SUMMARY
Protein Tyr nitration is a post-translational modification yielding 3-nitrotyrosine (NO2-Tyr). Formation of NO2-Tyr is generally considered as a marker of nitroxidative stress and is involved in some human pathophysiological disorders, but it has been poorly studied in plants. Leghemoglobin (Lb) is an abundant hemeprotein of legume nodules that plays an essential role as O2 transporter. Liquid chromatography coupled to tandem mass spectrometry was used for a targeted search and quantification of NO2-Tyr in Lbs. For all Lbs examined, Tyr30, located in the distal heme pocket, is the major target of nitration. Lower amounts were found for NO2-Tyr25 and NO2-Tyr133. Nitrated Lb and other as yet unidentified nitrated proteins were also detected in nodules of plants not receiving NO3− and were found to decrease during senescence. This demonstrates formation of nitric oxide (•NO) and NO2− by alternative means to nitrate reductase, probably via a NO synthase-like enzyme, and strongly suggests that nitrated proteins perform biological functions and are not merely metabolic byproducts. In vitro assays with purified Lbs revealed that Tyr nitration requires NO2− + H2O2 and that peroxynitrite is not an efficient inducer of nitration, possibly by isomerizing it to NO3−. Nitrated Lb is formed via oxoferryl Lb, which generates nitrogen dioxide and tyrosyl radicals. This mechanism is distinctly different from that involved in heme nitration. Formation of NO2-Tyr in Lbs is a consequence of active metabolism in functional nodules, where Lbs may act as a sink of toxic peroxynitrite and may play a protective role in the symbiosis.
Keywords: Glycine max, leghemoglobin, legume nodules, nitrogen dioxide, peroxynitrite, Phaseolus vulgaris, protein tyrosine nitration
INTRODUCTION
The molecular interaction of legumes and soil bacteria (rhizobia) culminates with the formation of nodules, generally on the roots. Legume nodules are unique symbiotic organs where atmospheric N2 is reduced to ammonia assimilable for the plant in exchange for photosynthetically produced sugars. This biological N2 fixation is highly beneficial for agriculture and the environment as it allows a reduction in the input of nitrogen fertilizers. Nodule function requires leghemoglobins (Lbs), hemeproteins that occur at concentrations of 1–5 mM in the cytosol of nodule host cells. These proteins transport and deliver O2 to the symbiosomes at a low but steady concentration that allows efficient bacteroid respiration while preventing nitrogenase inactivation (Appleby and Bergersen, 1980). Generally, there are several Lb isoproteins in the nodules. Soybean nodules contain four major Lbs and bean nodules one major Lb (Fuchsman and Appleby, 1979).
Only the ferrous form of Lbs binds O2 and under physiological conditions 20% of the ferrous Lb is oxygenated and 80% is deoxygenated. However, nonfunctional forms of Lb may be produced in nodules (Lee et al., 1995) by redox reactions involving reactive oxygen species (ROS). Ferric Lb may be generated by autoxidation of oxygenated Lb, and ferryl Lb (Lb4+=O, formally oxoFe4+) by oxidation of ferrous or ferric Lbs with H2O2 (Aviram et al., 1978; Moreau et al., 1995). In vitro studies have shown that the oxidative attack of H2O2 on Lbs gives rise to protein radicals, which are then quenched via formation of intramolecular (heme-protein) and intermolecular (dimers) cross-links (Moreau et al., 1995). Ferrous, ferric and ferryl Lbs can react also with reactive nitrogen species (RNS). Thus, ferrous Lb binds •NO avidly to form the nitrosyl complex (LbNO), which has been detected in intact nodules (Mathieu et al., 1998; Meakin et al., 2007). In vitro oxygenated ferrous Lb and ferric Lb are able to isomerize ONOO− to NO3− and ferryl Lb is reduced by nitric oxide (•NO) (Herold and Puppo, 2005a,b). Recently, we reported the presence in soybean nodules of green derivatives of Lbs having a nitro (NO2) group on the 4-vinyl of the heme (nitri-heme) (Navascués et al., 2012). By analogy with similar nitri-hemes found in vitro in myoglobin (Mb; Bondoc and Timkovich, 1989) and hemoglobin (Hb; Otsuka et al., 2010), and to avoid confusion with Lb nitrated in Tyr residues, we will refer to those Lbs as nitri-Lbs.
The nitration of Tyr residues in proteins involves the substitution of a H atom by a NO2 group to yield NO2-Tyr. This post-translational protein modification has attracted much attention in studies related to human health as it is involved in diverse pathological conditions (Schopfer et al., 2003; Radi, 2004). Our knowledge of protein nitration is much more limited in plants, where an increase in the amount of nitrated proteins is associated to nitroxidative stress (Corpas et al., 2007; Cecconi et al., 2009; Begara-Morales et al., 2013). Many of these studies are based on proteomic approaches, in which the proteins are separated in two-dimensional gels, detected with anti-NO2-Tyr antibody, and identified (Chaki et al., 2009; Galetskiy et al., 2011; Begara-Morales et al., 2013). In other cases, the plants are treated with •NO or ONOO− donors prior to proteomic analysis (Tanou et al., 2012). Detailed studies using immunoprecipitation and/or semiquantitative assays with antibodies led to the identification of o-acetylserine(thiol)lyase (Álvarez et al., 2011) and glutamine synthetase (Melo et al., 2011) as targets of nitration. However, to our knowledge there are no studies showing unequivocally the presence and site of NO2-Tyr residues in plant proteins in vivo. In an incisive work, Lozano-Juste et al. (2011) were able to detect a peptide of glyceraldehyde-3-phosphate dehydrogenase with an NH2-Tyr residue, and concluded that this was generated by reduction of NO2-Tyr during the mass spectrometry (MS)-based proteomic analysis, emphasizing that Tyr nitration analyses are prone to artifacts.
The major drawbacks for studying protein nitration in vivo are indeed the low abundance of nitrated proteins and the potential artifacts that may arise during processing and MS analysis of samples. Because Lb is essential for symbiotic N2 fixation and participates in multiple reactions involving ROS and RNS, we reasoned that this hemeprotein might be a target of nitration in vivo and that its high abundance in nodules would facilitate the quantification of NO2-Tyr by dedicated MS procedures. Further motivation for undertaking this research was the puzzling observation that nitri-Lbs have their apoproteins intact (Navascués et al., 2012), which suggests that nitration of hemes and of Tyr residues, if any, should obbey distinct mechanisms and/or occur at different developmental stages and/or stress conditions of nodules.
In this work, we demonstrate that Lbs bearing NO2-Tyr are present in legume nodules and that Tyr30, which is located in the heme distal pocket, is the major target of nitration. Nitrated Lb decreases during nodule senescence and is present also in nodules of plants that were not given any NO3− in the nutrient solution. This indicates that there is a steady-state level of nitrated Lb in vivo and that sources other than bacteroid or plant nitrate reductases, probably a •NO synthase (NOS)-like activity, also produce the NO2−required for nitration. Based on in vitro assays that generate nitrating molecules, such as nitrogen dioxide radicals (•NO2) and ONOO−-derived species, we propose a mechanism entailing ferryl Lb and •NO2, rather than ONOO−, that accounts for the Tyr nitration pattern of Lb observed in vivo. Finally, we compare the mechanisms involved in heme and Tyr nitration and discuss their biological relevance.
RESULTS
Physiological state of nodules during natural and induced senescence
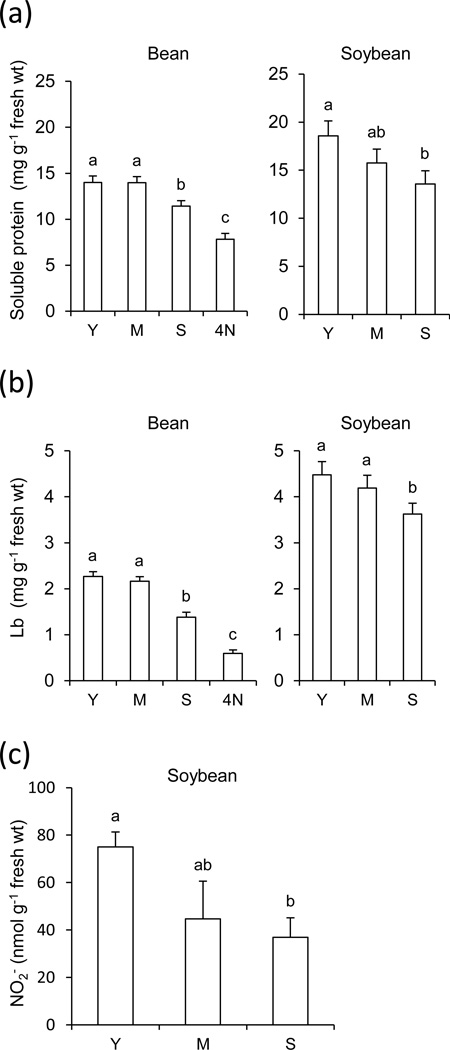
Two crop legumes, bean and soybean, were chosen to investigate Lb nitration because they have been extensively used by our group and others in studies of nodule natural and induced senescence (Pfeiffer et al., 1983; Evans et al., 1999; Escuredo et al., 1996; Gogorcena et al., 1997). Bean and soybean plants were grown in controlled-environment cabinets and under field conditions, respectively, and nodules were harvested at three developmental stages. Additional experiments were set up with bean plants grown in the complete absence of NO3− to compare nitration levels in nodules, as well as with bean plants treated with 10 mM NO3− for 4 days to induce senescence of young nodules. The total soluble protein and Lb of host cells are markers of the physiological state of nodules and were found to decrease by 19% and 39% in senescent bean nodules and by 27% and 20% in senescent soybean nodules; however, much larger decreases, 44% for soluble protein and 74% for Lb, were found in NO3−-treated bean nodules (Figures 1a,b). The NO2− content of nodules was also determined as it is relevant for our nitration studies. Bean nodules contained very low amounts, ~5–8 nmol g−1 fresh wt (detection limit ~1 nmol g−1 fresh wt), whereas the NO2− content of soybean nodules reached ~40–80 nmol g−1 fresh wt, showing a decreasing trend with aging (Figure 1c).
Figure 1. Physiological state of bean and soybean nodules.
Nodule contents of total soluble protein (a), Lb (b) and NO2− (c) during natural and NO3−-induced senescence. The NO2− content of bean nodules was ~5–8 nmol g−1 fresh wt, close to the detection limit of the assay, and therefore it was not shown in the figure. Y, young nodules; M, mature nodules; S, senescent nodules; 4N, young nodules after 4 days of NO3− treatment. Data are means ± SE of four to six biological replicates. Means denoted by the same letter do not significantly differ at P<0.05 based on the Duncan's multiple range test.
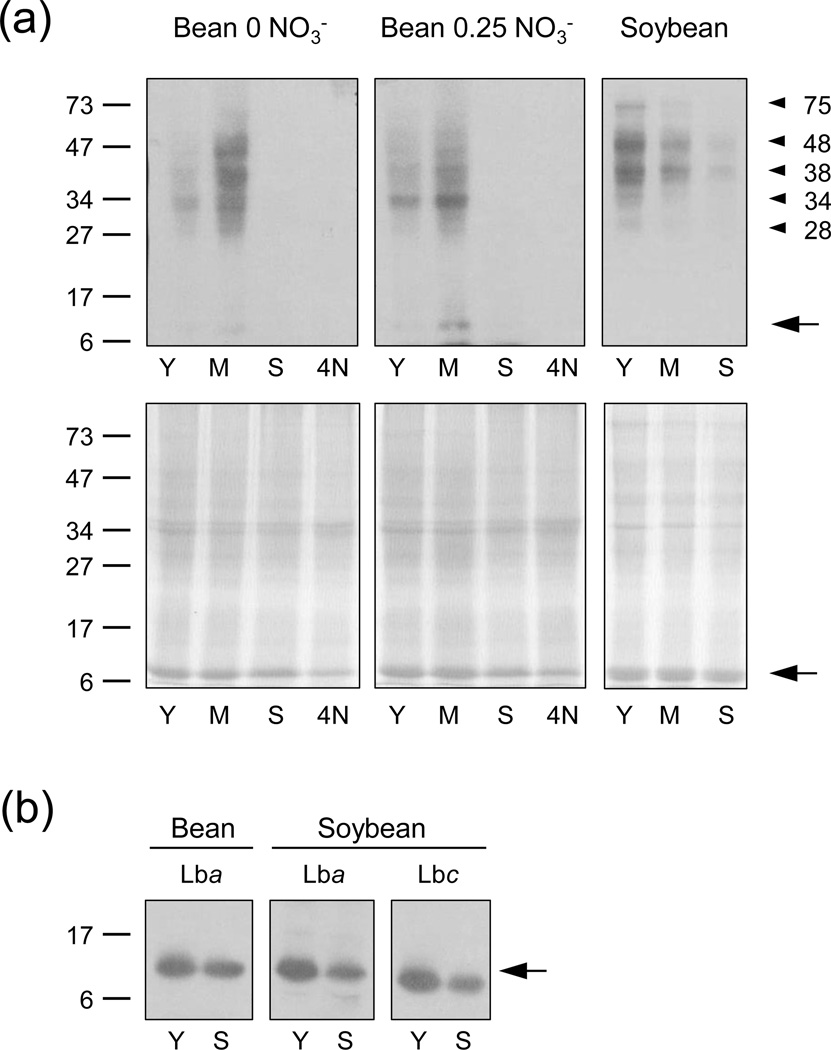
The protein nitration profiles were examined on immunoblots with an anti-NO2-Tyr antibody (Figure 2a,b). These allowed us to make a number of interesting observations. First, the nitration patterns of bean and soybean nodules were similar during senescence, and in fact nitrated proteins were virtually undetectable in aged or NO3−-treated bean nodules and hardly visible in senescent soybean nodules. Second, nitration was more intense in soybean nodules (readily visible bands with 20 µg protein) than in bean nodules (requiring 50 µg protein). Third, nodules of bean plants not receiving NO3− had substantial nitration levels. Fourth, comparison of immunoblots and the respective Coomassie-stained gels revealed that a few proteins with lower abundance than Lb were intensely nitrated. These proteins were not identified and had apparent molecular masses of ~32, 38 and 45 kDa in bean nodules and ~28, 34, 38, 48 and 75 kDa in soybean nodules. Finally, nitrated Lb was hardly detectable on immunoblots of bean nodules and not visible on immunoblots of soybean nodules. To verify the presence of nitrated Lb in nodule extracts using immunoblots, a preparative native gel was loaded with high amounts (~500 µg) of protein and the bands corresponding to bean Lba (PvLba) and soybean Lba and Lbc (GmLba and GmLbc) were excised, eluted and analyzed on SDS-gels and immunoblots. This demonstrated that nitrated Lb is present in nodules (Figure 2b). Direct in-gel trypsinization of the excised Lb bands and subsequent MS analysis or direct analysis of crude nodule extracts by MS with Orbitrap technology also confirmed the presence of very low levels of nitrated Lb.
Figure 2. Immunoblots showing the presence of nitrated proteins in bean and soybean nodules.
(a) Bean plants were grown in the absence of NO3− or under common conditions with 0.25 mM NH4NO3, and soybean plants were grown in the field. Nodule extracts were analyzed on 12.5% SDS-gels. Immunoblots (up) and Coomassie-stained gels (down) are shown. Protein loaded was 50 µg (bean) or 20 µg (soybean) per lane.
(b) Extracts from similar nodule samples as above were loaded on preparative native gels, then Lb bands were eluted and proteins were resolved on 15% SDS-gels and blotted. Protein loaded was 20 µg per lane.
Y, young nodules; M, mature nodules; S, senescent nodules; 4N, young nodules after 4 days of NO3− treatment. Molecular mass (kDa) markers are indicated on the left. Nitrated protein bands and nitrated Lb bands are marked with arrowheads and arrows, respectively. Immunoblots are representative of six (a) or three (b) blots obtained with different nodule samples, except that in a few cases nitration intensity was similar in Y and M nodules of bean plants grown on 0.25 mM NH4NO3.
Nitration of Lbs occurs in legume nodules and decreases during senescence
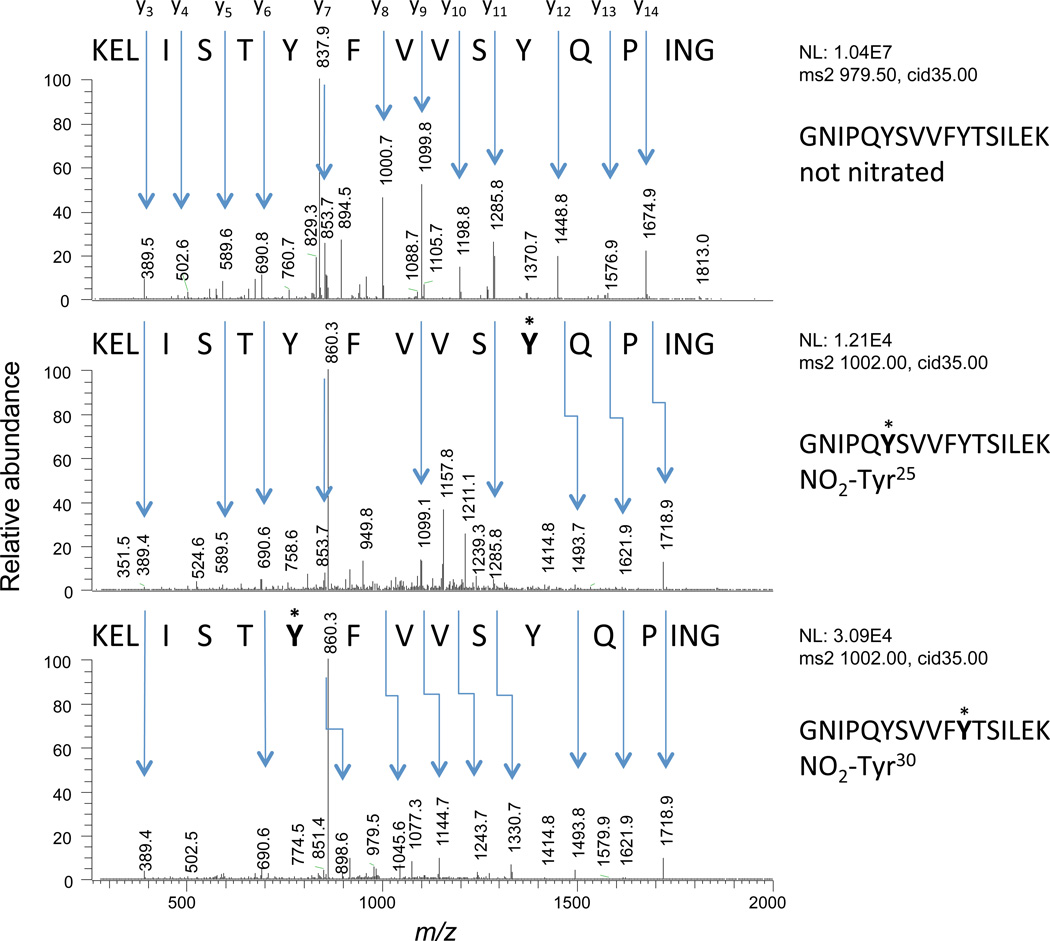
The tryptic peptides of Lbs obtained as indicated above were independently analyzed by µLC-ESI-MS/MS and nLC-ESI-MS/MS with similar results. Both methods were used in order to increase the reproducibility and confidence of the identification and quantification of the very low abundant modified peptides. The use of dithiothreitol or β-mercaptoethanol was avoided to prevent reduction of NO2-Tyr residues, which may be mediated by the hemeproteins themselves at 37°C and above (Balanbali et al., 1999; Söderling et al., 2007), as often practised in proteomic analyses. Because Lbs do not contain Cys residues, the alkylating reagent iodoacetamide was also omitted prior to trypsinization. An illustrative example is the tryptic peptide GNIPQYSVVFYTSILEK of PvLba and its mononitrated species GNIPQY*SVVFYTSILEK (NO2-Tyr25) and GNIPQYSVVFY*TSILEK (NO2-Tyr30). The three peptide species were fully resolved (Figure S1) and unequivocally identified by their MS/MS profiles (Figure 3). Likewise, we found in nodules the nitrated peptides ANIPQYSVVFY*TSILEK (NO2-Tyr30) of GmLba and ANIPQYSVVFY*NSILEK (NO2-Tyr30) of GmLbc1, as well as a small proportion (0.16–0.34%) of the C-terminal peptide AWEVAY*DELAAAIK (NO2-Tyr133) of GmLba. We were unable to detect in nodules the dinitrated species NO2-Tyr25 + NO2-Tyr30 or mononitrated NO2-Tyr133 in PvLba, and the same occurred for mononitrated NO2-Tyr25 in GmLba and GmLbc1.
Figure 3. Identification of nitrated peptides.
Fragmentation spectra of the unmodified GNIPQYSVVFYTSILEK peptide (up) of PvLba and of its NO2-Tyr25 (centre) and NO2-Tyr30 (down) modified forms. The spectra were obtained with the LTQ Velos system on a data-dependent mode.
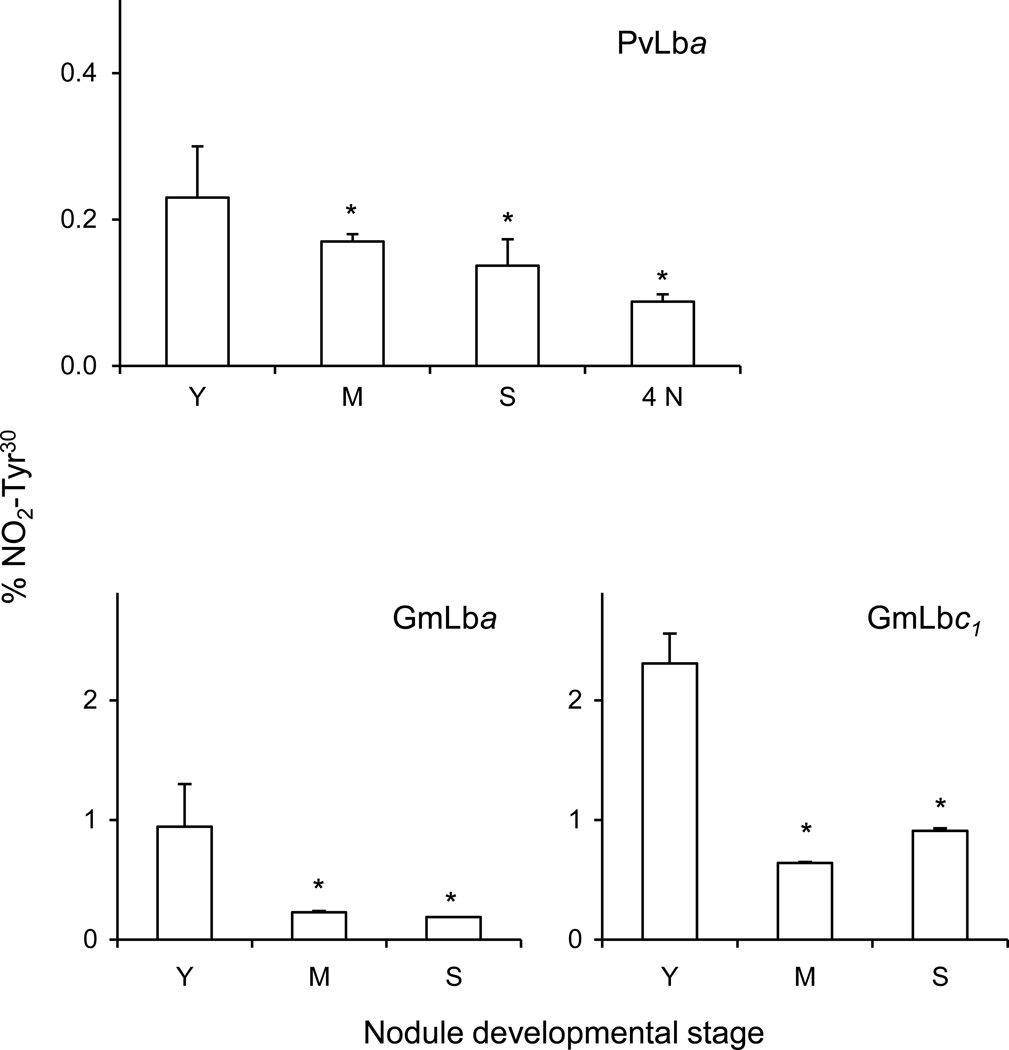
LC-MS/MS aproaches based on targeted precursors and selected reaction monitoring (SRM) were used to determine the proportion of NO2-Tyr30 in Lbs during natural and NO3−-induced nodule senescence. We chose Tyr30 to quantify nitration because it is the most abundant nitrated Tyr residue and is important for Lb function (Kundu et al., 2004). Figure 4 shows that young nodules contain 0.23% of nitrated PvLba, 0.95% of nitrated GmLba and 2.31% of nitrated GmLbc1. Similar values of Lb nitration were obtained for nodules of plants than had received 0 or 0.25 mM NH4NO3 in the nutrient solution. The proportions of nitrated Lb relative to total Lb decreased during the natural (~30%) and NO3−-induced (~60%) senescence of bean nodules as well as with advanced aging (~70%) of soybean nodules (Figure 4).
Figure 4. In vivo nitration of PvLba, GmLba and GmLbc1 during natural and nitrate-induced senescence of nodules.
Y, young nodules; M, mature nodules; S, senescent nodules; 4N, young nodules after 4 days of NO3− treatment. Data are given as percentage of NO2-Tyr30 relative to Tyr30, and represent the mean ± SE of two or three biological replicates corresponding to different protein preparations. Asterisks denote statistical differences relative to young nodules at P<0.05 based on the Student’s t-test. No differences in the NO2-Tyr30 content of PvLba were found in nodules treated with NO3− for 1, 2 or 3 days relative to untreated (control) nodules.
Nitration of Tyr residues in vitro: in search for a mechanism in vivo
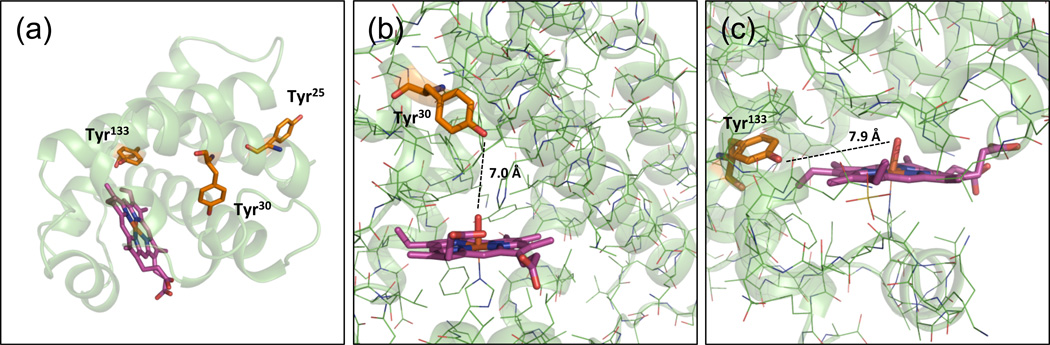
The model structure of GmLba shows that Tyr30 is located in the distal heme pocket, Tyr133 is also in the heme pocket but situated laterally, and Tyr25 is outside the heme crevice (Figure 5). Because Tyr30 is the main target of nitration in vivo, we surmised that the heme may participate in nitration of this Tyr residue. Considering that NO2− and •NO lack of sufficient reactivity to cause nitration but may give rise to the nitrating agents •NO2 and ONOO− in the presence of H2O2 (Herold, 2004; Nicolis et al., 2004), we assayed nitration using the estimated concentrations of ferric Lb (10 µM), H2O2 (20–100 µM) and NO2− (100 µM) in nodules or in other plant tissues (Evans et al., 1999; Becana and Klucas, 1992). Also, care was taken to perform all the reactions at physiological conditions of nodules (pH 6.5 and 26C) rather than at conditions commonly used for animal systems (pH 7.4 and 37C). At proportions of Lb:H2O2:NO2− of 1:2:2 and 1:2:10, nitration was less intense than at 1:10:10 or 1:10:100. The two last proportions were chosen for further studies because the high yields of the nitrated products facilitated their separation on preparative gels and the subsequent MS analyses (see below). Treatment of 10 µM of PvLba or GmLba with 100 µM H2O2 + 100 µM NO2− yielded 25–50% NO2-Tyr30 and ~0.5% NO2-Tyr25 (except for GmLbc1, which produced 13.5% NO2-Tyr25) (Table 1). Increasing NO2− concentration to 1 mM, while keeping H2O2 constant, produced ~70% of NO2-Tyr30 and 0.4–1.4% NO2-Tyr25 (Table 1). Thus, NO2-Tyr30 was the preferred target of Lb nitration by H2O2 + NO2− in vitro.
Figure 5. Molecular model of GmLba showing the three Tyr residues.
(a) Positions of Tyr25, Tyr30 and Tyr133 in relation to the heme.
(b) Tyr30 located in the distal heme pocket.
(c) Tyr133 in the edge of the heme, showing in both cases the distance between the Tyr hydroxyl and the oxoferryl. The GmLba crystal structure (PDB accession 1BIN) was used for modeling with PyMOL software (www.pmol.org).
Table 1.
In vitro nitration of Tyr residues of Lbs
| % Nitrationb | |||
|---|---|---|---|
| Lba | Tyr | 0.1 mM H2O2 + 0.1 mM NO2− |
0.1 mM H2O2 + 1 mM NO2− |
| PvLba | Tyr25 | 0.4 | 1.4 |
| Tyr30 | 54.4 | 75.9 | |
| GmLba | Tyr25 | 0.6 | 1.1 |
| Tyr30 | 49.9 | 77.7 | |
| GmLbc1 | Tyr25 | 13.5 | 0.9 |
| Tyr30 | 25.3 | 64.4 | |
| GmLbc2 | Tyr25 | 0.5 | 0.6 |
| Tyr30 | 38.3 | 65.6 | |
| GmLbc3 | Tyr25 | 0.5 | 0.4 |
| Tyr30 | 32.1 | 55.2 | |
All Lbs were used at a concentration of 10 µM.
Percentage of nitration = [NO2-Tyr/(NO2-Tyr + Tyr)] × 100. Values are means of two or three biological replicates with SE<10%.
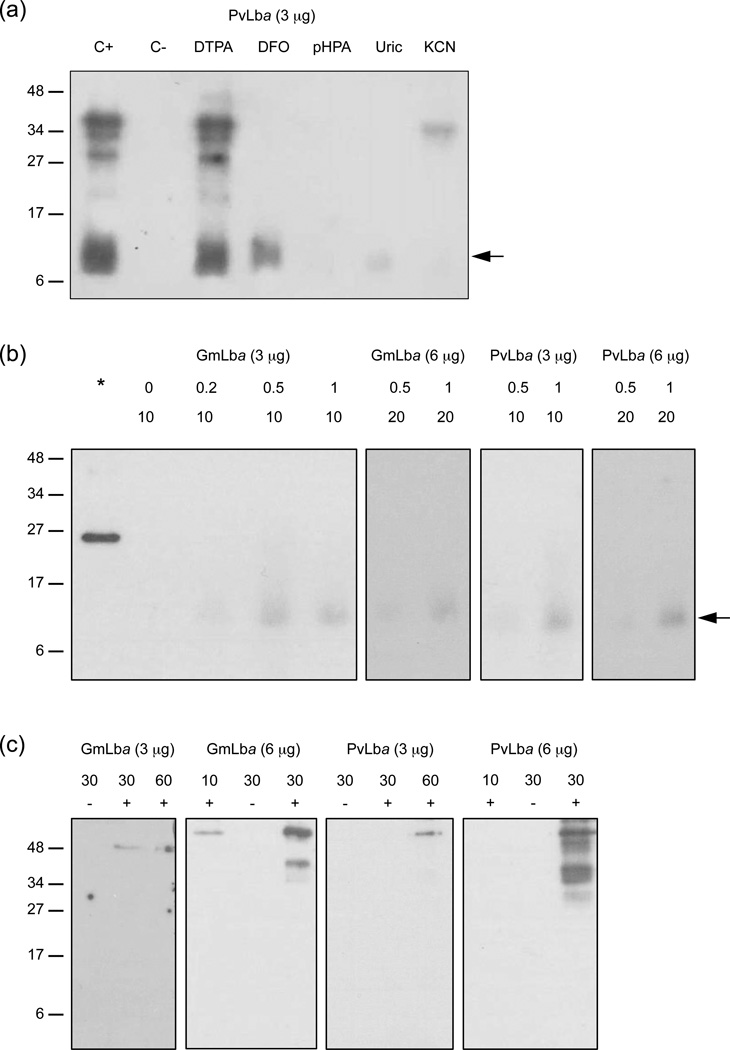
To gain further insights on the mechanism of the nitration reaction, Lbs were treated with H2O2 + NO2− in the presence of potential inhibitors (Bartesaghi et al., 2004, 2006; Castro et al., 2004) and the products were analyzed on immunoblots (Figure 6a). These show that nitration does not take place when H2O2 was omitted and that the reaction was inhibited by deferoxamine mesylate (DFO; powerful Fe3+chelator and •NO2 scavenger), 4-hydroxyphenylacetic acid (pHPA; hydrophylic structural analog of Tyr that intercepts •NO2), uric acid (scavenger of radicals derived from ONOO−) and cyanide (ligand of ferric hemeproteins). The addition of diethylenetriamine-pentaacetic acid (DTPA; metal ion chelator) had no effect. We then examined whether ONOO− could be also a nitrating agent in our system by treating Lbs with 3-mopholino-sydnonimine (SIN-1) (Figure 6b) or with synthetic ONOO− (Figure 6c) in the presence of 100 µM DTPA to suppress metal-catalyzed nitration. It has been shown that SIN-1 generates nearly equimolar amounts of •NO and superoxide radicals (O2−•), which then react with each other producing a flux of ONOO− for at least 2 h (Álvarez et al., 2002). At pH 6.5 and 26°C, we could only detect weak nitration on immunoblots when 10 µM of GmLba or PvLba were treated with 1 mM SIN-1 for 1 h (6 µg protein) or for 2 h (3 µg protein) (Figure 6b). Nitration was not visible when 1 mM SIN-1 was applied for 1 h and 3 µg of Lb was loaded. Nevertheless, significant nitration occurred when the pH was raised to 7.4 and the temperature to 37C. To further support the absence of nitration at relevant conditions for nodule cells, Lbs were exposed to pure ONOO− in the presence of a physiological concentration of CO2 (5 mM HCO3− at pH 6.5) because CO2 potentiates the nitrating effect of ONOO− (Gow et al., 1996). We could detect significant nitration only when 30 µM Lb was treated with a bolus of 500 µM ONOO− and 6 µg protein was loaded on the gels, and nitration was negligible when 60 µM Lb and 3 µg protein were used (Figure 6c). Interestingly, in contrast with the SIN-1 treatment, which produces a controlled flux of ONOO− over time, only Lb aggregates could be observed with the bolus addition of pure ONOO− (Figure 6c).
Figure 6. In vitro nitration of Lb.
(a) PvLba (10 µM) was treated with H2O2 (100 µM) + NO2− (1 mM), then immediately loaded on SDS-gels, blotted and probed with anti-NO2-Tyr antibody. (C+) positive control with complete mix. (C-) negative control omitting H2O2. Concentrations of compounds (added to the proteins before inclusion of H2O2 + NO2−): 100 µM DTPA, DFO and uric acid; 5 mM pHPA; 10 mM KCN. Similar results were obtained for GmLba.
(b) Effect of SIN-1 on GmLba and PvLba nitration. The asterisk is a positive control of nitrated iron-superoxide dismutase (1 µg; Castro et al., 2004). Numbers are the concentrations of SIN-1 (0.2–1 mM) and Lb (10–20 µM) used for the reactions and the amounts of Lb protein (3–6 µg) loaded per lane.
(c) Effect of ONOO− on GmLba and PvLba nitration. Lbs were treated with 500 µM ONOO− + HCO3− (5 mM) + DTPA (100 µM) for 5 min. Numbers are the concentrations of Lb (10–60 µM) used for the reactions and the amounts of Lb protein (3–6 µg) loaded per lane. Symbols +/− indicate addition or omission of ONOO−. In panels (a) and (b), arrows mark the position of the monomeric Lbs. In panels (a) and (c), immunoreactive protein bands with molecular masses >27 kDa correspond to dimers and aggregates of nitrated Lb. For all panels, experiments were performed in 20 mM potassium phosphate buffer (pH 6.5) at 26C, and molecular mass (kDa) markers are shown to the left.
Nitration of Tyr residues in vitro at preparative scale
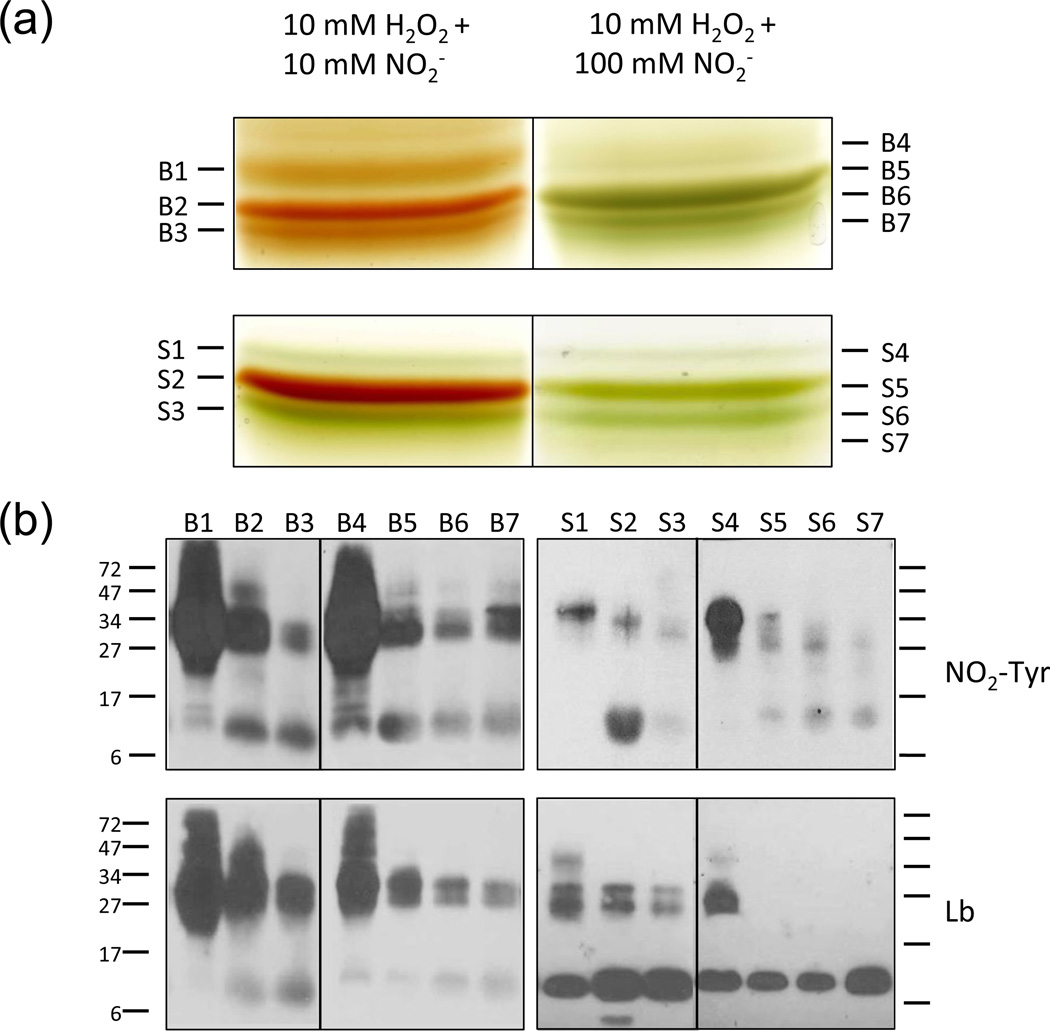
The nitration reaction was scaled up using 1 mM Lb and proportions of Lb: H2O2: NO2− of 1:10:10 and 1:10:100. The resulting Lb derivatives were resolved on preparative native gels (Figure 7a). PvLba and GmLba yielded three major products (B1–B3 and S1–S3) when nitration was performed with 10 mM H2O2 + 10 mM NO2−. The same Lbs produced four major derivatives (B4-B7 and S4-S7) upon nitration with 10 mM H2O2 + 100 mM NO2−. Some of these protein derivatives are green, indicating modification of the hemes. Because our results showed formation of nitrating species during the reactions, we searched for the presence of nitrated hemes. These can be identified by MS as they have molecular ions of m/z 661 (616 + 45 Da), corresponding to replacement of a H by a NO2 group. Tandem MS revealed that the m/z 661 ion exhibits a fragmentation pattern identical to that of the nitri-heme (Figure S2), suggesting that the NO2 is also on a vinyl group (Navascués et al., 2012). This peak was clearly detectable in proteins B1–B3 (red), B4 (green), S2 (red) and S3 (green). Therefore, the nitrated heme in those red proteins is at low levels, and the proteins B5-B7 (green) and S4–S7 (green), with virtually no peak at 661 Da, have other types of modified hemes as a result of the oxidative attack by H2O2.
Figure 7. Preparative separation and immunoblot analysis of Lb derivatives generated by nitration.
(a) Separation of Lb derivatives on preparative native gels. PvLba (upper gels) and GmLba (lower gels), both at 1 mM, were treated for 30 min with 10 mM H2O2 + 10 or 100 mM NO2− in 20 mM potassium phosphate buffer (pH 6.5). The protein products (bean, B1 to B7; soybean, S1 to S7) were eluted from gels and analyzed by MS.
(b) Immunoblot analyses of proteins eluted from (a) using antibodies against NO2-Tyr and Lb. Besides monomeric nitrated Lb, dimers and other Lb aggregates (>27 kDa) were formed with different levels of nitration. GmLba was more resilient to nitration than PvLba, as the former showed less intense signal with the NO2-Tyr antibody and maintained most Lb in monomeric form.
All the proteins were eluted from the gels and analyzed by immunoblots, low-resolution MS (MALDI-TOF/TOF-MS) and high-resolution MS (Orbitrap nESI-FT-MS). Immunoblot (Figure 7b) and MS (Table S1) analyses revealed that each protein band contains NO2-Tyr and is a mixture of monomers, dimers and in some cases trimers (B1, B4, S1 and S4), with molecular masses in the range of ~15300–15600, ~30500–31900 and ~46400–47900 Da, respectively. High-resolution MS analysis (± 0.2 Da) of the monomers (Table S1) confirmed the presence of NO2-Tyr (+45 Da) in most of the proteins, as well as dinitration (+90 Da) in B4. It also revealed changes in the monoisotopic molecular masses of the monomeric proteins compared with the control proteins (15479 for PvLba and 15233 for GmLba). These changes are +13 Da for B1 and −190 Da for B5, B6 and B7, but were not investigated further as their relevance in vivo is uncertain.
DISCUSSION
Legume nodule senescence is characterized by, among other factors, a decline in N2 fixation, soluble protein and Lb (for reviews see Puppo et al., 2005; Becana et al., 2010). Indeed, we confirmed the senescent stage of nodules harvested from fruiting plants or from young plants supplied with NO3− because the total soluble protein and Lb of bean and soybean nodules decreased markedly due to an increase in endoprotease activity (Pfeiffer et al., 1983; Pladys et al., 1988). In particular, treatment of bean plants with 10 mM NO3− for 4 days caused a major decrease of Lb and Lb/soluble protein ratio (from 16% to 7%), indicating that Lb is especially prone to degradation (Figures 1a,b). This is consistent with an early observation that proteolytic activity against Lb is augmented at the acid pH of senescent nodules (Pladys et al., 1988).
Bean nodules contained low amounts of NO2− but soybean nodules accumulated relatively high levels of this metabolite (Figure 1c). This is readily explained by the fact that bean nodule bacteroids (strain 3622) do not express nitrate reductase, whereas soybean nodule bacteroids (strain USDA110) display relatively high nitrate reductase activity, which is responsible for most NO2− produced in soybean nodules (Becana et al., 1989; Meakin et al., 2007). This NO2− accumulation may, in fact, be a contributing factor to the high nitration levels of soybean nodules relative to bean nodules (Figure 2a). However, nitration was clearly detectable in bean nodules that had not received any NO3− (Figure 2a), evidencing that alternative sources to bacteroid and plant nitrate reductases also exist in nodules to generate •NO and/or the NO2− derived therefrom. In fact, •NO and NO2− are precursors of nitrating RNS and the presence of the LbNO complex has been reported in soybean nodules grown without NO3− (Mathieu et al., 1998). A likely source of RNS is a NOS-like activity. In plants, this activity was originally described in lupin (Lupinus albus) roots and nodules and measured by the conversion of 14C-Arg into 14C-citrulline at a rate of 150–200 pmol •NO produced min−1 mg−1 protein (Cueto et al., 1996). We have observed similar values in bean nodules with a highly sensitive and specific assay in which •NO was detected by ozone chemiluminiscence (Corpas et al., 2007). Together, these observations give strong support to the presence of NOS-like activity in legume nodules.
The accumulation of nitrated proteins is assumed to be a marker of nitroxidative stress in animals (Schopfer et al., 2003; Radi, 2004) and plants (Corpas et al., 2007). Our finding that protein nitration decreases with nodule senescence was completely unexpected as nitrated proteins may accumulate during aging of leaves and roots of several plants, including legumes (Begara-Morales et al., 2013). At present we cannot offer an explanation for this other than it may be a feature of nodules and/or that nitrated proteins are preferential substrates for the nodule proteases induced during senescence. Consistent with this decreasing trend for nitrated proteins of nodules in general, we demonstrate here that Lb nitration occurs in vivo and is more intense in young than in senescent nodules (Figure 4). We had anticipated that Lb may be prone to nitration in nodules because it reacts rapidly in vitro with ROS and RNS (Becana and Klucas, 1992; Moreau et al., 1995, 1996). However, other still unknown proteins appear to be more nitrated than Lb, for they exhibit intense bands on immunoblots and are considerably less abundant than Lb. Our data also show that a steady-state level of nitrated Lb, roughly estimated in the range of 0.1–1%, occurs as a result of normal nodule metabolism. A few unindentified nitrated proteins have been detected also by immunoblots in other 'control' (unstressed, young) plant tissues (Chaki et al., 2011; Galetskyi et al., 2011; Tanou et al., 2012; Begara-Morales et al., 2013). Thus, the existence of a basal nitration level is a general phenomenon, strongly suggesting that nitrated proteins perform biological functions and are not merely metabolic byproducts.
Previous findings of oxidative damage of crucial biomolecules (Evans et al., 1999) and production of nitri-Lbs (Navascués et al., 2012) in senescing soybean nodules indicate that nitroxidative stress occurs at very late stages of senescence. We are therefore bound to conclude that NO2-Tyr in proteins is not a useful marker of nitroxidative stress, at least in nodules. Also, the decreased content of nitrated Lbs in senescing tissue strongly suggests that the two forms reported so far of modified Lbs, nitri-hemes and nitrated apoproteins, are generated by distinct mechanisms, as we will discuss below.
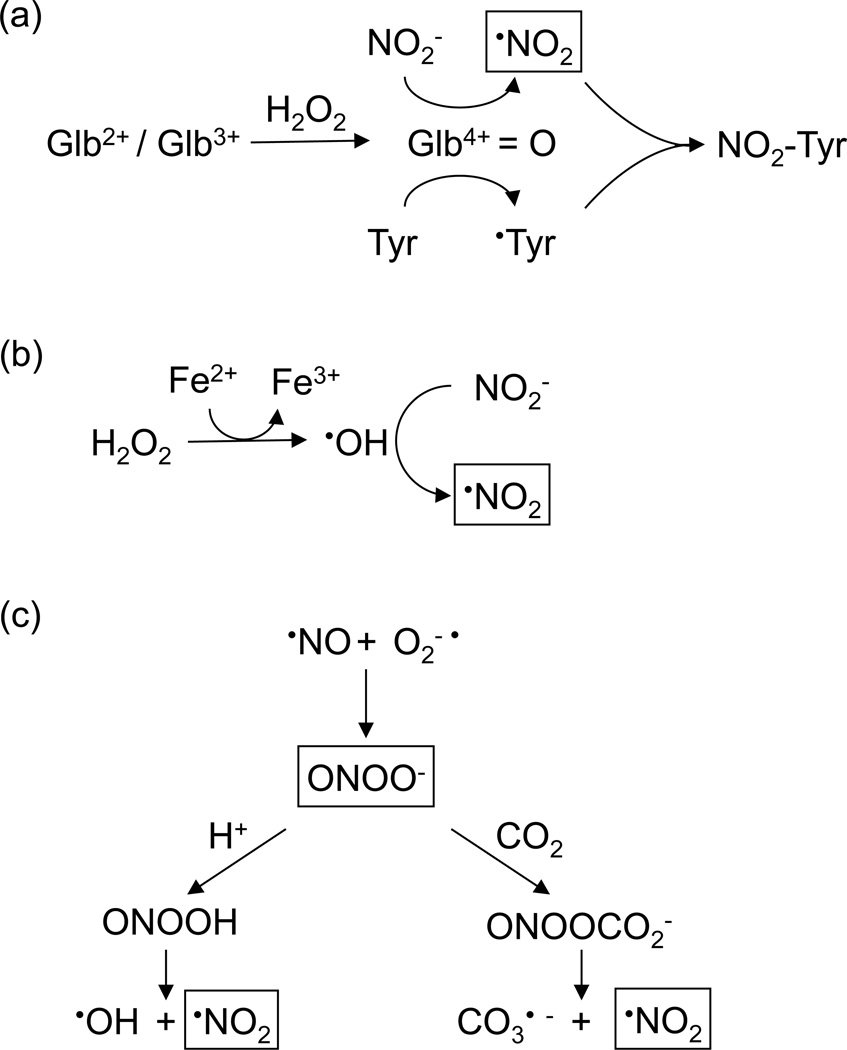
The mechanism of Tyr nitration in Lbs was investigated. Essentially, three types of mechanisms have been proposed for Tyr nitration of animal globins (Figure 8). (a) Formation of ferryl globins and their radical forms, which oxidize NO2− to •NO2 (Figure 8a; Van der Vliet et al., 1997; Herold, 2004; Sakamoto et al., 2004). (b) Fenton reaction, catalyzed by traces of free or protein-associated transition metals, in which the resulting •OH oxidizes NO2− to •NO2 (Figure 8b; Thomas et al., 2002). (c) Formation of ONOO− or its derived species, peroxynitrous acid (ONOOH) and nitrosoperoxocarboxylate (ONOOCO2−), which may undergo homolytic cleavage to •OH, •NO2 and carbonate radical (CO3−•) (Figure 8c; Radi, 2004; Bartesaghi et al., 2004, 2006). Mechanisms (a) and (b) require NO2− + H2O2, whereas mechanism (c) does not. In our experiments with Lb, the failure of DTPA to inhibit Tyr nitration rules out mechanism (b). However, ONOO− might be formed via •NO + O2−• (Radi, 2004), and this possibility was examined by treating Lbs with the ONOO−-generating compound SIN-1 or with synthetic ONOO−. In both cases, we found no significant nitration when the reactions were carried out at pH 6.5 and 26C, physiological conditions of nodules (Pladys et al., 1988). Instead, we did detect nitration when a high flux of ONOO− was generated from SIN-1 at pH 7.4 and 37C for 2 h. Under these conditions, the production and stability of ONOO− are higher and thus 1 mM SIN-1 generates an accumulated level equivalent to ~0.5 mM ONOO− in 1 h (Álvarez et al., 2002). This is an extremely high amount, unlikely to be produced in healthy plant tissues and certainly not in young, actively N2-fixing nodules. Taking into account that 500 µM of pure ONOO− does not cause appreciable nitration of Lb and that the pH of nodules decreases from 6.5 to 5.5 during nodule senescence, we conclude that direct nitration of Lb by ONOO− is probably irrelevant in vivo, although we cannot entirely preclude the possibility that some minor nitration occurs via ONOO−. This conclusion is consistent with previous in vitro studies showing that horse Mb and human Hb catalyze ONOO− isomerization without being significantly nitrated (Herold and Shivashankar, 2003).
Figure 8. Mechanisms of protein Tyr nitration.
(a) Nitration mediated by ferryl globin (Glb4+=O), which oxidizes NO2− to •NO2. The tyrosyl (•Tyr) radical cannot be generated directly from Tyr by reaction with H2O2 or ONOO− and its formation is very slow with •NO2 (Radi, 2004).
(b) Iron-catalyzed Fenton reaction followed by •OH-mediated NO2− oxidation.
(c) Nitration mediated by ONOO− and/or its derived radicals •NO2, •OH and CO3−•. The nitrating species are indicated within boxes. For further explanation see Discussion.
The inhibition of nitration by DFO, pHPA and uric acid (Figure 6a), which preferentially scavenge •NO2 (Bartesaghi et al., 2004, 2006; Castro et al., 2004), supports mechanism (a). Interestingly, DFO suppressed formation of dimers and other aggregates and decreased nitration of monomeric Lb, consistent with previous work showing that DFO inhibits dimerization and nitration of free Tyr by mechanisms that are independent of metal ions (Bartesaghi et al., 2004). In sharp contrast, cyanide completely inhibited nitration of the monomer, indicating the participation of the heme in the reaction. However, it did not inhibit dimerization, which may be attributed to side reactions of cyanide-derived radicals (Castro et al., 2004).
Studies of Tyr nitration of Mb and Hb with NO2− + H2O2 have led to the proposal that the nitrating compound is •NO2 and/or heme-bound ONOO−, depending of the relative concentrations of NO2− and H2O2 (Herold, 2004; Nicolis et al., 2004). Moreover, in all cases, a tyrosyl radical (•Tyr) must be formed from Tyr, a reaction that cannot be achieved by ONOO− per se (Castro et al., 2004; Radi, 2004). We conclude that Tyr nitration of Lb occurs by mechanism (a), requiring ferryl Lb to generate both •NO2 and •Tyr, which then recombine to form NO2-Tyr (Figure 8a). The inhibition of Lb nitration by cyanide further supports a ferryl-mediated, heme-dependent mechanism. This would explain why Tyr30, located in the distal heme pocket, is a preferred target for in vivo and in vitro nitration. However, the oxidation of Tyr30 by the oxoferryl intermediate is unlikely to be direct because the distance between the two structures is >7 Å (Figure 5b,c). Generally, Tyr residues with at least one carbon within 4–5 Å from the oxoferryl are assumed to be amenable for their direct one-electron oxidation to •Tyr (Prasad et al., 2007). Thus, in the case of Lb, Tyr30 oxidation may be mediated via intramolecular electron transfer (Petruk et al., 2012). Formation of •Tyr must also occur on Tyr25 and Tyr133, as they were found to be nitrated. Notably, a protein radical was detected by EPR in GmLba treated with H2O2 in the absence of NO2−, and the signal was assigned to Tyr133 (Moreau et al., 1996). This observation is not at odds with our results, considering that ferryl Lb is responsible for the H abstraction from Tyr according to mechanism (a).
The nitrated Lbs described here are different from nitri-Lbs (Navascués et al., 2012). Nitrated Lbs contain NO2-Tyr whereas nitri-Lbs do not, probably as a result of the different free radical mediated reactions implicated in their production. Formation of nitrated Lbs requires H2O2 to generate ferryl Lb and ultimately •Tyr, whereas formation of nitri-Lbs requires very high NO2− concentrations but not H2O2, and involves HNO2 as precursor of the nitrating agent. Treatment of Lbs with NO2− + H2O2 elicited not only Tyr nitration but also heme modifications and protein dimerization and aggregation, as shown by immunoblots (Figure 7b) and MS analysis (Table S1). These Lb derivatives are originated by intermolecular reactions of protein radicals, not necessarily entailing •Tyr. Notably, the dimers and aggregates contain high amounts of NO2-Tyr relative to monomers. In this respect, we noted that ONOO− also produced aggregates of nitrated Lb, whereas SIN-1 formed mainly monomers (Figures 6b,c). We attribute this to differences in the concentrations of •Tyr achieved with the different modes of exposure, which are much greater with the bolus addition of ONOO− than with SIN-1. Thus, in the first case, •Tyr radicals are more likely to recombine forming Tyr-Tyr bridges than with SIN-1.
The relative abundance of the two types of modified Lbs during nodule aging is also different. The content of nitrated Lbs is higher in young (very active) nodules, which evidences the production in vivo of ferryl Lb, •NO2 and •Tyr. Nitration of Tyr30 may interfere with Lb function because this amino acid residue allows for a proper delivery of O2 (Kundu et al., 2004). Our results show that a low steady-state level of nitrated Lbs is fully compatible with nodule activity and that Lb is nitrated by a mechanism involving oxyferryl heme rather than ONOO−, most likely as a result of the efficient capacity of Lb to isomerize ONOO− to NO3− (Herold and Puppo, 2005a). Taking these results together with other in vivo observations (Mathieu et al., 1998; Navascués et al., 2012), we propose that, in young nodules, Lbs may act as a sink of •NO and derived RNS such as ONOO− to keep the symbiosis active, preventing rejection of, or toxic effects on, the bacteroids. By contrast, the content of nitri-Lbs is higher in old (less active or inactive) nodules and may be a result of an excessive production of RNS associated to senescence and nitroxidative stress. Further work will be needed to determine if Lb dimers and other aggregates occur in vivo during legume nodule development and senescence.
EXPERIMENTAL PROCEDURES
Plant growth
Bean (Phaseolus vulgaris L. cv Contender) was inoculated with Rhizobium leguminosarum bv phaseoli strain 3622 and grown in vermiculite-containing pots under controlled environment conditions (Gogorcena et al., 1997) with either 0 or 0.25 mM NH4NO3 in the nutrient solution. This low NH4NO3 concentration stimulates nodulation of most legumes. Young, mature and senescent nodules were harvested from beans of ~1 month (vegetative stage), ~1.5 months (flowering stage) and ~2 months (fruiting stage), respectively. In another set of experiments, beans of ~1 month were supplied with 10 mM KNO3 for 4 days to induce nodule senescence. Soybean (Glycine max Merr. cv Williams) was inoculated with Bradyrhizobium japonicum strain USDA110 and grown in an experimental plot in Sevilla (Spain) from July to September. Young, mature and senescent nodules were harvested from soybeans of ~1 month (vegetative stage), ~2 months (late flowering-early fruiting stage) and ~3 months (fruiting stage), respectively. Bean and soybean nodules were frozen in liquid nitrogen and stored at −80°C.
Physiological state and immunoblot analyses of nodules
Total soluble protein and Lb were used as markers of the physiological state of nodules (Pfeiffer et al., 1983; Escuredo et al., 1996). Nodules (200 mg) were homogenized with 2 × 500 µl 20 mM potassium phosphate (pH 7.0), and the extracts were cleared by centrifugation (20000 g, 20 min). Soluble protein was determined by the dye-binding microassay (Bio-Rad; http://www.bio-rad.com) using bovine serum albumin as a standard. Lb concentration was determined by the alkaline pyridine method, based on the differential spectra of the dithionite-reduced versus ferricyanide-oxidized pyridine-hemochromes (556 nm minus 539 nm, extinction coefficient 23.4 mM−1 cm−1; Appleby and Bergersen, 1980).
Some biochemical markers relevant to our nitration studies, including NO2− concentrations and protein nitration profiles, were also determined in nodules. Nitrite was quantified by diazotization after rapid and complete deproteinization of nodule extracts (Becana et al., 1989; Arrese-Igor et al., 1998). Nodules (0.5 g) were ground in a mortar on melting ice with 5 ml of 1 M zinc acetate at 0°C. The precipitated proteins were removed by centrifugation and the supernatant mixed with an equal volume of ice-cold ethanol. After centrifugation, 500 µl of the clean extract was mixed with equal volumes of 1% sulfanilamide in 1.5 M HCl and 0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride. Blanks containing sample but 1.5 M HCl instead of sulfanilamide were run in parallel. After 20 min at room temperature, the absorbance at 540 nm was read and NO2− concentration calculated with a calibration curve (0–20 nmol).
The protein nitration profiles of nodules were obtained using immunoblots. Nodule extracts, SDS-gels and transfer of proteins to polyvinylidene difluoride membranes (FluoroTrans; Pall, http://www.pall.com) were performed following standard protocols, but omitting reductants in the sample buffer. Membranes were probed with a monoclonal anti-NO2-Tyr (Cayman, https://www.caymanchem.com) antibody and in some cases with a polyclonal affinity-purified anti-Lb antibody (see below), both at a dilution of 1:1000. The secondary antibodies were, respectively, goat anti-mouse IgG (Bio-Rad) and goat anti-rabbit IgG (Sigma, http://www.sigmaaldrich.com) peroxidase conjugates, which were used at a dilution of 1:20000. Immunoreactive proteins were detected by chemiluminescence using the SuperSignal West Pico kit (Pierce, http://www.piercenet.com).
The antiserum against PvLba was produced by injecting ~0.8 mg of protein in each of two white rabbits and was used to purify polyclonal monospecific antibodies by chromatography in CNBr-activated Sepharose 4 Fast Flow (GE Healthcare, www.gelifesciences.com). The immunoaffinity column was prepared with ~5 mg of protein. Conventional protocols for immunization and antibody purification were carried out by BioGenes (http://www.biogenes.de).
LC-MS/MS
For the identification and quantification of NO2-Tyr in Lbs in vivo, nodules (200 mg) were ground in a ice-cold mortar with a medium (400 µl) consisting of 50 mM Tris-HCl (pH 8.0), 0.5 mM EDTA, 1% polyvinylpyrrolidone-10 and protease inhibitor cocktail (Complete Mini; Roche, https://lifescience.roche.com/shop/home). Extracts were centrifuged (13000 g, 10 min) and the soluble proteins subjected to preparative native gel electrophoresis (12.5% acrylamide, 3 mm thick). Samples (400–500 µg of protein per lane) were mixed 1:4 (vol:vol) with loading buffer omitting SDS and reductants. Gels were run at 100 V for 2 h 15 min on a MGV-100 apparatus (CBS, http://www.cbsscientific.com). The bands of PvLba, GmLba and GmLbc were carefully excised from the gels, cut into small pieces and kept at −20°C until MS analysis, which usually took place one week later. In-gel digestion was performed with trypsin at 37°C for 8 h with an automatic device (DigestPro MS; Intavis, http://www.intavis.com). For some MS experiments, proteins were eluted overnight with 10 mM NH4HCO3 with identical results.
Two types of analyses were used to detect and quantify the low abundant nitrated peptides of Lbs. The first analysis involved liquid chromatography coupled to tandem MS with micro-electrospray ionization (µLC-ESI-MS/MS). In this case, the instrument was an LTQ Velos (Thermo Scientific; www.thermofisher.com). Aliquots of the tryptic digest were diluted to 20 µl with 5% methanol and 1% formic acid and loaded onto a chromatographic system consisting of a C18 preconcentration cartridge (Agilent Technologies; http://www.agilent.com) connected to a C18 tip column (15 cm × 150 µm, 5 µm; Nikkyo Technos, http://www.nikkyo-tec.co.jp). The separation was performed using a 40-min acetonitrile gradient from 3% to 40% (solvent A: 0.1% formic acid; solvent B: acetonitrile + 1% formic acid) at a flow rate of 1.0 µl min−1. The HPLC system comprised an Agilent 1200 capillary pump, a binary pump, a thermostatted micro-injector and a micro-switch valve. The MS/MS system was operated in the positive ion mode with a spray voltage of 2 kV. Initial identification of the non-nitrated and nitrated peptides in the chromatograms was carried out in the data dependent mode, acquiring a full scan followed by ten MS/MS scans of the ten most intense signals detected in the MS scan. An exclusion time of 30 s was included to avoid repetitive MS/MS analysis of the dominant MS signals. Peptides were identified by database search of the MS/MS spectra using SEQUEST (Proteome Discoverer 1.3; Thermo Scientific). Search was performed with the following parameters: peptide mass tolerance 5 ppm, fragment tolerance 0.8 Da, enzyme set as trypsin, allowance up to two missed cleavages, and dynamic modification for Met oxidation (+15.995 Da) and Tyr nitration (+44.985 Da). Relative quantification was carried out using SRM. The areas of the signals of the product ions were integrated and the ratios of the areas of the modified to unmodified peptides were calculated. The transitions used to integrate the peak areas are given in Table S2.
For the second analysis, peptide digests were applied to an EASY-Spray PepMap RSLC C18 column (15 cm × 50 µm; particle size, 2 µm; Thermo Scientific) coupled to a one-dimensional nano-flow LC (Dionex UltiMate 3000; Thermo Scientific). The peptides were separated using a 60-min acetonitrile gradient from 95% of solvent A (0.1% formic acid) to 50% of solvent B (50% acetonitrile + 0.1% formic acid) at a flow rate of 300 nl min−1. The nLC-ESI-MS/MS analysis was performed on a LTQ Orbitrap Elite (Thermo Scientific) at a resolution of 60,000. The repeat count was set to 1, repeat duration 20 sec, exclusion list size 500, exclusion duration 60 sec and exclusion mass width 10 ppm. Charge state screening was enabled with rejection of unassigned and 1+ charge states. Minimum signal threshold counts were set to 5,000. Peptide identification was carried out with SEQUEST as indicated for the first method. ProtMAX software was used for relative quantification of the identified nitrated peptides with respect to the unmodified ones by the relative abundances calculated from automated extraction of the summed ion intensities of the targeted peptide precursor masses (Egelhofer et al., 2013).
For both analyses, the monitored tryptic peptides with UniProt accession numbers of the proteins are given in Table S2.
Purification of Lbs and in vitro nitration
For in vitro nitration experiments, bean and soybean Lbs were purified essentially as described (Jun et al., 1994). Nodule extracts were prepared in 50 mM Tris-HCl (pH 7.5), containing 1 mM EDTA and protease inhibitor cocktail. The protein was fractionated with 50–85% ammonium sulfate, resuspended in 10 mM Tris-HCl (pH 9.2), and oxidized with excess ferricyanide. The protein was dialyzed overnight with 50 mM potassium phosphate (pH 7.0), and applied to a hydroxyapatite column (HTP; Bio-Rad). The column effluent was concentrated and the buffer exchanged to 20 mM Tris-HCl (pH 8.0) with Vivaspin devices (10 kDa cutoff; Sartorius, http://www.sartorius.de). The protein was loaded onto a weak anion-exchange column (Whatman DE-52 cellulose; GE Healthcare) equilibrated with 20 mM Tris-HCl (pH 8.0). PvLba was eluted with 80 mM NaCl in buffer, GmLba with buffer alone, and GmLbc (Lbc1 + Lbc2 + Lbc3) with a gradient of 0–100 mM NaCl in buffer.
Nitration of pure Lbs at analytical (10 µM ferric Lb) and preparative (1 mM ferric Lb) scales was conducted with relative proportions of Lb: H2O2: NO2− of 1:10:10 and 1:10:100. The assays were carried out in 20 mM potassium phosphate buffer (pH 6.5) at 26°C for 30 min. The proteins were then immediately loaded on SDS-gels and immunoblots performed with anti-NO2-Tyr and anti-Lb antibodies. At the analytical scale, the effects of some compounds on nitration were tested by adding them in the reaction mix after Lb, but prior to NO2− + H2O2, at the following concentrations: DTPA (100 µM), DFO (100 µM), pHPA (5 mM), uric acid (10 µM) and KCN (10 mM). Nitration of Lbs was also attempted by exposing the proteins to SIN-1 (0.2–1 mM) for 1–2 h or to synthetic ONOO− (100–500 µM) for 5 min, at pH 6.5 and 26°C. For comparison, additional assays were performed by exposing Lbs to SIN-1 at pH 7.4 and 37°C for 1–2 h. Synthetic ONOO− was prepared by the reaction between H2O2 and NO2− at acid pH following standard protocols (Gow et al., 1996; Bartesaghi et al., 2006). The ONOO− preparations had <20% NO2− contamination but were free of H2O2. Any possible effect of contaminating NO2− was ruled out by control experiments with decomposed ONOO− ('reverse-order addition'; Bartesaghi et al., 2004).
At the preparative scale, the Lb nitration products were excised from gels, eluted with 10 mM NH4HCO3 and analyzed by MS to determine molecular masses of the holoproteins and hemes and to quantify NO2-Tyr. Low-resolution MS was performed using different dilutions of the samples in 0.1% trifluoroacetic acid. The samples were spotted on a MALDI plate with sinapinic acid and analyzed using a MALDI-TOF/TOF mass spectrometer (4800 TOF/TOF; AB Sciex, http://www.absciex.com). High-resolution MS was performed by nLC-ESI-MS/MS with an LTQ XL-Orbitrap mass spectrometer (Thermo Scientific). For nESI-FT-MS analysis, 5 µl of sample diluted in 50% MeOH + 0.1% trifluoroacetic acid was loaded in the nanospray needle. Full MS spectra were acquired (50 microscans/sec) at a resolution of 100,000 using an energy on source of 35 (full range of m/z 300–1800). For heme analyses, the signals at m/z 616 and 661 were selected as precursor masses and product ion spectra were obtained by collision induced dissociation (isolation width 2.5 and collision energy 35). Spectra were deconvoluted using the Xtract tool included in the Xcalibur 2.0 software (Thermo Scientific).
Supplementary Material
ACKNOWLEDGMENTS
We thank Javier Luque (Universidad de Barcelona) for his invaluable help with Figure 5. M.S. was the recipient of a predoctoral contract (Programa Junta de Ampliación de Estudios) from CSIC. L.C.-B. was the recipient of a predoctoral contract (Formación de Personal Investigador) from Ministerio de Economía y Competitividad (MINECO). C.S. was funded by the Austrian Fonds zur Förderung der wis-senschaftlichen Forschung (P23441-B20). This work was mainly supported by MINECO-Fondo Europeo de Desarrollo Regional (AGL2011-24524 and AGL2014-53717-R) and CSIC (Proyecto Intramural Especial 201240E150). Additional funding was provided by grants of Agencia Nacional de Investigación e Innovación (Fondo Clemente Estable, FCE 6605) to S.B. and Universidad de la República and National Institutes of Health (RO1 AI095173) to R.R.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
Figure S1. Peak-base separation of non-nitrated and mono-nitrated peptides of PvLba.
Figure S2. Tandem MS analyses of the molecular ions (m/z 661) of nitrated hemes.
(a) Fragmentation profile of heme from the green Lb derivative S6 (terminology according to Figure 7).
(b) Fragmentation profile of nitri-heme (Navascués et al., 2012). Insets are close-up views of the spectra (range m/z ~480–660).
Table S1. Compilation of data obtained for Lb derivatives using immunoblots, low-resolution MS and high-resolution MS.
Table S2. MS/MS Transitions monitored for the calculation of the nitration ratios.
REFERENCES
- Álvarez MN, Trujillo M, Radi R. Peroxynitrite formation from biochemical and cellular fluxes of nitric oxide and superoxide. Methods Enzymol. 2002;359:353–366. doi: 10.1016/s0076-6879(02)59198-9. [DOI] [PubMed] [Google Scholar]
- Álvarez C, Lozano-Juste J, Romero LC, García I, Gotor C, León J. Inhibition ofArabidopsis O-acetylserine(thiol)lyase A1 by tyrosine nitration. J. Biol. Chem. 2011;286:578–586. doi: 10.1074/jbc.M110.147678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby CA, Bergersen FJ. Preparation and experimental use of leghaemoglobin. In: Bergersen FJ, editor. Methods for Evaluating Biological Nitrogen Fixation. Chichester: Wiley; 1980. pp. 315–335. [Google Scholar]
- Arrese-Igor C, Gordon AJ, Minchin FR, Denison RF. Nitrate entry and nitrite formation in the infected region of soybean nodules. J. Exp. Bot. 1998;49:41–48. [Google Scholar]
- Aviram I, Wittenberg BA, Wittenberg JB. The reaction of ferrous leghemoglobin with hydrogen peroxide to form leghemoglobin (IV) J. Biol. Chem. 1978;253:5685–5689. [PubMed] [Google Scholar]
- Balanbali B, Kamisaki Y, Martin E, Murad F. Requirements for heme and thiols for the nonezymatic modification of nitrotyrosine. Proc. Natl. Acad. Sci. USA. 1999;96:13136–13141. doi: 10.1073/pnas.96.23.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartesaghi S, Trujillo M, Denicola A, Folkes L, Wardman P, Radi R. Reactions of desferrioxamine with peroxynitrite-derived carbonate and nitrogen dioxide radicals. Free Radic. Biol. Med. 2004;36:471–483. doi: 10.1016/j.freeradbiomed.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Bartesaghi S, Valez V, Trujillo M, Peluffo G, Romero N, Zhang H, Kalyanaraman B, Radi R. Mechanistic studies of peroxynitrite-mediated tyrosine nitration in membranes using the hydrophobic probe N-t-BOC-L-tyrosinetert-butyl ester. Biochemistry. 2006;45:6813–6825. doi: 10.1021/bi060363x. [DOI] [PubMed] [Google Scholar]
- Becana M, Minchin FR, Sprent JI. Short-term inhibition of legume N2 fixation by nitrate. I. Nitrate effects on nitrate-reductase activities of bacteroids and nodule cytosol. Planta. 1989;180:40–45. doi: 10.1007/BF02411408. [DOI] [PubMed] [Google Scholar]
- Becana M, Klucas RV. Oxidation and reduction of leghemoglobin in root nodules of leguminous plants. Plant Physiol. 1992;98:1217–1221. doi: 10.1104/pp.98.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becana M, Matamoros MA, Udvardi M, Dalton DA. Recent insights into antioxidant defenses of legume root nodules. New Phytol. 2010;188:960–976. doi: 10.1111/j.1469-8137.2010.03512.x. [DOI] [PubMed] [Google Scholar]
- Begara-Morales JC, Chaki M, Sánchez-Calvo B, Mata-Pérez C, Leterrier M, Palma JM, Barroso JB, Corpas FJ. Protein tyrosine nitration in pea roots during development and senescence. J. Exp. Bot. 2013;64:1121–1134. doi: 10.1093/jxb/ert006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondoc LL, Timkovich R. Structural characterization of nitrimyoglobin. J. Biol. Chem. 1989;264:6134–6145. [PubMed] [Google Scholar]
- Castro L, Eiserich JP, Sweeney S, Radi R, Freeman BA. Cytochrome c: a catalyst and target of nitrite-hydrogen peroxide-dependent protein nitration. Arch. Biochem. Biophys. 2004;421:99–107. doi: 10.1016/j.abb.2003.08.033. [DOI] [PubMed] [Google Scholar]
- Cecconi D, Orzetti S, Vandelle E, Rinalducci S, Zolla L, Delledonne M. Protein nitration during defense response in Arabidopsis thaliana. Electrophoresis. 2009;30:2460–2468. doi: 10.1002/elps.200800826. [DOI] [PubMed] [Google Scholar]
- Chaki M, Valderrama R, Fernández-Ocaña AM, Carreras A, Gómez-Rodríguez MV, López-Jaramillo J, Begara-Morales JC, Sánchez-Calvo B, Luque F, Leterrier M, Corpas FJ, Barroso JB. High temperature triggers the metabolism ofS-nitrosothiols in sunflower mediating a process of nitrosative stress which provokes the inhibition of ferredoxin-NADP reductase by tyrosine nitration. Plant Cell Environ. 2011;34:1803–1818. doi: 10.1111/j.1365-3040.2011.02376.x. [DOI] [PubMed] [Google Scholar]
- Corpas FJ, del Río LA, Barroso JB. Need of biomarkers of nitrosative stress in plants. Trends Plant Sci. 2007;12:436–438. doi: 10.1016/j.tplants.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Cueto M, Hernández-Perera O, Martín R, Bentura ML, Rodrigo J, Lamas S, Golvano MP. Presence of nitric oxide synthase activity in roots and nodules of Lupinus albus. FEBS Lett. 1996;398:159–164. doi: 10.1016/s0014-5793(96)01232-x. [DOI] [PubMed] [Google Scholar]
- Egelhofer V, Hoehenwarter W, Lyon D, Weckwerth W, Wienkoop S. Using ProtMAX to create high-mass-accuracy precursor alignments from label-free quantitative mass spectrometry data generated in shotgun proteomics experiments. Nature Protocols. 2013;8:595–601. doi: 10.1038/nprot.2013.013. [DOI] [PubMed] [Google Scholar]
- Escuredo PR, Minchin FR, Gogorcena Y, Iturbe-Ormaetxe I, Klucas RV, Becana M. Involvement of activated oxygen in nitrate-induced senescence of pea root nodules. Plant Physiol. 1996;110:1187–1195. doi: 10.1104/pp.110.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PJ, Gallesi D, Mathieu C, Hernández MJ, de Felipe MR, Halliwell B, Puppo A. Oxidative stress occurs during soybean nodule senescence. Planta. 1999;208:73–79. [Google Scholar]
- Fuchsman WH, Appleby CA. Separation and determination of the relative concentrations of the homogenous components of soybean leghemoglobin by isoelectric focusing. Biochim. Biophys. Acta. 1979;579:314–324. doi: 10.1016/0005-2795(79)90059-x. [DOI] [PubMed] [Google Scholar]
- Galetskiy D, Lohscheider JN, Kononikhin AS, Popov IA, Nikolaev EN, Adamska I. Phosphorylation and nitration levels of photosynthetic proteins are conversely regulated by light stress. Plant Mol. Biol. 2011;77:461–473. doi: 10.1007/s11103-011-9824-7. [DOI] [PubMed] [Google Scholar]
- Gogorcena Y, Gordon AJ, Escuredo PR, Minchin FR, Witty JF, Moran JF, Becana M. N2 fixation, carbon metabolism, and oxidative damage in nodules of dark-stressed common bean plants. Plant Physiol. 1997;113:1193–1201. doi: 10.1104/pp.113.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow A, Duran D, Thom SR, Ischiropoulos H. Carbon dioxide enhancement of peroxynitrite-mediated protein tyrosine nitration. Arch. Biochem. Biophys. 1996;333:42–48. doi: 10.1006/abbi.1996.0362. [DOI] [PubMed] [Google Scholar]
- Herold S. Nitrotyrosine, dityrosine, and nitrotryptophan formation from metmyoglobin, hydrogen peroxide, and nitrite. Free Radic. Biol. Med. 2004;36:565–579. doi: 10.1016/j.freeradbiomed.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Herold S, Puppo A. Oxyleghemoglobin scavenges nitrogen monoxide and peroxynitrite: a possible role in functioning nodules? J. Biol. Inorg. Chem. 2005a;10:935–945. doi: 10.1007/s00775-005-0046-9. [DOI] [PubMed] [Google Scholar]
- Herold S, Puppo A. Kinetics and mechanistic studies of the reactions of metleghemoglobin, ferrylleghemoglobin, and nitrosylleghemoglobin with reactive nitrogen species. J. Biol. Inorg. Chem. 2005b;10:946–957. doi: 10.1007/s00775-005-0047-8. [DOI] [PubMed] [Google Scholar]
- Herold S, Shivashankar K. Metmyoglobin and methemoglobin catalyze the isomerization of peroxynitrite to nitrate. Biochemistry. 2003;42:14036–14046. doi: 10.1021/bi0350349. [DOI] [PubMed] [Google Scholar]
- Jun HK, Sarath G, Moran JF, Becana M, Klucas RV, Wagner FW. Characteristics of modified leghemoglobins isolated from soybean (Glycine max Merr.) root nodules. Plant Physiol. 1994;104:1231–1236. doi: 10.1104/pp.104.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu S, Blouin GC, Premer SA, Sarath G, Olson JS, Hargrove MS. Tyrosine B10 inhibits stabilization of bound carbon monoxide and oxygen in soybean leghemoglobin. Biochemistry. 2004;43:6241–6252. doi: 10.1021/bi049848g. [DOI] [PubMed] [Google Scholar]
- Lee KK, Shearman L, Erickson BK, Klucas RV. Ferric leghemoglobin in plant-attached leguminous nodules. Plant Physiol. 1995;109:261–267. doi: 10.1104/pp.109.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Juste J, Colom-Moreno R, León J. In vivo protein tyrosine nitration in Arabidopsis thaliana. J. Exp. Bot. 2011;62:3501–3517. doi: 10.1093/jxb/err042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu C, Moreau S, Frendo P, Puppo A, Davies MJ. Direct detection of radicals in intact soybean nodules: presence of nitric oxide leghemoglobin complexes. Free Radic. Biol. Med. 1998;24:1242–1249. doi: 10.1016/s0891-5849(97)00440-1. [DOI] [PubMed] [Google Scholar]
- Meakin GE, Bueno E, Jepson B, Bedmar EJ, Richardson DJ, Delgado MJ. The contribution of bacteroidal nitrate and nitrite reduction to the formation of nitrosylleghaemoglobin complexes in soybean root nodules. Microbiology. 2007;153:411–419. doi: 10.1099/mic.0.2006/000059-0. [DOI] [PubMed] [Google Scholar]
- Melo PM, Silva LS, Ribeiro I, Seabra AR, Carvalho HG. Glutamine synthetase is a molecular target of nitric oxide in root nodules of Medicago truncatula and is regulated by tyrosine nitration. Plant Physiol. 2011;157:1505–1517. doi: 10.1104/pp.111.186056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau S, Davies MJ, Puppo A. Reaction of ferric leghemoglobin with H2O2: formation of heme-protein cross-links and dimeric species. Biochim. Biophys. Acta. 1995;1251:17–22. doi: 10.1016/0167-4838(95)00087-b. [DOI] [PubMed] [Google Scholar]
- Moreau S, Davies MJ, Mathieu D, Hérouart D, Puppo A. Leghemoglobin-derived radicals. Evidence for multiple protein-derived radicals and the initiation of peribacteroid membrane damage. J. Biol. Chem. 1996;271:32557–32562. doi: 10.1074/jbc.271.51.32557. [DOI] [PubMed] [Google Scholar]
- Navascués J, Pérez-Rontomé C, Gay M, Marcos M, Yang F, Walker FA, Desbois A, Abián J, Becana M. Leghemoglobin green derivatives with nitrated hemes evidence production of highly reactive nitrogen species during aging of legume nodules. Proc. Natl. Acad. Sci. USA. 2012;109:2660–2665. doi: 10.1073/pnas.1116559109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolis S, Monzani E, Roncone R, Gianelli L, Casella L. Metmyoglobin-catalyzed exogenous and endogenous tyrosine nitration by nitrite and hydrogen peroxide. Chem. Eur. J. 2004;10:2281–2290. doi: 10.1002/chem.200304989. [DOI] [PubMed] [Google Scholar]
- Otsuka M, Marks SA, Winnica DE, Amoscato AA, Pearce LL, Peterson J. Covalent modifications of hemoglobin by nitrite anion: formation kinetics and properties of nitrihemoglobin. Chem. Res. Toxicol. 2010;23:1786–1795. doi: 10.1021/tx100242w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruk AA, Bartesaghi S, Trujillo M, Estrin DA, Murgida D, Kalyanaraman B, Marti MA, Radi R. Molecular basis of intramolecular electron transfer in proteins during radical-mediated oxidations: computer simulation studies in model tyrosine-cysteine peptides in solution. Arch. Biochem. Biophys. 2012;525:82–91. doi: 10.1016/j.abb.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer NE, Torres CM, Wagner FW. Proteolytic activity in soybean root nodules. Activity in host cell cytosol and bacteroids throughout physiological development and senescence. Plant Physiol. 1983;71:797–802. doi: 10.1104/pp.71.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pladys D, Barthe P, Rigaud J. Changes in intracellular pH in French-bean nodules induced by senescence and nitrate treatment. Plant Sci. 1988;56:99–106. [Google Scholar]
- Prasad JC, Goldstone JV, Camacho CJ, Vajda S, Stegeman JJ. Ensemble modeling of substrate binding to cytochromes P450: analysis of catalytic differences between CYP1A orthologs. Biochemistry. 2007;46:2640–2654. doi: 10.1021/bi062320m. [DOI] [PubMed] [Google Scholar]
- Puppo A, Groten K, Bastian F, Carzaniga R, Soussi M, Lucas MM, de Felipe MR, Harrison J, Vanacker H, Foyer CH. Legume nodule senescence: roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytol. 2005;165:683–701. doi: 10.1111/j.1469-8137.2004.01285.x. [DOI] [PubMed] [Google Scholar]
- Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc. Natl. Acad. Sci. USA. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto A, Sakurao SH, Fukunaga K, Matsubara T, Ueda-Hashimoto M, Tsukamoto S, Takahashi M, Morikawa H. Three distinct Arabidopsis hemoglobins exhibit peroxidase-like activity and differentially mediate nitrite-dependent protein nitration. FEBS Lett. 2004;572:27–32. doi: 10.1016/j.febslet.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Schopfer FJ, Baker PRS, Freeman BA. NO-dependent protein nitration: a cell signaling event or an oxidative inflammatory response? Trends Biochem. Sci. 2003;28:646–654. doi: 10.1016/j.tibs.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Söderling A, Hultman L, Delbro D, Højrup P, Caidahl K. Reduction of the nitro group during sample preparation may cause underestimation of the nitration level in 3-nitrotyrosine immunoblotting. J. Chromatography B. 2007;851:277–286. doi: 10.1016/j.jchromb.2007.02.036. [DOI] [PubMed] [Google Scholar]
- Tanou G, Filippou P, Belghazi M, Job D, Diamantidis G, Fotopoulos V, Molassiotis A. Oxidative and nitrosative-based signaling and associated post-translational modifications orchestrate the acclimation of citrus plants to salinity stress. Plant J. 2012;72:585–599. doi: 10.1111/j.1365-313X.2012.05100.x. [DOI] [PubMed] [Google Scholar]
- Thomas DD, Espey MG, Vitek MP, Miranda KM, Wink DA. Protein nitration is mediated by heme and free metals through Fenton-type chemistry: an alternative to the NO/O2− reaction. Proc. Natl. Acad. Sci. USA. 2002;99:12691–12696. doi: 10.1073/pnas.202312699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Vliet A, Eiserich JP, Halliwell B, Cross CE. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. J. Biol. Chem. 1997;272:7617–7625. doi: 10.1074/jbc.272.12.7617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.