Abstract
Phospholamban (PLB) inhibits the activity of cardiac sarcoplasmic reticulum (SR) Ca2+-ATPase (SERCA2a). Phosphorylation of PLB during sympathetic activation reverses SERCA2a inhibition, increasing SR Ca2+ uptake. However, sympathetic activation also modulates multiple other intracellular targets in ventricular myocytes (VMs), making it impossible to determine the specific effects of reversal of PLB inhibition on the spontaneous SR Ca2+ release. Therefore, it remains unclear how PLB regulates rhythmic activity in VMs. Here we used the Fab fragment of 2D12, a monoclonal anti-PLB antibody, to test how acute reversal of PLB inhibition affects the spontaneous SR Ca2+ release in normal VMs. Ca2+ sparks and spontaneous Ca2+ waves (SCWs) were recorded in the line-scan mode of confocal microscopy using the Ca2+ fluorescent dye Fluo-4 in isolated permeabilized mouse VMs. Fab, which reverses PLB inhibition, significantly increased the frequency, amplitude, and spatial/temporal spread of Ca2+ sparks in VMs exposed to 50 nM free [Ca2+]. At physiological diastolic free [Ca2+] (100–200 nM), Fab facilitated the formation of whole-cell propagating SCWs. At higher free [Ca2+], Fab increased the frequency and velocity, but decreased the decay time of the SCWs. cAMP had little additional effect on the frequency or morphology of Ca2+ sparks or SCWs after Fab addition. These findings were complemented by computer simulations. In conclusion, acute reversal of PLB inhibition alone significantly increased the spontaneous SR Ca2+ release, leading to the facilitation and organization of whole-cell propagating SCWs in normal VMs. PLB thus plays a key role in subcellular Ca2+ dynamics and rhythmic activity of VMs.
Keywords: Calcium, sarcoplasmic reticulum, phospholamban, spontaneous calcium waves
1. Introduction
The rate at which Ca2+ is pumped into the lumen of cardiac sarcoplasmic reticulum (SR) by the SR Ca2+-ATPase (SERCA2a) is tightly controlled by the regulatory protein phospholamban (PLB) [1, 2]. Dephosphorylated PLB inhibits SERCA2a activity while increased β-adrenergic stimulation phosphorylates PLB by cAMP–dependent protein kinase A (PKA) and Ca2+/calmodulin-dependent protein kinase (CaMKII), reversing SERCA2a inhibition, thus enhancing the Ca2+ uptake into cardiac SR [3, 4]. Multiple studies in living ventricular myocytes (VMs) treated with isoproterenol or the monoclonal anti-PLB antibody 2D12 [5, 6], or isolated from PLB transgenic and knockout (PLB-KO) mice [7–10] have demonstrated that PLB modulates intracellular Ca2+ dynamics, regulating both inotropy and lusitropy.
In diastole, SR Ca2+ may be released via cardiac ryanodine receptor channel (RyR2) as Ca2+ sparks or spontaneous Ca2+ waves (SCWs), a process that is important to physiological rhythm and pathophysiological conditions such as the formation of delayed afterdepolarizations (DADs) and the syndrome of catecholaminergic polymorphic ventricular tachycardia (CPVT) [11–13]. The regulation of the spontaneous SR Ca2+ release is very complicated, and involves many regulatory factors including both cytoplasmic and lumenal Ca2+ [14], multiple protein kinases (e.g., PKA and CaMKII) [15–17], and junctional regulatory protein complexes [18, 19]. Within this complex system, the specific role of PLB in regulation of spontaneous SR Ca2+ release during β-adrenergic stimulation in VMs remains unclear. One reason is that besides PLB, β-adrenergic stimulation also phosphorylates RyR2 and other Ca2+ handling proteins regulating the SR Ca2+ release, making it impossible to delineate whether reversal of PLB inhibition alone is sufficient to augment spontaneous SR Ca2+ release and cause cell wide SCWs. Previous studies using PLB-KO mice suggest that PLB ablation increases inotropy but not chronotropy [7]. However, the chronic absence of PLB induces multiple adaptive changes of intracellular Ca2+ handling proteins [20, 21]. The specific role of PLB in rhythmic control in VM remains unclear.
PLB, as a key component of the Ca2+ clock, has also been shown to influence rhythmic activity of sinoatrial node cells [22]. Recent studies also indicate that PLB plays a key role in modulating the rhythmic Ca2+ activity in VMs. In particular, Kapoor et al. demonstrated that expression of Tbx18 induced rhythmic intracellular Ca2+ cycling events in VMs, mimicking the “Ca2+ clock” of native sinoatrial node cells. In this process, phosphorylation of PLB was 65-fold higher than that in the control VMs, indicating the modulation of PLB helps to generate rhythmic activity[23]. Sirenko et al. also demonstrated that the permeabilized VMs showed increased spontaneous Ca2+ releases with the self-organized and partial synchronization of Ca2+ sparks after PLB inhibition by drugs or 2D12 [24]. On the other hand, Bai et al. demonstrated that despite severe SR Ca2+ leak with multiple Ca2+ sparks or small wavelets, VMs from PLB-KO mice break up the formation of organized and whole-cell propagating SCWs in triggering the DADs [25]. They further showed that PLB ablation actually suppressed triggered activity and stress-induced ventricular tachycardia in the mouse model of PLB-KO plus RyR2-mutation. While these studies point to regulation of the rhythmic Ca2+ activity by PLB in VM, an important question that remains to be addressed is whether accelerating SR Ca2+ uptake by specifically removing PLB inhibition of the Ca2+ pump is pro-arrhythmic (i.e. increasing the automaticity in normal VMs)[24] or anti-arrhythmic (i.e. suppressing the DADs in the CPVT model)[25] in the VMs.
In this study, we took advantage of the specific action of the Fab fragment of the monoclonal anti-PLB antibody 2D12 at blocking the interaction between PLB and SERCA2a in isolated permeabilized (skinned) murine VMs [5, 6, 26]. We demonstrate that acute and specific reversal of PLB inhibition can significantly increase the frequency, amplitude, and spatial/temporal spread of Ca2+ sparks, leading to the facilitation and organization of whole-cell propagating SCWs. These findings were complemented by computer simulations studying the effects of reversal of PLB inhibition in VMs [27, 28].
2 Materials and Methods
2.1 Myocyte preparation
The study protocols were approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine and the Methodist Research Institute, Indianapolis, Indiana. Hearts from adult C57Bl/6 mice were quickly excised by thoracotomy and retrogradely perfused on a Langendorff apparatus maintained at 37°C. The enzyme digestion step consisted of perfusing Tyrode's solution containing 1 mg/ml collagenase (Type II, 300 U/mg; Worthington) and 0.1 mg/ml protease (Type XIV, ≥3.5 U/mg; Sigma) for 6 min. Ventricular myocytes (VMs) were dissociated from digested ventricles by gentle mechanical dissociation and used within 3 hour. The modified Tyrode's solution contained (in mM) 136 NaCl, 5.4 KCl, 0.33 NaH2PO4, 1.0 MgCl2, 10 HEPES, and 10 glucose, pH 7.4 (NaOH) [27]. All chemicals were obtained from Sigma unless indicated otherwise.
2.2 Myocyte permeabilization
VM membranes were permeabilized with saponin (0.005% w/v) for 60 s in a mock internal solution composed of (in mM) 100 potassium aspartate, 20 KCl, 10 HEPES, 0.5 EGTA, and 0.75 MgCl2, pH 7.2 (KOH). Permeabilized VMs were then resuspended in a saponin-free mock internal solution composed of (in mM) 100 potassium aspartate, 20 KCl, 5 KH2PO4, 5 MgATP, 10 phosphocreatine, 5 U/ml creatine phosphokinase, 10 HEPES, 0.5 EGTA, 1 MgCl2 (free), 0.015 Fluo-4 (Invitrogen), and 8% w/v dextran (molecular weight ~40,000; prevents osmotic swelling), pH 7.2 (KOH) [27]. CaCl2 was added to make free [Ca2+] of 50 nM to 1 µM. Free Ca2+ concentration and Mg2+ concentration were calculated with the use of WebMaxC Extended (maxchelator.stanford.edu). All experiments were performed at room temperature.
2.3 Fab fragment of 2D12 antibody and labeling
Fab fragment of affinity purified 2D12 was made using commercial kit (Pierce). In some experiments, 2D12 and Fab were covalently labeled with Aexa-594 (Invitrogen).
2.4 Ca2+ spark/wave imaging and immunostaining imaging of the confocal microscopy
We imaged spontaneous Ca2+ activity by using the Leica TCS SP8 LSCM inverted microscope fitted with a ×40 1.42 NA oil immersion objective. The Ca2+ indicator dye Fluo-4 was excited at 488 nm wavelength with an argon/krypton laser with intensity attenuated to 1–3%. Emission wavelengths > 510 nm were detected by the photomultiplier. Fluorescence intensity space-time recordings were acquired in the line scan mode (1.69 ms/line, 3000 lines/recording) along the longitudinal axis of the myocyte and digitized into 1024 × 1024-pixel images (8-bit) line scan with nominal pixel dimensions of 98 nm. In some experiments, permeabilized VMs incubated in internal solution with Fluo-4 were stained with Fab or 2D12 label with Alexa Fluor 594nm at a concentration of 20 µg/mL for one hour. Samples were then directly examined by the microscopy using the ×40 1.42 NA oil immersion objective and a pixel size of 138 nm. The immuno-histological images of Fab-PLB or 2D12-PLB label with Alexa Fluor 594nm were obtained by illumination with 561nm laser light, while fluorescence was collected in the long-pass range of > 580 nm by the photomultiplier.
2.5 Ca2+ spark detection and analysis
The SparkMaster plugin for ImageJ software [29] was used to detect and analyze Ca sparks. The analysis parameters were as follows: scanning speed, 520.8 lines/s; pixel size, 0.08–0.13 µm; spark threshold criteria, 3.8; background, 550–1330; and analysis intervals, 5.[27] We measured Ca2+ spark cluster sizes (spatial widths in line scan) using a custom algorithm that defines a Ca2+ spark cluster as Ca2+ sparks separated by less than the single spark average full width in space and the single spark average full duration in time.
2.6 Ca2+-ATPase Assay
Ca2+-dependent ATPase activities of canine SR membranes were measured using an enzyme-coupled spectrophotometric assay [26]. The rate of NADH decay was measured at 340 nm in a SPECTRAmax® PLUS (Molecular Devices) microplate spectrophotometer at 37 °C with 2 µg of membrane protein in buffer containing 50 mM MOPS (pH 7.0), 3 mM MgCl2, 100 mM KCl, 5 mM NaN3, 3 µg/ml of the Ca2+ ionophore A23187, 3 mM ATP, and Ca/EGTA as indicated. Ca2+-ATPase activities were measured in the presence and absence of anti-PLB monoclonal antibody 2D12 or the Fab fragment of 2D12. All ATPase activities reported are Ca2+-dependent.
2.7 Computational Simulation
We used a VM Ca2+ cycling model to simulate the Ca2+ sparks and waves in VMs with PLB inhibition [30]. In brief, the model is a three-dimensional Ca2+ release unit (CRU) network (65×27×11=19,305 CRUs) with the CRUs coupled via Ca2+ diffusion in the myoplasmic space and SR. Each CRU contains a cluster of 100 RyR channels which were simulated using random Markov transitions. All simulations were carried out by clamping the membrane voltage at −80 mV. [Ca]o=10 mM was used to overload the cell to promote Ca waves. To simulate the effect of PLB inhibition by Fab, the KCa of the SR uptake was reduced. Computer simulations were performed on a single NVIDIA Tesla C2050 high performance Fermi-based graphics processing unit. Details regarding the numerical algorithms and implementation computing can be found in our recent publication [31].
2.8 Statistics
Data were expressed as means ± SEM. The statistical significance was evaluated by t-test and analysis of variance (ANOVA) followed by Bonferroni post hoc tests. A values of P<0.05 were considered statistically significant.
3. Results
3.1 Characterization of Fab fragment of 2D12
Compared to the absence of antibody (Con, squares), both Fab (+Fab, triangles) and 2D12 (+2D12, circles) shifted the Ca2+-activation curve of the Ca2+-dependent ATPase activity of cardiac SR membranes to the left, decreasing the KCa value from 0.30±0.04 µM to 0.15 ±0.03 µM and 0.11 ±0.02 µM (n=6, P = 0.02 compared to control), respectively (Fig. 1A), thus restoring the high apparent Ca2+ affinity of the Ca2+ pump. These results suggest that the Fab, similar to the well- studied 2D12 [26], almost completely reversed PLB inhibition. Fab increased the Ca2+-ATPase activity more than 2-fold at low free Ca2+ concentrations (from ~50 to ~200 nM) as compared to the absence of PLB inhibition. However, Fab did not affect the maximal enzyme velocity of Ca2+-ATPase activity at saturating Ca2+ concentrations. Similar results were obtained when Ca2+ uptake by SR vesicles was measured (Data not shown).
Figure 1.
Fab binding to native PLB. A. The effect of Fab or 2D12 on the Ca2+-dependent ATPase activity of cardiac SR membranes. 6 experiments were performed. See text for KCa values. B–G. Representative confocal immunofluorescence images showing binding of Fab or 2D12 to PLB in permeabilized VMs. 20 µg/ml Alexa-594 labelled 2D12 (B and C) or Fab (D to G) was added to permeabilized VM and incubated for 15 min at room temperature. A peptide containing PLB residue 1–31 was incubated with Fab-594 in experiments in Fig 1F and G. H to J, Fixed VMs were co-incubated with Fab (covalently labeled with Alexa-594) and the monoclonal anti-SERCA2a antibody 2A7-A1(covalently labeled with Alexa-488). Overlay image was shown in Fig. 1J. Images were obtained under the DIC (B, D, and F) and fluorescent (C, E, and G–J) mode of confocal microscopy, respectively. Bar represents 10 µm.
We tested the binding efficacy of Fab or 2D12 to PLB in permeabilized, semi-intact VMs. Fab or 2D12, covalently labeled with Alexa-594 (20 µg/ml), was added directly to the bath and permeabilized VMs were imaged with confocal microscopy (Figure 1B). After 15 min antibody incubation, we found strong immunofluorescent signals showing a characteristic cross-striated staining pattern at about 2 µm intervals, suggesting that Fab penetrated well into permeabilized VMs and efficiently bound to PLB. In contrast, 2D12 fluorescence was usually localized at the periphery of the VMs and did not penetrate deep into VMs. In control experiments, we incubated permeabilized VMs with Fab (covalently labeled with Alexa-594) and peptide containing PLB residue 1–31. As shown in Fig 1G, PLB1–31 completely blocked Fab binding to PLB, confirming the high specificity of Fab binding to PLB. In addition, co-incubation with Fab (covalently labeled with Alexa-594) and the monoclonal anti-SERCA2a antibody 2A7-A1(covalently labeled with Alexa-488) revealed co-localization of signals, consistent with close proximity of the two proteins (Fig. 1H to 1J). These results suggest that Fab, as compared to the 2D12, is a better reagent for penetrating into the SR myocytes, and binds to native PLB more completely in the SR membrane of permeabilized VMs.
3.2 Effect of Fab on Ca2+ sparks/SCWs
We next studied how Fab binding to PLB affects intracellular Ca2+ cycling in VMs. Figure 2 shows confocal images of the Ca2+ fluorescence from the Fluo-4 Ca2+ indicator and immunofluorescece from Fab in the same permeabilized VM before and after addition of the Alexa-594-labeled Fab. At the baseline, 50 nM free [Ca2+] generated multiple Ca2+ sparks (Figure 2A, left panel), which is consistent with the results of previous studies [16]. Approximately 15 min after Fab incubation, Ca2+ spark clusters and mini-waves increased significantly (from 0.7 ±0.1 to 1.9 ± 0.8 Hz) (Figure 2A, middle panel). The background Ca2+ signal decreased, which may result from a transient reduction in cytosolic Ca2+ by increased SERCA2a uptake until a new leak-uptake balance is reached for SR. At the same time, Figure 2B and C show the development of the strong immunofluorescent signals from Alexa-594-labeled Fab in the same VM, confirming that PLB was bound efficiently by Fab (Figure 2C). Furthermore, the SERCA inhibitor Thapsigargin (10 µM) completely abolished Ca2+ sparks and clusters (Figure 2A, right panel). These results suggest that Fab binding to PLB reverses its inhibition of SERCA2a. As the result, higher SR Ca2+ content caused increased spontaneous SR Ca2+ release events.
Figure 2.
Confocal Ca2+ and immunofluorescence imaging of a representative permeabilized VM. The Ca2+ activity with Fluo-4 Ca2+ indicator and PLB immunostaining by Fab covalently labeled with Alexa-594 in VMs were measured under the line scan mode (A), DIC (B), or fluorescence mode (C) of a confocal microscope at baseline (left panels), 15 min after antibody incubation (middle panels), and after Thapsigargin (right panels) (10 µM) consecutively. (D), Bar graphs showed the background fluorescence and the number of Ca2+ clusters and mini-waves before and after addition of Fab (n=3 cells, P<0.05). The free [Ca2+] in the bath was 50 nM.
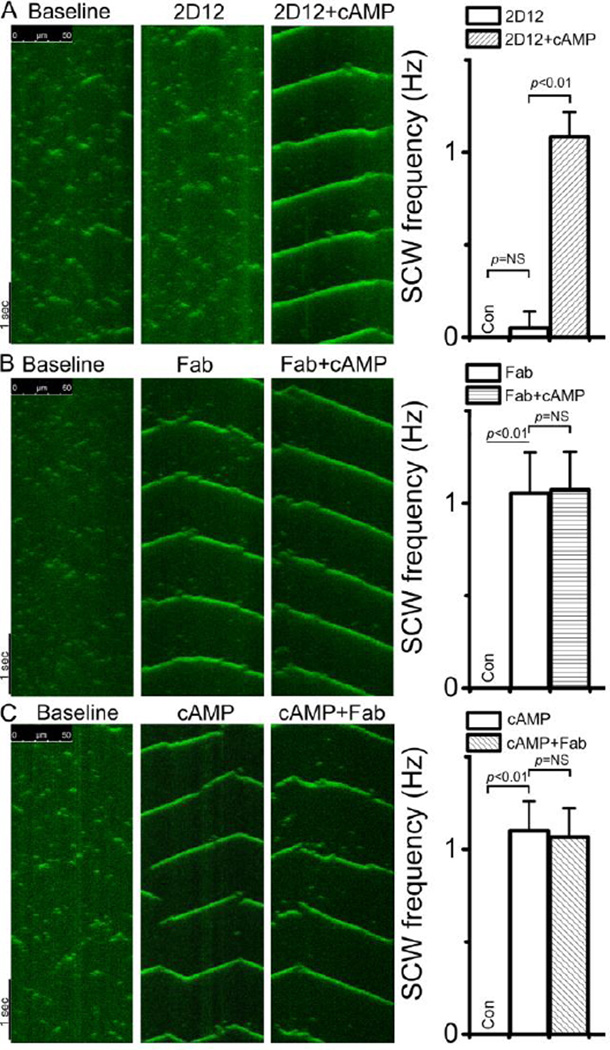
cAMP activates PKA, leading to phosphorylation of PLB at Ser16 and reversal of PLB inhibition (supplement Figure S1), thus augmenting SR Ca2+ content and spontaneous Ca2+ release [3, 24, 32]. We used the cAMP response as a control to verify the extent of the Fab effect. We sequentially added cAMP before or after Fab or 2D12 addition to myocytes. Figure 3A shows that with 200 nM free [Ca2+], 2D12 only slightly increased frequency of Ca2+ sparks but did not generate SCW, consistent with the finding of Sirenko et al [24]. However, 20 µM cAMP following 2D12 incubation caused the transition from stochastic Ca2+ sparks to periodic and whole-cell SCWs, consistent with previously reported effect of cAMP [24]. Importantly, as shown in Figure 3B, Fab alone changed the Ca2+ activity from sparks/marco-sparks into periodic and whole-cell propagating SCWs. Sequential addition of cAMP had little effect on the morphology or frequency of SCW in the VMs already treated with Fab (Figure 3B, right panel). In separate experiments in which we added cAMP first, addition of Fab had no further effect on SCWs generated by cAMP (Figure 3C). The above results demonstrate that Fab, which specifically dissociates PLB from the SERCA2a and reverses PLB inhibition, dramatically increases spontaneous Ca2+ release in VMs. Again, due to its poor binding efficiency in permeabilized VMs, 2D12 only marginally altered intracellular Ca2+ dynamics. These experiments show for the first time that acute elimination of PLB inhibition itself is sufficient to facilitate the formation of SCWs in VMs.
Figure 3.
The effect of 2D12 or Fab on the intracellular Ca2+ activity in the permeabilized VM. Confocal Ca2+ imaging of permeabilized VMs was obtained with the Fluo-4 Ca2+ indicator under the line-scan mode. The baseline (left panels) was recorded in 200 nM free [Ca2+]. Antibody (100µg/ml) or cAMP (20 µM) was added sequentially to the bath as indicated (Top). A. Ca2+ activity in a VM after 15 min of incubation with 2D12, and then after cAMP. B. Ca2+ activity in a VM after 15 min of incubation with Fab, and then after cAMP. C. Ca2+ activity in a VM after 15 min of incubation with cAMP, and then after Fab. Plots were average of at least 10 VMs.
The specificity of Fab in the dose- and time-dependent effect on spontaneous subcellular Ca2+ releases was further studied in VMs. Figure 4A shows the time course of the XT line-scan of a VM exposed to 200 nM of free [Ca2+] after addition of Fab (100 µg/ml). In 5 min, the Ca2+ activity evolved from small Ca2+ sparks at baseline into chains of Ca2+ clusters with increased amplitude, temporal and spatial spread. Fifteen min after addition of Fab, periodic and organized SCWs propagated over the entire VM (Figure 4A). In addition, Figure 4B shows the time interval of 15 min after Fab was gradually titrated up in the cell suspension. As exposure concentration of Fab increased, the Ca2+ activity evolved from rare small Ca2+ spark clusters at baseline into chains of Ca2+ sparks and SCWs. The maximal effect of Fab was saturated after 40 µg/ml. In control experiments, addition of the affinity-purified monoclonal anti-SERCA2a antibody, 2A7-A1, which was purified in the same buffer as for 2D12, had no effect on the spontaneous subcellular Ca2+ release activity over 1 hour (data not shown).
Figure 4.
Time and dose-dependent effects of Fab on the intracellular Ca2+ activity. Confocal Ca2+ imaging of permeabilized VMs used Fluo-4 Ca2+ indicator under the line-scan mode. Baseline was obtained in the absence of Fab under the free [Ca2+] of 200 nM. A. Ca2+ images was obtained at various time points (top) after Fab exposure (100 µg/ml). (B) Ca2+ images was obtained after addition of Fab sequentially (top) at the time interval of 15 min. Plots were average of at least 4 VMs.
3.3 The Ca2+-dependency of Fab effect in VMs
We recorded the local Ca2+ release in the XT line-scan in permeabilized VMs before and after Fab application (100 µg/ml) at different free [Ca2+](Figure 5). In each experiment, cAMP (20 µM) was added into the cell suspension 30 min after Fab application to test for any further change in local Ca2+ release. At 50 nM free [Ca2+], there were multiple stochastic spontaneous Ca2+ sparks in VMs at baseline (Figure 5A). After Fab application, the frequency of Ca2+ sparks was increased about 1.5-fold from 11.3 ± 5.4 sparks s−1 (100 µm)−1 at baseline to 17.1±4.8 sparks s−1 (100 µm)−1 (n = 12, P = 0.002). In addition, macrosparks and mini-waves were noted after Fab administration (Figure 5A). The properties of the Ca2+ sparks at baseline and after Fab are summarized in Table 1. In particular, the amplitude of sparks increased from 1.7 ± 0.4 in F/F0 at baseline to 2.9 ± 0.8 in F/F0 (P = 0.002); the full width at half-maximal amplitude (FWHM) increased from 2.2 ± 0.3 to 2.4 ± 0.3 µm (P = 0.04); the full duration at half-maximal amplitude (FDHM) increased from 20.5 ± 3.4 to 23.3 ± 3.4 ms (P = 0.002). There were no differences in the time constant of Ca2+ spark decay between the baseline vs Fab administration. cAMP (20 µM) after Fab application did not further change the frequency or morphology of Ca2+ sparks (Figure 5A and Table 1). All these results suggest that Fab inhibition of PLB increased the frequency and magnitude of local Ca2+ release.
Figure 5.
Effect of Fab on the intracellular Ca2+ activity at various Ca2+ concentrations. A–F, At each Ca2+ concentration indicated ([Ca]), confocal line-scan images of the same permeabilized VM were obtained at baseline (a), after addition of 100 µg/ml Fab (b), and after 20 µM cAMP (c) exposure consecutively. Bar graphs showed the frequency of Ca2+ sparks or waves at each condition. P value was indicated. Traces in B to F showed intensity of fluorescent signals (F) of SCWs. See Table 2 for the Ca2+ decay time and velocity of SCWs (as arrows). At least 12 VMs were imaged at each condition.
Table 1.
Ca2+ Spark Characteristics
| Total spark number |
Peak amplitude, F/F0 |
FWHM, µm |
FDHM, ms | Tau decay, ms |
|
|---|---|---|---|---|---|
| Baseline | 678 | 1.8±0.4 | 2.2±0.3 | 20.5±3.4 | 20.4±4.4 |
| Fab | 1020 | 2.9±0.8* | 2.4±0.3* | 23.3±3.3* | 22.3±7.9 |
| cAMP | 1014 | 2.8±0.9+ | 2.4±0.4+ | 23.8±3.5+ | 22.0±8.1 |
Ca2+ sparks were measured in permeabilized VMs at 50 nM free [Ca2+] as indicated in Figure 5. Ca2+ sparks characteristics at baseline, after Fab (100µg/ml), and after cAMP (20µM) are reported. Total numbers of Ca2+ sparks, amplitude, FWHM, FDHM, and the Ca2+ decay time of Ca2+ sparks (Tau decay) were compared using one way ANOVA (n= 12, * baseline vs Fab P < 0.05, + baseline vs cAMP P < 0.05). Data are mean±SEM.
With physiological diastolic free [Ca2+] of 100 and 200 nM in permeabilized VMs at baseline, there were multiple Ca2+ sparks but no whole-cell SCW (Figure 5B and C, panel a). After Fab administration, periodic SCWs formed and propagated in the whole-cell (P < 0.001). Table 2 shows characteristics of SCWs. Again, it should be noted that formation of SCWs was accompanied by significant reduction in background fluorescence, consistent with increased SR Ca2+ uptake and lowered cytoplasmic Ca2+ concentration after adding Fab (compare traces b to a in Figures 5B and C). At 400 nM free [Ca2+], Fab converted the fractured partial organized SCWs at baseline into highly synchronized and organized SCWs (Figure 5D). Further, in addition to increasing the amplitude (2.9 ± 0.3 vs 4.4 ± 0.4 in F/F0, P < 0.001) and shortening the Ca2+ decay time of SCWs from 89 ± 14 to 52 ± 8 ms after Fab addition (P < 0.001, Table 2), Fab significantly increased the frequency of the SCWs from 1.1 ± 0.4 to 1.6 ± 0.5 Hz (P = 0.001) (compare traces b to a in Figures D). At 500 nM free [Ca2+], there were already periodic and organized SCWs in the VMs at baseline (Figure 5E, panel a). As expected, Fab decreased the Ca2+ decay time of SCWs from 74 ± 12 to 54 ± 4 ms after Fab addition (P < 0.001, Figure 5E, red arrows), which is consistent with the idea that relief of PLB inhibition of SECA2a by Fab speeds up Ca2+ uptake. Importantly, Fab further increased the frequency of SCWs from 2.4 ± 0.1 at baseline to 2.8 ± 0.2 Hz, P < 0.001) (Figure 5E). In addition, Fab increased SCW velocity at these Ca2+ concentrations. At 1000 nM free [Ca2+], there was no significant difference in the frequency, velocity, or Ca2+ decay of SCWs between the baseline and after Fab application (Figure 5F, red arrows), compatible with the finding that PLB inhibition did not affect maximal Ca2+-ATPase activity in native SR vesicles at high micromolar free [Ca2+] (Figure 1A). Finally, for all free [Ca2+] concentrations tested, sequential addition of cAMP (20 µM) 30 min after Fab application did not further change the frequency or morphology of SCWs in VMs (Fig. 5, panel c), respectively.
Table 2.
Spontaneous Ca2+ wave Characteristics
| decay time (ms) | velocity (um/sec) | frequency (Hz) | ΔF/Fo | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Free [Ca2+] (nM) |
Baseline | Fab | Fab + cAMP |
Baseline | Fab | Fab + cAMP |
Baseline | Fab | Fab + cAMP |
Baseline | Fab | Fab + cAMP |
| 100 | -- | 44±6* | 47±8^ | -- | 133±21* | 138±25^ | -- | 0.44±0.16* | 0.46±0.13^ | -- | 4.68±0.54* | 4.84±0.69^ |
| 200 | -- | 45±7* | 46±9^ | -- | 140±26* | 142±20^ | -- | 1.05±0.21* | 1.07±0.21^ | -- | 4.49±0.63* | 4.45±0.57^ |
| 400 | 89±14 | 52±8* | 53±9^ | 112±26 | 144±13* | 146±21^ | 1.12±0.38 | 1.61±0.55* | 1.76±0.62^ | 2.89±0.31 | 4.41±0.41* | 4.38±0.38^ |
| 500 | 74±12 | 54±4* | 52±8^ | 123±10 | 143±23* | 150±25^ | 2.38±0.11 | 2.82±0.24* | 2.76±0.16^ | 2.39±0.51 | 2.91±1.02 | 2.88±0.71 |
| 1000 | 61±9 | 60±8 | 60±9 | 157±23 | 156±24 | 157±24 | 2.90±0.11 | 2.95±0.12 | 2.93±0.14 | 2.33±0.38 | 2.38±0.32 | 2.41±0.34 |
Spontaneous Ca2+ wave (SCWs) were measured in permeabilized VMs at various free [Ca2+] as indicated in Figure 5. SCWs characteristics at baseline, after Fab (100µg/ml), and after cAMP (20µM) are reported. SCWs Ca2+ decay time, velocity, frequency, and ΔF/F0 were compared using one way ANOVA (12 cells from 4 mice were chosen for comparison in each free [Ca2+], * baseline vs Fab P < 0.05, ^ baseline vs Fab+cAMP P < 0.05). Data are mean±SEM.
3.4 The computational modeling of the effects of reversal of PLB inhibition on Ca2+ cycling dynamics
To investigate whether Fab reversal of PLB inhibition alone can promote Ca waves, we carried out computer simulations using a VM model under Ca2+ overload condition for different KCa of the SERCA pump. Figure 6 shows the computer simulation results of the effects of PLB inhibition on Ca2+ waves and oscillations. When KCa > 0.5 µM (Fig.6A), the VMs predominantly exhibited single sparks, spark clusters, and miniwaves. As KCa was decreased to simulate reversal of PLB inhibition, more and more spark clusters and non-persistent waves form. At KCa = 0.35 µM (Fig.6B), persistent Ca2+ waves and periodic whole-cell Ca2+ oscillation occurred. As KCa was decreased further, the whole-cell Ca2+ oscillations became more periodic and the period decreased (compare Fig.6B and Fig.6C). As shown in the bottom panels in Fig.6, decreasing KCa increased the SR load which is the dominant cause promoting Ca waves and oscillations in the simulations. These results are fully consistent with the experimental findings observed in the murine VMs.
Figure 6.
Computer simulation results of intracellular Ca2+ activity under different KCa. Top panels: Space-time plots of cytosolic Ca concentration for KCa of 0.5 µM (A), 0.35 µM (B), and 0.17 µM (C). Middle panels: Whole-cell cytosolic Ca concentrations versus time for the corresponding KCa values. Bottom panels: Whole-cell SR Ca concentrations versus time for the corresponding KCa values.
4. Discussion
This present study shows that acute specific reversal of PLB inhibition by the anti-PLB monoclonal antibody had significant impact on the subcellular Ca2+ activity in normal VMs. The experimental results and computational simulations demonstrate that reversal of PLB inhibition alone, with or without activating RyR2, was sufficient to initiate cell wide SCWs in isolated permeabilized murine VMs.
4.1 New platform to investigate PLB in influencing the subcellular Ca2+ activity
Animal models (e.g., PLB-KO mice) provide valuable tools to study of the relationship between PLB and the subcellular Ca2+ activity. However, the chronic PLB-KO mouse model is associated with compensatory adaptations. In particular, in response to the chronically elevated Ca2+ contents in the SR in PLB-KO mouse, RyR2 expression is reduced by 30%.[20] Therefore, the SR Ca2+-release process in PLB-KO mice is altered, which makes it difficult to determine whether the observed phenotype is due to loss of PLB alone or to the accompanying compensatory mechanisms [20].
Pharmacological interventions have been used to study the effects of PLB inhibition, but these drugs may have off-target effects. For example, studies of the actions of PLB on the spontaneous SR Ca2+ release are complicated at the cellular level when using protein phosphatase inhibitor, cAMP, or kinase itself to activate PKA or CaMKII. Because these interventions simultaneously target multiple proteins (e.g. RyR2, Dihydropyridine Receptors, CaMKII, PLB) that affect intracellular Ca2+ dynamics and cellular function, it is not possible to define a specific role for PLB in intracellular Ca2+ handling.
Biochemical studies have demonstrated that the intact monoclonal antibody, 2D12, specifically reversed SERCA2a inhibition by completely disrupting PLB binding to SERCA2a in native SR vesicle [26, 33]. However, delivering 2D12 into living VMs remains challenging. Sham et al used patch pipette to inject 2D12 into intact VMs and demonstrated that 2D12 reversed SERCA2a inhibition in VMs and mimicked the effects induced by β-adrenergic stimulation on the Ca2+ transient, without any notable cytotoxicity or off-target effect [5]. Alternatively, saponin-permeabilized VMs provide a semi-intact system, allowing direct access of protein/reagents to the intracellular space [34]. Recently, Sirenko et al. reported that addition of 2D12 to permeabilized rabbit VMs induced a self-organized and partial synchronization of spontaneous Ca2+ releases [24]. However, being a relatively large sized molecule of ~150KD, 2D12 apparently has difficulty passing through the discrete pores of 30 Å diameter in permeabilized VMs created by saponin [35, 36], which is shown in our study (Figure 1). Here, we used the Fab fragment of 2D12, which is only ~1/3 size of 2D12, to achieve deeper penetration and more efficient binding to PLB, and observed robust effects on subcellular Ca2+ activity in VMs (Figure 1 to 3).
Specifically, Fab binding to PLB was directly monitored by immunofluorescent signal from the covalently labeled Aexa-594 fluorophore and correlated to increased intracellular Ca2+ release (Figure 2). Also, the dose and time-dependent changes of subcellular Ca2+ activity confirmed the specificity of Fab. To achieve the maximal reversal of PLB inhibition, we used a saturating dose of Fab (~100 µg/ml) in our experiments. Efficient Fab binding to PLB was verified by a strong correlation in Ca2+-dependency (i.e., large effect at low physiology concentration, small effect at high intracellular Ca2+ concentrations) between the effect of Fab on intracellular Ca2+ kinetics at the subcellular level and on Ca2+-ATPase enzyme activity in in vitro study. A significant decrease in SCWs decay time at free [Ca2+] below 500 nM after Fab application is consistent with the well characterized effects of reversal of PLB inhibition. High concentrations of cAMP (20 µM) after Fab application did not cause any further change in subcellular Ca2+ activity at different baseline levels of free [Ca2+], indicating that PLB is efficiently saturated by Fab and uncoupled from Ca2+-ATPase inhibition. Therefore, Fab appears to be a valuable new tool to reverse selectively PLB inhibition of SERCA2a in permeabilized VMs, and provides an attractive new system to delineate the role of PLB and other factors in regulation of spontaneous SR Ca2+ release.
4.2 PLB is the key regulator in the rhythmic regulation of VMs
PLB is well known to regulate excitation-contraction coupling in heart. Here, by showing that acute reversal of PLB inhibition increased the Ca2+ sparks and facilitated the formation of SCWs, we demonstrate that PLB is also a powerful regulator for the formation of rhythmic subcellular Ca2+ activity in VMs. Note that reversal of PLB inhibition may affect SCWs in two ways: by enhancing SERCA2a uptake, PLB may buffer the diffusion of Ca2+ from Ca2+ release site to Ca2+ release site, leading to less effective Ca2+-induced Ca2+ release and broken SCWs; on the other hand, reversal of PLB inhibition also increases Ca2+ SR content, leading to greater local Ca2+ releases favoring propagation of SCWs. Further, our data suggest that acute reversal of PLB inhibition increased SCW velocity (Table 2). This phenomenon can be explained by the “sensitization” wave-front hypothesis proposed by Keller et al [37]. Local increase in Ca2+ inside SR, by acute reversal of PLB inhibition, may sensitize nearby RyR2 clusters, speeding up SCW propagation. When we modeled the effect of increasing the Ca2+ affinity of SERCA2a (decreasing KCa value), we indeed found that the latter effect predominated and Ca2+ waves were promoted, consistent with our experimental data (Figures 5 and 6).
Our data may also be relevant to observations obtained using the PLB-KO mouse model [38, 39]. Unlike our results in which acute removal of PLB inhibition in normal VMs promoted propagating SCWs, quiescent PLB-KO VMs did not exhibit cell-wide propagating SCWs [8, 40]. When crossed with RyR2-R4496C mutant mice (a CPVT model) which show increased proclivity to SCWs, the double mutant VMs showed an increased frequency of Ca2+ sparks and miniwaves, but did not exhibit whole-cell propagating SCWs, suggesting the reversal of PLB inhibition might be therapeutic in CPVT. However, it should be noted that in order to compensate the abnormal SR Ca2+ homeostasis, PLB-KO mouse downregulates the expression of RyR2 [20], which is expected to inhibit Ca2+ wave propagation. In addition, crossing PLB-KO with other mouse models also known to cause various Ca2+ protein adaptations [21]. For example, the mice with PLB-KO and SERCA2a replacement by the high Ca2+ affinity SERCA2b were shown to develop severe cardiac hypertrophy [41]. Further, Louch et al found that PLB ablation caused further down-regulation of the Ca pump in these mice but normalized global Ca2+ homeostasis in cardiomyocytes [42]. They suggest that the Ca2+ affinity of SERCA plays a more important role than the expression level and maximal turnover rate. Here we showed that acute reduction in Ca2+ affinity of SERCA2a by reversing PLB inhibition, but with intact SERCA2a, caused significant increase in intracellular Ca2+ release, consistent with the importance of PLB modulation of SERCA2a Ca2+ affinity in regulation of intracellular Ca2+ dynamics.
PLB is a key modulator of subcellular Ca2+ kinetics and may serve as a therapeutic target to improve inotropic function of the heart. Our data here indicates that PLB could also be targeted to induce chronotropic effects. For example, as a potential future direction of bio-pacemaker design [24], selective reversal of PLB inhibition could initiate rhythmic whole-cell propagating Ca2+ activity, i.e., a “Ca2+ clock” under physiological diastolic [Ca2+] conditions (i.e. 100 nM in our study) in quiescent VMs. On the other hand, while the selective PLB inhibition provides a potentially promising way to enhance the SERCA2a activity and restores the normal SR Ca2+ load in patients with heart failure, the same intervention may potentially increase the risk of trigger-activity or automaticity, leading to ventricular arrhythmia in the acute phase.
5. Limitations
The present studies were performed at room temperature, which affects the rate of SERCA2a activity. However, the previous experiments with permeabilized myocytes were usually also performed at room temperature [16, 17]. The SR Ca content was not estimated in the study. We used the effect of cAMP as a positive control. We used permeabilized rather than intact VMs for these experiments. Permeabilization may cause lost accessory proteins or factors required for phosphorylation. Although similar results were obtained in intact and permeabilized VMs in previous studies [16, 17, 34], whether the results in permeabilized VMs can be directly extrapolated to intact myocytes is untested. To minimize this limitation, we also performed computer simulation studies using an intact VM model. There are differences between KCa values used in simulation using a whole cell model and in in vitro experiments using pure membranes in the test tubes (Fig 1). Different KCa values were used in various simulation approaches in the literatures. Because the results were consistent with that obtained with permeabilized VMs, these simulation studies strengthen the conclusions of our study.
Supplementary Material
Highlights.
The Fab fragment of an anti-phospholamban antibody relieves PLB inhibition of SERCA2a
Fab binds to PLB in semi-intact, permeabilized ventricular myocytes
Fab facilitates the formation of whole-cell propagating spontaneous Ca waves
Fab increases the frequency and velocity of spontaneous Ca waves
PLB regulates subcellular Ca dynamics and rhythmic activity of ventricular myocytes
Acknowledgments
We thank Jin Guo, Glen A Schmeisser, and Jian Tan for the technical support.
Funding Source
This study was supported in part by NIH Grants R37-HL049428 (LRJ), P01 HL78931 (JNW), R0171140, a Medtronic-Zipes Endowment (PSC), the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative and the Kawata and Laubisch endowments of the UCLA.
Abbreviations
- 2D12
anti-PLB monoclonal antibody
- CaMKII
Ca2+/calmodulin-dependent protein kinase
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- DAD
delay afterdepolarizations
- Fab
fab fragment of 2D12
- KCa
Ca concentration for half maximal effect
- PKA
cAMP–dependent protein kinase A
- PLB
phospholamban
- PLB-KO
PLB deficiency mice
- RyR2
cardiac ryanodine receptor channel
- SCW
spontaneous calcium wave
- SERCA2a
isoform of sarco(endo)plasmic reticulum Ca2+-ATPase in cardiac sarcoplasmic reticulum
- SR
sarcoplasmic reticulum
- VM
ventricular myocytes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Simmerman HK, Jones LR. Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol Rev. 1998;78:921–947. doi: 10.1152/physrev.1998.78.4.921. [DOI] [PubMed] [Google Scholar]
- 2.Kranias EG, Hajjar RJ. Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circ Res. 2012;110:1646–1660. doi: 10.1161/CIRCRESAHA.111.259754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindemann JP, Jones LR, Hathaway DR, Henry BG, Watanabe AM. beta-Adrenergic stimulation of phospholamban phosphorylation and Ca2+-ATPase activity in guinea pig ventricles. J Biol Chem. 1983;258:464–471. [PubMed] [Google Scholar]
- 4.Brittsan AG, Kranias EG. Phospholamban and cardiac contractile function. J Mol Cell Cardiol. 2000;32:2131–2139. doi: 10.1006/jmcc.2000.1270. [DOI] [PubMed] [Google Scholar]
- 5.Sham JS, Jones LR, Morad M. Phospholamban mediates the beta-adrenergic-enhanced Ca2+ uptake in mammalian ventricular myocytes. Am J Physiol. 1991;261:H1344–H1349. doi: 10.1152/ajpheart.1991.261.4.H1344. [DOI] [PubMed] [Google Scholar]
- 6.Mattiazzi A, Hove-Madsen L, Bers DM. Protein kinase inhibitors reduce SR Ca transport in permeabilized cardiac myocytes. Am J Physiol. 1994;267:H812–H820. doi: 10.1152/ajpheart.1994.267.2.H812. [DOI] [PubMed] [Google Scholar]
- 7.Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, et al. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res. 1994;75:401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- 8.Santana LF, Kranias EG, Lederer WJ. Calcium sparks and excitation-contraction coupling in phospholamban-deficient mouse ventricular myocytes. J Physiol. 1997;503(Pt 1):21–29. doi: 10.1111/j.1469-7793.1997.021bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolska BM, Stojanovic MO, Luo W, Kranias EG, Solaro RJ. Effect of ablation of phospholamban on dynamics of cardiac myocyte contraction and intracellular Ca2+ Am J Physiol. 1996;271:C391–C397. doi: 10.1152/ajpcell.1996.271.1.C391. [DOI] [PubMed] [Google Scholar]
- 10.Koss KL, Kranias EG. Phospholamban: a prominent regulator of myocardial contractility. Circ Res. 1996;79:1059–1063. doi: 10.1161/01.res.79.6.1059. [DOI] [PubMed] [Google Scholar]
- 11.Eisner DA, Kashimura T, O'Neill SC, Venetucci LA, Trafford AW. What role does modulation of the ryanodine receptor play in cardiac inotropy and arrhythmogenesis? J Mol Cell Cardiol. 2009;46:474–481. doi: 10.1016/j.yjmcc.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Priori SG, Chen SR. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res. 2011;108:871–883. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bers DM. Cardiac sarcoplasmic reticulum calcium leak: basis and roles in cardiac dysfunction. Annual Review of Physiology. 2014;76:107–127. doi: 10.1146/annurev-physiol-020911-153308. [DOI] [PubMed] [Google Scholar]
- 14.Chen W, Wang R, Chen B, Zhong X, Kong H, Bai Y, et al. The ryanodine receptor store-sensing gate controls Ca2+ waves and Ca2+-triggered arrhythmias. Nature medicine. 2014;20:184–192. doi: 10.1038/nm.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Kranias EG, Mignery GA, Bers DM. Protein kinase A phosphorylation of the ryanodine receptor does not affect calcium sparks in mouse ventricular myocytes. Circ Res. 2002;90:309–316. doi: 10.1161/hh0302.105660. [DOI] [PubMed] [Google Scholar]
- 17.Guo T, Zhang T, Mestril R, Bers DM. Ca2+/Calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ Res. 2006;99:398–406. doi: 10.1161/01.RES.0000236756.06252.13. [DOI] [PubMed] [Google Scholar]
- 18.Gyorke I, Hester N, Jones LR, Gyorke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J. 2004;86:2121–2128. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, et al. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu G, Luo W, Slack JP, Tilgmann C, Sweet WE, Spindler M, et al. Compensatory mechanisms associated with the hyperdynamic function of phospholamban-deficient mouse hearts. Circ Res. 1996;79:1064–1076. doi: 10.1161/01.res.79.6.1064. [DOI] [PubMed] [Google Scholar]
- 21.Sipido KR, Vangheluwe P. Targeting sarcoplasmic reticulum Ca2+ uptake to improve heart failure: hit or miss. Circ Res. 2010;106:230–233. doi: 10.1161/CIRCRESAHA.109.210740. [DOI] [PubMed] [Google Scholar]
- 22.Lakatta EG, Maltsev VA, Vinogradova TM. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart's pacemaker. Circ Res. 2010;106:659–673. doi: 10.1161/CIRCRESAHA.109.206078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapoor N, Liang W, Marban E, Cho HC. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nature biotechnology. 2013;31:54–62. doi: 10.1038/nbt.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sirenko S, Maltsev VA, Maltseva LA, Yang D, Lukyanenko Y, Vinogradova TM, et al. Sarcoplasmic reticulum Ca2+ cycling protein phosphorylation in a physiologic Ca2+ milieu unleashes a high-power, rhythmic Ca2+ clock in ventricular myocytes: relevance to arrhythmias and bio-pacemaker design. J Mol Cell Cardiol. 2014;66:106–115. doi: 10.1016/j.yjmcc.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai Y, Jones PP, Guo J, Zhong X, Clark RB, Zhou Q, et al. Phospholamban knockout breaks arrhythmogenic Ca2+ waves and suppresses catecholaminergic polymorphic ventricular tachycardia in mice. Circ Res. 2013;113:517–526. doi: 10.1161/CIRCRESAHA.113.301678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z, Akin BL, Jones LR. Mechanism of reversal of phospholamban inhibition of the cardiac Ca2+-ATPase by protein kinase A and by anti-phospholamban monoclonal antibody 2D12. The J Biol Chem. 2007;282:20968–20976. doi: 10.1074/jbc.M703516200. [DOI] [PubMed] [Google Scholar]
- 27.Nivala M, Ko CY, Nivala M, Weiss JN, Qu Z. Criticality in intracellular calcium signaling in cardiac myocytes. Biophys J. 2012;102:2433–2442. doi: 10.1016/j.bpj.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nivala M, Ko CY, Nivala M, Weiss JN, Qu Z. The emergence of subcellular pacemaker sites for calcium waves and oscillations. J Physiol. 2013;591:5305–5320. doi: 10.1113/jphysiol.2013.259960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Picht E, Zima AV, Blatter LA, Bers DM. SparkMaster: automated calcium spark analysis with ImageJ. Am J Physiol. 2007;293:C1073–C1081. doi: 10.1152/ajpcell.00586.2006. [DOI] [PubMed] [Google Scholar]
- 30.Restrepo JG, Weiss JN, Karma A. Calsequestrin-mediated mechanism for cellular calcium transient alternans. Biophys J. 2008;95:3767–3789. doi: 10.1529/biophysj.108.130419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nivala M, de Lange E, Rovetti R, Qu Z. Computational modeling and numerical methods for spatiotemporal calcium cycling in ventricular myocytes. Frontiers in Physiology. 2012;3:114. doi: 10.3389/fphys.2012.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wegener AD, Simmerman HK, Lindemann JP, Jones LR. Phospholamban phosphorylation in intact ventricles. Phosphorylation of serine 16 and threonine 17 in response to beta-adrenergic stimulation. J Biol Chem. 1989;264:11468–11474. [PubMed] [Google Scholar]
- 33.Akin BL, Jones LR. Characterizing phospholamban to sarco(endo)plasmic reticulum Ca2+-ATPase 2a (SERCA2a) protein binding interactions in human cardiac sarcoplasmic reticulum vesicles using chemical cross-linking. J Biol Chem. 2012;287:7582–7593. doi: 10.1074/jbc.M111.334987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lukyanenko V, Gyorke S. Ca2+ sparks and Ca2+ waves in saponin-permeabilized rat ventricular myocytes. J Physiol. 1999;521(Pt 3):575–585. doi: 10.1111/j.1469-7793.1999.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lukyanenko V. Permeabilization of cell membrane for delivery of nano-objects to cellular sub-domains. Methods in Molecular Biology. 2013;991:57–63. doi: 10.1007/978-1-62703-336-7_7. [DOI] [PubMed] [Google Scholar]
- 36.Jamur MC, Oliver C. Permeabilization of cell membranes. Methods in Molecular Biology. 2010;588:63–66. doi: 10.1007/978-1-59745-324-0_9. [DOI] [PubMed] [Google Scholar]
- 37.Keller M, Kao JP, Egger M, Niggli E. Calcium waves driven by "sensitization" wave-fronts. Cardiovascular Res. 2007;74:39–45. doi: 10.1016/j.cardiores.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Hoit BD, Khoury SF, Kranias EG, Ball N, Walsh RA. In vivo echocardiographic detection of enhanced left ventricular function in gene-targeted mice with phospholamban deficiency. Circ Res. 1995;77:632–637. doi: 10.1161/01.res.77.3.632. [DOI] [PubMed] [Google Scholar]
- 39.DeSantiago J, Maier LS, Bers DM. Frequency-dependent acceleration of relaxation in the heart depends on CaMKII, but not phospholamban. Journal of molecular and cellular cardiology. 2002;34:975–984. doi: 10.1006/jmcc.2002.2034. [DOI] [PubMed] [Google Scholar]
- 40.Huser J, Bers DM, Blatter LA. Subcellular properties of [Ca2+]i transients in phospholamban-deficient mouse ventricular cells. Am J Physiol. 1998;274:H1800–H1811. doi: 10.1152/ajpheart.1998.274.5.H1800. [DOI] [PubMed] [Google Scholar]
- 41.Vangheluwe P, Tjwa M, Van Den Bergh A, Louch WE, Beullens M, Dode L, et al. A SERCA2 pump with an increased Ca2+ affinity can lead to severe cardiac hypertrophy, stress intolerance and reduced life span. J Mol Cell Cardiol. 2006;41:308–317. doi: 10.1016/j.yjmcc.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 42.Louch WE, Vangheluwe P, Bito V, Raeymaekers L, Wuytack F, Sipido KR. Phospholamban ablation in hearts expressing the high affinity SERCA2b isoform normalizes global Ca2+ homeostasis but not Ca2+-dependent hypertrophic signaling. Am J Physiol Heart Circ Physiol. 2012;302:H2574–H2582. doi: 10.1152/ajpheart.01166.2011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.