Abstract
Currently the use of non-autologous cell culture media (e.g., animal-derived or allogeneic serum) for clinical applications of mesenchymal stem cells (MSCs) is criticized by regulatory agencies. Autologous platelet-rich plasma (PRP) is proposed as a safer alternative medium supplement for adipose-derived mesenchymal stem cells (AT-MSC) culture. To study its efficiency on cell proliferation, AT-MSCs were cultured for 10 days in media supplemented with different concentrations of autologous non-activated PRP (nPRP) or thrombin-activated PRP (tPRP) (1–60%). AT-MSC proliferation, cell phenotype, multipotency capacity, and chromosome stability were assessed and compared to AT-MSCs expanded in a classical medium supplemented with 10% of fetal bovine serum (FBS). Culture media supplemented with nPRP showed dose-dependent higher AT-MSC proliferation than did FBS or tPRP. Twenty percent nPRP was the most effective concentration to promote cell proliferation. This condition increased 13.9 times greater AT-MSC number in comparison to culture with FBS, without changing the AT-MSC phenotype, differentiation capacity, and chromosome status. We concluded that 20% autologous nPRP is a safe, efficient, and cost-effective supplement for AT-MSC expansion. It should be considered as an alternative to FBS or other nonautologous blood derivatives. It could serve as a potent substitute for the validation of future clinical protocols as it respects good manufacturing practices and regulatory agencies' standards.
Introduction
Mesenchymal stem cells (MSCs) currently represent a promising cell source for regenerative medicine and tissue engineering strategies,1,2 in particular for bone, cartilage, and soft tissue regeneration.3–5 These multipotent cells principally have the ability to differentiate to mesodermal lineages such as adipocytes, osteocytes, and chondrocytes.6–10 Bone marrow has been used as the main source of MSCs for many years. Presently, an increasing interest is devoted to MSC isolated from adipose tissue (AT-MSC).11,12 This source presents several advantages in comparison to bone marrow: (i) adipose tissue is easier to harvest, (ii) it is widely available, and (iii) it contains higher MSC concentration.12,13
Ex vivo cell culture is mandatory for most clinical applications of MSCs. Cell expansion requires a basal medium supplemented with proteins, growth factors, and enzymes to support cell attachment and proliferation. Classical protocols use culture media supplemented with xenogeneic additives (e.g., fetal calf serum or fetal bovine serum [FBS]),14,15 which present a potential risk of infection and immunological reaction. To reduce these risks, efforts are devoted toward the development of human allogeneic supplements (e.g., human serum, human platelet derivatives).16–19 The use of these nonautologous culture protocols still presents at least three main limitations: (i) potential risks of contamination (e.g., virus, prion),20 (ii) immune reactions due to nonautologous proteins internalization by MSC,21–24 and (iii) the suboptimal rate of cell proliferation.25,26 Therefore, a safe and effective culture supplement is urgently needed to comply at best with national and international regulatory agencies' requirements for clinical applications of MSCs.
Platelets are a natural reservoir of growth factors, which are efficient in promoting cell proliferation, differentiation, and tissue regeneration. When platelets are physiologically activated, their α-granules gradually secrete growth factors and cytokines such as platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), transforming growth factor-β (TGFβ),27 vascular endothelial growth factor (VEGF), and endothelial growth factor (EGF).28 However, platelet activation by thrombin or Ca2+ provokes complete non-orchestrated release of growth factors within the first few hours only.29,30 Currently, plasma rich in platelets obtained from patient's own blood is already used efficiently for wound healing, bone regeneration, or skin rejuvenation.31–33 We thus postulate that autologous platelet-rich plasma (PRP) can serve as a safe and effective biological supplement, substituting current nonautologous products for cell expansion.
To define an autologous system for AT-MSC proliferation, we assessed the efficiency of autologous PRP on AT-MSC proliferation in comparison to the classical FBS-supplemented medium. We investigated the optimal PRP concentration and compared nonactivated PRP (nPRP), containing intact platelets, to thrombin-activated PRP (tPRP). Furthermore, we assessed the platelet viability over time in PRP. We postulated that live platelets, delivering continuous growth factors to the media, could eliminate the need for medium changes during up to 10 days of AT-MSC culture.
Materials and Methods
Adipose tissue harvesting and PRP preparation
For each experiment, adipose tissue and blood were collected from the same patient who underwent abdominoplasty. All experiments were done in accordance with the established ethical standards, local ethics committee agreement, and patient consent.
Adipose tissue harvesting
Fat tissue was collected and purified from the subcutaneous abdomen layer of patients according to the Coleman technique as previously described.34 Briefly, 20 mL of fat tissue was harvested manually from each patient with a 3 mm cannula (Mentor, Santa Barbara, CA) connected to 10 mL Luer-Lok™ syringes (BD Biosciences, Franklin Lakes, NJ). The pure fat tissue was separated from blood, oil, and liquid after 3 min centrifugation at 3200 rpm at 1200G.
PRP and autologous thrombin preparation
For PRP preparation, specific tubes containing sodium citrate as anticoagulant and a specific gel separating platelets and plasma from other blood components (e.g., red and white blood cells) were used. Briefly, 8 mL of human peripheral blood was collected into a Regen-BCT tube (RegenKit®; RegenLab, Le Mont-sur-Lausanne, Switzerland). The collected blood was centrifuged for 5 min in a standard laboratory centrifuge at 1500G. Subsequently, the white and red blood cells accumulated in the bottom of the tube under the separator gel, whereas the plasma and platelets remained above the gel layer. Plasma containing platelets was homogenized by returning the tube five times to obtain 4 mL of nPRP, which was collected in a polypropylene tube (Becton-Dickinson, Franklin Lakes, NJ) until use. Platelets, red and white blood cells in whole blood were counted (KX-21N; Sysmex, Lincolnshire, IL) before centrifugation and in the prepared nPRP before addition to culture media.
For tPRP experiments, autologous thrombin was obtained with an ATS tube, a similar tube to that used for PRP preparation, but without the added anticoagulant. Briefly, 8 mL of blood was collected into the Regen-ATS tubes (RegenKit, RegenLab) and centrifuged for 10 min at 1500G. Similarly to the PRP preparation, red and most of the white blood cells were sequestrated below the separating gel, whereas the plasma over the gel formed a clot due to the lack of anticoagulant. The serum extracted from the clot, rich in thrombin, was added 1:10 to PRP to activate the platelets and obtain tPRP.
AT-MSC isolation
Pure fat was digested with 0.01% collagenase type I (Sigma-Aldrich, St. Louis, MO) for 45 min at 37°C with gentle agitation. The nondigested adipose tissue was removed after centrifugation at 1400 rpm for 10 min. The remaining pellet, called the stromal vascular fraction (SVF), was suspended in erythrocyte lysis buffer for 5 min (Qiagen, Hilden, Germany). It was then washed with the basal medium: Dulbecco's modified Eagle's medium (DMEM)-low glucose containing 1 g/L glucose, l-glutamine, 25 mM HEPES (Invitrogen, Carlsbad, CA), supplemented with penicillin and streptomycin 10,000 μg/mL (Bioconcept, Salem, NH), and 2 units/mL heparin (Liquemin 5000; Roche, Basel, Switzerland). After centrifugation at 1200 rpm for 5 min, SVF was then resuspended in DMEM and supplements, and filtered through a 100 μm nylon cell strainer (BD Biosciences). The mean cell density in the isolated SVF was 30×104 cells/mL.
AT-MSC culture
SVF cells were plated at 5000 cell/cm2 in a 48-well plate (BD Biosciences) and cultured in different media culture conditions: 10% FBS (Gibco, Carlsbad, CA) as control or 1%, 5%, 10%, 20%, 40%, and 60% of either nPRP or tPRP added to the basal DMEM and supplements (1 mL medium for each condition). The resulting plastic-adherent cell population after 24–48 h of culture was determined as AT-MSCs. Cells were cultivated at 37°C for 10 days in a standard incubator with 5% CO2 without changing the culture media for FBS and PRP conditions.
AT-MSC viability and proliferation
Cell viability and number was assessed after 10 days of culture using two different techniques, hematocytometer (Marienfeld, Emmendingen, Germany) and an image-based cytometer (Tali; Invitrogen). After dissociation with trypsin (trypsin-EDTA [1×] 0.05%; Invitrogen), cell viability was determined for each condition by Trypan blue exclusion (Sigma-Aldrich) or Propidium Iodide staining and fluorescence analysis (R&D Systems, Minneapolis, MN), respectively. The doubling time for the AT-MSC population in 10 days (240 h) was quantified for each patient.
To assess cell proliferation, an EdU cell proliferation assay (Click-iT EdU; Invitrogen) was performed.35 Briefly, 5000 cells at P1 were cultured per condition for 24 h and then incubated with EdU solution for 24 h. Thereafter, cells were fixed with 10% formalin, stained according to the manufacturer's instructions and scored for EdU-labeled proliferating cells. EdU dye integrates with newly formed DNA strands. The EdU assay thus provides the proliferation ratio expressed as a percentage: the number of EdU bright cells divided by the number of Hoechst bright cells (total number of cells)×100.
Cell phenotype assessment by flow cytometry
To confirm the bona fide MSC phenotype in 20% nPRP versus 10% FBS cultures after 10 days, immunophenotypic analysis was performed by flow cytometry, according to whether they show positive for CD73, CD105, CD90, and absence of CD45, CD19, and HLA-DR. Briefly, cells were trypsinized, resuspended in phosphate-buffered saline (PBS) containing 1% FBS, and marked with mouse anti-human antibodies: CD45-FITC (Diatec, Oslo, Norway), CD73-PE, CD90-Cy5, HLA-DR-FITC (Immunotech-Coulter, Marseille, France), CD105-FITC (ABD Serotec, Kidlington, United Kingdom), CD19-FITC (Life Technologies, Carlsbad, CA). Cell viability was quantified by 7-Aminoactinomycin D (Sigma-Aldrich). Mouse isotype antibodies (Beckman Coulter, Brea, CA) were used as a control. Ten thousand labeled cells were analyzed by a FACS Calibur flow cytometer using the CellQuest software (Becton-Dickinson).
AT-MSC differentiation analysis
To assess the influence of nPRP on conserving the multipotentiality of AT-MSCs, we induced adipogenic, osteogenic, and chondrogenic differentiation on P3. Cells were cultured on P0–P2 with either 20% nPRP or 10% FBS containing medium, and passed at 80% of confluency. For the third passage, a fresh nPRP was prepared from the same blood donor as initially used. Adipogenic, osteogenic, or chondrogenic differentiation were achieved by adding specific differentiation media: Stempro® Adipogenesis, Osteogenesis, or Chondrogenesis Differentiation Kits (Life Technologies), at the third passage and the medium was changed every 3 days.
Adipocytes were marked by Red oil O (Sigma-Aldrich) staining lipid droplets after 2 weeks of culture. Osteocytes were stained by Alizarin Red (Sigma-Aldrich), which marks calcium deposits after 3 weeks of culture, whereas chondrocytes were stained by Alcan Blue (Sigma-Aldrich), which reveals the presence of acid mucopolysaccharides and glycosaminoglycan after 2 weeks of culture.
Chromosome stability assessment and cytogenetic analysis
The stability of the chromosomes in 20% nPRP cultured cells was compared to 10% FBS cultured cells. The samples were prepared on the first passage from a 60% to 80% confluent culture flask of 25 cm2. Colcemid (Invitrogen) treatment was done for 15 h with concentration of 0.05 μg/mL as described previously.36 The colcemid was removed and the cultures were trypsinized with trypsin-EDTA (Invitrogen) to recover the cells. The cells were treated with 0.075 mM hypotonic lysis solution (KCl) at 37°C for 20 min and fixed in methanol:acetic acid solution (3:1, v/v). The metaphase spreads were analyzed after a G-banding (GTG) as previously described37 with an average resolution of 350 bph.
Platelet viability analysis
Platelet viability was assessed by Calcein/AM (Biotium, Inc., Hayward, CA), which marks viable platelets by producing a green fluorescent signal. Time course platelet viability (day 0, 4, 7, and 10) was established in the basal DMEM medium and supplements with 20% nPRP with and without AT-MSC culture at 37°C in the incubator. Briefly, at each time point 50 μL of 1 μM Calcein/AM in PBS was added to PRP followed by incubation for 30 min at 37°C. The fluorescence was measured on a fluorescence plate reader at 485 nm excitation and 530 nm emission wavelengths. Platelet viability in each time point was normalized by the platelet viability on day 0.
Statistical analysis
The Mann–Whitney U test was used for comparison between groups, with values of p<0.05 (*) being regarded as significant. Data are presented as mean±SEM.
Results
Platelet and blood cells counting
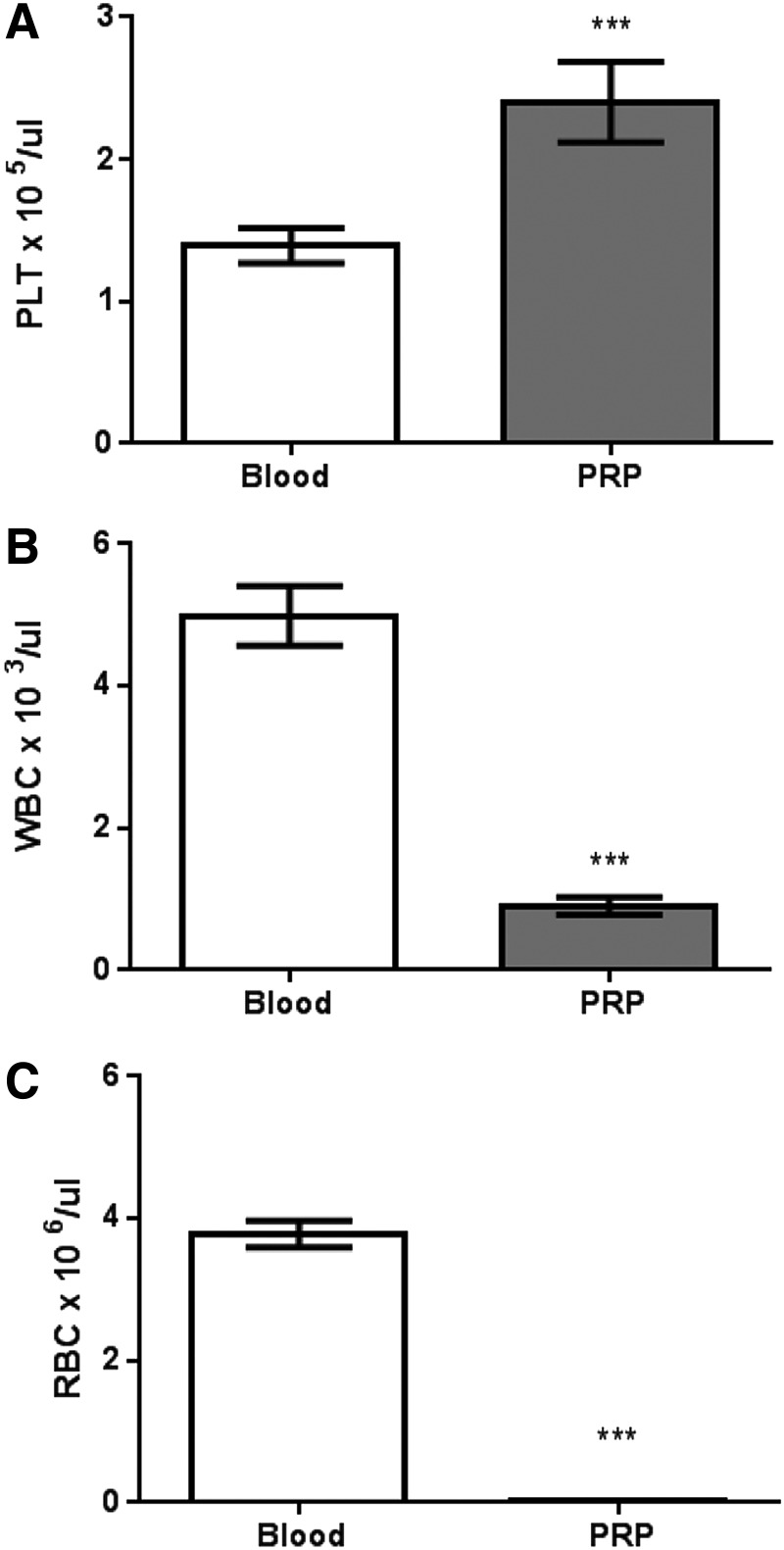
The platelet recovery rate in nPRP from the whole blood was 98%. Mean platelet concentration stuck over the separator gel (buffy coat) was 1.12×106±120.6 platelets/μL. As for these experiments, the platelets were resuspended in all 4 mL of plasma over the gel, the final mean platelet concentration of nPRP used in this study was 2.41×105±20.36 platelets/μL. This concentration is 1.7 times more than the whole blood before centrifugation (1.44×105±9.10 platelets/μL) (Fig. 1). Conversely, the mean white blood cell concentration was significantly lower in nPRP in comparison to whole blood (0.72×103±0.11 cells/μL vs. 4.87×103±0.46 cells/μL); the mean red blood cell concentration was similarly reduced (0.03×106±0.004 cells/μL vs. 3.86×106±0.21 cells/μL) (n=14).
FIG. 1.
Analysis of number of platelets (A), white blood cells (B), and red blood cells (C) in whole blood compared to purified platelet-rich plasma (PRP). n=14 patients (***p<0.001).
PRP enhances AT-MSC proliferation dose dependently
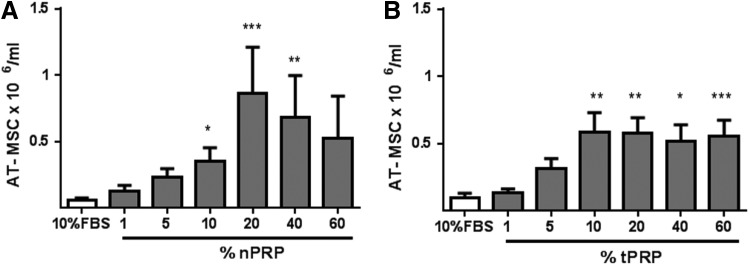
In all conditions, AT-MSCs kept their typical spindle fibroblast shape during the culture period. After 10 days culture, all media supplemented with different nPRP concentrations presented a higher AT-MSC number when compared to FBS-containing media (Fig. 2). This positive effect of nPRP followed a dose-dependent bell-shape curve. Media supplemented with 20% nPRP offered the optimal condition, with AT-MSC number being 13.9 times higher than in 10% FBS (n=14, p<0.001) after 10 days of culture. In comparison, other conditions were less effective [e.g., 10% and 40% nPRP media increased respectively 5.6 and 10.9 times the AT-MSC number when compared to 10% FBS (n=14, p<0.001)] (Fig. 3A).
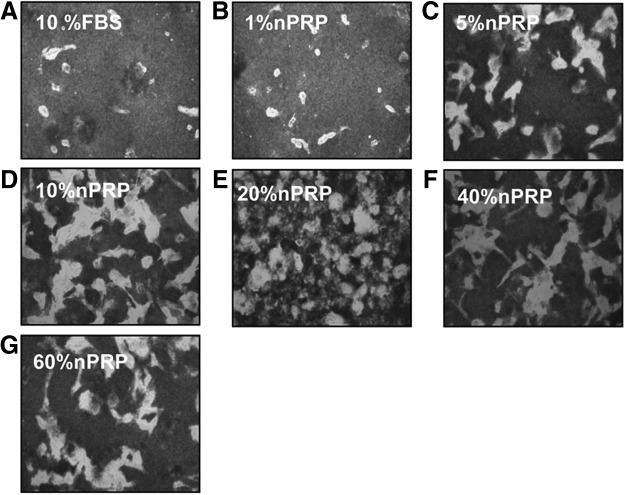
FIG. 2.
Bright-field micrographs of adipose-derived mesenchymal stem cell (AT-MSC). In the presence of 10% fetal bovine serum (FBS) (A), and 1–60% nonactivated PRP (nPRP) (B–G) after 10 days culture (P0). Magnification 20×. Pictures are representatives of one donor. High density of platelets produces a darker background.
FIG. 3.
Effect of different concentrations of nPRP (A, n=14) or thrombin-activated PRP (tPRP) (B, n=6) on the proliferation of AT-MSC cultured over 10 days without medium change. Ten percent FBS is used as control. *p<0.05; **p<0.01 and ***p<0.001.
The tPRP-containing media showed also a higher AT-MSC number in comparison to media with FBS. Compared to 10% FBS, the AT-MSC number was 5.9, 5.8, 5.2, and 5.6 times higher in 10%, 20%, 40%, and 60% tPRP (n=6, p<0.05), respectively (Fig. 3B). However, all tPRP conditions were consistently less effective than 20% nPRP.
In comparison with nPRP media, where a concentration of 20% presented the best and reproducible condition for all tested patient cells (n=14), tPRP-treated AT-MSC showed inconsistent results over six patients: three patients had the best AT-MSC proliferation rate with 40% tPRP, whereas two other patients had the highest AT-MSC number with 10% tPRP and one patient with 60% tPRP (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tec).
In summary, over 10 days culture, 20% nPRP and 10% tPRP produced the highest stimulatory effect on AT-MSC, causing them to multiply 230- and 155-fold versus 16.5-fold expansion in 10% FBS, respectively.
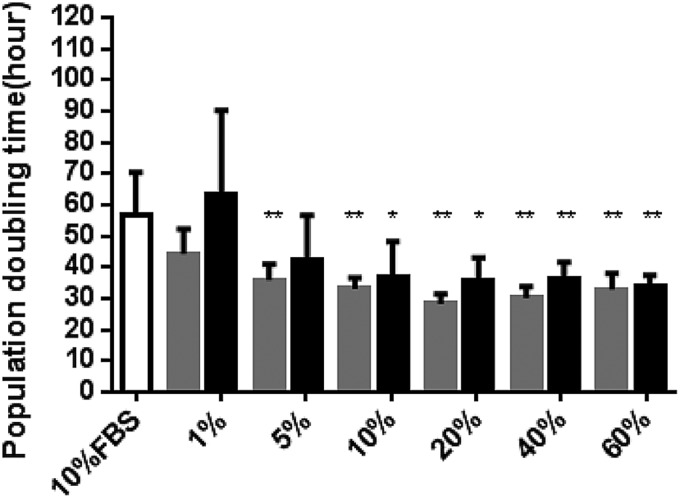
The EdU assay over 24 h demonstrated that 20% nPRP induces a higher proliferation rate of AT-MSCs (68%) in comparison to 10% FBS (54%) and other nPRP concentrations (Fig. 4). The population doubling time was significantly shorter for 20% nPRP, in comparison to other conditions (Fig. 5). AT-MSCs doubled their population every 28 h in 20% nPRP condition: twice as fast than with 10% FBS media condition (56 h, n=6, p<0.05) (Fig. 5).
FIG. 4.
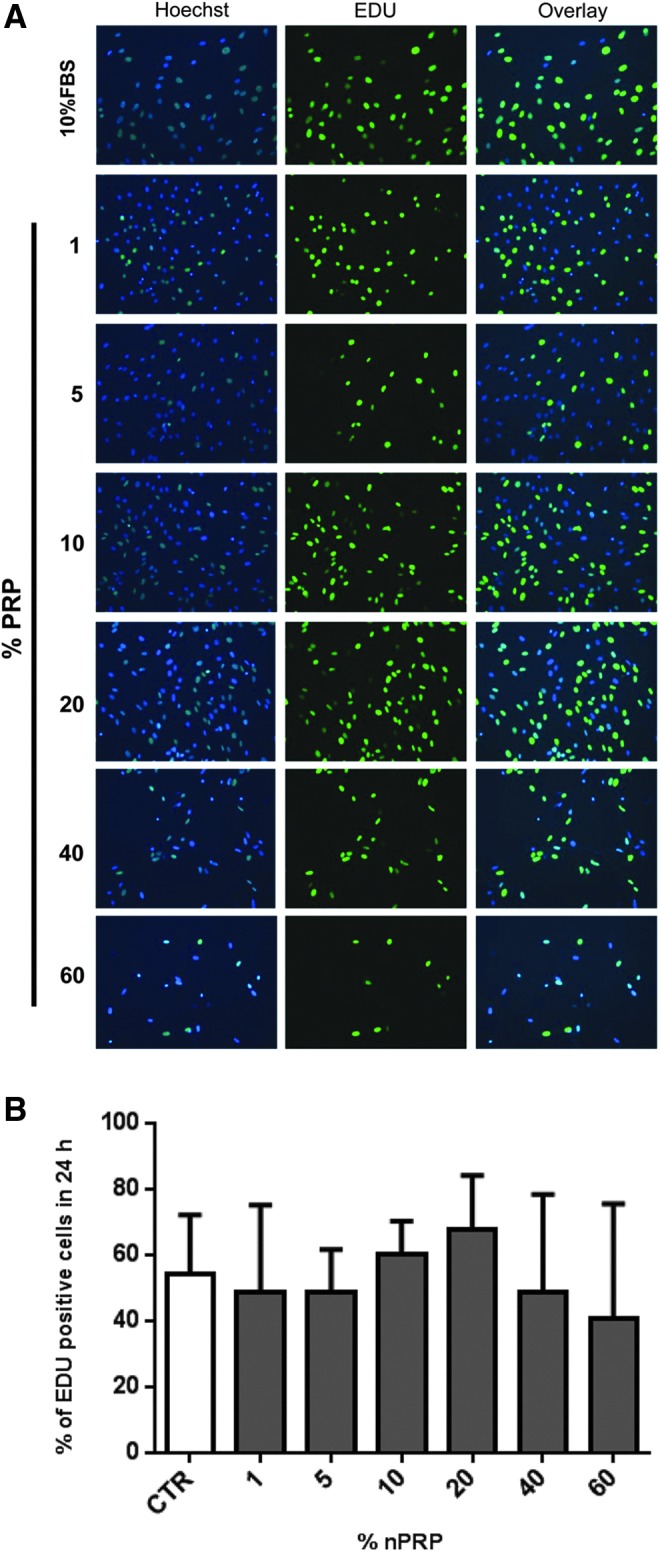
Assessment of 24 h-proliferation efficiency of AT-MSCs by EdU staining. Different concentrations of nPRP (1–60%) were compared to 10% FBS on AT-MSCs (P1) at the end of 10 days culture period. (A) Active proliferating cells are revealed by EdU-based green fluorescence nucleus staining, compared to total cells stained by Hoechst dye (blue nucleus staining). (B) Percentage of EdU positive cells was estimated by the formula: (green bright AT-MSC nucleus/total blue AT-MSC nucleus)×100. n=4.
FIG. 5.
Population doubling time in AT-MSC. Cells were cultured in media supplemented with 10% FBS as control (white bar), and increasing concentrations of nPRP (gray bars) or tPRP (black bars). *p<0.05 and **p<0.01 (n=6).
Cell characterization by surface marker expression
To confirm the stability of AT-MSC characteristics after 10 days culture, the typical surface marker expression of AT-MSCs cultured with nPRP or FBS was studied. Regardless of the supplement used in the medium, the AT-MSC marker profile remained unchanged: cells were positive for CD90 (82.5% of cells in FBS condition vs. 91.3% in nPRP), CD105 (82.5% vs. 91.3%), and CD73 (91.3% vs. 95.1%), whereas they were negative for CD45 (0.103% vs. 0.058%), CD19 (0.027% vs. 0.096%), and HLA-DR (1.35% vs. 0.81%) (Fig. 6).
FIG. 6.
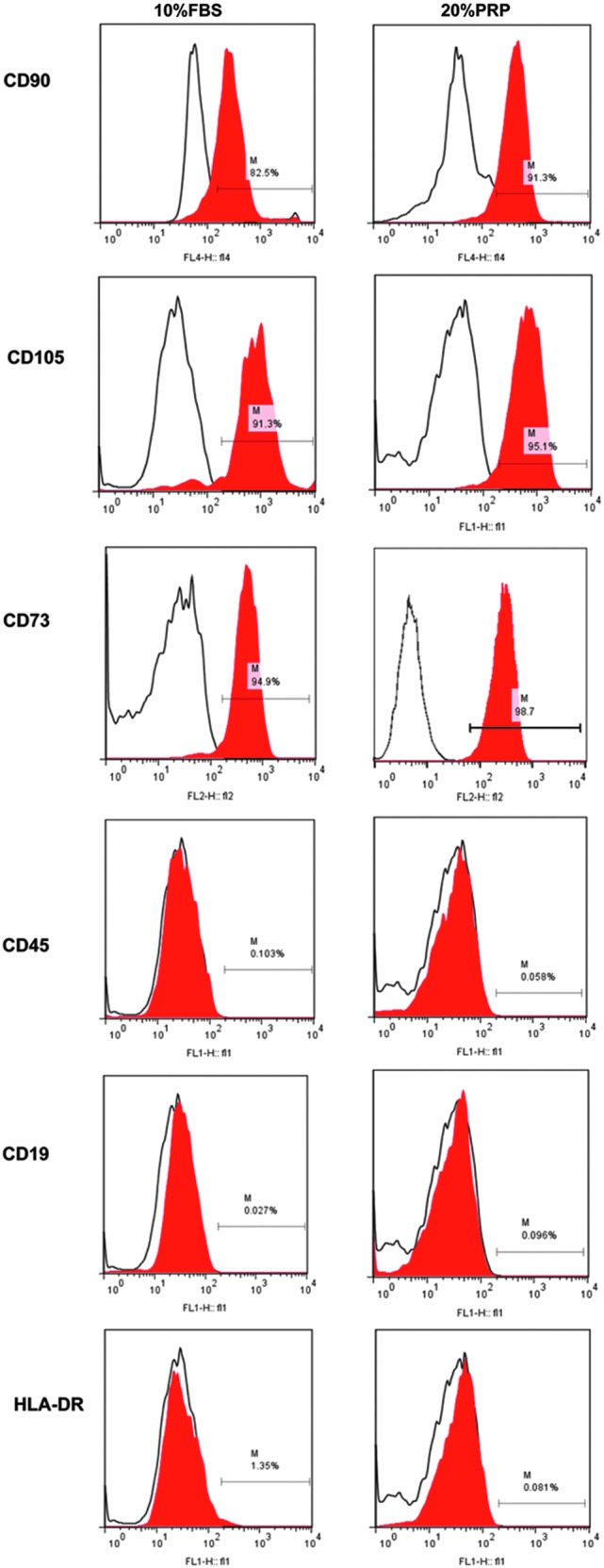
Analysis of surface marker expression. Phenotype of AT-MSCs cultured in 10% FBS or 20% nPRP was assessed by flow cytometry at passage 0. Isotype control antibody is shown by the black empty lines whereas the expressed markers are in red. “M bars” indicate the percentage of cells expressing the surface marker.
Differentiation capacity
We verified that the intrinsic differentiation potential of AT-MSCs into adipocytes, osteocytes, or chondrocytes was kept intact regardless of the supplements used for their proliferation. Indeed, we observed that nPRP did not adversely affect the differentiation capacity of AT-MSCs tested at passage 3. Adipogenic (Fig. 7A, B), chondrogenic (Fig. 7C, D), and osteogenic differentiation (Fig. 7E, F) were confirmed and were qualitatively comparable to 10% FBS.
FIG. 7.
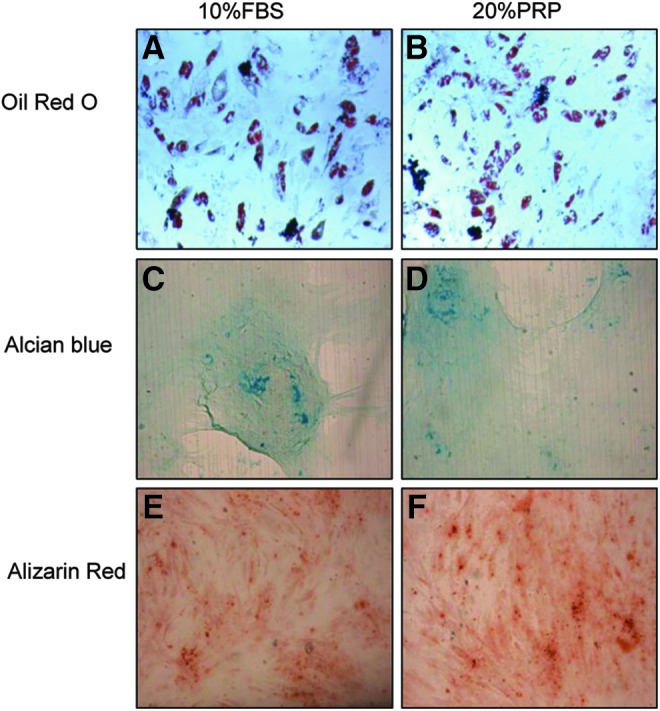
Representative qualitative evaluation of AT-MSC differentiation capacity. Differentiation toward adipocyte (A, B), chondrocyte (C, D), or (E, F) osteocyte phenotype after culture in 10% FBS or 20% nPRP.
Karyotyping analysis
Cytogenetic analysis of cells cultured in either 10% FBS or 20% PRP conditions, did not show any abnormal karyotype. FBS and nPRP culture conditions had numerical and structural stability. Thus, treating cells with nPRP do not modify the chromosomal stability (Fig. 8).
FIG. 8.
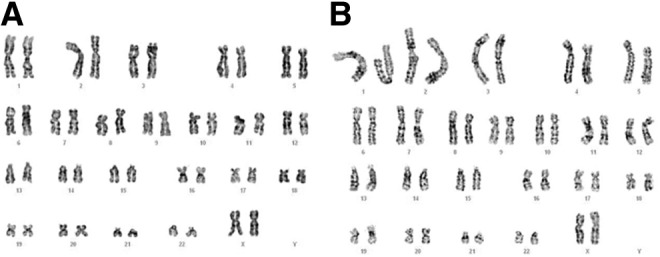
Representative chromosome karyotype by G-banding. (A) 10% FBS and (B) 20% nPRP cultured MSCs were compared for chromosome aberration.
Platelet viability
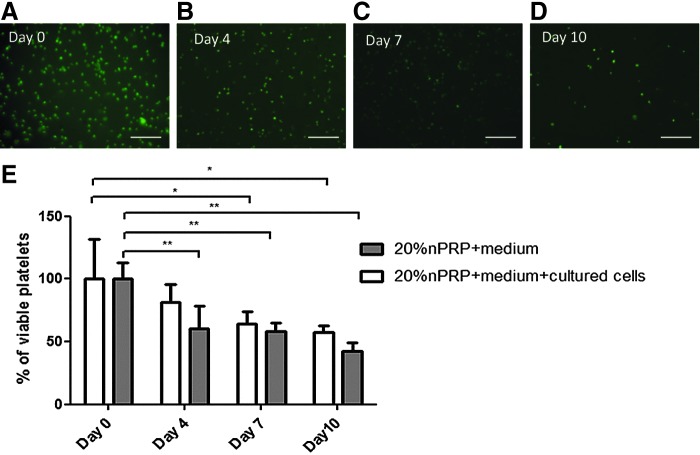
We then measured the time-dependent platelet survival rate in 20% nPRP-supplemented medium in the presence or absence of AT-MSCs. On day 4 and in the presence of AT-MSCs, more than 80% of platelets were viable when normalized to day 0. This percentage decreased to 64% platelet viability on day 7 and remained as high as 57% on day 10 (Fig. 9). The survival rate in the absence of AT-MSCs was instead lower: 60%, 58%, and 43% on days 4, 7, and 10, respectively.
FIG. 9.
Assessment of platelet viability by Fluorescence microscopy using calcein dye. (A–D) Fluorescence micrograph of viable, calcein-stained platelets monitored over a 10 days period in media containing 20% nPRP in contact with AT-MSC without medium change (magnification: 40×). (E) Mean viable platelet in the presence or absence of AT-MSC. Percentage of platelet viability in 20% nPRP was normalized to day 0 (n=5; *p<0.05, **p<0.01).
Discussion
The culture protocols for clinical application of AT-MSCs have to comply with good manufacturing practices (GMP) and regulatory agencies' standards. All steps including manufacturing, sampling, testing, storage, packaging, and distribution need to be standardized, safe, and efficient.38,39 In vitro cell expansion is one of the key steps of manufacturing. However, so far, none of the available protocols completely satisfy these requirements. Currently FBS, a complex mixture of proteins and nutrients, is typically used to isolate and expand MSCs. This xenogeneic media supplement is regarded critically by regulatory agencies because of its high risk of contamination (e.g., virus positivity reported to be as high as 20–50%) and its allergic reactions due to xenogeneic proteins internalization by MSCs.40 Obviously, all these disadvantages can compromise the therapeutic success.41
Several researchers have made efforts in substituting xenogeneic MSC culture media by human blood-derived products. As reviewed recently by Bieback, a range of human products have been tested: plasma, serum, umbilical cord blood serum, and platelet, derived from either a blood bank or fresh blood, but rarely autologous.41 Bieback et al. demonstrated that pooled human platelet lysate derived from several patients could be a substitute for FBS in bone marrow-derived MSC expansion.25 Kocaoemer et al. claimed that 10% nonautologous PRP activated by shock freezing or thrombin increased the AT-MSC proliferation after 11 days culture.26 Instead of directly adding platelets, others reported that the supernatant of activated PRP could increase MSC proliferation rate over other medium supplements.25,26,42 Others concluded that the addition of growth factors per se (e.g., PDGF, TGF, EGF) can greatly increase the cell migration and proliferation rate.43–45 However, as all of these studies applied nonautologous products, the potential risk of contamination and immunologic reactions remains.
Therefore, considerable efforts are presently devoted to defining an ideal medium which can substitute the current nonautologous systems. Recently, a few studies have suggested that media supplemented with autologous PRP could be used for AT-MSC expansion.42,46 In these studies, only the supernatant of PRP was added to the cell culture, and the media were free of platelets. Li et al., demonstrated that the frozen supernatant of autologous PRP activated with bovine thrombin had a potent effect on AT-MSC proliferation and neurogenic differentiation.46 Kakudo et al. compared the effect of different media supplemented with frozen supernatant obtained from whole blood, nonactivated or thrombin–calcium-activated autologous PRP on AT-MSC proliferation as well. They concluded that the media with 5% of activated-PRP supernatant offers the best effect on AT-MSC proliferation by increasing cell numbers of five-folds within 7 days of culture.42
In conformity with regulatory agencies and GMP standards, culture media should be, amongst other criteria, free from contamination risk,47 nonimmunogenic,48 nononcogenic, and effective in increasing cell proliferation rate.18,39 Importantly, it should maintain the MSC phenotype unmodified and retain its differentiation capacity over an extended time period.
According to these needs, our study demonstrates that nPRP can be used as an autologous biological medium supplement for AT-MSC proliferation in vitro. It could efficiently substitute current nonautologous products. In comparison with FBS used traditionally for cell culture, our data demonstrated that a media supplemented with 20% of autologous nPRP can significantly promote AT-MSC expansion, without affecting AT-MSC phenotype and multipotency.
In addition, to our knowledge, our study demonstrates for the first time the advantage of AT-MSC coculture with platelets, where the culture media is directly supplemented with autologous functional fresh and nonactivated platelets, instead of their supernatant.
Our data show that nPRP has a dose-dependent effect on AT-MSC proliferation. Media supplemented with 20% nPRP (2.41×105±20.36 platelets/μL) was the optimal condition for each patient, where AT-MSCs grew up to 230-fold within 10 days culture, significantly higher than with FBS (i.e., 16.5-fold) or other supplements. Furthermore, unlike other studies using chemically activated PRP with calcium or thrombin,26,42 our study reveals nPRP as more efficient than the nonphysiologically activated PRP such as tPRP.
We were also able to demonstrate that a majority of platelets remained viable after 10 days of culture, particularly in the presence of AT-MSCs. Therefore, it circumvents the need for a medium change every 3 days as required in classical cell culture protocols using FBS or PRP supernatant. So, as nPRP application decreases the need of medium replacement up to 10 days, we postulate that it would enable a safer system, thereby reducing risks inherent to cell manipulation, and also providing a more cost-effective and manpower-saving operational method.
We hypothesize that nonactivated platelets present in nPRP get gradually activated and secrete orchestrated growth factors up to 10 days. This could closely mimic the physiological activity of platelets in blood stream, where they gradually release their growth factors during their 10 days lifespan. This avoids high burst growth factors release at culture start49 and ensures the activity of secreted growth factors during longer time periods.50
As growth factors are essential for MSC proliferation and differentiation, a precise dose combination and time delivery are critical for optimal results.51,52 For instance, high growth factor concentrations may lead to unwanted differentiated cell phenotypes,28 as well as downregulation of surface receptors rendering cells insensible to such factors. This could explain why the highest nPRP concentrations (e.g., 40% and 60%), by containing excessive growth factor amounts, were less effective than 20% nPRP. Several studies have also proved that applying excessive platelet concentrations may bring paradoxical inhibitory effects.42,53–56 Graziani et al. concluded that the PRP containing a platelet concentration 2.5×higher than the whole blood had the maximum effect on fibroblasts and osteoblasts proliferation; whereas higher concentrations would decrease the proliferation effect.53 Weibrich et al. claimed that a concentration of 106 platelets/μL stimulate bone regeneration, whereas higher concentrations might reveal inhibitory effects.55 Similarly, we can speculate that physiological and controlled growth factor release by live platelets in nPRP may be more effective than tPRP, where a burst of growth factors are released at start. In line with this, Scherer et al. also observed that nPRP was more efficient than tPRP in wound healing.56
Our study demonstrates that nPRP did not change AT-MSCs marker phenotype and multipotency, important criteria for an ideal expansion medium. Unlike other studies claiming that platelet lysate or thrombin-activated platelets may change the differentiation capacity of MSCs,44,57 we proved, in this study, that AT-MSC differentiation capacity toward adipogenic, osteogenic, and chondrogenic lineages was not reduced in 20% nPRP, in comparison to FBS.
Furthermore, unlike several studies reporting some concern about genetic stability throughout cell expansion58–61 and the investigations that report the chromosomal aberration due to culture contamination,62,63 we demonstrated that media supplemented with nPRP do not modify the chromosome stability of AT-MSCs. This is a biosafety criterion that is mandatory for MSC's clinical administration and required by regulatory agencies.
Obviously, for the clinical application of PRP, there is still a lack of standardization and agreement on the efficiency of PRP and its method of preparation and application.64 Each autologous PRP could have different characteristics (e.g., platelet number and growth factor concentrations, platelet survival rate), thus achieving variable results. This variability is due to variations between individual donors (e.g., age, thrombocythemia) and to different PRP preparation techniques available on the market (e.g., device type, centrifugation number); therefore, efforts have to be made to standardize PRP products.
Clearly, further investigations are required to address the signaling pathways involved in PRP-dependent AT-MSCs proliferation and differentiation.
Conclusions
In this study, we demonstrate for the first time that, when compared to activated PRP or a xenogeneic supplement like FBS, autologous nPRP is a safe and efficient biological culture supplement capable of promoting AT-MSC proliferation while maintaining their phenotype, differentiation potential, and chromosome stability. The use of fresh autologous nPRP easily complies with regulatory agencies' standards for clinical protocols.
Supplementary Material
Acknowledgments
The authors gratefully thank Dr. Vincent Jaquet for his help with Calcein assay and Dr. Frederique Bena for performing karyotyping experiments. The authors also thank Dr. Veronique Serre Benier for her help with EdU assay experiments and Dr. Genta Plasari and Mrs. Solange Vischer for their kind technical support. This study was supported by the Swiss National Science Foundation (Grant #310030-120751).
Disclosure Statement
The Regen BCT and Regen ATS tubes were generously provided by Regen Lab.
References
- 1.Braghirolli D.I., Zamboni F., Chagastelles P.C., Moura D.J., Saffi J., Henriques J.A., et al. . Bio-electrospraying of human mesenchymal stem cells: An alternative for tissue engineering. Biomicrofluidics 7,44130, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahman M.M., Subramani J., Ghosh M., Denninger J.K., Takeda K., Fong G.H., et al. . CD13 promotes mesenchymal stem cell-mediated regeneration of ischemic muscle. Front Physiol 4,402, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zomorodian E., and Baghaban Eslaminejad M.Mesenchymal stem cells as a potent cell source for bone regeneration. Stem Cells Int 2012,980353, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Pham P., Bui K.H., Ngo D.Q., Vu N.B., Truong N.H., Phan N.L., et al. . Activated platelet-rich plasma improves adipose-derived stem cell transplantation efficiency in injured articular cartilage. Stem Cell Res Ther 4,91, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Y., Yan Z., Zhang H., Lu W., Liu S., Huang X., et al. . Expansion and delivery of adipose-derived mesenchymal stem cells on three microcarriers for soft tissue regeneration. Tissue Eng Part A 17,2981, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Schaffler A., and Buchler C.Concise review: adipose tissue-derived stromal cells—basic and clinical implications for novel cell-based therapies. Stem Cells 25,818, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Krause D.S.Plasticity of marrow-derived stem cells. Gene Ther 9,754, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Tuan R.S., Boland G., and Tuli R.Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther 5,32, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Haan G., and Van Zant G.Stem cells from birth to death: the history and the future. J Am Aging Assoc 25,79, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romanov Y.A., Darevskaya A.N., Merzlikina N.V., and Buravkova L.B.Mesenchymal stem cells from human bone marrow and adipose tissue: isolation, characterization, and differentiation potentialities. Bull Exp Biol Med 140,138, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Strem B.M., and Hedrick M.H.The growing importance of fat in regenerative medicine. Trends Biotechnol 23,64, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H., et al. . Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13,4279, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser J.K., Wulur I., Alfonso Z., and Hedrick M.H.Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol 24,150, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Ghorbani A., Jalali S.A., and Varedi M.Isolation of adipose tissue mesenchymal stem cells without tissue destruction: a non-enzymatic method. Tissue Cell 46,54, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Geissler S., Textor M., Schmidt-Bleek K., Klein O., Thiele M., Ellinghaus A., et al. . In serum veritas-in serum sanitas? Cell non-autonomous aging compromises differentiation and survival of mesenchymal stromal cells via the oxidative stress pathway. Cell Death Dis 4,e970, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phadnis S.M., Joglekar M.V., Venkateshan V., Ghaskadbi S.M., Hardikar A.A., and Bhonde R.R.Human umbilical cord blood serum promotes growth, proliferation, as well as differentiation of human bone marrow-derived progenitor cells. In Vitro Cell Dev Biol Anim 42,283, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Schallmoser K., Bartmann C., Rohde E., Reinisch A., Kashofer K., Stadelmeyer E., et al. . Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion 47,1436, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Stute N., Holtz K., Bubenheim M., Lange C., Blake F., and Zander A.R.Autologous serum for isolation and expansion of human mesenchymal stem cells for clinical use. Exp Hematol 32,1212, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Sun X., Gan Y., Tang T., Zhang X., and Dai K.In vitro proliferation and differentiation of human mesenchymal stem cells cultured in autologous plasma derived from bone marrow. Tissue Eng Part A 14,391, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Medicinal and other products and human and animal transmissible spongiform encephalopathies: memorandum from a WHO meeting. Bull World Health Organ 75,505, 1997 [PMC free article] [PubMed] [Google Scholar]
- 21.Gregory C.A., Reyes E., Whitney M.J., and Spees J.L.Enhanced engraftment of mesenchymal stem cells in a cutaneous wound model by culture in allogenic species-specific serum and administration in fibrin constructs. Stem Cells 24,2232, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Selvaggi T.A., Walker R.E., and Fleisher T.A.Development of antibodies to fetal calf serum with arthus-like reactions in human immunodeficiency virus-infected patients given syngeneic lymphocyte infusions. Blood 89,776, 1997 [PubMed] [Google Scholar]
- 23.Drach G., Maret A., Richard M.F., and Barbu E.[Transfer and induction of delayed hypersensitivity to methylated bovine serum albumin in the absence of adjuvant]. C R Acad Sci Hebd Seances Acad Sci D 284,2435, 1977 [PubMed] [Google Scholar]
- 24.Tuschong L., Soenen S.L., Blaese R.M., Candotti F., and Muul L.M.Immune response to fetal calf serum by two adenosine deaminase-deficient patients after T cell gene therapy. Hum Gene Ther 13,1605, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Bieback K., Hecker A., Kocaomer A., Lannert H., Schallmoser K., Strunk D., et al. . Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells 27,2331, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Kocaoemer A., Kern S., Kluter H., and Bieback K.Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem cells 25,1270, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Dahlin R.L., Ni M., Meretoja V.V., Kasper F.K., and Mikos A.G.TGF-beta3-induced chondrogenesis in co-cultures of chondrocytes and mesenchymal stem cells on biodegradable scaffolds. Biomaterials 35,123, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng F., Boucher S., Koh S., Sastry K.S., Chase L., Lakshmipathy U., et al. . PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood 112,295, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Roussy Y., Bertrand Duchesne M.P., and Gagnon G.Activation of human platelet-rich plasmas: effect on growth factors release, cell division and in vivo bone formation. Clin Oral Implants Res 18,639, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Su C.Y., Kuo Y.P., Nieh H.L., Tseng Y.H., and Burnouf T.Quantitative assessment of the kinetics of growth factors release from platelet gel. Transfusion 48,2414, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Cervelli V., Palla L., Pascali M., De Angelis B., Curcio B.C., and Gentile P.Autologous platelet-rich plasma mixed with purified fat graft in aesthetic plastic surgery. Aesthetic Plast Surg 33,716, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Daif E.T.Effect of autologous platelet-rich plasma on bone regeneration in mandibular fractures. Dent Traumatol 29,399, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Zapata M.J., Marti-Carvajal A.J., Sola I., Exposito J.A., Bolibar I., Rodriguez L., et al. . Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev 10,CD006899, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Coleman S.R.Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg 19,421, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Niclauss N., Bosco D., Morel P., Giovannoni L., Berney T., and Parnaud G.Rapamycin impairs proliferation of transplanted islet beta cells. Transplantation 91,714, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Muntion S., Sanchez-Guijo F.M., Carrancio S., Villaron E., Lopez O., Diez-Campelo M., et al. . Optimisation of mesenchymal stromal cells karyotyping analysis: implications for clinical use. Transfus Med 22,122, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Garcia J.L., Hernandez J.M., Gutierrez N.C., Fernandez P., and Rios A.[Cytogenetics in the study of malignant blood diseases]. Sangre (Barc) 41,289, 1996 [PubMed] [Google Scholar]
- 38.Sensebe L., and Bourin P.Producing MSC according GMP: process and controls. Biomed Mater Eng 18,173, 2008 [PubMed] [Google Scholar]
- 39.Dos Santos F., Campbell A., Fernandes-Platzgummer A., Andrade P.Z., Gimble J.M., Wen Y., et al. . A xenogeneic-free bioreactor system for the clinical-scale expansion of human mesenchymal stem/stromal cells. Biotechnol Bioeng 111,1116, 2014 [DOI] [PubMed] [Google Scholar]
- 40.Note for Guidance on the Use of Bovine Serum in the Manufacture of Human Medicinal Products. [cited CPMP/BWP/1793/02]. Available from: www.tga.gov.au/pdf/euguide/bwp179302en.pdf, 2003. (Last accessed 2014April04)
- 41.Bieback K.Platelet lysate as replacement for fetal bovine serum in mesenchymal stromal cell cultures. Transfus Med Hemother 40,326, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kakudo N., Minakata T., Mitsui T., Kushida S., Notodihardjo F.Z., and Kusumoto K.Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg 122,1352, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Kilian O., Flesch I., Wenisch S., Taborski B., Jork A., Schnettler R., et al. . Effects of platelet growth factors on human mesenchymal stem cells and human endothelial cells in vitro. Eur J Med Res 9,337, 2004 [PubMed] [Google Scholar]
- 44.Gruber R., Karreth F., Kandler B., Fuerst G., Rot A., Fischer M.B., et al. . Platelet-released supernatants increase migration and proliferation, and decrease osteogenic differentiation of bone marrow-derived mesenchymal progenitor cells under in vitro conditions. Platelets 15,29, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Tamama K., Fan V.H., Griffith L.G., Blair H.C., and Wells A.Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells 24,686, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Li H., Han Z., Liu D., Zhao P., Liang S., and Xu K.Autologous platelet-rich plasma promotes neurogenic differentiation of human adipose-derived stem cells in vitro. Int J Neurosci 123,184, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Unger C., Skottman H., Blomberg P., Dilber M.S., and Hovatta O.Good manufacturing practice and clinical-grade human embryonic stem cell lines. Hum Mol Genet 17,R48, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Sensebe L., Krampera M., Schrezenmeier H., Bourin P., and Giordano R.Mesenchymal stem cells for clinical application. Vox Sang 98,93, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Barry O.P., and FitzGerald G.A.Mechanisms of cellular activation by platelet microparticles. Thromb Haemost 82,794, 1999 [PubMed] [Google Scholar]
- 50.Marx R.E.Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 62,489, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Rodrigues M., Griffith L.G., and Wells A.Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res Ther 1,32, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho H.S., Song I.H., Park S.Y., Sung M.C., Ahn M.W., and Song K.E.Individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal stem cells. Korean J Lab Med 31,212, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Graziani F., Ivanovski S., Cei S., Ducci F., Tonetti M., and Gabriele M.The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res 17,212, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Yamaguchi R., Terashima H., Yoneyama S., Tadano S., and Ohkohchi N.Effects of platelet-rich plasma on intestinal anastomotic healing in rats: PRP concentration is a key factor. J Surg Res 173,258, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Weibrich G., Hansen T., Kleis W., Buch R., and Hitzler W.E.Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone 34,665, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Scherer S.S., Tobalem M., Vigato E., Hetit Y., Modarressi A., Hinz B., et al. . Nonactivated versus thrombin-activated platelets on wound healing and fibroblast to myofibroblast differentiation in vivo and in vitro. Plast Reconstr Surg 129,46e, 2012 [DOI] [PubMed] [Google Scholar]
- 57.Chevallier N., Anagnostou F., Zilber S., Bodivit G., Maurin S., Barrault A., et al. . Osteoblastic differentiation of human mesenchymal stem cells with platelet lysate. Biomaterials 31,270, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Han C., Zhang X., Xu W., Wang W., Qian H., and Chen Y.Cloning of the nucleostemin gene and its function in transforming human embryonic bone marrow mesenchymal stem cells into F6 tumor cells. Int J Mol Med 16,205, 2005 [PubMed] [Google Scholar]
- 59.Rubio D., Garcia S., Paz M.F., De la Cueva T., Lopez-Fernandez L.A., Lloyd A.C., et al. . Molecular characterization of spontaneous mesenchymal stem cell transformation. PLoS One 3,e1398, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rubio D., Garcia-Castro J., Martin M.C., de la Fuente R., Cigudosa J.C., Lloyd A.C., et al. . Spontaneous human adult stem cell transformation. Cancer Res 65,3035, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Wang Y., Huso D.L., Harrington J., Kellner J., Jeong D.K., Turney J., et al. . Outgrowth of a transformed cell population derived from normal human BM mesenchymal stem cell culture. Cytotherapy 7,509, 2005 [DOI] [PubMed] [Google Scholar]
- 62.de la Fuente R., Bernad A., Garcia-Castro J., Martin M.C., and Cigudosa J.C.Retraction: spontaneous human adult stem cell transformation. Cancer Res 70,6682, 2010 [DOI] [PubMed] [Google Scholar]
- 63.Garcia S., Bernad A., Martin M.C., Cigudosa J.C., Garcia-Castro J., and de la Fuente R.Pitfalls in spontaneous in vitro transformation of human mesenchymal stem cells. Exp Cell Res 316,1648, 2010 [DOI] [PubMed] [Google Scholar]
- 64.Sommeling C.E., Heyneman A., Hoeksema H., Verbelen J., Stillaert F.B., and Monstrey S.The use of platelet-rich plasma in plastic surgery: a systematic review. J Plast Reconstr Aesthet Surg 66,301, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.