Abstract
Although the human brain is exceptional in size and information processing capabilities, it is similar to other mammals with regards to the factors that promote its optimal performance. Three such factors are the challenges of physical exercise, food deprivation/fasting, and social/intellectual engagement. Because it evolved, in part, for success in seeking and acquiring food, the brain functions best when the individual is hungry and physically active, as typified by the hungry lion stalking and chasing its prey. Indeed, studies of animal models and human subjects demonstrate robust beneficial effects of regular exercise and intermittent energy restriction/fasting on cognitive function and mood, particularly in the contexts of aging and associated neurodegenerative disorders. Unfortunately, the agricultural revolution and the invention of effort-sparing technologies have resulted in a dramatic reduction or elimination of vigorous exercise and fasting, leaving only intellectual challenges to bolster brain function. In addition to disengaging beneficial adaptive responses in the brain, sedentary overindulgent lifestyles promote obesity, diabetes and cardiovascular disease, all of which may increase the risk of cognitive impairment and Alzheimer’s disease. It is therefore important to embrace the reality of the requirements for exercise, intermittent fasting and critical thinking for optimal brain health throughout life, and to recognize the dire consequences for our aging population of failing to implement such brain-healthy lifestyles.
Evolutionary perspective: why ‘hunger games’ bolster brainpower
Animals in the wild, particularly carnivores, survive by being able to locate and acquire food. As a corollary, evolution favored those individuals and species that were adept at outsmarting their prey and their competitors in the struggle for limited food sources. The brain is therefore geared for a high level of motivation and optimal sensory- motor and cognitive function when the individual experiences hunger / food scarcity, and the often vigorous exercise required to obtain food (Figure 1; and see Raichlen and Gordon, 2011). For most species, individuals would not survive if their brains and bodies were not functioning well when hungry. Unlike the ad libitum eating pattern of modern humans and their domesticated pets and farm animals, our human ancestors and wild animals ate/eat sporadically with inter-meal intervals that depend upon the availability of food sources. For example, many carnivores catch and eat prey only once a day, once every few days, or even less frequently (Gervasi et al., 2012). Extreme examples include the king and emperor penguins which typically fasts for 3–6 months every year (Cherel and Le Maho, 1985). In many areas of the world herbivores also experience extended periods of time with little or no food. For example, deer in northern regions of the world typically endure long periods with little food during the harsh cold winter months. Conversely, drought in the hot dry months of summer often means long fasting periods for a variety of animals in regions of Africa and Australia. Human populations in undeveloped countries continue to experience intermittent food shortages, with starvation occurring in some instances (Haile, 2005). Interestingly, hunter-gatherers experience less famine than agriculturalists (Berbesque et al., 2014) which may be explained, in part, by their greater experience with and knowledge of their environment and its complement of food sources. Indeed, the human populations that suffer the most from famine nowadays are those in sub-Saharan Africa that are reliant on agriculture (Haile, 2005).
Figure 1.
Images depicting the evolutionary basis for the importance of optimal brain function under conditions of food deprivation and physical exertion. Carnivorous animals in the wild, and our human ancestors, had to expend considerable effort to catch and kill prey. Advances in cognitive capabilities were driven, in large part, by the need to develop strategies and tools that enabled consistent success in the hunt for food. The upper image shows a tiger chasing two antelope (source: Wikemedia Commons). The lower image shows an American Indian hunting a bison (source: Wikipedia). In the case of the Indian, the human brain evolved the capabilities of making tools (bow and arrow), taming and training horses, and language for communication in planning and executing the hunt.
Whereas the need to acquire food was a major day-to-day challenge during much of hominid evolutionary history, for people in modern societies a constant reliable supply of food is the norm. Large-scale agriculture, and food processing and distribution, enables most people to spend their waking hours on occupations and leisure activities that involve highly advanced physical effort-sparing technologies. Intellectual challenges in modern societies are focused on occupation-specific tasks, rather than the challenge of acquiring food. Regular intellectual challenges are critical for brain development and a successful career, and recent findings suggest that intermittent exercise and energy restriction can further enhance and then sustain the functional capabilities of the brain during aging. Intermittent energetic challenges can prevent the development of metabolic phenotypes (obesity, insulin resistance and diabetes) that impair brain function. By tapping into the evolutionarily conserved cellular and molecular mechanisms described below, regular exercise and intermittent energy restriction during adult life can optimize brain function and may forestall age-related neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases.
Empirical findings demonstrate that exercise, energy restriction and intellectual enrichment enhance neuroplasticity
Laboratory rats and mice are usually fed ad libitum and so eat as much and as often as desired. In addition, they are typically maintained in small cages with no running wheels or other opportunities for exercise. Moreover, each cage usually contains 4 or 5 animals, which is a relatively small social group. Thus, it is reasonable to consider control laboratory animals as ‘couch potatoes’ that, compared to rodents in the wild, are sedentary, overeat and experience little cognitive stimulation (Martin et al., 2010). It is important to recognize that control laboratory rodents are in an unchallenging environment when considering the often quite profound effects of energy restriction, exercise and enriched housing environments on their brains. In this context it is reasonable to consider that findings from studies of these laboratory animals may extrapolate to humans with couch potato lifestyles, but perhaps not so much to those who exercise regularly, limit their energy intake, and engage in intellectual challenges.
The typical chow consumed by laboratory rodents is formulated to contain mostly carbohydrates, and less fat than the diets of many humans who become obese. Accordingly, when rats or mice are maintained on a high fat diet they accumulate much more fat and become insulin resistant, similar to humans. Interestingly, there is considerable variability in propensity for insulin resistance and obesity among strains of mice and rats maintained under the usual ad libitum feeding conditions. For example, at Charles River Laboratories 4 month-old male Wistar rats weight about 500 g, whereas 4 month-old Sprague-Dawley and Brown Norway rats typically weigh about 400 g and 300 g, respectively. As is usual for humans, female rats weigh less than males but maintain a higher percentage of their weight as fat. Nevertheless, experimental reductions in energy intake result in reductions in body weight and fat mass in all of these rat strains (Randerath et al., 1993; Shimokawa et al., 2003; Martin et al., 2007). With this in mind, I briefly describe some of the more salient effects of exercise, energy restriction and enriched environments on the function and structural plasticity of the brains of rats and mice. Recent reviews of the extensive literature on these topics include: Mattson, 2012; Redolat et al., 2012; Intlekofer and Cotman, 2013; Voss et al., 2013.
When rats or mice are provided with running wheels many of them run long distances, up to 10 – 20 kilometers per day; they typically run in many relatively short bouts of no more than a few minutes at a time (Stranahan et al., 2009). Mice that run exhibit improved spatial learning and memory, and increased synaptic strength in the hippocampus (long-term potentiation of synaptic transmission) (van Praag et al., 1999). The density of dendritic spines, an indicator of synapse number, is increased in hippocampal dentate granule neurons of runner mice compared to non-runners (Stranahan et al., 2009). Running also stimulates the proliferation of neural stem cells in the hippocampus, and increases the number of newly-generated neurons that integrate into functional neuronal networks (van Praag et al., 1999; 2002). Recent findings suggest that neurogenesis plays an important role in spatial pattern separation, a cognitive process dependent upon hippocampal dentate granule neurons (Creer et al., 2010). Learning and memory performance declines in many rats and mice during aging and such age-related cognitive deficits can be prevented and reversed by daily exercise (Marlatt et al., 2012; Spiesman et al., 2013). Moreover, consumption of a high fat diet impairs learning and memory performance in rodents, and running wheel exercise can ameliorate the adverse effects of a high fat diet (Molteni et al., 2004). Finally, an additional benefit of exercise experienced by humans is reduced anxiety and improved mood (Strohle, 2009). Controlled studies in rodents have also demonstrated anxiolytic and antidepressant effects of exercise (Duman et al., 2008). Altogether, the evidence is strong that intermittent exercise is one challenge that can improve cognitive function and mood.
Dietary energy restriction (daily caloric restriction or intermittent fasting) can enhance performance of rats and mice in tests of learning and memory, and can counteract age-related molecular and cellular alterations that impair cognition (Figure 2). For example, when 3 month-old mice were maintained for 12 months on a reduced calorie diet, they performed significantly better in the radial arm maze test compared to control mice fed ad libitum; the energy-restricted mice exhibited less accumulation of lipofuscin (a marker of cellular aging) in neurons in the frontal cortex and hippocampus (Idrobo et al., 1987). When senescence-accelerated (SAMP8) mice are maintained for 7 months on a reduced energy diet, they perform better on a passive avoidance test of learning and memory compared to SAMP8 mice fed ad libitum (Komatsu et al., 2008). Compared to mice fed ad libitum, young mice maintained on an alternate day fasting diet for 11 months perform significantly better in the Barnes maze and fear conditioning tests of learning and memory, and this improved cognitive function is associated with increased size of the CA1 pyramidal neuron region of the hippocampus (Li et al., 2013). Other studies have shown that alternate day fasting enhances hippocampal neurogenesis in mice by increasing the survival of newly-generated neurons (Lee et al., 2002). Importantly, exercise and energy restriction can have additive effects on neuroplasticity. During a 3 month period, both running wheel exercise and daily energy restriction results in increased dendritic spine density (an indicator of synapse numbers) in hippocampal dentate granule neurons, and mice that run and are calorie restricted exhibit a dendritic spine density greater than mice that only run or are only energy restricted (Stranahan et al., 2009). The latter study further showed that dendritic spine density is significantly reduced in diabetic mice, and that both running and caloric restriction increase spine density in the diabetic mice. From the evolutionary perspective of animals in the wild, these findings are consistent with the notion that the challenges of food deprivation and running result in structural and functional improvements of neuronal circuits in the brain that provide a survival advantage for the animal.
Figure 2.
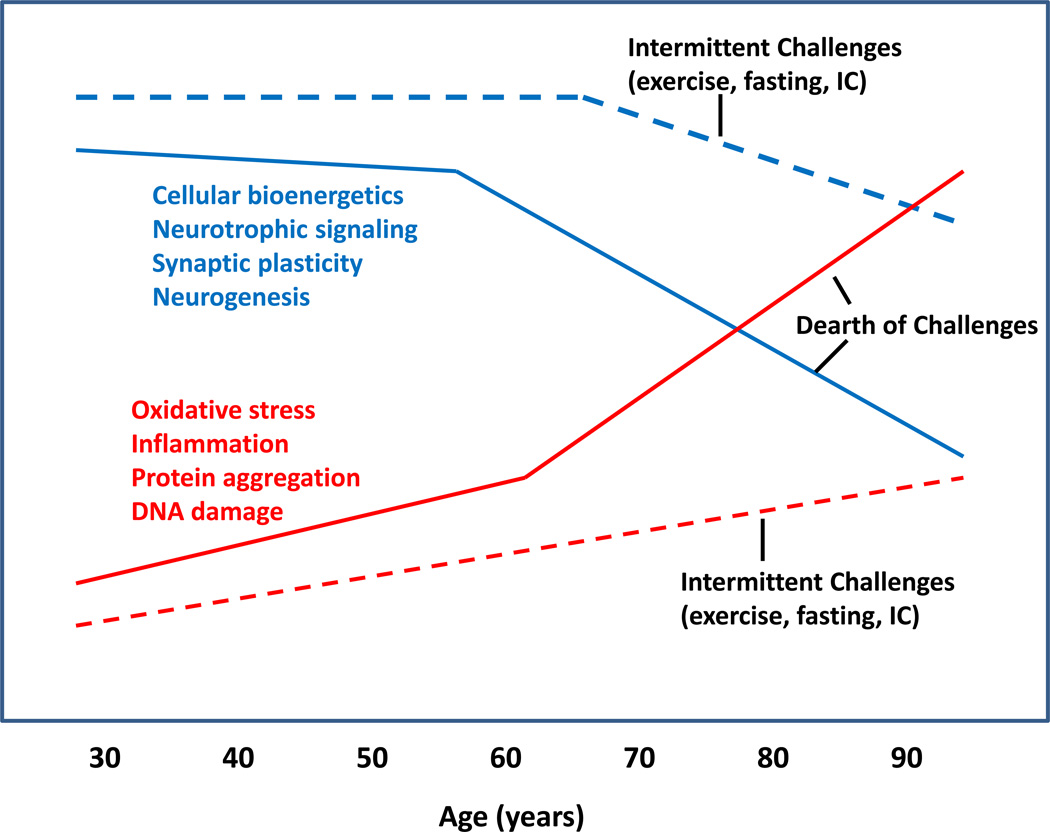
Model for the impact of aging and lifelong intermittent challenges on the trajectories of processes promoting and protecting against age-related neuronal dysfunction and cognitive impairment. During aging the ability of neurons to efficiently acquire energy substrates (e.g., glucose and ketones) and process them to produce ATP (mitochondrial function) is impaired. Neurotrophic support, synaptic plasticity and neurogenesis (the production of new neurons from stem cells) also decline during aging. During aging brain cells experience increased levels of oxidative stress, inflammation, protein aggregation and DNA damage. Intermittent exercise, energy restriction and intellectual challenges (IC) during adult life forestall age-related dysfunction of and damage to neurons (dashed lines).
Cognitive capabilities are best improved by cognitive challenges. Neuronal circuits benefit most when they are directly ‘exercised’ by engaging in intellectual challenges. In an attempt to simulate a more natural environment filled with intellectual challenges, investigators have implemented ‘enriched environments’ (EEs) which typically consist of relatively large cages of at least 1 square meter that include numerous climbing structures, burrows, running wheels, etc. The enriched environment is usually occupied by more animals (e.g., 8 – 12) than in the usual cages. While the specific features of EEs vary considerably among studies (e.g., differences in cage size, number and types of structures present, number of animals/cage, etc.), the overall results of studies of the effects of EEs on the brain are in general agreement. Animals maintained in EEs exhibit reduced anxiety and depression-like behaviors, and improved performance in tests of learning and memory compared to animals maintained in the usual housing condition (see Simpson and Kelly, 2011 for review). EEs can ameliorate age-related decrements in spatial memory in mice (Frick et al., 2003), and can stimulate a robust increase in hippocampal neurogenesis in old mice (Kempermann et al., 2002). While EEs promote neuroplasticity and improve cognitive function, the relative contributions of the different aspects of complex EEs (exploration, exercise and social interactions) to the changes in the brain are unclear. Indeed, some evidence suggests that it is the exercise component of EEs that enhances hippocampal neurogenesis and maze learning (Kobilo et al., 2011; Mustroph et al., 2012).
Signaling pathways and molecular mechanisms
There is considerable overlap in cellular and molecular mechanisms by which the challenges of exercise, energy restriction and intellectual enrichment enhance neuroplasticity and sustain cognitive performance during aging (Figure 3). Three general mechanisms are: 1) increased synaptic activity resulting in the production and release of neurotrophic factors which then activate signaling pathways that stimulate the formation and plasticity of synapses, and neurogenesis; 2) activation of adaptive cellular stress responses that result in mobilization of defenses against oxidative and metabolic stress, and inflammation; and 3) peripheral signals, such as ketone bodies and muscle-derived factors that help support the increased metabolic demands of active neuronal networks. The roles of neurotrophic factors, particularly brain-derived neurotrophic factor (BDNF), in the beneficial effects of exercise, energy restriction and EE have been heavily studied, and the reader is referred to recent review articles for in-depth information on this topic (Bekinschtein et al., 2011; Mattson, 2012; Vivar et al., 2013; Marosi and Mattson, 2014). In addition, emerging findings described below suggest that adaptive stress response pathways in brain cells and signals from the periphery also contribute to the enhancement of cognitive function by exercise, energy restriction and EE, and its resilience during aging.
Figure 3.
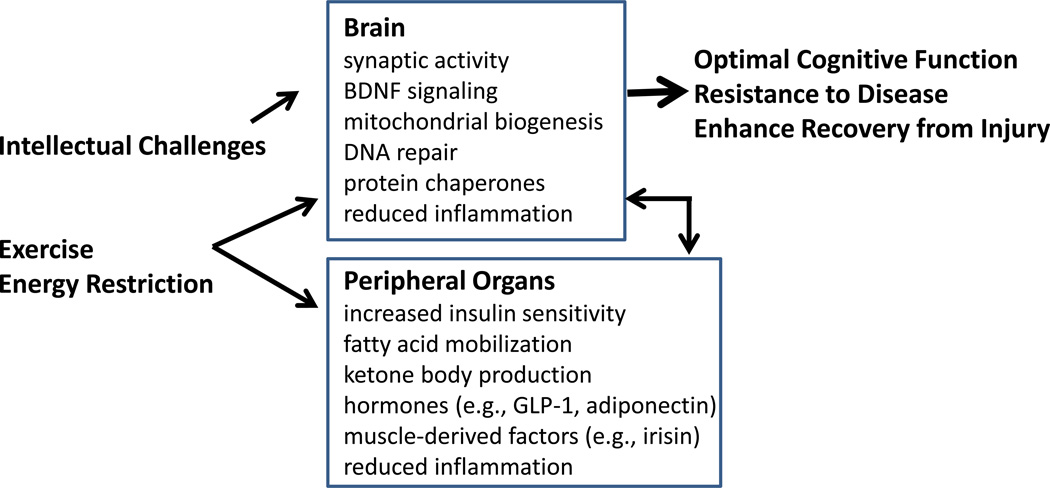
Intermittent intellectual challenges, exercise and energy restriction support optimal brain function and resistance to disease via of effects in the brain and peripheral organs. Intellectual challenges primarily affect the brain directly by increasing synaptic activity resulting in the production of neurotrophic factors such as BDNF, and by stimulating mitochondrial biogenesis, DNA repair and the production of cytoprotective protein chaperones. Cognitive stimulation may also promote enhance peripheral organ health by, for example, BDNF-mediated activation of the parasympathetic nervous system and increased insulin sensitivity of muscle and liver cells (Wan et al., 2014). Exercise and intermittent energy restriction/fasting elicit changes in brain signaling and gene expression that overlap considerably with those that occur in response to intellectual challenges. In addition, exercise and energy restriction reduce inflammation (microglial activation and pro-inflammatory cytokine production) in the brain. Energy restriction and exercise have profound effects on peripheral organs including a metabolic shift that mobilizes fatty acids (in adipose cells) which are then converted to ketone bodies (e.g., β-hydroxybutyrate) in the liver. Ketones enter the brain, are used as an energy source by neurons, and are neuroprotective. Fasting and exercise also increase insulin sensitivity and reduce inflammation in peripheral tissues. Some hormones produced in response to energy restriction and exercise are known to promote neuronal plasticity and survival, including glucagon-like peptide 1 (GLP-1) and adiponectin (Li et al., 2009, 2010; Qui et al., 2011). Moreover, recent findings suggest that muscle-derived factors can enter the brain and contribute to enhanced neuroplasticity and stress resistance. Altogether, the effects of intermittent challenges in the brain and periphery support optimal brain function, resistance to disease and improved recovery from injury.
Mature (active) BDNF is produced as part of a precursor protein called pro-BDNF. Pro-BDNF can be cleaved by a furin protease to generate mature BDNF intracellularly which is then secreted, or pro-BDNF can be secreted and cleaved extracellularly by plasmin (see Deinhardt and Chao, 2014 for review). BDNF binds to the high-affinity receptor tyrosine kinase TrkB which initiates intracellular signaling cascades involving several major kinases including phosphatidylinositol 3 kinase (PI3K), Akt and extracellular signal regulated kinases (ERKs). Evidence suggests that BDNF is released from neurons at or near synapses in response to synaptic activity. Studies in which BDNF signaling was manipulated genetically or pharmacologically revealed its important roles in synapse formation and plasticity, and learning and memory (for review see Marosi and Mattson, 2014). Moreover, BDNF plays important roles in neurogenesis by promoting the differentiation, growth and survival of newly-generated hippocampal neurons. Exercise, EEs and dietary energy restriction have all been shown to increase BDNF production and signaling in the hippocampus and other brain regions (Lee et al., 2002). Increased neuronal network activity and mild metabolic stress may mediate increased BDNF signaling in response to all three challenges. Indeed, even short periods of exercise improve spatial learning by a mechanism involving BDNF and the transcription factor CREB (cyclic AMP response element-binding protein) (Aguiar et al., 2011). Considerable evidence also suggests that the antidepressant effects of exercise are mediated by BDNF signaling (Sartori et al., 2011). In addition to more direct effects of exercise and energy restriction on the brain, recent findings suggest that signals from the periphery, such as the muscle-derived protein irisin can induce BDNF production in the brain (Wrann et al., 2013). Whether BDNF plays similar roles in humans remains to be established. However, consistent with translation to humans, aerobic exercise in human subjects improves cognitive function and also increases levels of circulating BDNF (Griffin et al., 2011).
The mechanisms by which BDNF promotes synapse formation and plasticity are being elucidated. Activation of TrkB results in a signaling cascade that affects the polymerization state of actin filaments which mediates growth of dendrites towards the BDNF source (Song et al., 1997). The acute effects of BDNF on growth cones involves polymerization of actin filaments by a mechanism involving Rho kinase and a protein called actin depolymerizing factor (Gehler et al., 2004). BDNF signaling stimulates protein translation in dendrites, which is believed critical for LTP (Lu et al., 2008). Direct application of BDNF to the hippocampus in vivo results in increased levels of the postsynaptic density protein 95 (PSD95), the glutamate receptor subunit GluR2, and the monocarboxylic acid transporter 2 (MCT2) (Robinet and Pellerin, 2011). PSD95 and GluR2 are known to play important roles in synapse formation and plasticity, while MCT2 may enhance local cellular bioenergetics at synapses. BDNF also up-regulates expression of the N-methyl-D-aspartate glutamate receptor subunits NR1 and NR2A, resulting in enhanced calcium influx upon synapse activation, which likely contributes to enhanced LTP (Glazner and Mattson, 2000). In addition, BDNF promotes the differentiation of neural stem cells into neurons, and the survival, growth and synapse formation of the newly generated neurons (Marosi and Mattson, 2014). Such effects of BDNF signaling likely play important roles in the processes by which exercise and intermittent fasting enhance cognitive function (Mattson, 2012; Noble et al., 2014). Interestingly, recent findings suggest that BDNF signaling in the brainstem enhances activation of cardiovagal (cholinergic) neurons resulting in a reduction in resting heart rate (Wan et al., 2014). The latter mechanism likely mediates the beneficial effects of endurance exercise and intermittent fasting on cardiovascular function and stress resistance (Wan et al., 2003, 2004; Mager et al., 2006).
BDNF promotes the survival of neurons under conditions of oxidative and metabolic stress. This was established in cell culture and animal models where treatment with BDNF protected neurons against excitotoxicity, energy deprivation and oxidative stress (Cheng and Mattson, 1994; Frim et al., 1994; Mattson et al., 1995). Intermittent fasting protects hippocampal neurons against seizure-induced excitotoxicity (Bruce-Keller et al., 1999), and this excitoprotective effect of intermittent fasting is mediated, at least in part, by BDNF signaling (Duan et al., 2001). Two additional mechanisms by which BDNF supports the survival and optimal function of neurons during aging are by bolstering cellular bioenergetics and enhancing repair of damaged DNA. BDNF signaling enhances glucose transport, amino acid transport and protein synthesis in cultured cerebral cortical neurons (Burkhalter et al., 2003). BDNF stimulates mitochondrial biogenesis in hippocampal neurons by a mechanism involving induction of expression of PGC-1α (Cheng et al., 2012). The latter study showed that the ability of BDNF to promote synapse formation and maintenance is critically dependent upon PGC-1α. In addition to BDNF signaling, mitochondrial biogenesis and the improvement of neuronal bioenergetics in response to exercise may involve AMP-activated protein kinase (AMPK) (Gomez-Pinilla et al., 2008).
Unrepaired damage to DNA accumulates in most cells, including those in the brain, during normal aging (Lu et al., 2004), and impaired DNA repair in brain cells occurs in mild cognitive impairment and Alzheimer’s disease (Weissman et al., 2007). As a result of activation of excitatory glutamate receptors, neurons experience intermittent increases in oxidative DNA damage which is normally repaired rapidly (Yang et al., 2010). Glutamate receptor activation results in calcium influx and activation of a calcium/calmodulin-dependent protein kinase and CREB which, in turn, induces the expression of the DNA repair enzyme APE1 (Yang et al., 2010). It was recently reported that BDNF and exercise also up-regulate APE1, suggesting that exercise can enhance DNA repair in neurons (Yang et al., 2014). Although yet to be determined, it is likely that other challenges that stimulate BDNF signaling, such as intermittent fasting and intellectual endeavors, also enhance neuronal DNA repair.
Exercise, energy restriction and EEs may also forestall brain aging by suppressing neuro-inflammation. As in other tissues, the brain often experiences local inflammation during aging as indicated by microglial cell activation and elevated levels of pro-inflammatory cytokines such as tumor necrosis factor (TNF) and interleukin-1β (IL-1β). While some aspects of immune cell activation in the brain are undoubtedly adaptive (Schwartz et al., 2013), sustained dysregulated activation of innate immune pathways is detrimental to the function and integrity of neurons (Norden and Godbout, 2013). Exercise was reported to inhibit microglial cell proliferation in the hippocampus of mice (Kohman et al., 2012). In a model relevant to cognitive impairment precipitated by infection in the elderly, voluntary exercise in old rats ameliorated infection-induced neuroinflammation and associated memory retention (Barrientos et al., 2011). Similarly, EEs improve learning and memory performance and lessen hippocampal inflammation in a mouse model of influenza infection (Jurgens and Johnson, 2012). Alternate day fasting reduces levels of pro-inflammatory cytokines (TNF and IL-1β) in the cerebral cortex and striatum in a mouse model of focal ischemic stroke (Arumugam et al., 2010). Future studies will be required to determine whether intermittent fasting, exercise and/or intellectual challenges also improve functional outcome in human patients affected by a chronic or acute neuroinflammatory state.
Recent findings suggest that several peripheral signals produced and released into the blood by peripheral organs play important roles in the beneficial effects of fasting and/or exercise on the brain. One prominent example is ketones, particularly β-hydroxybutyrate, which are generated in the liver from fatty acids released from adipose cells when liver glycogen stores are depleted, as occurs during fasting and extended periods of vigorous exercise. Ketones can be utilized as an energy source for neurons, and may also affect certain signaling pathways that enhance neuroplasticity (Maalouf et al., 2009). For example, β-hydroxybutyrate can inhibit certain protein deacetylases and thereby regulates gene expression (Newman and Verdin, 2014). By these, and perhaps yet to be discovered mechanisms, ketones can protect neurons against dysfunction and degeneration in animal models of epileptic seizures, Alzheimer’s disease and Parkinson’s disease (Tieu et al., 2003; McNally and Hartman, 2012; Kashiwaya et al., 2013).
Intermittent challenges may protect against neurodegenerative disorders
Epidemiological data suggest that individuals who exercise regularly during their adult life are at reduced risk of Alzheimer’s and Parkinson’s diseases. Population-based studies suggest that individuals who exercise in midlife are less likely to develop dementia later in life (Geda et al., 2010). Consistent with the possibility that regular exercise protects against Alzheimer’s disease are data showing that midlife cardiorespiratory fitness is associated with a lower risk of dementia (Defina et al., 2013). In a study of over 200,000 participants, individuals who reported high levels of physical activity from ages 15 – 39 years were less likely to have been diagnosed with Parkinson’s disease later in life (Xu et al., 2010). Intellectually challenging occupations and a socially engaged lifestyle may help forestall Alzheimer’s disease (Scarmeas and Stern, 2003; Sharp and Gatz, 2011). While there have been no studies to elucidate whether intermittent energy restriction protects against Alzheimer’s and/or Parkinson’s disease, there is accumulating evidence that overeating is a risk factor for both of these age-related neurodegenerative disorders. Several studies have reported that being overweight in midlife increases the risk of Alzheimer’s disease (Xu et al., 2011; Tolppanen et al., 2014). Altogether, the findings from studies of human populations are consistent with the notion that intermittent exercise, energy restriction and intellectual challenges throughout life can forestall age-related decrements in brain function and neurodegenerative disorders.
Studies of laboratory animals have shown that exercise can counteract disease processes in experimental models of Alzheimer’s disease, Parkinson’s disease and stroke (Duan and Mattson, 1999; Adlard et al., 2005; Gertz et al., 2006; Halagappa et al., 2007; Arumugam et al., 2010; Fredriksson et al., 2011). In a mouse model of Alzheimer’s disease, 6 months of running wheel exercise ameliorated synaptic dysfunction by a mechanism involving normalization of levels of glutamate and GABA receptors, and SIRT1, a histone deacetylase that has been shown to counteract cellular aging processes (Revilla et al., 2014). In a different mouse model relevant to Alzheimer’s disease and frontotemporal dementia, mice with running wheels in their cages exhibited less memory impairment and intracellular Tau accumulation, and maintained normal amounts of choline acetyltransferase in cholinergic septal neurons, compared to more sedentary mice (Belarbi et al., 2011). Hippocampal neurogenesis is impaired in mouse models of Alzheimer’s disease (Haughey et al., 2002), and running and EEs can restore neurogenesis in the mice (Rodriguez et al., 2011). Mice expressing a form of human apolipoprotein E4 that increases the risk of Alzheimer’s disease in humans exhibit impaired cognitive function, and running ameliorates the cognitive deficit (Nichol et al., 2009). Mice exercised on a treadmill 40 minutes/day, 5 days/week before, during and after low dose chronic treatment with a toxin (MPTP) that causes degeneration of dopaminergic neurons, exhibited reduced degeneration of dopaminergic neurons and improved motor function compared to MPTP-treated sedentary mice (Lau et al., 2011). One month of treadmill exercise also improved motor performance and enhanced dopaminergic neurotransmission when initiated 5 days after administration of a high dose of MPTP to mice (Petzinger et al., 2007). The mechanisms by which exercise counteracts the pathological processes of Alzheimer’s and Parkinson’s diseases may include up-regulation of neurotrophic factors and enhanced DNA repair (Coelho et al., 2014; Yang et al., 2014).
Intermittent energy restriction (alternate day fasting) has been reported to improve functional outcome and retard molecular and cellular disease processes in animal models of Alzheimer’s, Parkinson’s and Huntington’s diseases, and stroke. When rats were maintained for several months on the intermittent fasting diet and then administered the seizure-inducing excitotoxin kainic acid, their hippocampal neurons were resistant to degeneration and their learning and memory was preserved (Bruce-Keller et al., 1999). In a mouse model of Alzheimer’s disease, 1 year of intermittent fasting prevented cognitive deficits (Halagappa et al., 2007). Compared to mice fed ad libitum, dopaminergic neurons were preserved and motor function improved in mice maintained on an alternate day fasting diet prior to administration of MPTP (Duan and Mattson, 1999). In two different models of Huntington’s disease, one involving a neurotoxin (3-nitropropionic acid) that selectively damages striatal neurons and the other a genetic model (transgenic huntingtin mutant mice), intermittent fasting attenuated striatal neuron degeneration and lessened motor deficits (Bruce-Keller et al., 1999; Duan et al., 2003). Moreover, rats and mice maintained on an alternate day fasting diet prior to an experimental stroke exhibited reduced brain damage and improved neurological function compared to animals fed ad libitum (Yu and Mattson, 1999; Arumugam et al., 2010). Several mechanisms by which intermittent energy restriction protects brains cells have been proposed including activation of signaling pathways that increase the production of trophic factors (e.g., BDNF and FGF2), protein chaperones (e.g., HSP70 and GRP78) and antioxidant enzymes such as heme oxygenase 1 (Yu and Mattson, 1999; Guo et al., 2000; Arumugam et al., 2010). In addition, intermittent fasting suppresses neuro-inflammation (Figure 3). For example, when mice were deprived of food for 16 hours daily for 4 months and then subjected to experimental stroke, there was less activation of the so-called inflammasome and reduced levels of pro-inflammatory cytokines in brain cells in the ischemic cortex (Fann et al., 2014). Also, in a rat model of systemic bacterial infection alternate day fasting suppressed inflammation in the brain and lessened memory deficits (Vasconcelos et al., 2014).
Environmental enrichment has been reported to reduce amyloid accumulation in a mouse model of Alzheimer’s disease by a mechanism involving increased activity of the amyloid-degrading enzyme neprilysin (Lazarov et al., 2005). Others have shown that EEs improve cognitive function in a mouse model of Alzheimer’s disease (Jankowsky et al., 2005). Chronic uncontrollable stress can increase the risk of anxiety and depression, and possibly Alzheimer’s disease. EE prevents reductions in hippocampal neuron dendrite length and mitigates learning and memory deficits in rats (Hutchison et al., 2012). EE was also reported to ameliorate cognitive deficits in huntingtin mutant mice, an animal model of Huntington’s disease (Nithianantharajah et al., 2008). These findings suggest the intellectual challenges can counteract disease processes involved in several neurodegenerative disorders.
Finally, the results of studies of animal models suggest that individuals who challenge their brains intermittently with exercise, fasting or intellectual enrichment are more likely to have better outcomes from acute traumatic events. In a rat model of traumatic brain injury, rats that ran on running wheels after the brain injury recovered cognitive function compared to sedentary control rats, and the ability of exercise to improve the functional outcome was attenuated when BDNF signaling was blocked (Griesbach et al., 2009). Dietary energy restriction resulted in reduced brain damage and preservation of cognitive function a rat model of traumatic brain injury (Rich et al., 2010). When initiated after a surgical lesion to the fimbria-fornix (cholinergic neurons), EE lessened deficits in spatial learning (van Rijzingen et al., 1997). In a rat model of brain damage resulting from cardiac arrest, food restriction resulted in recovery of learning and memory deficits compared to rats fed ad libitum (Roberge et al., 2008). Compared to standard housing conditions, EE initiated after transient global cerebral ischemia also improves cognitive function in object recognition and water maze tests in rats (Gobbo et al., 2004). Recovery from spinal cord injuries can also be enhanced by exercise and intermittent fasting. For example, when initiated one day after a moderate spinal cord contusion, one hour of daily exercise in a transparent ball results in improved recovery of sensory function and reduced delayed damage to the spinal cord caudal to the lesion (Brown et al., 2011). Rats maintained on an alternate day fasting diet after an injury to the cervical spinal cord exhibit improved motor function and coordination, which is associated with reduced damage to the spinal cord and sprouting of axons (Plunet et al., 2008). Moreover, when initiated either prior to or soon after a contusion injury to the thoracic spinal cord, alternate day fasting improves recovery of ambulatory function in rats compared to rats fed ad libitum (Jeong et al., 2011). Collectively, these preclinical findings support the value of intermittent challenges in protecting the central nervous system against acute traumatic and ischemic injuries, and suggest that controlled clinical trials should be performed to establish the efficacy of intermittent fasting, exercise and enrichment alone and in combination in human patients with brain or spinal cord injuries.
It should be noted that while some of the mechanisms by which energy restriction and exercise can enhance brain health are the same or similar, there are likely to be qualitative or quantitative differences. One example comes from studies of hippocampal neurogenesis. Running wheel exercise can greatly increase the proliferation of neural stem cells as well as the survival of newly generated neurons (Voss et al., 2013), whereas intermittent fasting does not affect stem cell proliferation, but does increase the survival of newly generated neurons (Lee et al., 2002). With regards to neuroprotection, intermittent fasting appears to provide more robust beneficial effects compared to exercise in animal models of stroke (Marin et al., 2003; Arumugam et al., 2010), severe epileptic seizures (Bruce-Keller et al., 1999; Reiss et al., 2009) and Huntington’s disease (Duan et al., 2003; Potter et al., 2010). Comparisons of the effects of energy restriction and running wheel exercise on gene expression in various brain regions support the notion that these two different environmental factors have both overlapping and distinct effects on brain cell plasticity (Molteni et al., 2002; Xu et al., 2007; Stranahan et al., 2010). However, studies aimed at defining these differences and their roles in physiological responses to exercise and energy restriction are lacking.
Implications for a brain-healthy society
Advances in agriculture, food processing and transportation have contributed to a decline in the exposure of individuals in modern societies to the two major energetic challenges that sustain optimal brain health, namely, intermittent food deprivation/fasting and vigorous daily exercise. While many continue to engage in intellectual challenges that can enhance cognitive function, the available evidence suggests that such intellectual challenges are insufficient to mitigate age-related vulnerability to neurodegenerative disorders. It is therefore critical to establish in well-designed randomized controlled trials in human subjects ‘prescriptions’ for intermittent energy restriction and exercise that increase the likelihood of maintaining a high level of brain function and avoiding the major catastrophic brain disorders of stroke, Alzheimer’s and Parkinson’s diseases (Mattson, 2012; Mattson et al., 2014). Unfortunately, this is much easier said than done, for several reasons. First, the current eating pattern of three meals plus snacks each day has become engrained in our culture for many generations. Second, physical exertion is no longer required for most occupations, and door-to-door transportation is the norm, thereby making daily exercise an elective activity. Third, a high daily calorie intake is promoted by the processed food industry, resulting in a scenario in which many families consume large amounts of simple sugars and saturated fats on a daily basis (Stanhope, 2012; Montiero et al., 2013). Fourth, there is little focus by medical professionals on diet and lifestyle, in large part because of the emphasis on treating diseases with drugs and surgery. Fifth, the pharmaceutical industry is focused on treating diseases rather than preventing them, and so has no interest in effective preventative approaches such as dietary restriction and exercise. Finally, rigorous education of children on the importance of intermittent challenges for the brain (and general health) by schools and parents is largely lacking.
Clearly, it will be a Herculean task to surmount the aforementioned major barriers to implementing brain-healthy diets and lifestyles. Governments should take rigorous actions in the realms of education, medical training and practice, control of industries that promote unhealthy habits, and incentives for businesses to encourage brain-healthy practices in the workplace. Some countries have already recognized and taken substantive action upon some or all of these health-promoting functions of government, with Denmark and France being exemplary (Lachat et al., 2005). Medical training should emphasize disease prevention. Medical school curricula are focused almost exclusively on disease treatment rather than prevention. This certainly helps those who already suffer from a disease, but does nothing to reduce the disease risk for those who are as yet disease-free. The lack of emphasis on primary prevention in medical training and practice increases the burden of age-related diseases. Unfortunately, despite the overwhelming evidence that many major age-related diseases can be prevented or significantly delayed by lifestyles that include regular exercise and dietary energy restriction, there has been little meaningful effort to implement intermittent challenge-based prescriptions. The question then becomes: Who will initiate such major changes in our societies? Apparently, the impetus must come from the general public, through ‘grass roots’ efforts. Community efforts can be effective, as demonstrated by the construction of bicycle paths and other approaches to promoting daily exercise in some localities (Fenton, 2012). Although the evidence that intermittent fasting eating patterns improve health indicators and can forestall age-related diseases is strong (Longo and Mattson, 2014), it remains to be determined whether a change to such healthy eating patterns will become a reality.
Highlights.
Exercise, fasting and intellectual challenges improve brain function.
Energetic challenges stimulate synaptic plasticity and neurogenesis.
Intermittent challenges may protect the brain against injury and disease.
Society-wide changes will be required to implement brain-healthy lifestyles.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute on Aging, and the Glenn Foundation for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer's disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiar AS, Jr, Castro AA, Moreira EL, Glaser V, Santos AR, Tasca CI, Latini A, Prediger RD. Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech Ageing Dev. 2011;132:560–567. doi: 10.1016/j.mad.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol. 2010;67:41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HE, Campeau S, Watkins LR, Patterson SL, Maier SF. Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J Neurosci. 2011;31:11578–11586. doi: 10.1523/JNEUROSCI.2266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Oomen CA, Saksida LM, Bussey TJ. Effects of environmental enrichment and voluntary exercise on neurogenesis, learning and memory, and pattern separation: BDNF as a critical variable? Semin Cell Dev Biol. 2011;22:536–542. doi: 10.1016/j.semcdb.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Belarbi K, Burnouf S, Fernandez-Gomez FJ, Laurent C, Lestavel S, Figeac M, Sultan A, Troquier L, Leboucher A, Caillierez R, Grosjean ME, Demeyer D, Obriot H, Brion I, Barbot B, Galas MC, Staels B, Humez S, Sergeant N, Schraen-Maschke S, Muhr-Tailleux A, Hamdane M, Buée L, Blum D. Beneficial effects of exercise in a transgenic mouse model of Alzheimer's disease-like Tau pathology. Neurobiol Dis. 2011;43:486–494. doi: 10.1016/j.nbd.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Berbesque JC, Marlowe FW, Shaw P, Thompson P. Hunter-gatherers have less famine than agriculturalists. Biol Lett. 2014;10 doi: 10.1098/rsbl.2013.0853. 20130853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AK, Woller SA, Moreno G, Grau JW, Hook MA. Exercise therapy and recovery after SCI: evidence that shows early intervention improves recovery of function. Spinal Cord. 2011;49:623–628. doi: 10.1038/sc.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- Burkhalter J, Fiumelli H, Allaman I, Chatton JY, Martin JL. Brain-derived neurotrophic factor stimulates energy metabolism in developing cortical neurons. J Neurosci. 2003;23:8212–8220. doi: 10.1523/JNEUROSCI.23-23-08212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B, Mattson MP. NT-3 and BDNF protect CNS neurons against metabolic/excitotoxic insults. Brain Res. 1994;640:56–67. doi: 10.1016/0006-8993(94)91857-0. [DOI] [PubMed] [Google Scholar]
- Cheng A, Wan R, Yang JL, Kamimura N, Son TG, Ouyang X, Luo Y, Okun E, Mattson MP. Involvement of PGC-1α in the formation and maintenance of neuronal dendritic spines. Nat Commun. 2012;3:1250. doi: 10.1038/ncomms2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherel Y, Le Maho Y. Five months of fasting in king penguin chicks: body mass loss and fuel metabolism. Am J Physiol. 1985;249:R387–R392. doi: 10.1152/ajpregu.1985.249.4.R387. [DOI] [PubMed] [Google Scholar]
- Coelho FG, Vital TM, Stein AM, Arantes FJ, Rueda AV, Camarini R, Teodorov E, Santos-Galduróz RF. Acute aerobic exercise increases brain-derived neurotrophic factor levels in elderly with Alzheimer's disease. J Alzheimers Dis. 2014;39:401–408. doi: 10.3233/JAD-131073. [DOI] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci USA. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defina LF, Willis BL, Radford NB, Gao A, Leonard D, Haskell WL, Weiner MF, Berry JD. The association between midlife cardiorespiratory fitness levels and later-life dementia: a cohort study. Ann Intern Med. 2013;158:162–168. doi: 10.7326/0003-4819-158-3-201302050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinhardt K, Chao MV. Shaping neurons: Long and short range effects of mature and proBDNF signalling upon neuronal structure. Neuropharmacology. 2014;76(Pt C):603–609. doi: 10.1016/j.neuropharm.2013.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson's disease. J Neurosci Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Mattson MP. Brain-derived neurotrophic factor mediates an excitoprotective effect of dietary restriction in mice. J Neurochem. 2001;76:619–626. doi: 10.1046/j.1471-4159.2001.00071.x. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci U S A. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fann DY, Santro T, Manzanero S, Widiapradja A, Cheng YL, Lee SY, Chunduri P, Jo DG, Stranahan AM, Mattson MP, Arumugam TV. Intermittent fasting attenuates inflammasome activity in ischemic stroke. Exp Neurol. 2014;257C:114–119. doi: 10.1016/j.expneurol.2014.04.017. [DOI] [PubMed] [Google Scholar]
- Fenton M. Community design and policies for free-range children: creating environments that support routine physical activity. Child Obes. 2012;8:44–51. doi: 10.1089/chi.2011.0122. [DOI] [PubMed] [Google Scholar]
- Fredriksson A, Stigsdotter IM, Hurtig A, Ewalds-Kvist B, Archer T. Running wheel activity restores MPTP-induced functional deficits. J Neural Transm. 2011;118:407–420. doi: 10.1007/s00702-010-0474-8. [DOI] [PubMed] [Google Scholar]
- Frick KM, Stearns NA, Pan JY, Berger-Sweeney J. Effects of environmental enrichment on spatial memory and neurochemistry in middle-aged mice. Learn Mem. 2003;10:187–198. doi: 10.1101/lm.50703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frim DM, Uhler TA, Galpern WR, Beal MF, Breakefield XO, Isacson O. Implanted fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevent 1-methyl-4-phenylpyridinium toxicity to dopaminergic neurons in the rat. Proc Natl Acad Sci USA. 1994;91:5104–5108. doi: 10.1073/pnas.91.11.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geda YE, Roberts RO, Knopman DS, Christianson TJ, Pankratz VS, Ivnik RJ, Boeve BF, Tangalos EG, Petersen RC, Rocca WA. Physical exercise, aging, and mild cognitive impairment: a population-based study. Arch Neurol. 2010;67:80–86. doi: 10.1001/archneurol.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehler S, Shaw AE, Sarmiere PD, Bamburg JR, Letourneau PC. Brain-derived neurotrophic factor regulation of retinal growth cone filopodial dynamics is mediated through actin depolymerizing factor/cofilin. J Neurosci. 2004;24:10741–10749. doi: 10.1523/JNEUROSCI.2836-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz K, Priller J, Kronenberg G, Fink KB, Winter B, Schröck H, Ji S, Milosevic M, Harms C, Böhm M, Dirnagl U, Laufs U, Endres M. Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow. Circ Res. 2006;99:1132–1140. doi: 10.1161/01.RES.0000250175.14861.77. [DOI] [PubMed] [Google Scholar]
- Gervasi V, Nilsen EB, Sand H, Panzacchi M, Rauset GR, Pedersen HC, Kindberg J, Wabakken P, Zimmermann B, Odden J, Liberg O, Swenson JE, Linnell JD. Predicting the potential demographic impact of predators on their prey: a comparative analysis of two carnivore-ungulate systems in Scandinavia. J Anim Ecol. 2012;81:443–454. doi: 10.1111/j.1365-2656.2011.01928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazner GW, Mattson MP. Differential effects of BDNF, ADNF9, and TNFalpha on levels of NMDA receptor subunits, calcium homeostasis, and neuronal vulnerability to excitotoxicity. Exp Neurol. 2000;161:442–452. doi: 10.1006/exnr.1999.7242. [DOI] [PubMed] [Google Scholar]
- Gobbo OL, O'Mara SM. Impact of enriched-environment housing on brain-derived neurotrophic factor and on cognitive performance after a transient global ischemia. Behav Brain Res. 2004;152:231–241. doi: 10.1016/j.bbr.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci. 2008;28:2278–2287. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–115. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin ÉW, Mullally S, Foley C, Warmington SA, O'Mara SM, Kelly AM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. 2011;104:934–941. doi: 10.1016/j.physbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Guo Z, Ersoz A, Butterfield DA, Mattson MP. Beneficial effects of dietary restriction on cerebral cortical synaptic terminals: preservation of glucose and glutamate transport and mitochondrial function after exposure to amyloid beta-peptide, iron, and 3-nitropropionic acid. J Neurochem. 2000;75:314–320. doi: 10.1046/j.1471-4159.2000.0750314.x. [DOI] [PubMed] [Google Scholar]
- Haile M. Weather patterns, food security and humanitarian response in sub-Saharan Africa. Philos Trans R Soc Lond B Biol Sci. 2005;360:2169–2182. doi: 10.1098/rstb.2005.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, Mattson MP. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer's disease. Neurobiol Dis. 2007;26:212–120. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer's disease. J Neurochem. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- Hutchinson KM, McLaughlin KJ, Wright RL, Bryce Ortiz J, Anouti DP, Mika A, Diamond DM, Conrad CD. Environmental enrichment protects against the effects of chronic stress on cognitive and morphological measures of hippocampal integrity. Neurobiol Learn Mem. 2012;97:250–260. doi: 10.1016/j.nlm.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrobo F, Nandy K, Mostofsky DI, Blatt L, Nandy L. Dietary restriction: effects on radial maze learning and lipofuscin pigment deposition in the hippocampus and frontal cortex. Arch Gerontol Geriatr. 1987;6:355–362. doi: 10.1016/0167-4943(87)90014-8. [DOI] [PubMed] [Google Scholar]
- Intlekofer KA, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer's disease. Neurobiol Dis. 2013;57:47–55. doi: 10.1016/j.nbd.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Melnikova T, Fadale DJ, Xu GM, Slunt HH, Gonzales V, Younkin LH, Younkin SG, Borchelt DR, Savonenko AV. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer's disease. J Neurosci. 2005;25:5217–5224. doi: 10.1523/JNEUROSCI.5080-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong MA, Plunet W, Streijger F, Lee JH, Plemel JR, Park S, Lam CK, Liu J, Tetzlaff W. Intermittent fasting improves functional recovery after rat thoracic contusion spinal cord injury. J Neurotrauma. 2011;28:479–492. doi: 10.1089/neu.2010.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens HA, Johnson RW. Environmental enrichment attenuates hippocampal neuroinflammation and improves cognitive function during influenza infection. Brain Behav Immun. 2012;26:1006–1016. doi: 10.1016/j.bbi.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwaya Y, Bergman C, Lee JH, Wan R, King MT, Mughal MR, Okun E, Clarke K, Mattson MP, Veech RL. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer's disease. Neurobiol Aging. 2013;34:1530–1539. doi: 10.1016/j.neurobiolaging.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011;18:605–609. doi: 10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, DeYoung EK, Bhattacharya TK, Peterson LN, Rhodes JS. Wheel running attenuates microglia proliferation and increases expression of a proneurogenic phenotype in the hippocampus of aged mice. Brain Behav Immun. 2012;26:803–810. doi: 10.1016/j.bbi.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu T, Chiba T, Yamaza H, Yamashita K, Shimada A, Hoshiyama Y, Henmi T, Ohtani H, Higami Y, de Cabo R, Ingram DK, Shimokawa I. Manipulation of caloric content but not diet composition, attenuates the deficit in learning and memory of senescence-accelerated mouse strain P8. Exp Gerontol. 2008;43:339–346. doi: 10.1016/j.exger.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Lachat C, Van Camp J, De Henauw S, Matthys C, Larondelle Y, Remaut-De Winter AM, Kolsteren P. A concise overview of national nutrition action plans in the European Union Member States. Public Health Nutr. 2005;8:266–274. doi: 10.1079/phn2004691. [DOI] [PubMed] [Google Scholar]
- Lau YS, Patki G, Das-Panja K, Le WD, Ahmad SO. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson's disease with moderate neurodegeneration. Eur J Neurosci. 2011;33:1264–1274. doi: 10.1111/j.1460-9568.2011.07626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, Brossi A, Lahiri DK, Mattson MP, Hoffer BJ, Wang Y, Greig NH. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci USA. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Duffy KB, Ottinger MA, Ray B, Bailey JA, Holloway HW, Tweedie D, Perry T, Mattson MP, Kapogiannis D, Sambamurti K, Lahiri DK, Greig NH. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer's disease. J Alzheimers Dis. 2010;19:1205–1219. doi: 10.3233/JAD-2010-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang Z, Zuo Z. Chronic intermittent fasting improves cognitive functions and brain structures in mice. PLoS One. 2013;8(6):e66069. doi: 10.1371/journal.pone.0066069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89:312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59:293–315. doi: 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally MA, Hartman AL. Ketone bodies in epilepsy. J Neurochem. 2012;121:28–35. doi: 10.1111/j.1471-4159.2012.07670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager DE, Wan R, Brown M, Cheng A, Wareski P, Abernethy DR, Mattson MP. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J. 2006;20:631–637. doi: 10.1096/fj.05-5263com. [DOI] [PubMed] [Google Scholar]
- Marin R, Williams A, Hale S, Burge B, Mense M, Bauman R, Tortella F. The effect of voluntary exercise exposure on histological and neurobehavioral outcomes after ischemic brain injury in the rat. Physiol Behav. 2003;80:167–175. doi: 10.1016/j.physbeh.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Marlatt MW, Potter MC, Lucassen PJ, van Praag H. Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Dev Neurobiol. 2012;72:943–952. doi: 10.1002/dneu.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25:89–98. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Pearson M, Kebejian L, Golden E, Keselman A, Bender M, Carlson O, Egan J, Ladenheim B, Cadet JL, Becker KG, Wood W, Duffy K, Vinayakumar P, Maudsley S, Mattson MP. Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology. 2007;148:4318–4333. doi: 10.1210/en.2007-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Ji S, Maudsley S, Mattson MP. "Control" laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci USA. 2010;107:6127–6133. doi: 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Lovell MA, Furukawa K, Markesbery WR. Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca2+ concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J Neurochem. 1995;65:1740–1751. doi: 10.1046/j.1471-4159.1995.65041740.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16:706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse WJ, Mosley M, Notterpek L, Ravussin E, Scheer FA, Seyfried TN, Varady KA, Panda S. Meal frequency and timing in health and disease. Proc Natl Acad Sci U S A. 2014;111:16647–16653. doi: 10.1073/pnas.1413965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Ying Z, Gómez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16:1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gómez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–440. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Monteiro CA, Moubarac JC, Cannon G, Ng SW, Popkin B. Ultra-processed products are becoming dominant in the global food system. Obes Rev. 2013;2(14 Suppl):21–28. doi: 10.1111/obr.12107. [DOI] [PubMed] [Google Scholar]
- Mustroph ML, Chen S, Desai SC, Cay EB, DeYoung EK, Rhodes JS. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience. 2012;219:62–71. doi: 10.1016/j.neuroscience.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab. 2014;25:42–52. doi: 10.1016/j.tem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5:287–294. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithianantharajah J, Barkus C, Murphy M, Hannan AJ. Gene-environment interactions modulating cognitive function and molecular correlates of synaptic plasticity in Huntington's disease transgenic mice. Neurobiol Dis. 2008;29:490–504. doi: 10.1016/j.nbd.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Noble EE, Mavanji V, Little MR, Billington CJ, Kotz CM, Wang C. Exercise reduces diet-induced cognitive decline and increases hippocampal brain-derived neurotrophic factor in CA3 neurons. Neurobiol Learn Mem. 2014;114C:40–50. doi: 10.1016/j.nlm.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Godbout JP. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol. 2013;39:19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, Turnquist P, Vucković M, Fisher BE, Togasaki DM, Jakowec MW. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci. 2007;27:5291–5300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plunet WT, Streijger F, Lam CK, Lee JH, Liu J, Tetzlaff W. Dietary restriction started after spinal cord injury improves functional recovery. Exp Neurol. 2008;213:28–35. doi: 10.1016/j.expneurol.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Potter MC, Yuan C, Ottenritter C, Mughal M, van Praag H. Exercise is not beneficial and may accelerate symptom onset in a mouse model of Huntington's disease. PLoS Curr. 2010;2 doi: 10.1371/currents.RRN1201. RRN1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu G, Wan R, Hu J, Mattson MP, Spangler E, Liu S, Yau SY, Lee TM, Gleichmann M, Ingram DK, So KF, Zou S. Adiponectin protects rat hippocampal neurons against excitotoxicity. Age (Dordr) 2011;33:155–165. doi: 10.1007/s11357-010-9173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichlen DA, Gordon AD. Relationship between exercise capacity and brain size in mammals. PLoS One. 2011;6(6):e20601. doi: 10.1371/journal.pone.0020601. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath K, Zhou GD, Hart RW, Turturro A, Randerath E. Biomarkers of aging: correlation of DNA I-compound levels with median lifespan of calorically restricted and ad libitum fed rats and mice. Mutat Res. 1993;295:247–263. doi: 10.1016/0921-8734(93)90024-w. [DOI] [PubMed] [Google Scholar]
- Redolat R, Mesa-Gresa P. Potential benefits and limitations of enriched environments and cognitive activity on age-related behavioural decline. Curr Top Behav Neurosci. 2012;10:293–316. doi: 10.1007/7854_2011_134. [DOI] [PubMed] [Google Scholar]
- Reiss JI, Dishman RK, Boyd HE, Robinson JK, Holmes PV. Chronic activity wheel running reduces the severity of kainic acid-induced seizures in the rat: possible role of galanin. Brain Res. 2009;1266:54–63. doi: 10.1016/j.brainres.2009.02.030. [DOI] [PubMed] [Google Scholar]
- Revilla S, Suñol C, García-Mesa Y, Giménez-Llort L, Sanfeliu C, Cristòfol R. Physical exercise improves synaptic dysfunction and recovers the loss of survival factors in 3xTg-AD mouse brain. Neuropharmacology. 2014;81:55–63. doi: 10.1016/j.neuropharm.2014.01.037. [DOI] [PubMed] [Google Scholar]
- Rich NJ, Van Landingham JW, Figueiroa S, Seth R, Corniola RS, Levenson CW. Chronic caloric restriction reduces tissue damage and improves spatial memory in a rat model of traumatic brain injury. J Neurosci Res. 2010;88:2933–2939. doi: 10.1002/jnr.22443. [DOI] [PubMed] [Google Scholar]
- Roberge MC, Messier C, Staines WA, Plamondon H. Food restriction induces long-lasting recovery of spatial memory deficits following global ischemia in delayed matching and non-matching-to-sample radial arm maze tasks. Neuroscience. 2008;156:11–29. doi: 10.1016/j.neuroscience.2008.05.062. [DOI] [PubMed] [Google Scholar]
- Robinet C, Pellerin L. Brain-derived neurotrophic factor enhances the hippocampal expression of key postsynaptic proteins in vivo including the monocarboxylate transporter MCT2. Neuroscience. 2011;192:155–163. doi: 10.1016/j.neuroscience.2011.06.059. [DOI] [PubMed] [Google Scholar]
- Rodríguez JJ, Noristani HN, Olabarria M, Fletcher J, Somerville TD, Yeh CY, Verkhratsky A. Voluntary running and environmental enrichment restores impaired hippocampal neurogenesis in a triple transgenic mouse model of Alzheimer's disease. Curr Alzheimer Res. 2011;8:707–717. doi: 10.2174/156720511797633214. [DOI] [PubMed] [Google Scholar]
- Sartori CR, Vieira AS, Ferrari EM, Langone F, Tongiorgi E, Parada CA. The antidepressive effect of the physical exercise correlates with increased levels of mature BDNF, and proBDNF proteolytic cleavage-related genes, p11 and tPA. Neuroscience. 2011;180:9–18. doi: 10.1016/j.neuroscience.2011.02.055. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Stern Y. Cognitive reserve and lifestyle. J Clin Exp Neuropsychol. 2003;25:625–633. doi: 10.1076/jcen.25.5.625.14576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Kipnis J, Rivest S, Prat A. How do immune cells support and shape the brain in health, disease, and aging? J Neurosci. 2013;33:17587–17596. doi: 10.1523/JNEUROSCI.3241-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp ES, Gatz M. Relationship between education and dementia: an updated systematic review. Alzheimer Dis Assoc Disord. 2011;25:289–304. doi: 10.1097/WAD.0b013e318211c83c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa I, Higami Y, Tsuchiya T, Otani H, Komatsu T, Chiba T, Yamaza H. Life span extension by reduction of the growth hormone-insulin-like growth factor-1 axis: relation to caloric restriction. FASEB J. 2003;17:1108–1109. doi: 10.1096/fj.02-0819fje. [DOI] [PubMed] [Google Scholar]
- Simpson J, Kelly JP. The impact of environmental enrichment in laboratory rats--behavioural and neurochemical aspects. Behav Brain Res. 2011;222:246–264. doi: 10.1016/j.bbr.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- Speisman RB, Kumar A, Rani A, Foster TC, Ormerod BK. Daily exercise improves memory, stimulates hippocampal neurogenesis and modulates immune and neuroimmune cytokines in aging rats. Brain Behav Immun. 2013;28:25–43. doi: 10.1016/j.bbi.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhope KL. Role of fructose-containing sugars in the epidemics of obesity and metabolic syndrome. Annu Rev Med. 2012;63:329–343. doi: 10.1146/annurev-med-042010-113026. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19:951–961. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Becker KG, Zhang Y, Maudsley S, Martin B, Cutler RG, Mattson MP. Hippocampal gene expression patterns underlying the enhancement of memory by running in aged mice. Neurobiol Aging. 2010;31:1937–1949. doi: 10.1016/j.neurobiolaging.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ströhle A. Physical activity, exercise, depression and anxiety disorders. J Neural Transm. 2009;116:777–784. doi: 10.1007/s00702-008-0092-x. [DOI] [PubMed] [Google Scholar]
- Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, Naini A, Vila M, Jackson-Lewis V, Ramasamy R, Przedborski S. D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest. 2003;112:892–901. doi: 10.1172/JCI18797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolppanen AM, Ngandu T, Kåreholt I, Laatikainen T, Rusanen M, Soininen H, Kivipelto M. Midlife and late-life body mass index and late-life dementia: results from a prospective population-based cohort. J Alzheimers Dis. 2014;38:201–209. doi: 10.3233/JAD-130698. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijzingen IM, Gispen WH, Spruijt BM. Postoperative environmental enrichment attenuates fimbria-fornix lesion-induced impairments in Morris maze performance. Neurobiol Learn Mem. 1997;67:21–28. doi: 10.1006/nlme.1996.3735. [DOI] [PubMed] [Google Scholar]
- Vasconcelos AR, Yshii LM, Viel TA, Buck HS, Mattson MP, Scavone C, Kawamoto EM. Intermittent fasting attenuates lipopolysaccharide-induced neuroinflammation and memory impairment. J Neuroinflammation. 2014;11:85. doi: 10.1186/1742-2094-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar C, Potter MC, van Praag H. All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr Top Behav Neurosci. 2013;15:189–210. doi: 10.1007/7854_2012_220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013;17:525–544. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Camandola S, Mattson MP. Intermittent food deprivation improves cardiovascular and neuroendocrine responses to stress in rats. J Nutr. 2003;133:1921–1929. doi: 10.1093/jn/133.6.1921. [DOI] [PubMed] [Google Scholar]
- Wan R, Camandola S, Mattson MP. Dietary supplementation with 2-deoxy-D-glucose improves cardiovascular and neuroendocrine stress adaptation in rats. Am J Physiol Heart Circ Physiol. 2004;287:H1186–H1193. doi: 10.1152/ajpheart.00932.2003. [DOI] [PubMed] [Google Scholar]
- Wan R, Weigand LA, Bateman R, Griffioen K, Mendelowitz D, Mattson MP. Evidence that BDNF regulates heart rate by a mechanism involving increased brainstem parasympathetic neuron excitability. J Neurochem. 2014;129:573–580. doi: 10.1111/jnc.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman L, Jo DG, Sørensen MM, de Souza-Pinto NC, Markesbery WR, Mattson MP, Bohr VA. Defective DNA base excision repair in brain from individuals with Alzheimer's disease and amnestic mild cognitive impairment. Nucleic Acids Res. 2007;35:5545–5555. doi: 10.1093/nar/gkm605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18:649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Zhan M, Duan W, Prabhu V, Brenneman R, Wood W, Firman J, Li H, Zhang P, Ibe C, Zonderman AB, Longo DL, Poosala S, Becker KG, Mattson MP. Gene expression atlas of the mouse central nervous system: impact and interactions of age, energy intake and gender. Genome Biol. 2007;8:R234. doi: 10.1186/gb-2007-8-11-r234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Park Y, Huang X, Hollenbeck A, Blair A, Schatzkin A, Chen H. Physical activities and future risk of Parkinson disease. Neurology. 2010;75:341–348. doi: 10.1212/WNL.0b013e3181ea1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology. 2011;76:1568–1574. doi: 10.1212/WNL.0b013e3182190d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Tadokoro T, Keijzers G, Mattson MP, Bohr VA. Neurons efficiently repair glutamate-induced oxidative DNA damage by a process involving CREB-mediated up-regulation of apurinic endonuclease 1. J Biol Chem. 2010;285:28191–28199. doi: 10.1074/jbc.M109.082883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JL, Lin YT, Chuang PC, Bohr VA, Mattson MP. BDNF and exercise enhance neuronal DNA repair by stimulating CREB-mediated production of apurinic/apyrimidinic endonuclease 1. Neuromolecular Med. 2014;16:161–174. doi: 10.1007/s12017-013-8270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J Neurosci Res. 1999;57:830–839. [PubMed] [Google Scholar]