Abstract
While it is known that Schwann cells (SCs) provide cues to enhance regeneration following peripheral nerve injury, the effect of SC phenotypic memory (muscle or cutaneous nerve-derived) on enhancing axonal regeneration and functional recovery has been unclear in the literature. In particular, differences between muscle and cutaneous nerve-derived SC may encourage specific motor or sensory axonal guidance in cell/tissue transplantation therapies. Thus, the goal of this study was to determine whether phenotypically matched combinations of neurons and SCs stimulate greater axonal extension compared to mismatched combinations (i.e. motor neurons/muscle nerve-derived SCs vs. motor neurons/cutaneous nerve-derived SCs). Additionally, the effect of glial cell line-derived neurotrophic factor (GDNF) treatment on SC-neuron interaction was also evaluated. In order to examine these interactions, microfluidic devices were used to assess the effects of soluble factors secreted from SCs on neurons. Unlike traditional co-culture methods, the devices allow for easier quantification of single neurite extension over long periods of time, as well as easy cell and media sampling of pure populations for biochemical analyses. Results demonstrated longer neurite growth when neurons are cultured with phenotype matched SCs, suggesting that SCs are capable of retaining phenotypic memory despite a prolonged absence of axonal contact. Furthermore, the negative effect of mismatched cultures can be overcome when mismatched SCs are preconditioned with GDNF. These results suggest that treatment of SCs with GDNF could enhance their ability to promote regeneration through mismatched grafts frequently used in clinical settings.
Keywords: peripheral nerve regeneration, microdevices, growth factors, neurite extension, muscle and cutaneous phenotype
Introduction
Preferential motor reinnervation is a process described by Brushart in which regenerating motor axons preferentially reinnervate muscle over cutaneous tissue (Brushart et al., 1998; Brushart, 1988; Madison et al., 1996). The underlying mechanism of this effect has been investigated, and a two-stage process has emerged. A smaller, early response of motor axons to the motor pathway itself is observed, followed by a more robust response to retrograde signaling from the muscle end organ target (Redett et al., 2005). This observation led to the identification of differential gene and protein expression profiles between motor and sensory branches (Franz et al., 2005; Hoke et al., 2006; Martini et al., 1992), which was attributed to distinct phenotypic differences between motor and sensory Schwann cells (SCs) (Hoke et al., 2006). These differential expression patterns could prove to be important, as it has been shown when motor nerves are repaired using sensory nerve grafts, axonal regeneration and recovery was impaired (Brenner et al., 2006; Nichols et al., 2004). Thus, if SCs are to be studied and used for transplantation to enhance regeneration of clinical repair options, such as biomaterial conduits and acellular nerve allografts (ANAs), it is necessary to determine how these different phenotypes influence neurons and how we can potentially drive SCs toward phenotypes that promote functional motor recovery.
Following injury, SCs can dedifferentiate from a mature, myelinating phenotype into an immature, proliferative state (Arthur-Farraj et al., 2009; Arthur-Farraj et al., 2012; Parrinello et al., 2010; Woodhoo et al., 2009). In this state, SCs undertake the early removal of myelin debris at the distal nerve segment and recruit macrophages for later myelin clearance. SCs secrete the extracellular matrix proteins of the basal lamina and neurotrophic factors that guide regenerating axons to their distal targets (Ribeiro-Resende et al., 2009; Shakhbazau et al., 2013). Previous studies have examined the effect of SC transplantation in ANAs and conduits and showed improved axonal regeneration and functional recovery; yet results vary in their efficacy compared to isograft controls (Fox et al., 2005; Guenard et al., 1992; Jesuraj et al., 2014b).
Therefore, it may be important to study the phenotypes of SCs (muscle or cutaneous-nerve derived) that are being used and how these may be manipulated to improve the regenerative capacity of transplanted cells. As mentioned previously, SCs obtained from motor or sensory roots have shown differential gene expression patterns indicating different phenotypes (subtypes) of mature SCs (Brushart et al., 2013; Brushart, 1988; He et al., 2012; Hoke et al., 2006; Vyas et al., 2010). For example, sensory SCs show upregulated brain-derived neurotrophic factor (BDNF) and myelin basic protein (MBP) expression compared to motor SCs, whereas motor SCs have increased expression of vascular endothelial growth factor (VEGF) and protein kinase C iota (PRKCi) (Hoke et al., 2006). However, in the course of expanding SCs in vitro for up to 4 weeks to obtain sufficient cell numbers for transplantation, some of these phenotypic markers can become dysregulated (Jesuraj et al., 2012). It has yet to be determined, however, whether these “dysregulated” SCs maintain a “memory” of their original phenotype and therefore can promote specific interactions with phenotypically matched motor and sensory neurons, particularly when the SCs are not physically associated with the axon.
In addition, we are investigating the effect of glial cell line-derived neurotrophic factor (GDNF) preconditioning on the interaction between SCs and neurons. GDNF has been studied extensively for its effect on promoting axon regeneration in vitro and in vivo (Hoke et al., 1998; Hoke et al., 2002; Lin et al., 2011; Wood et al., 2009a; Wood et al., 2009b), and has been shown to aid in neuron survival and proper motor axon reinnervation leading to enhanced motor output (Hoke et al., 1998; Hoke et al., 2000; Hoke et al., 2002; Sakamoto et al., 2003). Just as important, however, may be the effect GDNF has on SCs after injury. GDNF is known to affect SC differentiation to the myelinating phenotype and be involved in SC maturation, migration, and proliferation (Hoke et al., 2003; Jesuraj et al., 2014a; Klemke et al., 1997; Meintanis et al., 2001; Morgan et al., 1991; Paratcha et al., 2003). Furthermore, recent work in our lab has shown application of exogenous GDNF helps SCs differentiate into their native muscle and cutaneous nerve-derived phenotypes through a Fyn kinase-mediated pathway, which may improve interaction with specific motor and sensory axons in transplantation therapies (Jesuraj et al., 2014a).
In this study, we examined the interaction of phenotypically matched and mismatched SCs and neurons through soluble factor signaling in a novel co-culture platform. Preferential neurite extension into microchannels was quantified in matched and mismatched SC co-culture conditions. The effect of exogenous GDNF preconditioning of SCs on neurite extension was also assessed. Endogenous GDNF mRNA and protein levels in SCs were monitored and analyzed over the culture period to determine the effect of exogenous GDNF treatment on SC phenotype as well as the amount of endogenously produced GDNF that would be capable of influencing neurite extension from the opposing neuronal chamber. Matched combinations of phenotype-specific SCs and neurons resulted in greater neurite extension indicating SCs seem to maintain some degree of specificity or phenotypic “memory” for matched neurons. Preconditioning of SCs with GDNF led to similar neurite lengths in mismatched and matched phenotype combinations. This finding may prove clinically valuable; as sensory nerves are often used to bridge motor nerve defects with inferior results. Treatment of mismatched SCs or grafts with GDNF may help lead to better motor function, regardless of nerve source.
Materials and Methods
2.1 SC Harvest and Isolation
Muscle and cutaneous nerve-derived SCs were collected from adult male Lewis rat muscle and cutaneous femoral nerve branches as previously described (Jesuraj et al., 2012). Femoral muscle branches were used to harvest muscle SCs as opposed to pure ventral motor roots in order to provide a clinically accessible cell source. To isolate the SCs, nerve branches were placed in Leibovitz's L-15 medium (Invitrogen, Carlsbad, CA) with 1% Collagenase (Fischer, Pittsburgh, PA) and 2.5% Tryspin-EDTA (Invitrogen) for 30 min at 37°C. Branches were centrifuged for 5 min at 130 g, supernatant removed, and then washed 3× with SC medium (Dulbecco's modified Eagle medium (DMEM), Invitrogen; 10 % fetal bovine serum (FBS), Sigma Aldrich, St. Louis, MO; 1% antibiotic antimitotic (ABAM), Invitrogen). Cells were then plated onto 10 μg/mL poly-L-lysine (PLL; Sigma Aldrich) coated 100 mm Petri dishes (Corning) with SC media. Cell proliferation was stopped at day 2 with 10 μM cytosine-beta-arabino furanoside HCl (Ara-C; Sigma-Aldrich) and fibroblasts were removed from culture at day 6 through complement killing using Thy1.1 antibody (Serotec, Raleigh , NC) and rabbit complement (Sigma Aldrich). Fresh media was supplied with 20 μg/mL pituitary extract (Biomedical Tech, Stoughton, MA) and 2 μM forskolin (Sigma Aldrich) to promote SCs proliferation. Complement killing was performed at all subsequent passages to remove any surviving fibroblasts and all experimental procedures were performed with SCs between passages 2 and 4.
2.2 Motor and Sensory Neuron Isolation
Motor and sensory neurons were harvested from embryonic day 15/16 Sprague-Dawely rat spinal cords and dorsal root ganglia, respectively, as previously described (Gingras et al., 2007; Graber and Harris, 2013). Sprague-Dawley rat neurons were used in co-culture with the Lewis rat-derived SCs to approximate the response of axons to allogeneic SCs similar to what is used for cell transplantation therapies. Briefly, dorsal root ganglia were collected and dissociated for 30 min with 0.25% Trypsin-EDTA at 37°C. After which, the cells were centrifuged for 5 min at 240 g and resuspended in neurobasal medium (Invitrogen). Cells were pipetted through a 10 μm cell sieve and 40 μm sieve, where the cells that passed through the sieve were discarded as glia and cells that remained within the sieve were collected for neurons. Motor neurons were isolated from the spinal cord as previously described (Gingras et al., 2007; Leach et al., 2011). Briefly, the spinal cords were dissociated in 0.25% Trypsin in L-15 medium for 40 min at 37°C. The cords were then allowed to settle and the supernatant was replaced with DMEM:F12 (3:1) media supplemented with 4 μg/mL of DNase I (Sigma Aldrich). The cords were mechanically dissociated at this point with a Pasteur pipette and the mixture was placed onto a 4 mg/mL BSA cushion and centrifuged at 100 g for 10 min. The pellet was collected and resuspended in the DMEM:F12/DNase I media and placed on a 7.42% Nycoprep density gradient (Axis Shield, Cosmo Bio USA) The gradient was centrifuged at 500 g for 20 min and the cell solution at the interface of the Nycoprep and media was collected. This cell solution was also pipetted through the cell sieves as described above and the motor neurons were collected.
2. 3 Microdevice Fabrication
The master silicone wafer for the co-culture microdevice was fabricated using standard soft photolithography (Lu et al., 2012; Taylor et al., 2005). In order to generate the master wafer, two steps were used to create the smaller 5 μm tall microchannels and then the larger 100 μm tall cell culture reservoirs. Briefly, to form the microchannels, a 5 μm tall layer of negative photoresist SU8-2 (Microchem, Newton, MA) was spun-coated onto a silicone wafer upon which the transparency was placed and exposed to UV light. Uncrosslinked areas were dissolved and washed using an SU8 developer (Microchem). Subsequently, the wafer underwent another spinning coating process with SU8 2050 (Microchem) to form a 100 μm layer and exposed to UV light with a transparency mask generating the neuronal and SC chambers. Again, the wafer was developed and baked to its final form.
Microdevices were formed using polydimethylsiloxane (PDMS) molded from the master wafer. In a ratio of 10:1 (base:curing agent) the elastomer, Sylgard 184 (Dow Corning, USA), was poured over the master wafer within a 100 mm Petri dish and placed in a vacuum desiccator for 2h to remove bubbles. The PDMS covered wafer was then placed in a 60 °C oven overnight to harden and autoclaved to complete any crosslinking. Microdevices were then cut from the molds and cell seeding chambers were created using 6 mm semicircular punches. Microdevices were placed in a plasma cleaner (Harrick Plasma, Ithaca, NY) for 2.5 min with glass cover slips attached to 35 mm dishes in order to form a tight seal and prevent any leakage. Once bonded, the devices were sterilized by UV exposure. The devices were coated with 10 μg/mL PLL for 1 h in the SC soma chamber and with 10 μg/mL poly-d-lysine and 10 μg/mL laminin in the neuronal and microchannel chambers.
2.4 Microdevice Cell Seeding and Culture
Once the microdevices had been prepared, muscle or cutaneous nerve-derived SCs were seeded into the PLL-coated SC chamber at 7 × 103 cells/cm2 in SC media or with SC media supplemented with 100 ng/mL of GDNF. SCs were then allowed to grow for 5 days prior to neuronal seeding, at which point the devices were washed 3× to remove any exogenous GDNF that could affect neurite growth. Motor and sensory neurons were seeded into the neuronal somal chambers of the microdevices at 2.5×105 cells/cm2 in neurobasal media supplemented with 1× B27, 1× penicillin/streptomycin (Invitrogen), and 10 μg/mL of fluorodeoxyuridine (FDU, Sigma Aldrich) to again remove any remaining endogenous glia. Motor neurons were also supplemented with 4 μg/mL of hydrocortisone in order to improve their survival after removal of supporting cell types based on previous reports in the literature, this is not commonly added to sensory neuron cultures (Banker and Goslin, 1998; Gingras et al., 2007). In order to ensure only factors secreted by SCs affect the neurons and not vice versa, a 30 μL volume difference was used between the two chambers such that the higher volume of media in the SC chamber allowed only for flow from the SC chamber into the neuronal chamber. Media was collected and replaced from the SC chambers at days 2 and 7 for protein analysis, while SC lysates were collected from the devices at day 7 for mRNA gene expression analysis. Finally, neurite extension of motor and sensory neurons into the microchannels was visualized by Calcein-AM dye (Invitrogen) and images were taken on a fluorescent microscope with a CCD Olympus camera at a 10× objective. Neurite extension length was measured by ImageJ software. The level of extensions into the microdevices allow for us to determine the degree of preferential growth toward the SC chamber. A schematic of the experimental paradigm is shown in Figure 1.
Figure 1.
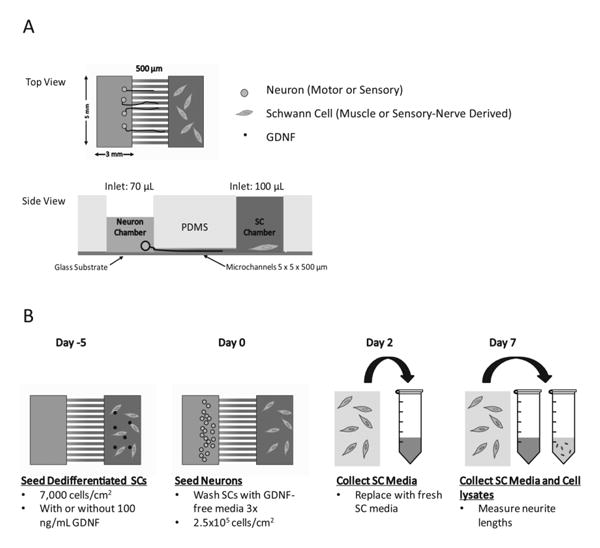
Schematic of study design and timeline for culture in microdevices. A) Microdevice and microchannels dimensions are 5 × 5 × 500 (L×W×H) μm. Volume difference allows for one way flow and diffusion of media and growth factors. B) Timeline of cell seeding, media/cell lysate collection, and neurite length analysis is shown.
2.5 Quantitative Real Time- Polymerase Chain Reaction (qRT-PCR)
SC lysates were collected from the microdevices and RNA was purified using RNeasy mini prep kit (Qiagen). The purity and concentration of mRNA was determined using a Nanodrop and then mRNA was converted to cDNA using High Capacity mRNA-to-cDNA kit (Life Technologies). Gene expression for S100b, Nestin, GDNF, and the housekeeping gene (β-actin was determined using Taqman gene expression assays (Life Technologies) and Fast Master Mix (Life Technologies) on the Step One Plus Real Time PCR system.
2.6 GDNF Protein Quantification
GDNF protein levels in collected media samples were determined using R&D System GDNF DuoSet ELSIA kit. Control microdevices were concurrently run in which devices with no cells were given identical GDNF treatments and washes in order to ensure no exogenous GDNF remained within the wells and GDNF detection was purely that of GDNF produced by the SCs.
2.7 Statistics
Statistical analysis was performed using Statistica software (Statsoft, Tulsa, OK) with comparative ANOVA analysis and a Scheffé's post hoc test with p<0.05. Data represented is shown as mean ± standard error of the mean. Data included n=4-6 microdevices per condition; n=14-239 neurites measured per condition.
3. Results
3.1 Effect of SC Muscle and Cutaneous Nerve-Derived Phenotype on Neurite Extension
To determine the specific phenotypic effect of SCs on neurons, neurite extension into the microchannels of motor and sensory neurons was measured when cultured in matched and mismatched combinations of muscle and cutaneous-derived SCs. Representative images of neurites cultured with matched SCs in the opposing chamber at day 7 are shown in Figure 2. These images demonstrate that the presence of SCs alone is capable of ensuring neuron survival and promoting neurite extension without any growth factors added to the serum-free neurobasal media. In contrast, devices lacking SCs resulted in low neuronal survival and failed to encourage neurites to extend into the microchannels thus remaining within the somal chamber (data not shown). As shown in Figure 3, SCs were capable of promoting neurite extension in a phenotype specific manner. Matched combinations of muscle and cutaneous-derived SCs with neurons resulted in significantly longer extension into the microchannels than mismatched combinations. For example, when motor neurons were cultured with muscle-derived SCs, neurite extension was significantly greater than when cultured with cutaneous SCs, ∼ 300 μm versus 175 μm, respectively. The same trend was observed with sensory neurons, with extension of 400 μm with cutaneous SC cultures versus ∼ 225 μm with muscle SC cultures. In addition to neurite length, the frequency of neurite extension into the microchannels was also affected. As shown in Table 1, when cells were cultured in matched combinations, as many as ∼240 individual neurites extending into the microchannels were capable of being measured. However, in mismatched conditions, only 14-24 individual neurites were measured within the channels. These results demonstrate the phenotypic “memory” of SCs is maintained in up to 6 weeks in culture, despite loss of axon contact and dysregulation of specific markers.
Figure 2.
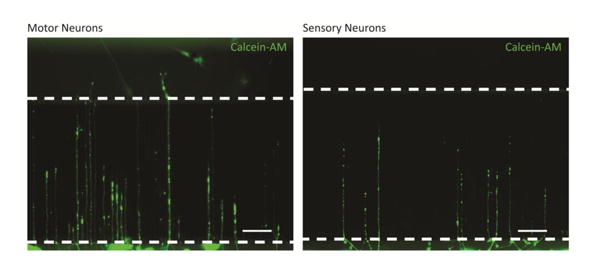
Representative images of motor (A) and sensory (B) neurites extending into microchannels with support from phenotypically matched SCs in the opposing chamber. Dotted lines mark microchannel ends. Neuronal chamber, microchannels, and SC chamber are oriented from bottom to top of image. Scale bar = 100 μm.
Figure 3.
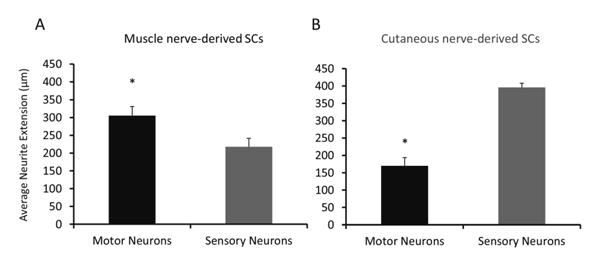
Response of neurons to phenotypically matched and mismatched SCs in microdevices. Average neurite extension into microchannels of motor (black bars) and sensory (gray bars) neurons was measured when cultured with muscle (A) and cutaneous (B) nerve-derived SCs. Statistical significance was determined by ANOVA with Scheffé's post hoc test. * denotes p<0.05 compared to sensory neurons. N=16-120 neurites measured.
Table 1. Number of neurites extending into microchannels.
| Muscle nerve-derived SCs | Cutaneous nerve-derived SCs | |||
|---|---|---|---|---|
| Motor Neurons | Sensory Neurons | Motor Neurons | Sensory Neurons | |
| Untreated SCs | 72 | 26 | 14 | 239 |
| GDNF-treated SCs | 63 | 9 | 46 | 161 |
3.2 Effect of GDNF Treatment on SCs and their Effect on Neurite Extension
In order to determine the effect of GDNF treatment on SCs and their subsequent interaction with neurons, SCs were pretreated with 100 ng/mL of GDNF for 5 days prior to neuronal seeding. The resulting neurite extension of motor and sensory neurons into the microchannels in response to the preconditioned muscle and cutaneous-derived SCs was measured at 7 days. In matched phenotype conditions, neurite extension trended higher but was not significantly different (∼300 to 350 μm for motor neurons and ∼400 to 410 μm for sensory neurons without and with GDNF pretreatment, respectively). However, neurite extension in the mismatched conditions treated with GDNF, with both motor and sensory neurons, was significantly longer than the untreated conditions (Figure 4). Motor neurite extension increased 100%, from ∼175 to 350 μm, when co-cultured with GDNF preconditioned cutaneous-derived SCs. Sensory neurons, likewise, showed a 56% increase, from ∼225 to 350 μm when cultured with muscle-derived SCs preconditioned with GDNF. The neuronal response of the mismatched conditions was rescued to approximately the same level as matched conditions. The number of neurites extending into microchannels wasn't greatly affected by preconditioning of SCs with GDNF (Table 1), compared to the significant changes in neurite extension. This suggests the role of phenotype match or mismatch, both preconditioned with GDNF or not, affects the degree of neurite extension into the channels rather than survival. This is a significant observation that may prove useful in overcoming the impaired regeneration seen in motor-sensory graft mismatch frequently used in clinical settings.
Figure 4.
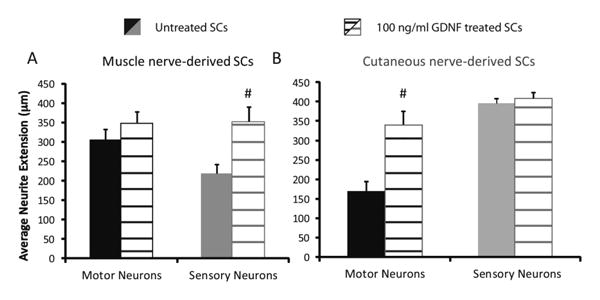
Response of neurons to phenotypically matched and mismatched SCs in microdevices after GDNF pretreatment. Average neurite extension into microchannels of motor (black bars) and sensory (gray bars) neurons was measured when cultured with muscle (A) and cutaneous (B) nerve-derived SCs (solid bars) or GDNF pretreated SCs (hashed bars). Statistical significance was determined by ANOVA with Scheffé's post hoc test. * denotes p<0.05 compared to sensory neurons; # denotes p<0.05 compared to untreated SCs. N=16-120 neurites measured.
3.3 Gene and Protein Expression of Growth Factors after GDNF Treatment
The effect of exogenous GDNF treatment on endogenous SC GDNF gene expression, as well as protein secretion, was analyzed to understand of how the SCs may be interacting with the neurons through soluble, secreted factors. First, GDNF mRNA levels in GDNF treated and untreated SCs were quantified using qRT-PCR. As shown in Figure 5, GDNF expression was upregulated in exogenously, pretreated cells compared to untreated controls. There was no significant difference in level of the response for muscle or cutaneous-derived SCs at the mRNA level. GDNF protein levels at days 2 and 7 were analyzed using an ELISA detection method. Exogenous GDNF was thoroughly washed from the microdevices to avoid any interference with detecting endogenously produced GDNF; in addition, measurements of devices treated with GDNF without cells present in the SC chamber were subtracted from the averages to remove any background levels. Pretreated muscle and cutaneous-derived SCs showed significantly higher levels of GDNF production compared to untreated SCs at days 2 and 7, approximately 100-fold and 10-fold higher respectively. Furthermore, muscle nerve-derived SCs produced significantly higher levels of GDNF compared to cutaneous nerve-derived SCs at day 2; however, by day 7, the amount of GDNF within the devices was equal between the two SC types (Figure 6). There was a significant decrease in GDNF production as time increased without supersaturated levels exogenous GDNF treatment. However, these results demonstrate that ability of GDNF to affect SC gene and protein expression levels in a positive feedback loop even 7 days after removal of exogenous GDNF, which could prove useful in an in vivo environment where pretreatment is performed prior to cell transplantation.
Figure 5.
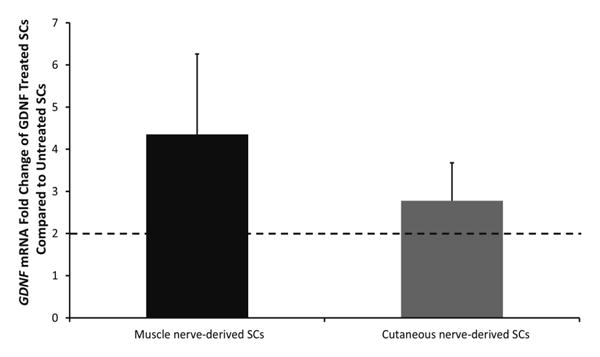
Gene expression of GDNF in exogenously treated muscle (black bar) and cutaneous (gray bar) nerve-derived SCs compared to untreated SCs. Cell lysates were collected at 7 days after GDNF treatment. Dotted line at 2 represents upregulation of GDNF mRNA of GDNF-treated SCs compared with untreated SCs. No difference is seen between muscle and cutaneous nerve-derived SCs. Statistical significance was determined by ANOVA with Scheffé's post hoc test (N≥4).
Figure 6.
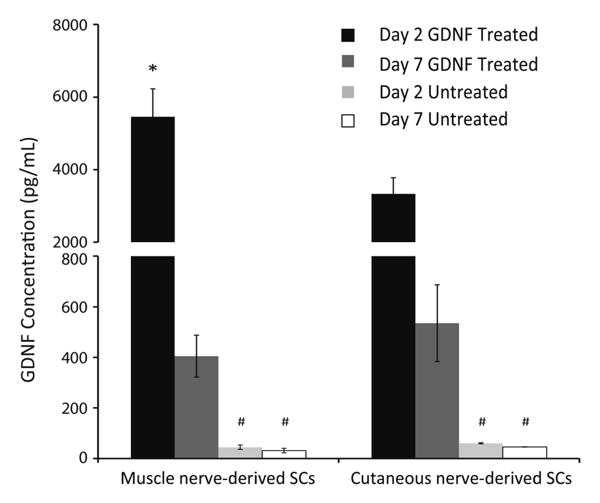
Protein levels of endogenously produced GDNF measured at days 2 and 7 for GDNF treated and untreated SCs. Data (mean ± SEM) are shown for muscle and cutaneous nerve-derived SCs with and without GDNF pretreatment. Statistical significance was determined by ANOVA with Scheffé's post hoc test (N≥4). * denotes p<0.05 compared to cutaneous SCs; # denotes p<0.05 compared to GDNF treated SCs at same time point.
Discussion
Much research has focused on the use of SCs for cell transplantation therapy in peripheral nerve injuries. SCs are a great source of growth factor production, which aids neuronal survival and axon regeneration, as well as the ability to remyelinate axons after injury (Parrinello et al., 2010). Yet, there still remains much to be understood about SC function and phenotype, which may explain insufficient results seen in biomaterial conduits and ANAs compared to autografts. Thus, gaining a better understanding of the interaction SCs have with neurons prior-to and after injury, as well as being able to modulate that interaction, may promote enhanced functional recovery.
Differences between muscle and sensory phenotypes arose from the concept of preferential motor reinnervation, a process in which motor axons will preferentially reinnervate muscle over skin (Brushart, 1988). Investigation into the cause of this process showed differential gene expression profiles between cutaneous branches and ventral roots, which may provide specific cues to properly guide regenerating axons. These cues were attributed to SCs as they make up the majority of the cellular content within nerves (Hoke et al., 2006; Oda et al., 1989). This was further shown in femoral muscle and cutaneous branches, as well as SCs immediately harvested from the branches (Brushart et al., 2013; Jesuraj et al., 2012). However, when SCs were expanded in vitro for 30 days for transplantation studies, the differential gene expression profiles between muscle and cutaneous SCs became dysregulated (Jesuraj et al., 2012). Thus, the use of mitogens for expansion of SCs can lead to significant changes in the transcriptional profile, and potential interaction with neurons. This may result in the dysreguation of genetic profiles seen in transplantation studies using expanded phenotype specific SCs, and perhaps why use of muscle SCs did not lead to improved functional motor recovery (Jesuraj et al., 2014b). Here we studied the interaction of motor and sensory neurons with expanded muscle and cutaneous-derived SCs in an in vitro system. The microdevice platform allows us to study the effect of SC secreted factors on neurons in matched and mismatched phenotypic conditions. This may help us understand why phenotype mismatch between autografts, or transplanted SCs often lead to limited functional recovery.
We assessed the preferential response of neurons to SCs in matched and mismatched phenotypic conditions through the degree of neurite extension into microchannels separating the SC and neuronal chambers in the microdevice. These results demonstrated that despite the long period of expansion and isolation from axonal contact, SCs were capable of promoting neurite extension in a phenotype specific manner both in neurite length and, perhaps more dramatically, the number of neurites extending into the microchannels. This result was unanticipated as SCs are known to dedifferentiate after loss of axon contact (Arthur-Farraj et al., 2012). An organotypic in vitro model was developed that showed growth in a phenotype specific manner (Vyas et al., 2010); however axon contact was maintained in this case and similar to that seen in vivo, in which studies showed branches of the same phenotype result in better functional and histological outcomes (Brenner et al., 2006; Moradzadeh et al., 2009). A more recent study demonstrated that SCs are capable of maintaining specific phenotype up to 2 weeks in vitro without axonal cues (Brushart et al., 2013), however this is the first study to show that SCs are capable of supporting growth in a phenotype specific manner without axonal contact after more than 4 to 6 weeks of expansion. Thus, despite mRNA changes observed in expanded SC cultures, there may be some phenotypic “memory” between SCs and neurons that is yet to be fully understood.
In addition, we examined how exogenous GDNF preconditioning affects the phenotypic interaction between SCs and neurons. Previous studies have shown GDNF treatment can affect muscle and cutaneous-derived SC gene expression profiles and maturation of expanded SCs (Jesuraj et al., 2014a). GDNF pathway signaling affects SC proliferation, migration, and differentiation (Hoke and Griffin, 1997, Hoke, et al., 2003, Jesuraj, et al., 2014a, Magill, et al., 2010, Morgan, et al., 1991). In this study, our results showed no difference neurite extension in matched phenotype conditions after GDNF treatment. Perhaps more interesting was the effect of GDNF in the mismatched phenotype conditions. If GDNF was to drive muscle and cutaneous nerve-derived SCs further into their distinct phenotypes, the mismatched conditions could be expected to show a further decrease in neurite extension. However, as the results demonstrate, GDNF treatment actually rescues the effect of phenotypic mismatch and promotes neurite extension to the same level of phenotypically matched conditions. This may lead to better therapies if sensory nerve grafts must be used to bridge motor nerve defects, they could be treated (or even pretreated with GDNF) to overcome mismatch effects. It should be noted that the neurons used in this study were embryonic-derived as opposed to post-natal or adult neurons. It should be noted that the neurons used in this study were embryonic-derived as opposed to post-natal or adult neurons. Embryonic neurons were chosen for this study because adult neurons can be very difficult and expensive to obtain, and they demonstrate poor survival in vitro (Brewer and Torricelli, 2007; Eide and McMurray, 2005). However, as adult neurons can respond differently to secreted growth factors and cues than embryonic cells (Goldberg, 2003), it would be beneficial to study the preferential growth response of adult sensory and motor neurons to cutaneous and muscle nerve-derived SCs.
In order to understand what may be changing in the SCs due to the exogenous GDNF treatment, we analyzed the gene expression and protein secretion levels of endogenous GDNF in both muscle and cutaneous-derived SCs. These results indicated increased levels of GDNF production even 7 days after exogenous GDNF treatment had been stopped, as well as upregulated GDNF expression in both muscle and cutaneous-derived SCs. GDNF levels initially showed a higher response from muscle-derived SCs than cutaneous at 2 days post treatment, but by 7 days, both protein and mRNA level were similar between the two phenotypes. These results support evidence presented by Iwase et al. that the GDNF pathway in SCs is a positive feedback system through activation of the Mek/Erk pathway (Iwase et al., 2005), which may be accounting for the recovery in neurite extension observed for the mismatched conditions. As both motor and sensory neurons respond to GDNF, an overall increase of GDNF in the system may be causing the neurite extension to overcome what was previously less growth promoting. Thus, developing a strategy that delivers GDNF in a sustained, controllable manner would be ideal in targeting endogenous GDNF production and overcoming phenotype mismatch.
Conclusion
Gaining a better understanding and characterization of the cells used in transplantation therapies is critical to understanding why grafts and transplants fail clinically. Here, we have developed a microdevice platform that allowed us to study interactions between SCs and neurons to determine why phenotypically mismatched grafts fail, as well as which cell may lead to better functional recovery following injury. In addition, this system allowed us to isolate the effect of neurotrophins, such as GDNF, on SCs and the subsequent effects on SC-neuron interaction. We have shown that, despite lack of axon contact, SCs are capable of interacting with neurons in a phenotype specific manner through soluble, secreted factors. However, by introducing exogenous GDNF to the system, SCs can help overcome phenotype mismatch and promote neurite extension of similar lengths to that of matched phenotypes.
Highlights.
Schwann cells (SCs) retain some phenotypic memory despite extended loss of axonal contact
Neurons respond in a phenotype specific manner toward SCs
GDNF preconditioning of SCs rescues negative effects of mismatched neurons
Exogenous GDNF treatment increases endogenous GDNF mRNA and protein expression levels
Acknowledgments
This research was funded in part by NIH RO1NS051706 and NSF DGE-1143954 (LMM). This work was performed in part at the Nano Research Facility (NRF), a member of the National Nanotechnology Infrastructure Network (NNIN), which is supported by the National Science Foundation under Grant no. ECS-0335765. The authors thank Sara Oswald for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arthur-Farraj P, Makwana M, Wilton D, Latouche-Hartman M, Acosta A, Cuthill D, Bherens A, Mirsky R, Raivich G, Jessen KR. In Injured Nerves, C-Jun in Schwann Cells Controls Demyelination, Neuronal Survival, Axonal Re-Growth and Functional Recovery. Journal of the Peripheral Nervous System. 2009;14:9–10. [Google Scholar]
- Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker G, Goslin K. Culturing nerve cells. Cellular and molecular neuroscience. 2nd 1998. [Google Scholar]
- Brenner MJ, Hess JR, Myckatyn TM, Hayashi A, Hunter DA, Mackinnon SE. Repair of motor nerve gaps with sensory nerve inhibits regeneration in rats. Laryngoscope. 2006;116:1685–1692. doi: 10.1097/01.mlg.0000229469.31749.91. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR. Isolation and culture of adult neurons and neurospheres. Nat Protocols. 2007;2:1490–1498. doi: 10.1038/nprot.2007.207. [DOI] [PubMed] [Google Scholar]
- Brushart TM, Aspalter M, Griffin JW, Redett R, Hameed H, Zhou C, Wright M, Vyas A, Hoke A. Schwann cell phenotype is regulated by axon modality and central-peripheral location, and persists in vitro. Experimental Neurology. 2013;247:272–281. doi: 10.1016/j.expneurol.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM, Gerber J, Kessens P, Chen YG, Royall RM. Contributions of pathway and neuron to preferential motor reinnervation. Journal of Neuroscience. 1998;18:8674–8681. doi: 10.1523/JNEUROSCI.18-21-08674.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TME. Preferential Reinnervation of Motor Nerves by Regenerating Motor Axons. Journal of Neuroscience. 1988;8:1026–1031. doi: 10.1523/JNEUROSCI.08-03-01026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide L, McMurray CT. Culture of adult mouse neurons. BioTechniques. 2005;38:99–104. doi: 10.2144/05381RR02. [DOI] [PubMed] [Google Scholar]
- Fox IK, Schwetye KE, Keune JD, Brenner MJ, Yu JW, Hunter DA, Wood PM, Mackinnon SE. Schwann-cell injection of cold-preserved nerve allografts. Microsurgery. 2005;25:502–507. doi: 10.1002/micr.20152. [DOI] [PubMed] [Google Scholar]
- Franz CK, Rutishauser U, Rafuse VF. Polysialylated neural cell adhesion molecule is necessary for selective targeting of regenerating motor neurons. Journal of Neuroscience. 2005;25:2081–2091. doi: 10.1523/JNEUROSCI.4880-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras M, Gagnon V, Minotti S, Durham HD, Berthod F. Optimized protocols for isolation of primary motor neurons, astrocytes and microglia from embryonic mouse spinal cord. Journal of Neuroscience Methods. 2007;163:111–118. doi: 10.1016/j.jneumeth.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Goldberg JL. How does an axon grow? Genes Dev. 2003;17:941–958. doi: 10.1101/gad.1062303. [DOI] [PubMed] [Google Scholar]
- Graber DJ, Harris BT. Purification and culture of spinal motor neurons from rat embryos. Cold Spring Harb Protoc. 2013;2013:319–326. doi: 10.1101/pdb.prot074161. [DOI] [PubMed] [Google Scholar]
- Guenard V, Kleitman N, Morrissey TK, Bunge RP, Aebischer P. Syngeneic Schwann-Cells Derived from Adult Nerves Seeded in Semipermeable Guidance Channels Enhance Peripheral-Nerve Regeneration. Journal of Neuroscience. 1992;12:3310–3320. doi: 10.1523/JNEUROSCI.12-09-03310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He QR, Man LL, Ji YH, Ding F. Comparison in the biological characteristics between primary cultured sensory and motor Schwann cells. Neuroscience Letters. 2012;521:57–61. doi: 10.1016/j.neulet.2012.05.059. [DOI] [PubMed] [Google Scholar]
- Hoke A, Bell R, Zochodne DW. GDNF and its receptors are upregulated in the denervated distal stump of injured sciatic nerves. Neurology. 1998;50:A28–A28. [Google Scholar]
- Hoke A, Cheng C, Zochodne DW. Expression of glial cell line-derived neurotrophic factor family of growth factors in peripheral nerve injury in rats. Neuroreport. 2000;11:1651–1654. doi: 10.1097/00001756-200006050-00011. [DOI] [PubMed] [Google Scholar]
- Hoke A, Gordon T, Zochodne DW, Sulaiman OAR. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Experimental Neurology. 2002;173:77–85. doi: 10.1006/exnr.2001.7826. [DOI] [PubMed] [Google Scholar]
- Hoke A, Ho T, Crawford TO, LeBel C, Hilt D, Griffin JW. Glial cell line-derived neurotrophic factor alters axon Schwann cell units and promotes myelination in unmyelinated nerve fibers. Journal of Neuroscience. 2003;23:561–567. doi: 10.1523/JNEUROSCI.23-02-00561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke A, Redett R, Hameed H, Jari R, Zhou C, Li ZB, Griffin JW, Brushart TM. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. Journal of Neuroscience. 2006;26:9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase T, Jung CG, Bae H, Zhang M, Soliven B. Glial cell line-derived neurotrophic factor-induced signaling in Schwann cells. Journal of Neurochemistry. 2005;94:1488–1499. doi: 10.1111/j.1471-4159.2005.03290.x. [DOI] [PubMed] [Google Scholar]
- Jesuraj NJ, Marquardt LM, Kwasa JA, Sakiyama-Elbert SE. Glial cell line-derived neurotrophic factor promotes increased phenotypic marker expression in femoral sensory and motor-derived Schwann cell cultures. Exp Neurol. 2014a;257C:10–18. doi: 10.1016/j.expneurol.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesuraj NJ, Nguyen PK, Wood MD, Moore AM, Borschel GH, Mackinnon SE, Sakiyama-Elbert SE. Differential gene expression in motor and sensory Schwann cells in the rat femoral nerve. J Neurosci Res. 2012;90:96–104. doi: 10.1002/jnr.22752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesuraj NJ, Santosa KB, Macewan MR, Moore AM, Kasukurthi R, Ray WZ, Flagg ER, Hunter DA, Borschel GH, Johnson PJ, Mackinnon SE, Sakiyama-Elbert SE. Schwann cells seeded in acellular nerve grafts improve functional recovery. Muscle Nerve. 2014b;49:267–276. doi: 10.1002/mus.23885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke RL, Cai S, Giannini AL, Gallagher PJ, deLanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. Journal of Cell Biology. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach MK, Feng ZQ, Gertz CC, Tuck SJ, Regan TM, Naim Y, Vincent AM, Corey JM. The culture of primary motor and sensory neurons in defined media on electrospun poly-L-lactide nanofiber scaffolds. Journal of visualized experiments : JoVE. 2011 doi: 10.3791/2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Ramadan M, Hronik-Tupaj M, Kaplan DL, Philips BJ, Sivak W, Rubin JP, Marra KG. Spatially Controlled Delivery of Neurotrophic Factors in Silk Fibroin-Based Nerve Conduits for Peripheral Nerve Repair. Annals of Plastic Surgery. 2011;67:147–155. doi: 10.1097/SAP.0b013e3182240346. [DOI] [PubMed] [Google Scholar]
- Lu X, Kim-Han JS, O'Malley KL, Sakiyama-Elbert SE. A microdevice platform for visualizing mitochondrial transport in aligned dopaminergic axons. J Neurosci Methods. 2012;209:35–39. doi: 10.1016/j.jneumeth.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison RD, Archibald SJ, Brushart TM. Reinnervation accuracy of the rat femoral nerve by motor and sensory neurons. Journal of Neuroscience. 1996;16:5698–5703. doi: 10.1523/JNEUROSCI.16-18-05698.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R, Xin Y, Schmitz B, Schachner M. The L2/Hnk-1 Carbohydrate Epitope Is Involved in the Preferential Outgrowth of Motor Neurons on Ventral Roots and Motor Nerves. European Journal of Neuroscience. 1992;4:628–639. doi: 10.1111/j.1460-9568.1992.tb00171.x. [DOI] [PubMed] [Google Scholar]
- Meintanis S, Thomaidou D, Jessen KR, Mirsky R, Matsas R. The neuron-glia signal beta-neuregulin promotes Schwann cell motility via the MAPK pathway. Glia. 2001;34:39–51. [PubMed] [Google Scholar]
- Moradzadeh A, Borschel GH, Luciano JP, Whitlock EL, Hayashi A, Hunter DA, Mackinnon aSE. The Impact of Motor and Sensory Nerve Architecture on Nerve Regeneration. Exp Neurol. 2009;212:370–376. doi: 10.1016/j.expneurol.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan L, Jessen KR, Mirsky R. The effects of cAMP on differentiation of cultured Schwann cells: progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP-, N-CAM-, NGF-receptor-) depends on growth inhibition. J Cell Biol. 1991;112:457–467. doi: 10.1083/jcb.112.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CM, Brenner MJ, Fox IK, Tung TH, Hunter DA, Rickman SR, Mackinnon SE. Effect of motor versus sensory nerve grafts on peripheral nerve regeneration. Experimental Neurology. 2004;190:347–355. doi: 10.1016/j.expneurol.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Oda Y, Okada Y, Katsuda S, Ikeda K, Nakanishi I. A Simple Method for the Schwann-Cell Preparation from Newborn Rat Sciatic-Nerves. Journal of Neuroscience Methods. 1989;28:163–169. doi: 10.1016/0165-0270(89)90032-0. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ledda F, Ibanez CF. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family Ligands. Cell. 2003;113:867–879. doi: 10.1016/s0092-8674(03)00435-5. [DOI] [PubMed] [Google Scholar]
- Parrinello S, Napoli I, Ribeiro S, Digby PW, Fedorova M, Parkinson DB, Doddrell RDS, Nakayama M, Adams RH, Lloyd AC. EphB Signaling Directs Peripheral Nerve Regeneration through Sox2-Dependent Schwann Cell Sorting. Cell. 2010;143:145–155. doi: 10.1016/j.cell.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redett R, Jari R, Crawford T, Chen YG, Rohde C, Brushart TM. Peripheral pathways regulate motoneuron collateral dynamics. Journal of Neuroscience. 2005;25:9406–9412. doi: 10.1523/JNEUROSCI.3105-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Resende VT, Koenig B, Nichterwitz S, Oberhoffner S, Schlosshauer B. Strategies for inducing the formation of bands of Bungner in peripheral nerve regeneration. Biomaterials. 2009;30:5251–5259. doi: 10.1016/j.biomaterials.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Kawazoe Y, Shen JS, Takeda Y, Arakawa Y, Ogawa J, Oyanagi K, Ohashi T, Watanabe K, Inoue K, Eto Y, Watabe K. Adenoviral gene transfer of GDNF, BDNF and TGF beta 2, but not CNTF, cardiotrophin-1 or IGF1, protects injured adult motoneurons after facial nerve avulsion. Journal of Neuroscience Research. 2003;72:54–64. doi: 10.1002/jnr.10558. [DOI] [PubMed] [Google Scholar]
- Shakhbazau A, Martinez JA, Xu QG, Kawasoe J, van Minnen J, Midha R. Evidence for a systemic regulation of neurotrophin synthesis in response to peripheral nerve injury. J Neurochem. 2013;122:501–511. doi: 10.1111/j.1471-4159.2012.07792.x. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nature Methods. 2005;2:599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Li ZB, Aspalter M, Feiner J, Hoke A, Zhou CH, O'Daly A, Abdullah M, Rohde C, Brushart TM. An in vitro model of adult mammalian nerve repair. Experimental Neurology. 2010;223:112–118. doi: 10.1016/j.expneurol.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MD, Borschel GH, Sakiyama-Elbert SE. Controlled release of glial-derived neurotrophic factor from fibrin matrices containing an affinity-based delivery system. Journal of Biomedical Materials Research Part A. 2009a;89A:909–918. doi: 10.1002/jbm.a.32043. [DOI] [PubMed] [Google Scholar]
- Wood MD, Moore AM, Hunter DA, Tuffaha S, Borschel GH, Mackinnon SE, Sakiyama-Elbert SE. Affinity-based release of glial-derived neurotrophic factor from fibrin matrices enhances sciatic nerve regeneration. Acta Biomaterialia. 2009b;5:959–968. doi: 10.1016/j.actbio.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhoo A, Alonso MBD, Droggiti A, Turmaine M, D'Antonio M, Parkinson DB, Wilton DK, Al-Shawi R, Simons P, Shen J, Guillemot F, Radtke F, Meijer D, Feltri ML, Wrabetz L, Mirsky R, Jessen KR. Notch controls embryonic Schwann cell differentiation, postnatal myelination and adult plasticity. Nature Neuroscience. 2009;12:839–U846. doi: 10.1038/nn.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]


