Abstract
Evidence is strong that a reduction in risk for breast cancer is associated with moderate to vigorous physical activity (PA); however, there is limited understanding of the role of type, intensity, duration, and frequency of PA and their mechanisms in accounting for this health benefit. The objective of this review is to stimulate investigations of candidate mechanisms that may account for the effects of the intensity and duration of aerobic PA on breast cancer risk and tumor burden. Three hypotheses are considered: 1) the mTOR network hypothesis: PA inhibits carcinogenesis by suppressing the activation of the mTOR signaling network in mammary carcinomas; 2) the hormesis hypothesis: the carcinogenic response to PA is non linear and accounted for by a physiological cellular stress response; and 3) the metabolic reprogramming hypothesis: PA limits the amount of glucose and glutamine available to mammary carcinomas thereby inducing apoptosis because tumor-associated metabolic programming is reversed. To link these hypotheses to systemic effects of PA, it is recommended that consideration be given to determining : 1) what contracting muscle releases into circulation or removes from circulation that would directly modulate the carcinogenic process in epithelial cells; 2) whether the effects of muscle contraction on epithelial cell carcinogenesis are exerted in an endocrine, paracrine, autocrine, or intracrine manner; and 3) if the effects of muscle contraction on malignant cells differ from effects on normal or pre-malignant cells that do not manifest the hallmarks of malignancy.
Keywords: physical activity, breast cancer, mTOR, hormesis, myokines, metabolic reprogramming
Introduction
National and international expert panels have concluded that increased physical activity (PA) plays a critical role in chronic disease prevention, which includes cancer, and those panels have underscored the need for understanding the role of type, intensity, duration, and frequency of PA and their mechanisms in accounting for health benefits (1-3). Investigations into the effects of physical activity (PA) on various aspects of carcinogenesis in human populations involve an effort to quantify a very heterogeneous set of PA exposures. While such efforts are difficult and many limitations are widely recognized, there is substantial evidence supporting the existence of an inverse relationship between PA and cancer incidence and cancer-related mortality. In a recently released 2008 NIH Report on Physical Activity and Health (3), it was concluded that the evidence is strong that a reduction in risk for breast cancer is associated with moderate to vigorous PA. Estimates of the affect size ranged from 20% to 80% reduction in risk from the majority of reported cohort studies and 20% to 70% from a number of population-based case-control studies. The expert panel concluded that breast risk reduction is likely to range from 20 to 40%. In the epidemiological literature, the effects of PA on breast cancer have been categorized as those associated with differences in body mass index (BMI), presumably due to increased energy expenditure, and those that are termed direct effects, i.e. they are observed in individuals with the same BMI but that vary in their levels of PA, and therefore are presumed to be independent of energy balance (3). The focus of this review is on candidate mechanisms that may account for the effects of the intensity and duration of aerobic PA on breast cancer risk and tumor burden that act apart from those mechanisms associated with BMI.
Evidence from laboratory animal models of the effects of PA on breast carcinogenesis
Our laboratory has designed and built computer controlled non motorized and motorized activity wheels that reinforce running behavior of rats using food reward in order to minimize the many difficulties encountered in pre-clinical animal studies of PA and cancer (4;5). Using these instruments and a pre-clinical model for breast cancer, we have shown that PA decreases the incidence and number of mammary cancers per rat and reduces tumor burden (6;7). While our initial reports studied effects of PA associated with regulation of body weight, recent work has shown direct effects of PA, i.e. body weight independent, that were detected in rats that ran at moderate intensity for less than 120 minutes per day; inhibitory activity was diminished at lower PA intensity and was also lower with running durations >120 min (8). Biomarker data have implicated IGF-1, IL-6, corticosterone and glucose as candidate affecter molecules possibly mediating the effects of PA on mammary carcinogenesis. Decreased tumor burden in response to PA appeared to be associated with higher rates of apoptosis with effects of PA also being detected on the G1/S transition of the cell cycle and on tumor vascularization. Emerging evidence from those studies (6;8;9) implies that protection against cancer is mediated in part by the mammalian target of rapamycin (mTOR) signaling network of which AMP activated protein kinase (AMPK) and protein kinase B (Akt) are components. Effects of PA on fork-head transcription factors (FOXO), sirtuins (SIRT1), and p53 were also observed in malignant tissues.
Candidate hypotheses for effects of PA on carcinogenesis
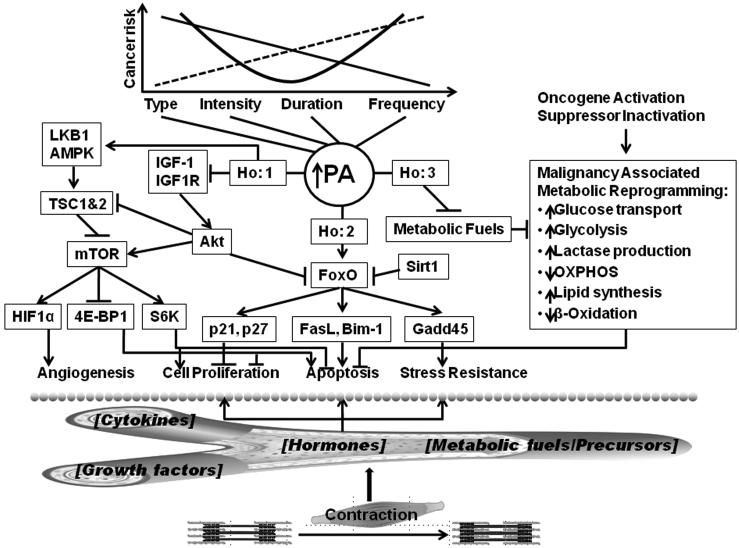
These observations prompt the question of how PA, which is defined as skeletal muscle contraction resulting in a quantifiable expenditure of energy (10), affects the development of malignancy in mammary epithelial cells. Three hypotheses are advanced to explain the effects of PA on mammary carcinogenesis. They are not developed in equal depth but are presented here as emergent ideas in order to stimulate new efforts in the investigation of mechanisms that underlie the protection against cancer afforded by PA. While these hypotheses are presented separately, the mechanisms they reference are likely to be interrelated, a perspective reflected by the diagram of mechanisms shown in Figure 1.
Figure 1. Summary of candidate mechanisms.
As shown at the top of the figure, physical activity (PA) is characterized by its four components: type, intensity, duration, and frequency; the dose dependent effects of each component on cancer risk have yet to be determined. Three hypotheses (Ho) are advanced to explain the effect of PA in reducing cancer risk and these Ho are likely to be interrelated. Ho:1 predicts that PA inhibits carcinogenesis by suppressing the activation of the mTOR signaling network in mammary carcinomas. Suppression is mediated through effects of PA on concentrations of the circulating growth factors and hormones that regulate the mTOR network. Ho:2 predicts that the carcinogenic response to PA is hormetic, i.e. non linear (J or U-shaped) and accounted for by a physiological cellular stress response. Ho:3 predicts that PA limits the amount of glucose and glutamine available to mammary carcinomas thereby inducing apoptosis because malignancy-associated metabolic programming is reversed; this serves to limit the accumulation of tumor cell mass. The bottom of the figure speculates that the effects of contracting muscle are conveyed to the target epithelial cell populations by effects of PA on circulating concentrations of cytokines, growth factors, hormones, and/or metabolic fuels/precursors.
mTOR network hypothesis: PA inhibits carcinogenesis by suppressing the activation of the mTOR signaling network in mammary carcinomas. Suppression is mediated through effects of PA on concentrations of the circulating growth factors and hormones that regulate the mTOR network but that are distinct from those affecting mTOR activity in contracting skeletal muscle as reviewed in (11;12). As a consequence, the drive for cell proliferation is reduced, a pro-apoptotic environment is maintained, and the stimulus for new blood vessel formation is suppressed in mammary carcinomas. The relevance of this hypothesis is that one or more elements of the mTOR network are misregulated in the majority of human breast cancers (13). Various components of this network are discussed in subsequent sections.
The mammalian target of rapamycin (mTOR) is an intracellular protein that plays a key role in integrating information received from the extracellular environment via the binding of growth factors and hormones with their cognate receptor tyrosine kinases (e.g.IGF-1: IGF1-R) with signals from metabolic checkpoints within the cell in a manner that affects cell growth, cell division, and cell survival (or death) (14). mTOR is an evolutionarily conserved serine-threonine kinase that is a key regulator of protein translation and synthesis. mTOR is centrally involved in cell growth, i.e. increase in cell size and cell mass, and these processes are tightly coupled to cell division (reviewed in (15)). The regulation of mTOR is multifaceted, and still being investigated. It has two biochemically and functionally distinct complexes, mTOR complex 1 (TORC1) and mTOR complex 2 (TORC2) (16). TORC1 is comprised of mTOR, regulatory associated protein of mTOR (Raptor) and G protein beta subunit-like (Gβ1/also known as mLST8) and its activity is nutrient/energy sensitive, whereas TORC2 is comprised of mTOR, rapamycin-insensitive companion of mTOR (Rictor), stress-activated-protein-kinase-interacting protein 1 (SIN1) and Gβ1) and plays a role in regulating the signaling pathway of which Akt is a component. A primary locus for control of mTOR is via the tuberous sclerosis protein complex (TSC), specifically TSC2, which is phosphorylated on different sites by either activated AMPK (Thr1227 and Ser1345 residues that correspond to Thr1271 and Ser1387 respectively in human TSC2) or activated Akt (Thr1462 and Ser939 residues). A second locus for control of mTOR via AMPK and Akt is via the phosphorylation of TORC1 complex components raptor and PRAD, respectively (14). mTOR mediates its effects on downstream targets via site specific phosphorylation. Relative to its effects on cell growth and cell division, two principal targets of mTOR are 70-kDa ribosomal protein S6 kinase (p70S6K) and 4E binding protein 1 (4E-BP1). Activated mTOR phosphorylates p70S6K and this leads to increased ribosomal biogenesis (17;18). 4E-BP1 is a repressor of translation initiation (19-21). Activated mTOR phosphorylates 4E-BP1 which inactivates the protein. When it is hypophosphorylated, 4E-BP1 binds to and inhibits the rate-limiting translation initiation factor eIF4E (eukaryotic translation initiation factor 4E). Upon phosphorylation, eIF4E is released from 4E-BP1, allowing eIF4E to assemble with other translation initiation factors to initiate cap-dependent translation (20). We have reported that PA decreases the levels of both phosphorylated S6K and 4E-BP1. Much remains to be done in understanding how PA regulates mTOR in mammary gland and mammary carcinomas, and a logical next step is investigation of effects on TORC1 and TORC2.
AMP activated protein kinase (AMPK) serves as a metabolic checkpoint, down regulating cell growth and cell division in the absence of an adequate supply of biosynthetic and energy substrates (14). In this respect, AMPK has been likened in its function to p53 that serves as a guardian of genome integrity. AMPK has been shown to be an exquisitely sensitive detector of small changes in the intracellular ratio of AMP to ATP, and some investigators have even proposed that AMPK plays a central role in homeostatic regulation of whole body energy metabolism (22). We have reported that PA results in AMPK activation (7). This suggests that PA is either altering substrate availability (the fuel mixture presented to tissues throughout the body) or that activation is being induced via a mechanism independent of the AMP to ATP ratio. In this regards, it is becoming clear that additional factors control the activation of AMPK, including various cytokines (23).
Protein kinase B (Akt) PA has been reported to decrease circulating levels of IGF-1 (24), an observation confirmed in our work under some conditions of PA (6;9). Lower levels of IGF-1 would be expected to down-regulate signaling via the pathway of which IGF-1 receptor, phosphoinositide kinase-3 (PI3K), and Akt are components. Of these proteins, activated Akt, a serine/threonine kinase, is the critical affecter molecule. Akt is activated by its phosphorylation on Ser473. Phospho-Akt serves important roles in cell proliferation, cell survival and new blood vessel formation that are associated with tumor development (25). While it is clear that reduced levels of activated Akt are likely to affect proliferation, apoptosis and angiogenesis by mechanisms independent of mTOR (reviewed in (26)), the finding that PA induced both AMPK activation and down regulation of growth factor signaling via Akt, points to mTOR as a downstream target mediating the effects of PA. That mTOR may represent a molecular target that is regulated by PA to inhibit carcinogenesis is a currently unappreciated possibility.
Insulin like growth factor-1 (IGF-1) The insulin-like growth factor (IGF) signaling system plays a critical role in the growth and development of many tissues and regulates overall growth ((27) and references therein). The IGF system has also been implicated in various pathophysiological conditions, and is thought to play a particularly prominent role in tumorigenesis. The IGF system is comprised of the IGF ligands (IGF-I and IGF-II), cell-surface receptors that mediate the biological effects of the IGFs, including the IGF-I receptor (IGF-IR), the IGF-II receptor (IGF-IIR), and the insulin receptor (IR), as well as a family of IGF-binding proteins (IGFBPs). IGFBPs affect the half-lives and bioavailability of the IGFs in the circulation, in extracellular fluids, and may exert IGF-independent effects under certain conditions. Most, if not all, of the effects of IGF-I result from its activation of the IGF-IR and lead to activation of the mitogen-activated protein kinase (MAP kinase) and PI3 kinase cascades. The ultimate targets of the MAP kinase and PI3 kinase cascades include members of mTOR network and forkhead transcription factor families. Regulation of transcription factors provides a mechanism by which IGF action at the cell surface can elicit changes in gene expression that eventually mediate the proliferative, differentiating, and apoptotic effects of IGF-1. In 1998, Hawkinson et al. reported that pre-menopausal, but not post-menopausal women in the highest tertile of serum IGF-I levels had a significantly increased risk of developing breast cancer (28). We have found that the level of plasma IGF-1 and signaling in mammary carcinomas via the pathway of which the cell surface receptor of IGF-1 is a component were reduced by PA (6;9).
The hormesis hypothesis
This hypothesis predicts that the carcinogenic response to PA is hormetic, i.e. non linear (J or U-shaped) and accounted for by a physiological cellular stress response.
Hormesis is a term used to refer to a biphasic dose-response to an environmental agent, frequently a physiological stressor, which PA is considered to be. A hormetic response is characterized by a low dose beneficial effect, illustrated by rats that ran at moderate intensity for a shorter period of time and that were protected against cancer, and by a high dose effect similar to that observed in the absence of exposure to the environmental agent (loss of protection against mammary cancer with increasing PA duration, (8)). While this is to our knowledge the first report of a hormetic response to PA relative to cancer, biphasic responses to PA have been reported for a number of physiological-pathophysiological processes (29). Examples of hormesis include findings that: 1) physical inactivity or strenuous exercise bouts increased the risk of infection, while moderate exercise up-regulated the immune system, 2) regular PA, with moderate intensity and duration improved cardiovascular function; whereas, PA inactivity or strenuous PA increased the risk of heart attacks and stroke, 3) mechanical damage-mediated adaptation resulted in increased muscle mass and increased resistance to stressors in comparison to inactivity or strenuous activity, and 4) single bouts of exercise increased, and regular exercise decreased the oxidative challenge to the body, whereas excessive exercise and overtraining led to enhanced oxidative stress (29;30). Recent findings have elucidated cellular signaling pathways and molecular mechanisms that mediate hormetic responses. They typically involve kinases, deacetylases, and transcription factors, many of which have been implicated as being involved in the carcinogenic process (31). Specific examples of such pathways for which we have preliminary data that PA modulates are sirtuins (SIRT), which are histone deacetylases, and the forkhead box sub group O (FoxO) family of transcription factors. The network of which SIRT1 and FoxO are components and the cellular processes regulated by this network are shown in Figure 1. The pathways of which NF-kappaB and the Nrf-2/ARE are components have also been shown to be involved in hormetic responses and both are implicated in carcinogenesis and affected by PA (31).
FoxO Forkhead transcription factors (FoxOs) are highly conserved among species and play a critical role in cell cycle control and cellular stress responses. FoxOs have diverse effects on differentiation, proliferation and cell survival by regulating genes involved in cell cycle passage (p27 and cyclins), apoptosis (FAS ligand and BIM), and cell survival and stress resistance (GADD45 and others)(32-35). While FoxOs are known to be regulated by the insulin signaling pathway, work recently reported by the Brunet lab (36;37), indicates that AMPK phosphorylates 6 specific residues on FoxO and phosphorylation of these sites opposes the phosphorylation of other FoxO sites by AKT. Phosphorylation of FoxO by AMPK affects the conformation of the protein such that sirtuin mediated deacetylation is also modified. How FoxO activation is influenced by PA requires additional investigation.
Sirtuins Silent information regulator 2 (Sir2) proteins, or sirtuins, are protein deacetylases/mono-ADP-ribosyltransferases found in organisms ranging from bacteria to humans (38). Sirtuins (SIRTs 1-7), or class III histone deacetylases (HDACs), are protein deacetylases/ADP ribosyltransferases that target a wide range of cellular proteins in the nucleus, cytoplasm, and mitochondria for post-translational modification by acetylation (SIRT1, -2, -3 and -5) or ADP ribosylation (SIRT4 and -6) (39). The orthologs of sirtuins in lower organisms play a critical role in regulating lifespan. For cancer, there are growing implications that sirtuins protect against cancer development since they regulate the cellular responses to stress and ensure that damaged DNA is not propagated and that mutations do not accumulate. However, there also is emerging evidence that sirtuins are direct participants in the growth of some cancers (39).
The dependence of sirtuins on nicotinamide adenine dinucleotide (NAD(+)) links their activity to cellular metabolic status. Emerging evidence indicates that deacetylation of FOXO by SIRT1 favors expression of cell survival/stress resistance and the down regulation of pro-apoptotic genes (40-44). SIRT1 also deacetylates the C-terminal Lys-382 of p53 and this down regulates p53 dependent apoptosis (45). In addition, cytokines such as those that we have found to be regulated by PA, have been reported to have direct and indirect effects on cellular stress responses modulated by acetylation/deacetylation reactions, and these effects can be further modified by cortical steroids that PA dramatically induces (46).
Cytokines That PA could exert effects on the carcinogenic process that are related to bodily movement per se, i.e. to muscle contraction, is consistent with an experiment reported over four decades ago indicating that contracting skeletal muscle produces a factor(s) that inhibits tumor cell growth (47). Moreover, emerging evidence indicates that contracting muscle releases cytokines, referred to as myokines, that have endocrine activity (48). This finding has important implications in that it suggests that skeletal muscle represents the largest endocrine organ in the body. A myokine is defined as a muscle contraction-induced humoral factor, an “exercise factor”, that could mediate some of the PA-induced changes in an endocrine or paracrine manner. Currently recognized myokines include Interleukin-6 (IL-6), IL-8, IL-15, and visfatin, although the list is likely to expand given that the “myokine field” is less than 2-years old. In recent decades, many in vitro and in vivo studies have increased the comprehension of the role of cytokines in breast oncology (49). Some cytokines (IL-1, IL-6, IL-11, TGF-β) stimulate while others (IL-12, IL-18, IFNs) inhibit breast cancer proliferation and/or invasion. Similarly, high circulating levels of some cytokines seem to be favorable (soluble IL-2R) while others are unfavorable (IL-1b, IL-6, IL-8, IL-10, IL-18, gp130) prognostic indicators. However, many cytokines have pleiotrophic effects that are context specific, TGF-β being a prominent example.
The metabolic reprogramming hypothesis PA alters the availability of glucose and amino acids such as glutamine that are available to mammary carcinomas thereby delaying cell cycle progression and inducing apoptosis because demands for precursor molecules dictated by tumor-associated metabolic programming are not met; this serves to limit the accumulation of tumor cell mass.
Metabolic Requirements of Skeletal Muscle versus Tumor Cells. In contracting skeletal muscle, aerobic metabolism involves glycolytic conversion of glucose via pyruvate into acetyl-CoA and its complete oxidation through the mitochondrion-localized tricarboxylic acid (TCA) cycle and oxidative phosphorylation to CO2 and H2O which generates 38 ATP molecules per molecule of glucose. Duration exercise, TCA cycle flux increases to meet requirements for ATP and can entail a 60 to 100 fold increase in the concentration of TCA cycle intermediates in part through anapleurotic mechanisms involving various glycolytic products and amino acids, prominent among which are pyruvate, glutamine, and alanine (50;51). Catapleurosis also occurs during exercise affecting circulating levels of pyruvate, alanine, as well as glucose and glutamine (52).
In contrast to contracting skeletal muscle’s requirements to support moderate to intense endurance activity, tumor cells use glucose, glutamine, and other metabolic substrates to meet not only energetic requirements but also the biosynthetic needs for cell growth and nucleic acid synthesis for rapid cell division (53). In tumor cells, glycolysis tends to be aborted at either of two steps. First, aerobic glycolysis in tumor cells implies conversion of glucose into pyruvate (which generates only two ATP molecules per molecule of glucose) and subsequently into the waste product lactic acid. Second, in tumor cells, acetyl-CoA tends to be introduced into a truncated TCA cycle, with the net result that acetyl-CoA is exported into the cytosol and serves as a building block for cell growth and proliferation. In this truncated TCA cycle, citrate is preferentially exported to the cytosol via the tricarboxy-late transporter. Once in the cytosol, citrate is cleaved by ATP citrate lyase (ACL) to generate oxaloacetate and acetyl-CoA. Oxaloacetate is reduced to malate, then reimported into mitochondria and reconverted to oxaloacetate in the matrix (while generating NADH that represses the TCA cycle), and it reacts with acetyl-CoA to complete the substrate cycle. Many of these alterations can be caused by the activation of HIF-1 and c-Myc (54-56).
As reviewed in (54) , the essential hallmarks of cancer (57) are intertwined with an altered cancer cell-intrinsic metabolism, either as a consequence or a cause (54). Commonly cited examples in support of this observation include the resistance of cancer cells to apoptosis induction that has been linked to altered tricarboxylic acid (TCA) metabolism in the mitochondria, specifically via effects on pyruvate dehydrogenase, pyruvate dehydrogenase kinase, and lactate dehydrogenase (58), and the profound impact and direct regulation of numerous steps in intermediary metabolism that occur as a component of the constitutive activation of signaling pathways that stimulate cell growth and proliferation while blocking cell death (55). These pathways are associated with the activation of specific oncogenes and/or loss of tumor suppressor function. As reviewed in (54), key oncogenes and tumor suppressors genes known to be involved in breast cancer (notable examples are p53, PI3 kinase, and HIF1α) regulate metabolism and associated alterations in metabolism have been directly linked to each of the hallmarks of malignancy (57), prompting the hypothesis that tumor cell metabolism is cancer’s Achilles heel.
It is well documented that contracting muscle preferentially utilizes glucose and glutamine as energy substrates and, depending on the degree of fitness and training, can alter the profile and amount of fatty acids in circulation (3;52). The impact of PA on other products of intermediary metabolism that enter the blood or are removed from it to support muscle biogenesis and/or contraction has yet to be studied other than for molecules like lactate. In view of the large shifts in metabolites and substrates that occur during PA, and that are affected by its intensity and duration, it is possible that shifts in metabolism induced by PA modulate the metabolic reprogramming that occurs during carcinogenesis to support cell growth and proliferation. These ideas imply that carcinogenesis can be inhibited or enhanced by effects on intermediary metabolism linked to PA, depending on its intensity and duration.
Systemic Considerations
An important consideration not yet discussed is how effects originating from skeletal muscle contraction are likely to be conveyed to breast epithelial cells. To address this issue, we suggest that consideration be given to the following questions: 1) what does contracting muscle release into circulation or remove from circulation that would directly modulate the carcinogenic process in epithelial cells (is the mechanism related to changes in the availability of substrates for metabolism or signaling molecules), 2) are the effects of muscle contraction on epithelial cell carcinogenesis exerted in an endocrine, paracrine, autocrine, or intracrine manner (is the mechanism due to direct effects on the target epithelial cells [autocrine or intracrine] or are the effects indirect or mediated through effects on tissues distant from target epithelial cells [endocrine] or via cells in immediate proximity to target epithelial cells [paracrine]), and 3) are the effects of muscle contraction on malignant cells different from effects on normal or pre-malignant cells that do not manifest the hallmarks of malignancy (do we expect cancer epithelial cells to respond differently than pre-malignant epithelial cells or normal cells) (57)? Addressing these questions should provide a basis by which to connect systemic effects of PA to intracellular events that regulate cell function and carcinogenesis and that PA appears to modulate.
Concluding Comments
Many experts contend that a primary factor that accounts for the global epidemic of overweight and obesity is physical inactivity (1). This has led to the public health recommendation that individuals should increase their level of PA (1-3). However, the focus on PA to control weight gain, while critical, tends to distract attention from the fact that approximately 40% of women are an appropriate weight for their height. Importantly, for those individuals who are maintaining body weight in an acceptable range for cancer risk reduction, but who also wish to increase their PA for further reduction in risk, the relative importance of PA intensity, duration, and frequency for cancer prevention is unclear and requires investigation. The hypotheses presented in this review specifically address this population of women. As noted in the recently released NIH report on Physical Activity and Health, key translational questions remain unanswered about the intensity and duration of PA that prevents and control cancer and the nature of the dose response is unclear. Given evidence that breast cancer mortality also appears to be decreased by PA and that physical activity is increasingly being recommended during and after cancer treatment, the questions considered herein have the potential, when resolved, to inform understanding not only of how PA reduces breast cancer risk but also of how PA affects the growth of established breast tumors and breast cancer metastasis, and to identify the molecular characteristics of the breast cancers that PA affects.
Footnotes
This work was supported by United States Public Health Services Grants R01-CA100693 and U54-CA116847 from the National Cancer Institute.
Reference List
- 1.IARC . 6. IARC Press. IARC Handbook of Cancer Prevention; Lyon: 2002. Weight Control and Physical Activity; pp. 1–355. [Google Scholar]
- 2.Wiseman MJ. The second AICR/WCRF international expert report: Food, nutrition, physical activity, and cancer prevention: a global perspective. Journal of Nutrition. 2007;135(12):3037S. [Google Scholar]
- 3.Physical Activity Guidelines Advisory Committee . Physical Activity guidelines Advisory Committee Report, 2008. US Department of Health and Human Services; Washington DC: 2008. [DOI] [PubMed] [Google Scholar]
- 4.Thompson HJ. Pre-clinical investigations of physical activity and cancer: a brief review and analysis. Carcinogenesis. 2006;27:1946–9. doi: 10.1093/carcin/bgl117. [DOI] [PubMed] [Google Scholar]
- 5.Thompson HJ. Effects of physical activity and exercise on experimentally-induced mammary carcinogenesis. Breast Cancer Res Treat. 1997;46:135–41. doi: 10.1023/a:1005912527064. [DOI] [PubMed] [Google Scholar]
- 6.Jiang W, Zhu Z, Thompson HJ. Effects of physical activity and restricted energy intake on chemically induced mammary carcinogenesis. Cancer Prev Res (Phila Pa) 2009;2:338–44. doi: 10.1158/1940-6207.CAPR-08-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu Z, Jiang W, Sells JL, Neil ES, McGinley JN, Thompson HJ. Effect of nonmotorized wheel running on mammary carcinogenesis: circulating biomarkers, cellular processes, and molecular mechanisms in rats. Cancer Epidemiol Biomarkers Prev. 2008;17:1920–9. doi: 10.1158/1055-9965.EPI-08-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson HJ, Neil ES, Sells JL, McGinley J, Jiang W, Zhu Z. Motorized wheel running and mammary carcinogenesis in rats: effects of physical activity duration. Nutrition and Cancer. 2008 doi: 10.1158/1055-9965.EPI-08-0175. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Z, Jiang W, McGinley JN, Thompson HJ. Energetics and mammary carcinogenesis: effects of moderate-intensity running and energy intake on cellular processes and molecular mechanisms in rats. J Appl Physiol. 2009;106:911–8. doi: 10.1152/japplphysiol.91201.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–31. [PMC free article] [PubMed] [Google Scholar]
- 11.Koh HJ, Brandauer J, Goodyear LJ. LKB1 and AMPK and the regulation of skeletal muscle metabolism. Curr Opin Clin Nutr Metab Care. 2008;11:227–32. doi: 10.1097/MCO.0b013e3282fb7b76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witczak CA, Sharoff CG, Goodyear LJ. AMP-activated protein kinase in skeletal muscle: from structure and localization to its role as a master regulator of cellular metabolism. Cell Mol Life Sci. 2008;65:3737–55. doi: 10.1007/s00018-008-8244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood LD, Parsons DW, Jones S, et al. The Genomic Landscapes of Human Breast and Colorectal Cancers. Science. 2007;318:1108–13. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 14.Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation--AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006;574:63–71. doi: 10.1113/jphysiol.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–34. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 17.Lee CC, Huang CC, Wu MY, Hsu KS. Insulin stimulates postsynaptic density-95 protein translation via the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway. J Biol Chem. 2005;280:18543–50. doi: 10.1074/jbc.M414112200. [DOI] [PubMed] [Google Scholar]
- 18.Martin KA, Rzucidlo EM, Merenick BL, et al. The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am J Physiol Cell Physiol. 2004;286:C507–C517. doi: 10.1152/ajpcell.00201.2003. [DOI] [PubMed] [Google Scholar]
- 19.Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–26. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- 20.Sonenberg N, Gingras AC. The mRNA 5' cap-binding protein eIF4E and control of cell growth. Curr Opin Cell Biol. 1998;10:268–75. doi: 10.1016/s0955-0674(98)80150-6. [DOI] [PubMed] [Google Scholar]
- 21.Martin KA, Blenis J. Coordinate regulation of translation by the PI 3-kinase and mTOR pathways. Adv Cancer Res. 2002;86:1–39. doi: 10.1016/s0065-230x(02)86001-8. [DOI] [PubMed] [Google Scholar]
- 22.Hardie DG. The AMP-activated protein kinase pathway--new players upstream and downstream. J Cell Sci. 2004;117:5479–87. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 23.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Z, Jiang W, McGinley J, Wolfe P, Thompson HJ. Effects of dietary energy repletion and IGF-1 infusion on the inhibition of mammary carcinogenesis by dietary energy restriction. Mol Carcinog. 2005;42:170–6. doi: 10.1002/mc.20071. [DOI] [PubMed] [Google Scholar]
- 25.Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–52. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- 26.Younes H, Leleu X, Hatjiharissi E, et al. Targeting the phosphatidylinositol 3-kinase pathway in multiple myeloma. Clin Cancer Res. 2007;13:3771–5. doi: 10.1158/1078-0432.CCR-06-2921. [DOI] [PubMed] [Google Scholar]
- 27.LeRoith D, Roberts CT. The insulin-like growth factor system and cancer. Cancer Letters. 2003;195:127–37. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 28.Hankinson SE, Willett WC, Colditz GA, et al. Circulating concentrations of insulin-like growth factor I and risk of breast cancer. The Lancet. 1998;351:1393–6. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 29.Radak Z, Kumagai S, Taylor AW, Naito H, Goto S. Effects of exercise on brain function: role of free radicals. Appl Physiol Nutr Metab. 2007;32:942–6. doi: 10.1139/H07-081. [DOI] [PubMed] [Google Scholar]
- 30.Goto S, Naito H, Kaneko T, Chung HY, Radak Z. Hormetic effects of regular exercise in aging: correlation with oxidative stress. Appl Physiol Nutr Metab. 2007;32:948–53. doi: 10.1139/H07-092. [DOI] [PubMed] [Google Scholar]
- 31.Mattson MP. Hormesis defined. Ageing Research Reviews. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouchard C, Lee S, Paulus-Hock V, Loddenkemper C, Eilers M, Schmitt CA. FoxO transcription factors suppress Myc-driven lymphomagenesis via direct activation of Arf. Genes Dev. 2007;21:2775–87. doi: 10.1101/gad.453107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakae J, Oki M, Cao Y. The FoxO transcription factors and metabolic regulation. FEBS Lett. 2008;582:54–67. doi: 10.1016/j.febslet.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 34.Brunet A. [The multiple roles of FOXO transcription factors] Med Sci (Paris) 2004;20:856–9. doi: 10.1051/medsci/20042010856. [DOI] [PubMed] [Google Scholar]
- 35.Carter ME, Brunet A. FOXO transcription factors. Curr Biol. 2007;17:R113–R114. doi: 10.1016/j.cub.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Greer EL, Oskoui PR, Banko MR, et al. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007;282:30107–19. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- 37.Greer EL, Dowlatshahi D, Banko MR, et al. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17:1646–56. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwer B, Verdin E. Conserved metabolic regulatory functions of sirtuins. Cell Metab. 2008;7:104–12. doi: 10.1016/j.cmet.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Saunders LR, Verdin E. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene. 2007;26:5489–504. doi: 10.1038/sj.onc.1210616. [DOI] [PubMed] [Google Scholar]
- 40.Brunet A, Sweeney LB, Sturgill JF, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 41.Brunet A, Kanai F, Stehn J, et al. 14-3-3 transits to the nucleus and participates in dynamic nucleocytoplasmic transport. J Cell Biol. 2002;156:817–28. doi: 10.1083/jcb.200112059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 43.Tran H, Brunet A, Griffith EC, Greenberg ME. The many forks in FOXO's road. Sci STKE. 2003 doi: 10.1126/stke.2003.172.re5. 2003:RE5. [DOI] [PubMed] [Google Scholar]
- 44.Tran H, Brunet A, Grenier JM, et al. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science. 2002;296:530–4. doi: 10.1126/science.1068712. [DOI] [PubMed] [Google Scholar]
- 45.Vaziri H, Dessain SK, Ng EE, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–59. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 46.Bodles AM, Barger SW. Cytokines and the aging brain - what we don't know might help us. Trends in Neurosciences. 2004;27:621–6. doi: 10.1016/j.tins.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 47.HOFFMAN SA, PASCHKIS KE, DEBIAS DA, CANTAROW A, WILLIAMS TL. The influence of exercise on the growth of transplanted rat tumors. Cancer Res. 1962;22:597–9. [PubMed] [Google Scholar]
- 48.Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol. 2007;103:1093–8. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- 49.Nicolini A, Carpi A, Rossi G. Cytokines in breast cancer. Cytokine Growth Factor Rev. 2006;17:325–37. doi: 10.1016/j.cytogfr.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Bowtell JL, Marwood S, Bruce M, Constantin-Teodosiu D, Greenhaff PL. Tricarboxylic acid cycle intermediate pool size: functional importance for oxidative metabolism in exercising human skeletal muscle. Sports Med. 2007;37:1071–88. doi: 10.2165/00007256-200737120-00005. [DOI] [PubMed] [Google Scholar]
- 51.Bowtell JL, Bruce M. Glutamine: an anaplerotic precursor. Nutrition. 2002;18:222–4. doi: 10.1016/s0899-9007(01)00795-x. [DOI] [PubMed] [Google Scholar]
- 52.Gleeson M. Dosing and Efficacy of Glutamine Supplementation in Human Exercise and Sport Training. J Nutr. 2008;138:2045S–2049. doi: 10.1093/jn/138.10.2045S. [DOI] [PubMed] [Google Scholar]
- 53.DeBerardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell. 2008;13:472–82. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 55.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 56.Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell. 2007;12:108–13. doi: 10.1016/j.ccr.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 58.Bonnet S, Archer SL, lalunis-Turner J, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]