Abstract
Positive emotions foster social relationships and motivate thought and action. Dysregulation of positive emotion may give rise to debilitating clinical symptomatology such as mania, risk-taking, and disinhibition. Neuroanatomically, there is extensive evidence that the left hemisphere of the brain, and the left frontal lobe in particular, plays an important role in positive emotion generation. Although prior studies have found that left frontal injury decreases positive emotion, it is not clear whether selective damage to left frontal emotion regulatory systems can actually increase positive emotion. We measured happiness reactivity in 96 patients with frontotemporal dementia, a neurodegenerative disease that targets emotion-relevant neural systems and causes alterations in positive emotion (i.e., euphoria and jocularity), and in 34 healthy controls. Participants watched a film clip designed to elicit happiness and a comparison film clip designed to elicit sadness while their facial behavior, physiological reactivity, and self-reported emotional experience were monitored. Whole-brain voxel-based morphometry analyses revealed that atrophy in predominantly left hemisphere fronto-striatal emotion regulation systems including left ventrolateral prefrontal cortex, orbitofrontal cortex, anterior insula, and striatum (pFWE < .05) was associated with greater happiness facial behavior during the film. Atrophy in left anterior insula and bilateral frontopolar cortex was also associated with higher cardiovascular reactivity (i.e., heart rate and blood pressure) but not self-reported positive emotional experience during the happy film (p< .005, uncorrected). No regions emerged as being associated with greater sadness reactivity, which suggests that left-lateralized fronto-striatal atrophy is selectively associated with happiness dysregulation. Whereas previous models have proposed that left frontal injury decreases positive emotional responding, we argue that selective disruption of left hemisphere emotion regulating systems can impair the ability to suppress positive emotions such as happiness.
Keywords: positive emotion, frontotemporal dementia, laterality, emotion regulation, approach
1. Introduction
Positive emotions refer to a family of emotions that includes happiness, amusement, attachment love, nurturant love, awe, and enthusiasm, among others (Shiota, Neufeld, Yeung, Moser, & Perea, 2011). These emotions serve important social functions, facilitating approach behavior, motivating social engagement, fostering new social connections (Fredrickson, 2004), and reversing the physiological activation caused by negative emotions (Fredrickson & Levenson, 1998). Certain levels of positive emotional reactivity are thought to be optimal; levels that are too low or too high can be problematic. For example, overly low levels of positive emotion underlie clinical symptoms such as anhedonia and depression whereas overly high levels can give rise to inappropriate interpersonal boundaries, risk-taking, and mania (Gruber, Harvey, & Purcell, 2011).
Distributed brain systems involved in both emotion generation and emotion regulation act in concert to produce observed levels of a positive emotional response (typically measured in terms of changes in facial behavior, physiology, and subjective experience). While emotion generating systems (i.e., projections from pregenual anterior cingulate cortex to the central nucleus of the amygdala, hypothalamus, and brainstem) initiate rapid emotional responses to positive emotional cues (Saper, 2002), emotion regulating systems (i.e., ventrolateral prefrontal cortex, orbitofrontal cortex, dorsomedial prefrontal cortex, and pre/supplementary motor area), with connections to striatum, thalamus, and subthalamic nuclei, promote down-regulation of affective responding in ways that are commensurate with individual goals and the social context (Aron, 2007; Ochsner & Gross, 2005; Wager, Davidson, Hughes, Lindquist, & Ochsner, 2008). Thus, whether an injury to neural systems that support positive emotion results in muted or intensified emotion should depend on the locus of the anatomical injury. In general, damage to emotion generating circuits should reduce positive emotional reactivity whereas damage to emotion regulating circuits should weaken inhibition and thus result in heightened positive emotion.
The extent to which positive emotion is lateralized in the brain has long been debated. While some argue that there is right hemisphere dominance for the perception and expression of both positive and negative emotion (Tucker, 1981), others propose that the left hemisphere plays a dominant role in positive emotion (Davidson & Fox, 1982). Previous studies have concluded that left-hemisphere damage typically diminishes positive emotion whereas right-hemisphere damage typically increases positive emotion. Two lines of evidence support this conclusion. In Wada studies that deactivate the right hemisphere (via unilateral intracarotid injection of sodium amytal) but preserve the left, patients frequently exhibit optimism and laughter (Perria, Rosadini, & Rossi, 1961; Sackeim et al., 1982). Similarly, numerous lesion studies, but not all (House, Dennis, Warlow, Hawton, & Molyneux, 1990), have found that right-hemisphere injury often results in laughing and smiling (Gainotti, 1972; Sackeim et al., 1982). Positive emotions are thought to persist in patients with right hemisphere damage or dysfunction because of preservation (and even release) of left-hemisphere circuits that produce positive emotion. Positive emotions produced by these circuits may be more apparent when right hemisphere negative emotion generators are attenuated.
Despite the advances in understanding the laterality of positive emotion, the ways that left hemisphere neural systems support positive emotion generation and regulation remain poorly understood. The majority of previous clinical studies that related asymmetric brain injury to positive emotional change did not directly relate lesion size or location with positive emotional behavior. Thus, it is difficult to know whether all left hemisphere lesions diminish positive emotion or whether the effects depend on lesion location. Electrophysiological studies of prefrontal activation asymmetry offer more anatomical specificity, pointing to the left frontal lobe as an integral left hemisphere hub for positive emotion generation (Davidson, 1992). However, in these studies, frontal asymmetry indices have typically been based on dorsolateral prefrontal cortex activity. Thus, they are not well-suited to shed light on the role of ventral frontal and subcortical structures in positive emotion (Davidson & Irwin, 1999) nor to tease apart the roles of left-dominant frontal systems that support positive emotion generation from those that support emotion regulation. Determining whether greater left frontal activity during positive emotion reflects the involvement of positive emotion generators, regulators, or both, is critical to our understanding of the ways that left frontal systems mount positive emotional responses. Although focal lesion, Wada test, and asymmetry studies have provided invaluable information regarding the neural architecture of positive emotion, we believe that further explication of this architecture will benefit greatly from the application of additional approaches.
Neurodegenerative diseases, which selectively disrupt distributed neural networks (Seeley, Crawford, Zhou, Miller, & Greicius, 2009), offer a powerful lesion-based approach for determining how lateralized brain systems promote positive emotion. Frontotemporal dementia (FTD) is a neurodegenerative disease that targets neural systems that are integral for emotion generation and regulation. In FTD, gradual degeneration of the frontal, anterior temporal, and insular cortex, and subcortical structures (i.e., striatum, amygdala, and hypothalamus) is accompanied by parallel declines in social behavior, emotion, speech, and language (Boxer & Miller, 2005). Many patients with FTD have bilateral atrophy, affecting the left and right hemispheres similarly, while others have asymmetric atrophy. Predominantly right-sided atrophy is associated with socioemotional impairment (e.g., loss of empathy and disinhibition); predominantly left-sided atrophy is associated with progressive deterioration of speech and language. Given that patients vary in the degree to which they have atrophy in left and right emotion-relevant networks and in the extent to which they exhibit change in positive emotion, FTD is a particularly useful population in which to test theories of positive emotion lateralization.
Positive emotional alterations in FTD have received relatively little attention to date. Although many patients with FTD lose interest in people and activities that were previously enjoyable and rewarding, behaviors that suggest a decline in positive emotion, other patients exhibit euphoria, impulsivity, disinhibition, smiling, laughing, overfamiliarity, and jocularity (Mendez, Chen, Shapira, Lu, & Miller, 2006; Woolley et al., 2007), behaviors that are suggestive of an increase in positive emotion, perhaps resulting from deficits in emotion regulation. In laboratory assessments, patients with FTD do poorly when asked to regulate negative emotions (Goodkind, Gyurak, McCarthy, Miller, & Levenson, 2010), but their control of positive emotions has not been evaluated. When watching happy film clips, patients with FTD (on average) show levels of happiness facial behavior and physiological reactivity comparable to those of healthy controls (Werner et al., 2007) despite having diminished emotional reactions to situations that are typically disgusting and embarrassing (Eckart, Sturm, Miller, & Levenson, 2012; Sturm, Ascher, Miller, & Levenson, 2008). To our knowledge, there have been no studies linking different patterns of atrophy in FTD with differences in positive emotional behavior.
The goal of the present study was to examine relationships between left-lateralized atrophy and positive emotional reactivity. We used a laboratory-based approach to measure emotional reactivity in individuals with FTD while they watched positive and negative emotional film clips. These film clips are effective elicitors of emotional facial expression, autonomic nervous system responding, and subjective emotional experience in patients with neurodegenerative disease (Levenson et al., 2008). Patients watched a film clip chosen to elicit happiness, a positive emotion characterized by smiling and laughing behavior and autonomic nervous system activation (Giuliani, McRae, & Gross, 2008) that occurs in response to playful situations (Panksepp, 2007). They also viewed a sad film clip, which provided a negative emotional comparison condition. Behavioral, autonomic, and experiential responses to these film clips were used as variables of interest in structural neuroimaging analyses.
Reflecting the foregoing discussion, we tested two competing hypotheses about the left frontal neural systems that support positive emotion: (1) atrophy in any left frontal area will be associated with diminished happiness reactivity, or (2) atrophy in left frontal emotion regulating systems (with relative preservation of left hemisphere emotion generating circuits) will be associated with heightened happiness reactivity.
2. Materials and Methods
2.1. Participants
Participants underwent a multidisciplinary team evaluation at the University of California, San Francisco Memory and Aging Center that included a clinical interview, neurological exam, functional assessment, and neuropsychological testing as well as structural magnetic resonance imaging (MRI). Neuropsychological testing included assessment of verbal and visual episodic memory, executive function (e.g., set-shifting, working memory, and fluency), language, and visuospatial functioning. The cognitive screening data were used to determine patients’ clinical and research diagnoses. The majority of participants completed neuropsychological testing in close proximity to the emotional assessment (within 5 months for patients and 12 months for healthy controls). Functional assessments of dementia severity were obtained using the Clinical Dementia Rating Scale (CDR; Morris, 1993). The CDR Total (scores range from 0 to 3) and Sum of the Boxes (CDR-SB) scores (scores range from 0 to 18, with higher scores on both CDR measures indicating greater functional impairment) were computed for each participant, providing indices of disease severity. The healthy controls were recruited from advertisements and were free of current or previous neurological or psychiatric disorders. Controls underwent an identical neurological, cognitive, and imaging work-up as the patients and were included as a comparison group for measures of emotional reactivity and brain volume. Table 1 presents the demographic, cognitive, and functional data for each group.
Table 1. Characteristics of participants classified by diagnostic group.
Means (M) and standard deviations (SD) are listed for each group unless otherwise noted.
|
Healthy
Controls |
FTD
(subtypes combined) |
bvFTD | svPPA | nfvPPA | |
|---|---|---|---|---|---|
| n | 34 | 96 | 47 | 33 | 16 |
| Age | 64.9 (9.3) | 61.9 (7.3) | 59.6 (7.5) | 63.2 (5.3) | 65.9 (7.9) |
| Sex: % Female | 50.0 | 39.6 | 31.9 | 39.4 | 62.5 |
| Education | 17.3 (2.2) | 15.8 (2.8) | 16.2 (2.6) | 15.8 (3.1) | 14.8 (2.5) |
| Handedness: % Right- handed |
91.2 | 89.6 | 93.6 | 81.8 | 93.8 |
| Study wave: % Wave 1 | 47.1 | 42.7 | 51.1 | 45.5 | 12.5 |
| CDR Total | 0.0 (0.1) | 0.9 (0.6) | 1.2 (0.6) | 0.8 (0.5) | 0.5 (0.5) |
| CDR-SB | 0.0 (0.1) | 5.1 (3.3) | 6.7 (2.8) | 3.9 (2.8) | 2.6 (3.4) |
| MMSE | 29.7 (0.5) | 24.4 (6.1) | 25.3 (4.9) | 23.8 (6.7) | 22.8 (7.7) |
| California Verbal Learning Test Short Form 10-Minute Recall (/9)† |
7.1 (1.8) | 3.5 (2.8) | 4.0 (2.7) | 2.3 (2.5) | 4.7 (3.2) |
| Benson Figure Copy 10- Minute Recall (/17) |
12.2 (2.6) | 7.3 (4.7) | 7.4 (4.5) | 6.4 (4.8) | 9.7 (4.4) |
| Modified Trails (correct lines per minute) |
35.0 (10.2) | 17.7 (12.6) | 16.4 (13.8) | 21.3 (10.9) | 12.2 (10.0) |
| Modified Trails Errors | 0.33 (0.7) | 1.4 (1.9) | 1.9 (2.2) | 0.4 (0.6) | 2.2 (2.1) |
| Phonemic Fluency (# correct in 60 seconds) |
16.6 (6.7) | 7.7 (5.6) | 8.7 (7.1) | 7.1 (2.8) | 5.0 (3.2) |
| Semantic Fluency (# correct in 60 seconds) |
23.2 (5.2) | 10.9 (5.0) | 12.4 (4.9) | 8.7 (4.1) | 10.3 (5.6) |
| Design Fluency Correct (# correct in 60 seconds) |
11.0 (3.1) | 6.8 (3.5) | 6.4 (3.6) | 7.0 (3.5) | 7.6 (3.6) |
| Design Fluency Repetitions | 1.4 (1.8) | 3.8 (5.1) | 5.1 (6.2) | 2.1 (2.8) | 2.7 (3.0) |
| Digits Backward | 5.5 (1.5) | 4.3 (1.5) | 4.3 (1.6) | 4.4 (1.1.) | 3.6 (1.8) |
| Benson Figure Copy (/17) | 15.7 (1.0) | 14.9 (1.6) | 14.6 (1.7) | 15.3 (1.5) | 15.1 (1.6) |
| Calculations (/5) | 5.0 (0.2) | 4.2 (1.2) | 3.9 (1.4) | 4.7 (0.5) | 4.3 (1.3) |
| Boston Naming Test Spontaneous Correct (/15) |
14.7 (0.6) | 10.1 (4.6) | 12.5 (3.0) | 5.7 (3.9) | 11.8 (3.5) |
| Peabody Picture Vocabulary Test (/16) |
15.7 (0.6) | 12.4 (3.9) | 14.4 (2.0) | 8.9 (4.2) | 13.7 (2.3) |
19/34 healthy controls got the California Verbal Learning Test-II (16-word list) instead of the Short-Form. Their performance on the 20-minute delay was also in the average range (M= 13.3, SD= 2.2). bvFTD = behavioral variant frontotemporal dementia, svPPA = semantic variant primary progressive aphasia, nfvPPA = non-fluent variant primary progressive aphasia, MMSE = Mini-Mental State Examination, CDR Total = Clinical Dementia Rating Total score, and CDR-SB = Clinical Dementia Rating Sum of Boxes.
FTD includes three clinical subtypes: behavioral variant frontotemporal dementia (bvFTD), semantic variant primary progressive aphasia (svPPA), and non-fluent variant primary progressive aphasia (nfvPPA). Each of the FTD subtypes has a unique symptom constellation that relates to an associated pattern of brain atrophy. In bvFTD, prominent socioemotional deficits occur due to neurodegeneration in predominantly right anterior insula and pregenual anterior cingulate cortex; in svPPA, loss of single-word knowledge arises secondary to anterior temporal lobe degeneration; and in nfvPPA, motor-speech impairment and agrammatism arise in relation to atrophy in left anterior insula, frontal operculum, and inferior frontal gyrus (Seeley et al., 2009).
The final sample of participants included 96 patients with FTD (47 patients with bvFTD, 33 patients with svPPA, and 16 patients with nfvPPA) who were diagnosed according to standard research criteria (Gorno-Tempini et al., 2011; Rascovsky et al., 2007) and 34 healthy controls. Patients were included in the study if they met research criteria for any of the three FTD clinical syndromes described above, completed the emotional assessment, and had a structural MRI within 5 months of the emotional evaluation. Four patients who fulfilled these criteria were excluded from the study because of poor MRI quality.
2.2. Emotional Evaluation
2.2.1. Procedure
Participants’ emotional functioning was assessed at the Berkeley Psychophysiology Laboratory at the University of California, Berkeley. Participants signed consent forms and were seated in a well-lit, 3 m × 6 m experiment room. All stimuli and instructions were presented on a 21-inch color television monitor at a distance of 1.75 m from the participant. Participants completed our standard day-long assessment of emotional functioning that assesses a number of aspects of emotional reactivity, regulation, and recognition/empathy using a variety of tasks including film viewing, social interaction, startle, and karaoke-style singing (Levenson et al., 2008). The data used in the present study were obtained from two study waves (one conducted between 2002 and 2007 and the other between 2007 and 2012); differences between the study waves will be noted and were controlled for statistically.
2.2.2. Laboratory Tasks
2.2.2.1. Happy Film
Participants were asked to relax during a 60-second pre-trial baseline during which an “X” appeared on the television monitor. Participants then saw a film clip that was chosen to elicit happiness. Participants saw either a film depicting Sarah Hughes ice skating and winning the gold medal in front of a large crowd at the Olympics (study wave 1) or a clip of the candy factory scene from I Love Lucy in which two women try to keep up with the rapid pace of a conveyer belt and stuff chocolate candies into their mouths (study wave 2). Despite differences in content, these films both elicit happiness behavior (as indicated by smiling and laughing), physiological reactivity, and self-reported positive emotional experience. These films averaged 2 minutes and 53 seconds in length.
2.2.2.2. Sad Film
After the 60-second pre-trial baseline (described above), participants viewed a well-validated film clip that elicits sadness as measured by sadness behavior, physiological reactivity, and self-reported sadness experience (Werner et al., 2007). The clip was excerpted from the film The Champ (used for both wave 1 and wave 2) and depicts a young boy crying as he watches his father die in the presence of several friends. The sad film was 2 minutes and 13 seconds in length.
2.2.3. Measures
2.2.3.1. Memory Control Question
In order to ensure that participants attended to, understood, and remembered the films, they answered a “memory” question a few minutes after each film had ended. Participants were asked, “What happened in this film?” and were given three multiple choice options. The question and responses were presented visually on a piece of paper or computer monitor in addition to being read aloud. Responses were coded as correct, incorrect, or no answer given.
2.2.3.2. Emotional Behavior
Participants’ behavior was videotaped continuously using a remote-controlled, high-resolution video camera. Participants’ facial behavior during an emotionally intense 30-second period of each film was later coded. A team of trained coders used a modified version of the Emotional Expressive Behavior coding system (Gross & Levenson, 1993) to code each second for nine emotional behaviors (anger, disgust, happiness/amusement, contempt, sadness, embarrassment, fear, surprise, and confusion) on an intensity scale ranging from 0 to 3. Happiness behavior was coded when the participant exhibited smiling and laughter, and sadness behavior was coded when the participant displayed downturned lip corners and upturned inner eyebrows. Inter-coder reliability for the coding system was high (intraclass correlation coefficient = .82). See Table 2 for mean levels of emotional behavior for each group. The intensity scores for each occurrence of happiness during the happy film and sadness during the sad film were summed to obtain a total score for the target emotion for each film.
Table 2. Behavioral data.
Means (M) and standard deviations (SD) for target emotional behavior (happiness and sadness) during the films stratified by diagnostic group. For each target emotional behavior, the mean and 95% confidence intervals (CI) are presented for each diagnostic group. In order to illustrate the heterogeneity of the groups’ facial behavior, the percentages of low, average, and high expressors during the happy and sad films are also presented. Low expressors’ mean target facial behavior fell below the healthy controls’ 95% CI, average expressors’ mean target facial behavior fell within the healthy controls’ 95% CI, and high expressors’ mean target facial behavior fell above the healthy controls’ 95% CI.
|
Healthy
Controls M(SD) |
FTD
(subtypes combined) M(SD) |
bvFTD
M(SD) |
svPPA
M(SD) |
nfvPPA
M(SD) |
|
|---|---|---|---|---|---|
| Happiness behavior |
29.3 (20.4) | 19.2 (21.8) | 16.8 (21.7) | 17.3 (20.0) | 30.0 (23.5) |
| 95% CI | 22.2 – 36.5 | 14.8 – 23.6 | 10.4 – 23.2 | 10.2 – 24.4 | 17.5 – 42.5 |
| Low expressor (%) |
32.4 | 60.4 | 63.8 | 66.7 | 37.5 |
| Average expressor (%) |
38.2 | 13.5 | 12.8 | 12.1 | 18.8 |
| High expressor (%) |
29.4 | 26.0 | 23.4 | 21.2 | 43.8 |
| Sadness behavior |
9.3 (13.9) | 6.5 (12.1) | 7.5 (12.4) | 4.7 (11.1) | 7.1 (13.4) |
| 95% CI | 4.5 – 14.1 | 4.0 – 8.9 | 3.9 – 11.2 | 0.8 – 8.6 | 0.0 – 14.3 |
| Low expressor (%) |
64.7 | 72.9 | 68.1 | 81.8 | 68.8 |
| Average expressor (%) |
2.9 | 4.2 | 4.3 | 0.0 | 12.5 |
| High expressor (%) |
32.4 | 22.9 | 27.7 | 18.2 | 18.3 |
2.2.3.3. Physiological Reactivity
Physiological measures were monitored continuously using a Grass Model 7 or Biopac polygraph, a computer with analog-to-digital capability, and an online data acquisition and analysis software package written by Robert W. Levenson. The software computed second-by-second averages for the following measures: (1) heart rate (Beckman miniature electrodes with Redux paste were placed in a bipolar configuration on opposite sides of the participant’s chest; the inter-beat interval was calculated as the interval, in milliseconds, between successive R waves); (2) finger pulse amplitude (a UFI photoplethysmograph recorded the amplitude of blood volume in the finger using a photocell taped to the distal phalanx of the index finger of the non-dominant hand); (3) finger pulse transmission time (the time interval in milliseconds was measured between the R wave of the electrocardiogram [EKG] and the upstroke of the peripheral pulse at the finger site, recorded from the distal phalanx of the index finger of the non-dominant hand); (4) ear pulse transmission time (a UFI photoplethysmograph attached to the right earlobe recorded the volume of blood in the ear, and the time interval in milliseconds was measured between the R wave of the EKG and the upstroke of peripheral pulse at the ear site); (5) systolic blood pressure, (6) diastolic blood pressure, and (7) mean arterial pressure (a blood pressure cuff was placed on the middle phalanx of the middle finger of the non-dominant hand and continuously recorded blood pressure using an Ohmeda Finapress 2300); (8) skin conductance (a constant-voltage device was used to pass a small voltage between Beckman regular electrodes [using an electrolyte of sodium chloride in unibase] attached to the palmar surface of the middle phalanges of the ring and index fingers of the non-dominant hand); (9) general somatic activity (an electromechanical transducer attached to the platform under the participant’s chair generated an electrical signal proportional to the amount of movement in any direction); (10) respiration period (a pneumatic bellows was stretched around the thoracic region and the inter-cycle interval was measured in milliseconds between successive inspirations); (11) respiration depth (the point of the maximum inspiration minus the point of maximum expiration was determined from respiratory tracing); and (12) finger temperature (a thermistor attached to the distal phalanx of the little finger of the non-dominant hand recorded temperature in degrees Fahrenheit). This array of measures was selected to sample from major autonomic (cardiovascular, electrodermal, respiratory) and somatic systems that are important for emotional responding. See Table 3 for mean physiological levels for each group.
Table 3. Physiological data and self-reported emotional experience.
Means (M) and standard deviations (SD) for individual physiological measures and self-reported positive emotional experience during the happy film.
|
Healthy
Controls M(SD) |
FTD
(subtypes combined) M(SD) |
bvFTD
M(SD) |
svPPA
M(SD) |
nfvPPA
M(SD) |
|
|---|---|---|---|---|---|
| Physiological measures | |||||
| Inter-beat interval (ms) | 913.1 (122.1) |
823.2 (139.6) | 790.4 (139.5) |
855.0 (152.0) |
854.7 (99.2) |
| Finger pulse amplitude (units) |
20.8 (23.5) |
24.9 (29.2) | 26.5 (36.6) |
20.8 (18.5) |
28.0 (22.9) |
| Finger pulse transmission time (ms) |
267.3 (29.7) |
264.2 (30.0) | 263.8 (32.1) |
266.2 (29.1) |
261.2 (27.8) |
| Ear pulse transmission time (ms) |
202.3 (37.3) |
191.4 (26.5) | 190.3 (21.7) |
198.4 (32.3) |
181.7 (25.4) |
| Systolic blood pressure (mmHg) |
143.8 (22.6) |
139.6 (21.4) | 137.9 (21.8) |
137.0 (20.8) |
149.3 (20.6) |
| Diastolic blood pressure (mmHg) |
79.4 (11.5) |
81.8 (12.2) | 83.3 (13.4) |
78.2 (10.5) |
84.0 (11.0) |
| Mean arterial pressure (mmHg) |
99.0 (14.0) |
98.5 (13.2) | 99.2 (14.8) |
95.3 (11.6) |
102.8 (10.4) |
| Skin conductance (μmhos) |
2.4 (2.1) |
2.1 (1.9) | 2.5 (2.1) |
1.4 (1.2) |
2.3 (1.9) |
| Somatic activity (units) | 1.3 (0.8) |
1.3 (0.8) | 1.7 (0.9) |
1.1 (0.6) |
0.9 (0.4) |
| Respiration period (ms) |
3315.2 (1065.9) |
3312.9 (1190.3) |
3240.3 (956.5) |
3402.7 (1547.4) |
3347.7 (1111.5) |
| Respiration depth (units) |
92.3 (114.3) |
81.5 (113.1) | 107.3 (123.7) |
75.2 (107.1) |
22.1 (66.5) |
| Finger temperature (°F) |
82.1 (6.0) |
83.3 (6.6) | 84.4 (6.6) |
81.7 (6.1) |
83.4 (7.2) |
| Self-reported emotional experience |
|||||
| Happiness or amusement |
1.7 (0.5) |
1.6 (0.6) | 1.6 (0.6) |
1.5 (0.7) |
1.6 (0.6) |
Physiological reactivity scores were computed for the happy and sad films. For each film trial, the average level of each physiological measure during the 60-second pre-film baseline was subtracted from the average level during an intense 30-second period during each film. Scores were normalized and reversed as needed (i.e., cardiac inter-beat interval, finger pulse transmission time, ear pulse transmission time, and respiration period) so that larger values reflected greater physiological arousal.
2.2.3.4. Self-Reported Emotional Experience
After each film, participants were asked to rate how intensely they experienced the target emotion for each film (i.e., happy/amused after the happy film and sad after the sad film). Participants were asked, “Did you feel ___ while watching the film?” and were given the response choices of “No,” “A little,” or “A lot.” These answers were given a numerical score of 0, 1, or 2, respectively. See Table 3 for mean self-reported emotion levels for each group.
2.3. Neuroimaging
2.3.1. Structural Neuroimaging Acquisition
Participants underwent research-quality structural MRI. 1.5T images were acquired on a 1.5T Siemens Magnetom VISION system (Siemens, Iselin, NJ) at the San Francisco Veterans Administration Hospital equipped with a standard quadrature head coil, using a magnetization prepared rapid gradient echo (MPRAGE) sequence (164 coronal slices; slice thickness = 1.5 mm; field of view [FOV] = 256 × 256 mm2; matrix 256 × 256; voxel size 1.0 × 1.5 × 1.0 mm3; repetition time [TR] = 10 ms; echo time [TE] = 4 ms; flip angle = 15°). 3T images were obtained on a 3.0 Tesla Siemens (Siemens, Iselin, NJ) TIM Trio scanner equipped with a 12-channel head coil located at the UCSF Neuroscience Imaging Center. Whole brain images were acquired using volumetric MPRAGE (160 sagittal slices; slice thickness = 1.0 mm; FOV = 256 × 230 mm2; matrix 256 × 230; voxel size 1.0 × 1.0 × 1.0 mm3; TR = 2300 ms; TE = 2.98 ms; flip angle = 9°). 4T images were acquired at the San Francisco Veterans Administration Hospital Bruker MedSpec system with an 8 channel head coil controlled by a Siemens Trio console, using an MPRAGE sequence (192 sagittal slices; slice thickness = 1 mm; FOV = 256 × 224 mm2; matrix = 256 × 224; voxel size = 1.0 × 1.0 × 1.0 mm3; TR = 2840 ms; TE = 3 ms; flip angle = 7°). Structural neuroimaging analyses utilizing images collected across different modes of hardware have robust effects (Abdulkadir et al., 2011) and, thus, are unlikely to cause artifacts at the level of strict statistical thresholds.
2.3.2. Preprocessing
Preprocessing was conducted according to previously described methods (Sturm et al., 2013). Structural T1 images were visually inspected for movement artifact, corrected for bias field, segmented into gray matter, white matter, and cerebrospinal fluid, and spatially normalized to MNI space (Ashburner & Friston, 2005) using Statistical Parametric Mapping (SPM) 5 (Friston, Ashburner, Kiebel, Nichols, & Penny, 2007). In all preprocessing steps, SPM5 default parameters were utilized with the exception of using the light clean-up procedure in the morphological filtering step. Default tissue probability priors (voxel size: 2.0 × 2.0 × 2.0 mm3) of the International Consortium for Brain Mapping were used. Segmented images were visually inspected for adequate gray-white segmentation, and the Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL) toolbox was then used. Gray and white matter maps were then summed, and these images were smoothed with an 8 mm full-width at half-maximum Gaussian kernel.
2.4. Analyses
2.4.1. Demographic and Clinical Analyses
We used analyses of variance (ANOVA) to compare the FTD group (subtypes combined) to the healthy controls in their age and functional status (CDR-SB). We used chi-square tests to determine whether there were similar proportions of men and women and study wave 1 or 2 participation rates among the patients and controls. We used those variables that were significantly different as covariates in our behavioral analyses.
We used ANOVA to examine group differences on cognitive screening measures. For the cognitive test scores, partial eta squared (ηp2) statistics are noted with .01-.05 representing a small effect, .06 to .13 representing a medium effect, and .14 or greater representing a large effect (Cohen, 1992). Means and standard deviations for the demographic and clinical measures for the combined FTD group as well as each clinical subtype are presented in Table 1.
2.4.2. Memory Control Question
We conducted chi-square tests to determine whether similar proportions of patients and controls responded to the memory control question correctly.
2.4.3. Emotional Measures: Group Comparisons
2.4.3.1. Emotional Behavior
We conducted one-way analyses of covariance (ANCOVA) to compare total happiness and sadness behavior during the films in the FTD group (subtypes combined) compared to the healthy controls (controlling for age and CDR-SB). We next conducted follow-up ANCOVAs comparing the healthy controls to the FTD clinical subtypes on total happiness and sadness behavior.
2.4.3.2. Distribution of Extreme Behavioral Scores
To examine the distribution of happiness and sadness behavior in the patients with FTD, we first computed the 95% confidence intervals (CI) for total happiness and sadness behavior in the healthy controls. We then coded each of the patients with 0 for those falling below the controls’ 95% CI (low expressor), 1 for those falling within the controls’ 95% CI (average expressor), or 2 for those falling above the controls’ 95% CI (high expressor).
2.4.4. Neuroimaging Analyses: Emotional Behavior
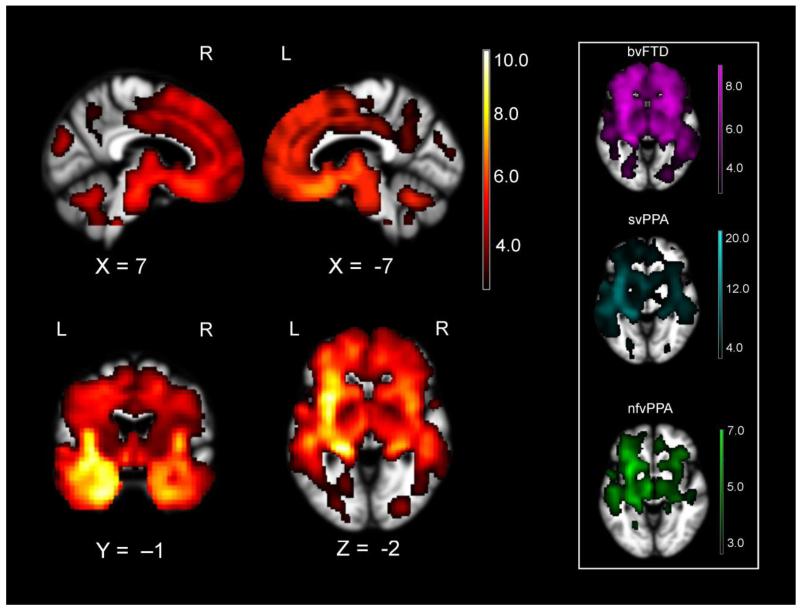
Taken together, the full FTD sample had significant variability in the extent to which left and right emotion generating and regulating systems were affected. Each FTD subtype exhibited frontotemporal atrophy with the expected subtype-specific variability (i.e., bilateral predominantly frontal atrophy in bvFTD, dominant left anterior temporal lobe atrophy in svPPA, and primarily left inferior frontal involvement in nfvPPA) that is consistent with the clinical syndrome (see Figure 1; Seeley et al., 2009). We harnessed this heterogeneity in behavior and brain atrophy to examine whether deterioration of lateralized neural systems correlated with happiness behavior across individuals. We conducted whole-brain voxel-based morphometry (VBM) analyses in the patients to correlate emotional behavior with combined gray/white matter structural maps, which provide a single measure of brain parenchyma and is a useful way to correlate atrophy with behavior in patients with neurodegenerative disease (Wilson et al., 2010).
Figure 1.
The full FTD sample (FTD subtypes combined) had significant atrophy in left and right hemisphere emotion generating (e.g., amygdala, hypothalamus, and brainstem) and emotion regulating (e.g., orbitofrontal and ventrolateral prefrontal cortex) systems as compared to a sample of healthy controls (n= 34). The atrophy pattern for each clinical subtype separately versus healthy controls is displayed in the box of the right (bvFTD in violet, svPPA in cyan, and nfvPPA in green). Color bar represents T-scores (hot= pFWE<.05 according to study-specific permutation analysis) for regions with smaller volume in FTD when controlling for age, sex, field strength, handedness, and total intracranial volume.
Our primary variable of interest in the whole-brain VBM analyses was total happiness behavior during the happy film. We included age, sex, CDR-SB, diagnosis (two variables for the three patient groups, parameterized 0 for the target diagnostic group and 1 for the remaining groups, to rule out the possibility that significant findings held true only in one group), study wave (in order to control for differences in data processing in the two waves of data collection), field strength (two variables for the three field strengths, parameterized 0 for the field strength of interest and 1 for the remaining field strengths), handedness (left= 0, right= 1), and total intracranial volume (a total of gray matter, white matter, and cerebrospinal fluid volume, to account for individual differences in head size) as nuisance covariates. To explore whether similar brain regions were also associated with sadness behavior during the sad film, we ran an additional whole-brain analysis using total sadness behavior during the sad film (same covariates as in the previous analyses).
In the whole-brain VBM analyses, a priori significance was established at uncorrected praw<.005. One thousand permutation analyses using combined peak and extent thresholds were run to derive a study-specific error distribution to determine the one-tailed T-threshold for multiple comparisons correction at pFWE<.05 (Nichols & Holmes, 2002). Permutation analysis is a resampling approach to significance testing by which a test statistic is compared to the null distribution derived from the present study’s dataset and thus is an accurate representation of Type 1 error at p< .05 across the entire brain (Kimberg, Coslett, & Schwartz, 2007). Images were overlaid with MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron/) on an average brain based on the gray and white matter templates used for DARTEL warping.
2.4.5. Neuroimaging Analyses: Physiological Reactivity and Subjective Experience
In these analyses, we restricted our search to brain areas that were significantly associated with happiness behavior at pFWE< .05 to offset the loss of power incurred by correcting for multiple comparisons. Results were considered significant at p<.005, uncorrected.
2.4.5.1. Physiological Reactivity
Emotional reactivity during film-viewing may be manifest by coordinated changes in subjective experience, facial behavior, and physiological activation (Giuliani et al., 2008; Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005). In order to constrain the scope of the neuroimaging analyses, we correlated total happiness behavior with individual physiological reactivity scores and planned to focus our exploratory VBM analyses on those physiological variables that were significantly associated with happiness behavior.
2.4.5.2. Subjective Experience
Total ratings of happiness or amusement experience during the happy film were also used as a variable of interest in an additional VBM analysis.
3. Results
3.1. Demographic and Clinical Analyses
There was a trend for the patients with FTD (subtypes combined) to be younger than the healthy controls, F(1, 128)= 3.8, p= .053. There were no differences in the proportions of men and women, χ2(1, N=130)= 1.1, p= .29, or study wave, χ2(1, N=130)= 0.2, p=.66, among the groups. By definition, the patients with FTD were more functionally impaired than the healthy controls, CDR-SB, F(1, 128)= 78.9, p< .001. Thus, we included age and CDR-SB as covariates in our analyses.
Overall, patients with FTD performed worse than the healthy controls on neuropsychological testing. Patients had most difficulty with tests of executive functioning including tests of generation: semantic fluency, F(1, 87)= 96.6, p< .001, ηp2= .53; phonemic fluency, F(1, 87)= 36.6, p< .001, ηp2= .30; and design fluency, F(3, 87)= 24.5, p< .001, ηp2= .22. Scores on Benson recall (Possin, Laluz, Alcantar, Miller, & Kramer, 2011; visual episodic memory), F(1, 87)= 21.1, p< .001, ηp2= .20; abbreviated Boston Naming Test (confrontational naming; Kaplan, Goodglass, & Weintraub, 1983), F(3, 87)= 20.2, p< .001, ηp2= .19; Modified Trails completion time (set-shifting), F(3, 87)= 19.9, p< .001, ηp2= .19; California Verbal Learning Test-Short Form (Delis, Kramer, Kaplan, & Ober, 2000) 10-minute delay (verbal episodic memory), F(3, 97)= 21.9, p< .001, ηp2= .18; Peabody Picture Vocabulary Test (semantic knowledge; Dunn, 1970), F(3, 87)= 14.4, p< .001, ηp2= .14; digits backward (working memory), F(3, 87)= 11.0, p< .05, ηp2= .11; calculations, F(3, 87)= 8.3, p< .01, ηp2= .09; Benson figure copy (Possin et al., 2011) (visuospatial processing), F(3, 87)= 4.2, p< .05, ηp2= .05, were also affected. In general, patients with FTD were in the mild to moderate stages of disease progression as indicated by their scores on functional and cognitive assessments. See Table 1 for means and standard deviations for these measures.
3.2. Memory Control Question
The FTD group (subtypes combined) did not differ from the healthy controls in the proportion of participants who answered the memory question correctly for the happy film, χ2(1, N=128)= 0.6, p= .50, or the sad film, χ2(1, N=128)= 1.5, p= 22. The healthy controls and each FTD subtype performed well on these questions and had little trouble identifying the correct response for the happy film (97.1% of healthy controls, 93.5% of patients with bvFTD, 90.6% of patients with svPPA, and 100% of patients with nfvPPA) and sad film (100.0% of healthy controls, 95.6% of patients with bvFTD, 93.9% of patients with svPPA, and 100% of patients with nfvPPA). We conclude from these findings that the patients had no difficulty comprehending or recalling the films’ content.
3.3. Emotional Measures: Group Comparisons
3.3.1. Emotional Behavior
One-way ANCOVAs (controlling for age and CDR-SB) found no differences between the FTD (subtypes combined) group and the healthy controls on total happiness, F(1, 126)= 1.1, p= .29, or sadness, F(1, 126)= 0.1, p= .72, behavior displayed during the films. Follow-up ANCOVAs comparing the clinical FTD subtypes (bvFTD, svPPA, and nfvPPA) to the healthy controls (controlling for age and CDR-SB) also revealed no main effect of diagnosis on happiness, F(3, 124)= 1.5, p= .21, or sadness, F(3, 124)= 0.7, p= .57, behavior.
3.3.2. Distribution of Extreme Behavioral Scores
The lack of a significant main effect of diagnosis on total happiness and sadness behavior prompted us to examine the distribution of facial expressivity in each of the diagnostic groups. Each of the FTD subtypes had significant proportions of patients who fell into the low and high extremes of facial expressivity during the happy and sad films as compared to the healthy controls, which speaks to the heterogeneity in positive and negative emotional reactivity in FTD. During the happy film, the majority of patients in the FTD (subtypes combined, bvFTD, and svPPA) groups were low expressors (60.4, 63.8, and 66.7%, respectively) with approximately twice as many patients in each of these groups showing minimal happiness behavior as compared to the healthy controls. Each of these groups had comparable proportions of high expressors (26.0, 23.4, 21.2%) as the healthy controls (29.4%), however, rendering group effects statistically non-significant. Interestingly, the nfvPPA group had the highest rate of high expressors during the happy film (43.8%). During the sad film, although the majority of participants in each of the groups displayed low levels of sadness behavior (percentages ranged from 64.7 to 81.8%), there was also a subset in each group who were high expressors during this film (percentages ranged from 18.2 to 32.4%). See Table 2 for the proportions of participants in each diagnostic group that were low, average, and high expressors as compared to the healthy controls.
3.4. Neuroimaging Analyses: Emotional Behavior
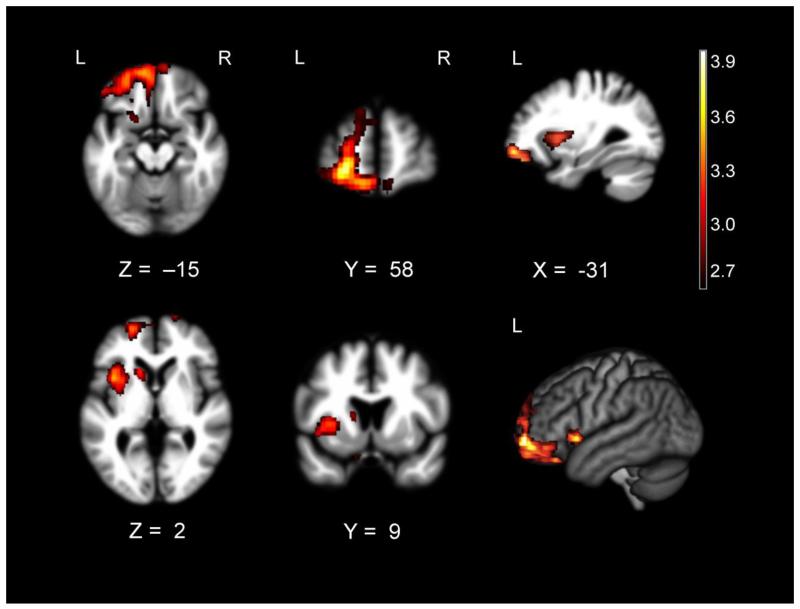
Whole-brain VBM analyses revealed multiple areas where atrophy was associated with greater happiness behavior during the happy film. These included a cluster that included left ventrolateral prefrontal cortex, orbitofrontal cortex, left anterior insula, left striatum, left rostromedial prefrontal cortex, and right orbitofrontal cortex (pFWE< .05). See Table 4 for T-scores and significance levels for all associated regions. Figure 2 displays the statistical maps.
Table 4. Anatomical correlates of happiness reactivity.
Volume loss in predominantly left hemisphere regions is associated with greater happiness behavior (whole-brain analysis) and greater cardiovascular reactivity (masked to the significant cluster found in the behavioral analysis) during the happy film in FTD when controlling for age, sex, CDR-SB, diagnosis, study wave, field strength, handedness, and total intracranial volume. Montreal Neurological Institute coordinates (x, y, z) given for maximum T-score for the cluster (cluster size > 70 mm3). Results are significant at praw<.005, uncorrected.
| Anatomical Region |
Cluster
Volume (mm3) |
x | y | z |
Maximum
T-score |
|---|---|---|---|---|---|
| Happiness behavior | |||||
| Left ventrolateral prefrontal cortex | 41136* | −22 | 62 | −4 | 3.98 |
| Left orbitofrontal cortex | † | ||||
| Left rostromedial prefrontal cortex | † | ||||
| Left striatum | † | ||||
| Left anterior insula | † | ||||
| Right orbitofrontal cortex | † | ||||
| Physiological reactivity Inter-beat interval |
|||||
| Left medial orbitofrontal cortex | 800 | −18 | 70 | −6 | 3.26 |
| Right medial orbitofrontal cortex | 600 | 12 | 72 | −2 | 3.23 |
| Left superior frontal gyrus | 536 | −6 | 64 | 30 | 3.06 |
| Right rostromedial prefrontal cortex | 112 | 6 | 64 | 8 | 3.24 |
| Left frontopolar cortex | 88 | −10 | 62 | −22 | 2.79 |
| Systolic blood pressure | |||||
| Left frontopolar cortex | 320 | −26 | 64 | −4 | 3.12 |
| Left anterior insula | 72 | −42 | 16 | −8 | 3.23 |
| Diastolic blood pressure | |||||
| Left frontopolar cortex | 104 | −22 | 56 | −20 | 2.79 |
| Left anterior insula | 96 | −42 | 16 | −8 | 3.11 |
| Mean arterial pressure | |||||
| Left anterior insula | 640 | −42 | 16 | −8 | 3.93 |
| Left rostromedial prefrontal cortex | 184 | −6 | 64 | 28 | 2.98 |
| 80 | −22 | 66 | 10 | 3.05 |
denotes the cluster significant at pFWE < .05.
signifies that these regions were included in the cluster above.
Figure 2.
T-score maps of brain areas for which volume loss was associated with higher levels of happiness behavior in patients with FTD (n= 96) when controlling for age, sex, CDR-SB, diagnosis, study wave, field strength, handedness, and total intracranial volume. Smaller volume in a cluster (Max T= 3.98) that included left ventrolateral and orbitofrontal cortex; left anterior insula, striatum, rostromedial prefrontal cortex, and superior frontal gyrus; and bilateral gyrus rectus was associated with higher happiness behavior after correction for Type 1 error (pFWE<.05). Color bar represents T-scores (hot= pFWE<.05 according to study-specific permutation analysis, T> 2.63). Results for all analyses are overlaid on the warping template from DARTEL.
At less stringent statistical thresholds (p< .005, uncorrected), smaller volume in other left-hemisphere regions including supplementary motor area (T= 4.44; MNI peak: -4, 14, 68; size 6232 mm3), lingual gyrus (T= 3.22; MNI peak: -16, -76, -10; size 1040 mm3), superior temporal gyrus (T= 2.98; MNI peak: -46, -20, 4; size 672 mm3), hypothalamus (T= 2.99; MNI peak: -4, -10, -22; size 552 mm3), precuneus (T= 3.08; MNI peak: -6, -56, 60; size 312 mm3), as well as right hemisphere regions including ventrolateral prefrontal cortex (T= 3.28; MNI peak: 50, 42, -8; size 4064 mm3 and T= 2.89; MNI peak: 46, 36, 8; size 552 mm3), postcentral gyrus (T= 3.68; MNI peak: 48, -22, 56; size 1512 mm3), and rolandic operculum (T= 3.31; MNI peak: 58, 8, 14; size 1272 mm3 and T= 2.80; MNI peak: 60, -6, 14; size 200 mm3) were also associated with greater happiness behavior during the happy film. In a separate whole-brain VBM analysis, there were no regions for which smaller volume was associated with greater sadness behavior during the sad film.
3.5. Neuroimaging Analyses: Physiological Reactivity and Subjective Experience
3.5.1. Physiological Reactivity
Greater happiness behavior during the happy film was associated with higher reactivity in heart rate, r(95)= .44, p< .001; somatic activity, r(95)= .44, p< .001; skin conductance, r(93)= .36, p< .001; respiration period, r(85)= .35, p< .01; systolic blood pressure, r(76)= .27, p< .05; diastolic blood pressure, r(76)= .42, p< .001; and mean arterial pressure, r(76)= .33, p< .01. Thus, these variables were used as independent variables in the VBM analyses.
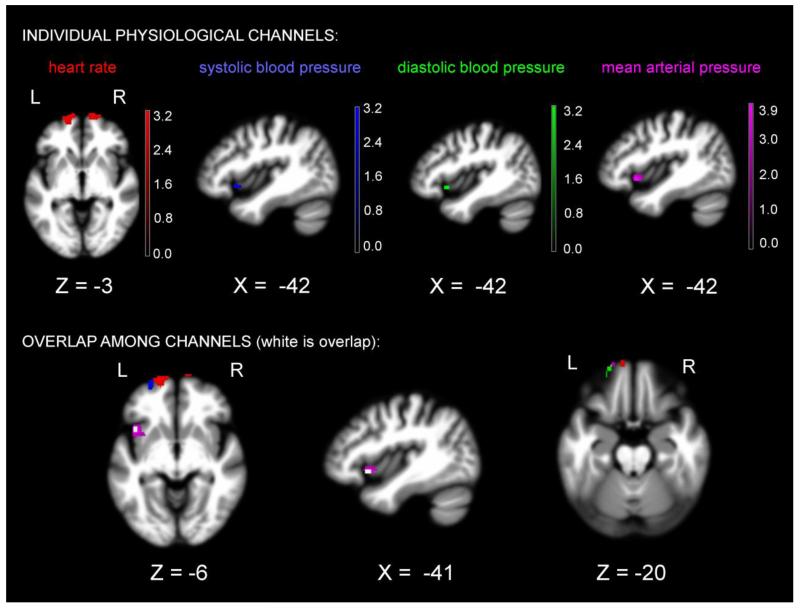
When controlling for the same covariates that were used in the behavioral analysis, smaller volume in left anterior insula was associated with greater reactivity during the happy film in systolic blood pressure, diastolic blood pressure, and mean arterial pressure (p< .005, uncorrected). Smaller volume in bilateral frontopolar cortex was associated with greater reactivity in heart rate during the happy film. See Table 4 for T-scores and significance levels for all associated regions. Figure 3 displays the statistical maps.
Figure 3.
T-score maps of brain areas for which volume loss was associated with higher reactivity in heart rate, systolic blood pressure, diastolic blood pressure, and mean arterial pressure in patients with FTD while they watched the happy film, controlling for multiple covariates (for list, see Figure 2). Color bar represents T-scores, praw<.005, uncorrected: red= heart rate (Max T= 3.26), blue= systolic blood pressure (Max T= 3.23), green= diastolic blood pressure (Max T= 3.11), violet= mean arterial pressure (Max T= 3.93), and white= overlap.
3.5.2. Subjective Experience
There were no regions that were significantly associated with greater happiness or amusement experience at p< .005, uncorrected, when controlling for the same covariates listed above.
4. Discussion
Previous studies have established that the left hemisphere, and the left frontal lobe in particular, plays an integral role in positive emotion. How left hemisphere emotion generating and regulating systems interact to produce positive emotion, however, is less well understood. Using a sample of patients with FTD, we found that atrophy in predominantly left fronto-striatal emotion regulation systems (i.e., left ventrolateral prefrontal cortex, orbitofrontal cortex, rostromedial prefrontal cortex, striatum, and anterior insula) was associated with higher levels of happiness behavior while watching a happy film. Tissue loss in frontopolar cortex and anterior insula were associated with higher attendant cardiovascular reactivity during the happy film. No brain regions were significantly associated with higher self-reported happiness or amusement experience. We investigated whether these results were specific to positive emotion by also examining whether a similar lateralized atrophy pattern was associated with greater negative emotion. Higher sadness behavior was not associated with atrophy in any of these regions, which suggests that atrophy in left-sided emotion regulatory systems may relate specifically to positive emotion dysregulation.
The results of the present study extend previous models of the neural systems that support positive emotion. Many studies suggest that the left frontal lobe plays a dominant role in positive emotion generation and that left-sided damage, therefore, reduces positive emotion (Davidson & Fox, 1982; Sackeim et al., 1982). However, these studies have not been able to determine whether left-lateralized damage that is relatively restricted to emotion generators or emotion regulators has different effects on positive emotion. Emotions are both automatic, allowing rapid responding to salient biological and social cues, and flexible, enabling nuanced emotional modulation. Thus, asymmetric damage that targets brain systems that support emotional reactivity or emotion regulation may result in valence-specific emotional loss or gain. Contrary to previous studies, our results suggest that left frontal damage does not always cause predictable deficits in positive emotion. Rather, our findings support a model of emotion in which relatively selective damage to left hemisphere emotion regulatory systems weakens positive emotion regulation and facilitates positive emotional responding to a happy film (consistent with our hypothesis 2).
Happiness is a positive emotion that is characterized by changes in facial expression and autonomic reactivity. The degree to which an individual displays happiness in response to a positive emotional stimulus such as a film clip depends on multiple factors (e.g., personality style, previous experience, and mood state) and likely is the product of activity in both emotion generating and regulating systems. Regions that we found to be important for controlling happiness behavior and cardiovascular reactivity overlap with areas known to be important for emotion regulation as well as for behavioral inhibition more broadly (Aron, 2007; Nee, Wager, & Jonides, 2007). For example, atrophy in orbitofrontal cortex, a region that promotes social regulation and socioemotional stimulus tracking (Beer, Heerey, Keltner, Scabini, & Knight, 2003; Goodkind et al., 2012), may also interfere with interoception, facial control, and cardiovascular responding to positive emotional stimuli (An, Bandler, Ongur, & Price, 1998; Ferry, Ongur, An, & Price, 2000), leading to dysregulated happiness. Neurodegeneration in the anterior insula, a region that integrates multi-modal interoceptive and sensory information (Craig, 2002; Menon & Uddin, 2010) and is important for expressive suppression, behavioral inhibition, and autonomic control (Giuliani, Drabant, Bhatnagar, & Gross, 2011; Jezzini, Caruana, Stoianov, Gallese, & Rizzolatti, 2012; Kurth, Zilles, Fox, Laird, & Eickhoff, 2010), may also diminish emotion regulation by degrading afferent representations of facial movement or impeding the translation of interoceptive signals into efferent inhibitory motor commands via the striatum. Left-lateralized atrophy in frontally anchored systems that promote emotion regulation, therefore, may make positive emotions more likely to be elicited and interfere with their downregulation.
Our findings also have clinical implications for FTD and other mental illnesses. Positive emotions play an essential role in human life by promoting approach behavior and affiliation (Fredrickson, 2004). Some individuals with FTD become overfamiliar, jocular, creative, and elated (Mendez et al., 2006), symptoms that may stem from positive emotion dysregulation and can lead to problematic behaviors (e.g., engagement in inappropriate social interactions such as touching strangers and high-risk/high-reward activities such as gambling). Bipolar disorder is characterized by chronically elevated positive emotion (in addition to heightened irritability or “mixed” emotional states in which there is a combination of euphoria and irritability), emotion dysregulation, and interpersonal difficulties. Although previous neuroimaging studies have found diminished activity in emotion regulating systems and enhanced activity in emotion generators in bipolar disorder (Brooks, Hoblyn, Woodard, Rosen, & Ketter, 2009), it is not clear whether there is lateralized network dysfunction in bipolar disorder. Given that the left frontal lobe plays a dominant role in positive emotion and in anger, a negative emotion that is unique in that it also promotes approach behavior (Harmon-Jones, Gable, & Peterson, 2010), left-lateralized frontal dysfunction may be a plausible explanation for the joint dysregulation of happiness and anger/irritability that defines bipolar disorder. Whether lateralized shrinkage in orbitofrontal cortex volume also relates to age-related increases in positive emotion in normal aging (Levenson, Carstensen, & Gottman, 1994) is a question that has not yet been investigated.
5. Limitations
There are some limitations of the present study that warrant consideration. First, we only examined happiness as an exemplar of positive emotion. Happiness, when accompanied by smiling and laughing is a high arousal positive emotion that may have different neural correlates than other positive emotions that are less activating (e.g., nurturant love, contentment, or compassion). Thus, atrophy in left hemisphere fronto-striatal systems may only be relevant to high arousal positive emotions. If this were true, then our findings would not generalize to low arousal positive emotions. Second, we do not know with certainty which hemisphere was responsible for the generation of positive emotion in the present study. Because ipsilateral frontal projections are more common than contralateral projections (Barbas, Hilgetag, Saha, Dermon, & Suski, 2005), it is most likely that damage to left-frontal emotion regulation systems would release activity in left hemisphere emotion generating systems, but this was not directly measured. Third, the patients with FTD did not significantly differ from the healthy controls in their mean level of happiness reactivity. While many patients with FTD showed little emotion to these films, some patients had a dysregulated reaction. Thus, our findings may only be relevant for a subset of patients with FTD who do not yet have extensive damage to emotion generating systems. It is likely that selective damage to left fronto-striatal emotion regulating systems will only lead to increased happiness in those patients who can still initiate a positive emotional response. Whether our results also have implications for other pathological forms of positive emotional dysregulation (i.e., mania), remains to be investigated.
6. Conclusions
The present study offers new insights into the neural systems that support positive emotion by offering evidence that selective damage to left hemisphere fronto-striatal emotion regulating circuits may be associated with gains in positive emotions such as happiness. Although previous emotional theories and neuroanatomical models have emphasized the importance of the left frontal lobe in positive emotion, these theories are less explicit about the roles that asymmetric emotion generating and regulating systems play in supporting valence-specific emotional behavior. This study has implications for basic affective neuroscience and has broad-reaching implications for understanding positive emotional alterations in both psychiatric and neurological disease as well as the emotional changes that occur with normal aging.
Acknowledgements
The authors would like to thank Stephen Wilson, Ph.D. (www.neuroling.arizona.edu) and Benno Gesierich, Ph.D. for their assistance with the neuroimaging processing and analyses.
Funding
This project was supported by grants from the NIH National Institute on Aging (P50AG023501, P01AG019724, 1R01AG032306-01A1, AG17766, AG19724, and 1K23AG040127), and The Larry L. Hillblom Foundation (2013-A-029-SUP, 2002/2J, 2007/2I, and 2005/2T).
References
- Abdulkadir A, Mortamet B, Vemuri P, Jack CR, Jr., Krueger G, Kloppel S. Effects of hardware heterogeneity on the performance of SVM Alzheimer’s disease classifier. NeuroImage. 2011;58(3):785–792. doi: 10.1016/j.neuroimage.2011.06.029. doi: 10.1016/j.neuroimage.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An X, Bandler R, Ongur D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. Journal of Comparative Neurology. 1998;401(4):455–479. [PubMed] [Google Scholar]
- Aron AR. The neural basis of inhibition in cognitive control. The Neuroscientist. 2007;13(3):214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Barbas H, Hilgetag CC, Saha S, Dermon CR, Suski JL. Parallel organization of contralateral and ipsilateral prefrontal cortical projections in the rhesus monkey. BMC Neurosci. 2005;6:32. doi: 10.1186/1471-2202-6-32. doi: 10.1186/1471-2202-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer JS, Heerey EA, Keltner D, Scabini D, Knight RT. The regulatory function of self-conscious emotion: Insights from patients with orbitofrontal damage. Journal of Personality and Social Psychology. 2003;85(4):594–604. doi: 10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Miller BL. Clinical features of frontotemporal dementia. Alzheimer Disease and Associated Disorders. 2005;19(Suppl 1):S3–6. doi: 10.1097/01.wad.0000183086.99691.91. [DOI] [PubMed] [Google Scholar]
- Brooks JO, 3rd, Hoblyn JC, Woodard SA, Rosen AC, Ketter TA. Corticolimbic metabolic dysregulation in euthymic older adults with bipolar disorder. Journal of Psychiatric Research. 2009;43(5):497–502. doi: 10.1016/j.jpsychires.2008.08.001. doi: 10.1016/j.jpsychires.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition. 1992;20(1):125–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Fox NA. Asymmetrical brain activity discriminates between positive and negative affective stimuli in human infants. Science. 1982;218(4578):1235–1237. doi: 10.1126/science.7146906. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3(1):11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: Adult version (CVLT-II): Manual. 2nd ed. Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- Dunn LM. Expanded manual for the Peabody Picture Vocabulary Test. American Guidance Service; Minneapolis, MN: 1970. [Google Scholar]
- Eckart JA, Sturm VE, Miller BL, Levenson RW. Diminished disgust reactivity in behavioral variant frontotemporal dementia. Neuropsychologia. 2012;50(5):786–790. doi: 10.1016/j.neuropsychologia.2012.01.012. doi: 10.1016/j.neuropsychologia.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry AT, Ongur D, An X, Price JL. Prefrontal cortical projections to the striatum in macaque monkeys: evidence for an organization related to prefrontal networks. Journal of Comparative Neurology. 2000;425(3):447–470. doi: 10.1002/1096-9861(20000925)425:3<447::aid-cne9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL. The broaden-and-build theory of positive emotions. Philosophical Transactions of the Royal Society of London Series B Biological Series. 2004;359(1449):1367–1378. doi: 10.1098/rstb.2004.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Levenson RW. Positive emotions speed recovery from the cardiovascular sequelae of negative emotions. Cognition and Emotion. 1998;12(2):191–220. doi: 10.1080/026999398379718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Kiebel S, Nichols T, Penny WD, editors. Statistical Parametric Mapping: The Analysis of Functional Brain Images. Academic Press; London: 2007. [Google Scholar]
- Gainotti G. Emotional behavior and hemispheric side of the lesion. Cortex. 1972;8(1):41–55. doi: 10.1016/s0010-9452(72)80026-1. [DOI] [PubMed] [Google Scholar]
- Giuliani NR, Drabant EM, Bhatnagar R, Gross JJ. Emotion regulation and brain plasticity: expressive suppression use predicts anterior insula volume. NeuroImage. 2011;58(1):10–15. doi: 10.1016/j.neuroimage.2011.06.028. doi: 10.1016/j.neuroimage.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani NR, McRae K, Gross JJ. The up- and down-regulation of amusement: experiential, behavioral, and autonomic consequences. Emotion. 2008;8(5):714–719. doi: 10.1037/a0013236. doi: 10.1037/a0013236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind MS, Gyurak A, McCarthy M, Miller BL, Levenson RW. Emotion regulation deficits in frontotemporal lobar degeneration and Alzheimer’s disease. Psychology and Aging. 2010;25(1):30–37. doi: 10.1037/a0018519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind MS, Sollberger M, Gyurak A, Rosen HJ, Rankin KP, Miller B, Levenson R. Tracking emotional valence: The role of the orbitofrontal cortex. Human Brain Mapping. 2012 doi: 10.1002/hbm.21251. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotional suppression: Physiology, self-report, and expressive behavior. Journal of Personality and Social Psychology. 1993;64(6):970–986. doi: 10.1037//0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- Gruber J, Harvey AG, Purcell A. What goes up can come down? A preliminary investigation of emotion reactivity and emotion recovery in bipolar disorder. Journal of Affective Disorders. 2011;133(3):457–466. doi: 10.1016/j.jad.2011.05.009. doi: 10.1016/j.jad.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, Peterson CK. The role of asymmetric frontal cortical activity in emotion-related phenomena: a review and update. Biological Psychology. 2010;84(3):451–462. doi: 10.1016/j.biopsycho.2009.08.010. doi: 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- House A, Dennis M, Warlow C, Hawton K, Molyneux A. Mood disorders after stroke and their relation to lesion location. A CT scan study. Brain. 1990;113(Pt 4):1113–1129. doi: 10.1093/brain/113.4.1113. [DOI] [PubMed] [Google Scholar]
- Jezzini A, Caruana F, Stoianov I, Gallese V, Rizzolatti G. Functional organization of the insula and inner perisylvian regions. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(25):10077–10082. doi: 10.1073/pnas.1200143109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston naming test. Lea & Febiger; Philadelphia, PA: 1983. [Google Scholar]
- Kimberg DY, Coslett HB, Schwartz MF. Power in Voxel-based lesion-symptom mapping. Journal of Cognitive Neuroscience. 2007;19(7):1067–1080. doi: 10.1162/jocn.2007.19.7.1067. doi: 10.1162/jocn.2007.19.7.1067. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure and Function. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson RW, Ascher E, Goodkind M, McCarthy M, Sturm V, Werner K. Laboratory testing of emotion and frontal cortex. In: Goldenberg G, Miller BL, editors. Handbook of clinical neurology: Vol. 88 (3rd series). Neuropsychology and behavioral neurology. Elsevier; Edinburgh: 2008. pp. 489–498. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Carstensen LL, Gottman JM. The influence of age and gender on affect, physiology, and their interrelations: a study of long-term marriages. Journal of Personality and Social Psychology. 1994;67(1):56–68. doi: 10.1037//0022-3514.67.1.56. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5(2):175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Chen AK, Shapira JS, Lu PH, Miller BL. Acquired extroversion associated with bitemporal variant of frontotemporal dementia. The Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18(1):100–107. doi: 10.1176/jnp.18.1.100. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5-6):655–667. doi: 10.1007/s00429-010-0262-0. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cognitive, Affective, and Behavioral Neuroscience. 2007;7(1):1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Science. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Neuroevolutionary sources of laughter and social joy: modeling primal human laughter in laboratory rats. Behavioural Brain Research. 2007;182(2):231–244. doi: 10.1016/j.bbr.2007.02.015. doi: 10.1016/j.bbr.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Perria L, Rosadini G, Rossi GF. Determination of side of cerebral dominance with amobarbital. Archives of Neurology. 1961;4:173–181. doi: 10.1001/archneur.1961.00450080055006. [DOI] [PubMed] [Google Scholar]
- Possin KL, Laluz VR, Alcantar OZ, Miller BL, Kramer JH. Distinct neuroanatomical substrates and cognitive mechanisms of figure copy performance in Alzheimer’s disease and behavioral variant frontotemporal dementia. Neuropsychologia. 2011;49(1):43–48. doi: 10.1016/j.neuropsychologia.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Kipps CM, Johnson JK, Seeley WW, Mendez MF, Miller BL. Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): Current limitations and future directions. Alzheimer Disease and Associated Disorders. 2007;21(4):S14–S18. doi: 10.1097/WAD.0b013e31815c3445. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Greenberg MS, Weiman AL, Gur RC, Hungerbuhler JP, Geschwind N. Hemispheric asymmetry in the expression of positive and negative emotions. Neurologic evidence. Archives of Neurology. 1982;39(4):210–218. doi: 10.1001/archneur.1982.00510160016003. [DOI] [PubMed] [Google Scholar]
- Saper CB. The central autonomic nervous system: Conscious visceral perception and autonomic pattern generation. Annual Review of Neuroscience. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota MN, Neufeld SL, Yeung WH, Moser SE, Perea EF. Feeling good: autonomic nervous system responding in five positive emotions. Emotion. 2011;11(6):1368–1378. doi: 10.1037/a0024278. doi: 10.1037/a0024278. [DOI] [PubMed] [Google Scholar]
- Sturm VE, Ascher EA, Miller BL, Levenson RW. Diminished self-conscious emotional responding in frontotemporal lobar degeneration patients. Emotion. 2008;8(6):861–869. doi: 10.1037/a0013765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, Yokoyama JS, Seeley WW, Kramer JH, Miller BL, Rankin KP. Heightened emotional contagion in mild cognitive impairment and Alzheimer’s disease is associated with temporal lobe degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(24):9944–9949. doi: 10.1073/pnas.1301119110. doi: 10.1073/pnas.1301119110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DM. Lateral brain function, emotion, and conceptualization. Psychological Bulletin. 1981;89(1):19–46. [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner KH, Roberts NA, Rosen HJ, Dean DL, Kramer JH, Weiner MW, Levenson RW. Emotional reactivity and emotion recognition in frontotemporal lobar degeneration. Neurology. 2007;69(2):148–155. doi: 10.1212/01.wnl.0000265589.32060.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, Gorno-Tempini ML. Connected speech production in three variants of primary progressive aphasia. Brain. 2010;133(Pt 7):2069–2088. doi: 10.1093/brain/awq129. doi: 10.1093/brain/awq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Wilson MR, Hung E, Gorno-Tempini ML, Miller BL, Shim J. Frontotemporal dementia and mania. Am J Psychiatry. 2007;164(12):1811–1816. doi: 10.1176/appi.ajp.2007.07061001. doi: 10.1176/appi.ajp.2007.07061001. [DOI] [PubMed] [Google Scholar]