Abstract
Background
Whether extracapsular extension of tumor (ECE) in the sentinel lymph node (SLN) is an indication for axillary dissection (ALND) in patients managed by ACOSOG Z0011 criteria is controversial. Here we examine the correlation between ECE in the SLN and disease burden in the axilla.
Methods
Patients meeting Z0011 clinicopathologic criteria (pT1-T2,cN0 with <3 positive SLNs) were selected from a prospectively maintained database (2006-2013). Chart review documented presence/extent of ECE. Neoadjuvant chemotherapy patients were excluded. Comparisons were made by presence/extent (≤2mm vs.>2mm) of ECE.
Results
Of 11,730 patients, 778 were pT1-T2, cN0 with <3 positive SLNs without ECE and 331 (2.8%) had ECE. Of these, 180 had ≤2mm, and 151 had >2mm ECE. Patients with ECE were older (57 vs54 yrs;p=0.001) and had larger (2.0cm vs 1.7cm,p<0.0001), multifocal (p=0.006), HR-positive tumors (p=0.0164), with LVI (p<0.0001). Presence and extent of ECE was associated with greater axillary disease burden; 20% and 3% of patients with and without ECE, respectively, had ≥4 additional positive nodes at cALND (p<0.0001) and 33% of patients with >2mm ECE had ≥4 additional positive nodes at cALND compared to 9% in the <2mm group(p<0.0001). On multivariate analysis,>2mm ECE was the strongest predictor of ≥4 positive nodes at cALND (OR 14.2).
Conclusions
Presence and extent of ECE were significantly correlated with nodal tumor burden at cALND, suggesting that >2mm of ECE may be an indication for ALND or RT when applying Z0011 criteria to patients with metastases in <3 SLNs. ECE reporting should be standardized to facilitate future studies.
Introduction
Axillary nodal involvement has long been recognized as a key prognostic factor in invasive breast cancer, and sentinel lymph node biopsy (SLNB) is the accepted standard of care for axillary staging.1-3 Recently, approaches to axillary management have undergone major changes, and there is great interest in identifying patients who do not require completion axillary dissection (ALND) despite the presence of positive sentinel nodes. Two randomized clinical trials have addressed this question. The American College of Surgeons Oncology Group (ACOSOG) Z0011 trial demonstrated no differences in locoregional recurrence or survival for women with T1-T2, clinically N0 tumors undergoing breast-conserving surgery (BCS) with whole-breast irradiation when metastases in 1 or 2 sentinel nodes were managed with SLNB alone vs SLNB and ALND.4,5 The After Mapping of the Axilla: Radiotherapy or Surgery? (AMAROS) trial showed no advantage for ALND compared to SLNB plus radiation to the axillary and medial supraclavicular fields (ART) in patients with <3 positive sentinel nodes.6 Thus, selection criteria for management with SLNB alone, or SLNB plus ART, is a major clinical controversy.
Extracapsular extension (ECE) is the growth or spread of tumor cells outside of the lymph node capsule. ECE is recognized as an indicator of poor prognosis.7 Retrospective analyses have shown that ECE is correlated with negative prognostic factors, including lymphovascular invasion (LVI) and macrometastases in the sentinel lymph node (SLN).8,9 ECE has also been demonstrated to predict the presence of non-SLN involvement.1,10,11
Whether the presence of ECE in the SLN is an indication for ALND or ART in patients otherwise eligible to be managed with SLNB alone according to ACOSOG Z0011 criteria is uncertain. Patients with gross ECE were excluded from ACOSOG Z0011, and the presence of microscopic ECE was not evaluated in that study. We sought to determine the correlation between the presence and extent of ECE in the SLN and disease burden in the axilla in clinically node-negative women with T1 and T2 breast carcinomas, and to identify factors associated with the presence of ECE in the SLN.
Methods
After institutional review board approval, retrospective review of prospective databases was undertaken to identify patients with clinical stage T1-2, node-negative breast cancer who underwent SLNB at Memorial Sloan Kettering Cancer Center (MSKCC) from 1/2006-3/2013. Patients receiving neoadjuvant therapy, those with pathologically negative or IHC-only positive SLN, and ≥3 positive SLNs were excluded, leaving a cohort of patients with early-stage breast cancer and 1 or 2 positive SLNs by routine hematoxylin and eosin (H&E) staining. Although the majority of patients were treated prior to the adoption of ACOSOG Z0011 criteria at MSKCC (8/2010), they were retrospectively considered eligible for this approach based on clinical and pathologic staging criteria regardless of the type of breast surgery performed.
Prior to the adoption of ACOSOG Z0011 criteria, patients with 1 or 2 positive SLNs typically underwent ALND. In the post-Z0011 era, patients having BCS were managed according to the Z0011 protocol with ALND performed for gross ECE and/or ≥3 positive SLNs. There was no defined policy for completion ALND based on microscopic ECE. Standard clinical and pathologic data, including the presence and extent of ECE in the SLN, defined as absent, ≤2mm, or >2mm of ECE, were abstracted from the medical record. Cases where this information was missing were reviewed by the study pathologist (AC). Comparisons were made between patients with and without ECE, and by extent of ECE using Fisher's, Wilcoxon rank sum, and Kruskal Wallis tests. All statistical analysis was done in SAS 9.2 (SAS Institute, Cary, NC). P-values <0.05 were considered significant. Univariate and multivariate logistic regression models were used to assess associations between selected factors and the involvement of ≥4 nodes in patients with completion ALND.
Results
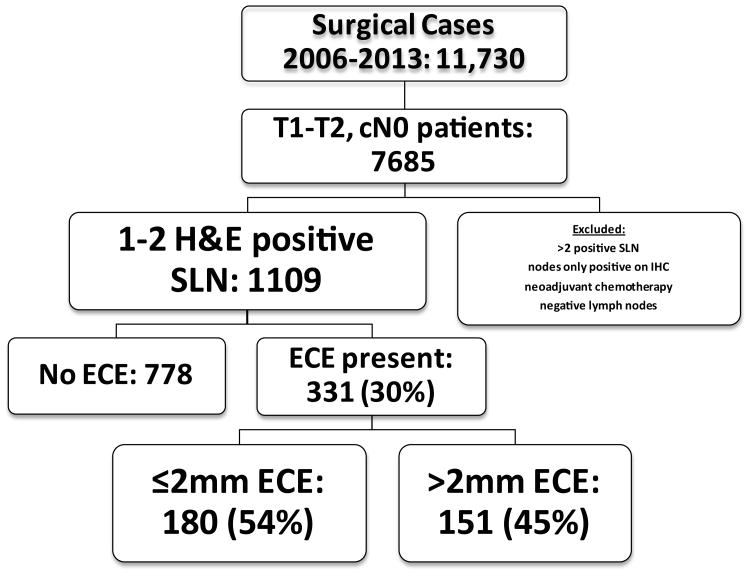
Between 1/2006 and 3/2013, 11,730 patients underwent surgical treatment for breast cancer; 1109 (9.5%) had clinical T1-T2, N0 breast cancer and 1 or 2 positive nodes. Of these, 331 (29.8%) had ECE in the SLN; 180 (54.4%) had ≤2mm of ECE, and 151 (45.6%) had >2mm of ECE (Figure 1).
Figure 1. Patient Selection.
The characteristics of patients with and without ECE in the SLN are compared in Table 1. Patients with ECE were older (median 57 vs 54 years; p=0.0012), and had larger (2.0cm vs 1.7cm; p≤0.0001) tumors which were more often hormone receptor-positive (91.8% vs 85.7%; p=0.0164). Multifocality/multicentricity and LVI were also significantly associated with ECE. There were no significant differences in nuclear grade or histologic differentiation between groups. The median number of SLNs removed in both groups was 3, and the majority of patients had one positive SLN; however, patients with ECE were more likely to have 2 positive sentinel lymph nodes (35% vs 19.5%; p<0.0001). Completion ALND was performed in 249 (75.2%) patients with ECE and in 513 (65.9%) patients without ECE. Additional positive nodes were found in 54.2% of patients with ECE compared to 21.8% of patients without ECE; patients with ECE were also more likely to have ≥4 positive nodes, 20.5% vs 2.5% (p<0.001), respectively (Table 1).
Table 1. Characteristics of patients with and without ECE.
| Factor | No ECE (n=778) | All ECE (n=331) | p-value |
|---|---|---|---|
|
| |||
| Median age, years (range) | 54 (20-96) | 57 (24-92) | 0.0012 |
|
| |||
| Median tumor size (range) | 1.7cm (0.09-5.0cm) | 2.0cm (0.3-5.0cm) | <0.0001 |
|
| |||
| Nuclear Grade | |||
| 1 | 85 (10.9%) | 50 (15.1%) | |
| 2 | 333 (42.8%) | 142 (42.9%) | |
| 3 | 276 (35.5%) | 111 (33.5%) | |
| Missing* | 84 (10.8%) | 28 (8.5%) | 0.1844 |
|
| |||
| Subtype | |||
| HR+/HER2- | 597 (76.7%) | 279 (84.3%) | |
| HR+/HER2+ | 70 (9.0%) | 25 (7.6%) | |
| HR-/HER2+ | 35 (4.5%) | 7 (2.1%) | |
| HR-/HER2- | 73 (9.4%) | 18 (5.4%) | |
| Missing* | 3 (0.4%) | 2 (0.6%) | 0.0164 |
|
| |||
| Multifocality | 231 (29.7%) | 126 (38.1%) | |
| Missing* | 0 (0%) | 1 (0.3%) | 0.0062 |
|
| |||
| LVI | 396 (50.9%) | 210 (63.4%) | <0.0001 |
|
| |||
| Differentiation | |||
| Poor | 412 (52.9%) | 173 (52.3%) | |
| Moderate | 234 (30.1%) | 103 (31.3%) | |
| Well | 36 (4.6%) | 6 (1.8%) | |
| Missing* | 96 (12.3%) | 49 (14.8%) | 0.0806 |
|
| |||
| Median number SLNs removed (range) | 3 (1-19) | 3 (1-14) | <0.0001 |
|
| |||
| Number positive SLNs removed | |||
| 1 | 626 (80.5%) | 215 (65%) | |
| 2 | 152 (19.5%) | 116 (35%) | <0.0001 |
|
| |||
| Completion ALND | 513 (65.9%) | 249 (75.2%) | 0.0023 |
|
| |||
| Positive nodes at cALND** | 112 (21.8%) | 135 (54.2%) | <0.0001 |
|
| |||
| Additional positive nodes at cALND** | |||
| 0 | 401 (78.2%) | 114 (45.8%) | |
| 1-3 | 99 (19.3%) | 84 (33.7%) | |
| ≥4 | 13 (2.5%) | 51 (20.5%) | <0.0001 |
ECE, extracapsular extension; HR, hormone receptor; LVI, lymphovascular invasion; ALND, axillary lymph node dissection; cALND, completion axillary lymph node dissection
Missing excluded for calculation of p-values
Among patients with cALND
Patient age, nuclear grade, or hormone receptor status did not differ based on extent of ECE (Table 2). Patients with ≤2mm ECE had smaller tumors, median 1.8cm vs 2.2cm (p=0.0004), and were more likely to have only one positive SLN, 73.9% vs 54.3% (p<0.0001, Table 2). Completion ALND was performed in 128 (71.1%) patients with ≤2mm ECE and in 121 (80.1%) patients with >2mm ECE. Additional positive nodes were found in 42.9% of patients with ≤2mm ECE compared to 66.1% of patients with >2mm ECE (p<0.0001); patients with >2mm ECE were also more likely to have ≥4 positive nodes, 33.1% vs 8.6% (p<0.0001), respectively (Table 2).
Table 2.
Characteristics and axillary disease burden of patients with no ECE, ≤2mm ECE, or >2mm ECE.
| Factor | No ECE (n=778) |
≤2mm (n=180) |
>2mm (n=151) |
p-value (≤ 2mm vs >2mm) |
p-value (≤ 2mm vs >2mm vs no ECE) |
|---|---|---|---|---|---|
|
| |||||
| Patient Characteristics | |||||
|
| |||||
| Median age, years (range) | 54 (20-96) | 57.0 (24-87) | 57.0 (31-92) | 0.8777 | 0.0049 |
|
| |||||
| Median tumor size (range) | 1.7cm (0.09-5.0cm) | 1.8cm (0.4-4.5cm) | 2.2cm (0.3-5cm) | 0.0004 | <0.0001 |
|
| |||||
| Nuclear Grade | |||||
| 1 | 85 (10.9%) | 23 (12.8%) | 27 (17.9%) | ||
| 2 | 333 (42.8%) | 82 (45.6%) | 60 (39.7%) | ||
| 3 | 276 (35.5%) | 63 (35.0%) | 48 (31.8%) | ||
| Missing* | 84 (10.8%) | 12 (6.7%) | 16 (10.6%) | 0.3394 | 0.2237 |
|
| |||||
| Subtype | |||||
| HR+/HER2- | 597 (76.7%) | 150 (83.3%) | 129 (85.4%) | ||
| HR+/HER2+ | 70 (9.0%) | 15 (8.3%) | 10 (6.6%) | ||
| HR-/HER2+ | 35 (4.5%) | 5 (2.8%) | 2 (1.3%) | ||
| HR-/HER2- | 73 (9.4%) | 9 (5.0%) | 9 (6.0%) | ||
| Missing* | 3 (0.4%) | 1 (0.6%) | 1 (0.6%) | 0.7422 | 0.0132 for HR+ vs HR- |
|
| |||||
| Patient axillary disease burden | |||||
|
| |||||
| Median number SLNs removed (range) | 3 (1-19) | 3 (1-14) | 2 (1-11) | 0.0095 | <0.0001 |
|
| |||||
| Number positive SLNs removed | |||||
| 1 | 626 (80.4%) | 133 (73.9%) | 82 (54.3%) | ||
| 2 | 152 (19.6%) | 47 (26.1%) | 69 (45.7%) | <0.0001 | <0.0001 |
|
| |||||
| Completion ALND | 513 (65.9%) | 128 (71.1%) | 121 (80.1%) | 0.0732 | 0.0015 |
|
| |||||
| Positive nodes at cALND** | 112 (21.8%) | 55 (42.9%) | 80 (66.1%) | <0.0001 | <0.0001 |
|
| |||||
| Number additional positive nodes at cALND* | |||||
| 0 | 401 (78.2%) | 73 (57.0%) | 41 (33.9%) | ||
| 1-3 | 99 (19.3%) | 44 (34.4%) | 40 (33.1%) | ||
| ≥4 | 13 (2.5%) | 11 (8.6%) | 40 (33.1%) | <0.0001 | <0.0001 |
ECE, extracapsular extension; HR, hormone receptor; SLN, sentinel lymph node; ALND, axillary lymph node dissection; cALND, completion axillary lymph node dissection
Missing excluded for calculation of p-values
Among patients with cALND
Table 3 examines the relationship between the number of positive SLNs, the extent of ECE, and additional nodal disease burden. Among patients with only one positive SLN, 38.5% of patients with ≤2mm ECE had additional positive nodes as compared to 57.2% of patients with >2mm ECE (p=0.0021). Similarly, among patients with two positive SLNs, 54% of patients with ≤2mm ECE had additional positive nodes compared to 76% of patients with >2mm ECE (p=0.0070). In contrast, within groups of patients with the same extent of ECE, the number of positive SLNs was not significantly associated with more extensive nodal burden. 28.6% of patients with one positive SLN and >2mm ECE, and 38% of patients with two positive SLNs and >2mm ECE had ≥4 additional positive nodes, respectively.
Table 3.
The relationship between number of positive SLNs, extent of ECE, and additional nodal burden among patients with cALND.
| No ECE (n=513) |
≤2mm (n=128) |
>2mm (n=121) |
Overall | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| No. of additional positive ALNs | 1 positive SLN n=396 |
2 positive SLNs n=117 |
1 positive SLN n=91 |
2 positive SLNs n=37 |
1 positive SLN n=63 |
2 positive SLNs n=58 |
1 positive SLN n=550 |
2 positive SLNs n=212 |
|
| ||||||||
| 0 | 311 (78.5%) | 90 (76.9%) | 56 (61.5%) | 17 (45.9) | 27 (42.8%) | 14 (24.1%) | 394 (71.6%) | 121 (57.1%) |
| 1-3 | 76 (19.2%) | 23 (19.7%) | 28 (30.8%) | 16 (43.2%) | 18 (28.6%) | 22 (37.9%) | 122 (22.2%) | 61 (28.8%) |
| >4 | 9 (2.3%) | 4 (3.4%) | 7 (7.7%) | 4 (10.8%) | 18 (28.6%) | 22 (37.9%) | 34 (6.2%) | 30 (14.2%) |
|
| ||||||||
| p-value | 0.7140 | 0.2390 | 0.0936 | <0.0001 | ||||
SLN, sentinel lymph node; ECE, extracapsular extension; cALND, completion axillary lymph node dissection; ALN, axillary lymph node
Univariate and multivariate analyses of clinicopathologic factors associated with involvement of ≥4 nodes at completion ALND are displayed in Table 4. Tumor size (OR 1.7), multifocality (OR 1.9), LVI (OR 2.1) and presence of ECE (OR 9.9) were all significantly associated with ≥4 positive nodes at ALND. When ECE was stratified by none vs. ≤2mm vs. >2mm, the greater extent of ECE was associated with an OR of 19 for >4 positive nodes, and ≤2mm of ECE was associated with an OR of 3.6. On multivariate analysis, >2mm ECE remains significantly associated (OR 14.2) with ≥4 positive nodes at completion ALND, after taking into account other clinicopathologic variables. Having two positive SLN was significantly associated with ≥4 additional positive nodes at ALND (OR 2.5; p = 0.0005) on univariate analysis, but was not a significant predictor of having >4 additional axillary nodes at completion ALND on multivariate analysis (p=0.1906).
Table 4.
Univariate and multivariate analyses of factors associated with involvement of 4 or more lymph nodes at completion ALND.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age | 1.0 (0.99-1.0) | 0.0779 | 1.0 (1.0-1.0) | 0.1104 |
| T size | 1.7 (1.3-2.2) | <0.0001 | 1.4 (1.1-1.9) | 0.0166 |
| Multifocality* | 1.9 (1.1-3.1) | 0.0170 | 1.8 (1.0-3.3) | 0.0437 |
| LVI | 2.1 (1.2-3.7) | 0.0106 | 1.1 (0.6-2.2) | 0.7178 |
| ECE | <0.0001 | <.0001 | ||
| None | Ref | Ref | ||
| ≤2mm | 3.6 (1.6-8.3) | 3.1 (1.3-7.2) | ||
| >2mm | 19.0 (9.7-37.1) | 14.2 (7.1-28.4) | ||
| No. of positive SLNs | 0.0005 | 0.1906 | ||
| 1 | Ref | Ref | ||
| 2 | 2.5 (1.5-4.2) | 1.5 (0.8-2.7) | ||
ECE, extracapsular extension; ALND, axillary lymph node dissection; OR, odds ratio; CI, confidence interval; LVI, lymphovascular invasion; SLN, sentinel lymph node
1 patient missing
Discussion
ECE is a common clinical finding as illustrated in our study where ECE was identified in the SLN in 30% of clinically node-negative patients with early-stage breast cancer. This is consistent with other contemporary studies reporting ECE in 19%-26% of SLNs.1,12 In a multi-center European study of 675 patients with involvement of 1-3 SLNs, the reported incidence of ECE varied significantly among centers, ranging from 21%-57% although other patient characteristics were similar.13 Previous studies have uniformly demonstrated that ECE was a predictor of a higher likelihood of non-SLN metastases.1,9,10,12,13 Until recently, this finding was of little practical import since ALND was the standard management approach for patients with macrometastases in the SLN. With the publication of the ACOSOG Z0011 trial indicating that clinically node-negative patients with T1 and T2 tumors found to have metastases in 1-2 SLN and undergoing BCS with whole-breast irradiation and systemic therapy can be managed without ALND4, ECE as a predictor of nodal disease burden takes on new significance.
The success of the ACOSOG Z0011 approach is predicated upon a limited burden of disease remaining in the axilla after the SLNs are removed, which is likely to be controlled with systemic therapy and RT. Only 27% of patients randomized to ALND in ACOSOG Z0011 had additional nodal disease.4 In our study, although limited to women who met ACOSOG Z0011 eligibility criteria, ECE was associated with non-SLN disease in 54.2% of cases compared to 21.8% in patients without ECE (p<0.0001). Additionally, ≥4 additional involved nodes were present in 20.5% of patients with ECE compared to 2.5% of those without ECE (p<0.0001).
Other studies support the presence of ECE as a predictor of a larger numbers of nodes with metastases. Rivers et al14 examined features associated with ≥4 non-sentinel node metastases in 285 patients with positive SLNs. Tumor size, LVI, ECE, size of SLN metastases, and ratio of number of positive to resected SLNs were all significantly associated with disease in ≥4 non-sentinel nodes. In our study, ECE was associated with larger tumor size, multifocality, LVI, and a greater number of positive SLNs.1,8,11,15,16 We also noted an association between ECE and older patient age, and hormone receptor-positive, HER2 negative tumors, characteristics not generally associated with poor prognosis. After adjusting for known prognostic variables, including age, tumor size, and multifocality, ECE remains a predictor of more extensive axillary nodal involvement, as demonstrated in the multivariate analysis where >2mm of ECE was the strongest predictor (OR 14.2) of ≥4 additional nodes at completion ALND. Data from Meretoja et al support this finding; using 675 patients with macrometastases in 1-3 SLNs, they developed a model to identify patients with involvement of ≥4 non-sentinel nodes and validated the model in an additional 760 patients. In this model, ECE was also a strong predictor (p<0.0001) of involvement of ≥4 additional nodes.13 In another study of 74 patients with tumor containing sentinel nodes, the mean number of involved non-SLNs was 2.5 for patients with no ECE compared to 7.6 in patients with ECE (p=0.0061). On multivariate analysis, only the presence of ECE was a significant predictor of non-SLN involvement.12 Mittendorf et al also found ECE to be a significant predictor of non-SLN involvement in a multivariate model constructed with 509 patients.17
We also examined the impact of the extent of ECE in the SLN, arbitrarily defined as ≤2mm or >2mm, on axillary disease burden. Patients with >2mm of ECE were significantly more likely than those with lesser amounts of ECE to have additional positive nodes (66.1% vs 42.9%; p<0.0001), and one-third of this group had ≥4 involved nodes compared to only 2.5% of patients with no ECE and 8.6% with ≤2mm of ECE. The extent of ECE was a significant predictor of residual disease in patients with 1 or 2 sentinel nodes containing tumor, indicating that it is not just a surrogate for involvement of a larger number of sentinel nodes. Few other studies have examined the importance of the extent of ECE. Palamba et al18 compared patients with no ECE (n=83) to those with ≤1mm of ECE (n=77) and those with >1mm of ECE (n=65). As in our study, the proportion of patients with ≥4 involved nodes differed significantly among groups, being 14.5%, 37.6%, and 84.6% (p<0.001), respectively. While these results suggest that the extent of ECE might be useful in further stratifying the risk of extensive involvement of non-sentinel nodes, the lack of a standardized method of measuring ECE makes the reproducibility of these findings among different pathologists uncertain.
Strengths of our study include a large, well-defined patient population and a standard method of reporting the presence of ECE which has been in place at our institution since 2006. Limitations include lack of centralized pathology review and possible unrecognized heterogeneity in the patient population since only patients seen after 2010 were actually treated according to the Z0011 approach. Although patients included between 2006-2010 appeared to meet study eligibility criteria, it is possible that some of these patients had a more extensive disease burden than evident in the medical record.
While our study and the published literature clearly demonstrate that the finding of ECE in SLNs is associated with a higher risk of additional nodal disease in the axilla, the immediate clinical implications of this finding are uncertain. Many patients with ECE undergo ALND due to the finding of metastases in ≥3 SLNs or the identification of grossly abnormal nodes intraoperatively. In the remainder, axillary recurrence risk is unknown since the ACOSOG Z0011 study did not include microscopic ECE as a stratification factor. In addition, a recent report examining the radiation fields among a subset of patients in the Z0011 trial suggests that approximately 19% of patients in both the ALND arm and the SLN-only arm received direct nodal irradiation, the significance of which remains unknown.19 In a prospective study conducted at MSKCC of managing patients meeting Z0011 eligibility criteria without ALND20, of the initial 287 patients, 111 had ECE. ALND was performed in 29 for involvement of ≥3 sentinel nodes, and in 16 due to surgeon preference based upon the presence of ECE.21 In the remaining 66 patients, no nodal recurrences have occurred after a median follow-up of 21 months. While this is reassuring, the follow-up is clearly too short to draw firm conclusions. Conversely, it may be premature to conclude that all patients with ECE require ALND. There is a lack of consensus in the literature on the impact of ECE on regional failure rates in patients treated with ALND7,22-24, and the nodal tumor burden which can be successfully managed without surgery in the setting of multimodality therapy is unknown, but it is clear that the risk of nodal recurrence is substantially lower than the incidence of disease left behind in the nodes.
There is an increasing body of evidence indicating that as improvements in systemic therapy prolong DFS and OS a similar improvement in locoregional control is observed.25 While awaiting additional data, recognition of the significance of ECE as a predictor of a heavy nodal tumor burden is useful in making decisions about the need for completion ALND or ART in patients who otherwise meet eligibility criteria for avoidance of ALND but have multiple unfavorable tumor characteristics, such as larger T2 tumors and LVI in the breast. Information from the AMAROS trial on the impact of ECE on patient outcomes, if available, will also help to clarify this issue. Determining the appropriate management of the patient with ECE in the SLN would be greatly facilitated by the adoption of a standard pathologic technique for measuring the extent of ECE to allow comparison among studies.
Conclusion
In this large series of consecutively treated patients meeting Z0011 criteria, the presence and extent of ECE was significantly correlated with nodal tumor burden at ALND. Factors that portend a more aggressive tumor phenotype, including LVI and larger, multifocal tumors were associated with the presence of ECE. These data, in conjunction with existing literature and emerging data from recent studies, suggest that >2mm of ECE may be an indication for ALND or regional node irradiation when applying Z0011 criteria to patients with metastases in <3 SLNs.
Synopsis.
ECE in 1-2 sentinel nodes is predictive of additional nodal metastases and a greater likelihood of disease in 4 or more nodes, even in patients with T1-2, cN0 cancers. One third of patients with >2mm of ECE have ≥4 involved nodes.
Acknowledgments
This study was presented at the 67th Annual Society of Surgical Oncology Cancer Symposium, March 2014, Phoenix, AZ, and funded in part through NIH/NCI Cancer Center Support Grant No. P30CA008748 and the Cary Grossman Breast Fellowship Fund.
Footnotes
Disclosures: The authors have no conflicts of interest to declare.
References
- 1.Goyal A, Douglas-Jones A, Newcombe RG, et al. Predictors of non-sentinel lymph node metastasis in breast cancer patients. Eur J Cancer. 2004;40(11):1731–7. doi: 10.1016/j.ejca.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11(10):927–33. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98(9):599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 4.Giuliano AE, Hunt KK, Ballman K, et al. Axillary Dissection vs. No Axillary Dissection in Women with Invasive Breast Cancer and Sentinel Node Metastasis: A Randomized Clinical Trial. JAMA. 2011;305(6):569–75. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252(3):426–32. doi: 10.1097/SLA.0b013e3181f08f32. discussion 32-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutgers EJ, Donker M, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer patients: Final analysis of the EORTC AMAROS trial (10981/22023) J Clin Oncol. 2013;31 doi: 10.1200/JCO.22.01565. suppl; abstr LBA1001. [DOI] [PubMed] [Google Scholar]
- 7.Bucci JA, Kennedy CW, Burn J, et al. Implications of extranodal spread in node positive breast cancer: a review of survival and local recurrence. Breast. 2001;10(3):213–9. doi: 10.1054/brst.2000.0233. [DOI] [PubMed] [Google Scholar]
- 8.Altinyollar H, Berberoglu U, Gulben K, et al. The correlation of extranodal invasion with other prognostic parameters in lymph node positive breast cancer. J Surg Oncol. 2007;95(7):567–71. doi: 10.1002/jso.20758. [DOI] [PubMed] [Google Scholar]
- 9.van la Parra RF, Peer PG, Ernst MF, et al. Meta-analysis of predictive factors for non-sentinel lymph node metastases in breast cancer patients with a positive SLN. Eur J Surg Oncol. 2011;37(4):290–9. doi: 10.1016/j.ejso.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Fujii T, Yanagita Y, Fujisawa T, et al. Implication of extracapsular invasion of sentinel lymph nodes in breast cancer: prediction of nonsentinel lymph node metastasis. World J Surg. 2010;34(3):544–8. doi: 10.1007/s00268-009-0389-4. [DOI] [PubMed] [Google Scholar]
- 11.Gorgulu S, Can MF, Yagci G, et al. Extracapsular extension is associated with increased ratio of metastatic to examined lymph nodes in axillary node-positive breast cancer. Clin Breast Cancer. 2007;7(10):796–800. doi: 10.3816/CBC.2007.n.042. [DOI] [PubMed] [Google Scholar]
- 12.Stitzenberg K, Meyer A, Stern S, et al. Extracapsular Extension of the Sentinel Lymph Node Metastasis: A Predictor of Nonsentinel Node Tumor Burden. Ann Surg. 2003;237(5):607–13. doi: 10.1097/01.SLA.0000064361.12265.9A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meretoja TJ, Audisio RA, Heikkila PS, et al. International multicenter tool to predict the risk of four or more tumor-positive axillary lymph nodes in breast cancer patients with sentinel node macrometastases. Breast Cancer Res Treat. 2013;138(3):817–27. doi: 10.1007/s10549-013-2468-3. [DOI] [PubMed] [Google Scholar]
- 14.Rivers AK, Griffith KA, Hunt KK, et al. Clinicopathologic features associated with having four or more metastatic axillary nodes in breast cancer patients with a positive sentinel lymph node. Ann Surg Oncol. 2006;13(1):36–44. doi: 10.1245/ASO.2006.03.080. [DOI] [PubMed] [Google Scholar]
- 15.Hetelekidis S, Schnitt S, Silver B, et al. The Significance of Extracapsular Extension of Axillary Lymph Node Metastases in Early-Stage Breast Cancer. Int J Radiat Oncol Biol Phys. 2000;46(1):31–4. doi: 10.1016/s0360-3016(99)00424-1. [DOI] [PubMed] [Google Scholar]
- 16.Neri A, Marrelli D, Roviello F, et al. Prognostic value of extracapsular extension of axillary lymph node metastases in T1 to T3 breast cancer. Ann Surg Oncol. 2005;12(3):246–53. doi: 10.1245/ASO.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 17.Mittendorf EA, Hunt KK, Boughey JC, et al. Incorporation of sentinel lymph node metastasis size into a nomogram predicting nonsentinel lymph node involvement in breast cancer patients with a positive sentinel lymph node. Ann Surg. 2012;255(1):109–15. doi: 10.1097/SLA.0b013e318238f461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palamba HW, Rombouts MC, Ruers TJ, et al. Extranodal extension of axillary metastasis of invasive breast carcinoma as a possible predictor for the total number of positive lymph nodes. Eur J Surg Oncol. 2001;27(8):719–22. doi: 10.1053/ejso.2001.1173. [DOI] [PubMed] [Google Scholar]
- 19.Jagsi R, Ballman K, Chadha M, et al. Radiation field design on the ACOSOG Z0011 trial. Cancer Res; Abstracts: Thirty-Sixth Annual CTRC-AACR San Antonio Breast Cancer Symposium - Dec 10-14, 2013; San Antonio, TX. 2013. Poster No. P5-14-9. [Google Scholar]
- 20.Dengel LT, Van Zee KJ, King TA, et al. Axillary dissection can be avoided in the majority of clinically node-negative patients undergoing breast-conserving therapy. Ann Surg Oncol. 2014;21(1):22–7. doi: 10.1245/s10434-013-3200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dengel L, Cody HS, King TA, et al. The presence and extent of extracapsular extension (ECE) and the need for axillary lymph node dissection (ALND) in patients who meet ACOSOG Z11 eligibility criteria. J Clin Oncol. 2013;31 suppl; abstr 1019. [Google Scholar]
- 22.Pierce LJ, Oberman HA, Strawderman MH, et al. Microscopic extracapsular extension in the axilla: is this an indication for axillary radiotherapy? Int J Radiat Oncol Biol Phys. 1995;33(2):253–9. doi: 10.1016/0360-3016(95)00081-9. [DOI] [PubMed] [Google Scholar]
- 23.Stranzl H, Mayer R, Ofner P, et al. Extracapsular extension in positive axillary lymph nodes in female breast cancer patients. Patterns of failure and indications for postoperative locoregional irradiation. Strahlenther Onkol. 2004;180(1):31–7. doi: 10.1007/s00066-004-1170-0. [DOI] [PubMed] [Google Scholar]
- 24.Strom EA, Woodward WA, Katz A, et al. Clinical investigation: regional nodal failure patterns in breast cancer patients treated with mastectomy without radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63(5):1508–13. doi: 10.1016/j.ijrobp.2005.05.044. [DOI] [PubMed] [Google Scholar]
- 25.Bouganim N, Tsvetkova E, Clemons M, et al. Evolution of sites of recurrence after early breast cancer over the last 20 years: implications for patient care and future research. Breast Cancer Res Treat. 2013;139(2):603–6. doi: 10.1007/s10549-013-2561-7. [DOI] [PubMed] [Google Scholar]