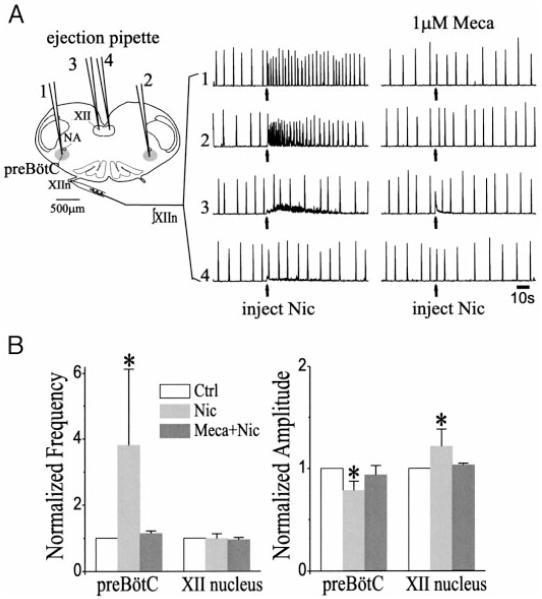
To determine the site of nicotinic action, we unilaterally pressure injected 10 nl nicotine (20 μM) into the preBötC and observed increases in frequency from 7.6 ± 2.0 to 29.4 ± 19.7/min (n = 6, paired t-test on the period, P = 0.0002), which were symmetric on both XII nerves (Fig. 2, A and B; data from both sides were pooled). We also observed a decrease in amplitude of inspiratory bursts (integrated) of XIIn from 143 ± 76 μV to 113 ± 67 μV (paired t-test, P = 0.006, Fig. 2, A and B). The effects were reduced or absent when the injections were in the vicinity of but outside the preBötC. When the same amount of nicotine was injected into the XII nucleus, there was an increase in tonic activity and an increase in amplitude of inspiratory bursts from 112 ± 80 μV to 127 ± 80 μV (P = 0.014, n = 5) of the ipsilateral but not the contralateral XIIn. There was no change in frequency of the rhythmic inspiratory bursts (Fig. 2, A and B). Bath application of the nicotinic antagonist Meca (1 μM) blocked the effects of nicotine injections into either preBötC or XII nucleus (Fig. 2, A and B).
FIG. 2.
Effects of microinjection of nicotine (Nic, 20 μM, 10 nl) on integrated XIIn activity (∫XIIn). A: arrows indicate the time of injection. The numbers 1, 2, 3, and 4 indicate injections into the ipsilateral preBötC, contralateral preBötC, ipsilateral XII nucleus, and contralateral XII nucleus, respectively. The injection pipettes were inserted into the loci 100–200 μm below the surface of the slice. Left XIIn activity was recorded with the medullary slice caudal side up. All data traces were from the same slice. First set of injections was prior to, and the 2nd set was at least 4.5 min after bath application of 1 μM mecamylamine (Meca). B: summary of the nicotine effects on respiratory frequency and amplitude (means ± SD). Respiratory frequency was determined as the reciprocal of the mean of 6–10 consecutive respiratory periods for each condition. The amplitude of inspiratory bursts was the averaged peak amplitude of 5 consecutive inspiratory bursts for each condition. * Statistical significance of Nic injection vs. control (paired t-test).
To further investigate the cellular mechanisms underlying nicotinic effects on respiratory frequency and pattern, we whole cell patch-clamped preBötC inspiratory neurons while simultaneously recording the respiratory-related motor output from XIIn. Bath application of 0.5 μM nicotine induced an inward current of −19.4 ± 13.4 pA (n = 16) in voltage-clamped (−60 mV) preBötC inspiratory neurons (Fig. 3A) and induced depolarization under current clamp (data not shown). There was also an increase in baseline noise (Fig. 3, A and B). The frequency of sEPSCs during the expiratory periods was 2.7 ± 1.2/s, and the amplitude was −19.6 ± 5.0 pA under control conditions with neurons voltage-clamped at −60 mV (Table 1). Nicotine increased the frequency of these sEPSCs to 4.3 ± 1.8/s and the amplitude to −22.2 ± 5.7 pA (n = 15, Fig. 3, A, B, D, and E, Table 1). Statistical analyses for each neuron were done assuming that each series of sEPSCs was a Poisson process (see methods). The increase in frequency was statistically significant in 10 of 15 neurons, and the increase in amplitude of sEPSCs was significant (Kolmogorov-Smirnov test) in 9 of 15 neurons. Most neurons (8 of 10) that showed a sEPSC frequency increase with nicotine also exhibited an increase in amplitude. All five neurons that did not show a sEPSC frequency increase with nicotine exhibited the nicotine-induced inward current and increase in baseline noise. Three pacemaker neurons (neurons that fired rhythmic bursts of action potentials during the normally silent expiratory period if depolarized to −45 to −55 mV) (Smith et al. 1991) were among these 15 inspiratory neurons. Nicotine increased the frequency and amplitude of sEPSCs in all three of these pacemaker neurons. In inspiratory neurons including the pacemaker neurons, nicotine decreased the amplitude from −57.7 ± 38.3 pA to −38.2 ± 26.8 pA (66 ± 12% of control) and the duration from 935 ± 191 ms to 824 ± 197 ms (89 ± 16% of control) of the inspiratory drive current in phase with the inspiratory bursts of XIIn (n = 17, Fig. 3C; Table 1). These effects on respiratory motor output and respiratory neurons were blocked by Meca (1–10 μM, n = 3, Fig. 3, A-E). The increase in frequency of sEPSCs induced by nicotine was decreased significantly by Meca (n = 3), and the average amplitude was decreased significantly in two of these three neurons.
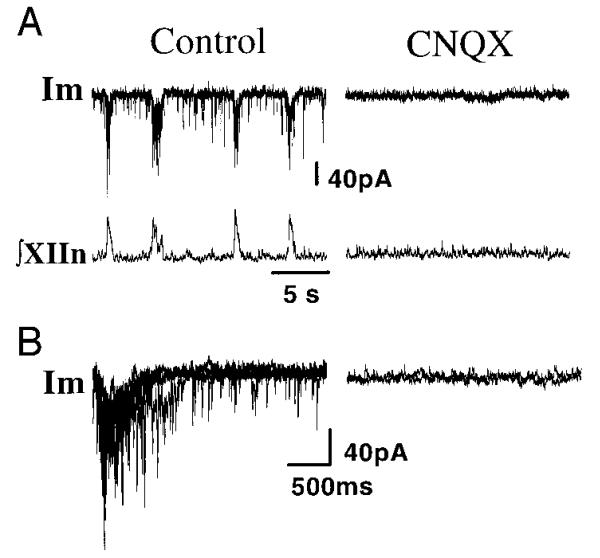
To identify the neurotransmitter systems for the endogenous excitatory input to the preBötC inspiratory neurons, we added CNQX to the perfusate (20 μM). Under control conditions, the sEPSC frequency was 2.6 ± 1.9/s (n = 6). CNQX completely eliminated sEPSCs (frequency = 0, Fig. 4, A and B); subsequent bath application of 0.5 μM nicotine did not induce sEPSCs. CNQX also eliminated the phasic inspiratory drive current in these neurons (n = 7, Fig. 4, A and B) and rhythmic motor activity from XIIn. The sEPSCs, phasic inspiratory drive current, and respiratory-related rhythmic motor activity from XIIn recovered after CNQX was washed out with fresh ACSF. One of these seven neurons had pacemaker-like properties: it generated rhythmic bursts during expiratory periods when it was moderately depolarized in the control condition under current clamp, and it also generated voltage-dependent rhythmic bursts after blockade of excitatory synaptic input by CNQX.
FIG. 4.
A: effects of bath application of 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) on sEPSCs during expiratory periods, on the phasic inspiratory drive current, and on the inspiratory bursting activity from XIIn. B: traces of 5 consecutive respiratory periods triggered by the up-stroke of the integrated inspiratory bursts from XIIn were superimposed for control. Five consecutive traces were superimposed for CNQX. Inspiratory neuron voltage-clamped at −60 mV. Im, membrane current.
With the bath solution containing TTX (0.5 μM), bath application of 0.5 μM nicotine did not induce any current or any increase in baseline noise, nor did it affect the whole cell input resistance in voltage-clamped inspiratory neurons (n = 7, Fig. 5). Two of these seven neurons were pacemaker neurons.
FIG. 5.
The membrane currents (Im) of an inspiratory neuron voltage-clamped at −60 mV before and during bath application of 0.5 μM nicotine with the bath solution containing 0.5 μM TTX. Insets: series of −5-mV voltage pulses were given to measure the input resistance of the neuron in control and nicotine conditions. The scales are the same for both insets.
DISCUSSION
By modulating synaptic transmission, nAChRs plays an important role in a variety of brain functions (McGehee et al. 1995; Role and Berg 1996; Wonnacott 1997). Low concentrations of nicotine enhance glutamatergic excitatory synaptic transmission in hippocampus by a presynaptic action (Gray et al. 1996). In this study, we showed that nicotine acted on the preBötC, resulting in increases in respiratory frequency and changes in respiratory-related motor output in vitro. Nicotine induced a tonic inward current, an increase in baseline noise, and an increase in sEPSC frequency and amplitude in inspiratory neurons including those with pacemaker-like properties. Nicotine did not induce these effects nor induce changes in input resistance when action potential–dependent synaptic transmission was blocked by TTX. These results suggest that nicotine affects inspiratory neurons primarily by modulating excitatory neurotransmission. By systematically analyzing sEPSCs during the expiratory period as well as the phasic inspiratory drive current, we demonstrated that activation of nAChRs enhanced tonic excitatory synaptic input to and inhibited excitatory coupling between inspiratory (including pacemaker) neurons. These results suggest that the nicotinic modulation of excitatory neurotransmission underlies cholinergic regulation of respiratory frequency and pattern.
Medullary sites of cholinergic modulation of respiratory pattern
Determination of the sites for cholinergic modulation of respiratory pattern has been difficult in in vivo experiments (Gesell et al. 1943; Nattie and Li 1990) as well as in the in vitro en bloc brain stem–spinal cord preparation (Murakoshi et al. 1985). Taking advantage of the slice preparation that retains functional respiratory networks and generates respiratory-related rhythmic motor output (Smith et al. 1991), we can locate the preBötC precisely and make targeted injections of cholinergic drugs. Unilateral injection of nicotine into the preBötC increased respiratory frequency. In contrast, injection of nicotine into the XII nucleus increased the tonic activity and the amplitude of inspiratory bursts of XIIn but did not affect the frequency of rhythmic activity (Fig. 2). These results are consistent with the hypothesis that the preBötC is the site for respiratory rhythm generation. The results from nicotine injection into the XII nucleus, in addition to serving as a control for the injection into the preBötC, consistent with the observation (Haxhiu et al. 1984) that nicotine injected intravenously, into the lateral ventricle or applied onto the ventral surface of the medulla increases the activity of XIIn and genioglossus muscle (a dilator upper airway muscle) in cat in vivo, provide a physiological rationale for the investigation of the clinical use of nicotinic agonists in the treatment of obstructive sleep apnea (Gothe et al. 1985), which involves sleep-related loss of tone in the genioglossus muscle.
Differential modulation of excitatory neurotransmission
Excitatory neurotransmission in the preBötC is essential for respiratory rhythm generation in vitro (Funk et al. 1993; Greer et al. 1991). Coupled glutamatergic inspiratory neurons with pacemaker properties are hypothesized as the kernel of respiratory rhythm generation (Rekling and Feldman 1998). The mutual phasic excitatory interactions between inspiratory neurons synchronize the bursting activity of these neurons. These neurons also receive tonic excitatory input that maintains the membrane potential in the range for oscillation and controls the oscillation frequency (Koshiya and Smith 1999). In this study, we showed that the phasic excitatory drive and the tonic excitatory input to preBötC inspiratory (including pacemaker) neurons can be differentially modulated. Low concentrations of nicotine decreased the amplitude and duration of the phasic inspiratory inward current, suggesting a suppression of the phasic excitatory interaction between inspiratory neurons. Nicotine also increased the frequency and amplitude of sEPSCs, indicating an enhancement of the tonic excitatory input. These effects on sEPSCs may be due to activation of nAChRs on neurons that provide tonic excitation to preBötC inspiratory neurons. Some of these sEPSCs may also come from other inspiratory neurons that are depolarized by nicotine and generate spikes randomly during expiration. The nicotine-induced inward current associated with an increase in baseline noise in inspiratory neurons also indicates a facilitation in tonic synaptic input since these effects disappeared in the presence of TTX (Fig. 5). The synaptic input is likely from distal dendrites; thus they cannot be identified as separate sEPSCs.
Butera et al. (1999) proposed computational models of rhythm generation based on the preBötC pacemaker hypothesis (Smith et al. 1991). Their model contains a heterogeneous population of voltage-dependent bursting neurons coupled by excitatory synapses and receiving tonic excitatory inputs. Simulation studies of this model suggest that 1) facilitation of the tonic excitatory input to pacemaker neurons increases respiratory frequency, and 2) decreases in the strength of excitatory synaptic coupling between pacemaker neurons (paradoxically) increases respiratory frequency. We observed that nicotine induced both 1) and 2) in inspiratory, including pacemaker, neurons concurrent with a dramatic increase in respiratory frequency. These observations are supportive of this model and suggest that nicotinic modulation of excitatory neurotransmissions at the cellular level can account for the cholinergic modulation of respiratory frequency observed at the systems level.
Bath application of nicotine decreased the amplitude and duration of the phasic inspiratory drive to preBötC inspiratory neurons, suggesting that nicotine inhibited excitatory coupling between inspiratory neurons. At the same time, we observed an increase in duration and a decrease in amplitude of the inspiratory bursts in XIIn; the decrease in amplitude was also observed when nicotine was locally injected into the preBötC. Inhibition of excitatory coupling between inspiratory neurons resulting in their desynchronizion could account for the observed decrease in amplitude and the increase in duration of inspiratory bursts in the respiratory motor output.
Both the excitatory coupling between inspiratory neurons and the tonic excitatory input to inspiratory neurons in the preBötC are proposed to be glutamatergic (Funk et al. 1993; Greer et al. 1991; Koshiya and Smith 1999). By systematically analyzing sEPSCs in preBötC inspiratory neurons, we found that sEPSCs during the expiratory period were blocked by CNQX (Fig. 4). There are two possibilities: 1) the sEPSCs are from various sources that use different excitatory neurotransmitters. However, the sEPSCs disappeared (frequency = 0) after application of CNQX, making this unlikely. 2) The tonic excitatory input arises from a source that connects to preBötC inspiratory neurons through an oligosynaptic pathway. If such a pathway exists, our results suggest that at least part of this pathway is glutamatergic and mediated by ionotropic non–N-methyl-d-aspartate (non-NMDA) receptors. Cholinergic synapses are unlikely to be one of the primary transmitters mediating the tonic excitatory input because the sEPSCs persisted in the presence of Meca.
In the presence of TTX, low concentrations of nicotine did not induce a postsynaptic current, increase the baseline noise, or affect the input resistance. This does not exclude the possibility that postsynaptic nicotine-gated channels are present in these neurons. The concentration of nicotine (0.5 μM) may have been too low to evoke a detectable current; alternatively, an evoked postsynaptic current may have quickly desensitized because bath application of nicotine was slow. However, we observed a long-lasting increase in respiratory frequency and other responses at this low concentration of nicotine without fast desensitization, suggesting that rapidly desensitizing postsynaptic nicotine-gated channels that mediate fast ACh synaptic transmission, if any, are not involved in this nicotinic regulation of respiratory frequency and respiratory pattern. Our results do not exclude the possibilities that electrical coupling through gap junctions or inhibitory neurotransmission may also be involved in the nicotinic modulation of respiratory pattern. Rekling et al. (2000) show that electrical coupling is present between preBötC rhythmogenic neurons. The inhibitory neurotransmitters GABA and glycine regulate respiratory pattern (Shao and Feldman 1997), and nicotine can modulate GABA release in the hippocampus (Alkondon et al. 1997) and in the lateral geniculate nucleus (Guo et al. 1998).
In summary, our major findings are as follows. 1) Low concentrations of nicotine act on the preBötC and regulate respiratory frequency and pattern. 2) Activation of nAChRs differentially modulates excitatory neurotransmission. The tonic excitatory input to inspiratory neurons including pacemaker neurons is enhanced, and the excitatory coupling between these neurons is inhibited by nicotine. 3) The tonic excitatory input to preBötC inspiratory neurons modulated by nicotine is likely glutamatergic and mediated by non-NMDA glutamate receptors. 4) Modulation of excitatory neurotransmission via nAChRs may be a mechanism that underlies the cholinergic regulation of respiratory frequency and pattern. Whether the modulatory effects of nicotine on excitatory neurotransmission in preBötC inspiratory neurons are pre- and/or postsynaptic or preterminal remains to be determined.
Acknowledgments
The authors thank Dr. Shane Saywell for valuable comments on the manuscript.
This research was supported by National Heart, Lung, and Blood Institute Grant HL-40959.
REFERENCES
- Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther. 1997;283:1396–1411. [PubMed] [Google Scholar]
- Benowitz NL. Pharmacology of nicotine: addiction and therapeutics. Annu Rev Pharmacol Toxicol. 1996;36:597–613. doi: 10.1146/annurev.pa.36.040196.003121. [DOI] [PubMed] [Google Scholar]
- Burton MD, Nouri M, Kazemi H. Acetylcholine and central respiratory control: perturbations of acetylcholine synthesis in the isolated brainstem of the neonatal rat. Brain Res. 1995;670:39–47. doi: 10.1016/0006-8993(94)01249-h. [DOI] [PubMed] [Google Scholar]
- Butera R, Rinzel J, Smith J. Models of respiratory rhythm generation in the pre-Bötzinger complex. II. Populations of coupled pacemaker neurons. J Neurophysiol. 1999;82:398–451. doi: 10.1152/jn.1999.82.1.398. [DOI] [PubMed] [Google Scholar]
- Cox DR, Lewis PAW. The Statistical Analysis of Series of Events. Spottiswoode, Ballantyne; London: 1966. Comparison of rates of occurrence; pp. 223–244. [Google Scholar]
- Dominguez DEL TORO E, Juiz JM, Peng X, Lindstrom J, Criado M. Immunocytochemical localization of the alpha 7 subunit of the nicotinic acetylcholine receptor in the rat central nervous system. J Comp Neurol. 1994;349:325–342. doi: 10.1002/cne.903490302. [DOI] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Generation and transmission of respiratory oscillations in medullary slices: role of excitatory amino acids. J Neurophysiol. 1993;70:1497–1515. doi: 10.1152/jn.1993.70.4.1497. [DOI] [PubMed] [Google Scholar]
- Gesell R, Hansen E, Worzniak J. Humoral intermediation of nerve cell activation in the central nervous system. Am J Physiol. 1943;138:776–791. [Google Scholar]
- Gillis R, Walton D, Quest J, Namath I, Hamosh P, Dretchen K. Cadiorespiratory effects produced by activation of cholinergic muscarinic receptors on the ventral surface of the medulla. J Pharmacol Exp Ther. 1988;247:765–773. [PubMed] [Google Scholar]
- Gothe B, Strohl KP, Levin S, Cherniack NS. Nicotine: a different approach to treatment of obstructive sleep apnea. Chest. 1985;87:11–17. doi: 10.1378/chest.87.1.11. [DOI] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL. Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. J Physiol (Lond) 1991;437:727–749. doi: 10.1113/jphysiol.1991.sp018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J-Z, Tredway TL, Chiappinelli VA. Glutamate and GABA release are enhanced by different subtypes of presynaptic nicotinic receptors in the lateral geniculated nucleus. J Neurosci. 1998;18:1963–1969. doi: 10.1523/JNEUROSCI.18-06-01963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund B, Cnattingius S. Cigarette smoking as a risk factor for sudden infant death syndrome: a population-based study. Am J Public Health. 1990;80:29–32. doi: 10.2105/ajph.80.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxhiu MA, Van LUNTEREN E, Van DE GRAAFF WB, Strohl KP, Bruce EN, Mitra J, Cherniack NS. Action of nicotine on the respiratory activity of the diaphragm and genioglossus muscles and the nerves that innervate them. Respir Physiol. 1984;57:153–169. doi: 10.1016/0034-5687(84)90090-2. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Stapleton JM, Benowitz NL, Grayson RF, London ED. Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug Alcohol Depend. 1993;33:23–29. doi: 10.1016/0376-8716(93)90030-t. [DOI] [PubMed] [Google Scholar]
- Klonoff-Cohen HS, Edelstein SL, Lefkowitz ES, Srinivasan IP, Kaegi D, Chang JC, Wiley KJ. The effect of passive smoking and tobacco exposure through breast milk on sudden infant death syndrome. J Am Med Assoc. 1995;273:795–798. doi: 10.1001/jama.1995.03520340051035. [DOI] [PubMed] [Google Scholar]
- Koshiya N, Smith JC. Neuronal pacemaker for breathing visualized in vitro. Nature. 1999;400:360–363. doi: 10.1038/22540. [DOI] [PubMed] [Google Scholar]
- Lotti M. Treatment of acute organophosphate poisoning. Med J Aust. 1991;154:51–55. doi: 10.5694/j.1326-5377.1991.tb112852.x. [DOI] [PubMed] [Google Scholar]
- Lydic R, Baghdoyan HA. Pedunculopontine stimulation alters respiration and increases ACh release in the pontine reticular formation. Am J Physiol Regulatory Integrative Comp Physiol. 1993;264:R544–R554. doi: 10.1152/ajpregu.1993.264.3.R544. [DOI] [PubMed] [Google Scholar]
- Mcgehee DS, Heath M, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1697. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- Metz B. Brain acetylcholinesterase and a respiratory reflex. Am J Physiol. 1958;192:101–105. doi: 10.1152/ajplegacy.1957.192.1.101. [DOI] [PubMed] [Google Scholar]
- Milerad J, Sundell H. Nicotine exposure and the risk of SIDS. Acta Paediatrica. 1993;82(Suppl 389):70–72. doi: 10.1111/j.1651-2227.1993.tb12882.x. [DOI] [PubMed] [Google Scholar]
- Murakoshi T, Suzue T, Tamai S. A pharmacological study on respiratory rhythm in the isolated brainstem-spinal cord preparation of the newborn rat. Br J Pharmacol. 1985;86:95–104. doi: 10.1111/j.1476-5381.1985.tb09439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie EE, Li A. Ventral medulla sites of muscarinic receptor subtypes involved in cardiorespiratory control. J Appl Physiol. 1990;69:33–41. doi: 10.1152/jappl.1990.69.1.33. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Shao XM, Feldman JL. Electrical coupling and excitatory synaptic transmission between rhythmogenic respiratory neurons in the preBötzinger complex. J Neurosci. 2000;20:RC113. doi: 10.1523/JNEUROSCI.20-23-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Role LW, Berg DK. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Bötzinger complex: differential roles of glycinergic and GABAergic neural transmission. J Neurophysiol. 1997;77:1853–1860. doi: 10.1152/jn.1997.77.4.1853. [DOI] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Acetylcholine modulates respiratory pattern: effects mediated by M3-like receptors in preBötzinger complex inspiratory neurons. J Neurophysiol. 2000;83:1243–1252. doi: 10.1152/jn.2000.83.3.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:716–719. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepans MBF, Wilkerson N. Physiologic effects of maternal smoking on breast-feeding infants. J Am Acad Nurse Practitioners. 1998;5:105–113. doi: 10.1111/j.1745-7599.1993.tb00850.x. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Roll D, Zilberman Y. An analysis of the respiratory stimulant effect of physostigmine and neostigmine in the conscious rabbit. Clin Exp Pharmacol Physiol. 1981;8:151–158. doi: 10.1111/j.1440-1681.1981.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Young TB, Bidwell TR, Badr MS, Palta M. Smoking as a risk factor for sleep-disordered breathing. Arch Intern Med. 1994;154:2219–2224. [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh recepters. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]