Abstract
Dorsal root ganglion (DRG) neurons transduce peripheral pain signals through small-diameter, non-myelinated C-fibers, which, when injured, can regenerate to restore pain sensation. Water channel aquaporin-1 (AQP1) is expressed at the plasma membrane of cell bodies and axons of DRG neurons, where it modulates the sensing of certain types of pain. Here, we found that AQP1 is also involved in DRG axonal growth and regeneration by a mechanism that may involve water transport-facilitated extension of axonal outgrowths. Spontaneous and nerve growth factor-stimulated axonal extension was reduced in cultures of AQP1-deficient DRG neurons and DRG explants compared to the wildtype. Axonal growth in AQP1-deficient DRG cultures was rescued by transfection with AQP1 or a different water-transporting AQP (AQP4), but not by a non-water-transporting AQP1 mutant. Following sciatic nerve compression injury AQP1 expression was increased in DRG neurons in wildtype mice, and DRG axonal growth was impaired in AQP1-deficient mice. Our results indicate AQP1 as a novel determinant of DRG axonal regeneration and hence a potential therapeutic target to accelerate neuronal regeneration.
Keywords: AQP1, DRG, neurite outgrowth, cell migration, nerve regeneration
Introduction
The dorsal root ganglion (DRG) contains cell bodies whose axonal projections in peripheral nerves carry sensory signals to the spinal cord. Peripheral pain signals are transduced by small-diameter, non-myelinated C-fibers arising from DRG neurons. Following peripheral nerve injury, damaged DRG axons can regenerate to restore pain sensation (Vogelaar et al., 2004). Many growth factors, transcription factors and extracellular matrix proteins have been implicated in the DRG response to injury and its associated axonal regeneration (Chen et al., 2007; Patodia and Raivich, 2012; Scheib and Hoke, 2013).
Most small-diameter DRG neurons prominently express aquaporin-1 (AQP1) on plasma membranes in their cell bodies and axonal projections (Oshio et al., 2006; Shields et al., 2007; Zhang and Verkman, 2010). We previously reported the involvement of AQP1 in the nociceptive function of DRG neurons (Zhang and Verkman, 2010). Patch-clamp of isolated DRG neurons showed impaired action potential firing in AQP1 deficiency, which was ascribed to reduced inward, Nav1.8-dependent Na+ currents. The altered DRG neuron function was associated with significant nociceptive impairment in AQP1−/− mice to inflammatory and cold pain. However, neuronal connections in general in AQP1−/− mice are probably not defective as there is no difference in thermal pain threshold between AQP1+/+ and AQP1−/− mice (Oshio et al., 2006; Shields et al., 2007; Zhang and Verkman, 2010).
During the course of our investigation of AQP1-dependent pain mechanisms in DRG neurons we noted the incidental finding that cultures of isolated adult DRG neurons from AQP1 null mice grew fewer and shorter axonal projections than corresponding cultures from wildtype mice. Motivated by this observation, and our previously discovered role of aquaporins in cell migration (Papadopoulos et al., 2008; Saadoun et al., 2005), here we investigated the involvement of AQP1 in axonal growth and regeneration in DRG neurons. We found significant impairment in DRG axonal regeneration in AQP1 deficiency in DRG cell and explant cultures, and in mice following sciatic nerve crush injury, with evidence supporting the involvement of AQP1 and axonal water permeability in DRG axonal elongation.
Material and methods
Mice
AQP1−/− mice in a CD1 genetic background were generated by targeted gene disruption as described (Ma et al., 1998). 8-to-10-week-old, litter-matched wildtype and AQP1−/− mice were used for comparative experiments; for other experiments 8-to-10-week-old age-matched mice were used. Investigators were blinded to genotype information in all experiments. Protocols were approved by the University of California, San Francisco Committee on Animal Research.
Isolation of DRG neurons
Adult mice were decapitated after anesthesia. L4-6 DRGs were removed and treated with collagenase (type IA, 1.5 mg/ml, Sigma) and trypsin (1 mg/ml, Sigma) in Dulbecco's modified Eagle's medium (DMEM) at 37 °C for 30 min. DRGs were then washed five times and gently triturated using fine fire-polished glass pipettes. Dissociated DRG neurons were plated onto poly-D-lysine (100 μg/mL) and laminin (5 μg/mL) -coated coverslips. For electroporation; cells from 15-20, 8-to-10-week-old age-matched AQP1−/− mice were centrifuged at room temperature for 5 min at 800 rpm. 3×105 cells per 20 μl and 2 μg plasmid were electroporated using the Neon Transfection System (Invitrogen, Carlsbad, CA) following the suggested protocol. Cells remained room temperature for 10 min after electroporation and were then plated onto 35-mm diameter wells at a density of 105 cells/well in 2 ml medium.
Compartmented chamber culture
Compartmented cultures were prepared as described (Campenot, 1982). After application of silicone grease (Dow Corning, Midland, MI), a Camp-10 Teflon divider (Tyler Research, Alberta, Canada) was seated into a collagen-coated 35-mm diameter tissue culture dish (Becton Dickinson Labware, Franklin Lakes, NJ). Before setting the divider, the collagen in the middle region of the dish was scraped with a pin rake to allow axon growth on the collagen between the scratches. One drop of medium containing 1% methylcellulose was placed in the scratched region to prevent the silicone grease from adhering to the collagen to allow axon growth under the silicon grease barrier without flow or diffusion of medium between compartments. The integrity of the seal was checked by filling the side compartments with medium and incubating the chambers at 37° overnight; only cultures without leakage were used for experiments (Tsui-Pierchala and Ginty, 1999). Dissociated DRG neurons (50,000/chamber) were plated in the middle compartment and maintained in 100 ng/mL NGF; after 3 days, 1 or 10 ng/mL recombinant NGF (2.5S, PeproTech, Rocky Hill, NJ) was added to the side compartment, and NGF concentration in the middle compartment was reduced to 1 ng/mL. After 7 days many axons had sprouted in the side compartment.
Explant cultures
Sensory ganglia were removed from adult wildtype or AQP1−/− mice, and the dorsal and peripheral roots were carefully trimmed and whole ganglia were cultured in a shallow layer of Matrigel (BD Bioscience, San Jose, CA) (Tonge et al., 1998) in multi-well plates containing 0.5 ml of RPMI medium and 50 ng/ml NGF. After 3 days in culture preparations were fixed with formaldehyde in PBS for 1 h. Axonal outgrowth was measured using Scion Image following immunofluorescence with the neurofilament marker Tuj1 (1:500, Millipore) and Alexa 555-conjugated secondary antibody. The longest axons growing from the cut ends of the peripheral nerves were quantified.
Sciatic nerve crush injury
Mice were anesthetized using 2.5% isoflurane and the skin was shaved. Under aseptic conditions a small incision was made on the skin at the mid-thigh level, followed by a small incision between the gluteus superficialis and biceps femoris muscles to expose the left sciatic nerve. The sciatic nerve was carefully separated from surrounding connective tissue and then crushed by a 15-s compression with a pair of fine Dumon #5 forceps (Fine Science Tools, Foster city, CA). Mice were monitored for 7 d after the injury (Bauder and Ferguson, 2012).
DRG cell line culture
ND7/23 DRG neuroblastoma cells (Wood et al., 1990) were obtained from the European Collection of Cell Cultures and cultured in DMEM supplemented with 10% fetal bovine serum, 50 units/ml penicillin, 50 μg/ml streptomycin, and 2 mM glutamine at 37 °C in humidified air with 5% CO2. In some studies cells were stably transfected with cDNA encoding human AQP1, AQP4, GFP-GPI or AQP1 C189F using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. AQP1 stably transfected cells were selected with 200 μg/ml G418 (Invitrogen). To initiate differentiation, cells were incubated in DMEM containing 0.5% FBS, 1 mM dibutyryl cAMP and 100 ng/mL NGF (Roobol et al., 1995; Wu et al., 2008). Cells were fixed 2 days after differentiation.
Immunohistochemistry
Mice were perfused with 4% paraformaldehyde and DRGs were postfixed for 24 h in 4% paraformaldehyde and processed in paraffin. DRG and sciatic nerve specimens of the control and injured sides from the same mouse were processed together. Sections (7 μm) were deparaffinized in xylene and rehydrated in graded ethanol. Epitope retrieval was done with citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6, 30 min, 95-100 °C). Staining was also done on isolated cells that were seeded onto 12-mm diameter coverslips and fixed with 4% paraformaldehyde. After blocking with 1% bovine serum albumin and 0.2% Triton X-100, samples were incubated with rabbit anti-AQP1 (1:200, Santa Cruz Biotechnology Inc., Santa Cruz, CA), rabbit anti-AQP4 (1:200, Santa Cruz Biotechnology Inc) or chicken anti-peripherin (1: 200, Millipore) antibody, followed by Alexa 488 or 555 conjugated secondary antibody. Nuclei were counterstained blue with 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA).
Neurite outgrowth assay
Neurite outgrowth was assayed in DRG neurons and differentiated ND7/23 cells. Neurons with processes were defined and neurite length measured using the NeuroJ plug-in of NIH ImageJ software (Meijering et al., 2004). The longest process of individual neurons in each replicate was measured and averaged.
Quantitative real-time reverse transcription-PCR
L4-6 DRGs (in control and sciatic nerve crush studies) were collected after euthanasia, total RNA was isolated by a PureLinkTM Micro-to-midi kit (Invitrogen), and cDNA was reverse-transcribed from mRNA with the Super-Script First Strand Synthesis System for reverse transcription-PCR (Invitrogen). Fluorescence-based quantitative real-time reverse transcription-PCR was carried out using the LightCycler 480 and with LightCycler FastStart DNA MasterPLUS SYBR Green I kit (Roche Diagnostics). Primers were as follows: 5′-TGTATGCCTCTGGTCGTACC-3′ (sense) and 5′-CAGGTCCAGACGCAGGATG-3′ (antisense) for GAPDH; 5′-CTCAACTACATGGTCTACATGTTCCA-3′ (sense) and 5′- CCATTTCGGCCTTGACTGT-3′ (antisense) for AQP1, 5′-CAGGAAAGATCCCAAGTCCA-3′ (sense) and 5′-GAACGGAACATTGCACACAC-3′ (antisense) for GAP-43. Data were analyzed by LightCycler software 4.0 (Roche Diagnostics) and reported as calibrated ratios normalized to GAPDH. Data were averaged from six mice.
Results
AQP1 expression in DRG and sciatic nerve
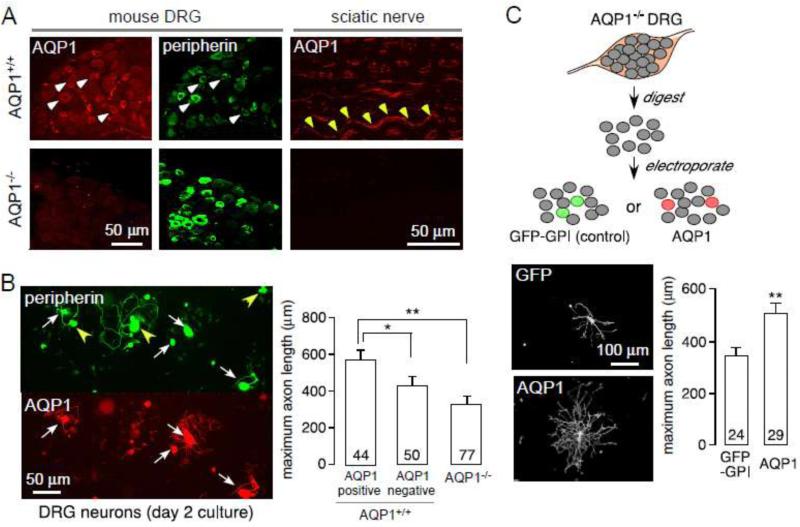
AQP1 immunofluorescence was seen mainly in the plasma membranes of small, <25-mm diameter neurons in mouse DRG (Fig. 1A, left) that project non-myelinated axons to the skin and spinal cord (Oshio et al., 2006; Shields et al., 2007). Double immunostaining with peripherin, a marker of small-diameter neurons (Lariviere et al., 2002), showed that >95 % of AQP1-positive neurons were peripherin-positive, indicating that nearly all AQP1-expressing cells are small nonmyelinated neurons. However, only ~70% peripherin-positive neurons were AQP1-positive, indicating different populations of small neurons in the DRG, which is consistent with previous data (Zhang and Verkman, 2010). AQP1 immunofluorescence was seen in a subpopoulation of axons in a longitudinal section of mouse sciatic nerve (Fig. 1A, right). AQP1 immunoreactivity was absent in DRG and sciatic nerve from AQP1−/− mice.
Figure 1.
AQP1-dependent axonal regeneration in DRG neuron cultures. A. AQP1 and peripherin immunofluorescence of DRG neurons and sciatic nerve (longitudinal section) from AQP1+/+ and AQP1−/− mice. White arrowheads, examples of AQP1 and peripherin colocalization; yellow arrowheads, AQP1 expression along sciatic nerve. B. (left) Peripherin and AQP1 immunofluorescence in DRG neuron cultures. White arrows, examples of peripherin and AQP1 colocalization; yellow arrowheads, examples of AQP1-negative cells. (right) Summary of maximal axon lengths, with numbers of cells studies shown in bars, * p < 0.01, ** p < 0.001. C. (top) Diagram of cell preparation. (bottom) Fluorescence of transfected cells and summary of maximal axon length, ** p < 0.001.
Impaired neurite outgrowth in cultures of AQP1-deficient DRG neurons
Adult DRG neurons from AQP1+/+ and AQP1−/− littermates were cultured on poly-D-lysine and laminin-coated coverglasses and grown in DRG media supplemented with 10 ng/mL NGF. After two days cells were stained for peripherin and AQP1, and maximal axon length of peripherin-positive cells was quantified. In DRG neuron cultures from AQP1+/+ mice ~70% of peripherin-positive cells were AQP1-positive, with the AQP1-positive neurons having significant longer neurites than the AQP1-negative neurons or neurites from DRG neuron cultures from AQP1−/− mice (Fig. 1B). AQP1 is strongly expressed on the soma (body) and axons of DRG neurons. Transfection of AQP1 but not GFP-GPI (as control) in the AQP1−/− neurons increased the number and length of neurites in the peripherin-positive population of neurons (Fig. 1C).
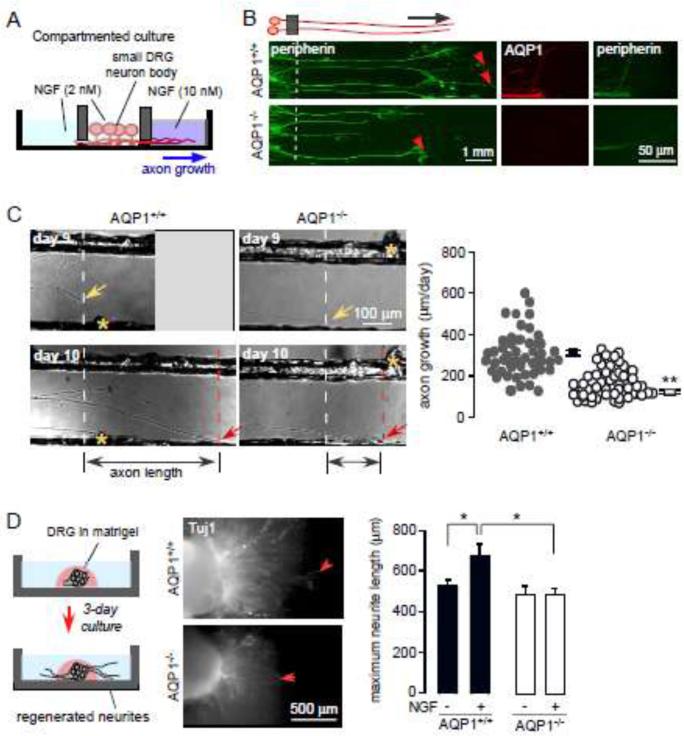
Impaired axonal growth in AQP1-deficient DRG neurons in compartmented chamber cultures
Compartmented DRG neuron culture was used to mimic the extension of axonal processes in DRG neurons that are far removed from the cell body, where target-derived growth factors are required for neuronal survival and function. Dissociated DRG cells (50,000/chamber) were plated in the middle compartment of the chamber and maintained in 100 ng/mL NGF for 3 days, after which 10 ng/mL NGF was added to the side compartment and NGF concentration was reduced to 1 ng/mL in the middle compartment (Fig. 2A). By seven days many axons had sprouted in the side compartment. Most of the axon bundles were both peripherin- and AQP1-positive in cultures from AQP1+/+ mice, with AQP1 expression seen all along the axon but with polarization to the tip of neuritis. After 10 days in culture the neurons from AQP1−/− mice showed much less total axon length (Fig. 2B). Repeated imaging of neurites on days 9 and 10 in culture showed significantly reduced axon growth in the AQP1−/− cultures (Fig. 2C).
Figure 2.
AQP1-dependent axonal regeneration in compartmented and explant DRG cultures. A. Diagram of compartmented DRG neuron culture. B. (left) Peripherin and AQP1 immunofluorescence of 10-day compartmented cultures from AQP1+/+ and AQP1−/− mice. White dashed line, location of barrier; red arrow, the axon tips. C. Axon growth over 24 h. Yellow star, reference point; white dashed line and yellow arrows, 0-h axon length; red dashed line and red arrow, 24-h axon length. Axon growth determined as the distance between white and red dashed lines. (right) Summary of 24-h axon growth, n=30-50, ** p < 0.001. D. (left) Diagram of DRG explant culture. (center) Tuj1 immunofluorescence of DRG explant cultures from AQP1+/+ and AQP1−/− mice. Red arrow, longest neurites. (right) Maximam neurite lengths (five longest neurites averaged), * p < 0.01.
Impaired neurite growth in AQP1-deficient DRG explant cultures
DRG explant cultures maintain the original DRG architecture with preserved neurons and Schwann cells, recapitulating the in vivo condition (Fig. 2D, left). After 3 days in culture in the presence of NGF, short lengths of intercostal nerves with attached DRGs start to bear neurites of all sizes, including those from small, AQP1-positive neurons as well as various middle and large size neurons. We found significantly increased neurite growth in NGF-treated DRG explant cultures from AQP1+/+ mice than from AQP1−/− mice (Fig. 2D, center and right), supporting the conclusion above that NGF-dependent axon elongation is AQP1-dependent.
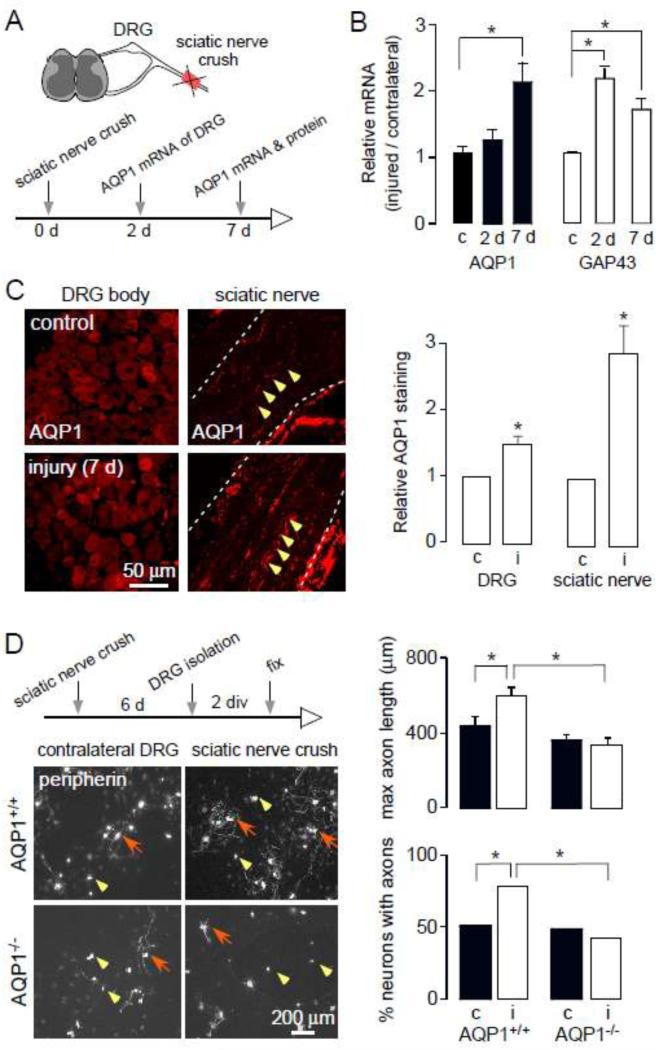
Upregulation of AQP1 during peripheral nerve regeneration
Following peripheral sciatic nerve crush injury (Fig. 3A), which injures axons projected from DRG neurons, the axons start to regenerate with upregulated expression of many genes such as GAP-43 (Chong et al., 1992). We found a significant increase in AQP1 and GAP-43 mRNA at 2 days and 7 days after injury in DRGs from the injured compared to the non-injured sides (Fig. 3B). Immuno-fluorescence showed significantly increased AQP1 protein at 7 days after crush injury both in the DRG cell body and in the sciatic nerve in the area of regeneration (Fig. 3C); AQP1 upregulation could come from both AQP1-positive and AQP1-silent. To investigate the growth of the injured DRG neurons, at 6 days after crush injury the ipsilateral and contralateral DRGs were isolated and cultured in vitro for 2 days with 10 ng/mL NGF (Fig. 3D, left). Cultures from AQP1−/− DRGs showed reduced regeneration as seen from maximal axon length and the percentage of neurons with axons (Fig. 3D, right). These results support AQP1 as an important determinant of small-neuron regeneration in vivo. AQP1 upregulation may be responsible for the greater axon regeneration or may be a secondary effect. We did not carry out behavioral studies in the crush-injured AQP1−/− mice because of baseline differences in nociception between AQP1+/+ and AQP1−/− mice (Zhang and Verkman, 2010), and potential confounding effects of polyuria and other non-neural phenotypes of the knockout mice (Ma et al., 1998).
Figure 3.
AQP1 upregulation following sciatic nerve crush injury. A. Diagram of sciatic nerve crush injury model. B. Relative AQP1 and GAP-43 mRNA expression, comparing injured to contralateral DRGs at days 2 and 7 after injury, n=6, * P < 0.05. C. AQP1 immunofluorescence of DRG neurons and sciatic nerve (longitudinal section) of contralateral and injury sides. White dashed line, sciatic nerve edge; yellow arrowhead, AQP1 expression on axons. (right) Relative AQP1 immunofluorescence in contralateral (c) and injured (i) sides, n=6, * p < 0.01. D. (left) Diagram of DRG neuron culture and peripherin immunofluorescence of cultures from AQP1+/+ and AQP1−/− mice. Yellow arrowheads, neurons without axons; red arrows, neuron with axons. (right) Summary of maximum axon length and percentage of neurons with axons, n=11, * p < 0.01.
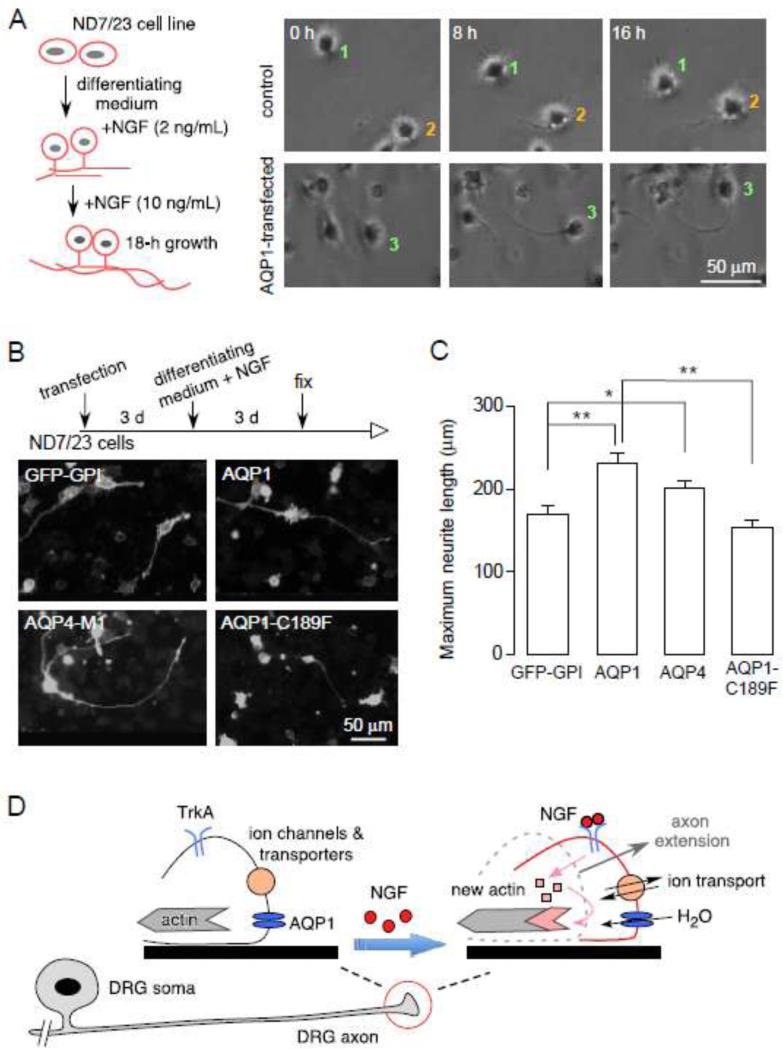
AQP1 water transport facilitates neurite growth in ND7/23 DRG cells
To investigate potential mechanisms of AQP1-dependent axonal regeneration, we studied neurite growth in the ND7/23 DRG cell line, which is a rat DRG / mouse fibroblast hybrid (Wood et al., 1990). ND7/23 cells do not natively express AQP1, but are easily transfected. After differentiation with db-cAMP and 0.5% FBS, the ND7/23 cells project neurites in the presence of NGF (Fig. 4A, and Supplementary Video 1). Neurite length was significantly greater in ND7/23 cells transfected with AQP1 compared to control (GFP-GPI transfected) cells (Fig. 4B, C). To test whether AQP1 water permeability is involved in neurite growth, the cells were transfected with a different water-transporting protein, AQP4-M1, and a non-water-transporting AQP1 mutant (AQP1-C189F). Neurite growth was increased with AQP4-M1, but not with AQP1-C189F.
Figure 4.
Mechanism of AQP1-dependent axon regeneration. A. (left) Diagram of ND7/23 cell differentiation. (right) Example of differentiated control and AQP1-transfected ND7/23 cells at 0, 8, 16 h. Numbers indicate the same cells in different frames. B. Immunofluorescence of ND7/23 cells transfected with AQP1, AQP4-M1, GFP-GPI or AQP1-C189F. C. Maximum neurite lengths, n=15-20, * p < 0.01; ** p < 0.001. D. Proposed mechanism of AQP1-dependent axon regeneration in pain-related sensory neurons.
Discussion
Our results provide evidence for a novel role of AQP1 in axonal growth and regeneration in DRG neurons. The data supporting this conclusion come from DRG cell cultures, DRG explant cultures and a sciatic nerve crush injury model, comparing AQP1+/+ and AQP1−/− mice. Also, transfection of AQP1 in the DRG-like cell line ND7/23 increased their neurite growth. Fig. 4D shows a proposed model of AQP1-dependent axon growth, which is inspired by similar mechanisms involved in lamellipodial extension during cell migration (Papadopoulos et al., 2008; Smith et al., 2014). After injury, locally released NGF activates TrkA receptors on DRG neurons, promoting actin synthesis and assembly near lamellipodia, with axonal extension facilitated by ion transporters, which establish osmotic driving forces, and AQP1 water channels, which facilitate water entry. AQP1 may facilitate neurite growth by direct or indirect interaction with axon guidance molecules, such as regulators of actin, focal adhesions and membrane trafficking regulators (Vitriol and Zheng, 2012).
We used several complementary in vitro and in vivo model systems to support the involvement of AQP1 on DRG neuron regeneration. Culture of single isolated DRG neurons is the most common model, which is well-suited for studies of individual modulators such as NGF. However, axon length is challenging to quantify because of rapid, tangled axon growth. The compartmented chamber model is useful to study NGF-dependent axon regeneration, as a grove on the dish surface restricts axon growth direction, allowing facile quantification of axon growth speed. However, both cell-based models lack potential modulating effects of adjacent Schwann cells and extracellular matrix signals. DRG explant culture is a good ex vivo model with adjacent Schwann cells intact, but is not useful to quantify the proportion of cells giving rise to outgrowths (Tucker and Mearow, 2008). Because the DRG neuron is non-dividing and difficult to efficiently transfect, the ND7/23 cell line provides a model system for investigating neurite development (Roobol et al., 1995). ND7/23 cells transform into a neuronal like cells in the presence of NGF after differentiation, which were used here to study the effect of transfected AQPs on neurite growth.
Axonal regeneration involves the intrinsic growth capacity of neurons as well as their environment, and is much more effective in the peripheral nervous system than in the central nervous system. AQP1 is not expressed on neurons in the CNS, but on the small neurons in DRG. AQP1 upregulation in DRG was seen on day 2 after injury, and significantly increased by day 7. Like many other upregulated DRG neuron genes in response to injury, AQP1 upregulation may involve the actions of multiple transcription factors, including AP-1, AFT3, CREB and nuclear factors of activated T cells (NFATs) (Chen et al., 2007). Multiple neurotrophic factors are implicated in peripheral axonal regeneration, including nerve growth factor (NGF), neurotrophin 3 (NT-3), glial cell line-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF) (Chen et al., 2007; Zhou et al., 2006). NGF binds to two types of receptors on DRG neurons: the low-affinity receptor p75 and the high-affinity receptor tyrosine kinase A (trkA) (Barbacid, 1994). trkA is primarily expressed on small DRG neurons (Fang et al., 2005) where AQP1 is expressed, which is why we used NGF in several of the in vitro experiments.
In adult mice the DRG contains a heterogeneous population of neurons, with about 50% large- and medium-diameter DRG cells that possess myelinated axons and respond principally to low threshold stimuli (McMahon et al., 1994), and about 50% small- diameter DRG cells with unmyelinated axons that respond principally to pain. The regeneration speed of different populations of neurons differs (Yamauchi et al., 1983), with more rapid recovery of touch sensation (related to the large neurons) than pain sensation (small neurons), suggesting different mechanisms of axonal regeneration in different populations of neurons. Our evidence here supports AQP1 water permeability as a new determinant of DRG axonal regeneration in the pain-related subset of neurons. In the trigeminal ganglion and the inferior vagal ganglion, AQP1 is also enriched in the small DRG neurons and axons, so AQP1 might also be involved in the regeneration of trigeminal nerve and nodose nerve.
In addition to the water-transporting role of AQP1 in facilitating DRG axon growth following nerve injury, there may be electrophysiological consequences of AQP1 upregulation following peripheral nerve injury. We previously demonstrated AQP1 regulation of neuronal excitability in small DRG neurons, which involved interaction with Nav1.8 sodium channels and modulation of action potential shape and firing rate (Zhang and Verkman, 2010). As peripheral nerve injury results in changes in excitability of cutaneous afferent DRG neurons (Devor, 2006; Gurtu and Smith, 1988), AQP1 upregulation in the injured nerve might stabilize nerve action potential properties and facilitate sensory functional recovery.
The involvement of AQP1 in DRG axon growth adds to the list of AQP-dependent cellular functions, some of which were unexpected (reviewed in ref. (Papadopoulos and Verkman, 2013). The water permeability function of AQPs is involved epithelial fluid secretion, brain water balance, neuroexcitatory phenomena, and, as discussed above, cell migration. A subset of AQPs that transport also glycerol (‘aquaglyceroporins’), including AQPs 3, 7 and 9, are involved in other functions including cell proliferation, epidermal hydration, fat metabolism and immune cell function. AQP-facilitated transport of gases such as CO2, H2O2, NH3 and NO may also occur, though their relevance to mammalian biology remains unproven. From the known biology of AQPs and the data here, it seems most likely that the AQP1-dependent growth of DRG axons involves AQP1-facilitated water transport at the axonal tips, though other mechanisms, such as AQP1-protein interactions or non-water-transporting functions, may be involved.
Conclusions
Our data implicate AQP1 as a novel determinant of DRG axonal regeneration, supporting an intriguing dual role of AQP1 in DRG neurons in nociception and axonal growth. Upregulation of AQP1 or other water transporting proteins in DRG neurons may promote axonal regeneration following injury, and, perhaps, AQP gene delivery to other types of neurons that do not express AQPs.
Supplementary Material
Highlights.
AQP1 deletion reduces DRG axonal growth.
AQP1 water permeability is a new determinant of DRG axonal regeneration.
AQP1 is a potential target to promote nerve regeneration.
Acknowledgments
Supported by grants DK35124, DK101373, DK72517, EB00415 and EY13574 from the National Institutes of Health, and a grant from the Guthy-Jackson Charitable Foundation. We thank L. Qian for mouse breeding and genotype analysis.
Abbreviations
- AQP
aquaporin
- BDNF
brain-derived neurotrophic factor
- CREB
cAMP response element-binding protein
- DMEM
Dulbecco's modified Eagle's medium (DMEM)
- DRG
dorsal root ganglion
- GDNF
glial cell line-derived neurotrophic factor
- NFATs
nuclear factors of activated T cells
- NGF
nerve growth factor
- NT-3
neurotrophin 3
- TrkA
tyrosine kinase A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- Bauder AR, Ferguson TA. Reproducible mouse sciatic nerve crush and subsequent assessment of regeneration by whole mount muscle analysis. J Vis Exp. 2012 doi: 10.3791/3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB. Development of sympathetic neurons in compartmentalized cultures. II. Local control of neurite survival by nerve growth factor. Dev Biol. 1982;93:13–21. doi: 10.1016/0012-1606(82)90233-0. [DOI] [PubMed] [Google Scholar]
- Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- Chong MS, Fitzgerald M, Winter J, Hu-Tsai M, Emson PC, Wiese U, Woolf CJ. GAP-43 mRNA in Rat Spinal Cord and Dorsal Root Ganglia Neurons: Developmental Changes and Re-expression Following Peripheral Nerve Injury. Eur J Neurosci. 1992;4:883–895. doi: 10.1111/j.1460-9568.1992.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Devor M. Sodium channels and mechanisms of neuropathic pain. J Pain. 2006;7:S3–S12. doi: 10.1016/j.jpain.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Fang X, Djouhri L, McMullan S, Berry C, Okuse K, Waxman SG, Lawson SN. trkA is expressed in nociceptive neurons and influences electrophysiological properties via Nav1.8 expression in rapidly conducting nociceptors. J Neurosci. 2005;25:4868–4878. doi: 10.1523/JNEUROSCI.0249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtu S, Smith PA. Electrophysiological characteristics of hamster dorsal root ganglion cells and their response to axotomy. J Neurophysiol. 1988;59:408–423. doi: 10.1152/jn.1988.59.2.408. [DOI] [PubMed] [Google Scholar]
- Lariviere RC, Nguyen MD, Ribeiro-da-Silva A, Julien JP. Reduced number of unmyelinated sensory axons in peripherin null mice. J Neurochem. 2002;81:525–532. doi: 10.1046/j.1471-4159.2002.00853.x. [DOI] [PubMed] [Google Scholar]
- Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J Biol Chem. 1998;273:4296–4299. doi: 10.1074/jbc.273.8.4296. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Meijering E, Jacob M, Sarria JC, Steiner P, Hirling H, Unser M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A. 2004;58:167–176. doi: 10.1002/cyto.a.20022. [DOI] [PubMed] [Google Scholar]
- Oshio K, Watanabe H, Yan D, Verkman AS, Manley GT. Impaired pain sensation in mice lacking Aquaporin-1 water channels. Biochem Biophys Res Commun. 2006;341:1022–1028. doi: 10.1016/j.bbrc.2006.01.062. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Saadoun S, Verkman AS. Aquaporins and cell migration. Pflugers Arch. 2008;456:693–700. doi: 10.1007/s00424-007-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci. 2013;14:265–277. doi: 10.1038/nrn3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patodia S, Raivich G. Role of transcription factors in peripheral nerve regeneration. Front Mol Neurosci. 2012;5:8. doi: 10.3389/fnmol.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roobol A, Holmes FE, Hayes NV, Baines AJ, Carden MJ. Cytoplasmic chaperonin complexes enter neurites developing in vitro and differ in subunit composition within single cells. J Cell Sci. 1995;108(Pt 4):1477–1488. doi: 10.1242/jcs.108.4.1477. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- Scheib J, Hoke A. Advances in peripheral nerve regeneration. Nat Rev Neurol. 2013;9:668–676. doi: 10.1038/nrneurol.2013.227. [DOI] [PubMed] [Google Scholar]
- Shields SD, Mazario J, Skinner K, Basbaum AI. Anatomical and functional analysis of aquaporin 1, a water channel in primary afferent neurons. Pain. 2007;131:8–20. doi: 10.1016/j.pain.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Jin BJ, Ratelade J, Verkman AS. Aggregation state determines the localization and function of M1- and M23-aquaporin-4 in astrocytes. J Cell Biol. 2014;204:559–573. doi: 10.1083/jcb.201308118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge D, Edstrom A, Ekstrom P. Use of explant cultures of peripheral nerves of adult vertebrates to study axonal regeneration in vitro. Prog Neurobiol. 1998;54:459–480. doi: 10.1016/s0301-0082(97)00072-5. [DOI] [PubMed] [Google Scholar]
- Tsui-Pierchala BA, Ginty DD. Characterization of an NGF-P-TrkA retrograde-signaling complex and age-dependent regulation of TrkA phosphorylation in sympathetic neurons. J Neurosci. 1999;19:8207–8218. doi: 10.1523/JNEUROSCI.19-19-08207.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker BA, Mearow KM. Peripheral sensory axon growth: from receptor binding to 0cellular signaling. Can J Neurol Sci. 2008;35:551–566. doi: 10.1017/s0317167100009331. [DOI] [PubMed] [Google Scholar]
- Vitriol EA, Zheng JQ. Growth cone travel in space and time: the cellular ensemble of cytoskeleton, adhesion, and membrane. Neuron. 2012;73:1068–1081. doi: 10.1016/j.neuron.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelaar CF, Vrinten DH, Hoekman MF, Brakkee JH, Burbach JP, Hamers FP. Sciatic nerve regeneration in mice and rats: recovery of sensory innervation is followed by a slowly retreating neuropathic pain-like syndrome. Brain Res. 2004;1027:67–72. doi: 10.1016/j.brainres.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Wood JN, Bevan SJ, Coote PR, Dunn PM, Harmar A, Hogan P, Latchman DS, Morrison C, Rougon G, Theveniau M, et al. Novel cell lines display properties of nociceptive sensory neurons. Proc Biol Sci. 1990;241:187–194. doi: 10.1098/rspb.1990.0084. [DOI] [PubMed] [Google Scholar]
- Wu D, Huang W, Richardson PM, Priestley JV, Liu M. TRPC4 in rat dorsal root ganglion neurons is increased after nerve injury and is necessary for neurite outgrowth. J Biol Chem. 2008;283:416–426. doi: 10.1074/jbc.M703177200. [DOI] [PubMed] [Google Scholar]
- Yamauchi S, Nomura S, Yoshimura M, Ueno T, Iwai Y, Shimamura K. A clinical study of the order and speed of sensory recovery after digital replantation. J Hand Surg Am. 1983;8:545–549. doi: 10.1016/s0363-5023(83)80122-1. [DOI] [PubMed] [Google Scholar]
- Zhang H, Verkman AS. Aquaporin-1 tunes pain perception by interaction with Na(v)1.8 Na+ channels in dorsal root ganglion neurons. J Biol Chem. 2010;285:5896–5906. doi: 10.1074/jbc.M109.090233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FQ, Walzer M, Wu YH, Zhou J, Dedhar S, Snider WD. Neurotrophins support regenerative axon assembly over CSPGs by an ECM-integrin-independent mechanism. J Cell Sci. 2006;119:2787–2796. doi: 10.1242/jcs.03016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.