Abstract
Chronic fatigue syndrome (CFS) is a chronic and extremely debilitating illness characterized by prolonged fatigue and multiple symptoms with unknown cause, diagnostic test, or universally effective treatment. Inflammation, oxidative stress, mitochondrial dysfunction, and CoQ10 deficiency have been well documented in CFS. We conducted an 8-week, randomized, double-blind placebo-controlled trial to evaluate the benefits of oral CoQ10 (200 mg/day) plus NADH (20 mg/day) supplementation on fatigue and biochemical parameters in 73 Spanish CFS patients. This study was registered in ClinicalTrials.gov (NCT02063126). A significant improvement of fatigue showing a reduction in fatigue impact scale total score (p<0.05) was reported in treated group versus placebo. In addition, a recovery of the biochemical parameters was also reported. NAD+/NADH (p<0.001), CoQ10 (p<0.05), ATP (p<0.05), and citrate synthase (p<0.05) were significantly higher, and lipoperoxides (p<0.05) were significantly lower in blood mononuclear cells of the treated group. These observations lead to the hypothesis that the oral CoQ10 plus NADH supplementation could confer potential therapeutic benefits on fatigue and biochemical parameters in CFS. Larger sample trials are warranted to confirm these findings. Antioxid. Redox Signal. 22, 679–685.
Introduction
Chronic fatigue syndrome (CFS) is a serious, complex, and extremely debilitating chronic illness with no known cause, diagnostic test, or universally effective treatment. It is characterized by prolonged, debilitating, and relapsing fatigue, often accompanied by numerous symptoms, resulting in significant disability for at least 6 months and often for years (3). There is currently no diagnostic laboratory test specific for the condition; this is why new potential diagnostic biomarkers are needed. The etiology of CFS remains unclear; however, recent studies have shown that oxidative stress and mitochondrial disturbances to energy requirements may be associated in its pathogenesis (3). It is uncertain whether oxidative stress and mitochondrial abnormality might be the common endpoint for all the dysfunctions present in the condition. Current treatments for CFS are largely aimed at symptoms management. The best evidence for treatment is cognitive behavioral therapy and graded exercise therapy; however, these are only moderately effective with a low recovery rate of only about 22% for those who have intensive treatment (2). Therefore, there is an urgent need for new treatments for this disabling condition. Better understanding on the multifactorial etiology and pathophysiology mechanisms will give insight into appropriate treatments for this illness. CFS is also associated with several related biochemical and immune abnormalities in multiple inflammatory, and oxidative and nitrosative stress (IO&NS) pathways. These pathways encompass: (i) immune activation; (ii) intracellular inflammation; (iii) dysfunctional mitochondria; (iv) lowered antioxidant status; (v) increased oxidative and nitrosative stress (O&NS), resulting into damage to DNA, essential fatty acids (lipids), proteins, mitochondria, and even red blood cells; and (vi) increased translocation of gram-negative bacteria (leaky gut) (8). All these important processes have shown to be involved in the development and progression of condition and may provide a tool for future CFS treatment approaches (8). Emerging data suggest that CoQ10 and NADH deficiencies have been described in patients with CFS and fibromyalgia (FMS) (1, 3, 5). Both CoQ10 and NADH play a critical role in mitochondrial ATP production and cellular metabolism homeostasis. It has been suggested that the combinations of natural antioxidant supplements can reduce significantly the fatigue and even naturally restore mitochondrial function, in long-term CFS patients with intractable fatigue (9). Preliminary data have shown a remarkable clinical improvement after initiating oral NADH or CoQ10 supplementation in patients with CFS and FMS (1, 5). Therefore, an 8-week, randomized, double-blind placebo-controlled trial (RCT) was conducted to evaluate the benefits of oral CoQ10 plus NADH supplementation on fatigue and biochemical parameters associated to oxidative stress, bioenergetics status, and mitochondrial dysfunction in 73 Spanish CFS patients.
Innovation.
Clinical reports in CFS suggest a role for mitochondrial dysfunction-dependent events and oxidative damage at the cellular level. Currently, drug therapy demonstrates limited effectiveness; all drugs have been focused on the management of disease symptoms. According to this study, the CoQ10 plus NADH cosupplementation could be a new alternative and complementary therapy in CFS patients. Furthermore, the CoQ10 plus NADH combination induced a significant reduction of fatigue, decrease in oxidative damage, improvement of mitochondrial function, and enhancement of energy showing a potential role in the physiopathology of the condition. The results described here may serve as a new way for designing experiments to better understand the effectiveness of mitochondrial targeted therapy in the treatment of CFS, FMS, and even other chronic conditions.
Results
Study participants
A total of 113 consecutive patients who had attended to CFS Clinical Unit (Vall d'Hebron Hospital, Barcelona, Spain) from January to December 2013 with a diagnosis of CFS according to the 1994 CDC Fukuda's criteria were initially evaluated and potential enrolled for eligibility in the study. Only 33 participants were excluded from intervention because they did not meet the inclusion criteria. Seventy-three women with CFS were eligible for the study and were randomized to either CoQ10 plus NADH or placebo group (mean age: 49.3±7.1 years) (Fig. 1). All patients' routine laboratory testing yielded normal results (data not shown). Demographic and clinical characteristics of intervention participants are provided in Table 1.
FIG. 1.
Flow diagram showing the distribution of the participants from initial assessment to analysis of study data. Details are given according to the Consolidated Standards of Reporting Trials (CONSORT) statement for reporting randomized controlled trials.
Table 1.
Demographic and Clinical Features of the Patient Population Recruited
| Randomization arms | ||||
|---|---|---|---|---|
| CoQ10+NADH group (n=39) | Placebo group (n=34) | |||
| Variables | Baseline | 8 weeks | Baseline | 8 weeks |
| Duration of illness (years) | 15.4±8.9 | 15.6±10.3 | 14.7±6.2 | 14.9±8.9 |
| Onset time from diagnosis (years) | 5.9±6.8 | 5.9±6.8 | 5.3±2.5 | 5.3±2.5 |
| Systolic BP (mmHg) | 139.8±19.5 | 136.4±19.3 | 137.1±18.4 | 134.5±17.2 |
| Diastolic BP (mmHg) | 72.6±14.1 | 71.2±13.2 | 69.8±12.1 | 70.3±11.6 |
| Body weight (kg) | 68.5±14.6 | 69.2±12.5 | 72.1±13.7 | 71.9±11.2 |
| BMI (kg/m2) | 26.7±5.2 | 27.1±3.7 | 25.9±2.4 | 27.2±1.8 |
Unless otherwise indicated, data are expressed as mean±SD. No significant differences were seen between both intervention groups.
BP, blood pressure; BMI, body mass index; SD, standard deviations.
Effect of CoQ10 plus NADH supplementation on clinical symptoms in CFS patients
A significant improvement of fatigue was observed in CFS patients after 8 weeks of CoQ10 plus NADH supplementation versus placebo, showing a reduction in the fatigue impact scale (FIS) total score (p<0.05) according to the assessment questionnaire as shown in Table 2. No adverse effects were seen or reported by patients.
Table 2.
Fatigue Index in 73 CFS Patients Based on Symptoms Assessment Questionnaire
| Randomization arms | ||||
|---|---|---|---|---|
| CoQ10+NADH group (n=39) | Placebo group (n=34) | |||
| Symptoms assessment | Baseline | 8 weeks | Baseline | 8 weeks |
| FIS | ||||
| FIS total score | 131.9±18.9 | 124.4±23.4a | 136.0±16.0 | 132.3±20.7 |
| Physical | 34.8±4.2 | 32.4±5.9 | 35.6±4.2 | 34.2±5.7 |
| Cognitive | 33.6±5.1 | 31.4±6.0 | 34.6±4.3 | 33.9±4.9 |
| Psychosocial | 63.5±10.9 | 60.6±12.6 | 65.7±9.8 | 64.2±10.9 |
Unless otherwise indicated, data are expressed as mean±SD.
p<0.05, significant differences versus placebo after 8 weeks of treatment.
FIS, fatigue impact scale.
Effect of CoQ10 plus NADH administration on NAD+/NADH in CFS patients
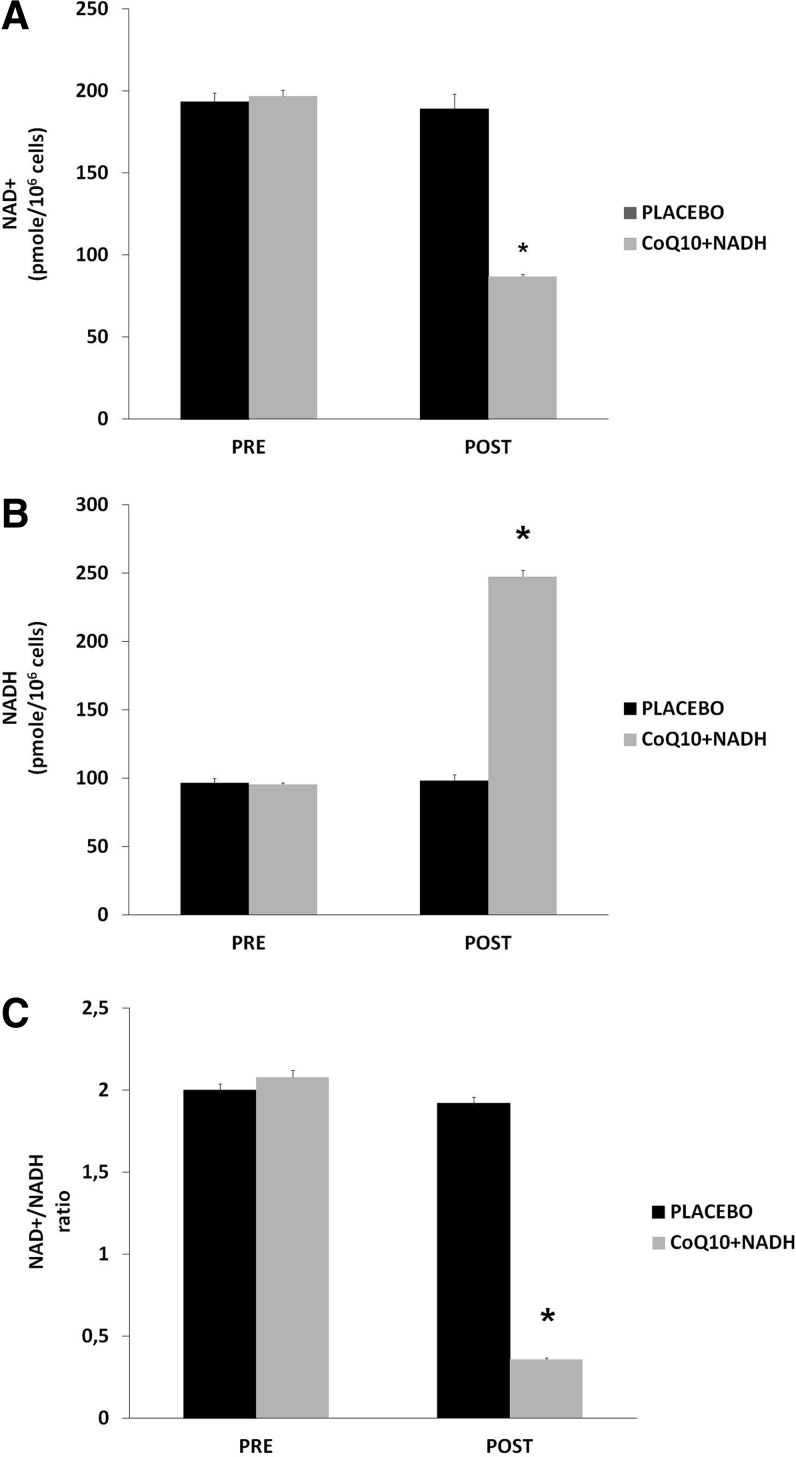
NADH is a coenzyme that can stimulate energy production by replenishing depleted cellular stores of ATP. The NAD+/NADH ratio plays an omnipresent role in regulating the intracellular redox status and therefore represents a function of the metabolic state. Given the major function of these two nucleotides in maintaining normal cellular homeostasis during inflammation, further studies into the biological roles of NAD+/NADH may increase our understanding for the potential role related with therapies in CFS and other chronic fatiguing illnesses (9). To the best of our knowledge, there is no well-documented information regarding the role of NAD+/NADH status in the pathogenesis of CFS. NAD+/NADH levels were measured in blood mononuclear cells (BMCs) from 73 CFS patients treated with CoQ10 plus NADH and placebo. All patients were in a clinically stable phase. After 8 weeks of treatment, patients treated with a combination of CoQ10 plus NADH showed significantly lower levels of NAD+ compared with placebo (86.9±1.4 vs. 189.4±8.9 pmol/106 cells; p<0.001) (Fig. 2A). We also observed significantly higher levels of NADH (247.3±5.1 vs. 98.2±4.6 pmol/106 cells; p<0.001) (Fig. 2B) and a significantly lower NAD+/NADH ratio (0.36±0.009 vs. 1.92±0.038 pmol/106 cells; p<0.001) (Fig. 2C) in CFS patients after CoQ10 plus NADH administration versus placebo, respectively. These results show that NAD+/NADH levels in BMCs are associated with impairment of the bioenergetic status and mitochondrial dysfunction in CFS. Given the importance of NAD+/NADH homeostasis in the maintenance of normal cellular function, it is likely that this molecule is of therapeutic relevance in CFS and other chronic fatiguing conditions. According to the results of the present study, impaired pyridine nucleotide metabolism may be a consequence of impaired mitochondrial respiratory function and may, at least in part, be attributed to increased NAD+/NADH ratio inducing free radical production. In summary, these results clearly indicate an association between BMCs NAD+ and NADH levels in CFS. Considering the vital role of NAD+ not only for maintaining mitochondria energy production but also genomic integrity, it is highly likely that this pyridine nucleotide may be pathogenically important in CFS. The main question as to whether altered NAD+/NADH levels in CFS is actively and directly associated with the pathogenesis of illness or represents an epiphenomenon of mitochondrial dysfunction and impairment of immune cell-mediated pathways remains unclear. The results from this study clearly indicate significant systemic energy depletion in BMCs from CFS patients.
FIG. 2.
NAD+/NADH content in blood mononuclear cells (BMCs) from chronic fatigue syndrome (CFS) patients at baseline and after 8 weeks of treatment. (A) NAD+ levels, (B) NADH levels, and (C) NAD+/NADH ratio were measured as noted in the Notes section. Data represent the mean±SD of duplicate separate assays. *p<0.001 significant differences between before and after 8 weeks of treatment.
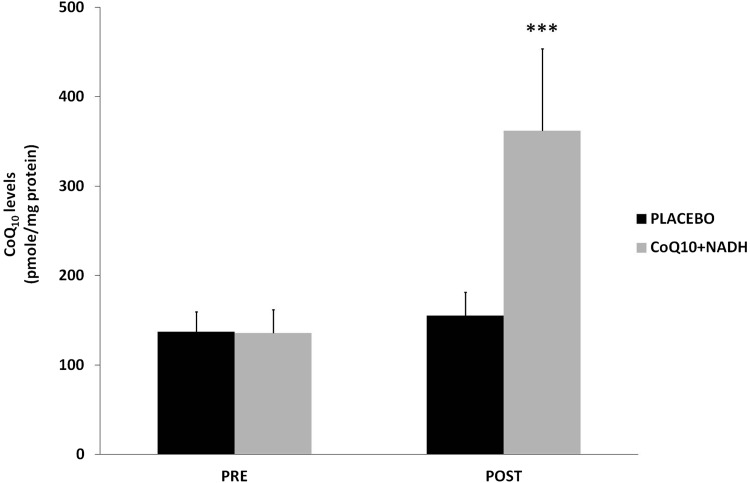
CFS patients treated with CoQ10 plus NADH show pronounced increase of CoQ10 levels
CoQ10 deficiency has been associated with a variety of human disorders, some of them caused by a direct defect of CoQ10 biosynthesis genes or as a secondary consequence of other diseases (9). Since it has been suggested that CoQ10 levels could be a potentially useful biological marker of mitochondrial dysfunction (3, 4), we measured CoQ10 levels in BMCs from CFS patients treated versus placebo. A significant increase of CoQ10 levels was observed in patients treated with the combination of CoQ10 plus NADH compared with the placebo group (361.8±37 vs. 155.1±26 pmol/mg proteins, p<0.05, respectively) (Fig. 3).
FIG. 3.
Coenzyme Q10 levels in BMCs from CFS patients were measured by high-performance liquid chromatography, as described in the Notes section. Data represent the mean±SD of three separate experiments. ***p<0.05 significant differences between before and after 8 weeks of treatment.
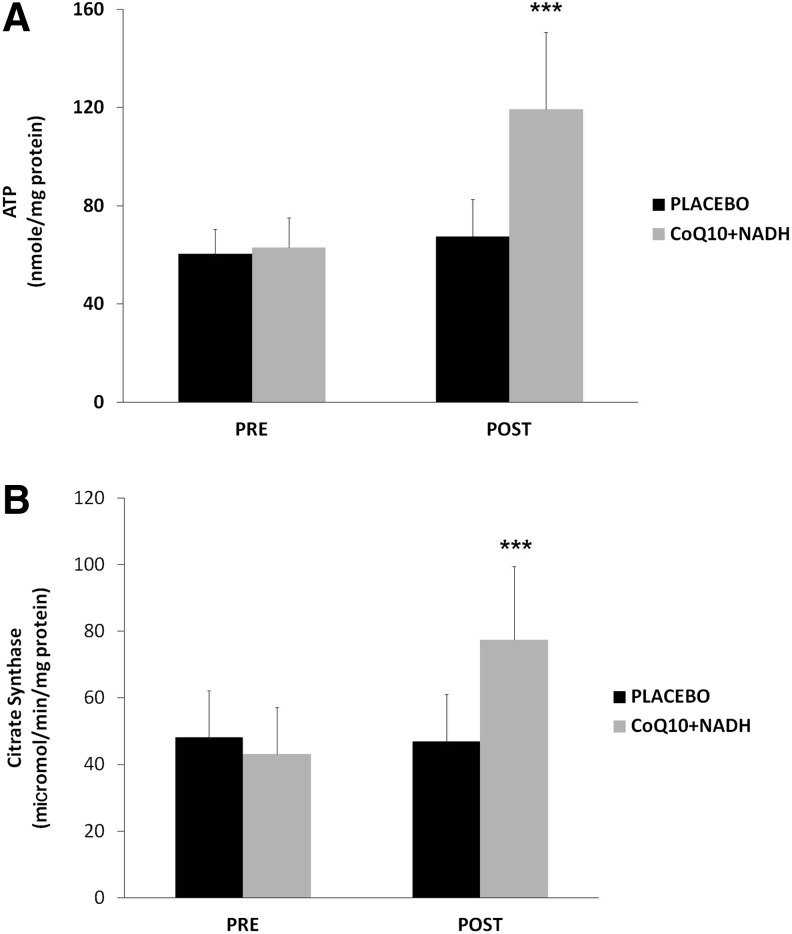
CoQ10 plus NADH combination shows significant changes in mitochondrial function of CFS patients
As an indicator of cellular bioenergetic status, we measured total ATP levels in BMCs from all CFS patients. On average, CFS patients treated with CoQ10 plus NADH showed significantly higher levels of ATP production than placebo (119.24±31.43 vs. 67.45±15.1 nmol/mg protein, p<0.05, respectively) (Fig. 4A). Furthermore, we measured the citrate synthase activity, an enzyme marker of mitochondrial mass in BMCs from CFS patients in both intervention groups. Interestingly, we observed a significant increase in the citrate synthase activity from CFS subjects treated versus placebo (77.4±22 vs. 46.9±14.1 μmol/min/mg protein, p<0.05, respectively) (Fig. 4B). These data show an interesting improvement on the mitochondrial function and mass in CFS patients after 8 weeks of treatment with a combination with two important bioenergetics cofactors (CoQ10 and NADH).
FIG. 4.
Intracellular ATP and mitochondrial citrate synthase in BMCs from CFS patients were measured, as described in the Notes section. (A) ATP content and (B) specific activity of mitochondrial citrate synthase were performed, as described in Notes section. Data represent the mean±SD of three separate experiments. ***p<0.05 significant differences between before and after 8 weeks of treatment.
CoQ10 and NADH combination attenuate oxidative damage through the reduction of lipoperoxides in CFS patients
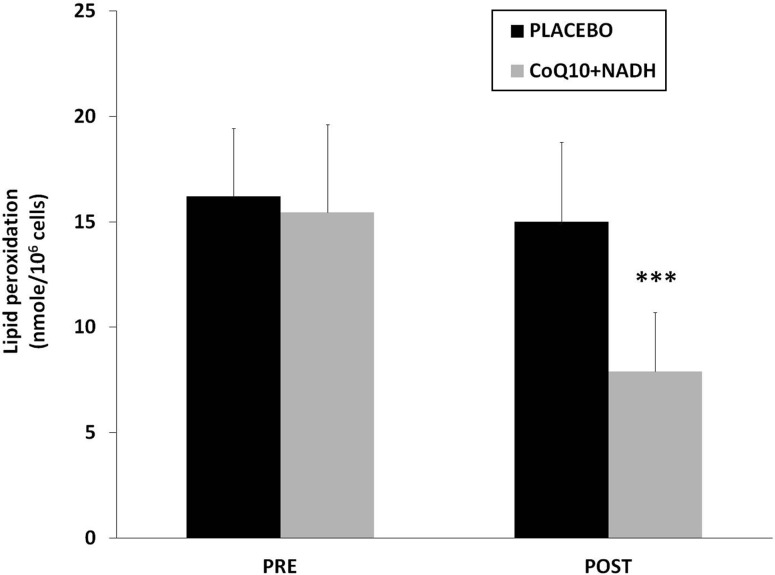
On one hand, it is known that mitochondrial dysfunction is often associated with an increased production of mitochondria reactive oxygen/nitrogen species (ROS/RNS). On the other hand, oxidative stress has been proposed as a relevant event in the pathogenesis of CFS and FMS (3, 4, 6, 9). Because both CoQ10 and NADH have shown to have an important antioxidant power, we have determined the effect of the combination in oxidative stress damage compared with placebo. To assess the oxidative stress, lipoperoxides levels in BMCs from CFS patients treated versus placebo were analyzed. CFS patients treated with CoQ10 plus NADH showed significantly lower levels of lipid peroxidation versus placebo (7.9±2.7 vs. 14.9±3.9 nmol/106 cells, p<0.05, respectively) (Fig. 5).
FIG. 5.
Lipid peroxidation levels in BMCs from CFS patients were measured by TBARS assay, as described in the Notes section. Data represent the mean±SD of three separate experiments. ***p<0.05 significant differences between before and after 8 weeks of treatment.
Discussion and Future Directions
CoQ10 and NADH could be the two potential drug candidates in the treatment of CFS and perhaps other chronic fatiguing illnesses for at least three main reasons. First, CoQ10 and NADH are bioenergetic cofactors with the potential to boost mitochondrial function. Second, CoQ10 plus NADH are powerful free radical scavengers that can mitigate lipid peroxidation and DNA damage caused by oxidative stress, and finally, it is plausible that the potential benefits demonstrated in this study (energy production and enhancement of fatigue) may be due in part to its powerful antioxidant properties. The present study demonstrates for first time that NAD+ and NADH levels in BMCs could be significantly altered in CFS patients. One of the major contributing factors toward NADH depletion in CFS is chronic oxidative stress and poor mitochondrial function leading to reduce cellular viability. Oxidative stress can be mediated by increased ROS/RNS and subsequently worsen when the cellular capability of detoxifying ROS/RNS is compromised. During the past few years, several pathophysiological events in CFS have been elucidated (3–7). Increasing evidence implicates oxidative damage, mitochondrial dysfunction, and bioenergetics metabolism impairments in the pathogenesis of CFS. According to these results, we reported for the first time that the oral CoQ10 plus NADH supplementation can result in a safe and effective therapy to reduce the fatigue, help restore mitochondrial function and bioenergetic metabolism, and ameliorate oxidative damage in CFS. We can hypothesize a pivotal role in combination of natural antioxidants supplements (CoQ10 plus NADH) as recommended and beneficial in fatigue and insight into pathogenesis of this condition. However, according to our results, the alterations of NAD+/NADH content, CoQ10 levels, lipid peroxidation, intracellular ATP, and activity of mitochondrial citrate synthase may explain that cellular bioenergetic disturbances, oxidative stress, and mitochondrial dysfunction-dependent mechanisms are common events in CFS patients and may be implicated in the severity of the symptoms (3, 6, 9). Therefore, CoQ10 plus NADH as natural supplementation could be used as a possible alternative and complementary therapy for this condition and even in other chronic illnesses associated to fatigue. However, further investigations will be required to clarify the more precise mechanisms by which the combination of antioxidant supplements may contribute to the pathogenesis and potential therapeutic mechanisms in CFS and other chronic illnesses.
Notes
The study was registered in ClinicalTrials.gov (NCT02063126). The study protocol was reviewed and approved by the Institutional Committee Review Board of Biomedical Research (Vall d'Hebron University Hospital, Barcelona, Spain). All the participants gave their written informed consent before initiating the study. This study was carried out in compliance with the Declaration of Helsinki and all the International Conference on Harmonisation and Good Practice Guidelines.
Clinical study design
We conducted an 8-week, randomized, double-blind placebo-controlled trial to evaluate the benefits of oral CoQ10 (200 mg/day) plus NADH (20 mg/day) supplementation on fatigue and biochemical parameters in 73 eligible CFS patients who fulfilled the 1994 CDC Fukuda's criteria. Exclusion criteria were acute infectious diseases in the previous 4 weeks, past or present neurological, psychiatry (depression/anxiety), metabolic, autoimmune, allergy-related diseases, dermal or chronic inflammation disorders, undesired habits (e.g., smoking, alcohol abuse), oral diseases (e.g., periodontitis), medical conditions that required glucocorticoids use, statins, or antidepressant/anxiolytics drugs, past or current breastfeeding. All patients had a sedentary life style. Treatment with simple analgesic (NSAID drugs, aspirin, and COX-2 inhibitors) was permitted during the study. Only 2 patients reported that they used analgesics (e.g., paracetamol/ibuprofen) on demand through the study.
Intervention
Seventy-three women with CFS who fulfilled the 1994 CDC Fukuda's criteria were evaluated and enrolled in the study from the outpatient CFS Clinical Unit (Vall d'Hebron University Hospital, Barcelona, Spain). Participants were randomized in a double-blind manner with a 1:1 ratio to CoQ10 plus NADH arm or placebo arm. Thirty-nine CFS subjects received oral Coenzyme Q10 (200 mg/day) plus NADH (20 mg/day) supplementation (VITAE NATURAL NUTRITION, S.L.) in soft gel capsules and thirty-four CFS subjects received a matching placebo, divided into two daily doses for 8 weeks.
Outcome measurements
The main objectives of the study were changes from baseline to 8 weeks of intervention in fatigue index and levels of biochemical parameters in BMCs associated to oxidative stress status and mitochondrial dysfunction, respectively, in study participants. Safety was assessed by interviewing subjects and informants about adverse events, measuring vital signs, brief physical examination at baseline and 8 weeks. Adverse effects were recorded at each study visits and defined according to the Medical Dictionary for Regulatory Activities terminology. Fatigue index was assessed through the symptoms questionnaire (FIS-40) at baseline and 8 weeks of treatment; scores range for fatigue (FIS total score, range from 0 to 160). Participants with higher total score indicate more severe symptoms, while lower total scores indicate less severe symptoms.
Patients' enrollment and blood collection
Blood samples of all participants were collected at baseline and 8 weeks of treatment for immediate biochemical assays and isolation of BMCs. Samples from 73 women with CFS were used for the analysis of biochemical parameters in the intervention study.
BMCs isolation
A 15 ml heparinized blood sample was collected after 12 h fasting from all CFS patients before (baseline) and at the end of treatment (8 weeks). Blood samples were drawn in the morning. Peripheral BMCs were isolated and purified by isopycnic centrifugation using Histopaque-1119 and Histopaque-1077 (Sigma). After harvesting, the BMCs were washed twice in sterile PBS and frozen at −80°C until analysis.
Quantification of intracellular NAD+/NADH
Total cellular NAD+ and NADH concentrations were determined independently in whole BMCs extracts (1×106 cells) using the NAD+/NADH cycling assay (Abcam ab65348, Inc.), as described previously. NAD+/NADH levels and NAD+/NADH ratio in all CFS patients pre- and post-treatment were plotted. NAD+ and NADH levels were expressed as pmol/106 cells.
CoQ10 determination
CoQ9 was used as an internal standard in all human assays. BMCs were lysed with 1% sodium dodecyl sulfate (SDS) and vortexed for 1 min. A mixture of ethanol: isopropanol (95:5) was added, and the samples were vortexed again for 1 min. To recover CoQ, 5 ml of hexane was added, and the samples were centrifuged at 1000 g for 5 min at 4°C. The upper phases from three extractions were recovered and dried on a rotator evaporator. The lipid extract was resuspended in 1 ml of ethanol, dried in a speed vac, and kept at −20°C until analysis. Samples were resuspended in a suitable volume of ethanol before a high-performance liquid chromatography (HPLC) injection. Lipid components were separated by a Prominence Shymadzu HPLC system (Shimadzu Scientific Instruments) equipped with a Shimadzu Shim-pack XR-ODS column. CoQ levels were analyzed using an ultraviolet SPD-20A detector. CoQ10 contents in BMCs were analyzed by HPLC (Beckman Coulter; 166-126 HPLC) with ultraviolet detection (275 nm) according to the method described (6).
Lipid peroxidation
Thiobarbituric acid reactive substances (TBARS) levels in BMCs were determined by a method based on the reaction with thiobarbituric acid (TBA) at 90°C–100°C using a commercial kit according to the instructions of the manufacturer (Cayman Chemical). TBARS were expressed as malondialdehyde (MDA) levels. In these assays, a MDA standard was used to construct a standard curve against which unknown samples can be plotted.
Intracellular ATP
Total ATP content in BMCs extracts was determined by a bioluminescence assay using an ATP determination kit (Invitrogen-Molecular Probes) according to the instructions of the manufacturer.
Citrate synthase assay
The specific activity of mitochondrial citrate synthase in whole-cell extracts prepared from BMCs was measured at 412 nm minus 360 nm (13.6 mM−1 cm−1) using 5,5′-di-thiobis (2-nitrobenzoic acid) to detect free sulfhydryl groups in Coenzyme A, as previously described (6).
Statistical analysis
Continuous variables were expressed as the mean±SD. Qualitative variables were expressed as a percentage. Data in figures are given as mean±SD. Data between different groups were analyzed statistically by using ANOVA on Ranks with Sigma Plot and Sigma Stat statistical software (SPSS for Windows, 19, 2010, SPSS, Inc.). To compare the study results from CFS patients treated with CoQ10 plus NADH supplementation or placebo between post-treatment versus baseline, a two-way variance (ANOVA) analysis was used. Differences with a p-value <0.05 were considered significant. Descriptive statistics were performed with the SAS/STAT® (v9.2) statistical software pack.
Abbreviations Used
- BMCs
blood mononuclear cells
- BMI
body mass index
- CFS
chronic fatigue syndrome
- CoQ10
Coenzyme Q10
- CS
mitochondrial citrate synthase
- FIS
fatigue impact scale
- HPLC
high-performance liquid chromatography
- MDA
malondialdehyde
- ROS/RNS
reactive oxygen/nitrogen species
- TBARS
thiobarbituric acid reactive substances
Acknowledgments
The authors thank all patients for participating in this study. This work has been supported by VITAE NATURAL NUTRITION, S.L. (Sant Cugat de Vallès, Barcelona, Spain). We are grateful to Dr. Esther M. Crawley (School of Social and Community Medicine, University of Bristol, UK) and Dr. Eva Balada (Systemic Autoimmune Diseases Unit, Vall d'Hebron University Hospital Research Institute, Barcelona, Spain) for their critical comments and suggestions on the article.
References
- 1.Alegre J, Roses JM, Javierre C, Ruiz-Baques A, Segundo MJ, and De Sevilla TF. [Nicotinamide adenine dinucleotide (NADH) in patients with chronic fatigue syndrome]. Rev Clin Esp 210: 284–288, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Baker R. and Shaw EJ. Diagnosis and management of chronic fatigue syndrome or myalgic encephalomyelitis (or encephalopathy): summary of NICE guidance. BMJ 335: 446–448, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castro-Marrero J, Cordero MD, Sáez-Francàs N, Jimenez-Gutierrez C, Aguilar-Montilla FJ, Aliste L, and Alegre-Martin J. Could mitochondrial dysfunction be a differentiating marker between chronic fatigue syndrome and fibromyalgia? Antioxid Redox Signal 19: 1855–1860, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Cordero MD, Alcocer-Gomez E, Culic O, Carrion AM, De Miguel M, Diaz-Parrado E, Perez-Villegas EM, Bullon P, Battino M, and Sanchez-Alcazar JA. NLRP3 Inflammasome is activated in fibromyalgia: the effect of Coenzyme Q. Antioxid Redox Signal 20: 1169–1180, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cordero MD, Alcocer-Gomez E, De Miguel M, Culic O, Carrion AM, Alvarez-Suarez JM, Bullon P, Battino M, Fernandez-Rodriguez A, and Sanchez-Alcazar JA. Can coenzyme q10 improve clinical and molecular parameters in fibromyalgia? Antioxid Redox Signal 19: 1356–1361, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Cordero MD, Cotan D, Del-Pozo-Martin Y, Carrion AM, De Miguel M, Bullon P, and Sanchez-Alcazar JA. Oral coenzyme Q10 supplementation improves clinical symptoms and recovers pathologic alterations in blood mononuclear cells in a fibromyalgia patient. Nutrition 28: 1200–1203, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Curriu M, Carrillo J, Massanella M, Rigau J, Alegre J, Puig J, Garcia-Quintana AM, Castro-Marrero J, Negredo E, Clotet B, Cabrera C, and Blanco J. Screening NK-, B- and T-cell phenotype and function in patients suffering from Chronic Fatigue Syndrome. J Transl Med 11: 68, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris G. and Maes M. A neuro-immune model of Myalgic Encephalomyelitis/Chronic fatigue syndrome. Metab Brain Dis 28: 523–540, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Nicolson GL. Mitochondrial dysfunction and chronic disease: treatment with natural supplements. Altern Ther Health Med 20Suppl 1: 18–25, 2014 [PubMed] [Google Scholar]