Abstract
Research indicates that female risk of developing Alzheimer’s disease (AD) is greater than that of males. Moderate reduction of calorie intake, known as calorie restriction (CR), reduces pathology in AD mouse models, and is a potentially translatable prevention measure for individuals at-risk for AD, as well as an important tool for understanding how the brain endogenously attenuates age-related pathology. Whether sex influences the response to CR remains unknown. In this study, we assessed the effect of CR on beta-amyloid peptide (Aβ) pathology and hippocampal CA1 neuron specific gene expression in the Tg2576 mouse model of cerebral amyloidosis. Relative to ad libitum (AL) feeding, CR feeding significantly reduced hippocampal Aβ burden in 15 month old female, but not age-matched male, Tg2576 mice. Sustained CR also significantly reduced expression of presenilin enhancer 2 (Psenen) and presenilin 1 (Ps1), components of the γ-secretase complex, in Tg2576 females. These results indicate that long-term CR significantly reduces age-dependent female Tg2576 Aβ pathology, which is likely to involve CR-mediated reductions in γ-secretase-dependent amyloid precursor protein (APP) metabolism.
Keywords: Abeta, aging; Alzheimer’s disease; amyloid-beta precursor protein; CA1 pyramidal neurons; calorie restriction; entorhinal cortex; hippocampus; microarray; mouse model; qPCR
INTRODUCTION
Alzheimer’s disease (AD) is a high-prevalence neurodegenerative disorder characterized by progressive loss of memory and executive function resulting from synaptic dysfunction and neurodegeneration within vulnerable brain regions (Almeida et al., 2005; Hsieh et al., 2006; Jacobsen et al., 2006; Snyder et al., 2005). Human studies indicate that women are at a greater risk of developing AD (Thies et al., 2013), and in mouse models of β-amyloidosis, female mice also show increased pathology earlier in the lifespan (Callahan et al., 2001; Hirata-Fukae et al., 2008; Lee et al., 2002; Wang et al., 2003). Although the mechanisms underlying this gender divergence are unclear, increased AD risk in women is likely associated with hormonal, metabolic, and/or nutritional status (Azad et al., 2007; Gustafson et al., 2003; Hayden and Zandi, 2006; Viña and Lloret, 2010; Wolf et al., 2012; Yue et al., 2005). Currently, more than 5 million Americans over the age of 65 have AD, of which 3.2 million are women (Thies et al., 2013), yet no highly effective AD treatments or preventive measures are available to address this immense public health issue, independent of gender status.
Aggregation and accumulation of beta-amyloid peptide (Aβ), a 39–42 amino acid peptide derived from sequential processing of the APP holoprotein, induces synaptic dysfunction (Almeida et al., 2005; Hsieh et al., 2006; Jacobsen et al., 2006; Snyder et al., 2005), oxidative damage (Bartley et al., 2012; Wan et al., 2011), tau aggregation (Chabrier et al., 2012; Oddo et al., 2006), and other types of cellular injury that ultimately drive neuropathology associated with AD (Goedert and Spillantini, 2006; Götz et al., 2009; Hardy and Allsop, 1991; Hyman et al., 1984; von Gunten et al., 2006). Sustained, moderate reduction (20–40%) of calorie intake without malnutrition, known as calorie restriction (CR), reduces Aβ neuropathology compared to ad libitum (AL) feeding in several AD mouse models (Mouton et al., 2009; Patel et al., 2005; Wang et al., 2005), in addition to extending healthspan and lifespan in many species (Colman et al., 2009; Hart et al., 1999; Kealy et al., 2002; Klass, 1977; Mattison et al., 2012; McCay et al., 1935; Roe et al., 1995; Rous, 1914). The pathways that the brain endogenously employs to blunt Aβ pathology in response to CR are likely complex, and are not well characterized. Determination of these changes will provide translationally-relevant information about potential targets for drug design and discovery.
Aβ is produced by amyloidogenic APP processing by β- and γ-secretases, whereas Aβ production is precluded by non-amyloidogenic APP processing via α- and γ-secretases (Rajendran and Annaert, 2012; Zhang et al., 2011). The constitutive γ-secretase cleavage event is mediated by a protein complex composed of presenilin 1 (Ps1) or presenilin 2 (Ps2) (Dewachter et al., 2002; Zhang et al., 2000), nicastrin (Ncstn) (Li et al., 2003), anterior pharynx defective 1 homolog A (Aph1a) (Francis et al., 2002; Goutte et al., 2002), and presenilin enhancer 2 (Psenen) (Francis et al., 2002; Steiner et al., 2002). Ps1/Ps2 serves as the γ-secretase catalytic active site (Esler et al., 2000; Li et al., 2000), while substrate interaction and stabilization is maintained by Ncstn and Aph1a (Francis et al., 2002; Li et al., 2003; Marlow et al., 2003). Psenen is required for γ-secretase complex maturation and additional stabilization. Psenen depletion reduces total γ-secretase complex levels (Steiner et al., 2002), and Psenen overexpression increases Aβ production (Marlow et al., 2003; Shiraishi et al., 2004). As γ-secretase constitutively cleaves the truncated APP fragment following α-or β-cleavage, increasing γ-secretase activity promotes production of pathogenic Aβ, while decreasing γ-secretase activity reduces Aβ (Chow et al., 2010; Zhang et al., 2011). Accordingly, γ-secretase inactivation in APP overexpression mouse models of AD pathology reduces Aβ burden (Dewachter et al., 2002; Saura et al., 2005).
In this study, we tested the hypothesis that 30% CR would attenuate amyloid pathology in both sexes of an aged AD mouse model via a multidisciplinary approach. We employed the Tg2576 mouse model of APP overexpression, which exhibits age-dependent increases in Aβ plaque load and concomitant memory deficits driven by overexpression of a human APP variant (APPswe) responsible for early-onset AD (Callahan et al., 2001; Citron et al., 1992; Good and Hale, 2007; Hsiao et al., 1996; Mullan et al., 1992). AD pathology exhibits a spatial and temporal occurrence in which specific neuron types in defined brain regions display pathology, while leaving other cell types and areas relatively spared (de LaCoste and White, 1993; Fan et al., 2008). Hence, we determined Aβ burden and assessed APP metabolite levels in aged Tg2576 mice (at a timepoint where Aβ deposition and accumulation has occurred) within the hippocampal formation and the adjacent entorhinal cortex, temporal lobe structures that are crucial for memory formation and recall that are affected early and severely during the progression of AD (Ginsberg et al., 2000; Hyman et al., 1984; Morrison and Hof, 2002) following CR or AL feeding. We also explored the influence of CR on age-dependent gene expression changes underlying Aβ pathology by qPCR and single population microarray analyses in area CA1 of the hippocampus.
MATERIALS AND METHODS
Tg2576 mouse model
Animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the Nathan Kline Institute/NYU Langone Medical Center and were in full accordance with NIH guidelines. Tg2576 mice (n=77), which harbor a human variant of APP termed the ‘Swedish’ mutation (APPswe), (Lys670→Asn and Met671→Leu), and nontransgenic (ntg; n=67) littermates on a Swiss Webster × DBA/C57BL6 F1 background were employed for these studies. For female mice, estrous cycle was not monitored. Initial analyses of Aβ species, APP holoprotein levels, and transcriptional levels via microarray were conducted in both Tg2576 and ntg mice. We present our datasets on Tg2576 mice because ntg mice did not develop detectable Aβ-pathology (serving as a negative control). Neither sex- nor diet-dependent changes in murine APP holoprotein levels were found in ntg mice.
Calorie restriction (CR) and ad libitum (AL) feeding regimens
Beginning at approximately 2.5 months of age, female and male Tg2576 mice and ntg littermates were randomly assigned with equal numbers to AL or 30% CR dietary regimens. The CR group consumed 30% fewer total calories than the AL group with the CR allotment adjusted weekly based on the AL group’s daily intake to maintain a uniform 30% reduction throughout the study. Specifically, for every 1 g AL of diet consumed, on average, by the age-matched AL group of mice, 0.71 g of CR diet was allotted to the CR mice. All CR mice were fed their daily food allotment in the morning. The dietary component of restriction was carbohydrates. Representative CR and AL dietary regimens, prepared by Research Diets Inc. (New Brunswick, NJ), are described in Supplemental Table 1. Mice were maintained on the CR or AL feeding regimens for approximately 2.8 or 12.5 months (Suppl. Fig. 1).
Tissue accession and processing
Tg2576 and ntg mice from both the AL and CR groups were sacrificed at approximately 5.3 and 15 months of age (prior to cerebral Aβ deposition and once Aβ deposition has already occurred, respectively). Mice were overdosed with ketamine (80 mg/kg) and xylazine (13 mg/kg) and perfused transcardially with ice-cold 0.1 M phosphate buffer. Brains were rapidly removed, and one hemi-brain was immediately drop-fixed in 4% paraformaldehyde in 0.1 M phosphate buffer for 24 hours at 4 °C, paraffin embedded, and sectioned at 6 µm for single cell isolation. From the remaining hemi-brain, the hippocampus or CA1-enriched portions of the hippocampus and entorhinal-enriched cortex were dissected, frozen on dry ice, and stored at −80 °C until RNA extraction or tissue homogenization for qPCR, ELISA, or immunoblot experiments (Alldred et al., 2012). All sacrifices took place in mid-afternoon. For protein analysis, frozen tissue was homogenized in tissue homogenization buffer (THB) containing 250 mM sucrose, 20 mM Tris-HCl, 1 mM EGTA, 1 mM EDTA, and protease inhibitors (10% weight by volume, pH 7.4). Homogenates were used for immunoblot analysis or subjected to formic acid (FA) extraction and Aβ sandwich ELISAs (Schmidt et al., 2012).
Aβ enzyme-linked immunosorbent assays (ELISAs)
Human Aβ levels were quantified via colorimetric sandwich ELISA from 10% sucrose homogenates of frozen cortex from 15 month old CR- and AL-fed Tg2576 mice subjected to FA extraction as previously described (Schmidt et al., 2012). Briefly, 96-well plates were coated with monoclonal capture antibodies JRF/cAβ40/10 (epitope specificity to the carboxyl-terminal 5 residues of Aβ1–40), and JRF/cAβ42/26 (epitope specificity to the carboxyl-terminal 10 residues of Aβ1–42), both recognizing human and mouse Aβ species. Post incubation, blocking, and washes, FA extracted samples and standard curve samples were applied to wells containing the capture antibody. After incubation, wells were washed, and a HRP-coupled monoclonal detection antibody JRF/AβN25 (epitope specificity to residues 1–7 of human Aβ) was added to the well. Peroxide substrate solution was applied, and the colorimetric signal was determined via optical density (OD450) reading using a microplate spectrophotometer. ELISA results were reported as fmol Aβ per g of total protein, based on standard curves using synthetic murine and human Aβ1–40 and Aβ1–42 peptide standards (American Peptide, Sunnyvale, CA) as described previously (Mathews et al., 2002; Morales-Corraliza et al., 2009; Schmidt et al., 2012).
Immunohistochemistry and stereological analysis of Aβ plaque burden
Paraffin-embedded, 6 µm hemi-brain horizontal sections from 15 month old CR- and AL-fed Tg2576 mice were deparaffinized in a xylene and ethanol series and boiled in citrate buffer (10 mM, pH 6.0), followed by incubation in ammonium chloride (80 mM in TBS), then 88% FA, with TBS washes between incubations. Sections were blocked using Mouse on Mouse (MOM) kit blocking reagent (Vector Laboratories, Burlingame, CA). Following TBS washes, sections were incubated with monoclonal 4G8 primary antibody (epitope specificity to Aβ 17–24 that recognizes APP, αCTF, Aβ1–40, Aβ1–42) (Covance, Princeton, NJ) diluted in MOM diluting reagent in TBS. Visualization was achieved through incubation with diaminobenzidine (DAB) in 0.03% hydrogen peroxide and 0.01 M imidazole in Tris buffer, as described previously (Alldred et al., 2012). Following TBS washes, sections were subjected to dehydration through an ethanol and xylene series and were coverslipped using Cytoseal (ThermoScientific, Waltham, MA).
Whole-slide images of 4G8-stained sections were acquired using a slide scanner (SCN400, Leica Microsystems, Buffalo Grove, IL), equipped with a 40×/0.7 NA objective (0.25 µm/pixel final image resolution). Stereological assessment was conducted by Sinq Systems (Silver Spring, MD). Anatomical delineation of hippocampal CA1 included all layers with borders as previously described (Calhoun et al., 1998), and borders for the entire EC with perirhinal cortex, parasubiculum and other neighboring regions were determined based upon detailed cytoarchitectonic differentiation. Section sampling was pseudo-random across the dorsoventral extent of included regions (approximate systematic-random sampling where sections were not damaged or part of other analyses). Volume-fraction was computed using stereological methods (Gundersen et al., 1988). A point-grid was superimposed on the image in a random XY location, and points were characterized as being over diffuse or dense amyloid (plaque load), or vascular amyloid. As the amount of vascular amyloid was very low in this model, it was not further analyzed. Point-spacing was optimized based upon pre-screening of the amount of amyloid per mouse (either 15 or 25 microns for hippocampal CA1 region), and 25, 30, or 45 microns for EC), resulting in an average of 355 and 193 positive points counted for CA1 and EC respectively. Stereological sampling error (CE) averaged 0.032 for CA1 and 0.035 for EC (Gundersen et al., 1999), and was well below biological variability. Morphological identification of points was performed by two independent raters, with each counting alternating sections from every mouse. Strong concordance was found on a subset of sections (n=17) counted by both raters (R2=0.94; F(1,16)=247.3, p<0.0001), and mean values for the two were used for these sections in final data analysis.
Immunoblot analysis
10 µg of protein homogenates were loaded into a gel electrophoresis apparatus, subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE; 4–20% gradient acrylamide gels; Bio-Rad, Hercules, CA), and transferred to nitrocellulose by electroblotting (Mini Transblot, Bio-Rad). Nitrocellulose membranes were blocked in 5% milk blocking buffer prior to being incubated with primary antibodies in blocking buffer. Following HRP-secondary antibody incubation, protein signals were detected using enhanced chemiluminescence or X-ray film. Signals were quantified using ImageJ software (NIH, Bethesda, MD). β-tubulin (β-Tub) immunoreactivity signal was used as a loading control (Choi et al., 2009; Mathews et al., 2002; Morales-Corraliza et al., 2009). Monoclonal C1/6.1 antibody, which recognizes the carboxyl-terminal cytoplasmic domain of APP, was employed for detection of APP holoprotein. For quantification of sAPP metabolites, soluble proteins were extracted from hemi-brain or EC-enriched homogenates using 0.2% diethylamine followed by centrifugation (Schmidt et al., 2012). Centrifugation supernatants were migrated, transferred to nitrocellulose membranes, and probed with monoclonal 6A1 antibody (1:1000), which is epitope-specific to the C-terminus portion of the human Swedish variant of sAPPβ (IBL, Japan), or monoclonal 6E10 antibody (1:1000), which is epitope-specific to human Aβ1–17 and recognizes APP, sAPPα, βCTF, αCTF, Aβ1–40, Aβ1–42 (Covance). Immunoblots were prepared 2–3 times per condition (Mathews et al., 2002; Morales-Corraliza et al., 2009).
Real-time qPCR
qPCR was performed on microdissected tissue samples of the hippocampal CA1 region, which were stored at −80 °C in microfuge tubes unti l use. Phenol-chloroform extracted RNA was subjected to bioanalysis (Bioanalyzer 2100, Agilent Biotechnologies, Santa Clara, CA) to quantify RNA concentration and quality. TaqMan hydrolysis probes (Life Technologies, Grand Island, NY) were employed for γ-secretase complex genes, including Psenen (Mm00727761_s1), Ps1 (Mm00501191_m1), Aph1a (Mm00778687_s1), and Ncstn (Mm01293326_g1), and housekeeping control genes, including hypoxanthine phosphoribosyltransferase 1 (Hprt1) (Mm01318747_g1) and succinate dehydrogenase complex, subunit A (Sdha) (Mm01352360_m1) (Life Technologies). Samples were assayed on a real-time qPCR thermal cycler (7900HT, Life Technologies) in 96-well optical plates. Standard curves and cycle threshold (Ct) were generated, and the ddCT method was employed to determine relative gene level differences between test and control genes. Negative controls consisted of the reaction mixture without input RNA (Alldred et al., 2009, 2014; Ginsberg et al., 2012).
Single-cell type expression profiling
Paraffin embedded 6 µm hemi-brain dissections were immunohistochemically (IHC) labeled through the use of a biotin-streptavidin-horseradish peroxidase protocol. An antineurofilament (NF) antibody (RMDO20, 1:200 dilution) (Lee et al., 1987) was employed to identify hippocampal CA1 pyramidal neurons, as described previously (Ginsberg et al., 2000; Alldred et al., 2012, 2014). Following IHC, CA1 pyramidal neurons were extracted through a computer-assisted LCM system (LCM, Arcturus PixCell IIe, Life Technologies) system as described previously (Alldred et al., 2008, 2014;Ginsberg et al., 2012) (Suppl. Fig. 2). The isolated cell bodies were immediately deposited in tubes containing TRIzol (Life Technologies), inverted, and frozen in order to isolate RNA through a phenol-chloroform extraction protocol.
An RNA amplification method termed terminal continuation (TC) amplification was employed to amplify total RNA from LCM-extracted CA1 pyramidal neurons in a linear fashion through in vitro transcription (Suppl. Fig. 2). This amplification approach produces RNA in quantities that are a linear representation of original starting material and readily detectible by microarray analysis. For these studies, 100 LCM-extracted, NF-immunoreactive CA1 pyramidal neurons from a single mouse were pooled per TC-reaction. During the initial step in the TCreaction, purified mRNAs were reverse transcribed to produce a cDNA template. Two oligonucleotide primers were used for cDNA synthesis, a poly d(T) primer and a TC primer, which included a sequence that is antisense to the T7 bacteriophage transcriptional promoter. During in vitro transcription, 33P-UTP was incorporated into newly synthesized RNA species complementary to the cDNA templates (Alldred et al., 2008, 2009; Ginsberg, 2008).
A custom-designed microarray platform of 648 genes was used to interrogate structural, receptor, ion channel, AD-related, cell cycle, metabolism, stress response, growth factors, and known CR-responsive genes, among others. Interrogated genes consisted of sequence-verified, linearized mouse and human cDNAs or expressed sequence-tagged cDNAs (ESTs) (1 µg) adhered to high-density nitrocellulose membranes (Alldred et al., 2012, 2014; Ginsberg and Che, 2005; Ginsberg et al., 2012). For microarray profiling, arrays were prehybridized to prevent nonspecific binding, probed with the radiolabeled TC reaction products, and sequentially washed to eliminate all non-stringently bound material. Hybridized arrays were incubated in a phosphor screen cassette and developed in a Storm 840 phosphor imager (GE Healthcare, Piscataway, NJ). ImageQuant software (GE Healthcare) was used to quantitate hybridization signal intensity (Suppl. Fig. 2). For this study, 162 custom-designed microarrays were used, which includes sample sizes of n=5 per genotype, diet, sex, age group, each assessed 3–5 times.
Statistical analysis
For qPCR, immunoblot, ELISA, and IHC stereology, results were subjected to statistical analysis in which the comparisons between diet and age groups were based on mixed-effect model analyses (McCulloch et al., 2011). Briefly, each metric was modeled as a function of dietary treatment and age, using mixed-effect models with random mouse effect to account for the correlation between repeated assays on the same mouse. Significant findings were followed by pair-wise comparisons between the means for the two dietary treatments of each age group. All analyses were performed using Proc Mixed in SAS software (SAS Institute Inc, 2009). For qPCR, immunoblot, ELISA, and IHC analysis, we report significant results with p<0.05. Statistical procedures for custom-designed microarray analysis have been described in detail (Alldred et al., 2014; Ginsberg, 2008; Ginsberg et al., 2006). Multiple arrays (n=3–5) were assayed per subject and nested to each subject for increased statistical stringency. A global normalization approach was utilized whereby hybridization signal intensity for each feature is subtracted from the background intensity and normalized as a ratio of the total array hybridization signal intensity. Signal intensities were compared with negative control arrays, which were hybridized with TC reaction products that contained no initial input RNA. A global normalization procedure effectively minimizes variation due to differences in specific activity and absolute quantity of individual probes (Ginsberg, 2005, 2008; Hemby et al., 2003; Kacharmina et al., 1999). In this analysis scheme, an expression profile of relative changes in mRNA levels was enabled. For microarray analysis, we report significant results with p<0.001 and adjusted pvalues of q<0.01 after controlling false discovery rate (FDR) due to the multiple-testing (Alldred et al., 2014; Benjamini and Hochberg, 1995; Efron, 2007).
RESULTS
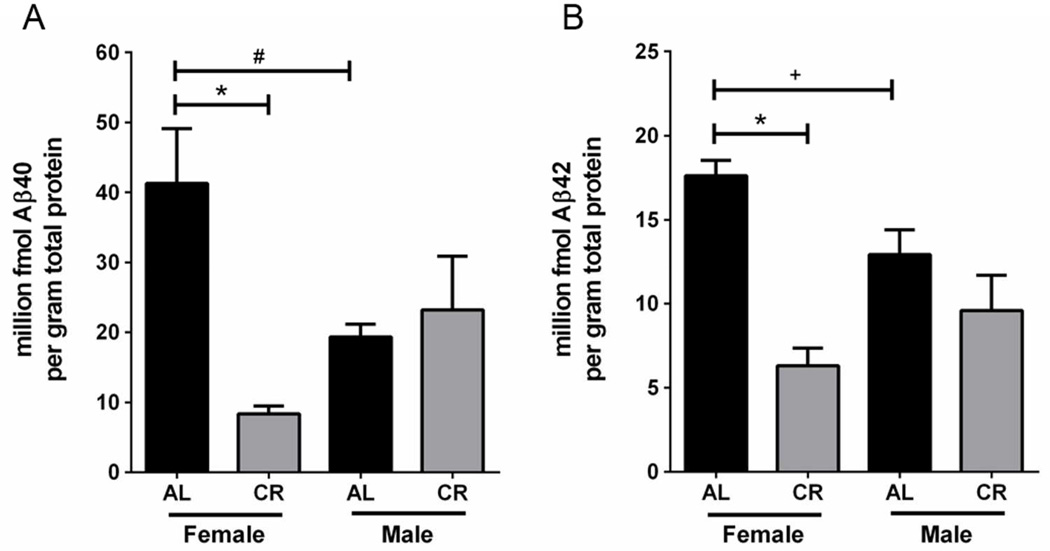
To test the influence of dietary intervention on Aβ pathology in Tg2576 mice, we conducted quantitative analysis of Aβ40 and Aβ42 peptide levels in EC using ELISA-based methods. Sustained CR feeding significantly reduced Aβ40 protein levels by 79.7% (p<0.0005) and Aβ42 protein levels by 64.2% (p<0.0005) within 15 month old female Tg2576 mice, relative to AL-feeding (Fig. 1). CR feeding did not significantly alter 15 month old male Aβ40 or Aβ42 protein levels (Fig. 1). Upon male and female comparison of 15 month old Tg2576 mice fed the control AL diet, we observed that males displayed 53.2% less Aβ40 protein (p=0.01) and 26.6% less Aβ42 protein (p=0.04) than female mice on the AL diet (Fig. 1).
Figure 1.
CR reduces cortical Aβ levels within 15 month old female Tg2576 mice. CR feeding significantly reduced female, but not male, Aβ40 (A) and Aβ42 (B) levels. Female AL Tg2576 mice had significantly higher levels of Aβ40 and Aβ42, relative to AL males (A, B); linear mixed model, entorhinal cortex; n=7–8 per condition, *p<0.0005, #p=0.01, +p=0.04.)
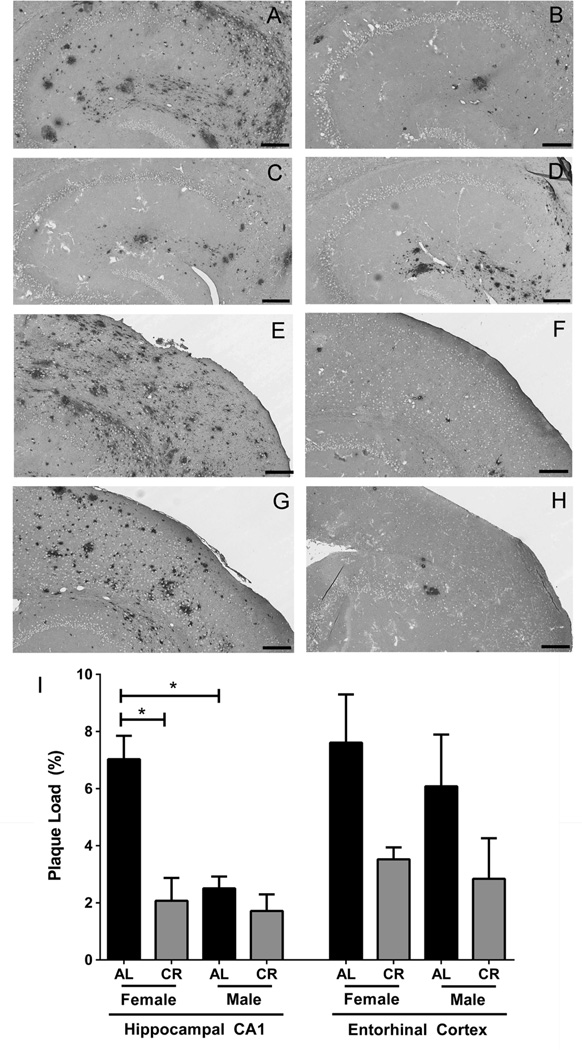
We corroborated ELISA-based results by assessing Aβ plaque burden in tissue sections of the EC and area CA1 of the hippocampus. Using the 4G8 antibody to detect deposited Aβ by IHC in combination with unbiased stereological analysis, we confirmed that compared to AL-feeding, sustained CR-feeding reduced Aβ plaque burden in the CA1 hippocampal region of 15 month old female Tg2576 mice by 70.4% (p<0.0001) (Fig. 2A, 2B, and 2I) and observed a similar trend in the EC (Fig. 2E, 2F, and 2I), noting that variation in Aβ plaque burden in the EC is greater than plaque load variation in the hippocampal CA1 region for AL- and CR-fed males and AL-fed females. In contrast to females, and consistent with the ELISA findings, CR feeding did not significantly alter Aβ plaque burden within age-matched male Tg2576 mice at the 15 month timepoint (Fig. 2G, 2H, and 2I). In Tg2576 mice maintained on the control AL diet, female Tg2576 mice had approximately 64.4% greater hippocampal CA1 plaque burden than agematched AL-fed males (p<0.0001; Fig. 2A, 2C, 2I).
Figure 2.
Long-term CR-feeding reduces Aβ plaque load within 15 month old female Tg2576 mice. Aβ immunoreactivity is depicted in tissues sections from area CA1 of the hippocampus (A–D) and EC (E–H) of 15 month old Tg2576 mice of the following diets and sexes: AL female (A, E), CR female (B, F), AL male (C, G), CR male (D, H). Stereological assessments are depicted (I; mean ± SEM). Scale bar: 200 µm. (linear mixed model, hippocampus, n=5–6 per condition, *p<0.0001; entorhinal cortex, n=4–6 per condition). Note that greater variation in plaque load was observed in the EC as compared to the hippocampal CA1 region for AL- and CR-fed males and AL-fed females.
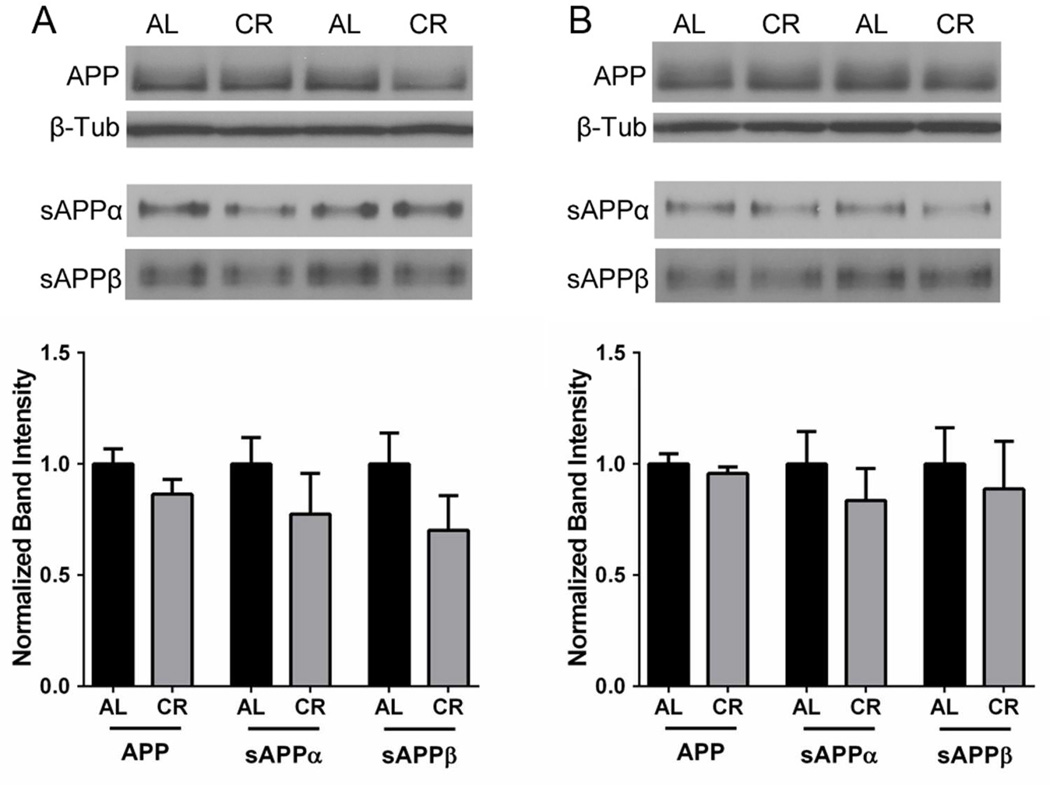
APP and APP metabolite levels were evaluated in the brains of Tg2576 mice maintained on AL or CR diets by immunoblot analysis. We observed no changes in total APP holoprotein levels, regardless of sex or diet (Suppl. Fig. 3). The two sAPP fragments, sAPPα and sAPPβ, are known to be stable APP metabolites in the Tg2576 mouse brain, and are informative of α-and β-cleavage rates (Morales-Corraliza et al., 2009). Hence, we examined sAPPα and sAPPβ, levels, focusing on female mice, which showed CR-dependent reductions in Aβ accumulation (Figs. 1, 2). Results indicate that CR-feeding did not significantly alter either sAPPα or sAPPβ levels in the EC of 15 month old Tg25769 mice (Fig. 3A). To determine whether α- or β-cleavage is altered at an earlier timepoint that would affect subsequent β-amyloid deposition, we examined 5.3 month old Tg2576 mice that were CR- or AL-fed for 2.8 months. Similar to the aged mice, CR feeding did not alter sAPPα or sAPPβ protein levels within hemi-brain dissections isolated from 5.3 month old female Tg2576 mice (Fig. 3B), suggesting that both α-and β-cleavage of APP is not altered by CR in the Tg2576 brain.
Figure 3.
CR does not alter sAPPα or sAPPβ levels in female Tg2576 mice. Sustained CR feeding did not significantly alter sAPPα or sAPPβ in the EC of 15 month old (A) or hemi-brains of 5.3 month (B) old female Tg2576 mice, relative to AL feeding (normalized mean ± SEM). β-Tub levels are shown as a control (linear mixed model, n=6–7).
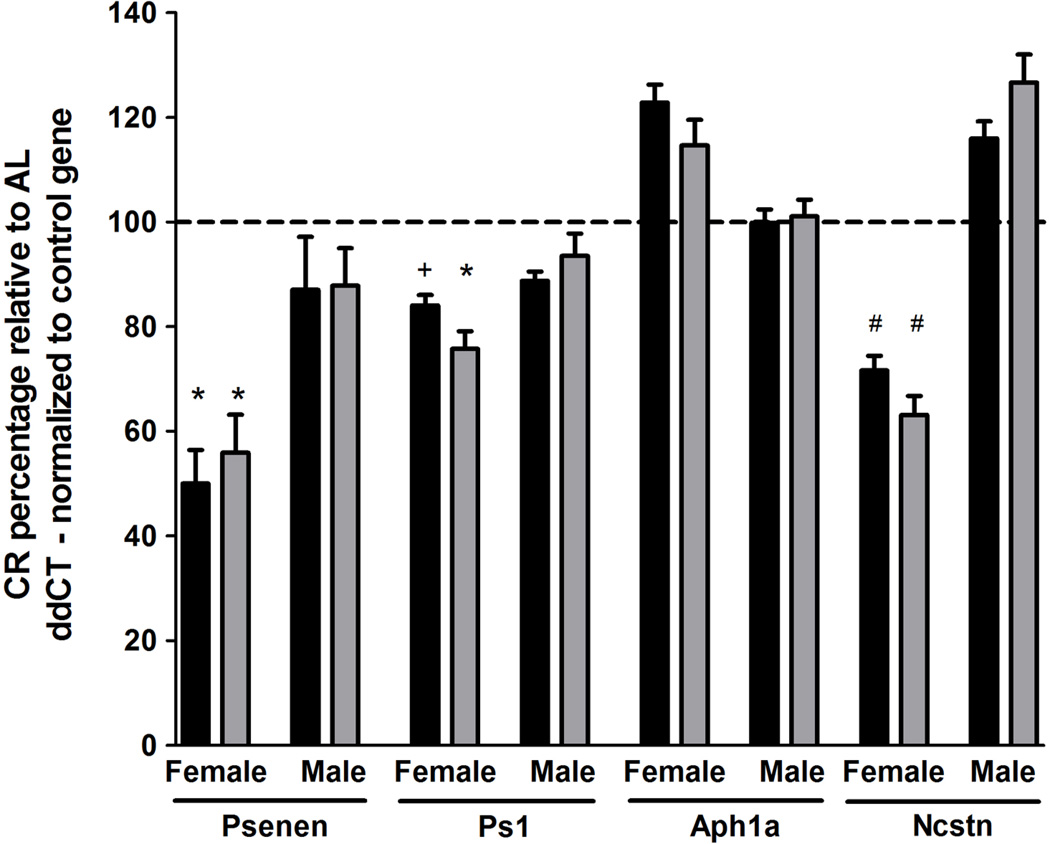
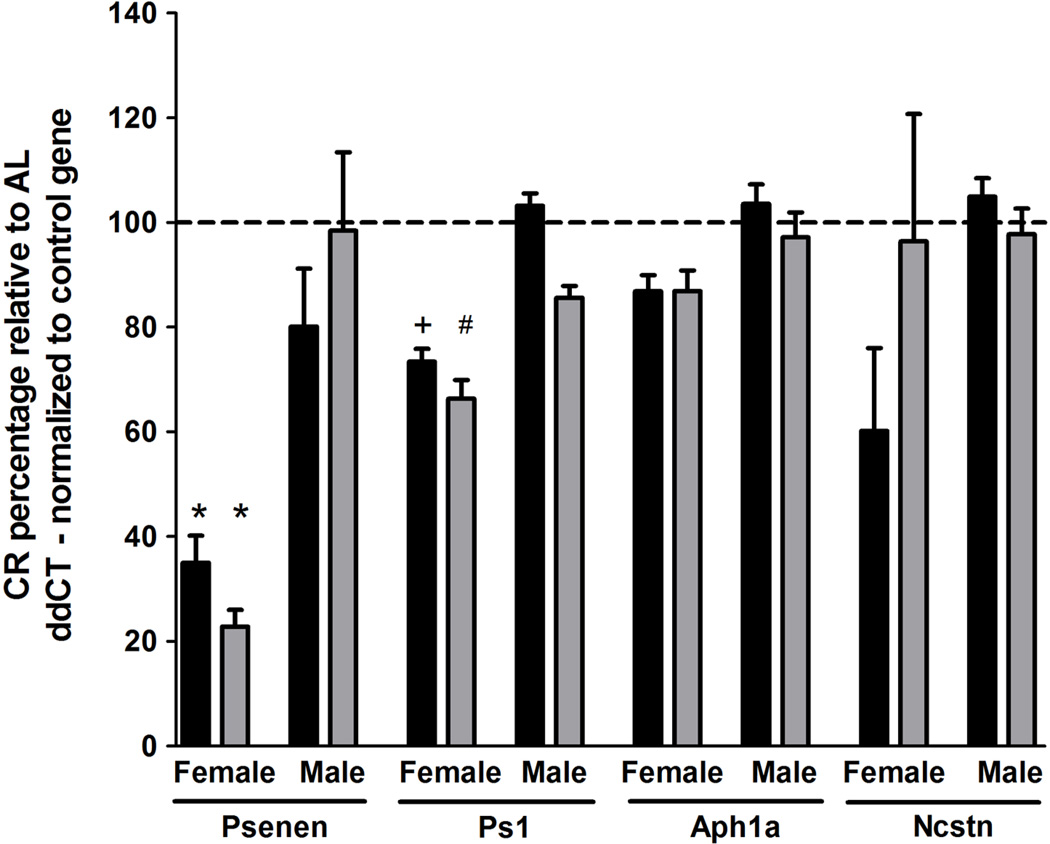
Based on these observations, we postulated that decreased γ-secretase processing of APP may be responsible for the observed Aβ reductions following CR. To test this, we performed qPCR analysis of γ-secretase complex subunit levels using hippocampal CA1 dissections. Target gene expression levels were normalized to two independent control genes, Sdha and Hprt1. Expression levels of Sdha and Hprt1 did not vary as a function of genotype or diet (data not shown). Within 15 month old female Tg2576 mice, we observed significant reductions within core components of the γ-secretase complex in CR-fed mice compared to AL-fed mice. Specifically, CR feeding decreased Psenen mRNA levels by 50.0% (p<0.03, normalized to Sdha) and 44.1% (p<0.03 normalized to Hprt1) and Ps1 mRNA levels by 16.0% (p=0.06, normalized to Sdha) and 24.3% (p<0.02 normalized to Hprt1), relative to sex- and agematched AL-fed mice (Fig 4). Ncstn mRNA levels were also reduced by 28.4% (p<0.005, normalized to Sdha) and 36.9% (p<0.005 normalized to Hprt1) within CR-fed 15 month old female Tg2576 mice, relative to AL-feeding (Fig. 4). To determine whether CR-dependent γ-secretase subunit reductions were consistent in both animals at this older age and for younger animals prior to Aβ deposition, we conducted qPCR analysis within 5.3 month old Tg2576 mice and observed reductions in Psenen mRNA levels of 65.0% (p<0.0005, normalized to Sdha) and 77.2% (p<0.0005 normalized to Hprt1) and reductions in Ps1 mRNA levels of 26.6% (p=0.06, normalized to Sdha) and 33.7% (p<0.04, normalized to Hprt1) within CR-fed females, relative to AL-fed females (Fig. 5). Interestingly, we observed no CR-dependent reductions in Psenen, Ps1, Ncstn, or Aph1a within male Tg2576 mice at 5.3 or 15 months of age (Figs. 4, 5). These findings implicate regulation of the γ-secretase complex by CR as a potential female-specific mechanism underlying modulation of Aβ pathology.
Figure 4.
CR reduces CA1 hippocampal Psenen and Ps1 transcript levels within 15 month old female, but not male, Tg2576 mice, compared to AL. γ-secretase subunit ddCT values were normalized to housekeeping gene ddCT levels, Sdha (black) and Hprt1 (gray). Normalized CR expression levels are depicted as a percentage relative to AL expression levels of the matched sex and age group (mean ± SEM). (linear mixed model, n=5–7 per condition, *p<0.03, +p=0.06, #p<0.005).
Figure 5.
qPCR analysis of γ-secretase subunit transcript levels. qPCR analysis was conducted using hippocampal CA1 regional dissections from 5.3 month old, AL- and CR-fed, male and female Tg2576 mice. γ-secretase subunit ddCT values were normalized to housekeeping gene ddCT levels, Sdha (black) and Hprt1 (gray). Normalized CR expression levels are depicted as a percentage, relative to AL expression levels of the matched sex and age group (mean ± SEM). (linear mixed model, n=5–7 per condition, *p<0.0005, +p=0.06, #p<0.04).
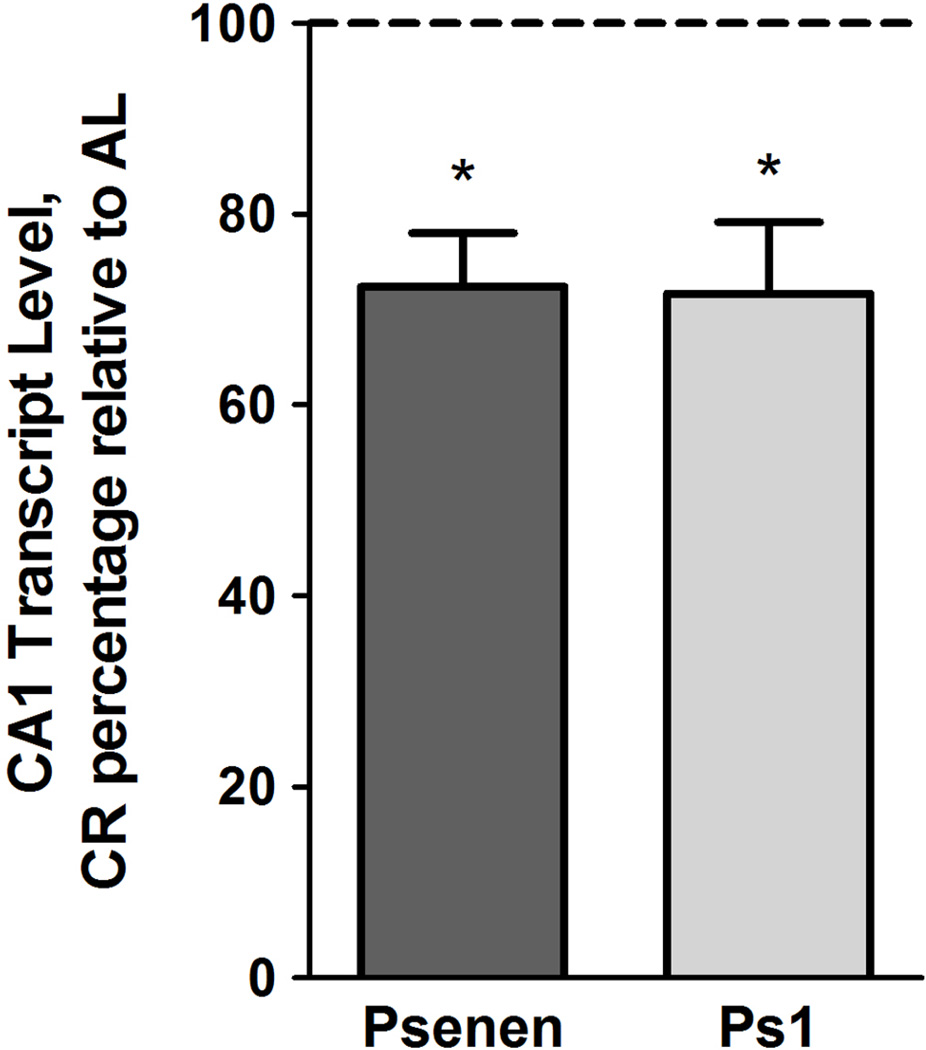
Within the Tg2576 hippocampus and cortex, APPswe transgene expression is primarily neuronal (Irizarry et al., 2001). We sought to validate observed CR-dependent reductions in γ-secretase subunit transcript levels by cell-type specific microarray analysis using populations of CA1 pyramidal neurons isolated from 5.3 month old female Tg2576 mice maintained on AL or CR feeding regimens (Suppl. Fig. 2). By this approach, we confirmed that expression of Psenen and Ps1 was significantly reduced by 27.6% (p<0.001, q<0.01) and 28.9% (p<0.001, q<0.01), respectively, within CA1 pyramidal neurons of 5.3 mo. old female Tg2576 mice maintained on a CR diet, relative to AL-fed mice of the same sex, genotype, and age group (Fig. 6). Interrogation of additional candidate genes relevant to APP processing and Aβ dynamics is presented in Supplemental Table 2.
Figure 6.
Microarray analysis demonstrates Psenen and Ps1 transcript level reduction within CA1 pyramidal neurons isolated from CR-fed, Tg2576 females. Normalized CR expression levels of 5.3 month old Tg2576 females are depicted as a percentage, relative to AL expression levels (mean ± SEM). CR feeding significantly reduced Psenen and Ps1 mRNA levels. (linear mixed model, n=5–7 per condition, *p<0.001, q<0.01).
DISCUSSION
Through a multidisciplinary approach, we demonstrate that continuous CR feeding significantly reduces Aβ pathology and expression of Psenen and Ps1, γ-secretase subunits responsible for constitutive cleavage of APP, within female Tg2576 mice. Interestingly, we observed no significant differences in Aβ burden or γ-secretase subunit levels as a function of the CR diet in age-matched male Tg2576 mice. This indicates that 15 month old Tg2576 females respond selectively to the CR diet, resulting in reduced Aβ pathology. To our knowledge, this is the first study to address the differential influence of CR on pathology and underlying gene expression within male and female mice.
It is well-established that AD pathology manifests differently among females and males (Callahan et al., 2001; Hirate-Fukae et al., 2008; Lee et al., 2002; Thies et al., 2013, Vina and Lloret, 2010; Wang et al., 2003) and that pronounced female pathology may be associated with metabolic or nutritional status (Azad et al.,2007; Gustafson et al., 2003; Hayden and Zandi, 2006; Wolf et al., 2012; Yue et al., 2005). It is possible, however, that a threshold of Aβ must be reached before CR will have an effect. In future studies, it would be beneficial to assess pathology indicators at later timepoints in Tg2576 mice, when Aβ deposition and accumulation is more prominent, to confirm sex differences in CR pathology responses. Similarly, assessments of the sex-specific influence of CR in distinct AD mouse models in which Aβ pathology develops more rapidly, such as the TgCRND8, 5XFAD, and 3xTg mouse lines (Hall and Roberson, 2012), would also be useful in dissecting sex differences in CR responses at varying pathology thresholds, as well as evaluating the effects of CR on tau pathology. As sex-specific differences in age-dependent Aβ pathology development and responses to CR are likely influenced by hormonal cycles, future studies should also incorporate estrous cycle synchronization, ovariectomy, and hormone replacement therapies (Ding et al., 2013, Kunzler et al., 2014). The present study provides insight into potential mechanisms underlying Aβ pathology sex divergence, implicating nutritional status and APP processing via γ-secretase in Tg2576 female mice. Additional studies are warranted to confirm the time course of CR-dependent pathology prevention in females as well as males, and to incorporate hormonal and behavioral characterizations, as well as more in-depth analysis of APP metabolites.
We did not identify any significant differences in expression levels of total APP holoprotein, regardless of diet or sex. We postulated, therefore, that Aβ reductions might be driven by altered APP metabolism. Increased A disintegrin and metalloproteinase domain (Adam)-dependent α-secretase function through activation of sirtuin 1 (Sirt1), a protein deacetylase that is stimulated by CR, has been previously implicated as a potential mechanism underlying CR-dependent Aβ reductions (Qin et al., 2006). sAPPα and sAPPβ, produced by α-and β-secretase cleavage events, respectively, possess relatively long half-lives within the Tg2576 brain (Morales-Corraliza et al., 2009) and are therefore, stable indicators of APP metabolism by α- and β-secretase. By immunoblot analysis, we found that CR did not significantly alter sAPPα and sAPPβ protein levels within 5.3 or 15 month old Tg2576 mice, relative to AL feeding, suggesting that Aβ reductions were not likely attributable to augmented α- or β-secretase activity. Furthermore, we did not observe CR-dependent increases in Sirt1 levels and observed modest downregulation of Adam9 and Adam10 levels, in addition to no change in Adam17 levels, as measured by microarray analysis of CA1 pyramidal neurons (Supplemental Table 2). It is possible that CR-dependent activation of Sirt1 signaling and subsequent α-secretase function may alter Aβ pathology in other relevant models of AD. Our findings, however, suggest that changes in α- or β-cleavage are not a primary mechanism of altered APP metabolism and subsequent Aβ reduction following a dietary regimen of CR in Tg2576 mice.
The γ-secretase complex is required for coordinated cleavage of APP leading to Aβ production. We detected significant decreases in transcript levels of Psenen and Ps1, rate-limiting components of the γ-secretase complex, at 5.3 and 15 months of age within CA1 hippocampal dissections from CR-fed female Tg2576 mice, relative to AL-feeding. We also identified CR-specific reductions in Ncstn mRNA levels in 15 month old Tg2576 females, potentially indicative of more pronounced downregulation of γ-secretase in aged mice. Interestingly, we did not identify any CR-induced γ-secretase subunit mRNA reductions within young or old Tg2576 males. Our laboratory has published several prior studies in both human postmortem AD brains and in relevant AD models within the CA1 hippocampal region that have demonstrated expression signatures of homogeneous cell type populations are more sensitive detectors of neuronal gene expression changes than regional dissections that contain an admixture of cellular populations (Ginsberg et al., 2000, 2004, 2010, 2012; Ginsberg and Che, 2005). This point is not trivial, as γ-secretase subunits may be differentially regulated in nonneuronal cells. Furthermore, hippocampal expression of APPswe is highest within neuronal cell types (Irizarry et al., 2001), so we chose to validate our qPCR findings using a technique that would focus analysis on RNA isolated solely from CA1 pyramidal neurons. Our microarray results confirmed that CR feeding significantly reduced female Tg2576 Psenen and Ps1 mRNA levels within CA1 neurons.
The present multidisciplinary findings dovetail with several animal model and human studies from independent laboratories establishing that γ-secretase is a potential target for AD intervention. Pharmacological manipulation of γ-secretase function is an effective method for reducing Aβ pathology and has been applied to several AD clinical trials, although off-target effects have hampered study success, raising a question whether Aβ monotherapy is an effective treatment strategy (Crump et al., 2013; Golde et al., 2013; Mikulca et al., 2014). Despite these concerns, γ-secretase inhibition in the Tg2576 AD mouse model abrogates agedependent pathology indicators, including Aβ burden, spatial memory deficits, abnormal dendritic spine morphology, and microglial activation (Sivilia et al., 2013) and interestingly, also blunts early impairments in younger Tg2576 mice (Balducci et al., 2011). Furthermore, work by Placanica et al. demonstrates that pronounced female Aβ pathology may be driven by increased γ-secretase activity, relative to males (Placanica et al., 2009). Commensurate with these observations, we found that CR endogenously reduces γ-secretase subunit levels in female, but not male, Tg2576 mice. The CR regimen we employed reduced calorie intake through carbohydrate reduction (Supplemental Table 1). Whether sustained CR through a combination of reduction in carbohydrates, fats, and/or proteins would show similar sex-specific reductions in Aβ pathology remains to be determined. Since high fat/cholesterol diets have been demonstrated to exacerbate Aβ pathology and other AD-like changes in relevant mouse models (Pedrini et al., 2009; Julien et al., 2010), reduction of fats or proteins as part of a continuous CR dietary intervention may have similar beneficial effects to the observed reduction through carbohydrates, although testing this hypothesis requires significant additional research.
In summary, our multifaceted datasets implicate altered APP cleavage by γ-secretase as one, but not necessarily the only, mechanism by which CR profoundly reduces Aβ pathology throughout aging in female Tg2576 mice. These findings highlight the importance of sex, age, and dietary considerations within animal model studies of AD, as well as translational considerations for human AD treatment.
Supplementary Material
RESEARCH HIGHLIGHTS.
ELISA, stereology, qPCR, and microarrays assessed calorie restriction in Tg2576 mice. Calorie restriction (CR) reduces Aβ levels in aged female, but not male Tg2576 mice. CR reduces expression of Psenen and Ps1, γ-secretase subunits in aged Tg2576 females. Long-term CR reduces age-dependent Tg2576 Aβ pathology in a sex-specific manner.
ACKNOWLEDGMENTS
Support for this project comes from NIH grants GM007238, RR029893, AG043375, AG014449, AG017617, and HD057564 and the Alzheimer’s Association (IIRG-12-237253). We would like to thank Irina Elarova, M.S., Jose Morales-Corraliza, Ph.D., Arthur Saltzman, M.S., and Harrison Wong, B.S. for expert technical assistance. We thank Paul E. Fraser, Ph.D. for his generous donation of reagents. We acknowledge Helen E. Scharfman, Ph.D. and Edward B. Ziff, Ph.D. for advice on the experimental paradigm, and Efrat Levy, Ph.D. for critically reviewing the manuscript. We would also like to thank the NYU Experimental Pathology Histology Core Lab and the NKI animal facility staff for unparalleled technical assistance. We gratefully acknowledge the NYU Clinical and Translational Science Institute (NYULMC CTSI) and Cellular and Molecular Biology (NYULMC CMB) training programs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
Dr. Calhoun is an employee and owner of Sinq Systems. The authors have no other disclosures. The authors have no actual or potential conflicts of interest.
REFERENCES
- Alldred MJ, Che S, Ginsberg SD. Terminal Continuation (TC) RNA amplification enables expression profiling using minute RNA input obtained from mouse brain. Int. J. Mol. Sci. 2008;9:2091–2104. doi: 10.3390/ijms9112091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alldred M, Che S, Ginsberg S. Terminal continuation (TC) RNA amplification without second strand synthesis. J. Neurosci. Methods. 2009;177:381–385. doi: 10.1016/j.jneumeth.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alldred MJ, Duff KE, Ginsberg SD. Microarray analysis of CA1 pyramidal neurons in a mouse model of tauopathy reveals progressive synaptic dysfunction. Neurobiol. Dis. 2012;45:751–762. doi: 10.1016/j.nbd.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alldred MJ, Lee SH, Petkova E, Ginsberg SD. Expression profile analysis of hippocampal CA1 pyramidal neurons in aged Ts65Dn mice, a model of Down syndrome (DS) and Alzheimer's disease (AD) Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, Gouras GK. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol. Dis. 2005;20:187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Azad NA, Al Bugami M, Loy-English I. Gender differences in dementia risk factors. Gend Med. 2007;4:120–129. doi: 10.1016/s1550-8579(07)80026-x. [DOI] [PubMed] [Google Scholar]
- Balducci C, Mehdawy B, Mare L, Giuliani A, Lorenzini L, Sivilia S, Giardino L, Calzà L, Lanzillotta A, Sarnico I, Pizzi M, Usiello A, Viscomi AR, Ottonello S, Villetti G, Imbimbo BP, Nisticò G, Forloni G, Nisticò R. The γ-secretase modulator CHF5074 restores memory and hippocampal synaptic plasticity in plaque-free Tg2576 mice. J. Alzheimers. Dis. 2011;24:799–816. doi: 10.3233/JAD-2011-101839. [DOI] [PubMed] [Google Scholar]
- Bartley MG, Marquardt K, Kirchhof D, Wilkins HM, Patterson D, Linseman DA. Overexpression of amyloid-β protein precursor induces mitochondrial oxidative stress and activates the intrinsic apoptotic cascade. J. Alzheimers. Dis. 2012;28:855–868. doi: 10.3233/JAD-2011-111172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995;57:289–300. [Google Scholar]
- Callahan MJ, Lipinski WJ, Bian F, Durham RA, Pack A, Walker LC. Augmented senile plaque load in aged female beta-amyloid precursor protein-transgenic mice. Am. J. Pathol. 2001;158:1173–1177. doi: 10.1016/s0002-9440(10)64064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabrier MA, Blurton-Jones M, Agazaryan AA, Nerhus JL, Martinez-Coria H, LaFerla FM. Soluble Aβ promotes wild-type tau pathology in vivo. J. Neurosci. 2012;32:17345–17350. doi: 10.1523/JNEUROSCI.0172-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JHK, Berger JD, Mazzella MJ, Morales-Corraliza J, Cataldo AM, Nixon RA, Ginsberg SD, Levy E, Mathews PM. Age-dependent dysregulation of brain amyloid precursor protein in the Ts65Dn Down syndrome mouse model. J. Neurochem. 2009;110:1818–127. doi: 10.1111/j.1471-4159.2009.06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow VW, Mattson MP, Wong PC, Gleichmann M. An overview of APP processing enzymes and products. Neuromolecular Med. 2010;12:1–12. doi: 10.1007/s12017-009-8104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M, Oltersdorf T, Haass C. Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production. Nature. 1992;360:672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump CJ, Johnson DS, Li Y-M. Development and mechanism of γ-secretase modulators for Alzheimer’s disease. Biochemistry. 2013;52:3197–3216. doi: 10.1021/bi400377p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De LaCoste MC, White CL. The role of cortical connectivity in Alzheimer’s disease pathogenesis: a review and model system. Neurobiol. Aging. 1993;14:1–16. doi: 10.1016/0197-4580(93)90015-4. [DOI] [PubMed] [Google Scholar]
- Dewachter I, Reversé D, Caluwaerts N, Ris L, Kuipéri C, Van den Haute C, Spittaels K, Umans L, Serneels L, Thiry E, Moechars D, Mercken M, Godaux E, Van Leuven F. Neuronal deficiency of presenilin 1 inhibits amyloid plaque formation and corrects hippocampal long-term potentiation but not a cognitive defect of amyloid precursor protein [V717I] transgenic mice. J. Neurosci. 2002;22:3445–3453. doi: 10.1523/JNEUROSCI.22-09-03445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding F, Yao J, Zhao L, Mao Z, Chen S, Brinton RD. Ovariectomy induces a shift in fuel availability and metabolim in the hippocampus of the female transgenic model of familial Alzheimer's. PLoS One. 2013;8:e59825. doi: 10.1371/journal.pone.0059825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B. Correlation and large-scale simultaneous significance testing. J. Am. Stat. Assoc. 2007;102:93–103. [Google Scholar]
- Esler WP, Kimberly WT, Ostaszewski BL, Diehl TS, Moore CL, Tsai JY, Rahmati T, Xia W, Selkoe DJ, Wolfe MS. Transition-state analogue inhibitors of gammasecretase bind directly to presenilin-1. Nat. Cell Biol. 2000;2:428–434. doi: 10.1038/35017062. [DOI] [PubMed] [Google Scholar]
- Fan Y, Batmanghelich N, Clark CM, Davatzikos C. Spatial patterns of brain atrophy in MCI patients, identified via high-dimensional pattern classification, predict subsequent cognitive decline. Neuroimage. 2008;39:1731–1743. doi: 10.1016/j.neuroimage.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis R, McGrath G, Zhang J, Ruddy DA, Sym M, Apfeld J, Nicoll M, Maxwell M, Hai B, Ellis MC, Parks AL, Xu W, Li J, Gurney M, Myers RL, Himes CS, Hiebsch R, Ruble C, Nye JS, Curtis D. aph-1 and pen-2 are required for Notch pathway signaling, gamma-secretase cleavage of betaAPP, and presenilin protein accumulation. Dev. Cell. 2002;3:85–97. doi: 10.1016/s1534-5807(02)00189-2. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Alldred MJ, Counts SE, Cataldo AM, Neve RL, Jiang Y, Wuu J, Chao MV, Mufson EJ, Nixon RA, Che S. Microarray analysis of hippocampal CA1 neurons implicates early endosomal dysfunction during Alzheimer's disease progression. Biol. Psychiatry. 2010;68:885–893. doi: 10.1016/j.biopsych.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg SD. RNA amplification strategies for small sample populations. Methods. 2005;37:229–237. doi: 10.1016/j.ymeth.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD. Transcriptional profiling of small samples in the central nervous system. Methols Mol. Biol. 2008;439:147–158. doi: 10.1007/978-1-59745-188-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg SD, Alldred MJ, Che S. Gene expression levels assessed by CA1 pyramidal neuron and regional hippocampal dissections in Alzheimer’s disease. Neurobiol. Dis. 2012;45:99–107. doi: 10.1016/j.nbd.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg SD, Che S. Expression profile analysis within the human hippocampus: comparison of CA1 and CA3 pyramidal neurons. J. Comp. Neurol. 2005;487:107–118. doi: 10.1002/cne.20535. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Che S, Wuu J, Counts SE, Mufson EJ. Down regulation of Trk but not p75NTR gene expression in single cholinergic basal forebrain neurons mark the progression of Alzheimer’s disease. J. Neurochem. 2006;97:475–487. doi: 10.1111/j.1471-4159.2006.03764.x. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Elarova I, Ruben M, Tan F, Counts SE, Eberwine JH, Trojanowski JQ, Hemby SE, Mufson EJ, Che S. Single-cell gene expression analysis: implications for neurodegenerative and neuropsychiatric disorders. Neurochem. Res. 2004;29:1053–1064. doi: 10.1023/b:nere.0000023593.77052.f7. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Hemby SE, Lee VM-Y, Eberwine JH, Trojanowski JQ. Expression profile of transcripts in Alzheimer’s disease tangle-bearing CA1 neurons. Ann. Neurol. 2000;48:77–87. [PubMed] [Google Scholar]
- Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- Golde TE, Koo EH, Felsenstein KM, Osborne BA, Miele L. γ-Secretase inhibitors and modulators. Biochim. Biophys. Acta. 2013;1828:2898–2907. doi: 10.1016/j.bbamem.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good MA, Hale G. The "Swedish” mutation of the amyloid precursor protein (APPswe) dissociates components of object-location memory in aged Tg2576 mice. Behav. Neurosci. 2007;121:1180–1191. doi: 10.1037/0735-7044.121.6.1180. [DOI] [PubMed] [Google Scholar]
- Götz J, Schonrock N, Vissel B, Ittner LM. Alzheimer’s disease selective vulnerability and modeling in transgenic mice. J. Alzheimers. Dis. 2009;18:243–251. doi: 10.3233/JAD-2009-1143. [DOI] [PubMed] [Google Scholar]
- Goutte C, Tsunozaki M, Hale VA, Priess JR. APH-1 is a multipass membrane protein essential for the Notch signaling pathway in Caenorhabditis elegans embryos. Proc. Natl. Acad. Sci. U.S.A. 2002;99:775–779. doi: 10.1073/pnas.022523499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kiêu K, Nielsen J. The efficiency of systematic sampling in stereology--reconsidered. J. Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ. The new stereological tools: Disector, fractionator, nucleator and point sampled intercepts and their use. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch. Intern. Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- Hall AM, Roberson ED. Mouse models of Alzheimer’s disease. Brain Res. Bull. 2012;88:3–12. doi: 10.1016/j.brainresbull.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- Hart RW, Dixit R, Seng J, Turturro A, Leakey JE, Feuers R, Duffy P, Buffington C, Cowan G, Lewis S, Pipkin J, Li SY. Adaptive role of caloric intake on the degenerative disease processes. Toxicol. Sci. 1999;52:3–12. doi: 10.1093/toxsci/52.2.3. [DOI] [PubMed] [Google Scholar]
- Hayden K, Zandi P. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis. Assoc. Disord. 2006;20:93–100. doi: 10.1097/01.wad.0000213814.43047.86. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Trojanowski JQ, Ginsberg SD. Neuron-specific age-related decreases in dopamine receptor subtype mRNAs. J. Comp. Neurol. 2003;456:176–183. doi: 10.1002/cne.10525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata-Fukae C, Li H-F, Hoe H-S, Gray AJ, Minami SS, Hamada K, Niikura T, Hua F, Tsukagoshi-Nagai H, Horikoshi-Sakuraba Y, Mughal M, Rebeck GW, LaFerla FM, Mattson MP, Iwata N, Saido TC, Klein WL, Duff KE, Aisen PS, Matsuoka Y. Females exhibit more extensive amyloid, but not tau, pathology in an Alzheimer transgenic model. Brain Res. 2008;1216:92–103. doi: 10.1016/j.brainres.2008.03.079. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:7–10. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, Locascio JJ, Hyman BT. beta-site APP cleaving enzyme mRNA expression in APP transgenic mice: anatomical overlap with transgene expression and static levels with aging. Am. J. Pathol. 2001;158:173–177. doi: 10.1016/s0002-9440(10)63955-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J, Wu C, Redwine J, Comery T, Arias R, Bowlby M, Martone R, Morrison J, Pangalos M, Reinhart P, Bloom F. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien C, Tremblay C, Phivilay A, Berthiaume L, Emond V, Julien P, Calon F. High-fat diet aggravates amyloid-beta and tau pathologies in the 3xTg-AD mouse model. Neurobiol. Aging. 2010;31:1516–1531. doi: 10.1016/j.neurobiolaging.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Kacharmina JE, Crino PB, Eberwine J. Preparation of cDNA from single cells and subcellular regions. Methods Enzymol. 1999;303:3–18. doi: 10.1016/s0076-6879(99)03003-7. [DOI] [PubMed] [Google Scholar]
- Kealy RD, Lawler DF, Ballam JM, Mantz SL, Biery DN, Greeley EH, Lust G, Segre M, Smith GK, Stowe HD. Effects of diet restriction on life span and age-related changes in dogs. J. Am. Vet. Med. Assoc. 2002;220:1315–1320. doi: 10.2460/javma.2002.220.1315. [DOI] [PubMed] [Google Scholar]
- Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech. Ageing Dev. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Kunzler J, Youmans KL, Yu C, LaDu MJ, Tai LM. APOE modulates the effect of estrogen therapy on Aβ accumulation. Neurosci. Lett. 2014;560:131–136. doi: 10.1016/j.neulet.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-Y, Cole TB, Palmiter RD, Suh SW, Koh J-Y. Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 2002;99:7705–7710. doi: 10.1073/pnas.092034699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VM, Carden MJ, Schlaepfer WW, Trojanowski JQ. Monoclonal antibodies distinguish several differentially phosphorylated states of the two largest rat neurofilament subunits (NF-H and NF-M) and demonstrate their existence in the normal nervous system of adult rats. J. Neurosci. 1987;7:3474–3488. doi: 10.1523/JNEUROSCI.07-11-03474.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Ma G, Cai H, Price DL, Wong PC. Nicastrin is required for assembly of presenilin/gamma-secretase complexes to mediate Notch signaling and for processing and trafficking of beta-amyloid precursor protein in mammals. J. Neurosci. 2003;23:3272–3277. doi: 10.1523/JNEUROSCI.23-08-03272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YM, Xu M, Lai MT, Huang Q, Castro JL, DiMuzio-Mower J, Harrison T, Lellis C, Nadin A, Neduvelil JG, Register RB, Sardana MK, Shearman MS, Smith AL, Shi XP, Yin KC, Shafer JA, Gardell SJ. Photoactivated gamma-secretase inhibitors directed to the active site covalently label presenilin 1. Nature. 2000;405:689–694. doi: 10.1038/35015085. [DOI] [PubMed] [Google Scholar]
- Marlow L, Canet RM, Haugabook SJ, Hardy JA, Lahiri DK, Sambamurti K. APH1, PEN2, and Nicastrin increase Aβ levels and γ-secretase activity. Biochem. Biophys. Res. Commun. 2003;305:502–509. doi: 10.1016/s0006-291x(03)00797-6. [DOI] [PubMed] [Google Scholar]
- Mathews PM, Jiang Y, Schmidt SD, Grbovic OM, Mercken M, Nixon RA. Calpain activity regulates the cell surface distribution of amyloid precursor protein. Inhibition of calpains enhances endosomal generation of beta-cleaved C-terminal APP fragments. J. Biol. Chem. 2002;277:36415–36424. doi: 10.1074/jbc.M205208200. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCay C, Crowell M, Maynard L. The effect of retarded growth upon the length of life span and upon the ultimate body size. J. Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- McCulloch CE, Searle SR, Neuhaus JM. Generalized, Linear, and Mixed Models. Second Edition. New York: John Wiley & Sons; 2011. [Google Scholar]
- Mikulca JA, Nguyen V, Gajdosik DA, Teklu SG, Giunta EA, Lessa EA, Tran CH, Terak EC, Raffa RB. Potential novel targets for Alzheimer pharmacotherapy: II. Update on secretase inhibitors and related approaches. J. Clin. Pharm. Ther. 2014;39:25–37. doi: 10.1111/jcpt.12112. [DOI] [PubMed] [Google Scholar]
- Morales-Corraliza J, Mazzella MJ, Berger JD, Diaz NS, Choi JHK, Levy E, Matsuoka Y, Planel E, Mathews PM. In vivo turnover of tau and APP metabolites in the brains of wild-type and Tg2576 mice: greater stability of sAPP in the beta-amyloid depositing mice. PLoS One. 2009;4:e7134. doi: 10.1371/journal.pone.0007134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Selective vulnerability of corticocortical and hippocampal circuits in aging and Alzheimer’s disease. Prog. Brain Res. 2002;136:467–486. doi: 10.1016/s0079-6123(02)36039-4. [DOI] [PubMed] [Google Scholar]
- Mouton PR, Chachich ME, Quigley C, Spangler E, Ingram DK. Caloric restriction attenuates amyloid deposition in middle-aged dtg APP/PS1 mice. Neurosci. Lett. 2009;464:184–187. doi: 10.1016/j.neulet.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullan M, Crawford F, Axelman K, Houlden H. A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of Beta–amyloid. Nat. Genet. 1992;1:345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Tran L, Lambert MP, Glabe CG, Klein WL, LaFerla FM. Temporal profile of amyloid-beta (Abeta) oligomerization in an in vivo model of Alzheimer disease. A link between Abeta and tau pathology. J. Biol. Chem. 2006;281:1599–1604. doi: 10.1074/jbc.M507892200. [DOI] [PubMed] [Google Scholar]
- Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, Morgan DG, Morgan TE, Finch CE. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol. Aging. 2005;26:995–1000. doi: 10.1016/j.neurobiolaging.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Pedrini S, Thomas C, Brautigam H, Schmeidler J, Ho L, Fraser P, Westaway D, Hyslop PS, Martins RN, Buxbaum JD, Pasinetti GM, Dickstein DL, Hof PR, Ehrlich ME, Gandy S. Dietary composition modulates brain mass and solubilizable Abeta levels in a mouse model of aggressive Alzheimer's amyloid pathology. Mol. Neurodegener. 2009;4:40. doi: 10.1186/1750-1326-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placanica L, Zhu L, Li Y-M. Gender- and age-dependent gamma-secretase activity in mouse brain and its implication in sporadic Alzheimer disease. PLoS One. 2009;4:e5088. doi: 10.1371/journal.pone.0005088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, Puigserver P, Sadoshima J, Deng H, Pedrini S, Gandy S, Sauve AA, Pasinetti GM. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J. Biol. Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- Rajendran L, Annaert W. Membrane trafficking pathways in Alzheimer’s disease. Traffic. 2012;13:759–770. doi: 10.1111/j.1600-0854.2012.01332.x. [DOI] [PubMed] [Google Scholar]
- Roe FJ, Lee PN, Conybeare G, Kelly D, Matter B, Prentice D, Tobin G. The Biosure Study: influence of composition of diet and food consumption on longevity, degenerative diseases and neoplasia in Wistar rats studied for up to 30 months post weaning. Food Chem. Toxicol. 1995;33(Suppl 1):1S–100S. doi: 10.1016/0278-6915(95)80200-2. [DOI] [PubMed] [Google Scholar]
- Rous P. The influence of diet on transplanted and spontaneous mouse tumors. J. Exp. Med. 1914;20:433–451. doi: 10.1084/jem.20.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura CA, Chen G, Malkani S, Choi S-Y, Takahashi RH, Zhang D, Gouras GK, Kirkwood A, Morris RGM, Shen J. Conditional inactivation of presenilin 1 prevents amyloid accumulation and temporarily rescues contextual and spatial working memory impairments in amyloid precursor protein transgenic mice. J. Neurosci. 2005;25:6755–6764. doi: 10.1523/JNEUROSCI.1247-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt SD, Mazzella MJ, Nixon RA, Mathews PM. Abeta measurement by enzyme-linked immunosorbent assay. Methods Mol. Biol. 2012;849:507–527. doi: 10.1007/978-1-61779-551-0_34. [DOI] [PubMed] [Google Scholar]
- Shiraishi H, Sai X, Wang H-Q, Maeda Y, Kurono Y, Nishimura M, Yanagisawa K, Komano H. PEN-2 enhances gamma-cleavage after presenilin heterodimer formation. J. Neurochem. 2004;90:1402–1413. doi: 10.1111/j.1471-4159.2004.02597.x. [DOI] [PubMed] [Google Scholar]
- Sivilia S, Lorenzini L, Giuliani A, Gusciglio M, Fernandez M, Baldassarro VA, Mangano C, Ferraro L, Pietrini V, Baroc MF, Viscomi AR, Ottonello S, Villetti G, Imbimbo BP, Calzà L, Giardino L. Multi-target action of the novel anti-Alzheimer compound CHF5074: in vivo study of long term treatment in Tg2576 mice. BMC Neurosci. 2013;14:1–14. doi: 10.1186/1471-2202-14-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat. Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Steiner H, Winkler E, Edbauer D, Prokop S, Basset G, Yamasaki A, Kostka M, Haass C. PEN-2 is an integral component of the gamma-secretase complex required for coordinated expression of presenilin and nicastrin. J. Biol. Chem. 2002;277:39062–39065. doi: 10.1074/jbc.C200469200. [DOI] [PubMed] [Google Scholar]
- Thies W, Bleiler L. Alzheimer's Association, Alzheimer's disease facts and figures. Alzheimers Dement. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Viña J, Lloret A. Why women have more Alzheimer’s disease than men: gender and mitochondrial toxicity of amyloid-beta peptide. J. Alzheimers. Dis. 2010;20(Suppl 2):S527–S533. doi: 10.3233/JAD-2010-100501. [DOI] [PubMed] [Google Scholar]
- Von Gunten A, Kövari E, Bussière T, Rivara C-B, Gold G, Bouras C, Hof PR, Giannakopoulos P. Cognitive impact of neuronal pathology in the entorhinal cortex and CA1 field in Alzheimer’s disease. Neurobiol. Aging. 2006;27:270–277. doi: 10.1016/j.neurobiolaging.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Wan L, Nie G, Zhang J, Luo Y, Zhang P, Zhang Z, Zhao B. β-Amyloid peptide increases levels of iron content and oxidative stress in human cell and Caenorhabditis elegans models of Alzheimer disease. Free Radic. Biol. Med. 2011;50:122–129. doi: 10.1016/j.freeradbiomed.2010.10.707. [DOI] [PubMed] [Google Scholar]
- Wang J, Ho L, Qin W, Rocher A, Seror I, Humala N, Maniar K, Dolios G, Wang R, Hof PR, Pasinetti GM. Caloric restriction attenuates β-amyloid neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2005;18:1–18. doi: 10.1096/fj.04-3182fje. [DOI] [PubMed] [Google Scholar]
- Wang J, Tanila H, Puoliväli J, Kadish I, van Groen T. Gender differences in the amount and deposition of amyloidβ in APPswe and PS1 double transgenic mice. Neurobiol. Dis. 2003;14:318–327. doi: 10.1016/j.nbd.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Wolf AB, Braden BB, Bimonte-Nelson H, Kusne Y, Young N, Engler-Chiurazzi E, Garcia AN, Walker DG, Moses GSD, Tran H, LaFerla F, Lue L, Emerson Lombardo N, Valla J. Broad-based nutritional supplementation in 3xTg mice corrects mitochondrial function and indicates sex-specificity in response to Alzheimer’s disease intervention. J. Alzheimers. Dis. 2012;32:217–232. doi: 10.3233/JAD-2012-120478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R. Brain estrogen deficiency accelerates Aβ plaque formation in an Alzheimer’s disease animal model. Proc. Natl. Acad. Sci. U.S.A. 2005;102:19198–19203. doi: 10.1073/pnas.0505203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Thompson R, Zhang H, Xu H. APP processing in Alzheimer’s disease. Mol. Brain. 2011;4:3. doi: 10.1186/1756-6606-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Nadeau P, Song W, Donoviel D, Yuan M, Bernstein A, Yankner BA. Presenilins are required for gamma-secretase cleavage of beta-APP and transmembrane cleavage of Notch-1. Nat. Cell Biol. 2000;2:463–465. doi: 10.1038/35017108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.