Abstract
Background
Impaired functional and cognitive status is an important outcome for older adults undergoing major cardiac surgery. We conducted this pilot study to gauge feasibility of assessing these outcomes longitudinally, from pre-operatively up to two time-points post-operatively to assess for recovery.
Methods
We interviewed patients ≥ age 65 years pre-operatively and repeated functional and cognitive assessments at 4–6 weeks and 4–6 months post-operatively. Simple unadjusted linear regression was used to test whether baseline measures changed at each follow-up time point. Then we used a longitudinal model to predict post-operative recovery overall, adjusting for co-morbidity.
Results
62 patients (age 74.7 ± 5.9) underwent scheduled cardiac surgery. Pre-operative ADL impairment was associated with poorer functional recovery at 4–6 weeks post-operatively with each baseline ADL impairment conferring recovery of 0.5 fewer ADLs (p<.05). By 4–6 months, we could no longer detect a difference in recovery. Pre-operative cognition and physical activity was not associated with post-operative changes in these domains.
Conclusion
A pre- and post-operative evaluation of function and cognition was integrated into the surgical care of older patients. Pre-operative impairments in ADLs may be a means to identify patients who might benefit from careful post-operative planning, especially in terms of assistance with self-care during the first 4–6 weeks after cardiac surgery.
Keywords: Aging, Elderly, Cardiac Surgery, Outcomes, Functional Status, Cognitive Function
1. INTRODUCTION
In selected patients, cardiac surgery can improve quality-of-life [1] and long-term survival [2]. Today, procedures such as coronary artery bypass grafting (CABG) [3,4] and valve replacement are routinely performed in older adults [5,6]. However, since older patients are at higher risk for complications such as post-operative delirium and prolonged immobility, a growing body of literature supports the evaluation of basic [7] and instrumental [8] activities of daily living (ADLs) and cognitive function to enhance surgical risk assessment for older patients undergoing scheduled surgery [9–21], including cardiac surgery [17,22–27]. In addition, postoperative evaluation of function has been proposed as an important outcome in older surgical patients [10]. Pre and post-surgical evaluation of physical and cognitive function is not currently a formal component of usual cardiac surgery care at most institutions.
We conducted an interdisciplinary, prospective pilot study to test the feasibility of measuring both cognitive and physical function in a longitudinal fashion among cardiac surgery patients ≥ 65 years of age. We asked the question of whether components of a comprehensive geriatric assessment could be incorporated into the workflow of the standard cardiac surgery pre-operative process, and whether we could capture functional recovery using post-operative telephone interviews as a potential outcome measure. Finally, as a secondary question, we tested whether specific components of the geriatric pre-operative assessment could help identify older patients at higher risk for short- and intermediate-term functional loss, which could potentially be valuable for counseling older surgical candidates and manage their expectations of postoperative recovery.
2. MATERIALS AND METHODS
2.1. Overview
The University of Michigan Health System (UMHS) is a 931-bed, tertiary care medical center with a free-standing cardiovascular hospital. Approximately 250 cardiac procedures are performed annually in older patients (age ≥ 65 years). Our pre-specified goal was to prospectively identify and evaluate 80 older adults concurrent to their usual pre-operative care for scheduled CABG, valve replacement or repair, or aortic surgery. All surgeries were performed with an open approach (i.e., none were “minimally-invasive”). We used a part-term cardiac research nurse who recruited from the scheduled patients of a weekly pre-operative cardiac surgery clinic. Feasibility was defined as completing geriatric evaluations without disrupting the normal flow of pre-operative surgical care, and contacting at least half of the patients at least once by telephone. Our initial time-frame was a 6-month enrollment period, however, we extended the time frame to 14 months due to higher-than-expected refusal rate. This study was approved by the University of Michigan Institutional Review Board. All subjects provided written informed consent.
We recruited patients ≥ age 65 years who had upcoming pre-operative visits using a combination of letters, telephone calls, and face-to-face contact. We enrolled the majority of patients at the preoperative testing visit scheduled within 4 weeks prior to the planned operative procedure. Patients were excluded if they did not undergo surgery.
At the time of enrollment, baseline assessment of cognitive and physical function was performed. All assessments were done in a separate room before or after the standard pre-operative evaluations. Usual clinical care was uninterrupted. Results of the assessment were not conveyed to the clinical team.
We conducted follow-up interviews at 4–6 weeks and 4–6 months post-operatively. We aimed to conduct the 4–6 week interview in person during the routine post-surgical follow-up visit; if this was not possible then this interview as well as the 4–6 month interview was conducted over the telephone. We selected these two time points (referred to as the “short-term” and “intermediate” time-points), based on the assumption that most patients would be discharged from the acute hospitalization by 4–6 weeks, and that any patients requiring post-acute rehabilitation would be home by 4–6 months. Interviews could be conducted with a proxy respondent if necessary for the functional status portion of the interview only.
2.2. Functional and Cognitive Measures
Physical function was assessed by patients’ self-reported ability to perform 9 basic and instrumental activities of daily living (ADLs): shopping, finances, light housework, meal preparation, driving, use of alternative transportation, telephone use, bathing, and walking across a room. Of these 9 ADLs, 7 are instrumental ADLs (IADLs), which are typically more complex tasks [8]. We focused on IADLs because older patients with dependence in basic ADLs [7] are generally not offered elective cardiac surgery. We considered an ADL as impaired if the patient had either (1) difficulty with the ADL severe enough to require help from another person or (2) inability to perform it due to personal health limitations [28]. We considered an ADL as “able” if neither of these criteria were met, then summarized overall functional ability as the number of ADLs classified as “able”. This approach has been previously validated in older ambulatory care [29,30] and surgical populations [31,32]. For this study, we clarified during the follow-up interviews that routine lifting and driving restrictions (prescribed at the time of discharge until the first follow-up visit) should not be interpreted as inability, for example for the driving and light housework (“lifting”) items.
We also assessed pre-operative physical activity level based on patients’ response to three questions from the Health and Retirement Survey [33] regarding the frequency and intensity of activity in a typical week [34]. For example, a moderately-active adult who reports vigorous activity (ex. running) one day per week, plus moderate activity (ex. walking at a moderate pace) a few days per week and mild activity (ex. light housework) a few days per week would have a score of 480 weekly-activity points [34].
Last, we evaluated short and longer-term cognitive performance using the modified-Telephone Interview for Cognitive Status (TICS-m), a validated cognitive screening instrument patterned on the Mini-Mental State Examination. This instrument was selected because it is validated for telephone use [35,36]. The TICS-m test items include 1) orientation; 2) an immediate and delayed 10-word recall test to measure memory; 3) a serial seven subtraction test to measure working memory; 4) a counting backward test to measure speed of mental processing [35]. This 35-item instrument classifies an individual as having moderate-to-severe dementia at ≤6 points, mild dementia for 7–8 points, and not demented ≥ 9 points [37].
2.3. Co-variables, intermediate outcomes, and other characteristics
Using medical record and administrative data review, we collected as potential intermediate outcomes: in-hospital death, discharge destination, defined as home (with or without skilled home care services) versus all other non-home discharges (nursing home, subacute rehabilitation, and long-term acute care facility), post-operative delirium, defined as new symptoms of confusion, agitation, and/or altered mental status, or new need for anti-psychotic medications, and care utilization (initial length of stay, 30-day readmission, 12-month total inpatient days, and 12-month outpatient visits). As a control variable, we collected the Charlson-Deyo [38] co-morbidity index from chart review, calculated using coded diagnoses from all inpatient and outpatient visits in the year prior to surgery. As additional descriptive variables, we collected age, gender, time on bypass pump (minutes), operation type (primary aortic valve replacement or repair, primary mitral valve versus all other valves, isolated CABG procedure, and additional vascular procedure).
2.4. Statistical Analysis
Our primary analyses focused on the functional and cognitive measures of surviving participants. We asked whether or not the pre-operative count of ADL abilities (range 0–9) predicted change in the count of post-operative ADL abilities. For example, if a patient had 9 ADL abilities at baseline, but 7 at follow-up, then the outcome was −2 ADLs, indicating failure to recover back to baseline by 2 ADLs. Similarly, we analyzed change in TICS-m score and physical activity level at the two follow-up time points, also with negative change scores indicating failure to recover baseline cognitive function and baseline activity level, respectively.
We used simple unadjusted linear regression to analyze our continuous outcomes (total number of ADL abilities, estimated weekly activity, and the TICs-m score). All models tested the corresponding baseline score as a co-variable. We also included as potential predictors all of the baseline characteristics, intermediate variables) in univariate analyses. Last, we focused on the ADL count in a longitudinal multivariable model, using up to two follow-up time points per participant to predict post-operative functional recovery, where we considered patients as random intercepts to account for between-patient differences. Using this model, we tested two main effects (baseline function and an indicator variable for longer-term versus short-term follow-up time period, representing difference in recovery between the two time points) and an interaction term that tested whether recovery between the two follow-up time points was modified by baseline ADL count (a difference in slopes). We considered co-morbidity and age group (≥ versus < 74, the median age) to test as main effects and interaction with time, retaining only if statistically significant. We used the final model to predict recovery across both follow-up time points for graphical display. All statistical analyses were performed using used STATA 12.0 (College Station, PA).
3.0 RESULTS
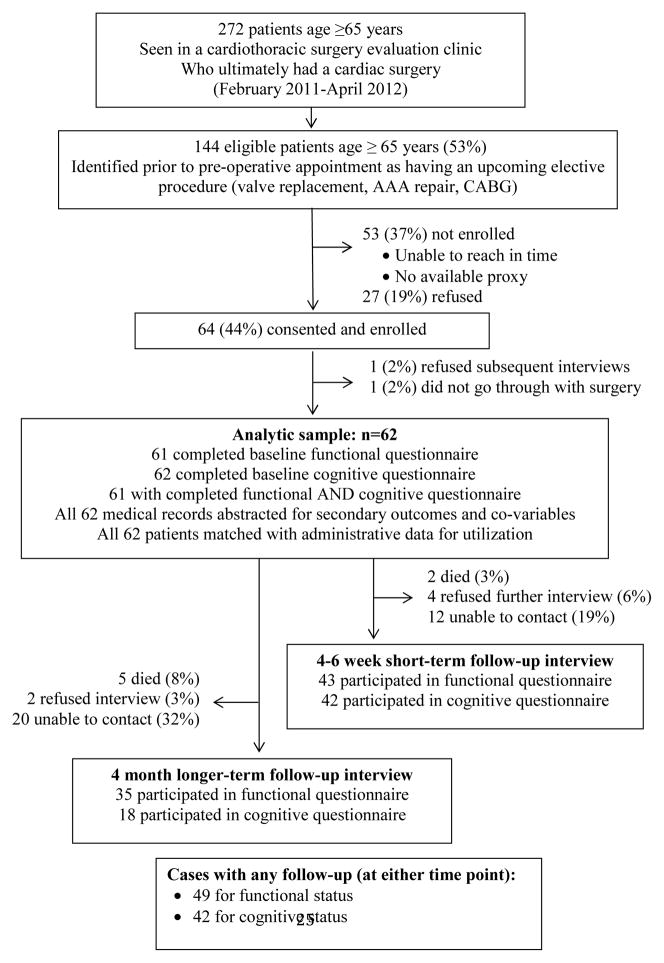
Of 272 patients age 65 and older who ultimately underwent cardiac surgery during the 14-month study period (February 15, 2011 to April 18, 2012), we were able to prospectively identify 144 (52.9%) with an upcoming cardiac surgery within 30 days and attempt to recruit prior to (via mail/telephone) or on the day of their pre-operative visit. Among these 144 patients, we were unable to contact or meet with 53 (36.8%) and 27 (18.8%) refused. For this pilot study, we recruited 64 (44.4%), but 2 patients dropped out after providing consent. The final study group (n=62) had some differences compared to those who did not enroll (n=80): shorter postoperative length of stay (7.4 versus 10.5 days, p<.01), fewer with chronic pulmonary disease (8% versus 25%, p<.01), and more with rheumatologic disease (7% versus 0%, p<.05). There were no significant differences in age, gender, presence of heart failure, peripheral vascular disease, diabetes, Charlson-Deyo score, discharge destination, or utilization of outpatient or inpatient visits before or after the index surgery.
The study population had a mean age of 74.7 ± 5.9 years (range 65 to 90). Baseline sample characteristics (Table 1) are notable for high mean pre-operative function status (8.7 of 9 possible ADLs) and moderate physical activity level (461 weekly-activity points). Only 12 (19.4%) patients reported ADL impairment at baseline. The most common impairments were the cognitively-intense activities of driving (n=9) and shopping (n=4). No patients had impairment in the two basic ADLs, bathing and walking. The majority of patients had valve replacement by open approach as the primary procedure (n=58), with more than half of the sample requiring mitral valve replacement or repair (n=34). There were no in-hospital deaths, but delirium occurred in 9 patients (14.5%) (Table 2). Most patients were discharged home with home care services (72.6%), followed by nursing home or other facility (19.4%).
Table 1.
Baseline characteristics of patients undergoing formal geriatric assessment prior to scheduled cardiac surgery (N=62)
| Continuous variables | Mean (±SD, range) | ||
|---|---|---|---|
| Age (years) | 74.7 (5.9, 65–90) | ||
| Pump time (minutes) | 102.4 (36.8, 51–237) | ||
| Charlson-Deyo Score [38] | 2.1 (1.4, 0–9) | ||
| ADL ability (shopping, managing money, walking, doing light housework, bathing or showering, preparing meals, driving, using transportation, using telephone) | 8.7 (0.8, 4–9) | ||
| Modified Telephone Interview Cognition Survey (TICS-m) score | 23.6 (4.1, 11–35) | ||
| Self-reported weekly physical activity points * | 460.9 (389.3, 24–1680) | ||
| Inpatient visits 6 months prior to pre-operative evaluation | 0.1 (0.4, 0–2) | ||
| Total inpatient days 6 months prior to pre-operative evaluation | 0.5 (1.9, 0–10) | ||
| Outpatient visits 6 months prior to pre-operative evaluation | 1.8 (2.4, 0–9) | ||
| Categorical variables | Frequency (%) | ||
| Male | 44 (71%) | ||
| Any on-pump time | 62 (100%) | ||
| Primary Procedure** | Valve | Primary aortic valve replacement/repair n=19 (30.6%) | 10 (16.1%) Aortic valve only |
| 4 (6.5%) plus additional other valve repair/replacement and/or CABG | |||
| 1 (1.6%) plus CABG | |||
| 4 (6.5%) plus vascular repair | |||
| Primary mitral valve replacement/repair, n=34 (54.8%) | 18 (29.0%) mitral valve only | ||
| 12 (19.4%) plus additional other valve repair/replacement and/or CABG | |||
| 4 (6.5%) plus CABG | |||
| Primary non-specified valve replacement/repair | 5 (8.1%) | ||
| Coronary artery bypass and/or other major vascular procedure (thoracic or abdominal aorta) | 4 (6.5%) | ||
Self-reported weekly physical activity summarizes points [in brackets] from three questions about vigorous physical activity [e.g., running, swimming, cycling, tennis; every day [840], more than once per week [360], once a week [120], 1–3 times per month [60], hardly ever or never [12]), moderate activity (e.g., gardening, cleaning the car, walking at a moderate pace, dancing; every day [525], more than once per week [225], once a week [75], 1–3 times per month [37.5], hardly ever or never [7.5]) and mild activity (e.g., vacuuming, laundry, home repairs; every day [315], more than once per week [135], once a week [45], 1–3 times per month [22.5], hardly ever or never [4.5]). If a patient reported vigorous activity as never [12], moderate activity as once per week [75], and mild activity more than once per week [135], then his/her estimated weekly physical activity would be 222 points.
All procedures were performed via open approach (i.e., none were “minimally invasive”).
ADL=activities of daily living
CABG=coronary artery bypass grafting
Table 2.
Clinical outcomes of interest among older adults undergoing scheduled cardiac surgery
| A. Intermediate outcomes and other descriptive variables (n=62) | ||
|---|---|---|
| Continuous variable | Mean (±SD, range) | |
| Length of stay (days) | 7.4 (3.54, 4–19) | |
| Categorical variables | Frequency (%) | |
| Any delirium | 9 (14.5%) | |
| Disposition | Home | 5 (8.1%) |
| Home with home care | 45 (72.6%) | |
| Post-hospital nursing home | 10 (16.1%) | |
| Long-term care | 1 (1.6%) | |
| Other | 1 (1.6%) | |
| In-hospital mortality | 0 (0%) | |
| B. Primary and secondary short-term outcomes (4–6 weeks) | |
|---|---|
| Continuous variables | Mean (±SD, range) |
| ADL ability count (out of 9) (n=39) | 8.4 (1.4, 3–9) |
| * Change in ADL ability count (compared to baseline) (n=38) | −0.3 (1.1, −4 to +1) |
| Self-reported weekly activity level points (n=43) | 563 (466, 24–1680) |
| ** Change in activity level (compared to baseline) (n=42) | 92 (535, −96 to +1458) |
| TICS-m score (n=41) | 23.7 (4.6, 11–35) |
| ** Change in TICS-m score (compared to baseline) (n=41) | −0.1 (3.5, −12 to +8) |
| C. Primary and secondary Intermediate-term outcomes | |
|---|---|
| Continuous variables | Mean (±SD, range) |
| ADL ability count (out of 9) at 4 months (n=34) | 8.6 (1.0, 5–9) |
| * Change in ADL ability count (compared to baseline) (n=34) | −0.2 (0.9, −3 to +1) |
| Self-reported weekly activity points at 4 months (n=35) | 618 (393, 24–1680) |
| ** Change in activity level score (compared to baseline) (n=35) | 147 (447, −960 to +1458) |
| TICS-m score at 4 months (n=18) | 24.6 (2.8, 20–29) |
| ** Change in TICS-m score (compared to baseline) (n=18) | 0.7 (3.0, −3 to + 6) |
ADL = activities of daily living, total count of abilities
TICS-m = modified Telephone Interview of Cognitive Status
Our primary outcome
Secondary outcomes
At short-term follow-up (4–6 weeks post-discharge) (Table 2) in 43 participants (Figure 1), the mean ADL count dropped from 8.7 at baseline to 8.4 (p=.07 for paired t-test). The two most common new ADL impairments at this time point occurred in the area of driving (n=3) and cleaning (n=3), both unrelated to driving and lifting restrictions. Patients also had new impairments in bathing (n=2) and walking (n=2). There was no change in TICS-m score (0.1 points, p=.8), and no significant change in activity level (gain of 92 weekly-activity points, p=.3).
Figure 1.
Data flow
For long-term outcome measures (4 or more months post-surgery) among 36 participants (Figure 1), the mean ADL score was not different from baseline (8.6 versus 8.7, p=.2), nor was TICS-m score (23.6 vs 24.6, p=.3). Physical activity increased by 147 weekly-activity points above baseline, but the difference failed to achieve statistical significance (p=.06).
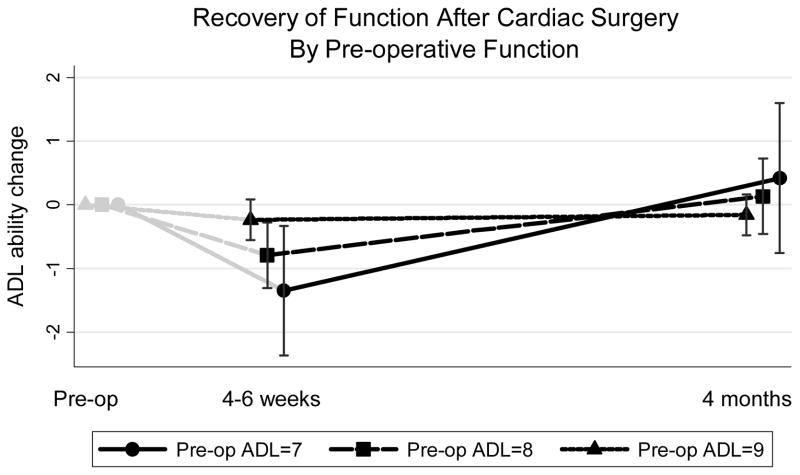
Of the primary outcomes, only baseline ADL count predicted poorer recovery to baseline at the time of short-term follow-up (4–6 weeks after surgery). On univariate analysis, ADL count had a large effect size (every additional ADL impairment at baseline was associated with poorer recovery, by .54 ADLs) but the association did not meet statistical significance (p=.11). Using both follow-up time-points for the outcome in a random-effects model controlling for co-morbidity and the interaction between baseline function and the slope of recovery between the two time points, the results were unchanged from the univariate analysis (each baseline ADL impairment associated with .56 fewer ADLs recovered, p<.05) at 4–6 weeks. Other model parameters are described in Table 3. Because patients with baseline impairment declined more by 4–6 weeks, this group had a significantly steeper recovery back to baseline by 4–6 months (Figure 2 and time-ADL interaction term in Table 3, p=.002). Co-morbidity also predicted an overall poorer recovery as well, with each additional point (Charlson-Deyo) conferring .2 ADLs less recovery (p=.03) on average at either follow-up time point. None of the other co-variables (age category, or co-morbidity or age interactions with time) substantively nor statistically changed the results of the final model and therefore were not retained.
Table 3.
Multi-level random-effects regression model to predict ADL change (n=72 follow-up interviews for 49 patients)
| Predictor variables | Coefficient | P value |
|---|---|---|
| Baseline ADL ability | 0.56 | 0.048 |
| Time | 7.67 | 0.002 |
| Time*Baseline ADL ability | −0.84 | 0.002 |
| Charlson-Deyo Co-morbidity Score | −0.21 | 0.028 |
| Constant | −4.81 | 0.054 |
ADL = Activities of Daily Living, range 7–9 abilities
Time = Evaluation of recovery occurring at the longer-term versus shorter-term follow-up time point (1 versus 0).
Time*Baseline ADL ability = interaction between Time and ADL ability
The interpretation of the co-efficients are in units of ADLs. For example, each additional point in co-morbidity is associated, on average, with .2 fewer ADLs recovered towards baseline. The effect of baseline ADLs and the ADL interaction with follow-up time is best seen graphically in Figure 2.
Figure 2.
Change in functional status at 4 weeks and 4 months, compared to pre-operative baseline. (n=72 follow-up interviews for 49 patients)
ADL = count of 9 Activities of Daily Living abilities.
We display the mean trajectories of recovery back to pre-operative function (count of 9 ADL abilities) for 49 individuals with a complete baseline functional interview and at least one follow-up functional status interview postoperatively at 4–6 weeks (first black marker) or 4 months (second black marker). This is a random-intercept model of estimated recovery (count of ADL abilities at follow-up minus baseline) for up to two time points per individual, controlling for co-morbidity, and including a fixed-effect for the interaction between baseline ADL and the time-point (difference in slopes). The co-variables were set at their mean or mode to obtain this graphical display. The three trajectories are the mean counts at the two follow-up time periods for those with 7 ADLs (n=2), 8 ADLs (n=7), and 9 ADLs (n=40) at baseline. The confidence intervals were obtained from the linear combination of the predicted outcome at each time point. To better illustrate the overall trajectory, we display the baseline ADL (zero change) as gray markers at the pre-operative time point (not modeled).
Better TICS-m score at baseline exhibited a floor effect associated with more decline in TICS-m score at both the short-term follow-up (each point at baseline associated with loss of .27 points, p<=05) and longer-term follow-up (.54 points, p=.01). As a predictor of TICS-m recovery, hospital delirium was associated with 4 fewer points (p<.01) at the short but not longer-term outcome (1.6 points, p=.3). Greater physical activity at baseline was also paradoxically associated with poorer return to baseline physical activity at short and longer-term follow-up (each weekly-activity point at baseline associated with loss of .78 points, p<.001, and .65 points, p<.001, respectively).
We did not observe any associations between any of the baseline geriatric assessments and development of intermediate outcomes of delirium, discharge destination, readmission, care utilization, or mortality, with the exception of TICS-m. For the univariable model predicting discharge to home, each TICS-m point (out of 35 points total) conferred 1.16 greater odds of discharge to home versus nursing home, however, this effect could have been found due to chance (p=.08).
4.0. DISCUSSION
We tested the feasibility of adding formal geriatric assessment to the standard pre-operative evaluation of older patients undergoing scheduled cardiac surgery. Although conducting the assessments during the pre-operative visit was feasible, we noted challenges with enrollment and follow-up of patients for short- and long-term telephone assessments. We also observed an association between baseline impairment and development of new functional impairment in the weeks following surgery. Among patients surviving to 4–6 months, almost everyone in this small cohort returned to baseline.
Our main results regarding functional recovery of ADL abilities have several clinical implications. First, the short-term post-operative course (4–6 weeks after surgery) appears to be a very critical time frame, with significant variation in the need for assistance with self-care. Second, we identified new impairments due to underlying difficulty with physical recovery. These activities include walking and bathing, activities that independent adults should be able to perform even under prescribed restrictions. Third, older adults with any pre-operative ADL impairment should be counseled to consider the potential need for assistance with self-care for 2 or more months postoperatively.
With regard to recovery of weekly activity level among this older group of patients who underwent major cardiac surgery, we were underpowered to capture a statistical improvement. Two larger studies, one of younger patients [39] and another that utilized post-operative interviews only [40], have used other questions to determine activity, and found overall improvement. However, the mean improvement we observed from 460 weekly-activity points pre-operatively to above 600 points at 4 months was substantial - suggesting that patients on average may regain ability to do regular moderate physical activities after major surgery – despite the advanced age of this group. A larger study using these questions regarding weekly activity, which are easily administered, is needed.
With regard to cognitive function, our findings are consistent with a larger study of 222 younger (mean age 61) cardiac surgery patients by Newman and colleagues [41]. They also observed a floor effect in the pre-operative cognitive score in that those with better scores had more to lose post-operatively, resulting in a paradoxical effect. Newman et al followed patients over a much longer 5-year follow-up for changes in cognitive function (far longer than in our study), but their short and intermediate-term findings (a small but statistically insignificant decline at 6 weeks and statistically insignificant improvement at 6 months) are similar to ours [41]. Our results are also consistent with prior work finding association between delirium and poorer follow-up cognitive function in the intermediate-term (at 4–6 weeks but not 4–6 months), a finding that was consistent with a study of 190 patients by Rudolph et al, where delirium was associated with poorer return to prior functional status at the earlier follow-up but not long-term follow-up [22]. These findings reinforce the need for vigilance during the post-discharge period, such as increased social support, for patients post-delirium as they may not have fully returned to baseline cognitive function. Testing patients for failure to return to baseline cognitive function - which requires some form of cognitive testing at baseline and post-operatively - is a care process that has been recommended for older, vulnerable hospital and surgical patients [42]. Our experience suggests that cardiac surgery patients are a potentially important population where this additional effort to screen for failure to return to baseline may be worthwhile. Further interventional research in the cognitively-vulnerable cardiac surgery patients is needed [23].
We experienced a number of challenges with patient recruitment, due in part to a higher-than expected refusal rate. Most patients cited fatigue as the reason for refusal. We also speculate that older patients may be reluctant to admit ADL impairment for fear that they could be denied surgery. In order to improve patient acceptance of geriatric screening, clinicians should emphasize that these screens are being used to facilitate pre-operative planning (ex. helping to determine post-operative care needs), in contrast to the traditional notion of pre-operative screening to determine surgical candidacy. These concerns are perhaps even more significant in the context of cognitive screening due to the stigma (real or perceived) associated with cognitive impairment.
As a result of our experience, we offer a number of suggestions to other programs contemplating the development of pre-operative screening paradigms that include functional and cognitive measures. First, reducing the overall burden of screening—both for patients and clinicians—would likely improve sustainability in clinical practice. For example, rather than assessing all ADLs, one option would be to assess baseline function in complex ADLs that are most likely to be impaired pre-operatively which were the more sensitive to cognition such as driving, medication management, and financial management. Second, pre-operative cognitive screening should be performed. This has been operationalized in previous work by Rudolph et al [21] and Harrington et al. [17] By incorporating cognition into the pre-operative evaluation there may be better opportunity to utilize social work or case management services for those at higher risk. For example, patients and their families could pre-emptively select a post-operative rehabilitation facility or arrange for family members to stay for an extended time in the patient’s home. Extra caregiving should be considered for weeks following delirium. One future approach would be to utilize more sensitive cognitive tests (alternating Trails B test to better test concentration, executive function, and mental processing) [43] along with screening of global cognitive function. Last, physical performance measures such as the Timed Up and Go (TUG) test [44] can be feasibly implemented in pre-operative evaluation of older general non-cardiac [18,45,46] and orthopedic [11] surgery patients. One study found that the TUG outperformed traditional surgical risk calculators at predicting mortality and complications of general surgery.[46] Physical measures such as TUG could be added to the pre-operative evaluation of cardiac surgery patients as a predictor cardiac surgery outcomes, such as recovery of physical mobility.
A strength of this study is the more real-world clinical setting which is uncommon among prospective studies of trajectories over time in surgical outcomes research. As the main goal of this study was to test feasibility, we report on the difficulty of determining from the cardiac surgery clinic schedule whether patients were truly pre-operative, then recruiting and retaining them for longitudinal study. Our analytic method (random-effects longitudinal model) is well-suited for small longitudinal follow-up studies, where patients with at least one follow-up interview can be retained in the analysis, i.e., despite incomplete participation in all planned interviews.
This work also has several major limitations, most notably the small sample. There is a very high likelihood of Type II error, assuming that some elements of geriatric screening does not predict post-operative complications when in actuality such screening does. A second limitation is that we may have selectively enrolled the healthier patients able to participate, as suggested by the shorter length of stay among those enrolled compared to those not enrolled. However, because the range of sickness at baseline was truncated (i.e., missing the sicker patients), we expect that this resulted in a “bias towards the null”, or a lessening of effect in our analysis. A third limitation involves how we measured cognition. There has been extensive research on post-operative cognitive impairment after cardiac surgery, however, with a wide variation in the measures used [24]. The TICS-m of cognitive performance is validated for use in community dwelling older adults and discriminates well between individuals with dementia and those with no cognitive impairment [37]. However, the TICS-m cognitive measures may have insufficiently focused on the cognitive domains affected in individuals with undergoing cardiac surgery. Streamlining the interview to items of most value is of highest importance for future measurement efforts.
Fourth, we had a substantial loss-to-follow-up for the functional and cognitive status interviews that is not uncommon among longitudinal studies of sick patients [32,41]. However, because those lost to follow-up were more likely to be the sicker patients, we also expect this limitation to bias our results toward the null. To address potential bias, we analyzed - and did not find – that those lost-to-follow-up differed from the studied patients in measures of sickness that we could measure (age, overall co-morbidity, specific co-morbidities, and health care utilization).
Last, as a single-center study at a tertiary referral center, the generalizability of our results may be limited to similar venues. Our patients may have been referred from outside centers due to advanced age, multiple or severe co-morbidities. However, capturing the clinical complexity of our patients in comparison to surgical candidates at regional centers was beyond the scope of this feasibility study. In addition, one indication for surgery, severe heart failure, has been found to be associated with similar outcome measures, including cognitive [47–50] and physical function [51], in community samples. Therefore, future research should include the collection of clinical details such as indication for surgery, degree of symptom severity, and expected goal of the surgery (survival, symptom management, etc.).
In conclusion, for older adults undergoing scheduled cardiac surgery, pre-operative functional status and cognitive status can be measured in a clinical setting and holds the potential for improving care in the short-term post-operative course. Further research is needed to understand the role of this potentially powerful tool into routine clinical practice.
Acknowledgments
This research was supported by the University of Michigan, Frankel Cardiovascular Center Inaugural Grant (PI Malani). Dr. Min was supported by the Older Americans Independence Act Claude D. Pepper Center (NIA AG AG024824 Research Career Development Core) and the Hartford Foundation Center of Excellence. Dr. Cigolle was supported by a National Institute on Aging K08 Award (K08 AG031837). Drs. Min and Cigolle were supported by the Geriatric, Research, Education, and Clinical Center (GRECC).
Footnotes
Conflict of Interest Disclosures: The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Author Contributions: Drs. Malani and Min had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Min, Gure, Cigolle, Lee, Romano, Nallamothu, Langa, Prager, Malani
Acquisition of data: Min, Mazzurco, Bloem, Malani
Analysis and interpretation of data: Min, Chan, Malani
Drafting of the manuscript: Min, Chan, Malani
Critical revision of the manuscript for important intellectual content: Mazzurco, Gure, Cigolle, Lee, Bloem, Romano, Nallamothu, Langa, Prager
Statistical analysis: Min, Chan
Obtained funding: Romano, Nallamothu, Langa, Prager, Malani
Administrative, technical, or material support: Chan, Cigolle, Bloem, Langa, Nallamothu
Study supervision: Malani
Additional Contributions: The authors acknowledge the clinical support of the faculty and staff of the University of Michigan Health System’s Department of Cardiac Surgery.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krane M, Voss B, Hiebinger A, Deutsch MA, Wottke M, Hapfelmeier A, et al. Twenty years of cardiac surgery in patients aged 80 years and older: risks and benefits. Ann Thorac Surg. 2011 Feb;91(2):506–13. doi: 10.1016/j.athoracsur.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 2.Likosky DS, Sorensen MJ, Dacey LJ, Baribeau YR, Leavitt BJ, DiScipio AW, et al. Long-term survival of the very elderly undergoing aortic valve surgery. Circulation. 2009 Sep 15;120(11 Suppl):S127–33. doi: 10.1161/CIRCULATIONAHA.108.842641. [DOI] [PubMed] [Google Scholar]
- 3.Yanagawa M, Umegaki H, Uno T, Oyun K, Kawano N, Maeno H, et al. Association between improvements in insulin resistance and changes in cognitive function in elderly diabetic patients with normal cognitive function. Geriatrics & gerontology international. 2011 Jul;11(3):341–7. doi: 10.1111/j.1447-0594.2011.00691.x. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010 Feb 23;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 5.Brennan JM, Edwards FH, Zhao Y, O’Brien SM, Douglas PS, Peterson ED. Long-term survival after aortic valve replacement among high-risk elderly patients in the United States: insights from the Society of Thoracic Surgeons Adult Cardiac Surgery Database, 1991 to 2007. Circulation. 2012 Sep 25;126(13):1621–9. doi: 10.1161/CIRCULATIONAHA.112.091371. [DOI] [PubMed] [Google Scholar]
- 6.Ivanov J, Weisel RD, David TE, Naylor CD. Fifteen-year trends in risk severity and operative mortality in elderly patients undergoing coronary artery bypass graft surgery. Circulation. 1998 Feb 24;97(7):673–80. doi: 10.1161/01.cir.97.7.673. [DOI] [PubMed] [Google Scholar]
- 7.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of Illness in the Aged. The Index of Adl: A Standardized Measure of Biological and Psychosocial Function. JAMA. 1963 Sep 21;185:914–9. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 8.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. The Gerontologist. 1969 Autumn;9(3):179–86. [PubMed] [Google Scholar]
- 9.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010 Jun;210(6):901–8. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 10.Malani PN. Functional status assessment in the preoperative evaluation of older adults. JAMA. 2009 Oct 14;302(14):1582–3. doi: 10.1001/jama.2009.1453. [DOI] [PubMed] [Google Scholar]
- 11.Harari D, Hopper A, Dhesi J, Babic-Illman G, Lockwood L, Martin F. Proactive care of older people undergoing surgery (‘POPS’): designing, embedding, evaluating and funding a comprehensive geriatric assessment service for older elective surgical patients. Age and ageing. 2007 Mar;36(2):190–6. doi: 10.1093/ageing/afl163. [DOI] [PubMed] [Google Scholar]
- 12.Kothari A, Phillips S, Bretl T, Block K, Weigel T. Components of geriatric assessments predict thoracic surgery outcomes. The Journal of surgical research. 2011 Mar;166(1):5–13. doi: 10.1016/j.jss.2010.05.050. [DOI] [PubMed] [Google Scholar]
- 13.Pol RA, van Leeuwen BL, Visser L, Izaks GJ, van den Dungen JJ, Tielliu IF, et al. Standardised frailty indicator as predictor for postoperative delirium after vascular surgery: a prospective cohort study. European journal of vascular and endovascular surgery: the official journal of the European Society for Vascular Surgery. 2011 Dec;42(6):824–30. doi: 10.1016/j.ejvs.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Robinson TN, Wallace JI, Wu DS, Wiktor A, Pointer LF, Pfister SM, et al. Accumulated frailty characteristics predict postoperative discharge institutionalization in the geriatric patient. J Am Coll Surg. 2011 Jul;213(1):37–42. doi: 10.1016/j.jamcollsurg.2011.01.056. discussion -4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson TN, Eiseman B, Wallace JI, Church SD, McFann KK, Pfister SM, et al. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg. 2009 Sep;250(3):449–55. doi: 10.1097/SLA.0b013e3181b45598. [DOI] [PubMed] [Google Scholar]
- 16.Robinson TN, Wu DS, Stiegmann GV, Moss M. Frailty predicts increased hospital and six-month healthcare cost following colorectal surgery in older adults. Am J Surg. 2011 Nov;202(5):511–4. doi: 10.1016/j.amjsurg.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrington MB, Kraft M, Grande LJ, Rudolph JL. Independent association between preoperative cognitive status and discharge location after cardiac surgery. American journal of critical care: an official publication, American Association of Critical-Care Nurses. 2011 Mar;20(2):129–37. doi: 10.4037/ajcc2011275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cronin J, Livhits M, Mercado C, Chen F, Foster N, Chandler C, et al. Quality improvement pilot program for vulnerable elderly surgical patients. Am Surg. 2011 Oct;77(10):1305–8. [PubMed] [Google Scholar]
- 19.Dasgupta M, Rolfson DB, Stolee P, Borrie MJ, Speechley M. Frailty is associated with postoperative complications in older adults with medical problems. Arch Gerontol Geriatr. 2009 Jan-Feb;48(1):78–83. doi: 10.1016/j.archger.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 20.American College of Surgeons and American Geriatrics Society. ACS NSQIP/AGS Best Practice Guidelines: Optimal Pre-operative Assessment of the Geriatric Surgical Patient. 2012 http://site.acsnsqip.org/wp-content/uploads/2011/12/ACS-NSQIP-AGS-Geriatric-2012-Guidelines.pdf.
- 21.Rudolph JL, Jones RN, Rasmussen LS, Silverstein JH, Inouye SK, Marcantonio ER. Independent vascular and cognitive risk factors for postoperative delirium. Am J Med. 2007 Sep;120(9):807–13. doi: 10.1016/j.amjmed.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 22.Rudolph JL, Inouye SK, Jones RN, Yang FM, Fong TG, Levkoff SE, et al. Delirium: an independent predictor of functional decline after cardiac surgery. J Am Geriatr Soc. 2010 Apr;58(4):643–9. doi: 10.1111/j.1532-5415.2010.02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao L, Taha R, Gauvin D, Othmen LB, Wang Y, Blaise G. Postoperative cognitive dysfunction after cardiac surgery. Chest. 2005 Nov;128(5):3664–70. doi: 10.1378/chest.128.5.3664. [DOI] [PubMed] [Google Scholar]
- 24.Rudolph JL, Schreiber KA, Culley DJ, McGlinchey RE, Crosby G, Levitsky S, et al. Measurement of post-operative cognitive dysfunction after cardiac surgery: a systematic review. Acta Anaesthesiol Scand. 2010 Jul;54(6):663–77. doi: 10.1111/j.1399-6576.2010.02236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afilalo J, Mottillo S, Eisenberg MJ, Alexander KP, Noiseux N, Perrault LP, et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circulation Cardiovascular quality and outcomes. 2012 Mar 1;5(2):222–8. doi: 10.1161/CIRCOUTCOMES.111.963157. [DOI] [PubMed] [Google Scholar]
- 26.Sundermann S, Dademasch A, Praetorius J, Kempfert J, Dewey T, Falk V, et al. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2011 Jan;39(1):33–7. doi: 10.1016/j.ejcts.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Sundermann S, Dademasch A, Rastan A, Praetorius J, Rodriguez H, Walther T, et al. One-year follow-up of patients undergoing elective cardiac surgery assessed with the Comprehensive Assessment of Frailty test and its simplified form. Interactive cardiovascular and thoracic surgery. 2011 Aug;13(2):119–23. doi: 10.1510/icvts.2010.251884. discussion 23. [DOI] [PubMed] [Google Scholar]
- 28.Saliba D, Elliott M, Rubenstein LZ, Solomon DH, Young RT, Kamberg CJ, et al. The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. Journal of the American Geriatrics Society. 2001 Dec;49(12):1691–9. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 29.Min LC, Elliott MN, Wenger NS, Saliba D. Higher vulnerable elders survey scores predict death and functional decline in vulnerable older people. J Am Geriatr Soc. 2006 Mar;54(3):507–11. doi: 10.1111/j.1532-5415.2005.00615.x. [DOI] [PubMed] [Google Scholar]
- 30.Min L, Yoon W, Mariano J, Wenger NS, Elliott MN, Kamberg C, et al. The vulnerable elders-13 survey predicts 5-year functional decline and mortality outcomes in older ambulatory care patients. J Am Geriatr Soc. 2009 Nov;57(11):2070–6. doi: 10.1111/j.1532-5415.2009.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Min L, Ubhayakar N, Saliba D, Kelley-Quon L, Morley E, Hiatt J, et al. The Vulnerable Elders Survey-13 predicts hospital complications and mortality in older adults with traumatic injury: A pilot study. Journal of the American Geriatrics Society. 2011 Aug;59(8):1471–6. doi: 10.1111/j.1532-5415.2011.03493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tillou A, Kelley-Quon L, Burruss S, Morley E, Cryer H, Cohen M, et al. Long-term Postinjury Functional Recovery: Outcomes of Geriatric Consultation. JAMA Surg. 2014 Jan 1;149(1):83–9. doi: 10.1001/jamasurg.2013.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Health and Retirement Survey. Biennial Interview Questionnaires: Functional Limitations, ADL/IADL, Helpers. available at hrsonline.isr.umich.edu.
- 34.Cigolle CT, Ofstedal MB, Tian Z, Blaum CS. Comparing models of frailty: the Health and Retirement Study. J Am Geriatr Soc. 2009 May;57(5):830–9. doi: 10.1111/j.1532-5415.2009.02225.x. [DOI] [PubMed] [Google Scholar]
- 35.Ofstedal MB, Fisher GG, Herzog AR. Documentation of Cognitive Functioning Measures in the Health and Retirement Study. 2005 [Google Scholar]
- 36.Plassman BL, Newman TT, Welsh KA, Helms M, Breitner JCS. Properties of the Telephone Interview for Cognitive Status - Application in Epidemiologic and Longitudinal-Studies. Neuropsy Neuropsy Be. 1994 Jul;7(3):235–41. [Google Scholar]
- 37.Langa KM, Chernew ME, Kabeto MU, Herzog AR, Ofstedal MB, Willis RJ, et al. National estimates of the quantity and cost of informal caregiving for the elderly with dementia. Journal of general internal medicine. 2001 Nov;16(11):770–8. doi: 10.1111/j.1525-1497.2001.10123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992 Jun;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 39.Jenkins CD, Stanton BA, Savageau JA, Ockene IS, Denlinger P, Klein MD. Physical, psychologic, social, and economic outcomes after cardiac valve surgery. Arch Intern Med. 1983 Nov;143(11):2107–13. [PubMed] [Google Scholar]
- 40.Chaturvedi RK, Blaise M, Verdon J, Iqbal S, Ergina P, Cecere R, et al. Cardiac surgery in octogenarians: long-term survival, functional status, living arrangements, and leisure activities. Ann Thorac Surg. 2010 Mar;89(3):805–10. doi: 10.1016/j.athoracsur.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001 Feb 8;344(6):395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 42.Arora VM, McGory ML, Fung CH. Quality indicators for hospitalization and surgery in vulnerable elders. J Am Geriatr Soc Oct. 2007;55 (Suppl 2):S347–58. doi: 10.1111/j.1532-5415.2007.01342.x. [DOI] [PubMed] [Google Scholar]
- 43.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc Apr. 2005;53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 44.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc Feb. 1991;39(2):142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 45.Miller AL, Min LC, Diehl KM, Cron DC, Chan CL, Sheetz KH, et al. Analytic morphomics corresponds to functional status in older patients. The Journal of surgical research Nov. 2014;192(1):19–26. doi: 10.1016/j.jss.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson TN, Wu DS, Sauaia A, Dunn CL, Stevens-Lapsley JE, Moss M, et al. Slower walking speed forecasts increased postoperative morbidity and 1-year mortality across surgical specialties. Ann Surg Oct. 2013;258(4):582–8. doi: 10.1097/SLA.0b013e3182a4e96c. discussion 8–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zuccala G, Cattel C, Manes-Gravina E, Di Niro MG, Cocchi A, Bernabei R. Left ventricular dysfunction: a clue to cognitive impairment in older patients with heart failure. Journal of neurology, neurosurgery, and psychiatry Oct. 1997;63(4):509–12. doi: 10.1136/jnnp.63.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jefferson AL, Poppas A, Paul RH, Cohen RA. Systemic hypoperfusion is associated with executive dysfunction in geriatric cardiac patients. Neurobiology of aging Mar. 2007;28(3):477–83. doi: 10.1016/j.neurobiolaging.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jerskey BA, Cohen RA, Jefferson AL, Hoth KF, Haley AP, Gunstad JJ, et al. Sustained attention is associated with left ventricular ejection fraction in older adults with heart disease. Journal of the International Neuropsychological Society: JINS Jan. 2009;15(1):137–41. doi: 10.1017/S1355617708090073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Festa JR, Jia X, Cheung K, Marchidann A, Schmidt M, Shapiro PA, et al. Association of low ejection fraction with impaired verbal memory in older patients with heart failure. Archives of neurology Aug. 2011;68(8):1021–6. doi: 10.1001/archneurol.2011.163. [DOI] [PubMed] [Google Scholar]
- 51.Hobbs FD, Kenkre JE, Roalfe AK, Davis RC, Hare R, Davies MK. Impact of heart failure and left ventricular systolic dysfunction on quality of life: a cross-sectional study comparing common chronic cardiac and medical disorders and a representative adult population. Eur Heart J Dec. 2002;23(23):1867–76. doi: 10.1053/euhj.2002.3255. [DOI] [PubMed] [Google Scholar]