Abstract
Everyday behaviors require a high degree of flexibility, in which prior knowledge is applied to inform behavior in new situations. Such flexibility is thought to be supported in part by memory integration, a process whereby related memories become interconnected in the brain through recruitment of overlapping neuronal populations. Recent advances in cognitive and behavioral neuroscience highlight the importance of a hippocampal–medial prefrontal circuit in memory integration. Emerging evidence suggests that abstracted representations in medial prefrontal cortex guide reactivation of related memories during new encoding events, thus promoting hippocampal integration of related experiences. Moreover, recent work indicates that integrated memories are called upon during novel situations to facilitate a host of behaviors, from spatial navigation to imagination.
Introduction
Decades’ worth of research documents the involvement of the hippocampus in rapidly encoding new episodes, which are then transferred (i.e., consolidated) to neocortex over time. However, memory is a dynamic phenomenon. The once widely accepted view that such consolidated memories are immune to modification has since been refuted. Consolidated memories may be reactivated during new experiences, at which point they become susceptible to distortion, deletion, or updating [1–3]. Conversely, reactivated memories may also influence how new content is encoded [4••,5]. Here, we review the recent work in cognitive and behavioral neuroscience that investigates the complex ways in which memories influence one another and change over time. One way such mutual influence may occur is through memory integration.
Memory integration refers to the idea that memories for related experiences are stored as overlapping representations in the brain, forming memory networks that span events and support the flexible extraction of novel information (Figure 1a). The notion that new encoding and prior knowledge interact with one another is by no means new [6, 7]; yet, the neural mechanisms and behavioral implications of memory integration have only recently become the subject of empirical investigation. The field’s growing interest in understanding these complex, real-world aspects of episodic memory has been realized thanks to the introduction of elegant behavioral paradigms and advanced analysis methods for neural data (see example in Figure 1b). We first review evidence for the neural mechanisms that sup-port memory integration. We then turn to a discussion of the range of behaviors that might be supported by integration, from flexible navigation to imagination and creativity. Finally, we set forth questions for future research.
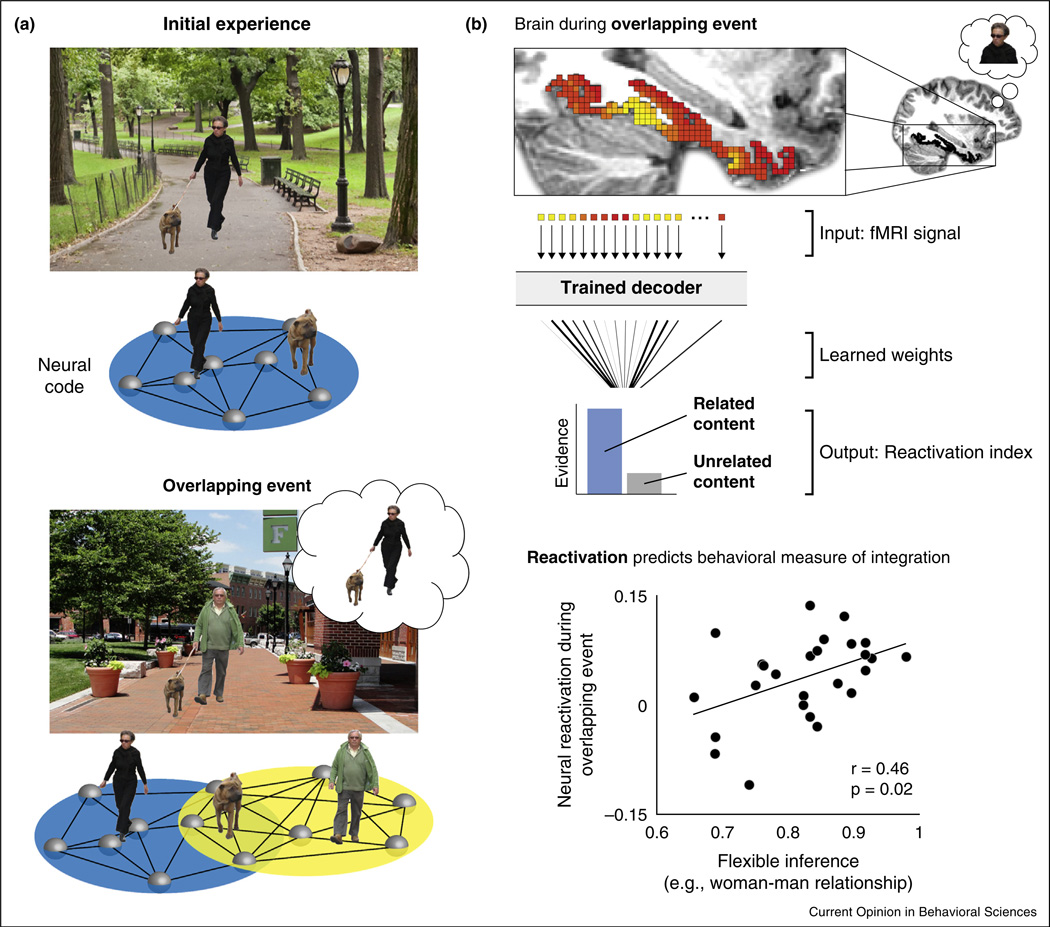
Figure 1.
Schematic depiction of memory integration. (a) Example overlapping events that might lead to integration and their associated neural codes. One day while walking in the park, you encounter a woman and her dog (initial experience, top panel). Connections are formed among a group of simultaneously activated neurons, coding the woman–dog association (blue network). A few days later, you encounter the same dog in town, this time with a man (overlapping event, bottom panel). The dog (overlapping element) triggers reactivation of your initial experience in the park (woman–dog association). Such reactivation enables connections to be formed among neural representations of the woman, dog, and man, linking the related events across time (overlapping blue and yellow networks). The resulting integrated memories are hypothesized to support novel judgments that require consideration of both events; here, for instance, you may infer a relationship between the woman and the man despite never having seen them together. (b) Top panel, depiction of a neural decoding approach quantifying the degree of memory reactivation during learning. The neural pattern evoked during the overlapping event is hypothesized to reflect reinstatement of the related — but not presently viewed — element (the woman). The fMRI signal is extracted for each voxel in a region of interest (here, ventral temporal cortex is used as an example). This information is then input into a neural decoder trained to recognize activation patterns associated with different kinds of stimuli (e.g., faces). On the basis of the weights for each voxel learned during training, the decoder outputs a value reflecting the degree to which the neural pattern reflects reactivation of the related versus unrelated content. These evidence scores can then be used as an index of reactivation. Bottom panel, evidence indicating that reactivation during encoding of overlapping events predicts later flexible inference (woman–man association), a behavioral index of memory integration. Adapted from Ref. [4••].
Neural mechanisms of memory integration
Human and animal lesion work highlights the critical roles of the hippocampus [8] and medial prefrontal cortex (mPFC [9, 10]) in memory integration (Figure 2). Damage to these structures impairs the ability to combine information acquired during different episodes despite intact memory for previously learned events. However, while these data underscore the importance of hippocampus and mPFC in memory integration, the precise mechanisms by which these regions contribute have only recently started to become clear.
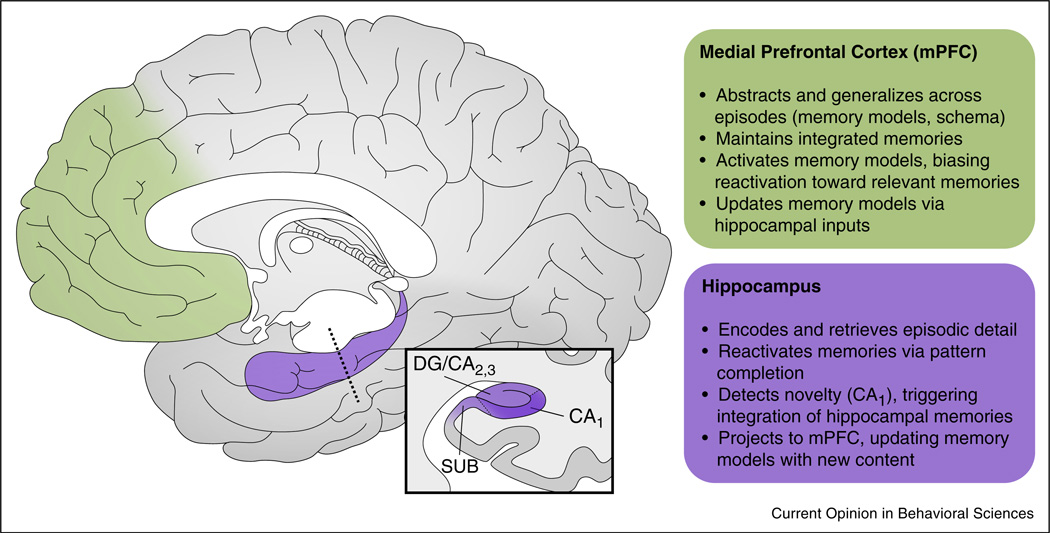
Figure 2.
Locations and hypothesized functions of regions critical for memory integration in the human brain. Green, medial prefrontal cortex; purple, hippocampus. Here, we intentionally provide a broad definition of mPFC due to high variability in the precise location of effects reported across studies. For instance, we include anterior cingulate cortex, which has been implicated in memory integration [60] and the formation of memory models [20]. Inset, cross section through the hippocampus (purple) highlighting area CA1 (dark purple portion). Approximate hippocampal subfield boundaries are indicated with thin dashed lines. Location of cross section along hippocampal axis is indicated with a thick dashed line. mPFC, medial prefrontal cortex; CA1, Cornu ammonis field 1; DG/CA2,3, dentate gyrus and Cornu ammonis fields 2 and 3; SUB, subiculum.
One period during which memory integration may take place is when new learning experiences share content (e.g., a person, place, or thing) with existing memory traces (Figure 1a). For a discussion of specific factors that impact the likelihood of integration, see Box 1. During the new experience, pattern completion mechanisms supported by the hippocampus reactivate the previously stored, overlapping memory [11, 12]. Empirical support for reactivation of prior memories during overlapping learning experiences has recently been garnered using neural decoding of fMRI data (Figure 1b) [4••, 5, 13].
Box 1 Manipulating integration.
A number of studies have investigated the various factors that influence integration. For instance, while there is evidence that integration can occur in the absence of conscious awareness [34, 38••,52, 53], studies have shown that integration may be facilitated when subjects become aware of the task structure (either via instructional manipulations or spontaneously) [54]. In fact, one experiment [54] demonstrated that such knowledge specifically benefitted judgments that spanned episodes with no effect on memory for the individual episodes themselves, suggesting that integration does not necessarily emerge with effective encoding of the underlying experiences. One possibility is that awareness constrains mental models in prefrontal regions, which in turn biases hippocampal reactivation during learning toward task-relevant memories, allowing for integration across events.
It has been hypothesized that being reminded of related memories prior to a new learning experience also increases the likelihood of integration, as the reactivated memories are labile and readily updated. Consistent with this idea, behavioral work in humans [55] found more intrusions (see Box 2) from a second learned list (List 2) when recalling the initial list (List 1) if participants had been reminded of List 1 before encoding List 2. This finding was recently replicated in rodents using ‘lists’ of ordered feeder locations [56], with animals that learned two lists in the same relative to different spatial contexts producing more intrusions. These findings are consistent with the proposal that integration occurs via reactivation of prior memories; here, this work further highlights that integration can be encouraged by reminding the learner of the original encoding context.
Other factors hypothesized to impact integration include (1) the nature of the underlying memory representations — with more distributed as opposed to localized representations proposed to promote integration [57]; and (2) the degree of competition between new content and prior memories (i.e., whether or not the two memories can coexist), with integration preferentially occurring in cases when competition is minimal [58].
With the related content reinstated in the brain, hippocampal area CA1 (Figure 2) is thought to compare prior memories with incoming information from the environment [14]. CA1 may signal the presence of novelty (i.e., when new experiences violate memory-based predictions) and facilitate new encoding by increasing the plasticity of neighboring CA3 neurons [15]. Recent high-resolution fMRI work has shown that activation in human CA1 during the encoding of events that overlap with prior experiences relates to a behavioral measure of memory integration [14], consistent with the notion that CA1 triggers integration. The resulting integrated memories are highly structured, with shared elements coded similarly across experiences [16•, 17]. One recent study [16•] has shown that hippocampal CA field firing patterns for overlapping events reflect a hierarchy of features coded according to their behavioral relevance. This organization scheme could then be exploited to extract commonalities across episodes and support a host of behaviors, as discussed below.
Medial PFC may influence memory integration by biasing reactivation toward behaviorally relevant memories [12, 18, 19]. Across a number of domains, mPFC is thought to represent mental models that guide behavior [20, 21]. While its specific role in memory is only starting to be uncovered, some suggest that mPFC forms mental models based on mnemonic content (i.e., memory models) [22•, 23], which may include features such as behavioral relevance and appropriate response [19]. These memory models may be activated when incoming information relates to existing knowledge, with mPFC selecting specific task-relevant memories for reactivation [18, 19, 21], perhaps via white matter projections to the medial temporal lobe (MTL) cortical structures that provide the major input to hippocampus [24]. Hippocampus may then bind reactivated content to current experience, resulting in an integrated trace. Following integration in hippocampus, memory models may be updated with new content as needed through direct hippocampal inputs to mPFC [18]. Through this process, mPFC may come to represent integrated memories that have been abstracted away from individual episodes (i.e., schema) over time [18, 25].
A number of studies suggest that memory integration persists into post-encoding rest [26] and sleep [27], with offline consolidation processes facilitating generalization across episodes. Specifically, hippocampus-driven reactivation during slow-wave sleep is thought to transform memories, allowing connections to be formed among representations co-activated in neocortex [28]. This process is thought to promote both the integration of new information into existing memories and abstraction across episodes in neocortical regions, particularly mPFC [28].
Behavioral implications
Memory integration has largely positive effects on behavior (though see Box 2 for examples of negative behavioral consequences). Below, we review recent work highlighting these benefits across a number of cognitive domains.
Box 2 Integration and memory distortion.
While the effects of integration on behavior are largely beneficial, a few studies have uncovered negative consequences of integration. For example, integration may lead to false memories (i.e., through overgeneralization) [59•], and memory misattributions [5, 22•, 55, 56]. Interestingly, patients with ventral mPFC lesions show reduced false memories relative to healthy control participants for words that were never seen but are thematically related to a studied word list [59•], consistent with the notion that ventral mPFC constructs generalized memory representations.
Integration may also explain the phenomenon of memory misattribution, in which an episodic experience is incorrectly attributed to a different encoding context than the one in which it occurred (e.g., as measured by intrusions; Box 1). Misattributions may occur when prior knowledge is reactivated and updated with the current experience to the detriment of memory accuracy. One fMRI study [5] used neural decoding to quantify the neural reinstatement of the context associated with prior memories (List 1) during new learning (List 2). Results showed that greater evidence for reactivation of the List 1 context was associated with more misattributions of List 2 words to List 1. Another study [22•] showed that when participants reactivated a prior experience during new encoding, ventral mPFC and hippocampal engagement was associated with later memory misattributions, consistent with a role for these regions in linking experiences across time.
Spatial navigation
Perhaps the most familiar and widely studied form of memory integration stems from Tolman’s seminal work on cognitive maps [7]. Tolman proposed that navigation relies on the coherent representation of spatial layouts, which can flexibly give rise to new inferences about the relative locations of landmarks in the environment [7]. Recent work in humans has demonstrated a relationship between hippocampal volumes and the ability to infer novel spatial relationships among a set of trained landmarks [29], consistent with the idea that the hippocampus constructs integrated spatial maps. A behavioral study further found sleep-related increases in spatial relational inference [27], indicating that early phase consolidation processes may facilitate the construction of cognitive maps.
Moreover, work in rodents demonstrates that the firing patterns of hippocampal CA1 neurons predict animals’ future routes [30]. These trajectories can represent even novel paths [30, 31], suggesting that the hippocampus — perhaps guided by mPFC [32] — may support flexible navigation by simulating and evaluating possible trajectories in the context of current goals.
Inferring relationships
Integrated memories may facilitate a host of novel judgments that require knowledge of the relationships among events, such as in associative inference, transitive inference, and acquired equivalence paradigms [11] (though see Ref. [33]). These judgments tap memory flexibility, requiring participants to make novel inferences on the basis of trained associations; for simplicity, we group these behaviors under the term ‘inference.’ Because integrated memories code for the relationships among learned associations (Figure 1a), they may be reinstated and the new information directly extracted during an inference judgment itself [34].
Recent work has directly linked learning-phase reactivation of related memories to subsequent behavior. For instance, the degree to which previously encoded content is reactivated during new events has been shown to predict both subsequent memory for the reactivated content [35] and later inference (Figure 1b [4••]), consistent with the notion that reactivation supports memory strengthening and flexibility via integration. One study [4••] also demonstrated that activation in hippocampus and ventral mPFC related to later inference performance. Moreover, that study observed functional connectivity enhancements, suggesting that memories bound in hippocampus may come to depend on mPFC as they are integrated and strengthened [4••]. Within the hippocampus, CA1 engagement during overlapping events has been shown to predict subsequent inference [14]. The degree to which learning-phase CA1 patterns are reinstated during inference has also been shown to relate to speed and accuracy, consistent with ideas regarding this region’s role in integration [14].
Recent work has also shown that inference is impaired in patients with lesions to ventral mPFC [10]. Furthermore, like spatial navigation, novel inference judgments are selectively facilitated following sleep [36, 37], emphasizing the importance of offline processes in integration.
Decision making
Integrated memories may also influence non-mnemonic decision making. For example, one recent fMRI study [38••] suggests that the hippocampus supports the transfer of monetary value across related experiences through additional recruitment of reward regions. The researchers showed greater reactivation of prior related knowledge during encoding of new reward information for stimuli that showed more evidence of subsequent preference shifts, a behavioral index of value transfer. Hippocampal–striatal functional coupling was also associated with value-related preference changes [38••], suggesting that hippocampus may interact with domain-specific regions (e.g., striatum in value learning tasks) in service of integration.
Consistent with a domain-general role for hippocampus in memory integration, rodent work [39] has found that the hippocampus was necessary for updating a known goal location with new value information. These updated memories may then be transferred to neocortex, as mPFC was necessary for retaining the updated knowledge to support performance on the next day [39]. Thus, integrated memories incorporating value information may be maintained as memory models in mPFC that will later bias behavior. We note that this role for mPFC is likely also domain-general given its documented involvement in a number of tasks lacking an explicit value component.
Schema
Recent attention has focused on the behavioral benefits conferred by memory schema. For instance, research in rodents has shown that prior knowledge of a spatial layout (i.e., a spatial schema) can both facilitate acquisition of new related memories and speed their consolidation [40, 41]. Echoing these results, a number of human studies have reported behavioral benefits in learning and memory when new information can be incorporated into an existing schema [42•, 43, 44]. Application of a schema to a new scenario has also been shown to recruit hippocampus [45, 46]. For example, one fMRI study [46] found that while engagement and connectivity of hippocampus and ventral mPFC was enhanced during generation of a task schema, the application of schema to guide behavior in a novel but similarly structured task selectively recruited hippocampus.
Rodent [41] and human [26, 42•, 43] work further suggests that mPFC may be activated along with hippocampus during learning of schema-related information. Recent empirical data indicate that one factor that may influence the relative engagement of MTL and mPFC is the degree of consistency between new information and existing schema. Specifically, one study [42•] demonstrated that mPFC engagement was more predictive of subsequent memory for information congruent with existing schema, perhaps reflecting direct encoding1of new content into prior knowledge. By contrast, MTL engagement was more predictive of successful encoding of incongruent information.
One theory [18] of schema suggests that with increasing congruency, mPFC becomes increasingly able to bias reactivation toward related memories. Increasing congruency would also be associated with decreasing novelty, which may result in decreased reliance on hippocampal integration triggered by area CA1. In such cases, mPFC memory models may guide reactivation and be updated directly, thus bypassing hippocampal involvement. By contrast, when an existing memory model is weak or nonexistent, mPFC would play no role in guiding memory retrieval. In this case, new content would be encoded by hippocampus. Across multiple related experiences (i.e., when forming a new schema), mPFC may come online [4••], reflecting the emergence of guided reactivation and the abstraction across experiences. However, in many cases, new events are likely to be neither entirely novel nor identical replications of prior experience. These events will instead share a moderate level of congruency with existing memory models, and would thus be expected to involve both mPFC and hippocampus.
Creativity and imagination
Memory integration may also underlie the ability to recombine prior memories to construct new ideas and imagine future scenarios [23]. Consistent with this notion, recent work [47] has demonstrated that hippocampal damage results in impaired performance on creativity tasks in which participants generate novel responses on the basis of existing knowledge. Medial PFC may also support performance in such tasks; one recent fMRI study [48] showed that individual differences in resting state functional connectivity of mPFC with posterior cingulate cortex predicted creativity.
Hippocampus and mPFC are also engaged during imagination [49•, 50], particularly when imagined scenarios are rich in episodic detail. One human fMRI showed enhanced connectivity between hippocampus and mPFC during imagination of future scenarios that were later remembered [50], consistent with the notion that these regions are important for creating and maintaining integrated memories — even those representing imagined events. Another study [49•] required participants to construct mental representations of novel foods from two familiar ingredients. Using an fMRI adaptation paradigm, researchers found that imagining novel foods engaged the same neuronal populations as did the ingredients in both hippocampus and mPFC, reflecting retrieval and recombination of prior memories during mental construction. The ingredient items themselves also came to recruit overlapping neuronal populations, perhaps reflecting integration of the simultaneously reactivated memories (Figure 1a). Interestingly, the degree of representational overlap of the ingredients in hippocampus and mPFC tracked across participants with subjective value of the imagined foods, suggesting that integration may be enhanced according to behavioral relevance (here, for high value items).
Conclusions
The findings reviewed here collectively suggest the importance of a hippocampal–mPFC circuit for linking related experiences. Memory integration may support a host of flexible behaviors, from navigating our environment to imagining our future. While recent years have brought a surge of attention to this area of study, we believe this is just the beginning of a rich scientific enterprise. What are the factors that influence integration (Box 1)? How do neural representations simultaneously support the maintenance of episodic detail and generalization across experiences? How do memory integration and behavioral flexibility change across the lifespan [51]? These are merely examples of the many important questions that remain the subject of future investigation.
Acknowledgements
This work was supported by the National Institute of Mental Health of the National Institutes of Health (R01 MH100121 to A.R.P.); by the National Science Foundation CAREER award (1056019 to A.R.P.); and by the Department of Defense (DoD) through the National Defense Science & Engineering Graduate Fellowship (NDSEG) Program (to M.L.S.).
Footnotes
This idea contrasts with standard views of consolidation, which propose that hippocampal memories are transferred to neocortex after long time periods; however, recent work suggests the possibility of neocortical encoding of new information independent of the hippocampus [61] (see however [62, 63]).
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
• • of outstanding interest
- 1.Nadel L, Hupbach A, Gomez R, Newman-Smith K. Memory formation, consolidation and transformation. Neurosci Biobehav Rev. 2012;36:1640–1645. doi: 10.1016/j.neubiorev.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Nadel L, Hardt O. Update on memory systems and processes. Neuropsychopharmacology. 2011;36:251–273. doi: 10.1038/npp.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKenzie S, Eichenbaum H. Consolidation and reconsolidation: two lives of memories? Neuron. 2011;71:224–233. doi: 10.1016/j.neuron.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zeithamova D, Dominick AL, Preston AR. Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron. 2012;75:168–179. doi: 10.1016/j.neuron.2012.05.010. This fMRI study employs multivoxel pattern analysis to demonstrate neural reactivation of prior related memories during new learning experiences. Moreover, the degree of reactivation during learning tracks across participants with the ability to make novel inferences. The authors also show that activation changes across learning in hippocampus and ventral mPFC relate to subsequent inference that hippocampal–ventral mPFC connectivity increases across repeated presentations of overlapping events.
- 5.Gershman SJ, Schapiro AC, Hupbach A, Norman KA. Neural context reinstatement predicts memory misattribution. J Neurosci. 2013;33:8590–8595. doi: 10.1523/JNEUROSCI.0096-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett F. Remembering: A Study in Experimental and Social Psychology. Cambridge University Press; 1932. [Google Scholar]
- 7.Tolman EC. Cognitive maps in rats and men. Psychol Rev. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- 8.Bunsey M, Eichenbaum H. Conservation of hippocampal memory function in rats and humans. Nature. 1996;379:255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- 9.DeVito LM, Lykken C, Kanter BR, Eichenbaum H. Prefrontal cortex: role in acquisition of overlapping associations and transitive inference. Learn Mem. 2010;17:161–167. doi: 10.1101/lm.1685710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koscik TR, Tranel D. The human ventromedial prefrontal cortex is critical for transitive inference. J Cogn Neurosci. 2012;24:1191–1204. doi: 10.1162/jocn_a_00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeithamova D, Schlichting ML, Preston AR. The hippocampus and inferential reasoning: building memories to navigate future decisions. Front Hum Neurosci. 2012:6. doi: 10.3389/fnhum.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 2013;23:R764–R773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhl BA, Bainbridge WA, Chun MM. Neural reactivation reveals mechanisms for updating memory. J Neurosci. 2012;32:3453–3461. doi: 10.1523/JNEUROSCI.5846-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlichting ML, Zeithamova D, Preston AR. CA1 subfield contributions to memory integration and inference. Hippocampus. 2014 doi: 10.1002/hipo.22310. http://dx.doi.org/10.1002/hipo.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larkin MC, Lykken C, Tye LD, Wickelgren JG, Frank LM. Hippocampal output area CA1 broadcasts a generalized novelty signal during an object-place recognition task. Hippocampus. 2014;24:773–783. doi: 10.1002/hipo.22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McKenzie S, Frank AJ, Kinsky NR, Porter B, Riviére PD, Eichenbaum H. Hippocampal representation of related and opposing memories develop within distinct, hierarchically organized neural schemas. Neuron. 2014 doi: 10.1016/j.neuron.2014.05.019. http://dx.doi.org/10.1016/j.neuron.2014.05.019. Applying a neural pattern similarity approach developed for fMRI to the analysis of simultaneous recordings from hippocampal neurons, McKenzie and colleagues provide a compelling demonstration of the hierarchical organization of rodent schema. They show that memories are organized according to behavioral relevance, such that events that occur within the same behavioral context are integrated (i.e., form a schema), while events in different behavioral contexts are represented in a distinct hierarchy (i.e., a different schema).
- 17.McKenzie S, Robinson NTM, Herrera L, Churchill JC, Eichenbaum H. Learning causes reorganization of neuronal firing patterns to represent related experiences within a hippocampal schema. J Neurosci. 2013;33:10243–10256. doi: 10.1523/JNEUROSCI.0879-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Kesteren MTR, Ruiter DJ, Fernández G, Henson RN. How schema and novelty augment memory formation. Trends Neurosci. 2012;35:211–219. doi: 10.1016/j.tins.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Kroes MCW, Fernández G. Dynamic neural systems enable adaptive, flexible memories. Neurosci Biobehav Rev. 2012;36:1646–1666. doi: 10.1016/j.neubiorev.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Roy M, Shohamy D, Wager TD. Ventromedial prefrontal–subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16:147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson RC, Takahashi YK, Schoenbaum G, Niv Y. Orbitofrontal cortex as a cognitive map of task space. Neuron. 2014;81:267–279. doi: 10.1016/j.neuron.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. St. Jacques PL, Olm C, Schacter DL. Neural mechanisms of reactivation-induced updating that enhance and distort memory. Proc Natl Acad Sci U S A. 2013;110:19671–19678. doi: 10.1073/pnas.1319630110. This study uses fMRI coupled with a task incorporating photographs from participants’ real episodic experiences touring a museum. The researchers find evidence that the quality of memory reactivation prior to presentation of new information is related to both memory for the reactivated content and integration of the new information into prior memories. In this study, integration leads to memory misattributions and is linked to engagement of ventral mPFC and hippocampus.
- 23.Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. The future of memory: remembering, imagining, and the brain. Neuron. 2012;76:677–694. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavada C, Compañy T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suárez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- 25.Richards BA, Xia F, Santoro A, Husse J, Woodin MA, Josselyn SA, Frankland PW. Patterns across multiple memories are identified over time. Nat Neurosci. 2014;17:981–986. doi: 10.1038/nn.3736. [DOI] [PubMed] [Google Scholar]
- 26.Van Kesteren MTR, Fernández G, Norris DG, Hermans EJ. Persistent schema-dependent hippocampal–neocortical connectivity during memory encoding and postencoding rest in humans. Proc Natl Acad Sci U S A. 2010;107:7550–7555. doi: 10.1073/pnas.0914892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coutanche MN, Gianessi CA, Chanales AJH, Willison KW, Thompson-Schill SL. The role of sleep in forming a memory representation of a two-dimensional space. Hippocampus. 2013;23:1189–1197. doi: 10.1002/hipo.22157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis PA, Durrant SJ. Overlapping memory replay during sleep builds cognitive schemata. Trends Cogn Sci. 2011;15:343–351. doi: 10.1016/j.tics.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Schinazi VR, Nardi D, Newcombe NS, Shipley TF, Epstein RA. Hippocampal size predicts rapid learning of a cognitive map in humans. Hippocampus. 2013;23:515–528. doi: 10.1002/hipo.22111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfeiffer BE, Foster DJ. Hippocampal place-cell sequences depict future paths to remembered goals. Nature. 2013;497:74–81. doi: 10.1038/nature12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta AS, van der Meer MAA, Touretzky DS, Redish AD. Hippocampal replay is not a simple function of experience. Neuron. 2010;65:695–705. doi: 10.1016/j.neuron.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Bruin JPC, Sànchez-Santed F, Heinsbroek RPW, Donker A, Postmes P. A behavioural analysis of rats with damage to the medial prefrontal cortex using the morris water maze: evidence for behavioural flexibility, but not for impaired spatial navigation. Brain Res. 1994;652:323–333. doi: 10.1016/0006-8993(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 33.Kumaran D. What representations and computations underpin the contribution of the hippocampus to generalization and inference? Front Hum Neurosci. 2012:6. doi: 10.3389/fnhum.2012.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shohamy D, Wagner AD. Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60:378–389. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuhl BA, Shah AT, DuBrow S, Wagner AD. Resistance to forgetting associated with hippocampus-mediated reactivation during new learning. Nat Neurosci. 2010;13:501–506. doi: 10.1038/nn.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Werchan DM, Gómez RL. Generalizing memories over time: sleep and reinforcement facilitate transitive inference. Neurobiol Learn Mem. 2013;100:70–76. doi: 10.1016/j.nlm.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Ellenbogen JM, Hu PT, Payne JD, Titone D, Walker MP. Human relational memory requires time and sleep. Proc Natl Acad Sci U S A. 2007;104:7723–7728. doi: 10.1073/pnas.0700094104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wimmer GE, Shohamy D. Preference by association: how memory mechanisms in the hippocampus bias decisions. Science. 2012;338:270–273. doi: 10.1126/science.1223252. In this study, participants incidentally learn pairs of visual stimuli. One of the paired items is then associated with a reward in a different task. Behaviorally, the researchers find that the positive value of reward spreads to the related but non-rewarded items, as participants are biased to chose the non-rewarded items over novel items in a subsequent preference task. Moreover, using fMRI, the researchers show that the degree of decision bias is predicted by both hippocampal engagment during the reward task as well as connectivity between hippocampus and reward regions.
- 39.Blanquat PDS, Hok V, Save E, Poucet B, Chaillan FA. Differential role of the dorsal hippocampus, ventro-intermediate hippocampus, and medial prefrontal cortex in updating the value of a spatial goal. Hippocampus. 2013;23:342–351. doi: 10.1002/hipo.22094. [DOI] [PubMed] [Google Scholar]
- 40.Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RGM. Schemas and memory consolidation. Science. 2007;316:76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- 41.Tse D, Takeuchi T, Kakeyama M, Kajii Y, Okuno H, Tohyama C, Bito H, Morris RGM. Schema-dependent gene activation and memory encoding in neocortex. Science. 2011;333:891–895. doi: 10.1126/science.1205274. [DOI] [PubMed] [Google Scholar]
- 42. Van Kesteren MTR, Beul SF, Takashima A, Henson RN, Ruiter DJ, Fernández G. Differential roles for medial prefrontal and medial temporal cortices in schema-dependent encoding: from congruent to incongruent. Neuropsychologia. 2013;51:2352–2359. doi: 10.1016/j.neuropsychologia.2013.05.027. Using fMRI, this study examines how the congruency between new events and preexisting knowledge impacts the relative engagment of MTL and mPFC during new encoding. Participants learn object-scene pairs of varying levels of congruency, exhibiting better memory for congruent than for incongruent pairs. Neurally, the researchers find that encoding-related activity increases in mPFC with increasing congruency, whereas MTL activation decreases with increasing congruency. The results suggest that mPFC may play a greater role in episodic encoding when new events can be incorporated into preexisting knowledge. When new events are incongruent with prior experiences, new encoding may be more reliant on MTL regions.
- 43.Van Kesteren MTR, Rijpkema M, Ruiter DJ, Morris RGM, Fernández G. Building on prior knowledge: schema-dependent encoding processes relate to academic performance. J Cogn Neurosci. 2014 doi: 10.1162/jocn_a_00630. http://dx.doi.org/10.1162/jocn. [DOI] [PubMed] [Google Scholar]
- 44.Kumaran D. Schema-driven facilitation of new hierarchy learning in the transitive inference paradigm. Learn Mem. 2013;20:388–394. doi: 10.1101/lm.030296.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Hoz L, Martin SJ. Double dissociation between the contributions of the septal and temporal hippocampus to spatial learning: the role of prior experience. Hippocampus. 2014 doi: 10.1002/hipo.22285. http://dx.doi.org/10.1002/hipo.22285. [DOI] [PubMed] [Google Scholar]
- 46.Kumaran D, Summerfield JJ, Hassabis D, Maguire EA. Tracking the emergence of conceptual knowledge during human decision making. Neuron. 2009;63:889–901. doi: 10.1016/j.neuron.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duff MC, Kurczek J, Rubin R, Cohen NJ, Tranel D. Hippocampal amnesia disrupts creative thinking. Hippocampus. 2013;23:1143–1149. doi: 10.1002/hipo.22208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, Kawashima R. The association between resting functional connectivity and creativity. Cereb Cortex. 2012;22:2921–2929. doi: 10.1093/cercor/bhr371. [DOI] [PubMed] [Google Scholar]
- 49. Barron HC, Dolan RJ, Behrens TEJ. Online evaluation of novel choices by simultaneous representation of multiple memories. Nat Neurosci. 2013;16:1492–1498. doi: 10.1038/nn.3515. In this study, participants are asked to construct novel experiences from multiple independent memories. The researchers find that generated experience activates the same pattern of response in both hippocampus and mPFC as the underlying memories from which it was created. The underlying memories also come to recruit overlapping sets of hippocampal and mPFC voxels, consistent with memory integration. The degree of neural overlap among the underlying memories further tracks participants’ ratings of subjective value for constructed events, suggesting that integrated memories also contain information about behavioral relevance.
- 50.Martin VC, Schacter DL, Corballis MC, Addis DR. A role for the hippocampus in encoding simulations of future events. Proc Natl Acad Sci U S A. 2011;108:13858–13863. doi: 10.1073/pnas.1105816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brod G, Werkle-Bergner M, Shing YL. The influence of prior knowledge on memory: a developmental cognitive neuroscience perspective. Front Behav Neurosci. 2013:7. doi: 10.3389/fnbeh.2013.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henke K, Reber TP, Duss SB. Integrating events across levels of consciousness. Front Behav Neurosci. 2013:7. doi: 10.3389/fnbeh.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Munnelly A, Dymond S. Relational memory generalization and integration in a transitive inference task with and without instructed awareness. Neurobiol Learn Mem. 2014;109:169–177. doi: 10.1016/j.nlm.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 54.Kumaran D, Melo HL. Transitivity performance, relational hierarchy knowledge and awareness: results of an instructional framing manipulation. Hippocampus. 2013;23:1259–1268. doi: 10.1002/hipo.22163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hupbach A, Gomez R, Hardt O, Nadel L. Reconsolidation of episodic memories: a subtle reminder triggers integration of new information. Learn Mem. 2007;14:47–53. doi: 10.1101/lm.365707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones B, Bukoski E, Nadel L, Fellous J-M. Remaking memories: reconsolidation updates positively motivated spatial memory in rats. Learn Mem. 2012;19:91–98. doi: 10.1101/lm.023408.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schiller D, Phelps EA. Does reconsolidation occur in humans? Front Behav Neurosci. 2011:5. doi: 10.3389/fnbeh.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hupbach A. The specific outcomes of reactivation-induced memory changes depend on the degree of competition between old and new information. Front Behav Neurosci. 2011:5. doi: 10.3389/fnbeh.2011.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Warren DE, Jones SH, Duff MC, Tranel D. False recall is reduced by damage to the ventromedial prefrontal cortex: implications for understanding the neural correlates of schematic memory. J Neurosci. 2014;34:7677–7682. doi: 10.1523/JNEUROSCI.0119-14.2014. Warren and colleagues examine the relationship between memory integration and false memories in a population of individuals with ventral mPFC damage. Patients and healthy controls perform the Deese–Roediger–McDermott (DRM) paradigm, in which they study lists of words that share a common theme (e.g., cold, blizzard, winter). False memories in this task are measured by the degree to which participants endorse related, non-studied lures (e.g., snow) as having been on the studied list. While healthy participants show high levels of false recall of non-studied lures, patients with ventral mPFC damage show reduced false memory. This finding suggests that ventral mPFC cortex plays a critical role in the deployment and use of preexisting memory schema when encoding new information.
- 60.Wang S-H, Tse D, Morris RGM. Anterior cingulate cortex in schema assimilation and expression. Learn Mem. 2012;19:315–318. doi: 10.1101/lm.026336.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharon T, Moscovitch M, Gilboa A. Rapid neocortical acquisition of long-term arbitrary associations independent of the hippocampus. Proc Natl Acad Sci U S A. 2011;108:1146–1151. doi: 10.1073/pnas.1005238108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Warren DE, Duff MC. Not so fast: hippocampal amnesia slows word learning despite successful fast mapping. Hippocampus. 2014 doi: 10.1002/hipo.22279. http://dx.doi.org/10.1002/hipo.22279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith C, Urgolites Z, Hopkins RO, Squire LR. Comparison of explicit and incidental learning strategies in memory-impaired patients. Proc Natl Acad Sci U S A. 2014;111:475–479. doi: 10.1073/pnas.1322263111. [DOI] [PMC free article] [PubMed] [Google Scholar]