Abstract
Background: Optimal glucose-lowering therapy in type 2 diabetes mellitus requires a patient-specific approach. Although a good framework, current guidelines are insufficiently detailed to address the different phenotypes and individual needs of patients seen in daily practice. We developed a patient-specific decision support tool based on a systematic analysis of expert opinion.
Materials and Methods: Based on the American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) 2012 position statement, a panel of 12 European experts rated the appropriateness (RAND/UCLA Appropriateness Method) of treatment strategies for 930 clinical scenarios, which were permutations of clinical variables considered relevant to treatment choice. These included current treatment, hemoglobin A1c difference from individualized target, risk of hypoglycemia, body mass index, life expectancy, and comorbidities. Treatment options included addition of a second or third agent, drug switches, and replacement by monotherapies if the patient was metformin-intolerant. Treatment costs were not considered. Appropriateness (appropriate, inappropriate, uncertain) was based on the median score and expert agreement. The panel recommendations were embedded in an online decision support tool (DiaScope®; Novo Nordisk Health Care AG, Zürich, Switzerland).
Results: Treatment appropriateness was associated with (combinations of) the patient variables mentioned above. As second-line agents, dipeptidyl peptidase-4 inhibitors were considered appropriate in all scenarios, followed by glucagon-like peptide-1 receptor agonists (50%), insulins (33%), and sulfonylureas (25%), but not pioglitazone (0%). Ratings of third-line combinations followed a similar pattern. Disagreement was highest for regimens including pioglitazone, sulfonylureas, or insulins and was partly due to differences in panelists' opinions and in drug availability and reimbursement across European countries (although costs were disregarded in the rating process).
Conclusions: A novel decision support tool based on the ADA/EASD 2012 position statement and a systematic analysis of expert opinion has been developed to help healthcare professionals to individualize glucose-lowering therapy in daily clinical situations.
Introduction
The complexity of glycemic management in type 2 diabetes mellitus (T2DM) has increased dramatically over the past 20 years. In 1995, the drugs available for treatment of T2DM in Europe were insulin, metformin, and sulfonylureas (SU). In 2012, nine glucose-lowering drug (GLD) classes were available, significantly increasing the number of treatment options.1 Therefore, new combinations of agents with complementary mechanisms of action are possible, facilitating individualized, patient-centered care as proposed in the latest position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD).1 Besides lifestyle modification measures, the statement recommends setting individual glycemic targets and treatment selection based on patient characteristics and properties of the glucose-lowering agents. However, phenotypes in T2DM vary widely, with substantial heterogeneity in clinical outcomes. Therefore, healthcare professionals now have many pharmacological approaches available to tailor treatment to individual patient needs. However, the expansion in clinical options is accompanied by a general lack of long-term comparative effectiveness studies to inform clinical decision-making, as well as new uncertainties regarding the long-term benefits of new drugs, for example, on macrovascular complications.2–4 Consequently, many clinicians are uncertain when faced with the task of finding the most suitable strategy for any given clinical scenario.
Despite the wide range of glucose-lowering options and the availability of treatment guidelines, observational studies in T2DM consistently report clinical inertia, defined as failure to initiate or intensify therapy according to evidence-based guidelines, along with poor hemoglobin A1c (HbA1c) levels.5 A retrospective cohort study based on 81,573 people with T2DM in the United Kingdom between 2004 and 2011 showed significant delays in intensifying treatment, with patients remaining in poor glycemic control for more than 7 years before intensification with insulin.6 In patients taking one, two, or three oral GLDs, median time from initiation of treatment to intensification with an additional GLD or insulin exceeded 7.2 years. The mean HbA1c level at intensification with an GLD or insulin for people taking one, two, or three GLDs was 8.7%, 9.1%, and 9.7%, respectively.6 In another retrospective database study in primary care in Germany and the United Kingdom, the time to insulin therapy significantly increased in T2DM patients from 2005 to 2010.7 The last HbA1c values before insulin initiation were high and slightly increased during the study period (Germany, from 8.2% in 2005 to 8.4% in 2010; United Kingdom, from 9.5% to 9.8%, respectively).7
One reason for clinical inertia includes treatment complexity. Therefore, there is a need for better translating scientific knowledge to everyday practice decisions.8 The use of clinical decision support systems (CDSSs) may be one solution because they can provide patient-specific recommendations at the point of care, through the input of patient data in an electronic or nonelectronic system with a use of algorithms that can match pieces of information from a knowledge database.
In order to simplify decision-making for glucose-lowering therapy in T2DM for primary care physicians and nonspecialists, we conducted a European expert panel study to translate the ADA/EASD position statement into recommendations at the patient-specific level, combining the evidence from clinical trials and expert opinion. The study focused on treatment choice for patients insufficiently controlled by or intolerant to metformin.
Materials and Methods
The study followed a systematic methodology with well-defined steps, previously used to develop decision support tools in other disease areas.9–11
RAND/UCLA Appropriateness Method
The appropriateness of treatment for a variety of clinical scenarios was studied using the RAND/UCLA Appropriateness Method (RUAM).12 The RUAM, a modified Delphi method, encompasses a highly structured approach to develop patient-specific treatment recommendations, based on the best available evidence from clinical studies and practice experience.12,13 A treatment is considered appropriate if its expected benefits exceed its potential negative consequences by a sufficient margin. Studies on reliability, internal consistency, and (predictive) validity of the RUAM have shown favorable results.14
Literature review
An extensive literature review was conducted to shape the research question and study design and to ensure that panel members had access to the same body of evidence during the rating process. For details, see supporting information at http://diascope.org/sites/default/files/RAND-DM_Literature.pdf
Panel composition and process
Based on their scientific and clinical expertise in the field of diabetes, 12 experts from eight European countries were selected: 11 diabetologists and one general practitioner with special expertise in diabetes. The panel met in November 2012 to discuss the literature review and starting points of the study. These included criteria for the patient population to be considered (T2DM, insufficient glycemic control with metformin mono-/dual therapy or intolerance to metformin, age ≥18 years, and absence of specific conditions such as pregnancy and severe renal impairment), as well as selection of seven clinical variables that formed the basis for developing a comprehensive set of clinical scenarios or patient profiles (current treatment, disease duration, HbA1c difference from individual target, risk of hypoglycemia, body mass index [BMI], life expectancy, and comorbidities relevant to antidiabetes treatment). Treatment options consisted of the addition of a second or a third drug (SU, pioglitazone, dipeptidyl peptidase-4 inhibitors [DPP-4i], glucagon-like peptide-1 receptor agonists [GLP-1 RA], or insulins) and drug switches. Insulin intensification was not considered in this initial phase of the study, and sodium/glucose cotransporter 2 inhibitors were not included, as they were not widely available in Europe at the time of this project. Using an electronic program, panelists individually performed 1,398 appropriateness ratings on a 9-point scale (reference values: 1=very inappropriate; 5=uncertain; 9=very appropriate). Panelists were instructed to take only the clinical perspective into consideration and to disregard costs and other potential constraints.
After the first rating round, the panel convened to discuss the results (January 2013). Panelists received feedback on their own ratings in comparison with the anonymous results of their colleagues. The discussion revealed that the variable “disease duration” was not discriminative for treatment choice and could therefore be removed from the model. Furthermore, the panel made several refinements to the definitions of criteria, variables, and treatment options. Thereafter, a second individual rating round, including 930 assessments, was conducted (May 2013). An overview of clinical variables and treatment options used in the second round is given in Table 1.
Table 1.
Overview of Clinical Variables and Treatment Options Used in the Second Rating Round During the Development of a Clinical Decision Support System for the Management of Hyperglycemia in Type 2 Diabetes Mellitus
| Variable | Number of categories | Description |
|---|---|---|
| Previous treatment | 5 | Metformin monotherapy or dual therapy with SU, PIO, DPP-4i, or GLP-1 RA |
| Difference from individualized HbA1c target | 2 | <1%; ≥1% |
| Risk of hypoglycemia | 2 | Low; moderate–high |
| Body mass index | 3 | 25; 25–29.9; ≥30 kg/m2 |
| Life expectancy | 2 | ≥2 years; <2 years |
| Comorbidities | 6 | Coronary heart disease, heart failure, advanced microvascular complications, renal impairment, liver dysfunction, dementia |
| Decision areas | Number of treatment options | Description |
|---|---|---|
| Insufficient control of metformin monotherapy | 5 | Two-drug regimen including SU, PIO, DPP-4i, GLP-1 RA, or insulins |
| Insufficient control of dual therapy with metformin | 27 | Addition of third drug or replacement by other two-drug regimen including metformin |
| Intolerance to metformin | 5 | Replacement by another monotherapy (SU, PIO, DPP-4i, GLP-1 RA, or insulins) |
| Comorbidities | 6 | Metformin, SU, PIO, DPP-4i, GLP-1 RA, insulins |
DPP-4i, dipeptidyl peptidase-4 inhibitors; GLP-1 RA, glucagon-like peptide-1 receptor agonists; HbA1c, hemoglobin A1c; PIO, pioglitazone; SU, sulfonylureas.
Statistical analysis
For calculating the appropriateness of treatments, the mathematical rules that are typically applied in RUAM studies were used.12 The outcome was appropriate if the median panel score was between 7 and 9 and inappropriate if the median was between 1 and 3, both without disagreement between panelists. Disagreement was defined when at least four out of 12 panelists scored in each of the sections 1–3 and 7–9. All other situations were deemed uncertain. Frequency tables and cross-tabulations were used to describe and analyze the appropriateness of treatments by clinical variables.
Electronic decision tool
The results of the second round were embedded in an electronic decision support tool (DiaScope®; Novo Nordisk Health Care AG, Zürich, Switzerland), which shows the appropriateness of treatments for any given patient profile. Where applicable, the results of separate conditions were combined with overall panel recommendations using the principle that the final outcome is determined by the lowest appropriateness category of the separate conditions.
Results
Insufficient glycemic control with metformin monotherapy
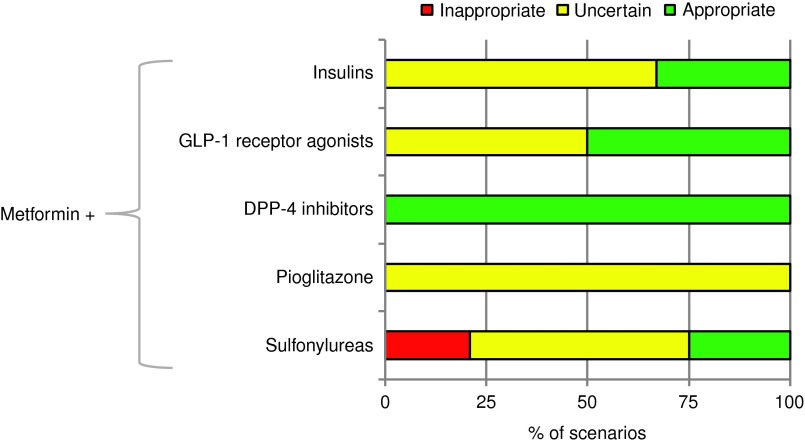
For patients insufficiently controlled with metformin monotherapy, second-line regimens with DPP-4i were rated appropriate for all scenarios (Fig. 1), followed by those with GLP-1 RA (50%), insulins (33%), and SU (25%). Analysis of appropriateness by clinical variables showed that the appropriateness of GLP-1 RA decreased if life expectancy was <2 years and BMI was <25 kg/m2 (Fig. 2). In contrast, the appropriateness of GLP-1 RA was high if the difference from the individualized HbA1c target was ≥1% and the BMI was ≥25 kg/m2. Regarding insulin, the dominant factor driving its appropriateness was also the difference from HbA1c target, whereas other variables (hypoglycemia risk, BMI, life expectancy) showed a negative association with appropriateness. The only class for which inappropriate outcomes were seen was SU. These apply exclusively to patients with difference from HbA1c target of <1% and moderate/high risk of hypoglycemia (details not shown). The outcomes for pioglitazone were always uncertain, predominantly because of disagreement between panelists.
FIG. 1.
Appropriateness of second-line regimens with metformin (percentage of clinical scenarios). A treatment is considered appropriate if its expected benefits exceed its potential negative consequences by a sufficient margin. DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1.
FIG. 2.
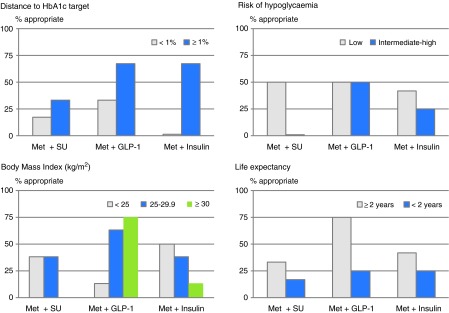
Appropriateness of second-line treatments (sulfonylureas [SU], glucagon-like peptide-1 receptor agonists [GLP-1 RA], and insulins, all with metformin [Met]) by clinical variables: distance to hemoglobin A1c (HbA1c) target, risk of hypoglycemia; body mass index, and life expectancy. Data are percentages of scenarios considered appropriate. Met+dipeptidyl peptidase-4 inhibitors was always considered appropriate, and Met+pioglitazone was never considered appropriate; therefore these combinations are not shown here.
Insufficient control with metformin dual therapy
Addition of a third drug in patients insufficiently controlled by dual therapy showed a hierarchy of appropriateness that was comparable to dual therapies (Table 2). Replacement by another two-drug regimen (switch) was in general less often considered appropriate than addition of a third drug (Table 2). The few situations in which a switch was perceived more relevant than addition was for GLP-1 RA after dual therapy with DPP-4i and for initiation of insulin after dual therapy with pioglitazone. Analysis of appropriateness of triple therapies in relation to clinical variables (data not shown) revealed similar underlying patterns as described for second-line treatments.
Table 2.
Appropriateness of Treatment Regimens in Cases of Insufficient Control by Dual Therapy with Metformin (Percentage of Clinical Scenarios)
| Current (failing) dual therapy (%) | ||||
|---|---|---|---|---|
| Met+SU | Met+PIO | Met+DPP-4i | Met+GLP-1 RA | |
| Change to three-drug regimen | ||||
| Add SU | NA | 13 | 17 | 33 |
| Add PIO | 0 | NA | 0 | 0 |
| Add DPP-4i | 83 | 100 | NA | NA |
| Add GLP-1 RA | 42 | 54 | NA | NA |
| Add insulin | 33 | 8 | 38 | 42 |
| Replace by other two-drug regimen | ||||
| Metformin+SU | NA | 0 | 0 | NA |
| Metformin+PIO | 0 | NA | 0 | NA |
| Metformin+DPP-4i | 0 | 46 | NA | NA |
| Metformin+GLP-1 RA | 33 | 33 | 33 | NA |
| Metformin+insulin | 38 | 50 | 25 | 38 |
DPP-4i, dipeptidyl peptidase-4 inhibitors; GLP-1 RA, glucagon-like peptide-1 receptor agonists; NA, not applicable; PIO, pioglitazone; SU, sulfonylureas.
Intolerance to metformin
For patients displaying intolerance to metformin monotherapy, the appropriateness of replacement by other monotherapies was rated separately. For SU, pioglitazone, and DPP-4i, the results were identical to those of adding the same drug when metformin alone was insufficient. For GLP-1 RA and insulin monotherapies, the appropriateness was somewhat lower (GLP-1 RA, 33% monotherapy vs. 50% dual therapy; insulins, 17% vs. 33%, respectively).
Comorbidities
Appropriateness of treatment in relation to comorbidities (see Table 1) was rated separately from other conditions. The panel considered pioglitazone to be inappropriate (absolute contraindication) in patients with heart failure. Relative contraindications (“use with caution”) were reported for coronary heart disease and stroke (SU, pioglitazone), stable heart failure and moderate renal impairment (metformin, SU), liver dysfunction (metformin, SU, pioglitazone), and dementia (SU, insulin).
Uncertainty and disagreement
The outcomes were uncertain for 60% of scenarios if all ratings were taken together. Out of these uncertain cases, 59% were due to ratings in the middle of the 9-point scale, reflecting either equilibrium of potential benefits and risks or uncertainty of the experts. For the remaining 41%, uncertainty was ascribed to disagreement among the 12 panelists. Disagreement was highest for regimens including pioglitazone (39%), SU (31%), and insulins (27%). Discussions during face-to-face meetings revealed that this was partly due to different personal experiences and opinions and to differences in drug availability and reimbursement conditions across European countries (although costs were supposed to be disregarded in the rating process).
Electronic decision support tool
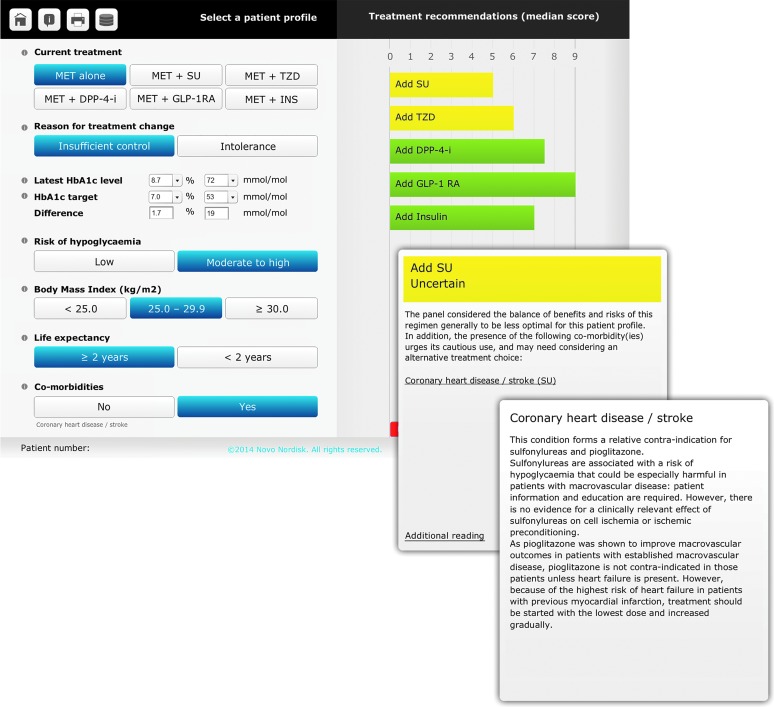
The results of the second rating round were embedded in an electronic decision support tool, called DiaScope. The heart of this decision tool is an interface that allows the user to create a patient profile and to see the appropriateness of the various treatment options (Fig. 3). Clicking through on a treatment shows the considerations behind the panel recommendation and provides detailed additional information on, for example, comorbidities. The tool is available in both online and offline formats and is accessible for physicians after registration (http://diascope.org).
FIG. 3.
User interface of the DiaScope: patient profile and display of treatment recommendations and additional information. DPP-4-i, dipeptidyl peptidase-4 inhibitors; GLP-1RA, glucagon-like peptide-1 receptor agonists; HbA1c, hemoglobin A1c; INS, insulins; MET, metformin; SU, sulfonylureas; TZD, thiazolidinediones.
Discussion
Adequate glucose-lowering therapy in subjects with T2DM requires repeated follow-up, with frequent adjustments of goals and treatment as the disease progresses. Real-life audits show that approximately 40% of patients in Europe do not have good glycemic control despite a large therapeutic armamentarium and numerous guidelines.15
The ADA/EASD 2012 position statement1 was used as the starting point for our discussions. It was assumed that lifestyle changes and diet should be recommended throughout the disease duration and that all patients should start with metformin monotherapy. In line with the RAND/UCLA method, patient variables were selected on their relevance to treatment decisions, and clinical scenarios had to be mutually exclusive. In accordance with the ADA/EASD statement, our model included different HbA1c targets, hypoglycemia risk, life expectancy, important comorbidities, and the presence of established vascular complications. However, BMI was preferred over weight in order to differentiate lean, overweight, and obese groups. In addition, life expectancy was considered a highly important variable for clinical decision-making. Finding an appropriate cutoff point separating short and long life expectancies in diabetes was not possible because of a lack of clinical evidence and of the difficulty in predicting life expectancy with confidence. However, it is generally easier to identify patients with a very short life expectancy (e.g., in the case of advanced and progressive cancer) and for whom treatment is primarily aimed at preserving patient comfort and avoiding aggressive strategies. The final consensus was a cutoff point of 2 years, as anything beyond is more difficult to predict.
Regarding comorbidities relevant to clinical decision-making, we added dementia to the list recommended by ADA/EASD. Dementia limits the patient in managing complex regimens or even injections, and cognitive dysfunction may also delay the recognition of symptoms of hypoglycemia and increase the risk in falls and fractures.16
In contrast, some other variables from the ADA/EASD statement were not included after detailed discussions. “Disease duration” proved not to be a differentiating criterion in the ratings, and we agreed that reaching an individualized HbA1c target in an effective and safe manner was more important than diabetes duration by itself. Moreover, T2DM is often diagnosed incidentally (e.g., after a check-up or diagnosis in a family relative), and the actual disease duration is unknown. Risks associated with “older age” in the position statement are reflected in the variables used in our model (higher atherosclerotic disease burden, reduced renal function, other comorbidities, hypoglycemia). Therefore, age was not retained as a separate variable as we considered that life expectancy and absence/presence of comorbidities were more important than age per se and that these variables are good proxies of a patient's overall functional status. Some drug classes were not included because they are used less in Europe (glinides, α-glucosidase inhibitors) or had only recently been approved (sodium/glucose cotransporter 2 inhibitors) so that real-life experience was too limited at the time of this study. Sodium/glucose cotransporter 2 inhibitors will be included in the next update of DiaScope.
Regimens with DPP-4i were considered appropriate in all scenarios because of their low side effect profile and oral administration, followed by those with GLP-1 RA and with insulins. However, these figures apply to a theoretical set of scenarios, and their distribution in real-life practice is yet to be determined. Sixty percent of all treatment options were labeled as uncertain, of which 41% were deemed as such because they received opposite (rather than equivocal) ratings. These opposite ratings reflect differences in expert opinions and experiences and hence the complexity of GLD management.
Similar to most other RAND/UCLA studies, we took the clinical perspective as a starting point, disregarding costs and other potential constraints to treatment choice. Despite extensive discussions, we were unable to totally eliminate the influence of country-specific perspectives on the ratings, resulting in disagreement for a substantial number of scenarios. As this reflects the “personal” character of the ratings, it may be questioned whether another panel, with another (geographic) composition or of different size, would have reached the same conclusions. Results from other RAND/UCLA studies comparing different panels for one same topic have shown that interpanel agreement is generally very satisfactory14 and is highest in panels composed of the same medical specialty.17–19
DiaScope is the first CDSS for the management of T2DM developed using the RAND/UCLA method. Treatment recommendations are displayed at a patient-specific level, based on published evidence and the collective judgment of a panel of 12 European experts in diabetes. Appropriateness levels reflect both the median value of the individual ratings and the extent of agreement between the experts. In addition, pop-ups provide detailed clarification on the key clinical variables (e.g., comorbidities) and show the considerations behind the panel recommendations. Other advantages include the display of all common treatment options for each patient profile (not only those considered appropriate), the gradation (median score) of the recommendations as opposed to categories only, and the inclusion of educational comments. The strength of DiaScope is the overview of a wide range of treatment options for multiple clinical presentations that physicians face in daily practice. The recommendations reflect the expert panel's opinion on their efficacy, tolerability, and ease of use, for given patient profiles, and are never in contradiction with the ADA/EASD position statement.
This tool may offer individual healthcare professionals an opportunity to assess a relevant clinical situation, draw their own conclusions, and then compare them with the panel recommendation. Although the panelists were almost all diabetologists, they took the RAND/UCLA perspective, “an average patient presenting to an average physician in an average care-providing facility,”13 as the starting point for their considerations, making the tool applicable for decision-making in most common healthcare settings. By providing a “second opinion” directly at the point of care, DiaScope should be viewed as an educational tool that could help promote the adoption and utilization of the ADA/EASD statements by presenting them in the context of interactive clinical scenarios.
Each time a CDSS is developed, the inevitable question is whether it will affect clinical care. Several CDSSs for the management of T2D for healthcare providers already exist, with large differences in terms of development methods, algorithms, content and sophistication level.20–24 In-depth literature reviews of CDSSs, one of which is in primary T2DM care, found that these tools can indeed be effective in improving the process of care, although few have shown improvements in patient outcomes.25,26 However, as many factors may influence health outcomes, measuring the effectiveness of CDSSs on these end points is difficult.
On the other hand, there is a risk that CDSSs could generate erroneous advice or misinterpretations. DiaScope is a simple and user-friendly tool that displays experts' opinions for all common treatment options instead of a single solution. Likewise, patient treatment preference was not retained to avoid excluding prematurely valuable options, as preference may be managed by physician–patient discussion or patient education as a subsequent step.
Most important is that a decision support system is only as effective as its underlying knowledge base, which changes rapidly as medical science evolves.27–29 Therefore we plan annual updates of DiaScope to reflect knowledge progression, changing guidelines, and increased expertise, making the tool as “evidence-adaptive” as possible.
Conclusions
Using the RAND/UCLA approach, an expert panel formulated patient-specific recommendations for glucose-lowering therapy in T2DM across numerous clinical scenarios, all embedded in an electronic tool. With the evolving complexity of T2DM management and the increasingly important role of general practitioners in managing these patients, the DiaScope tool may facilitate decision-making and eventually help to reduce clinical inertia. Further research will evaluate its applicability in primary care practice.
Acknowledgments
The panel study and development of the decision tool were funded by Novo Nordisk Health Care AG, Zürich, Switzerland, as were the services of the medical writer for editorial support. The authors wish to express their respectful memory of Prof. Michaela Diamant who passed away during the panel study on April 9, 2014. They also wish to thank Jessa Yperman from Ismar Healthcare (Lier, Belgium) for performing the literature review and Dr. Sylvie Picard (Dijon, France) for her valuable advice in finalizing the electronic tool. Thanks are given to Deborah Nock (Medical WriteAway, United Kingdom) for some editorial support.
Author disclosure statement
F.J.A.-B. has received honoraria as speaker and/or consultant from Abbott, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, LifeScan, Lilly, Madaus, MannKind Corp., Medtronic, Menarini, Merck Farma y Química, SA, MSD, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi, Schering-Plough, and Solvay. P.Y.B. has received honoraria as advisory board member and/or lecturer from Sanofi, Novo Nordisk, Eli Lilly, Abbott, Roche, MSD, and Lifescan. G.C. has received lecture fees from Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi, is a member of advisory boards of Janssen, Johnson & Johnson, Medtronic, Novo Nordisk, and Sanofi, and has received research support from Abbott, Novo Nordisk, Pfizer, and Sanofi. A.C. has received lecture fees from AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, Novartis, Sanofi, and Takeda, is a member of advisory boards of AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceuticals, Novo Nordisk, Merck Sharp & Dohme, and Sanofi, and has received research support from Eli Lilly and Novo Nordisk. B.G. has received honoraria as an advisory board member from AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Janssen, Lilly, Merck, Novartis, Novo Nordisk, Roche, and Sanofi and has received honoraria for lectures from these companies as well as from Abbott and Berlin Chemie. K.K. has acted as a consultant and speaker for AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, MSD, Novartis, Novo Nordisk, and Sanofi. He has received grants in support of investigator-initiated studies from AstraZeneca, Boehringer Ingelheim, Lilly, Novartis, Novo Nordisk, Roche, and Sanofi. C.M. is or has been advisor or speaker for and University Hospital of Leuven and has received research support or honoraria from Novo Nordisk, Merck/MSD, Eli Lilly, Sanofi, Novartis, AstraZeneca, Bristol-Myers Squibb, Janssen Pharmaceuticals, Pfizer, Boehringer Ingelheim, Medtronic, Roche, and Servier. M.R. is an employee of Steno Diabetes Center A/S, has served on advisory boards for Boehringer Ingelheim, Glaxo SmithKline, Novo Nordisk, Roche, and Sanofi, and has received honoraria for lectures from Astra Zeneca, Eli Lilly, Glaxo SmithKline Beecham, Novo Nordisk, Roche, and Sanofi. J.S. has received honoraria as an advisory board member and/or lecturer from Takeda, Bayer, Novartis, Merck Sharp & Dohme, AstraZeneca, Bristol-Myers Squibb, Novo Nordisk, Sanofi, Berlin Chemie, Eli Lilly, Boehringer Ingelheim, Merck, Roche, Ipsen, Pfizer, Janssen, and Lifescan. C.T. (or the institution with which he is associated) has received grant support from Novo Nordisk and fees for educational/advisory activities from AstraZeneca/Bristol-Myers Squibb, Merck Sharp & Dohme, Novo Nordisk, and Johnson & Johnson. T.V. has received lecture fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, Novartis, Sanofi, and Zealand Pharma and is a member of advisory boards for Eli Lilly, Novo Nordisk, Merck Sharp & Dohme, Takeda, and Bristol-Myers Squibb/AstraZeneca. T.-M.P. is an employee of Novo Nordisk Health Care AG and owns stocks/shares in the company. H.S. has received honoraria from Novo Nordisk for his advice to the design of the study and data analysis.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. : Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–1596 [DOI] [PubMed] [Google Scholar]
- 2.Turnbull FM, Abraira C, Anderson RJ, et al. : Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52:2288–2298 [DOI] [PubMed] [Google Scholar]
- 3.Scirica BM, Bhatt DL, Braunwald E, et al. : Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–1326 [DOI] [PubMed] [Google Scholar]
- 4.White WB, Cannon CP, Heller SR, et al. : Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327–1335 [DOI] [PubMed] [Google Scholar]
- 5.Aujoulat I, Jacquemin P, Rietzschel E, et al. : Factors associated with clinical inertia: an integrative review. Adv Med Educ Pract 2014;5:141–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khunti K, Wolden ML, Thorsted BL, et al. : Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care 2013;36:3411–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kostev K, Rathmann W: Changes in time to insulin initiation in type 2 diabetes patients: a retrospective database analysis in Germany and UK (2005–2010). Prim Care Diabetes 2013;7:229–233 [DOI] [PubMed] [Google Scholar]
- 8.Grimshaw JM, Thomas RE, MacLennan G, et al. : Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess 2004;8(6):1–72 [DOI] [PubMed] [Google Scholar]
- 9.Burmester G, Lanas A, Biasucci L, et al. : The appropriate use of non-steroidal anti-inflammatory drugs in rheumatic disease opinions of a multidisciplinary European expert panel. Ann Rheum Dis 2011;70:818–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moro E, Allert N, Eleopra R, et al. : A decision tool to support appropriate referral for deep brain stimulation in Parkinson's disease. J Neurol 2009;256:83–88 [DOI] [PubMed] [Google Scholar]
- 11.Reinisch W, Chowers Y, Danese S, et al. : The management of iron deficiency in inflammatory bowel disease—an online tool developed by the RAND/UCLA appropriateness method. Aliment Pharmacol Ther 2013;38:1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brook RH, Chassin MR, Fink A, et al. : A method for the detailed assessment of the appropriateness of medical technologies. Int J Technol Assess Health Care 1986;2:53–63 [DOI] [PubMed] [Google Scholar]
- 13.Fitch K, Bernstein SJ, Aguilar MD, et al. : The RAND/UCLA Appropriateness Method. Santa Monica, CA: RAND, 2001 [Google Scholar]
- 14.Lawson EH, Gibbons MM, Ko CY, et al. : The appropriateness method has acceptable reliability and validity for assessing overuse and underuse of surgical procedures. J Clin Epidemiol 2012;65:1133–1143 [DOI] [PubMed] [Google Scholar]
- 15.de Pablos-Velasco P, Parhofer KG, Bradley C, et al. : Current level of glycaemic control and its associated factors in patients with type 2 diabetes across Europe: data from the PANORAMA study. Clin Endocrinol (Oxf) 2014;80:47–56 [DOI] [PubMed] [Google Scholar]
- 16.Yaffe K, Falvey CM, Hamilton N, et al. : Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA Intern Med 2013;173:1300–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonnell J, Stoevelaar HJ, Bosch JL, et al. : The appropriateness of treatment of benign prostatic hyperplasia: a comparison of Dutch and multinational criteria. Health Policy 2001;57:45–56 [DOI] [PubMed] [Google Scholar]
- 18.Quintana JM, Arostegui I, Azkarate J, et al. : Evaluation of explicit criteria for total hip joint replacement. J Clin Epidemiol 2000;53:1200–1208 [DOI] [PubMed] [Google Scholar]
- 19.Vader JP, Burnand B, Froehlich F, et al. : Appropriateness of upper gastrointestinal endoscopy: comparison of American and Swiss criteria. Int J Qual Health Care 1997;9:87–92 [PubMed] [Google Scholar]
- 20.Wilkinson MJ, Nathan AG, Huang ES: Personalized decision support in type 2 diabetes mellitus: current evidence and future directions. Curr Diab Rep 2013;13:205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cleveringa FG, Gorter KJ, van den-Donk M, et al. : Combined task delegation, computerized decision support, and feedback improve cardiovascular risk for type 2 diabetic patients: a cluster randomized trial in primary care. Diabetes Care 2008;8:2273–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodbard D, Vigersky RA: Design of a decision support system to help clinicians manage glycemia in patients with type 2 diabetes mellitus. J Diabetes Sci Technol 2011;5:402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vigersky RA, Galen RS, Horne D, et al. : Computer assisted decision support (CADS) for primary care of diabetes [abstract]. Telemed J E Health 2007;13(2):168 [Google Scholar]
- 24.Ceriello A, Gallo M, Candido R, et al. : Personalized therapy algorithms for type 2 diabetes: a phenotype-based approach. Pharmgenomics Pers Med 2014;7:129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cleveringa FG, Gorter KJ, van den Donk M, et al. : Computerized decision support systems in primary care for type 2 diabetes patients only improve patients' outcomes when combined with feedback on performance and case management: a systematic review. Diabetes Technol Ther 2013;15:180–192 [DOI] [PubMed] [Google Scholar]
- 26.Garg AX, Adhikari NK, McDonald H, et al. : Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA 2005;293:1223–1238 [DOI] [PubMed] [Google Scholar]
- 27.Purcell GP: What makes a good clinical decision support system. BMJ 2005;330:741–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawamoto K, Houlihan CA, Balas EA, et al. : Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ 2005;15:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sim I, Gorman P, Greenes RA, et al. : Clinical decision support systems for the practice of evidence-based medicine. J Am Med Inform Assoc 2001;8:527–534 [DOI] [PMC free article] [PubMed] [Google Scholar]