Abstract
Importance
Muscle pain, fatigue, and weakness are common adverse effects of statin medications and may decrease physical activity in older men.
Objective
Determine whether statin use is associated with physical activity, longitudinally and cross-sectionally.
Design, Setting, and Participants
Men participating in the Osteoporotic Fractures in Men Study, a multicenter prospective cohort study of community-living men age 65+, enrolled between March 2000-April 2002.
Exposure
Statin use as determined by an inventory of medications (taken within last 30 days). In cross-sectional analyses, statin use categories were: users and nonusers. In longitudinal analyses, categories were: prevalent users (baseline use and throughout study), new users (initiated use during the study) and nonusers (never used).
Main Outcomes and Measure
Self-reported physical activity at baseline and 2 follow-up visits using the Physical Activity Scale for the Elderly (PASE). At the third visit, an accelerometer measured metabolic equivalents (METs; kcal/kg/hr) and minutes of moderate activity (METs ≥3.0), vigorous activity (METs ≥6.0), and sedentary behavior (METs ≤1.5).
Results
At baseline, 989 men (24%) were users and 3,148 (76%) were nonusers. The adjusted difference in baseline PASE between users and nonusers was −5.8 points (95% CI, −10.9 to −0.7). A total of 3,039 men met the inclusion criteria for longitudinal analysis: 727 (24%) prevalent users, 845 (28%) new users, 1,467 (48%) nonusers. PASE declined by an average of 2.5 points/year (2.0–3.0) for nonusers and 2.8 points/year (2.1, 3.5) for prevalent users, a nonstatistical difference (0.3 point, −0.5–1). For new users, annual PASE score declined at a faster rate than nonusers (0.9 point difference; 0.1–1.7). 3,071 men had adequate accelerometry data, 1,542 (50%) were statin users. Statin users expended less METS (0.03 kcal/kg/hr less; 0.02–0.04); engaged in less moderate physical activity (5.4 fewer minutes/day; 1.9–8.8), less vigorous activity (0.6 fewer minutes/day; 0.1–1.1), and more sedentary behavior (7.6 greater minutes/day; 2.6–12.4).
Conclusion and Relevance
Statin use was associated with modestly lower physical activity among community-living men, even after accounting for medical history and other potentially confounding factors. The clinical significance of these findings deserves further investigation.
Introduction
Physical activity is vital for older adults to maintain health, physical function, and independence.1–3 One objective of Healthy People 2020 is to increase the amount of leisure-time physical activities among older adults.4 Understanding factors that influence physical activity in older men is both clinically important and of major public health interest.
Muscle symptoms are the most common adverse effects experienced by patients taking statins. Symptoms include diffuse muscle pain, muscle fatigue, and weakness.5–7 If present, these symptoms are most often observed after initiation of statin therapy, and may cause a decline in physical activity.8 Several short-term studies have suggested that prevalent use or initiation of a statin use is linked to less physical activity in older adults followed for up to a year.8,9 Other studies have demonstrated that initiation of moderate physical activity increased muscle pain and symptoms in statin users.9–11 Finally, a recent 12-week aerobic exercise study showed that cardiorespiratory fitness and respiratory markers in the muscles were improved for statin nonusers, but did not improve in patients randomized to 40 mg of simvastatin.12 These studies underscore the possibility that statins may decrease physical activity in older adults, but long-term studies are needed to evaluate if these effects are sustained.
Using a large observational study in older men, the Osteoporotic Fractures in Men Study (MrOS), we evaluated the cross-sectional and longitudinal relationship between self-reported physical activity and statin use up to 6.9 years after baseline. This longer follow-up period allowed us to estimate changes in physical activity in prevalent statin users and initiators of statin medications compared to nonusers. We also evaluated the cross-sectional association between use of statin medication and physical activity measured objectively by an accelerometer.
Methods
Participants
The MrOS study recruited 5,994 community-living men aged 65 years and older from six geographical areas around the United States (Birmingham, AL, Minneapolis, MN, Palo Alto, CA, Pittsburgh, PA, Portland, OR, and San Diego, CA). A baseline examination was completed from March 2000 to April 2002.13 The MrOS is a study of healthy aging with a focus on osteoporosis and fractures. Men were eligible if they were able to walk without assistance of another person, did not have bilateral hip replacements, had no medical condition expected to cause imminent death, and were able to provide consent. Follow-up clinic visits occurred an average of 4.6 (±0.4) and 6.9 (±0.4) years after baseline, visits 2 and 3 respectively. The MrOS design, rationale, and recruitment have been published elsewhere.13 The institutional review board at each center approved the study protocol, and written informed consent was obtained from all men.
Medications
At each clinic visit, men were asked to bring all the medications they had taken in the past 30 days. Only prescription medications were included at baseline. Follow-up visits additionally included over-the-counter medications. All medications recorded by study staff were stored in an electronic medications inventory database (San Francisco Coordinating Center, San Francisco, CA). Each medication was matched to its ingredient(s) based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA).13
Demographic and health measurements
At all visits, men completed a self-administered questionnaire to ascertain their age, self-identified race, education, marital status, smoking status, self-perceived health, dizziness, and selected self-reported physician-diagnosed conditions, including previous myocardial infarction (MI), stroke, angina, heart failure, hypertension, diabetes, lung disease, rheumatoid arthritis, and Parkinson’s disease. Variables were categorized for this study based on clinically important cut-points as follows: self-identified race as non-white and white; education as some high school or less and high school or more; marital status as married and not married; smoking status as never smoker, past smoker, and current smoker; self-perceived health as very poor, poor, fair, good, and excellent. All self-reported physician-diagnosed conditions were categorized as present and absent. Measured height and weight were used to calculate body mass index. Serum samples were collected at the baseline visit after an overnight fast and total cholesterol was calculated from chemistry assays performed using a standard clinical automated analyzer.
PASE Analyses
At each visit men were asked to complete the Physical Activity Scale for the Elderly (PASE) questionnaire. PASE is a validated, self-administered questionnaire that inquires about the performance of occupational, household, and leisure items over a one-week period.14 Total PASE score is the weighted sum of participant responses regarding 12 various activities. For this analysis, PASE was adjusted by clinical site and season to reduce their possible effects on physical activity. An average seasonal PASE was calculated according to site. The difference between these mean values and the overall mean PASE at each visit was added to participants’ PASE score.
Analytic sample
The primary analytic method was a complete case analysis and the sample consisted of those men with no missing exposure, outcome, or covariates data. Men were excluded for lacking statin use or PASE at baseline (n=239 and n=3, respectively), lacking follow-up statin or PASE information (n=966), or discontinuing statin use during follow-up (n=319 total; 11% of baseline statin users stopped before visit 2 and 9% of visit 2 users stopped before visit 3). An additional 330 men were excluded from the cross-sectional and 1,428 from the longitudinal analysis because of missing covariates.
Statin use
Men were classified as either statin users or nonusers according to their use at baseline for our cross-sectional analysis. For the longitudinal analysis, men were categorized as follows: Prevalent users used a statin at all visits; Nonusers never reported using a statin; New users first reported using a statin either at visit 2 or at visit 3.
Statistical analysis
In order to characterize our baseline analytic sample, t-tests and chi-square tests were used to compare characteristics between baseline statin users and nonusers.
Multivariable linear regression modeling was used to assess the baseline cross-sectional association between statin use and PASE. First, we created a model controlling for age and site only. To account for potential confounding effects, we then constructed a fully adjusted model in which known or suspected risk factors for reduced physical activity were added: fixed-in-time: age, site, and baseline total cholesterol; time-varying: MI, stroke, hypertension, diabetes, perceived health, and body mass index.
Mixed effects linear regression modeling was used to determine the association between statin use and longitudinal changes in PASE. Of interest in our longitudinal models was a significant statin × time interaction, which estimated the difference in changing PASE scores between the two statin user groups and nonusers. Time was defined as calendar years since baseline, and was used as a continuous variable. As described above, we created an age and site- and a fully adjusted model. Using mixed effects regression allowed us to use both fixed-in-time and time-varying variables in the fully adjusted model. Time-varying variables were considered to account for changes in health status during the follow-up period.
Secondary analysis
To address potential bias in this analysis as a result of missing values, we used data imputation on statin use, PASE, and covariates in fully adjusted models. Data was imputed using the multiple imputation by chained equation (MICE) approach and 20 imputation cycles were performed to generate the data set. Imputation increased the sample size of men to 4,467.
Statistical tests were conducted at the 0.05, two-tailed level of significance. Analyses were performed using STATA version 13.0 (StataCorp, College Station, Texas).
Accelerometer Analyses
As part of visit 3, men were asked to wear an accelerometer, which collected physiological data every minute over a seven-day period (SenseWear® Pro3 Armband, by Body Media, Inc., Pittsburgh, PA). From these data, as well as height, weight, age, handedness, and smoking status, activity level (sedentary, moderate, and vigorous) and time spent at each level were estimated.15 Outcome variables used in this analysis were daily: 1) Metabolic equivalents (METs; kcal/kg/hr); 2) minutes of moderate physical activity (METs ≥3.0); 3) minutes of vigorous physical activity (METs ≥6.0); and, 4) minutes of sedentary behavior (METs ≤1.5).
Analytic sample
Only men with accelerometer data for at least 90% of the time for at least one 24-hour period were included.
Statin use
Men were classified as statin users or nonusers based on their medication use information collected at visit 3.
Statistical analysis
Multivariable linear regression was used to determine the cross-sectional association between accelerometer outcome measures and statin use. Minutes of moderate physical activity, minutes of vigorous physical activity, and minutes of sedentary behavior were log-transformed to normalize their distributions. For each outcome, a minimally-adjusted model using season, age, and site was created. Fully adjusted models were then created to account for confounding as described above. In models using log-transformed minutes, we reported the ratio of the medians for users versus nonusers and this ratio was interpreted in the text as a percent difference and absolute difference based on the median value.
Results
Statin use and PASE score
At baseline 4,137 men met the criteria of our cross-sectional analytic sample. Nearly a quarter of these men were statin users (N=989) and 76% were nonusers (n=3,148). The average age of users (±SD) was 72.9 (±5.3) and was 72.9 (±5.5) for nonusers. Of these men, statin users were more likely to report a previous MI, a previous stroke, hypertension, diabetes, lower total cholesterol and a lower self-perceived health (Table 1). The fully adjusted estimated difference in baseline PASE between users and nonusers was −5.8 points (95% CI, −10.9 to −0.7) (Table 2).
Table 1.
Baseline characteristics of statin users and nonusers in the cross-sectional PASE analytic sample (N=4,137)a
| Statin Users (N=989) |
Statin Nonusers (N=3,148) |
p-value b | |
|---|---|---|---|
| Age, mean (SD), years d | 72.9 (5.3) | 72.9 (5.5) | .98 |
| Non-white race/ethnicity | 70 (7.1) | 257 (8.2) | .27 |
| Education | |||
| Some High School or less | 53 (5.4) | 188 (6.0) | .47 |
| Married | 854 (86.4) | 2623 (83.3) | .02 |
| Clinical site d | |||
| Birmingham, AL | 171 (17.3) | 546 (17.3) | |
| Minneapolis, MN | 160 (16.2) | 525 (16.7) | |
| Pittsburg, PA | 173 (17.5) | 443 (14.1) | .007 |
| Palo Alto, CA | 175 (17.7) | 574 (18.2) | |
| Portland, OR | 121 (12.2) | 516 (16.4) | |
| San Diego, CA | 189 (19.1) | 544 (17.3) | |
| Smoking Status c | |||
| Never smoker | 363 (36.7) | 1249 (39.7) | |
| Past smoker | 608 (61.5) | 1791 (56.9) | .003 |
| Current smoker | 17 (1.7) | 108 (3.4) | |
| Body Mass Index, mean(SD), kg/m2 d | 27.8 (3.8) | 27.4 (3.9) | .003 |
| Total cholesterol, mean (SD), mg/dL d | 177.8 (29.1) | 199.7 (33.9) | <.001 |
| Self-reported medical history | |||
| Myocardial Infarction d | 294 (29.7) | 195 (6.2) | <.001 |
| Stroke d | 76 (7.7) | 110 (3.5) | <.001 |
| Angina | 297 (30.0) | 236 (7.5) | <.001 |
| Heart Failure | 65 (6.6) | 98 (3.1) | <.001 |
| Hypertension d | 526 (53.2) | 1,172 (37.2) | <.001 |
| Diabetes d | 136 (13.8) | 254 (8.1) | <.001 |
| Lung Disease | 100 (10.1) | 297 (9.4) | .53 |
| Rheumatoid Arthritis | 42 (4.3) | 159 (5.1) | .30 |
| Parkinson’s Disease | 5 (0.5) | 19 (0.6) | .72 |
| Dizziness | 269 (27.2) | 728 (23.1) | .009 |
| ACE Inhibitors & ARB | 335 (33.9) | 591 (18.8) | <.001 |
| Beta-blockers | 333 (33.7) | 380 (12.1) | <.001 |
| Calcium channel blockers | |||
| Dihydropyridine | 110 (11.1) | 192 (6.1) | <.001 |
| Non-dihydropyridine | 84 (8.5) | 141 (4.5) | |
| Fibrates c | 18 (1.8) | 48 (1.6) | .59 |
| Niacin c | 18 (1.8) | 51 (1.7) | .75 |
| Total number medications c | |||
| 0–3 | 300 (30.3) | 1,863 (60.9) | |
| 4–7 | 453 (45.8) | 860 (28.1) | <.001 |
| 8–11 | 182 (18.4) | 248 (8.1) | |
| 12+ | 54 (5.5) | 86 (2.8) | |
| Self-rated health d | |||
| Very Poor | 3 (0.3) | 3 (0.1) | |
| Poor | 18 (1.8) | 27 (0.9) | |
| Fair | 143 (14.5) | 282 (9.0) | <.001 |
| Good | 568 (57.4) | 1609 (51.1) | |
| Excellent | 257 (26.0) | 1227 (39.0) |
Abbreviations: ACE, angiotensin-converting-enzyme; ARB, angiotensin II receptor blockers; PASE, Physical Activity Scale for the Elderly; SD, standard deviation
Data are presented as number (%) unless otherwise indicated
p-values are from chi-square tests comparing statin user and nonusers for categorical variables and from t-tests for continuous variables
Denominator less than 4137 for these measures due to missing values: smoking status, n=4136; fibrates, n=4046; niacin, n=4046; total number of medications, n=4046
Variables in the fully adjusted PASE models
Table 2.
Cross-sectional associations between statins and PASE at baseline (n= 4,137).
| Age & Site Adjusted | Fully Adjusted1 | |||||
|---|---|---|---|---|---|---|
| Mean PASE (95% CI) | Estimated diff. (95% CI) |
p- value2 |
Mean PASE (95% CI) | Estimated diff. (95% CI) |
p- value2 |
|
| Nonuser | 153.3 (151.0, 155.7) | ref | 152.0 (149.7, 154.4) | ref | ||
| User | 142.1 (138.0, 146.3) | −11.2 (−15.9, −6.4) | <.001 | 146.3 (141.9, 150.6) | −5.8 (−10.9, −0.6) | .03 |
Abbreviations: CI, confidence intervals; Diff, difference; PASE, Physical Activity Scale for the Elderly; ref, reference group
Model controlled for the following variables: age, site, myocardial infarction, stroke, hypertension, diabetes, perceived health, body mass index, and total cholesterol
p-value for the estimate difference in PASE at baseline
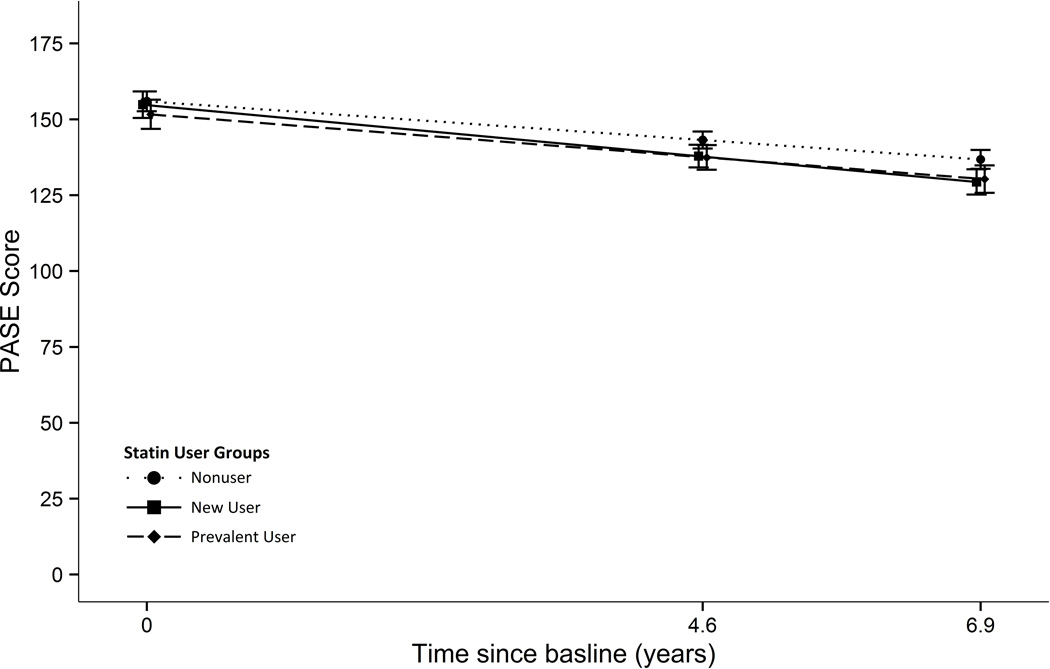
In our longitudinal analysis of statin use and PASE, 3,039 men were included in the analytic sample. Twenty-four percent (n=727) were prevalent statin users and 48% (n=1467) never used a statin over the approximate 7 years of follow-up. Slightly more than a quarter of men (n=845) first reported statin use during follow-up. On average, a decrease in physical activity was observed in all groups during follow-up (Table 3; Figure 1). According to the fully adjusted model, PASE score for prevalent users declined by roughly the same number of points annually as nonusers. The difference in the annual decline in the two groups (estimated by an interaction term in our fully adjusted model) was 0.3 points (95% CI, −0.5 to 1.1). In new users PASE score declined at a faster rate than nonusers; the difference between groups was 0.9 points/year (95% CI, 0.1 to 1.7). While PASE declined at a statistically significant greater rate than nonusers, the overall difference in PASE decline among the three statin use groups was not significant.
Table 3.
Longitudinal associations between statins and decline in PASE (n= 3,039).
| Age & Site Adjusted | Fully Adjusted1 | |||||
|---|---|---|---|---|---|---|
| Annual decline in PASE (95% CI) |
Estimated diff. in decline (95% CI)2 |
p- value3 |
Annual decline in PASE (95% CI) |
Estimated diff. in decline (95% CI)2 |
p-value3 | |
| Nonuser | 2.8 (2.3, 3.2) | ref | 2.5 (2.0, 3.0) | ref | ||
| Prevalent User |
2.9 (2.2, 3.6) | 0.1 (−0.7, 0.9) | 0.02 | 2.8 (2.1, 3.5) | 0.3 (−0.5, 1.1) | 0.07 |
| New User | 3.9 (3.3, 4.5) | 1.1 (0.3, 1.9) | 3.4 (2.8, 4.0) | 0.9 (0.1, 1.7) | ||
Abbreviations: CI, confidence intervals; Diff, difference; PASE, Physical Activity Scale for the Elderly; ref, reference group
Model controlled for the following variables: age, site, and baseline total cholesterol (fixed-in-time); myocardial infarction, stroke, hypertension, diabetes, perceived health, and body mass index (time-varying)
Estimate of the statin use × time interaction with nonusers as a reference group
Type III p-value for the statin use × time interaction
Figure 1.
Mean Physical Activity Scale in the Elderly (PASE) scores according to stain user groups as estimated by mixed effects linear regression adjusted for age, site, and baseline total cholesterol (fixed-in-time), myocardial infarction, stroke, hypertension, diabetes, perceived health and body mass index (time-varying). The error bars represent 95% confidence intervals for the estimated mean PASE at each visit (n=3,039).
Of the men who used a statin at baseline, 11% discontinued use prior to visit 2 and, of the men who used at visit 2, 9% discontinued use before visit 3. These men had fewer self-reported MIs than men who, during the same timeframe, did not stop. These men also had a nominally greater decline in PASE when compared to their proper counterparts, although no formal statistical tests were performed. All other characteristics were similar.
Our primary analytic approach used complete case analysis for both the cross-sectional and longitudinal analyses of statin use and PASE. To assess the impact of using complete cases, we performed multiple imputation of missing data and reran our models. Multiple imputation of missing data leads to similar estimates of association and did not change our conclusions. In particular, based on the fully adjusted models, cross-sectional difference in baseline PASE was −6.3 points (95% CI, −11.2 to −1.4); and the fully adjusted longitudinal difference in annual decline between persistent and nonusers was 0.2 points (95% CI, to −0.6 to 0.9) and between new and nonusers was 0.8 points (95% CI, 0.1 to 1.5).
Statin use and accelerometer measures
While 4,682 men returned for visit 3, only 3,071 men wore the accelerometer for at least 90% of the time, of which 1,542 (50%) were statin users. As estimated by the fully adjusted models, daily METS was lower in statin users by 0.03 kcal/kg/hr (95% CI, 0.02 to 0.04) (Table 4). Statin users engaged in 9.6% fewer minutes/day of moderate physical activity (95% CI, 3.1% to 16.4%) compared to nonusers; this translates into a difference of 5.4 fewer minutes/day (95% CI, 1.9 to 8.8), given the median minutes/day of moderate physical activity was 62.0 in nonusers. Similarly, statin users engaged in 9.0% fewer minutes of vigorous activity (95% CI, 1.7% to 16.8%) than nonusers or 0.6 minutes/day less (95% CI, 0.1 to 1.1), given the median minutes/day of vigorous activity was 7.4 minutes/day in nonuser. Statin users were involved in 0.6% more minutes/day of sedentary behavior (95% CI, 0.2% to 1.0%) than nonusers. This was 7.6 more minutes/day of sedentary behavior (95% CI, 2.6 to 12.4), since the median sedentary behavior was 1299.4 minutes/day (21.7 hours/day) in nonusers.
Table 4.
Cross-sectional association between statin use and accelerometer outcomes (n=3,071).
| Age & Site Adjusted | Fully Adjusted 1 | |||
|---|---|---|---|---|
| Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | |
| Mean METS, kcal/kg/hr | ||||
| Nonuser | 1.25 (1.24–1.26) | 1.23 (1.22–1.24) | ||
| User | 1.19 (1.18–1.20) | 1.20 (1.19–1.21) | ||
| Difference (User-Nonuser) | −0.06 (−0.07- −0.04) | <.001 | −0.03 (−0.04- −0.02) | <.001 |
| Median Moderate physical activity, minutes/day 2 | ||||
| Nonuser | 64.9 (62.1–67.7) | 62.0 (59.5–64.7) | ||
| User | 53.9 (51.7, 56.3) | 56.6 (54.3–59.1) | ||
| User/Nonuser 3 | 0.83 (0.78–0.88) | <.001 | 0.91 (0.86–0.97) | .003 |
| Median Vigorous physical activity, minutes/day | ||||
| Nonuser | 7.7 (7.3–8.1) | 7.4 (7.0–7.8) | ||
| User | 6.5 (6.2–6.8) | 6.8 (6.5–7.1) | ||
| User/Nonuser | 0.84 (0.79–0.90) | <.001 | 0.92 (0.86–0.98) | .01 |
| Median Sedentary behavior, minutes/day | ||||
| Nonuser | 1297.0 (1293.6–1300.5) | 1299.4 (1296.0–1302.8) | ||
| User | 1309.5 (1306.1–1312.9) | 1306.9 (1303.5–1310.3) | ||
| User/Nonuser | 1.010 (1.006–1.013) | <.001 | 1.006 (1.002–1.010) | 0.003 |
Abbreviations: CI, confidence intervals, METS, metabolic equivalents,
Covariates include: season, age, site, body mass index, beta-blocker use, ACE inhibitor/ARB use
Estimated medians are reported for outcomes measured in minutes/day since these outcomes were log-transformed for modeling purposes and interpretation of the model coefficients are relative median values
The ratio of user/nonuser was calculated from multivariable linear regression model coefficients estimating the association between statin use and each outcome; since the outcomes were log-transformed, the exponent of model coefficients are a ratio of the median value for users versus nonusers and are interpreted in the text as a percent difference in the outcome
Discussion
In this large observational study in older men, we examined the cross-sectional differences and longitudinal changes in physical activity by statin use. Overall, physical activity declined at similar rates to those observed in a prior study.16 While short-term studies suggest that statins decrease physical activity in older adults for up to one year, it was unclear if this effect was sustained.8,9 Our long-term study, that followed men up to an average of 6.9 years, suggests that statins are associated with less physical activity for as long as statins are used. In cross sectional analyses, statin users started with lower physical activity levels compared to nonusers. Longitudinally, prevalent statin users declined at similar rates as nonusers while new statin users declined more rapidly. This association was observed even after adjusting for time-varying health factors, such as MI or stroke.
The exact mechanism by which statins affect muscles is not known. There are a number of possible causes. For example, statins may disrupt mitochondrial function and interfere with ATP production, contributing to fatigue and muscle weakness.8,18–20 Disruption of mitochondria may also cause myopathy by increasing the production of reactive oxygen species, inducing DNA damage, and initiating apoptosis. Recent studies have also indicated these same mechanisms are precipitated or exacerbated during exercise in statin users.9–11 If exercise-induced myopathy occurs in older adults taking statin medications, this may explain why we observed prevalent statin users engaged in less physical activity in this study. In addition, new statin users had the largest drop in physical activity; starting off with physical activity similar to nonusers, but ending up with physical activity similar to prevalent statin users.
Prevalent statin use was associated with less physical activity, but perhaps reassuringly, was not associated with a more rapid decline compared to nonusers. While we hypothesized that prevalent statin use would result in a more rapid decline in physical activity, there are two possible reasons we did not observe this. First, those most susceptible to muscle symptoms may have stopped using a statin during this study. Secondly, a decline in one’s health may precipitate stopping a statin. Of baseline statin users, 9% stopped before visit 2, and of statin users at visit 2, 11% stopped before visit 3. Of those that stopped, we observed a nominal decrease in physical activity.
We also examined physical activity measured objectively by accelerometry. One measure from the accelerometer included METS, which is a global measure of physical activity. Statin users expended 0.03 fewer METS per day. In clinical terms, and using average body weight of the men in this study (78 kg), the average decrease in energy expenditure was approximately 56.2 kcal/day, or approximately 151 minutes/week of walking at a typical pace for older adults (2 miles/hour).21
The other objective measures included minutes of physical activity during moderate or vigorous activity and minutes of sedentary behavior. While the daily amount of moderate or vigorous activity was modestly less in statin users, it equates to approximately 37.8 minutes/week of less exercise. For comparison, the 2013 American Heart Association and the American College Cardiology (AHA/ACC) Guideline Lifestyle Managements recommends an average of 40 minutes of moderate to vigorous activity for 3–4 sessions per week.22,23 Finally, more sedentary behavior was observed in statin users, on average about 53 minutes/week, an increase to 21.8 hours/week. Sedentary time is associated with all-cause and CVD mortality.1,2,24 For example, in one study, >23 hours/week of sedentary behavior was associated with an adjusted hazard ratio of 1.37 (1.01–1.87) compared to <11 hours/week.24 While it should be noted that 53 more minutes of sedentary behavior per week may not be substantial in terms of CVD risk, clinicians and patients should be aware that more sedentary behavior and less physical activity may be observed with statin use. While accelerometer measurements were only performed once, these are an objective assessment of activity and support the differences observed with self-reported physical activity measured by PASE.
The recent 2013 AHA/ACC Guideline on the Treatment of Blood Cholesterol acknowledges that there are few data available in adults >75 years old and did not clearly support using high-intensity statin therapy in secondary prevention.17 For the same reason, they also recommend the initiation of statins for primary prevention in this population requires considering comorbidities, safety, and priorities of care. Thus, possible adverse effects on physical activity should be considered.
Limitations
This study was a study of older men, and generalization to older women may not be appropriate. PASE is a self-administered questionnaire and could be subject to measurement error or recall bias. The accelerometer data was only collected at visit 3, thus longitudinal changes could not be assessed for this measure.
As in any observational study of the effect of an intervention, control for confounding by indication was important. It can be difficult to predict whether factors associated with physical activity and statin use might bias the findings toward or away from the null. For example, health issues related to lower physical activity could also relate to statin intolerance or noncompliance, resulting in a weaker apparent effect of statin use on physical activity (bias toward the null). Similarly, low cholesterol in older adults is also associated with worse health and would coincide with lower physical activity and a lack of statin use.25–27 If residual confounding by health status remains, our results are likely to underestimate the true strength of association between statin use and physical activity. In contrast, the potential that our results overestimate the strength of association also exists. For example, if our adjustments for cardiovascular risk factors are incomplete, there is the possibility that it is these risk factors, and not statin use, that is responsible for the associations we observe with physical activity. Because of these potential biases, we took care to examine self-reported cardiovascular events that could reasonably be associated both with the use of a statin and physical activity. The collection of these variables over the course of follow-up allowed for adjustment of time-varying confounders, such as MI and stroke, which would be expected to both reduce physical activity and increase the probability of new statin use. Adjustment for cardiovascular events and medications, diabetes, and BMI, which were identified as confounders, minimizes the possibility that the results reported here were due to confounding by indication. However, as in all observational studies, the risk of residual confounding remains. For example, imperfect reporting of cardiovascular events and measurement of cardiovascular risk factors that could affect physical activity leaves room for the possibility of additional, uncontrolled confounding in these analyses.
Information about the duration of statin use was not known prior to the initiation of this study. Consequently, information about prior statin use was not known and participants classified as nonusers may have been former statin users. Classifying participants as new statin users allowed us to explore the association between statin initiation and physical activity. Unfortunately, this does not preclude the possibility that some men were new statin users, then experienced muscular adverse effects, and stopped using a statin prior to the initiation of this study or between visits. Thus, those recorded as being a prevalent or new statin user may have been users that didn’t experience muscular symptoms, and perhaps less susceptible to declines in physical activity.
Conclusion
In this prospective observational study in community-living older men, statin use was associated with modestly lower physical activity even after accounting for medical history and other potentially confounding factors. In addition, new statin use was associated with a more rapid decline in physical activity than nonuse. While similar effects have been reported in older adults in short-term studies, this study shows that although physical activity levels remain lower in prevalent statin users than nonusers, they do not continue to decline more rapidly than nonusers over time. The possible reasons for lower physical activity levels in statin users may be general muscle pain caused by statins, a well-known adverse effect; exercise-endured myopathy; or muscular fatigue. The clinical significance of these findings deserves further investigation.
Acknowledgments
Funding/Support:
The Osteoporotic Fractures in Men Study (MrOS) is supported by NIH funding; the following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01 AG027810, and UL1 RR024140. This analysis was funded by a grant from the Medical Research Foundation of Oregon.
Role of the Sponsor: NIH and the Medical Research Foundation of Oregon had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Prior presentations: This study was presented, in part, at the American Society of Consultant Pharmacists 2013 Annual Meeting, November 2013, Seattle Washington.
Authors’ contributions:
Dr Lee had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: DSHL, SM, CGL, EE, CW, RF, EO, CN
Acquisition of data: EO, CN
Analysis and interpretation of data: DSHL, SM, LG, CGL, EE, CW, RF, EO, PMC, MLS, DM, DB, CN
Drafting of the manuscript: DSHL, SM
Critical revision of the manuscript for important intellectual content: LG, CGL, EE, CW, RF, EO, PMC, DM, DB, CN
Statistical analysis: SM, RF
Obtained funding: DSHL, EO
Administrative, technical, or material support: DSHL, SM, LG, CGL, EE, CW, RF, EO, PMC, MLS, DM, DB, CN
Study supervision: CN
Conflict of interest disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Additional Contributions: The authors would like to thank Angie Mettie, B.S. (OSU/OHSU College of Pharmacy) for her suggested edits to this manuscript. We would also like to thank the participants of the Osteoporotic Fractures in Men Study (MrOS), without whom this research would not be possible.
References
- 1.Gregg EW, Cauley JA, Stone K, et al. Relationship of changes in physical activity and mortality among older women. JAMA. 2003 May 14;289(18):2379–2386. doi: 10.1001/jama.289.18.2379. [DOI] [PubMed] [Google Scholar]
- 2.Kujala Um KJSSKM. Relationship of leisure-time physical activity and mortality: The finnish twin cohort. JAMA: The Journal of the American Medical Association. 1998;279(6):440–444. doi: 10.1001/jama.279.6.440. [DOI] [PubMed] [Google Scholar]
- 3.Paffenbarger RS, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The Association of Changes in Physical-Activity Level and Other Lifestyle Characteristics with Mortality among Men. New England Journal of Medicine. 1993;328(8):538–545. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 4. [Accessed December 10, 2012];Healthy People 2020 Topic and Objectives: Older Adults Objectives. 2012 2012; http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=31.
- 5.Golomb B, Evans M, Dimsdale J, White H. Effects of statins on energy and fatigue with exertion: Results from a randomized controlled trial. Arch Intern Med. 2012;172(15):1180–1182. doi: 10.1001/archinternmed.2012.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Writing Committee M. Pasternak RC, Smith SC, et al. ACC/AHA/NHLBI Clinical Advisory on the Use and Safety of Statins. Circulation. 2002 Aug 20;106(8):1024–1028. doi: 10.1161/01.cir.0000032466.44170.44. 2002; [DOI] [PubMed] [Google Scholar]
- 7.Golomb BA, Dimsdale JE, White HL, Ritchie JB, Criqui MH. Reduction in blood pressure with statins: results from the UCSD Statin Study, a randomized trial. Arch Intern Med. 2008 Apr 14;168(7):721–727. doi: 10.1001/archinte.168.7.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker BA, Capizzi JA, Grimaldi AS, et al. Effect of Statins on Skeletal Muscle Function. Circulation. 2013 Jan 1;127(1):96–103. doi: 10.1161/CIRCULATIONAHA.112.136101. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to Moderate Muscular Symptoms with High-Dosage Statin Therapy in Hyperlipidemic Patients: The PRIMO Study. Cardiovascular Drugs and Therapy. 2005;19(6):403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 10.Meador BM, Huey KA. Statin-associated myopathy and its exacerbation with exercise. Muscle Nerve. 2010;42(4):469–479. doi: 10.1002/mus.21817. [DOI] [PubMed] [Google Scholar]
- 11.Semple S. Statin therapy, myopathy and exercise--a case report. Lipids in Health and Disease. 11(1):40. doi: 10.1186/1476-511X-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikus CR, Boyle LJ, Borengasser SJ, et al. Simvastatin impairs exercise training adaptations. Journal of the American College of Cardiology. 2013;(0) doi: 10.1016/j.jacc.2013.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005 Oct;26(5):569–585. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the elderly (PASE): Development and evaluation. Journal of Clinical Epidemiology. 1993;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 15.Cawthon PMBT, Cauley JA, Ensrud KE, Dam TT, Harrison SL, Peters KWMD. Objective assessment of activity, energy expenditure, and functional limitations in older men: The Osteoporotic Fractures in Men Study (MrOS) J Gerontol A Biol Sci Med Sci. 2013 doi: 10.1093/gerona/glt054. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janney CA, Cauley JA, Cawthon PM, Kriska AM for the Osteoporotic Fractures in Men Study G. Longitudinal Physical Activity Changes in Older Men in the Osteoporotic Fractures in Men Study. Journal of the American Geriatrics Society. 58(6):1128–1133. doi: 10.1111/j.1532-5415.2010.02861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013 Nov 7; doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Dirks AJ, Jones KM. Statin-induced apoptosis and skeletal myopathy. American Journal of Physiology - Cell Physiology. 2006 Dec 1;291(6):C1208–C1212. doi: 10.1152/ajpcell.00226.2006. 2006; [DOI] [PubMed] [Google Scholar]
- 19.Trapani L, Melli L, Segatto M, et al. Effects of myosin heavy chain (MHC) plasticity induced by HMGCoA-reductase inhibition on skeletal muscle functions. The FASEB Journal. 2011 Nov 1;25(11):4037–4047. doi: 10.1096/fj.11-184218. [DOI] [PubMed] [Google Scholar]
- 20.Wu JS, Buettner C, Smithline H, Ngo LH, Greenman RL. Evaluation of skeletal muscle during calf exercise by 31-phosphorus magnetic resonance spectroscopy in patients on statin medications. Muscle Nerve. 2011 Jan;43(1):76–81. doi: 10.1002/mus.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA : the journal of the American Medical Association. 2011 Jan 5;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013 Nov 7; doi: 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007 Aug 28;116(9):1094–1105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- 24.Warren TY, Barry V, Hooker SP, Sui X, Church TS, Blair SN. Sedentary behaviors increase risk of cardiovascular disease mortality in men. Med Sci Sports Exerc. May;42(5):879–885. doi: 10.1249/MSS.0b013e3181c3aa7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brescianini S, Maggi S, Farchi G, et al. Low total cholesterol and increased risk of dying: are low levels clinical warning signs in the elderly? Results from the Italian Longitudinal Study on Aging. Journal of the American Geriatrics Society. 2003 Jul;51(7):991–996. doi: 10.1046/j.1365-2389.2003.51313.x. [DOI] [PubMed] [Google Scholar]
- 26.Weverling-Rijnsburger AW, Blauw GJ, Lagaay AM, Knook DL, Meinders AE, Westendorp RG. Total cholesterol and risk of mortality in the oldest old. Lancet. 1997 Oct 18;350(9085):1119–1123. doi: 10.1016/s0140-6736(97)04430-9. [DOI] [PubMed] [Google Scholar]
- 27.Zuliani G, Cherubini A, Atti AR, et al. Low cholesterol levels are associated with short-term mortality in older patients with ischemic stroke. The journals of gerontology. Series A, Biological sciences and medical sciences. 2004 Mar;59(3):293–297. doi: 10.1093/gerona/59.3.m293. [DOI] [PubMed] [Google Scholar]