Abstract
We aimed to determine whether network-level functional connectivity differs in two clinical variants of Alzheimer’s disease: logopenic primary progressive aphasia (lvPPA) and dementia of the Alzheimer’s type (DAT). Twenty-four lvPPA subjects with amyloid deposition on PET and task-free fMRI were matched to 24 amyloid-positive DAT subjects and 24 amyloid-negative controls. Independent-component analysis and spatial-temporal dual-regression were used to assess functional connectivity within the language network, left and right working memory networks and ventral default mode network. lvPPA showed reduced connectivity in left temporal language network and inferior parietal and prefrontal regions of the left working memory network compared to controls and DAT. Both groups showed reduced connectivity in parietal regions of the right working memory network compared to controls. Only DAT showed reduced ventral default mode network connectivity compared to controls. Aphasia severity correlated with connectivity in left working memory network within lvPPA. Patterns of network dysfunction differ across these two clinical variants of Alzheimer’s disease, with lvPPA particularly associated with disruptions in the language and left working memory networks.
Keywords: Alzheimer’s disease, primary progressive aphasia, language, MRI, functional imaging
1. INTRODUCTION
The logopenic variant of primary progressive aphasia (lvPPA) is a neurodegenerative language disorder characterized by poor repetition of sentences, phonemic paraphasias, and anomia with preserved word comprehension (Gorno-Tempini et al., 2004, Gorno-Tempini et al., 2011), with atrophy and hypometabolism observed in left posterior temporal and inferior parietal lobes, spreading into posterior frontal lobe (Gorno-Tempini et al., 2004, Madhavan et al., 2013, Rohrer et al., 2013). While patterns of atrophy and hypometabolism have been well characterized in lvPPA, it is unknown how functional connectivity within the brain is disrupted in these subjects. Given the primary language deficit in lvPPA, one could hypothesize that connectivity within the language network would be disrupted in these subjects. Indeed, patterns of atrophy in lvPPA are topographically similar to the distribution of the language network which involves the posterior superior temporal gyrus and inferior frontal lobe, as well as adjoining prefrontal, temporal and parietal regions (Turken and Dronkers, 2011, Tomasi and Volkow, 2012, Lehmann et al., 2013). However, the deficits observed in lvPPA have also been ascribed to dysfunction in the phonological loop (Gorno-Tempini et al., 2004, Gorno-Tempini et al., 2008), a component of the working memory network, suggesting that this network may also play a central role in the syndrome. Phonological loop functions have been associated particularly with the left inferior parietal lobule (including supramarginal gyrus), but also the superior and middle temporal gyri (Paulesu et al., 1993, Vallar et al., 1997, Baldo et al., 2012).
The majority of lvPPA subjects have underlying Alzheimer’s disease (AD) pathology and deposition of beta-amyloid (Mesulam et al., 2008, Rabinovici et al., 2008, Leyton et al., 2011, Whitwell et al., 2013), yet in contrast to subjects with dementia of the Alzheimer’s type (DAT) (McKhann et al., 2011) they typically show relatively preserved episodic memory, particularly early in the disease course. The default mode network (DMN) has been shown to be disrupted in subjects with DAT and has been hypothesized to be associated with episodic memory impairment. It has become clear, however, that the DMN is composed of a number of different sub-components and the medial temporal components, involving retrospenial cortex and medial temporal structures, are specifically associated with episodic memory loss (Andrews-Hanna et al., 2010, Andrews-Hanna et al., 2014). It is unclear to what extent the medial temporal components of the DMN may be involved in lvPPA and whether involvement of this network would differ between lvPPA and DAT.
The aim of this study was to use task-free functional MRI (fMRI) to assess functional connectivity in lvPPA, with particular emphasis on the language, working memory networks, and DMN, and to compare it to DAT to determine if network-level connectivity differs between these two syndromic variants of AD. We also aimed to determine if functional connectivity correlates to the severity of language impairment in lvPPA.
2. MATERIAL AND METHODS
2.1. Subjects
We prospectively recruited 24 subjects who presented to the Mayo Clinic Department of Neurology between July 1st 2010 and May 1st 2014, fulfilled diagnostic criteria for lvPPA (Gorno-Tempini et al., 2011), and showed beta-amyloid deposition on Pittsburgh Compound B PET (PiB-PET) imaging, suggesting AD pathology. Each subject underwent a neurological examination by a Behavioral Neurologist (KAJ), neuropsychological testing, a detailed speech and language examination performed by one of two Speech-Language Pathologists (JRD and EAS) and volumetric MRI, as previously described (Josephs et al., 2012). The speech and language assessment included the Western Aphasia Battery (Kertesz, 2007) that provides the Aphasia Quotient which is a measure of aphasia severity and includes a repetition subscore, the Boston Naming Test (Lansing et al., 1999), and the Pyramids and Palm Trees Test (Howard and Patterson, 1992). The presence/absence of phonological errors and agrammatism in speech were assessed during consensus review of recorded conversation as well as spoken picture description and word and sentence repetition responses during the formal test battery. Agrammatism in writing was also assessed by consensus using written descriptions of the picture description task. Clinical diagnosis of lvPPA was rendered by consensus between two speech-language pathologists (JRD and EAS) based solely on data from speech-language assessments without any reference to neurological or neuroimaging results. Criteria for the diagnosis of lvPPA required both anomia and impaired sentence repetition (Gorno-Tempini et al., 2011). The presence of phonemic paraphasias and no other features suggestive of another speech and language disorder were supportive.
The 24 lvPPA subjects were matched 1:1 using age, gender and disease duration to a cohort of 24 subjects that fulfilled diagnostic criteria for DAT (McKhann et al., 2011) that also showed beta-amyloid deposition on PiB-PET imaging. The DAT subjects had all been prospectively recruited into the Mayo Clinic Alzheimer’s Disease Research Center (ADRC) between February 8th, 2010 and October 13th, 2011, and were identified from the ADRC database. The lvPPA subjects were also matched by age and gender to a cohort of 24 cognitively normal subjects who showed absent beta-amyloid deposition on PiB-PET imaging. The cognitively normal subjects had been prospectively recruited into the Mayo Clinic Study of Aging (MCSA) (Roberts et al., 2008), and had all been evaluated by a Neurologist to ensure that they had normal neurological and neurocognitive examinations, and were not taking any medications that would affect cognition. All DAT and cognitively normal subjects had undergone the same MRI protocol as the lvPPA subjects.
The study was approved by the Mayo Clinic IRB and all subjects were consented for enrollment into the study.
2.2 PiB-PET assessment
All subjects underwent PiB-PET scanning on a PET/CT scanner (GE Healthcare, Milwaukee, Wisconsin) operating in 3D mode in order to assess for the presence of beta-amyloid. A cortical-to-cerebellar (SUVR) global uptake ratio was calculated, and a cut-point of 1.5 was used to determine PiB positivity, as previously described in detail (Jack et al., 2008).
2.3 Image Acquisition
All subjects underwent a standardized MRI imaging protocol at 3.0 Tesla, that included a 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence (TR/TE/T1, 2300/3/900 ms; flip angle 8°, 26-cm FOV; 256 × 256 in-plane matrix with a phase FOV of 0.94, slice thickness of 1.2 mm, in-plane resolution 1) and task-free fMRI data gradient EPI (TR/TE = 3000/30 ms, 90° flip angle, slice thickness 3.3mm, in-plane resolution 3.3mm, and 103 volumes). Subjects were instructed to keep their eyes open during task-free fMRI scanning.
2.4 Task-free fMRI analysis
Preprocessing and data analysis were performed utilizing a combination of the Statistical Parametric Mapping (SPM8) software (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/), the Resting-State fMRI Data Analysis Toolkit (REST) v1.5 (http://www.restfmri.net) (Song et al., 2011), Data Processing Assistant for Resting- State fMRI (DPARSF) v2.0 (http://www.restfmri.net) (Chao-Gan and Yu-Feng, 2010), and in-house developed software implemented in MATLAB v7.11 (Mathworks Inc., Natick, MA, USA). All task-free fMRI data sets with greater than 3 mm of translational movement, 3° of rotational movement, or that failed visual inspection for obvious artifacts were excluded from analysis. We also avoid the commonly overlooked problem of spectral misspecification in our nuisance regression of motion parameters, and therefore reduce the commonly reported subtle motion confound (Hallquist et al., 2013). Furthermore, no significant differences were observed across groups (controls, lvPPA, DAT) on six motion parameters (yaw=0.4, 0.4, 0.3, p=0.14; roll=0.3, 0.3, 0.3, p=0.70; pitch=0.4, 0.7, 0.6, p=0.10; mmz=0.5, 0.7, 0.7, p=0.72; mmy=0.4, 0.3, 0.3, p=0.55; mmx=0.2, 0.2, 0.1, p=0.27).
Preprocessing steps included discarding the first 3 volumes to obtain steady state magnetization, slice time correction, realignment, normalization to SPM8 EPI template, smoothing with 4 mm full-width half maximum Gaussian kernel, linear detrending to correct for signal drift, and 0.01–0.08 Hz bandpass filtering to reduce non-neuronal contributions to blood-oxygenation-level-dependent (BOLD) signal fluctuations. In addition, linear regression correction for spurious variables included rigid body transformation motion effects and the first seven principle components of the voxel-wise time courses within white matter and cerebral spinal fluid regions of interest derived from their respective template space priors (Behzadi et al., 2007).
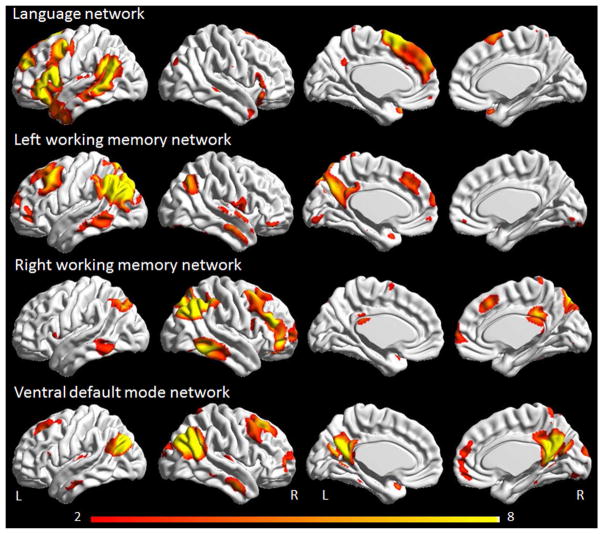
In order to identify networks-of-interest, group independent component analysis (ICA) using GIFT software was performed in an independent cohort of 892 cognitively normal subjects that had been recruited into the MCSA (Roberts et al., 2008, Jones et al., 2012). High-dimensional estimation of 54 components was applied where the number of components was based on the components estimation package in the GIFT software package. To ensure stability, the ICA analysis was run with 100 iterations using the ICASSO (Himberg et al., 2004) function within the GIFT software package. Each independent component was visually assessed and assigned a network name according to the anatomical location and a functional meta-analysis, as previously described (Jones et al., 2012). The high-dimensional group spatial ICA by its nature was created enforcing maximal spatial independence across the components (Jones et al., 2012). This procedure produced a population based network atlas. For the current study, four independent component networks of interest were analyzed: the language network, left and right working memory networks, and the ventral DMN (Figure 1). The ventral DMN was chosen since it is the ICA component that shows the closest spatial concordance with the medial temporal subsystem of the DMN that has been specifically associated with episodic memory impairments (Andrews-Hanna et al., 2010) that are typically observed in DAT. The ventral DMN, and the previously reported medial temporal subsystem of the DMN (Andrews-Hanna et al., 2010), both show involvement of ventral medial prefrontal cortex, posterior inferior parietal lobule, retrosplenial cortex, parahippocampal cortex and the hippocampus. The population based network atlas was used to back reconstruct the networks onto each subject in the current study using the spatial-temporal dual regression (STR) procedure as implemented in the GIFT software package. The first step in STR estimates the time course of the network of interest to be used as the regressor of interest in the subsequent estimate of the spatial extent of the network for an individual subject. A multivariate approach including four DMN subsystems (posterior, anterior ventral, anterior dorsal and ventral DMN) was used in order to specifically isolate the effects of the ventral DMN. The resulting spatial maps are then transformed to z-scores and used as the final parameter estimates of both the spatial extent and magnitude of functional connectivity for the network of interest.
Figure 1.
Networks of interest identified using a group independent component analysis in the MCSA cohort of 892 cognitively normal subjects. Renders were generated using the BrainNet Viewer (http://www.nitrc.org/projects/bnv/).
Voxel-level statistical analysis was performed in SPM. One-sample t-tests were used to display the voxel-wise connectivity map of each network in controls. Two-sample two-sided t-tests were performed to compare voxel-wise connectivity in each network between lvPPA and controls, DAT and controls, and lvPPA and DAT. In order to assess only within-network connections the group comparisons were inclusively masked by the group maps identified in the one sample t-tests of controls. All analyses were assessed corrected for multiple comparisons at the cluster level using family-wise error correction (p<0.001 for comparisons between disease groups and controls and p<0.05 for comparisons between disease groups) calculated using the AlphaSim function in the REST software package (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf). Regional functional connectivity values for each network were calculated and used to correlate to clinical measures within the lvPPA group. Z scores were averaged for each network across all voxels that showed significant differences between lvPPA and controls.
2.5 Statistical analysis
Demographic, cognitive and regional imaging variables were compared across all three groups using Chi-square or Kruskal-Wallis tests with post-hoc pairwise testing performed with Wilcoxon Rank Sum tests. Pair-wise correlations were performed to assess associations between regional functional connectivity in each network and performance on the Western Aphasia Battery Aphasia Quotient and repetition subscore, and Boston Naming Test within the lvPPA subjects. Statistical analyses were performed utilizing JMP computer software (JMP Software, version 10.0.0; SAS Institute Inc., Cary, NC) with significance assessed at p<0.05.
3. RESULTS
3.1 Subject demographics
By design, the lvPPA, DAT and control groups did not differ in age at scan or gender, and the lvPPA and DAT groups did not differ in time from onset-to-scan (Table 1). Both lvPPA and DAT showed significantly poorer performance on the Mini-Mental State Examination, Boston Naming Test, Trail Making Test A and B and the Auditory Verbal Learning Test delayed recall than controls. The lvPPA group showed significantly worse performance on the Boston Naming Test than DAT, and DAT showed significantly worse performance on the Mini-mental state examination and Auditory Verbal Learning Test delayed recall than lvPPA (Table 1).
Table 1.
Subject demographics
| Controls (n = 24) | lvPPA (n = 24) | DAT (n = 24) | P values | |
|---|---|---|---|---|
| Female, n (%) | 11 (46%) | 11 (46%) | 12 (50%) | 0.95 |
| Age, years | 65 ± 8 [59–71] | 66 ± 9 [58–72] | 68 ± 10 [59–75] | 0.45 |
| Age onset, years | NA | 62 ± 9 [55–68] | 64 ± 9 [57–71] | 0.46 |
| Time from disease onset to imaging, years | NA | 3.5 ± 1.4 [2–4] | 4.1 ± 1.2 [3–5] | 0.10 |
| Mini Mental State Exam score (/30) | 28 ± 1* [28–29] | 25 ± 3 [24–28] | 19 ± 6* [17–24] | <0.001†‡§ |
| Boston Naming Test (% correct)** | 93 ± 6 [88–97] | 60 ± 23 [50–80] | 80 ± 16 [73–89] | <0.001†‡§ |
| Western Aphasia Battery Aphasia Quotient (100) | NA | 85.3 ± 7.2 [83–88] | NA | NA |
| Western Aphasia Battery Repetition subscore (/10) | NA | 8.2 ± 1.0 [7.6–8.9] | NA | NA |
| Phonological errors (%) | NA | 24 (100%) | NA | NA |
| Pyramids and Palm Trees Test (/50) | NA | 48 ± 3 [47, 50] | NA | NA |
| Agrammatism in speech (%) | NA | 0 (0%) | NA | NA |
| Agrammatism in writing (%) | NA | 4 (20%) | NA | NA |
| Trail Making Test A*** | 32 ± 9 [27–35] | 59 ± 36 [35–71] | 85 ± 49 [42–128] | <0.001†‡ |
| Trail Making Test B*** | 74 ± 24 [52–90] | 180 ± 91 [106–250] | 208 ± 93 [138–300] | <0.001†‡ |
| Auditory Verbal Learning Test delayed recall | 8.5 ± 3.7 [6–11] | 4.2 ± 4.5 [0–7] | 0.5 ± 1.1 [0–0] | <0.001†‡§ |
| Rey-Osterrieth complex figure test**** | NA | 20 ± 12 [10–31] | 14 ± 12 [5–23] | 0.11 |
Data shown as mean ± standard deviation [inter-quartile range]. P values represent comparison across all three groups. NA = Not available
Significant difference between lvPPA and controls;
Significant difference between DAT and controls;
Significant difference between lvPPA and DAT
Short Test of Mental Status scores were converted to MMSE scores in the subjects recruited from the ADRC/ADPR using an algorithm developed at our center.
Boston Naming Test scores are shown as % of words correct out of total. DAT and control subjects received 60-item BNT and lvPPA subjects received 15-item BNT.
Seconds required to complete test
Raw score based on Taylor scoring method
3.2 Task-free fMRI
3.2.1 Language network
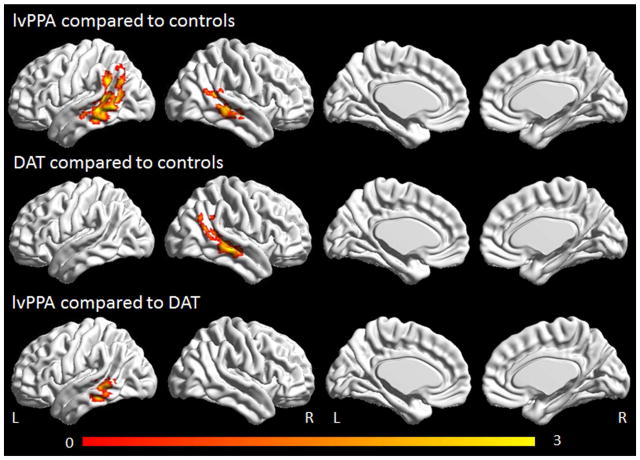
The lvPPA group showed reduced connectivity in the left lateral middle temporal gyrus and angular gyrus of the language network compared to controls (Figure 2). Reductions were also observed in the right middle temporal gyrus. In contrast, the DAT group showed reduced connectivity only in the right middle temporal gyrus compared to controls. The lvPPA group showed reduced connectivity in the left middle temporal gyrus of the language network compared to the DAT group (Figure 2). No regions showed increased connectivity in lvPPA or DAT compared to controls.
Figure 2.
Task-free fMRI results for the language network. Regions showing decreased connectivity in lvPPA compared to controls and DAT compared to controls are shown corrected for multiple comparisons at p<0.001. Regions showing reduced connectivity in lvPPA compared to DAT are shown corrected for multiple comparisons at p<0.05. Renders were generated using the BrainNet Viewer (http://www.nitrc.org/projects/bnv/).
3.2.2 Working memory networks
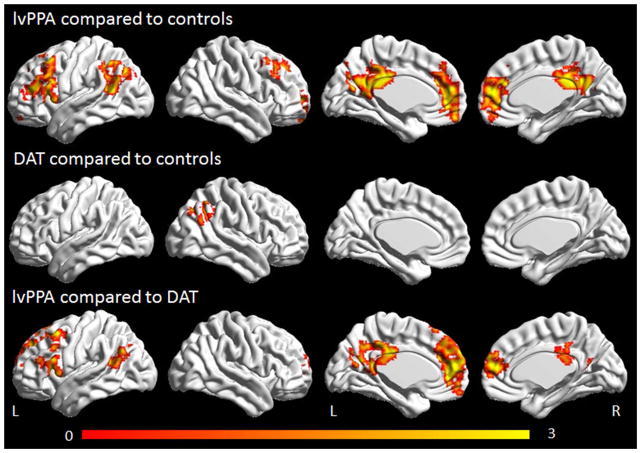
The lvPPA group showed reduced connectivity bilaterally in the medial and lateral prefrontal cortex and posterior cingulate and in the left angular and supramarginal gyrus, in the left working memory network compared to controls (Figure 3). Reduced connectivity was only observed in the right inferior parietal lobe in the DAT group compared to controls. The lvPPA group showed reduced connectivity bilaterally in the medial and lateral prefrontal cortex and posterior cingulate and in the left angular gyrus compared to the DAT group.
Figure 3.
Task-free fMRI results for the left working memory network. Regions showing decreased connectivity in lvPPA compared to controls and DAT compared to controls are shown corrected for multiple comparisons at p<0.001. Regions showing reduced connectivity in lvPPA compared to DAT are shown corrected for multiple comparisons at p<0.05. Renders were generated using the BrainNet Viewer (http://www.nitrc.org/projects/bnv/).
No differences were observed between the lvPPA and DAT groups in the right working memory network, with lvPPA showing reduced connectivity in the right parietal lobe and DAT showing reduced connectivity bilaterally in the posterior cingulate and precuneus, compared to controls (Figure 4). No regions showed increased connectivity in lvPPA or DAT compared to controls in either the left or right working memory networks.
Figure 4.
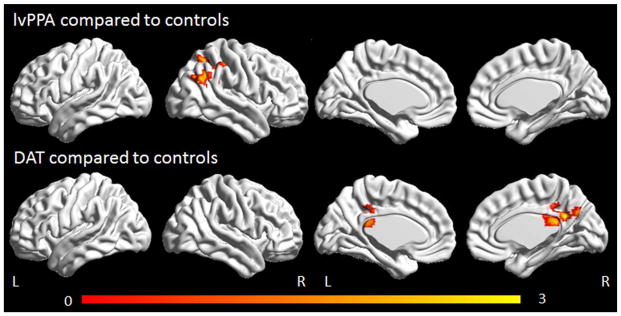
Task-free fMRI results for the right working memory network. Regions showing decreased connectivity in lvPPA compared to controls and DAT compared to controls are shown corrected for multiple comparisons at p<0.001. Renders were generated using the BrainNet Viewer (http://www.nitrc.org/projects/bnv/).
3.2.3 Ventral DMN
Only the DAT group showed altered connectivity in the ventral DMN, with reduced connectivity observed in the retrosplenial cortex bilaterally compared to controls (Figure 5). No differences were observed between the DAT and lvPPA groups. No regions showed increased connectivity in lvPPA or DAT compared to controls.
Figure 5.
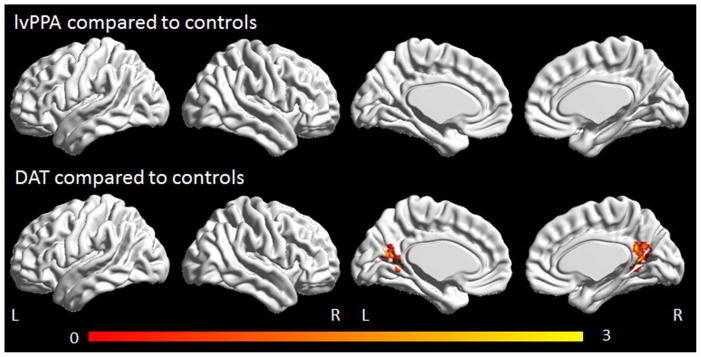
Task-free fMRI results for the ventral default mode network. Regions showing decreased connectivity in lvPPA compared to controls and DAT compared to controls are shown corrected for multiple comparisons at p<0.001. Renders were generated using the BrainNet Viewer (http://www.nitrc.org/projects/bnv/).
The reductions in functional connectivity observed across the four networks in lvPPA and DAT remained the same after correction for atrophy.
3.3 Language correlations in lvPPA
A trend was observed for functional connectivity in the left working memory network to correlate to both the Western Aphasia Battery Aphasia Quotient (R=0.37, p=0.08) and the repetition subscore of the Western Aphasia Battery (R=0.37, p=0.08) (Figure 6). No correlations were identified between functional connectivity in the language network, right working memory network, or ventral DMN and performance on the Western Aphasia Battery Aphasia Quotient, repetition, or Boston Naming Test.
Figure 6.
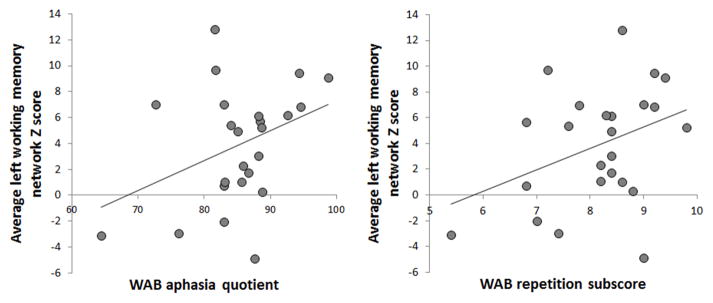
Scatter-plots showing the relationship between functional connectivity in the left working memory network and the WAB aphasia quotient and repetition sub score
4. DISCUSSION
This study demonstrates that lvPPA is particularly associated with disrupted functional connectivity in both the language and left working memory networks. The fact that these networks differed across lvPPA and DAT suggests that they play a fundamental role in determining clinical phenotype, rather than being associated with underlying pathology.
The language network includes the two main language centers, Broca’s area in the inferior frontal lobe, and Wernicke’s area in the posterior superior temporal gyrus, as well as other functionally correlated regions that are all thought to be associated with language comprehension (Xiang et al., 2010, Turken and Dronkers, 2011, Tomasi and Volkow, 2012). The network is typically left-lateralized, although does extend into homologous regions in the right hemisphere (Turken and Dronkers, 2011). Given its presumed central role in language function, it is fitting that disruption in this network would be observed in lvPPA. Reduced connectivity was observed throughout left posterior temporal and inferior parietal regions, as well as in the right posterior temporal lobe. The language deficits in lvPPA, including phonemic paraphasias, have indeed been localized to the lateral temporal lobe (Amici et al., 2007, Corina et al., 2010, Rogalski et al., 2011). Deficits arising from other areas in the language network, such as Broca’s area which is associated with agrammatic aphasia, are not typically observed in lvPPA; consistent with the fact that this region of the language network was spared in lvPPA. In contrast, the left hemisphere language network was relatively spared in DAT concurring with the fact that DAT subjects do not show a predominant or striking early language dysfunction, and performed better on the Boston Naming Test than our lvPPA subjects. Involvement of the language network could therefore potentially be a useful marker to differentiate lvPPA and DAT.
Disruption in the left working memory network was also striking in the lvPPA subjects, with decreased connectivity observed throughout left frontal and parietal regions compared to both controls and DAT subjects. Working memory allows us to hold and manipulate information in the mind over short periods of time, and relies heavily on attention processes. A component of working memory responsible for dealing with auditory or verbal information is known as the phonological loop (Baddeley, 1988). Deficits in this phonological loop have been suggested to be a core mechanism underlying lvPPA (Gorno-Tempini et al., 2004, Gorno-Tempini et al., 2008), with subjects showing deficits in repeating complex sentences and problems with sentence comprehension (Gorno-Tempini et al., 2008). The phonological store, responsible for brief storage of verbal information, has been localized to the left hemisphere, particularly the supramarginal gyrus (Paulesu et al., 1993, Vallar et al., 1997, Baldo et al., 2012); concurring with the inferior parietal regions of reduced connectivity observed in our lvPPA cohort. Regions in the left prefrontal cortex, which also showed reduced connectivity in our cohort, have been associated with an articulatory rehearsal component, responsible for refreshing the verbal information and keeping it active (D’Esposito and Postle, 1999, Baldo and Dronkers, 2006). Our findings therefore support the notion that disruption of the phonological loop is a central process in lvPPA. However, the left working memory network analysis also showed reduced connectivity in lvPPA bilaterally in the medial frontal and parietal regions. Further research will be needed to determine the role of these regions, although they have been implicated in attentional as well as working memory processes (Huang et al., 2013). The fact that the lvPPA subjects showed greater abnormalities in the left compared to the right working memory network also suggest that the phonological component of working memory may be preferentially impaired in lvPPA, rather than, for example, the component of working memory that deals with visual and spatial information. Disrupted connectivity in parietal regions of the left and right working memory networks were also observed in DAT, as has been previously shown (Filippi et al., 2013), although the lvPPA subjects showed greater involvement of the left working memory network than the DAT subjects. These findings concord with the fact that verbal short term memory has been shown to be affected to a greater degree than visuospatial short term memory in lvPPA, and to a greater degree than subjects with DAT (Foxe et al., 2013).
Importantly, our data suggested that connectivity within the left working memory network correlated with the degree of aphasia severity, as measured with the Western Aphasia Battery Aphasia Quotient, and with the degree of repetition deficits in the lvPPA subjects. No correlations were observed between the clinical measures and the language network. This suggests that disruption in the left working memory network in particular is central to the disease process in lvPPA and is related to the clinical abnormalities observed in these subjects. Our findings are concordant with current theories concerning the neural underpinnings of sentence repetition that implicate fronto-parietal networks, as well as the posterior superior temporal and inferior parietal lobes (Majerus, 2013). Atrophy of superior temporal and inferior parietal regions has been particularly implicated in sentence repetition in PPA (Amici et al., 2007, Rogalski et al., 2011, Leyton et al., 2012), although our findings also point to the importance of the frontoparietal working memory network.
In contrast to the findings in the language and working memory networks, functional connectivity within the ventral DMN was only abnormal in the DAT subjects, with reduced connectivity observed in the retrosplenial cortex. This finding conforms to the notion that the ventral component of the DMN is associated with episodic memory impairment (Andrews-Hanna et al., 2010, Andrews-Hanna et al., 2014), and hence shows abnormalities in DAT, a disorder characterized by episodic memory impairment. Indeed, as expected, the DAT subjects in this study performed significantly worse on delayed recall from the Auditory Verbal Learning Test than the lvPPA subjects. Although not prominent in Figure 1, our ventral DMN did indeed include the hippocampus, as well as a network of regions that have been associated with episodic memory impairment. It is therefore clear that despite the fact that both lvPPA and DAT have beta-amyloid deposition and the same underlying pathology, network dynamics differ across the two syndromes and likely play a role in the differing clinical presentations.
The results of this study increase understanding of the disease mechanisms underlying lvPPA and suggest that disruption to the language and working memory networks are important and characteristic features. It is possible that measurements from these networks may prove to be useful disease biomarkers in lvPPA. Our study is somewhat limited since it is cross-sectional in design. Further work will be needed to understand the progression of functional connectivity changes over time and to determine whether our results will generalize to other stages of the disease. It is likely that measures from the language and working memory networks may best differentiate lvPPA and DAT at early stages of the disease, with an eventual merging of clinical features and network disruption as the disease progresses and with advancing age (Jones et al., 2011).
HIGHLIGHTS.
Network dysfunction differs between logopenic aphasia and Alzheimer’s dementia
Logopenic aphasia shows greater reductions in language and working memory networks
Alzheimer’s dementia shows reductions in ventral default mode network
Our findings increase understanding of underlying disease mechanisms
Acknowledgments
The study was funded by NIH grants R01 DC010367 (PI Josephs), R01-AG11378 (PI Jack), and P50-AG016574 (PI Petersen) and Alzheimer’s Association grant NIRG-12-242215 (PI Whitwell). The sponsor played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. We would like to acknowledge Dr.’s Bradley Boeve, David Knopman, Daniel Drubach, Robert Ivnik and Glenn Smith, Mayo Clinic Rochester, MN, who performed clinical or neuropsychological evaluations on some of the DAT and healthy control subjects included in the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amici S, Ogar J, Brambati SM, Miller BL, Neuhaus J, Dronkers NL, et al. Performance in specific language tasks correlates with regional volume changes in progressive aphasia. Cognitive and behavioral neurology : official journal of the Society for Behavioral and Cognitive Neurology. 2007;20(4):203–11. doi: 10.1097/WNN.0b013e31815e6265. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65(4):550–62. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences. 2014;1316(1):29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Cognitive psychology and human memory. Trends in neurosciences. 1988;11(4):176–81. doi: 10.1016/0166-2236(88)90145-2. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Dronkers NF. The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology. 2006;20(5):529–38. doi: 10.1037/0894-4105.20.5.529. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Katseff S, Dronkers NF. Brain Regions Underlying Repetition and Auditory-Verbal Short-term Memory Deficits in Aphasia: Evidence from Voxel-based Lesion Symptom Mapping. Aphasiology. 2012;26(3–4):338–54. doi: 10.1080/02687038.2011.602391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova R, Srikanth R, Baer A, Laurienti PJ, Burdette JH, Hayasaka S, et al. Biological parametric mapping: A statistical toolbox for multimodality brain image analysis. NeuroImage. 2007;34(1):137–43. doi: 10.1016/j.neuroimage.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Frontiers in systems neuroscience. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corina DP, Loudermilk BC, Detwiler L, Martin RF, Brinkley JF, Ojemann G. Analysis of naming errors during cortical stimulation mapping: implications for models of language representation. Brain and language. 2010;115(2):101–12. doi: 10.1016/j.bandl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR. The dependence of span and delayed-response performance on prefrontal cortex. Neuropsychologia. 1999;37(11):1303–15. doi: 10.1016/s0028-3932(99)00021-4. [DOI] [PubMed] [Google Scholar]
- Filippi M, Agosta F, Scola E, Canu E, Magnani G, Marcone A, et al. Functional network connectivity in the behavioral variant of frontotemporal dementia. Cortex; a journal devoted to the study of the nervous system and behavior. 2013;49(9):2389–401. doi: 10.1016/j.cortex.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Foxe DG, Irish M, Hodges JR, Piguet O. Verbal and visuospatial span in logopenic progressive aphasia and Alzheimer’s disease. J Int Neuropsychol Soc. 2013;19(3):247–53. doi: 10.1017/S1355617712001269. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71(16):1227–34. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55(3):335–46. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallquist MN, Hwang K, Luna B. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage. 2013;82:208–25. doi: 10.1016/j.neuroimage.2013.05.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himberg J, Hyvarinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage. 2004;22(3):1214–22. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. The Pyramids and Palm Trees Test: A Test of Semantic Access from Words and Picture. Thames Valley Test Company; 1992. [Google Scholar]
- Huang S, Seidman LJ, Rossi S, Ahveninen J. Distinct cortical networks activated by auditory attention and working memory load. NeuroImage. 2013;83:1098–108. doi: 10.1016/j.neuroimage.2013.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain : a journal of neurology. 2008;131(Pt 3):665–80. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Machulda MM, Vemuri P, McDade EM, Zeng G, Senjem ML, et al. Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology. 2011;77(16):1524–31. doi: 10.1212/WNL.0b013e318233b33d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Vemuri P, Murphy MC, Gunter JL, Senjem ML, Machulda MM, et al. Non-stationarity in the “resting brain’s” modular architecture. PloS one. 2012;7(6):e39731. doi: 10.1371/journal.pone.0039731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain : a journal of neurology. 2012;135(Pt 5):1522–36. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. Western Aphasia Battery (Revised) San Antonio, Tx: PsychCorp; 2007. [Google Scholar]
- Lansing AE, Ivnik RJ, Cullum CM, Randolph C. An empirically derived short form of the Boston naming test. Arch Clin Neuropsychol. 1999;14(6):481–7. [PubMed] [Google Scholar]
- Lehmann M, Madison CM, Ghosh PM, Seeley WW, Mormino E, Greicius MD, et al. Intrinsic connectivity networks in healthy subjects explain clinical variability in Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(28):11606–11. doi: 10.1073/pnas.1221536110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton CE, Piguet O, Savage S, Burrell J, Hodges JR. The neural basis of logopenic progressive aphasia. Journal of Alzheimer’s disease : JAD. 2012;32(4):1051–9. doi: 10.3233/JAD-2012-121042. [DOI] [PubMed] [Google Scholar]
- Leyton CE, Villemagne VL, Savage S, Pike KE, Ballard KJ, Piguet O, et al. Subtypes of progressive aphasia: application of the International Consensus Criteria and validation using beta-amyloid imaging. Brain. 2011;134(Pt 10):3030–43. doi: 10.1093/brain/awr216. [DOI] [PubMed] [Google Scholar]
- Madhavan A, Whitwell JL, Weigand SD, Duffy JR, Strand EA, Machulda MM, et al. FDG PET and MRI in logopenic primary progressive aphasia versus dementia of the Alzheimer’s type. PloS one. 2013;8(4):e62471. doi: 10.1371/journal.pone.0062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus S. Language repetition and short-term memory: an integrative framework. Frontiers in human neuroscience. 2013;7:357. doi: 10.3389/fnhum.2013.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2011;7(3):263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63(6):709–19. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993;362(6418):342–5. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol. 2008;64(4):388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson CK, Weintraub S, et al. Anatomy of language impairments in primary progressive aphasia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(9):3344–50. doi: 10.1523/JNEUROSCI.5544-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Caso F, Mahoney C, Henry M, Rosen HJ, Rabinovici G, et al. Patterns of longitudinal brain atrophy in the logopenic variant of primary progressive aphasia. Brain and language. 2013 doi: 10.1016/j.bandl.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PloS one. 2011;6(9):e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Resting functional connectivity of language networks: characterization and reproducibility. Molecular psychiatry. 2012;17(8):841–54. doi: 10.1038/mp.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Frontiers in systems neuroscience. 2011;5:1. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar G, Di Betta AM, Silveri MC. The phonological short-term store-rehearsal system: patterns of impairment and neural correlates. Neuropsychologia. 1997;35(6):795–812. doi: 10.1016/s0028-3932(96)00127-3. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Lowe VJ, Duffy JR, Strand EA, Machulda MM, Kantarci K, et al. Elevated occipital beta-amyloid deposition is associated with widespread cognitive impairment in logopenic progressive aphasia. Journal of neurology, neurosurgery, and psychiatry. 2013 doi: 10.1136/jnnp-2013-305628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang HD, Fonteijn HM, Norris DG, Hagoort P. Topographical functional connectivity pattern in the perisylvian language networks. Cerebral cortex. 2010;20(3):549–60. doi: 10.1093/cercor/bhp119. [DOI] [PubMed] [Google Scholar]