Abstract
Objective
To explore the temporal expression in granulosa and theca cells of key members of the MMP and ADAMTS families across the periovulatory period in women in order to gain insight into their possible roles during ovulation and early luteinization.
Design
Experimental prospective clinical study and laboratory-based investigation.
Setting
University Medical Center and private IVF center.
Animal and Patient(s)
Thirty eight premenopausal women undergoing surgery for tubal ligation and 6 premenopausal women undergoing ART.
Intervention(s)
Administration of hCG and harvesting of follicles by laparoscopy and collection of granulosa-lutein cells at oocyte retrieval.
Main Outcome Measure(s)
Expression of mRNA for MMPs and ADAMTSs in human granulosa cells and theca cells collected across the periovulatory period of the menstrual cycle and in cultured granulosa-lutein cells after hCG. Localization of MMPs and ADAMTSs by immunohistochemistry.
Result(s)
Expression of MMP1 and MMP19 mRNA increased in both granulosa and theca cells after hCG administration. ADAMTS1 and ADAMTS 9 mRNA increased in granulosa cells after hCG treatment, however thecal cell expression for ADAMTS1 was unchanged while ADAMTS9 expression was decreased. Expression of MMP8 and MMP13 mRNA was unchanged. Immunohistochemistry confirmed the localization of MMP1, MMP19, ADAMTS1 and ADAMTS9 to the granulosa and thecal cell layers.
Conclusion(s)
The collection of the dominant follicle throughout the periovulatory period has allowed the identification of proteolytic remodeling enzymes in the granulosa and theca compartments that may be critically involved in human ovulation. These proteinases may work in concert to regulate breakdown of the follicular wall and release of the oocyte.
Keywords: ovulation, granulosa cell, theca cell, matrix metalloproteinase, ADAMTS
INTRODUCTION
The human ovarian follicle is supported by a complex network of extracellular matrix (ECM) proteins. The ECM composition of the ovarian follicle, with its granulosa, theca, and stromal cell compartments, is dependent upon the cell type and this composition changes throughout the different stages of follicular growth, ovulation and luteinization (1).
In the human, the granulosa cell compartment is comprised of steroidogenic cells supported by the ECM proteins laminin (2), type IV collagen (3), and type VI collagen (3, 4). This granulosa cell layer is separated from the theca interna by a basement membrane, or basal lamina. This basement membrane is composed of a lattice-type network of type IV collagen intertwined with a mesh of laminin (5, 6) and is stabilized by the binding of other proteins such as entactin, nidogen, perlecan, collagen type XVIII and the glycoprotein usherin (5). In the theca cell compartment, collagen type III is present in both the theca interna and the theca externa while collagen type I is only present in the theca externa of the human follicle (6). In the stroma outside of the theca, collagens I and III are distributed in concentric layers in the capsular stroma with bundles of collagens connecting these layers to form a lattice (7). Collagen type I is present in larger quantities in the outer layers of the follicle wall while collagen type III showed the inverse distribution with higher abundance in the more central parts of the capsular stroma (6).
The abundance of extracellular matrix proteins in the follicular wall has led to the hypothesis that their degradation is paramount for follicular rupture to occur (6, 8, 9). This concept has been supported by morphological observations that as ovulation approaches in the human, there is a decrease or fragmentation in the immunostaining intensity of type I, III and VI collagens in the perifollicular stroma (4, 6). This fragmented or discontinuous immunostaining for type VI collagen was evident predominantly in the apical area rather than in the base of the preovulatory follicle (4, 10). Okamura and colleagues examined the human apical wall by electron microscopy and observed a loss of collagen in the theca externa and tunica albuginea at the follicular apex. After rupture, the theca and tunica albuginea are occupied by “scattered fibrillar substance” with a loss of collagen bundles (11).
These morphological changes at the apex of the human follicle are postulated to occur through the actions of a broad array of proteinases including metallo-, serine and thiol proteinases (9, 12-17). The expression and activity of these proteinases are set in motion by the mid-cycle surge of luteinizing hormone (LH) in numerous species (9, 12-17). However, little is known about the expression of these proteinases in human ovulation due to the difficulties of collecting human ovarian tissues across the periovulatory period. The human data on proteinase expression and function in ovulation has mostly come from studies of granulosa-lutein cells from IVF, which represent cells from an artificial hyperstimulated cycle, with no possibilities to compare expression to earlier stages of the follicle. In the present study, we have utilized separated granulosa cells and theca cells of the dominant follicle at timed intervals across the periovulatory period to investigate the expression and localization of members of the matrix metalloproteinase (MMPs) and the A Disintegrin And Metalloproteinase with ThromboSpondin-like motifs (ADAMTS) families associated with the ovulatory process in the human. The hCG responsiveness of the in vivo cells was compared to in vitro models using granulosa-lutein cells collected at the time of IVF or virally transformed granulosa cells.
MATERIALS AND METHODS
Materials
Unless otherwise noted, all chemicals and reagents were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Molecular biological enzymes, culture media and additives, Trizol™, TaqMan primers and mastermix, were purchased from Life Technologies, Inc. (Grand Island, NY). Immunohistochemistry reagents for the Starr Trek Avidin-AP labeling system were purchased from Biocare (Concord, CA).
Human Follicles Collected across the Periovulatory Period
Human granulosa and theca cells from periovulatory follicles were collected as previously described (18). The study was approved by the regional human ethics committee of Gothenburg and informed written consent was obtained from all patients. To obtain high quality patient material, only women with proven fertility, regular menstrual cycles and without hormonal medications for at least 3 months were included. The women were monitored with repeated transvaginal ultrasound (TVU) for an average of 2 cycles to enable planning of follicle collection at one of four time points; pre-, early, late or postovulatory.
For samples collected at the preovulatory phase, surgery was performed prior to the LH surge when the dominant follicle was ≥14mm and ≤17.5mm. The remaining patients received an injection of human chorionic gonadotropin (s.c., 250 g rhCG, Ovitrelle®, Merck Serono, Geneva, Switzerland) to mimic the endogenous LH surge and underwent surgery after varying lengths of time following rhCG injection: early ovulatory phase (12 to ≤ 18h), late ovulatory phase (>18 to ≤ 34h), and post ovulatory phase (>44 to ≤70h). Frequent TVU examinations after rhCG administration have determined that rupture occurs approximately 36-38h after rhCG (19, 20). Samples for the measurement of serum levels of progesterone and estradiol were taken immediately before surgery to confirm the patients ovulatory phase category (7). The entire intact dominant follicle was excised from the ovary using scissors and without use of diathermy, and placed inside a laparoscopic sac to be retrieved through a suprapubic trocar incision and processed intact for immunohistochemistry or bisected for the collection of granulosa and theca cells.
The intact follicles for immunohistochemistry were fixed in 4% paraformaldehyde overnight, embedded in paraffin and sectioned at 7μm. For cell isolation, granulosa cells were collected by dissecting the follicle to release the loosely attached cells. The mural granulosa cells were then gently scraped from the follicle wall and pooled with the loosely attached granulosa cells. Theca cells of the interna layer were harvested mechanically from the remnant of the follicle by separating the theca interna layer from the theca externa layer. This theca interna cell layer also contains vascular cells, leukocytes and fibroblasts (6). The cells were frozen in liquid nitrogen for subsequent processing and analysis of mRNA expression for key MMPs and ADAMTS associated with ovulation (MMP1, MMP8, MMP13, MMP19, ADAMTS1 and ADAMTS9) by real time RTPCR. Theca cells were obtained from all four periovulatory phases, but granulosa cells could not be collected from the postovulatory group since large quantities had been lost at follicular rupture.
In Vitro Fertilization (IVF) Granulosa-Lutein Cell Experimentation
Due to the scarcity of human follicles, studies were performed to examine expression of MMP and ADAMTS in human granulosa-lutein cells from women undergoing in vitro fertilization (IVF). The study was approved by the human Institutional Review Board (IRB) of the University of Kentucky. Women (n = 6) were treated with a GnRH agonist (Lupron; TAP Pharmaceutical Products, Inc., Lake Forest, IL), given recombinant FSH (Gonal-f; Serono, Inc., Rockland, MA) to induce follicular growth, and monitored by ultrasound and serum estradiol levels.
When the two largest follicles reached an average diameter of ≥18 mm, rhCG (250 g, Ovitrelle®; Merck Serono, Inc.) was administrated s.c. and granulosa-lutein cells were collected 34-36h post hCG administration by ultrasound guided aspiration. After oocyte removal, follicular aspirates containing granulosa-lutein cells with red blood cells (RBCs) were pooled from multiple follicles of the same patient and placed in OptiMEM I media. The cells were pelleted by centrifugation (2000xg for 5 min), resuspended in 1 ml of OptiMEM I media, and Percoll gradient centrifugation was used to separate the RBCs from the granulosa-lutein cells. The isolated granulosa-lutein cells were cultured (2×104 cells/ml in a 6-well tissue culture plate) in OptiMEM media containing 10% FBS for 6-7 days with the media being changed every 24 hours. This 6-7 day acclimation culture period allowed the cells to regain responsiveness to hCG after being desensitized by the ovulatory dose of hCG as previously shown (21). For experimentation, these cells were serum starved for 1 hour and then treated with or without hCG (1 IU) and collected at 6, 12, and 24 hours.
HGL5 Cell Experimentation
The HGL5 cell line is a virally transformed luteinized granulosa cell line that has been well-characterized (22). The use of the HGL5 cell line overcomes a major problem of cell variability in primary human granulosa cells (23) and was used to examine the regulation of the MMPs and the ADAMTSs.
HGL5 cells were cultured in DMEM/F12 medium supplemented with 10% Ultra-low IgG fetal bovine serum, 1% ITS (insulin-transferrin-selenium), 1% Pen-Strep, and 10 mg/ml gentamicin. Cells were plated in 12-well plates at a density of 1.5×105 cells per ml per well. For experimentation, these cells were serum starved for 1 hour and then treated with or without forskolin (FSK; in ethanol, 10μM) plus phorbol-12-myristate-13-acetate (PMA; in DMSO, 20nM) and collected at 6, 12, and 24 hours.
RNA Isolation and Real-Time Quantitative Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from granulosa, theca, granulosa-lutein, or HGL5 cells using Trizol™ reagent and reversed transcribed according to the manufacturer's protocol. Messenger RNA expression levels for the collagenase subfamily of MMPs (MMP1, MMP8, MMP13) along with MMP19 and select ADAMTS were analyzed by real time PCR using TaqMan methodologies. TaqMan primers are as follows: human MMP1 (Hs00899658_m1), human MMP8 (Hs01029057_m1), human MMP13 (Hs00233992_m1), human MMP19 (Hs00275699_m1), human ADAMTS1 (Hs00199608_m1) and human ADAMTS9 (Hs00332069_m1). Human GAPDH (VIC® MGB Probe, #4326317E) was used as endogenous control gene. PCR reactions were performed on a Mx3000P® QPCR System (Agilent Technologies, Inc., Santa Clara, CA). The thermal cycling steps were programmed as follows: 2 min at 50°C to permit AmpErase® uracil-N-glycosylase optimal activity, a denaturation step for 10 min at 95°C, and then 15 sec at 95°C and 1 min at 60°C for 45 cycles, followed by 1 min at 95°C, 30 sec at 58°C and 30 sec at 95°C for ramp dissociation. The relative amount of mRNA in each sample was calculated following the 2-ΔΔCT method and normalized to GAPDH. The number of samples used in each experiment is indicated in the figure legends.
Immunohistochemistry of Human Follicles Collected across the Periovulatory Period
Immunohistochemistry was performed as previously described (24). Briefly, sections containing human follicles (n=a minimum of 2-3 sections from 3 follicles collected at each periovulatory time point) were deparaffinized, dehydrated, washed in Tris-buffered saline (TBS) and then quenched with Peroxidazed 1 to block endogenous peroxide activity. Antigen retrieval utilized DakoCytomation Target Retrieval solution (Dako North America, Inc., Carpinteria, CA) for 20 min at 100°C. The slides were washed and then were blocked with Background Sniper. Sections were incubated with antibodies for either MMP1 (ab4043 at 1:100 dilution; Abcam, Cambridge, MA); MMP19 (RP1MMP19 at 1:500 dilution; Triple Point Biologics Inc., Forest Grove, OR); ADAMTS1 (RP4ADAMTS-1 at 1:500 dilution; Triple Point Biologics Inc.); or ADAMTS9 (RP5ADAMTS-9 at 1:250 dilution; Triple Point Biologics Inc.); overnight at 4°C, washed and incubated with biotinylated secondary antibody for 1h at room temperature (Trekkie Biotinylated Rabbit Link). To amplify the reaction signal, the sections were treated with a conjugated streptavidin alkaline phosphatase (TrekAvidin-AP label) and visualized using a Vulcan Fast Red chromogen, counterstained with hematoxylin and coverslipped using Permount® mounting media (Thermo Fisher, Waltham, MA). In control sections, the primary antibody was omitted.
Statistical Analysis
Results are expressed as mean ± SEM. Differences in mRNA were analyzed by Kruskal-Wallis test (follicles) or ANOVA (IVF and HGL5 cells), and post-hoc comparisons were performed when appropriate using Dunn's Multiple Comparison test (follicles) or Tukey's (IVF and HGL5 cells), P<0.05 was considered significant. Additional analysis was performed comparing the thecal expression in the preovulatory time period to the pooled early and late times by Student's t-test.
RESULTS
Expression of MMP and ADAMTS mRNA in the human ovary
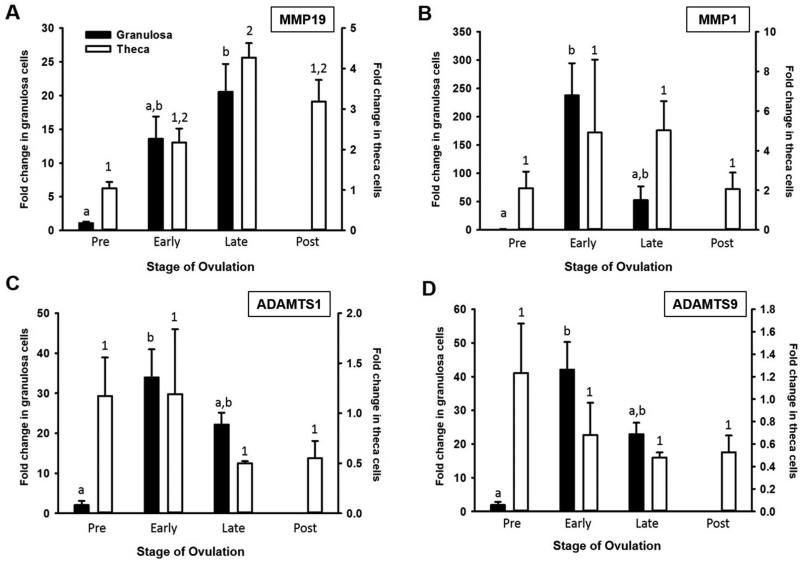
Administration of hCG induced the mRNA expression of MMP19, MMP1, ADAMTS1, and ADAMTS9 in the granulosa cell compartment (Fig 1). The temporal mRNA expression pattern of the various MMP and ADAMTS in granulosa cells differed slightly among the proteinases. Expression of MMP19 (Fig 1A) was elevated during the late ovulatory period whereas expression of MMP1 (Fig 1B), ADAMTS1 (Fig 1C), and ADAMTS9 (Fig 1D) increased during the early ovulatory period in granulosa cells. For MMP8 and MMP13, mRNA expression was extremely low and did not change throughout the periovulatory period in either granulosa or theca cells (data not shown).
Figure 1.
MMP and ADAMTS mRNA expression in human granulosa and theca cells from timed follicles collected across the periovulatory period. (A) MMP19, (B) MMP1, (C) ADAMTS1, (D) ADAMTS 9 (n=5 per group). Different letters or numbers denote significant differences between groups (P<0.05).
In theca cells, MMP19 was the only proteinase that was also induced by hCG with mRNA expression increasing during the late ovulatory period and remaining elevated during the postovulatory period (Fig 1A). Due to the variability present in the theca samples, the early and late periods were combined, reanalyzed, and compared to the preovulatory period. Pooled analysis revealed an increase in MMP1 mRNA and a decrease in ADAMTS9 mRNA after hCG administration. The postovulatory phase is not presented for the granulosa cells due to difficulties in collecting granulosa cells following ovulation.
Localization of MMPs and ADAMTSs in human follicles
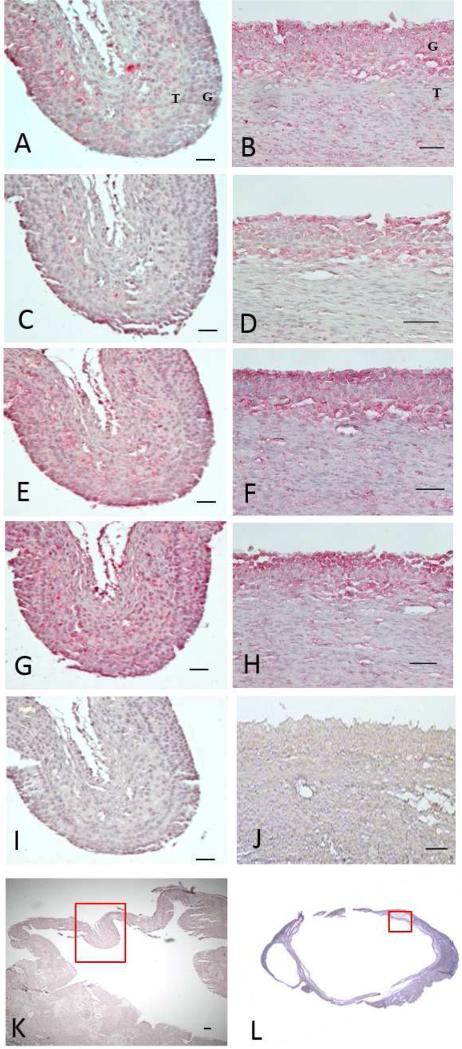
To explore the pattern of protein expression for the MMPs and ADAMTSs, proteins were localized in human follicles. For comparison, immunohistochemistry for all of the MMPs and ADAMTSs is depicted on adjacent sections from the same area of an early follicle (Fig 2A, 2C, 2E, 2G, 2I, and 2K) and a late ovulatory follicle (Fig 2B, 2D, 2F, 2H, 2J, and 2L). An overall theme regarding the expression of the various proteinases was observed with increased staining intensity present in the granulosa and theca cell layers after hCG administration (Fig 2). At 12-18h after hCG, there was diffuse staining in the granulosa, theca and stroma for MMP19 (Fig 2A), MMP1 (Fig 2C), and ADAMTS1 (Fig 2E), whereas ADAMTS9 staining was observed throughout the follicular wall (Fig 2G). At 18-34h after hCG administration, intense staining was present for all the proteinases, MMP19 (Fig 2B), MMP1 (Fig 2D), ADAMTS1 (Fig 2F), and ADAMTS9 (Fig 2H) in the granulosa cell layer with minimal staining in the adjacent stroma. There was no staining in control sections where the primary antibody was omitted (Fig 2I, 2J). These findings correlate with the observations of the mRNA expression, in which these proteinases are highly abundant throughout the follicular cell layers prior to ovulation.
Figure 2.
Immunohistochemical localization of MMP and ADAMTS expression in follicles collected during the early (A, C, E, G, I, K) and late (B, D, F, H, J, L) ovulatory time periods (n=4-5 per group). (A, B) MMP19, (C, D) MMP1, (E, F) ADAMTS1, (G, H) ADAMTS 9, (I, J) no primary antibody. Low magnification photomicrographs of the unstained follicle with red box indicating where the proceeding photomicrographs were taken (K, L). MMP and ADAMTS proteins were visualized by the chromogen Vulcan red, hematoxylin was used as the counterstain. Bars indicate 50μm, except (K) where bar indicates 100μm. Panel (L) is a composite of 4 pictures taken at 4x magnification. G=granulosa layer, T=theca layer.
Expression of MMP and ADAMTS in Granulosa-Lutein Cells
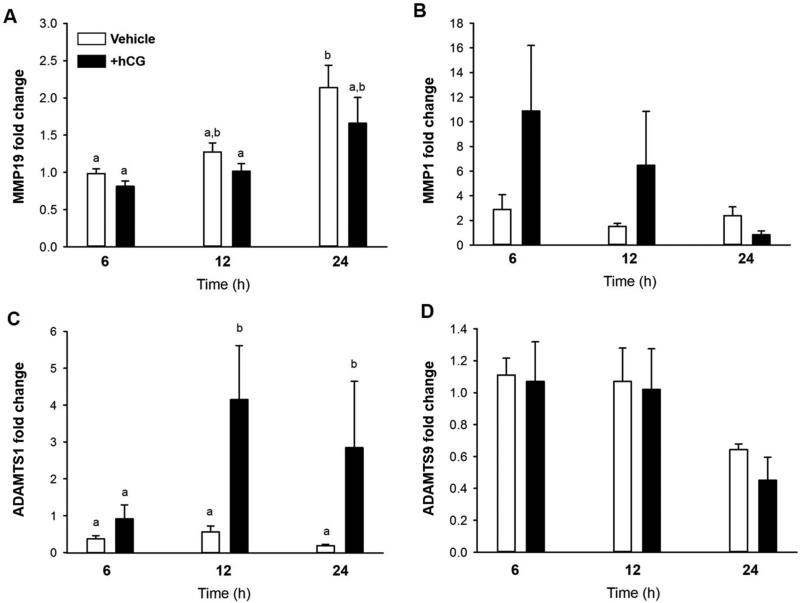
Previous studies have demonstrated that granulosa-lutein cells can be acclimatized to regain responsiveness to hCG to express certain genes associated with the ovulatory process (21). To determine if cultured cells could be used as a model to mimic our in vivo findings, granulosa-lutein cells from IVF were cultured for 6-7 days and then stimulated with hCG. As seen in Fig 3C, there was an induction of ADAMTS1 mRNA at 12 and 24 hours after hCG. However, there was no induction of MMP19 (Fig 3A), MMP1 (Fig 3B), or ADAMTS9 (Fig 3D) by hCG stimulation although there was a time dependent increase in MMP19 mRNA expression.
Figure 3.
Induction of MMP and ADAMTs mRNA expression in human granulosa-lutein cells from IVF patients. Granulosa-lutein cells were cultured for 6-7 days, stimulated with hCG and mRNA expression examined at 6, 12 and 24 hours (n=3). Different letters denote significant differences between groups (P<0.05). (A) MMP19, (B) MMP1, (C) ADAMTS1, (D) ADAMTS 9.
Expression of MMP and ADAMTS in HGL5 Cells
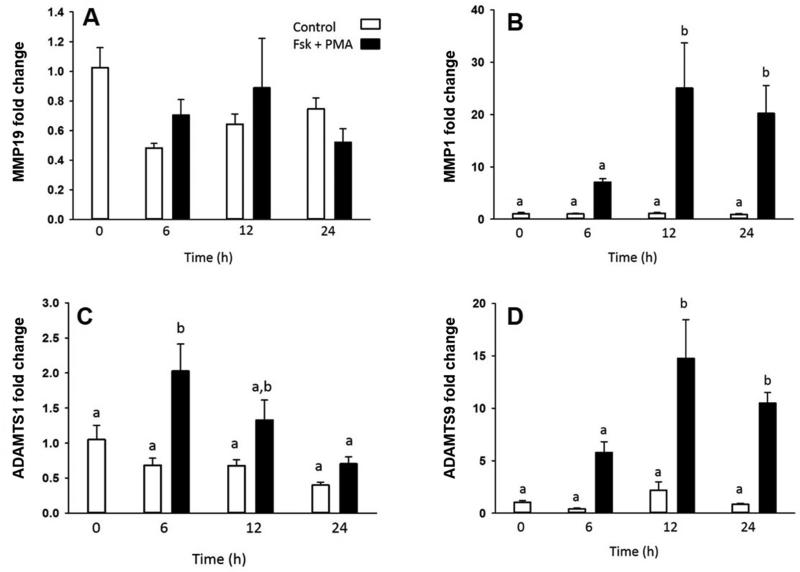
Investigators have used virally transformed granulosa cells to gain insight into granulosa cell function (23). Thus, we used this model to explore the expression of the MMPs and ADAMTSs in HGL5 cells. Treatment with FSK+PMA resulted in an increase in the mRNA levels of MMP1 (Fig 4B), ADAMTS1 (Fig 4C), and ADAMTS9 (Fig 4D), although the magnitude and temporal sequence of this induction varied among the proteinases. ADAMTS1 mRNA increased by 6 hours after FSK+PMA treatment and returned to control levels by 12 hours (Fig 4C). Expression of MMP1 and ADAMTS9 increased at 12 hours after FSK+PMA and remained elevated through 24 hours (Fig 4B and 4D respectively). There was no change in the expression of MMP19 mRNA levels after FSK+PMA administration (Fig 4A).
Figure 4.
MMP and ADAMTS mRNA expression in HGL5 cells cultured in the presence or absence of forskolin plus PMA for up to 24 hours (n=3). Different letters denote significant differences between groups (P<0.05). (A) MMP19, (B) MMP1, (C) ADAMTS1, (D) ADAMTS 9.
DISCUSSION
The present study identifies for the first time key MMPs and ADAMTS that are stimulated after hCG administration in the dominant follicle of women across the periovulatory period of the menstrual cycle. This hCG induction of MMP1, MMP19, ADAMTS1 and ADAMTS9 in the human mimics the periovulatory induction of these proteinases in other species, such as the monkey, cow, rabbit, rat and mouse (discussed below), suggesting there may be a commonality in the action of these proteinases in the ovulatory process.
Exploration of the members of the collagenase subfamily of MMPs in the present study (MMP1, MMP8, MMP13) revealed an increase in mRNA for MMP1 with no change in MMP8, the neutrophil collagenase, or collagenase 3, MMP13. The current findings of an induction of MMP1 mRNA expression by hCG in the human mimics the changes in this collagenase reported in many other species. For example in the rhesus macaque, hCG administration stimulates granulosa cell MMP1 mRNA by 12 hours after hCG and levels remained elevated at 24 and 36 hours. This hCG induction of MMP1 mRNA was mediated by progesterone as demonstrated by ablation and replacement studies (25).
A slightly different pattern of MMP1 mRNA expression was observed in intact follicles collected from rhesus macaques undergoing a controlled ovulation protocol (26). When the entire follicle was analyzed, Peluffo and colleagues observed a stimulation of MMP1 mRNA at 12 hours after hCG which declined at 24 and 36 hours, unlike the isolated granulosa cells where MMP1 mRNA remained elevated (25). This difference in the pattern of expression may be related to analysis of isolated granulosa cells compared to the intact follicle. In the present study, granulosa cell expression of MMP1 mRNA was highly induced and approximately 100 fold higher than expression in the theca. Therefore, the possibility exists that analysis of the intact follicle (26) may dilute the overall granulosa cell expression reported in the monkey (25).
MMP19 has been of interest in the ovulatory process because, like MMP1, it is induced by hCG in numerous species (26-31). In the present study, we observed that expression of MMP19 mRNA was stimulated in both the granulosa and theca cells and remained elevated prior to and following follicular rupture. Likewise in the rhesus macaque, MMP19 mRNA was elevated at 12 h after hCG in the dominant follicle and remained elevated across the periovulatory period and in the postovulatory follicle (26). Similar findings that LH induces the expression of MMP19 have been reported in the rat (27), mouse (28), and fish (29). Interestingly, there appears to be temporal differences in MMP19 expression in the bovine as the hCG induction of MMP19 occurs after ovulation (30, 31).
The importance of ADAMTS1 in the ovulatory process is highlighted by the observation that mice lacking ADAMTS1 are subfertile (32, 33). These animals showed a 90% decrease in ovulation rate due to a decline in follicular development, changes in lymphangiogenesis, and alterations in the final steps of the ovulatory process. These alterations include a compromise in the thinning and rupture of the follicle wall as well as an inability to cleave versican in the expanded COC matrix (32-34). As in the mouse, ADAMTS1 has been reported to be associated with follicular growth in the human (35) and reflect oocyte competence (35, 36). For example, ADAMTS1 expression was 3 fold higher in cumulus cells from oocytes that underwent successful fertilization than in cumulus cells of oocytes that were not fertilized (35) suggesting a role for ADAMTS1 in human cumulus function. However, the present findings provide the first evidence that ADAMTS1 is increased after hCG during the periovulatory period in the human and provide further support for an important role of this proteinase in the ovulatory process. Yet, these observations in the human are in contrast to reports in the macaque where there was no change in ADAMTS1 mRNA expression in the dominant follicle until after ovulation (26) suggesting a role in corpus luteum function. This difference points out the dissimilarities that exist in ovarian physiology between primate species and highlights the importance of also including human studies in experiments aimed to elucidate the complex physiology of reproduction.
Few reports exist on the expression of ADAMTS9 in the ovary. In the present study, we observed an increase in ADAMTS9 mRNA in granulosa cells during the early ovulatory period which remained elevated until the time of ovulation. These findings are similar to reports in preovulatory follicles from the macaque, where there was a 4 fold increase in ADAMTS9 within 12 hours after hCG administration which remained elevated throughout the periovulatory period (26). Immunohistochemistry confirmed localization to the granulosa and theca cells as well as the stroma (26). In PCOS patients, the expression of ADAMTS9 was lower in cumulus cells derived from oocytes with a higher developmental competence to develop into a blastocyst compared to oocytes with a lower developmental competence suggesting that ADAMTS9 may play a role during the oocyte nuclear maturation progress (37).
Due to the difficulties in obtaining timed in vivo human ovarian tissue, we explored in vitro models in an attempt to recapitulate the in vivo situation which could then be experimentally manipulated to understand MMP regulation. The granulosa-lutein cell model takes advantage of the easy access to cells collected immediately prior to ovulation during IVF protocols, however, these cells have been stimulated with hCG. To allow the granulosa-lutein cells to regain responsiveness to hCG after being desensitized by the ovulatory dose of hCG, cells were cultured for 6-7 days. Freimann and colleagues reported that cells regain their responsiveness as seen by the induction of amphiregulin (AREG) and epiregulin (EREG) in this model (21). Somewhat surprisingly, in the granulosa lutein cells only ADAMTS1 was increased whereas we have observed that this model mimics expression of known hCG induced ovulatory genes such as PTGS2, CCL20 and PGR (unpublished observations).
The HGL5 cells are a virally transformed human luteinized granulosa cell line that has been used by numerous investigators to query granulosa cell function (22, 23, 38). These cells are not LH or FSH responsive but will respond to forskolin or dibutyryl cAMP to produce progesterone. However, HGL5 cells do not mimic all of the actions of granulosa cells such as production of inhibin alpha (23). We observed that HGL5 cells were able to respond to forskolin with an induction of three of the four MMPs observed in vivo. These findings indicate that the granulosalutein cell model does not mimic the in vivo expression of the MMPs whereas the HGL5 cells more closely approximates the in vivo MMP expression.
In conclusion, the collection of the dominant follicle throughout the periovulatory period, has allowed us to identify key proteolytic remodeling enzymes involved in human follicular rupture. For example, MMP1 cleaves triple helical type I collagen which is a major component of the extracellular matrix (ECM) of the follicle wall while MMP19 acts on the ECM, other MMPs (activates MMP9), and urokinase receptors. ADAMTS1 cleaves follicular versican and ADAMTS9 acts on aggrecan core protein. We would propose that the presence of these proteinases in both the granulosa and thecal cell layers work in concert to regulate breakdown of the follicular wall and release of the oocyte.
Capsule.
Administration of hCG induces granulosa cell expression of mRNA for the proteinases MMP1, MMP19, ADAMTS1 and ADAMTS9 in the dominant follicle collected across the periovulatory period of the menstrual cycle.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Bruce Carr (University of Texas Southwestern) for the use of the HGL5 cells. This work was supported by National Institutes of Health HD057446, HD71875, UL1TR000117 (TEC) and the Swedish Research Council (11607, M.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rodgers RJ, Irving-Rodgers HF, Russell DL. Extracellular matrix of the developing ovarian follicle. Reproduction. 2003;126:415–24. doi: 10.1530/rep.0.1260415. [DOI] [PubMed] [Google Scholar]
- 2.Fujiwara H, Honda T, Ueda M, Nakamura K, Yamada S, Maeda M, et al. Laminin suppresses progesterone production by human luteinizing granulosa cells via interaction with integrin alpha 6 beta 1. Journal of Clinical Endocrinology and Metabolism. 1997;82:2122–8. doi: 10.1210/jcem.82.7.4095. [DOI] [PubMed] [Google Scholar]
- 3.Yamada S, Fujiwara H, Honda T, Higuchi T, Nakayama T, Inoue T, et al. Human granulosa cells express integrin alpha2 and collagen type IV: possible involvement of collagen type IV in granulosa cell luteinization. Mol Hum Reprod. 1999;5:607–17. doi: 10.1093/molehr/5.7.607. [DOI] [PubMed] [Google Scholar]
- 4.Iwahashi M, Muragaki Y, Ooshima A, Nakano R. Type VI collagen expression during growth of human ovarian follicles. Fertil Steril. 2000;74:343–7. doi: 10.1016/s0015-0282(00)00618-x. [DOI] [PubMed] [Google Scholar]
- 5.Irving-Rodgers HF, Rodgers RJ. Extracellular matrix of the developing ovarian follicle. SeminReprodMed. 2006;24:195–203. doi: 10.1055/s-2006-948549. [DOI] [PubMed] [Google Scholar]
- 6.Lind AK. Ph.D. The Sahlgrenska Academy; 2006. Human Ovulation: Studies on Collagens, gelatinases and tissue inhibitors of metalloproteinases. pp. 1–108. [Google Scholar]
- 7.Lind AK, Weijdegard B, Dahm-Kahler P, Molne J, Sundfeldt K, Brannstrom M. Collagens in the human ovary and their changes in the perifollicular stroma during ovulation. Acta obstetricia et gynecologica Scandinavica. 2006;85:1476–84. doi: 10.1080/00016340601033741. [DOI] [PubMed] [Google Scholar]
- 8.Espey LL. Ovulation as an inflammatory reaction-a hypothesis. Biology of Reproduction. 1980;22:73–106. doi: 10.1095/biolreprod22.1.73. [DOI] [PubMed] [Google Scholar]
- 9.Curry TE, Jr., Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocrine Reviews. 2003;24:428–65. doi: 10.1210/er.2002-0005. [DOI] [PubMed] [Google Scholar]
- 10.Dahm-Kahler P, Lofman C, Fujii R, Axelsson M, Janson PO, Brannstrom M. An intravital microscopy method permitting continuous long-term observations of ovulation in vivo in the rabbit. Hum Reprod. 2006;21:624–31. doi: 10.1093/humrep/dei394. [DOI] [PubMed] [Google Scholar]
- 11.Okamura H, Takenaka A, Yajima Y, Nishimura T. Ovulatory changes in the wall at the apex of the human Graafian follicle. J Reprod Fertil. 1980;58:153–5. doi: 10.1530/jrf.0.0580153. [DOI] [PubMed] [Google Scholar]
- 12.Curry TE, Jr., Smith MF. Impact of extracellular matrix remodeling on ovulation and the folliculo-luteal transition. SeminReprodMed. 2006;24:228–41. doi: 10.1055/s-2006-948552. [DOI] [PubMed] [Google Scholar]
- 13.Ohnishi J, Ohnishi E, Shibuya H, Takahashi T. Functions for proteinases in the ovulatory process. Biochimica et Biophysica Acta. 2005;1751:95–109. doi: 10.1016/j.bbapap.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Goldman S, Shalev E. MMPS and TIMPS in ovarian physiology and pathophysiology. Front Biosci. 2004;9:2474–83. doi: 10.2741/1409. [DOI] [PubMed] [Google Scholar]
- 15.Murdoch WJ, Gottsch ML. Proteolytic mechanisms in the ovulatory folliculo-luteal transformation. Connective Tissue Research. 2003;44:50–7. [PubMed] [Google Scholar]
- 16.Ny T, Wahlberg P, Brandstrom IJ. Matrix remodeling in the ovary: regulation and functional role of the plasminogen activator and matrix metalloproteinase systems. MolCell Endocrinol. 2002;187:29–38. doi: 10.1016/s0303-7207(01)00711-0. [DOI] [PubMed] [Google Scholar]
- 17.Smith MF, Ricke WA, Bakke LJ, Dow MPD, Smith GW. Ovarian tissue remodeling: role of matrix metalloproteinases and their inhibitors. Molecular and Cellular Endocrinology. 2002;191:45–56. doi: 10.1016/s0303-7207(02)00054-0. [DOI] [PubMed] [Google Scholar]
- 18.McCord LA, Li F, Rosewell KL, Brannstrom M, Curry TE. Ovarian expression and regulation of the stromelysins during the periovulatory period in the human and the rat. Biology of Reproduction. 2012;86:78. doi: 10.1095/biolreprod.111.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen AG, ls-Nielsen B, Hornnes PJ, Franch AL. Time interval from human chorionic gonadotrophin (HCG) injection to follicular rupture. Human Reproduction. 1995;10:3202–5. doi: 10.1093/oxfordjournals.humrep.a135888. [DOI] [PubMed] [Google Scholar]
- 20.Lind AK, Dahm-Kahler P, Weijdegard B, Sundfeldt K, Brannstrom M. Gelatinases and their tissue inhibitors during human ovulation: increased expression of tissue inhibitor of matrix metalloproteinase-1. Molecular Human Reproduction. 2006;12:725–36. doi: 10.1093/molehr/gal086. [DOI] [PubMed] [Google Scholar]
- 21.Freimann S, Ben-Ami I, Dantes A, Ron-El R, Amsterdam A. EGF-like factor epiregulin and amphiregulin expression is regulated by gonadotropins/cAMP in human ovarian follicular cells. Biochem Biophys Res Commun. 2004;324:829–34. doi: 10.1016/j.bbrc.2004.09.129. [DOI] [PubMed] [Google Scholar]
- 22.Rainey WH, Sawetawan C, Shay JW, Michael MD, Mathis JM, Kutteh W, et al. Transformation of human granulosa cells with the E6 and E7 regions of human papillomavirus. Journal of Clinical Endocrinology and Metabolism. 1994;78:705–10. doi: 10.1210/jcem.78.3.8126145. [DOI] [PubMed] [Google Scholar]
- 23.Havelock JC, Rainey WE, Carr BR. Ovarian granulosa cell lines. Mol Cell Endocrinol. 2004;228:67–78. doi: 10.1016/j.mce.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Rosewell KL, Li F, Puttabyatappa M, Akin JW, Brannstrom M, Curry TE., Jr Ovarian expression, localization, and function of tissue inhibitor of metalloproteinase 3 (TIMP3) during the periovulatory period of the human menstrual cycle. Biology of Reproduction. 2013;89:121. doi: 10.1095/biolreprod.112.106989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaffin CL, Stouffer RL. Expression of matrix metalloproteinases and their tissue inhibitor messenger ribonucleic acids in Macaque periovulatory granulosa cells: Time course and steroid regulation. Biology of Reproduction. 1999;61:14–21. doi: 10.1095/biolreprod61.1.14. [DOI] [PubMed] [Google Scholar]
- 26.Peluffo MC, Murphy MJ, Talcott BS, Stouffer RL, Hennebold JD. Systematic analysis of protease gene expression in the rhesus macaque ovulatory follicle: metalloproteinase involvement in follicle rupture. Endocrinology. 2011;152:3963–74. doi: 10.1210/en.2011-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jo M, Curry TE., Jr Regulation of matrix metalloproteinase-19 messenger RNA expression in the rat ovary. Biology of Reproduction. 2004;71:1796–806. doi: 10.1095/biolreprod.104.031823. [DOI] [PubMed] [Google Scholar]
- 28.Hagglund AC, Ny A, Leonardsson G, Ny T. Regulation and localization of matrix metalloproteinases and tissue inhibitors of metalloproteinases in the mouse ovary during gonadotropin-induced ovulation. Endocrinology. 1999;140:4351–8. doi: 10.1210/endo.140.9.7002. [DOI] [PubMed] [Google Scholar]
- 29.Crespo D, Pramanick K, Goetz FW, Planas JV. Luteinizing hormone stimulation of in vitro ovulation in brook trout (Salvelinus fontinalis) involves follicle contraction and activation of proteolytic genes. Gen Comp Endocrinol. 2013;188:175–82. doi: 10.1016/j.ygcen.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 30.Kliem H, Welter H, Kraetzl WD, Steffl M, Meyer HH, Schams D, et al. Expression and localisation of extracellular matrix degrading proteases and their inhibitors during the oestrous cycle and after induced luteolysis in the bovine corpus luteum. Reproduction. 2007;134:535–47. doi: 10.1530/REP-06-0172. [DOI] [PubMed] [Google Scholar]
- 31.Berisha B, Steffl M, Welter H, Kliem H, Meyer HH, Schams D, et al. Effect of the luteinising hormone surge on regulation of vascular endothelial growth factor and extracellular matrix-degrading proteinases and their inhibitors in bovine follicles. Reproduction, Fertility, and Development. 2008;20:258–68. doi: 10.1071/rd07125. [DOI] [PubMed] [Google Scholar]
- 32.Brown HM, Dunning KR, Robker RL, Pritchard M, Russell DL. Requirement for ADAMTS-1 in extracellular matrix remodeling during ovarian folliculogenesis and lymphangiogenesis. Developmental Biology. 2006;300:699–709. doi: 10.1016/j.ydbio.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Shozu M, Minami N, Yokoyama H, Inoue M, Kurihara H, Matsushima K, et al. ADAMTS-1 is involved in normal follicular development, ovulatory process and organization of the medullary vascular network in the ovary. Journal of Molecular Endocrinology. 2005;35:343–55. doi: 10.1677/jme.1.01735. [DOI] [PubMed] [Google Scholar]
- 34.Russell DL, Doyle KM, Ochsner S, Sandy JD, Richards JS. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem. 2003;278:42330–9. doi: 10.1074/jbc.M300519200. [DOI] [PubMed] [Google Scholar]
- 35.Yung Y, Maman E, Konopnicki S, Cohen B, Brengauz M, Lojkin I, et al. ADAMTS-1: a new human ovulatory gene and a cumulus marker for fertilization capacity. Mol Cell Endocrinol. 2010;328:104–8. doi: 10.1016/j.mce.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Guzman L, Adriaenssens T, Ortega-Hrepich C, Albuz FK, Mateizel I, Devroey P, et al. Human antral follicles <6 mm: a comparison between in vivo maturation and in vitro maturation in non-hCG primed cycles using cumulus cell gene expression. Mol Hum Reprod. 2013;19:7–16. doi: 10.1093/molehr/gas038. [DOI] [PubMed] [Google Scholar]
- 37.Huang X, Hao C, Shen X, Zhang Y, Liu X. RUNX2, GPX3 and PTX3 gene expression profiling in cumulus cells are reflective oocyte/embryo competence and potentially reliable predictors of embryo developmental competence in PCOS patients. Reproductive biology and endocrinology : RB&E. 2013;11:109. doi: 10.1186/1477-7827-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peluso JJ, DeCerbo J, Lodde V. Evidence for a genomic mechanism of action for progesterone receptor membrane component-1. Steroids. 2012;77:1007–12. doi: 10.1016/j.steroids.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]