Abstract
Background
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) used to treat peritoneal surface disease (PSD) from appendiceal cancer have shown variability in survival outcomes. The primary goal of this study was to determine predictors of surgical morbidity and overall survival. The secondary goal was to describe the impact of nodal status on survival after CRS/HIPEC for PSD from low-grade appendiceal (LGA) and high-grade appendiceal (HGA) primary lesions.
Methods
A retrospective analysis of 1,069 procedures from a prospective database was performed. Patient characteristics, tumor grade, nodal status, performance status, resection status, morbidity, mortality, and survival were reviewed.
Results
The study identified 481 CRS/HIPEC procedures: 317 (77.3 %) for LGA and 93 (22.7 %) for HGA lesions. The median follow-up period was 44.4 months, and the 30-day major morbidity and mortality rates were respectively 27.8 and 2.7 %. Major morbidity was jointly predicted by incomplete cytoreduction (p = 0.0037), involved nodes (p < 0.0001), and comorbidities (p = 0.003). Multivariate negative predictors of survival included positive nodal status (p = 0.003), incomplete cytoreduction (p < 0.0001), and preoperative chemotherapy (p = 0.04) in LGA patients and incomplete cytoreduction (p = 0.0003) and preoperative chemotherapy (p = 0.0064) in HGA patients. After complete cytoreduction, median survival was worse for patients with positive nodes than for those with negative nodes in LGA (85 months vs not reached [82 % alive at 90 months]; p = 0.002) and HGA (30 vs 153 months; p < 0.0001).
Conclusions
Positive nodes are associated with decreased survival not only for HGA patients but also for LGA patients even after complete cytoreduction. Nodal status further stratifies histologic grade as a prognostic indicator of survival. Patients with node-negative HGA primary lesions who receive a complete cytoreduction may experience survival comparable with that for LGA patients.
Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) has become an accepted and promising therapy for cases of peritoneal dissemination from appendiceal primary lesions. Observed outcomes vary greatly based on histologic type, tumor grade, and disease volume, with the best survival benefit observed in peritoneal surface disease (PSD) from low-grade appendiceal (LGA) primary lesions. However, even within the LGA group, significant variability was observed. Thus, all appendiceal cancer is not equal.1–4
The primary aim of this study was to determine factors predictive of surgical morbidity and overall survival for patients with PSD from appendiceal cancer. The secondary goal was to describe specifically the impact of nodal status on the overall survival of patients who have undergone CRS/HIPEC for PSD from LGA and high-grade appendiceal (HGA) primary lesions.
METHODS
This retrospective analysis investigated a prospectively maintained database of 1,069 CRS/HIPEC procedures performed between 1991 and 2013. Institutional review board approval was obtained. Data analysis included demographics, age, race, gender, Eastern Cooperative Oncology Group (ECOG) performance status, R status of resection, type of malignancy, histologic grade, nodal status, comorbidities, use of preor postoperative chemotherapy, volume of peritoneal disease, morbidity, mortality, and survival. Appendiceal primary lesions were grouped in cohorts based on histologic grade (low or high)5 and further subclassified based on lymph nodal status. Any well-differentiated primary lesion or histology consistent with mucinous carcinoma peritonei was considered low grade, whereas any moderately to poorly differentiated lesion or anything with signet-ring cells was considered high grade.
Nodes were evaluated in all resected specimens. Right hemicolectomy was routinely performed for high-grade lesions but for low-grade lesions only in cases for which a complete cytoreduction could not otherwise be obtained. Appendiceal cancers with neuroendocrine features were excluded.
The eligibility criteria for CRS/HIPEC specified histologic or cytologic diagnosis of peritoneal carcinomatosis, complete recovery from prior systemic chemotherapy or radiation treatments, a resectable or resected primary lesion, debulkable peritoneal disease, and no extraabdominal disease. The presence of peripheral liver metastases, if readily resectable, was not considered a contraindication. All patients had a complete history and physical exam, tumor markers, and computed tomography (CT) of the chest, abdomen, and pelvis before CRS/HIPEC procedures.
The CRS/HIPEC procedure was performed as previously described by our group.6 The administration of HIPEC was performed using the closed abdominal technique. The majority of the patients were perfused with mitomycin-C (MMC), 40 mg for 2 h, whereas a smaller group was perfused with oxaliplatin (200 mg/m2) within the context of an ongoing prospectively randomized clinical trial. Surgical morbidity and mortality were recorded according to the Clavien and Dindo classification system.7 The R0 and R1 resections were grouped together as complete cytoreductions. Cytoreductions with residual macroscopic disease were characterized as R2 and subdivided based on the size of residual disease as follows: R2a (≤5 mm), R2b (≤2 cm), R2c (>2 cm).
Descriptive statistics including medians and ranges for continuous data and frequencies and percentages for categorical data were calculated. Fisher’s exact tests were used to test for statistically significant differences in categorical variables, and Wilcoxon rank sum tests were used to test for group differences in continuous variables. Overall survival (OS) was calculated from the date of CRS/HIPEC (or first CRS/HIPEC in cases wherein a patient underwent more than one procedure) to the last known date of follow-up evaluation or the date of death. Survival was estimated using the Kaplan–Meier (product-limit) method. Group comparisons of OS were performed using the approximate χ2 statistic for the log-rank test. Cox’s proportional hazards models were fitted to assess uni- and multivariate relationships through regression analysis. Statistical significance was defined as a p value lower than 0.05. All analyses were performed using SAS 9.3 (SAS, Cary, NC, USA).
RESULTS
The review of 1,069 CRS/HIPEC procedures identified 481 (430 patients) performed for appendiceal cancer of epithelial origin. Of those procedures, 317 (77.3 %) were performed for LGA and 93 (22.7 %) for HGA lesions. The median follow-up period for the entire cohort was 44.4 months. The demographic details are presented in Table 1.
TABLE 1.
Cohort characteristics
| Characteristic | All surgeries (n = 481) |
|---|---|
| LGA (node positive/negative) | 317 (19/149) |
| HGA (node positive/negative) | 93 (40/32) |
| Median age: years (range) | 53 (20–87) |
| Male: n (%) | 217 (45.1) |
| Race: n (%) | |
| White | 411 (85.8) |
| Black | 55 (11.5) |
| Other | 13 (2.7) |
| Heart disease (n = 470): n (%) | 45 (9.6) |
| Lung disease (n = 469): n (%) | 19 (4.1) |
| Diabetes (n = 469): n (%) | 42 (9.0) |
| Median BMI (n = 445): kg/m2 (range) | 26.7 (16.6–63.3) |
| Smoker (n = 463): n (%) | |
| Current | 47 (10.2) |
| Past | 73 (15.8) |
| No | 343 (74.1) |
| Median preoperative albumin (range) (n = 463) |
3.9 (1.5–5.3) |
| ECOG performance status (n = 469): n (%) | |
| 0/1 | 407 (86.8) |
| 2 | 49 (10.4) |
| 3 | 13 (2.8) |
| Median no. of organs resected: n (range) | 3 (0–10) |
| Colon/rectal resection: n (%) | 266 (55.3) |
| Preoperative ascites (n = 461): n (%) | 111 (24.1) |
| Grade: n (%) | |
| Low | 317 (77.3) |
| High | 93 (22.7) |
| Resection status (n = 475): n (%) | |
| R0/1 | 211 (44.4) |
| R2a | 133 (28.0) |
| R2b | 90 (19.0) |
| R2c | 41 (8.6) |
| Prior surgical score (n = 470): n (%) | |
| 0 | 77 (16.4) |
| 1 | 144 (30.6) |
| 2 | 210 (44.7) |
| 3 | 39 (8.3) |
BMI body mass index, ECOG Eastern Cooperative Oncology Group
Morbidity and Mortality
For the entire cohort, the 30- and 90-day Clavien III/IV major morbidity rates were respectively 27.8 and 37.1 %, whereas the 30- and 90-day minor morbidity rates were 21.4 and 28.0 %. The median number of days spent in the intensive care unit was 1, and the median hospital stay was 9 days. The 30-day mortality rate was 2.7 %, whereas the 90-day mortality rate was 5.6 %. Multivariate analysis evaluating predictors of morbidity demonstrated that incomplete cytoreduction (p = 0.003), comorbidities (p = 0.003), and node involvement (p < 0.001) were associated with an increase in the risk for the development of a postoperative complication (Table 2).
TABLE 2.
Predictors of major and minor morbidity in appendiceal primary lesions treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) according to uni- and multivariate analyses
| Variable | Univariate analysis |
Multivariate analysis (final reduced model) |
||
|---|---|---|---|---|
| OR (95 % CI) | p value | OR (95 % CI) | p value | |
| Grade | 0.001 | |||
| High vs low | 3.2 (1.7–6.0) minor vs none | |||
| 2.1 (1.06–4.1) major vs none | ||||
| Resection status | <0.001 | 0.004 | ||
| R2a vs R0/R1 | 2.6 (1.5–4.5) minor vs none | 3.5 (1.7–7.4) minor vs none | ||
| 2.5 (1.4–4.6) major vs none | 3.2 (1.4–7.0) major vs none | |||
| R2b vs R0/R1 | 3.3 (1.6–6.5) minor vs none | 1.7 (0.7–4.4) minor vs none | ||
| 4.5 (2.2–9.0) major vs none | 3.3 (1.3–8.1) major vs none | |||
| R2c vs R0/R1 | 2.4 (1.04–5.8) minor vs none | 3.2 (0.5–18.6) minor vs none | ||
| 2.7 (1.1–6.6) major vs none | 2.7 (0.98–34.5) major vs none | |||
| Lymph nodes | <0.001 | <0.001 | ||
| Positive vs negative | 4.3 (2.1–8.5) minor vs none | 4.3 (2.1–8.9) minor vs none | ||
| 1.5 (0.6–3.3) major vs none | 1.1 (0.5–2.7) major vs none | |||
| Age | 0.39 | |||
| Race | 0.98 | |||
| Gender | 0.24 | |||
| Preoperative chemotherapy | 0.086 | |||
| No vs yes | 0.52 (0.28–0.94) minor vs none | |||
| 0.79 (0.41–1.53) major vs none | ||||
| Albumin | 0.044 | |||
| Per 1-unit increase | 0.57 (0.37–0.89) minor vs none | |||
| 0.70 (0.44–1.11) major vs none | ||||
| ECOG performance status | <0.001 | |||
| 0/1 vs 2/3 | 0.48 (0.20–1.13) minor vs none | |||
| 0.22 (0.10–0.50) major vs none | ||||
| Comorbidities | 0.001 | 0.003 | ||
| Per each 1 | 1.86 (1.28–2.72) minor vs none | 1.89 (1.17, 3.06) minor vs none | ||
| 1.91 (1.29–2.83) major vs none | 1.55 (0.93, 2.59) major vs none | |||
| Organs resected | 0.002 | |||
| Per each 1 | 1.17 (1.01–1.35) minor vs none | |||
| 1.31 (1.13–1.52) major vs none | ||||
| PCI score | 0.044 | |||
| Per 1-unit increase | 1.02 (0.98–1.05) minor vs none | |||
| 1.05 (1.01–1.09) major vs none | ||||
OR odds ratio, CI confidence interval, ECOG Eastern Cooperative Oncology Group, PCI Peritoneal Carcinomatosis Index
Survival
When survival was recorded as a function of resection for the entire cohort, the patients with R0/R1 complete macroscopic cytoreduction had a survival rate significantly better than the patients with R2a, R2b, or R2c resections (respective medians of 175, 73, 29, and 17 months; p < 0.001) (Fig. 1).
FIG. 1.
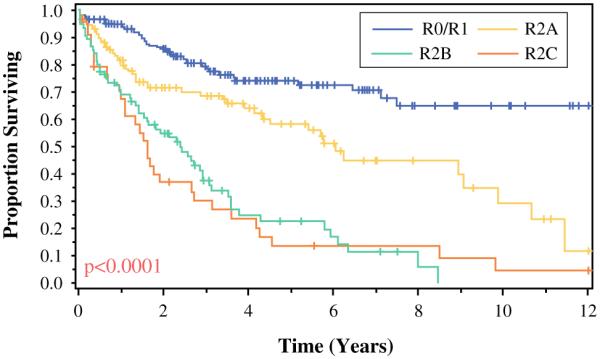
Effect that completeness of cytoreductive surgery has on survival of patients with peritoneal surface disease from appendiceal primary lesions
Univariate analyses were constructed initially, with multivariate models fitted using variables with a p value lower than 0.25 in the single-variable models. Multivariate analysis demonstrated that the jointly negative predictors of survival for patients with LGA were positive nodal status (hazard ratio [HR], 3.6; p = 0.003), incomplete cytoreduction (p < 0.001), and administration of preoperative chemotherapy (HR, 2.2; p = 0.04) (Table 3). Subgroup analysis of the patients who had a complete cytoreduction demonstrated that the median OS for the node-positive patients was less for both LGA lesions (85 months vs not reached [82 % alive at 90 months]) and HGA lesions (30 vs 153 months) (p < 0.001). In addition, the node-positive LGA patients had worse long-term survival than the node-negative HGA subjects, even after an R0/R1 complete cytoreduction (Fig. 2). Administration of postoperative chemotherapy had no effect on OS for LGA primary lesions (p = 0.88).
TABLE 3.
Factors predictive of survival with low- and high-grade appendiceal primary lesions (uni-/multivariate analysis)
| Variable | Low-grade appendiceal |
High-grade appendiceal |
||
|---|---|---|---|---|
| Univariate p value |
Multivariate p value | Univariate p value |
Multivariate p value | |
| Resection status | <0.001 | <0.001 HR = 2.5 (R2a vs R0/1), 9 (R2b vs R0/1), 3.8 (R2c vs R0/1) |
<0.001 | <0.001 HR = 3.8 (R2a vs R0/1), 4.9 (R2b vs R0/1), 5.9 (R2c vs R0/1) |
| Lymph nodes | <0.001 | 0.003 HR = 3.6 P vs N | 0.02 | |
| Age | 0.004 | 0.64 | ||
| Race | 0.22 | 0.44 | ||
| Gender | 0.19 | 0.79 | ||
| Albumin | 0.002 | 0.99 | ||
| ECOG performance status | <0.001 | 0.82 | ||
| Repeat CRS-HIPEC | 0.12 | 0.56 | ||
| No. of comorbidities | 0.19 | 0.42 | ||
| Minor vs no morbidity | 0.66 | 0.04 | ||
| Major vs no morbidity | 0.18 | <0.001 | ||
| No. of organs resected | <0.001 | 0.04 | ||
| Peritoneal carcinomatosis index | 0.001 | 0.008 | ||
| Preoperative chemotherapy | 0.003 | 0.05 HR = 2.2 Y vs N | 0.02 | 0.006 HR = 2.5 Y vs N |
| Postoperative chemotherapy | 0.88 | <0.001 | ||
HR hazard ratio, ECOG Eastern Cooperative Oncology Group, CRS-HIPEC cytoreductive surgery with hyperthermic intraperitoneal chemotherapy, P positive, N negative, Y yes, N no
FIG. 2.
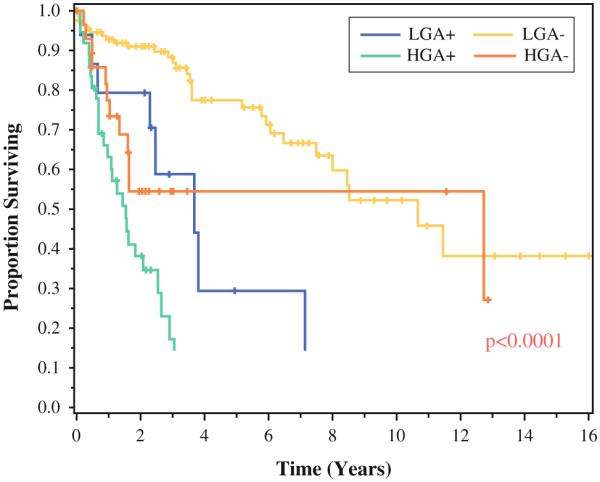
Survival of appendiceal primary lesions after complete cytoreduction based on grade (low grade [LGA]/high grade [HGA]) and nodal status (+ node-positive/− node-negative)
The predictors of poorer OS for the HGA patients in the multivariate analysis were incomplete cytoreduction (HR: 3.8 for R2a, 4.9 for R2b, and 5.9 for R2c; p < 0.001) and administration of preoperative chemotherapy (HR, 2.5; p = 0.006). Factors such as the presence of positive nodes (p = 0.02), major morbidity (p < 0.001), and peritoneal carcinomatosis index (PCI) (p = 0.007) were important only in the univariate analysis (Table 3). The HGA patients receiving postoperative chemotherapy experienced dramatically increased median OS (32 vs 6 months; p < 0.001). In contrast, the delivery of chemotherapy before CRS/HIPEC was associated with a significantly worse median OS (17 vs 30 months; p = 0.02).
To determine the effect of improvements in chemotherapy over time, a separate analysis was performed with HGA patients who received a complete cytoreduction after the year 2000. The median OS for those receiving upfront chemotherapy (n = 18) was 30.5 months versus the median OS not yet reached, with a median follow-up period of 55 months for those receiving CRS/HIPEC before chemotherapy (n = 7) (p = 0.03).
DISCUSSION
The presented data indicate that despite a significant disease burden at presentation, PSD from appendiceal primary lesions can be treated using CRS/HIPEC with acceptable morbidity and mortality. As previously reported, surgical complications were associated with increased volume of disease, preexisting medical comorbidities, poor functional status, and suboptimal nutrition.8 Grade 3/4 morbidity was threefold higher for the patients with incomplete cytoreduction. Incomplete cytoreduction was more frequent for the patients who had a higher volume of disease treated with extensive multivisceral resections. This must be taken into consideration, especially in the case of HGA primary lesions, for which heroic attempts to resect high-volume disease when complete cytoreduction cannot be obtained have the very real potential for increasing major surgical complications without improving survival.9 Increased morbidity also was found in node-positive patients, possibly as a result of more infiltrative biologic behavior.
To study OS, the cohort was divided into LGA and HGA groups and further subclassified based on nodal involvement. Complete cytoreduction was important for both LGA and HGA lesions in predicting improved OS. This is in agreement with prior reports demonstrating that even for HGA lesions, long-term survival is possible if a complete cytoreduction is obtained.10–12
Lymph node involvement was important for the patients with either LGA or HGA lesions who had a complete cytoreduction, indicating that node-positive appendiceal primary lesions, regardless of grade, represent entities with more aggressive biologic behavior than their node-negative counterparts. In addition, the node-negative HGA patients who had a complete cytoreduction exhibited better long-term survival than the node-positive LGA patients. Together, these results emphasize the importance of early diagnosis and referral for CRS/HIPEC when the disease volume permits complete macroscopic cytoreduction. Furthermore, a universally nihilistic approach to low-volume HGA primary lesions is not justified given that a complete cytoreduction offered to node-negative HGA patients demonstrated survival comparable with that of patients who had a complete cytoreduction for LGA primary lesions.
Delivery of preoperative chemotherapy was associated with a significant decrease in OS, not only for LGA patients but also for HGA patients. For LGA node-negative primary lesions, protracted treatment with chemotherapy, which has minimal or no tumor activity, results in a chemotherapy-associated drop in their functional status. Decreased functional status represents a well-documented factor for decreased survival.4,8 In addition, no data exist to suggest that chemotherapy offered to node-negative LGA patients after CRS/HIPEC has any effect either. Therefore, we see a very limited role for systemic chemotherapy among node-negative LGA patients.
For HGA patients, it seems that preoperative systemic delivery of chemotherapy does not achieve adequate peritoneal concentration to control the volume of PSD.1,13 Therefore, for patients with low-volume HGA lesions, if feasible, cytoreduction should be attempted first, followed by systemic chemotherapy, as indicated by the sixfold increase in survival for patients treated in the adjuvant setting. Patients should be selected carefully, however, because surgical complications may delay adjuvant systemic chemotherapy. We currently do not operate on high-volume, high-grade patients unless they have received preoperative chemotherapy and have not progressed with it.
For patients with intermediate volume of disease (PCI 10–18), we lean toward upfront systemic chemotherapy given that no imaging technique is available for accurate evaluation of the volume and distribution of disease. We currently refer our patients for adjuvant chemotherapy only if they have HGA primary lesions, regardless of nodal status, or node-positive LGA primary lesions.
Although the current study represents a large cohort with a very rare disease and is the first to identify nodal disease as a negative prognostic indicator of patients with PSD from LGA cancer, it has several limitations inherent to single-institution retrospective reviews. The selection of patients appropriate for CRS/HIPEC evolved during the study period, and our institution has seen a learning curve in our outcomes during the same time.6,7 Therefore, selection bias inevitably has played a role in the observed outcomes. We frequently see patients after attempted cytoreduction or after decisions about preoperative chemotherapy have already been made. The fact that worse outcomes were observed in patients with high-grade lesions receiving preoperative chemotherapy could reflect a selection bias whereby patients not responding to chemotherapy were referred for CRS/HIPEC. Finally, until quite recently, the PCI has not been collected intraoperatively, so we have used the total number of visceral resections per CRS/HIPEC as a surrogate of disease volume and extent of resection.
In conclusion, incomplete cytoreduction, comorbidities, and nodal involvement predict surgical morbidity for patients with appendiceal primary lesions treated with CRS/HIPEC. Nodal status further stratifies tumor grade as a prognostic indicator of survival. Positive nodes are associated with decreased survival in both HGA and LGA patients even after a complete cytoreduction. However, completely cytoreduced node-negative HGA patients exhibit OS comparable with that for node-positive LGA primary lesions. Early diagnosis and prompt referral for CRS/HIPEC is essential to optimize outcomes for patients with PSD from appendiceal primary lesions.
ACKNOWLEDGMENT
This study was supported by Wake Forest University Biostatistics shared resource NCI CCSG P30CA012197.
Footnotes
DISCLOSURE
Nothing to disclose
REFERENCES
- 1.Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449–56. doi: 10.1200/JCO.2011.39.7166. [DOI] [PubMed] [Google Scholar]
- 2.Elias D, Gilly F, Quenet F, et al. Pseudomyxoma peritonei: a French multicentric study of 301 patients treated with cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol. 2010;36:456–62. doi: 10.1016/j.ejso.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Baratti D, Kusamura S, Nonaka D, et al. Pseudomyxoma peritonei: clinical pathological and biological prognostic factors in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) Ann Surg Oncol. 2008;15:526–34. doi: 10.1245/s10434-007-9691-2. [DOI] [PubMed] [Google Scholar]
- 4.Stewart JHt, Shen P, Russell GB, et al. Appendiceal neoplasms with peritoneal dissemination: outcomes after cytoreductive surgery and intraperitoneal hyperthermic chemotherapy. Ann Surg Oncol. 2006;13:624–34. doi: 10.1007/s10434-006-9708-2. [DOI] [PubMed] [Google Scholar]
- 5.Bradley RF, Stewart JHt, Russell GB, et al. Pseudomyxoma peritonei of appendiceal origin: a clinicopathologic analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol. 2006;30:551–9. doi: 10.1097/01.pas.0000202039.74837.7d. [DOI] [PubMed] [Google Scholar]
- 6.Levine EA, Stewart JHt, Russell GB, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: experience with 501 procedures. J Am Coll Surg. 2007;204:943–53. doi: 10.1016/j.jamcollsurg.2006.12.048. discussion 953–5. [DOI] [PubMed] [Google Scholar]
- 7.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6,336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine EA, Stewart JHt, Shen P, et al. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg. 2014;218:573–85. doi: 10.1016/j.jamcollsurg.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glehen O, Mohamed F, Sugarbaker PH. Incomplete cytoreduction in 174 patients with peritoneal carcinomatosis from appendiceal malignancy. Ann Surg. 2004;240:278–85. doi: 10.1097/01.sla.0000133183.15705.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Halabi H, Gushchin V, Francis J, et al. The role of cytoreductive surgery and heated intraperitoneal chemotherapy (CRS/ HIPEC) in patients with high-grade appendiceal carcinoma and extensive peritoneal carcinomatosis. Ann Surg Oncol. 2012;19:110–4. doi: 10.1245/s10434-011-1840-y. [DOI] [PubMed] [Google Scholar]
- 11.Halabi HE, Gushchin V, Francis J, et al. Prognostic significance of lymph node metastases in patients with high-grade appendiceal cancer. Ann Surg Oncol. 2012;19:122–5. doi: 10.1245/s10434-011-1903-0. [DOI] [PubMed] [Google Scholar]
- 12.Lieu CH, Lambert LA, Wolff RA, et al. Systemic chemotherapy and surgical cytoreduction for poorly differentiated and signet ring cell adenocarcinomas of the appendix. Ann Oncol. 2012;23:652–8. doi: 10.1093/annonc/mdr279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugarbaker PH, Bijelic L, Chang D, et al. Neoadjuvant FOLFOX chemotherapy in 34 consecutive patients with mucinous peritoneal carcinomatosis of appendiceal origin. J Surg Oncol. 2010;102:576–81. doi: 10.1002/jso.21679. [DOI] [PubMed] [Google Scholar]


