Abstract
Healthy aging individuals are more likely to suffer profound memory impairments following an immune challenge than are younger adults. These challenges produce a brain inflammatory response that is exaggerated with age. Sensitized microglia found in the normal aging brain are responsible for this amplified response, which in turn interferes with processes involved in memory formation. Here, we examine factors that may lead aging to sensitize microglia. Aged rats exhibited higher CORT levels in the hippocampus, but not in plasma, throughout the daytime (diurnal inactive phase). These elevated hippocampal CORT levels were associated with increased hippocampal 11β-HSD1 protein expression, the enzyme that catalyzes glucocorticoid formation, and greater hippocampal glucocorticoid receptor (GR) activation. Intracisternal administration of mifepristone, a GR antagonist, effectively reduced immune-activated proinflammatory responses, specifically from hippocampal microglia, and prevented E. coli-induced memory impairments in aged rats. Voluntary exercise as a therapeutic intervention significantly reduced total hippocampal GR expression. These data strongly suggest that increased GR activation in the aged hippocampus plays a critical role in sensitizing microglia.
Keywords: normal aging, microglial sensitization, microglial immunophenotype, memory impairments, GR activation, mifepristone, voluntary exercise, in vivo microdialysis, automated blood sampling
1. Introduction
Cognitive declines that occur with aging have recently been linked to age-related potentiation of neuroinflammatory responses to challenge, largely mediated by sensitized microglia (Norden and Godbout, 2013). In particular, aging sensitizes hippocampal microglia such that immune challenges result in a potentiated and protracted inflammatory response in the hippocampus, relative to that in young adult subjects (Barrientos, et al., 2009a, Cunningham, et al., 2005, Frank, et al., 2010a, Godbout, et al., 2005). This potentiated inflammatory response in aged rats has been associated with impairments in hippocampal long-term potentiation (Chapman, et al., 2010), reduced expression of brain-derived neurotrophic factor (BDNF) (Chapman, et al., 2012, Cortese, et al., 2011), and long-lasting impairments in contextual and spatial forms of memory that depend on an intact hippocampus (Barrientos, et al., 2006). In the following series of experiments we investigated the factors that are responsible for aging-induced sensitization of hippocampal microglia. Our findings have led us to the somewhat paradoxical conclusion that age-related increases in basal hippocampal corticosterone (CORT), the principal glucocorticoid in rodents, is a key mediator of a microglia-dependent proinflammatory state in the hippocampus.
CORT is involved in metabolic function, immune reactions, and stress responses, and is well-known for its anti-inflammatory and immunosuppressant effects in both humans and animals (Selye, 1955). However, a more complex view that CORT actually has pleiotropic actions has emerged over the last dozen years (Busillo, et al., 2011, Sorrells, et al., 2009, Sorrells and Sapolsky, 2007). A series of papers (Dinkel, et al., 2003, MacPherson, et al., 2005, Munhoz, et al., 2006, Sapolsky, 1999, Sapolsky and Pulsinelli, 1985) demonstrated that chronic exposure to stress levels of CORT exacerbated, rather than inhibited, the neuroinflammatory response to challenge. Others have since reported similar findings (de Pablos, et al., 2006, Johnson, et al., 2002). More recently, Frank and colleagues reported that CORT administered acutely prior to a bacterial immune challenge facilitated the inflammatory response to the challenge, both in the periphery and in the brain. In contrast, CORT administered after the same immune challenge resulted in suppression of the inflammatory response (Frank, et al., 2010b). Together, these findings suggest that the temporal relationship between CORT elevations and immune challenge may be an important factor in determining whether CORT facilitates or suppresses the inflammatory response. They further demonstrated that prior in vivo administration of CORT potentiated the pro-inflammatory response of isolated hippocampal microglia exposed, ex vivo, to an immune challenge (Frank, et al., 2010b). These data established that elevations in CORT can be a key factor in sensitizing hippocampal microglia.
These findings led us to hypothesize that aging might increase basal concentrations of CORT, and that this factor may be causally related to aging-induced microglial sensitization and cognitive decline. To investigate this possibility, we employed a multi-disciplinary approach to fully characterize basal CORT and GR activation in young and old rats across times of day. Reducing GR activation with central mifepristone administration normalized hippocampal microglial immunophenotype and immune-activated proinflammatory responses, and prevented E. coli-induced memory impairments in aged rats. Finally, voluntary exercise was shown to be an effective behavioral therapeutic to reduce hippocampal GR expression. Taken together, this series of studies strongly suggest that greater GR activation in the hippocampus of aged rats is a key factor in sensitizing hippocampal microglia, as reduction of this activation reduced neuroinflammatory responses, and prevented long-lasting memory deficits following an immune challenge.
2. Materials and Methods
2.1 Subjects
Subjects were male F344xBN F1 rats obtained from the National Institute on Aging Rodent Colony maintained by Harlan (Indianapolis, IN). Upon arrival at our facility, aged rats were 24 mos. old and weighed approximately 550 g. Young adult rats were 3 mos. old and weighed approximately 275 g. All rats were age-matched and housed 2 to a cage (52 L × 30 W × 21 H, cm). The animal colony was maintained at 22±1° C on a 12-h light/dark cycle (lights on at 07:00 h). All rats were allowed free access to food and water and were given at least 1 week to acclimate to colony conditions before experimentation began. All experiments were conducted in accordance with protocols approved by the University of Colorado Animal Care and Use Committee. All efforts were made to minimize the number of animals used and their suffering.
2.2 Jugular vein cannulation
Rats were anaesthetized with inhaled isoflurane (5% initial and 2% maintenance). The right jugular vein was exposed and a silastic- (Dow Corning, Midland, MI) tipped polythene cannula (i.d. 0.5 mm o.d. 0.93 mm; Portex, Hythe, UK) filled with heparinized (10 IU/mL), pyrogen-free, isotonic saline was inserted into the vein until it was positioned close to the right atrium. The other end of the cannula was exteriorized through a scalp incision, and the neck incision was sutured. The free end of the cannula was inserted through a protective spring, which was fixed to the parietal bones using two stainless steel screws and dental cement. Following recovery from anesthesia the rats were individually housed in a room housing the automated blood sampling system. The free end of the protective spring was attached to a liquid swivel (Instech Laboratories, Inc, Plymouth Meeting, PA) that rotated through 360° in the horizontal plane and up to 180° in the vertical plane, giving the rats maximum freedom of movement. The rats recovered for 5 d prior to the experiment and each day during the recovery period the jugular cannulae were flushed with heparinized saline to maintain patency.
2.3 Automated blood sampling
Automated blood sampling (ABS) procedures were used to collect blood samples for measurement of plasma corticosterone concentrations as previously described (Lowry, et al., 2009). Five days following surgery the free ends of the jugular cannulae were attached to the ABS system using a liquid swivel. Flow of blood or heparinized saline between the rat, a reservoir, and a fraction collector was achieved by cooperative actions of a peristaltic pump and a 3-way valve controlled by a computer outside of the procedure room. Rats were connected to the system and ~50 ul of diluted blood was taken every 30 min for 24 h. Following every sample the blood volume that was removed from the rat was replaced with heparinized saline in order to prevent reductions in blood volume. Experimenters did not enter the room throughout the collection period.
Due to limitations of the ABS system there were variations in the amount of blood collected in individual samples. To account for this intra-sample variation the proportion of blood, relative to heparinized saline, that was collected for each sample was assessed. A volume of 3 µL was removed from each sample and, using a spectrophotometer (Synergy HT, BioTek Instruments, Winooski, VT), compared the samples to a standard curve of known blood proportions in sterile heparinized saline (0%, 12.5%, 25%, 37.5%, 50%, 62.5%, 75%, 87.5 %, 100%). Samples and standard curve were diluted 1:90 in sterile heparinized saline and loaded into 96-well plates in triplicate and read at 630 nm wavelength. A linear regression line was fit to the standard curve to produce an equation that was used to solve for the unknown sample dilutions. The dilution of each sample was transformed into a coefficient that was applied to the hormone assay results in order to correct for different blood dilutions in samples. Blood samples containing less than 15% blood were not considered reliable for corticosterone enzyme immunoassay and were excluded from the analysis.
2.4 In vivo hippocampal microdialysis
A guide cannula for the microdialysis probe (CMA/Microdialysis, North Chelmsford, MA) was stereotaxically placed unilaterally within the dorsal hippocampus of young & aged rats using the following coordinates relative to Bregma: AP: −5.60 mm, ML: +4.9 mm, DV: −3.4 mm. The guide cannula was secured to the top of the skull with dental acrylic. A screw cap of a 15 mL conical centrifuge tube, whose central lid portion was removed, was also affixed to the skull with dental acrylic so that its threads were exposed and it encircled the cannula. This was done so that the skull assembly could be protected during microdialysis. Rats were given 1 week to recover before experimentation began, and until that time, they were housed 2 to a cage. The night before sampling began, each animal was placed individually in a Plexiglas bowl (Bioanalytical Systems, West Lafayette, IN) to give it time to acclimate to the new conditions. A ~ 5 cm portion of a 15 mL Eppendorf tube was screwed onto the skull-mounted screw cap, through which the dialysis tubing, protected within a metal spring, entered and attached to the probe. Rats were able to move freely within the Plexiglas bowl via a freely moving swivel arm attached to this assembly. The microdialysis probe (0.5 mm in diameter, 1 mm membrane with a 20-kDa molecular weight cut-off; Eicom Corporation, Kyoto, Japan) was inserted through the guide cannula at this time. The tip of the probe extended 4 mm past the end of the guide cannula terminating at −7.4 mm. Isotonic Ringer's solution was infused at a rate of 0.2 µL/min overnight. At 9:00 h the next day, the flow rate was increased to 1.0 µL min and a 60-min stabilization period was allowed. The infusion flow remained constant throughout the experiment and samples were collected every 30 min over 24 h. Samples were collected automatically using a refrigerated, automated fraction collector (Microdialysis CMA 470, Holliston, MA), eliminating the need for experimenters to enter the room.
2.5 Corticosterone measurement
Dialysates and plasma samples were assayed for CORT using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Enzo Life Sciences, Farmington, NY), as per kit instructions. Hippocampal dialysate samples were run using a 1:2 dilution, and plasma samples were run using a 1:25 dilution.
2.6 Corticosterone binding globulin (CBG) measurement
Plasma samples were assayed for CBG levels using a commercially available ELISA kit (Uscn Life Science, Hubei, China), as per kit instructions, using a 1:1000 dilution.
2.7 Western blot
To examine GR expression in the hippocampus at the two times of day, young and aged rats were rapidly decapitated shortly after lights on or after lights off. Hippocampi were dissected on ice and processed immediately (fresh). For whole cell preparations, one hemisphere of the hippocampus was mechanically sonicated in 0.3 mL buffer containing 50 mM Tris base and an enzyme inhibitor cocktail (100 mM amino-n-caproic acid, 1 mM EDTA, 5 mM benzamidine HCl, and 0.2 mM phenylmethyl sulfonyl fluoride). Each tissue was mechanically sonicated for ~20 sec using an ultrasonic cell disrupter (ThermoFisher Scientific), centrifuged at 10,000 rpm at 4°C for 10 min, and supernatants transferred into a clean tube. To obtain nuclear and cytosolic fractions, the other hemisphere of the hippocampus was homogenized in a hand-held glass homogenizer, and fractionation was carried out using a commercially available kit (ThermoFisher Scientific #78835). Bradford protein assays were performed on all samples prior to the first freeze to determine total protein concentrations in each sample, with the goal of loading equal amounts of total protein into each lane. Small aliquots of samples (~15 µL) were then frozen at −80°C until western blots were performed, so that each aliquot was only used once, and was never refrozen and thawed. Hippocampal tissue harvested from several rats not associated with this study was processed as just described (for whole cell, nuclear, and cytosolic preparations) and pooled to create a large quantity of a standard sample. This standard sample was loaded into one lane of every gel used in the project, to account for variations that may occur from blot to blot. This standard was used to normalize every other band on the same blot, and this technique afforded us the liberty to then compare across multiple blots since every band in the dataset was normalized to the same standard. This was in addition to using a loading-control protein band on each gel. Using both a standard and a loading control allowed us to correct for differences in total protein loaded into a lane, and for differences in exposure between gels. To make fair comparisons between each preparation type (whole, nuclear, and cytosolic), an equal amount of total protein of these samples (5ug) was loaded into each lane. NuPAGE Bis-Tris (10-well, 4–12%; 1.5 mm) gel electrophoresis was used under reducing conditions (Life Technologies, Grand Island, NY). Gels were transferred to nitrocellulose membranes electrophoretically using the iBlot dry-blotting system (Life Technologies, Grand Island, NY). Nonspecific binding was blocked with Odyssey blocking solution (1 h at RT). Primary antibodies (in Odyssey blocking solution containing 0.1% Tween20 overnight at 4°C) were: rabbit anti-GR 1:5000 (Santa Cruz Biotechnology #1004, Dallas, TX), rabbit anti-11βHSD1 1:400 (Abcam #39364, Cambridge, MA). The loading control for whole cell samples was mouse anti- Bactin 1:500,000 (Sigma-Aldrich #A5316), for cytosolic fractions was mouse anti-Gapdh 1:500,000 (Abcam #8245, Cambridge, MA), and for the nuclear fractions was rabbit anti-p84 1:1000 (Abcam #102684, Cambridge, MA). Blots were washed 4×5min with TBS-T, and probed with the appropriate fluorescent secondary antibodies (goat anti-rabbit IgG (Li-Cor #926-32211, Lincoln, NE) or goat antimouse IgG (Li-Cor #926-32210, Lincoln, NE) both at 1:10,000) for 1 h at RT. Each band was detected and quantified by the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE).
2.8 GR antagonism
To test the hypothesis that greater GR activation sensitizes hippocampal microglia in aged rats, the GR antagonist mifepristone (25ng/rat; Sigma-Aldrich), or its vehicle (saline solution containing 2% ethanol) was administered to aged rats via the cisterna magna in a volume of 2.5 µL each day, for two sequential days. Vehicle-treated young adult rats served as a control group. A dose on the lower end of the spectrum was chosen to reduce but not eliminate all brain CORT action, so as to avoid elevations of endogenous CORT release due to total impairment of negative feedback inhibition. A review of the literature revealed that a range from 20–100 ng/rat was routinely administered i.c.v. (Garcia-Garcia, et al., 1998, Song, et al., 2004), and so a dose of 25 ng/rat was chosen.
2.9 Ex vivo immune stimulation of hippocampal microglia with LPS
Rats that had either received mifepristone or vehicle were killed 24 h following the second icm administration by a lethal dose of sodium pentobarbital and were transcardially perfused with ice-cold saline (0.9%). Brains were then rapidly extracted and placed on an ice-cold frosted glass plate and hippocampi dissected and homogenized in hand-held glass homogenizers containing 3 mL of 0.2% glucose. Hippocampal microglia were isolated using a Percoll density gradient as previously described (Frank, et al., 2006b), as this procedure yields highly pure microglia (Iba-1+/MHCII+/CD163−/GFAP−). Microglia were suspended in DMEM+10% FBS and microglia concentration determined by trypan blue exclusion. Microglia concentration was adjusted to a density of 3.5 × 103 cells/100 µl and 100 µl added to individual wells of a 96-well v-bottom plate. LPS was then utilized to challenge microglia in vitro. We have previously determined the optimal in vitro conditions under which LPS stimulates a proinflammatory cytokine response in these cells (Frank, et al., 2006a). Cells were incubated with 3 escalating doses of LPS (0.1, 1, and 10 ng/mL) or media alone, for 4 h at 37°C, 5% CO2. At the end of the incubation, the plate was centrifuged at 1000 × g for 10 min at 4°C to pellet cells. Cells were then washed 1× with 100 µL ice cold PBS and centrifuged at 1000 × g for 10 min at 4°C. Cell lysis/homogenization, and cDNA synthesis were performed using the SuperScript III CellsDirect cDNA Synthesis System (Life Technologies, Grand Island, NY). Four hours of incubation was optimal for detection of mRNA, but was not sufficient for protein measurement, therefore only mRNA is reported here.
2.10 Real time RT-PCR measurement of gene expression
Real time RT-PCR was used to detect gene expression of three proinflammatory cytokines, IL-1β, IL-6, and TNFα, and the microglial activation markers ionized calcium-binding adapter molecule 1 (Iba-1) and major histocompatibility complex class II (MHCII) as measures of microglial state. Moreover, expression of the glucocorticoid-induced leucine zipper (GILZ) gene was used as a measure of GR action to verify the effectiveness of mifepristone administration. A detailed description of the PCR amplification protocol has been published previously (Frank, et al., 2006a). cDNA sequences were obtained from Genbank at the National Center for Biotechnology Information (NCBI; www.ncbi.nlm.nih.gov). Primer sequences were designed using the Eurofins MWG Operon Oligo Analysis & Plotting Tool (http://www.operon.com/technical/toolkit.aspx) and tested for sequence specificity using the Basic Local Alignment Search Tool at NCBI (Altschul, et al., 1997). Primers were obtained from Invitrogen. Primer specificity was verified by melt curve analysis. All primers were designed to exclude amplification of genomic DNA. Primer description and sequences are presented in Table 1. PCR amplification of cDNA was performed using the Quantitect SYBR Green PCR Kit (Qiagen, Valencia, CA). Formation of PCR product was monitored in real time using the MyiQ Single-Color Real-Time PCR Detection System (BioRad, Hercules, CA). Samples were run in duplicate. Relative gene expression was determined by taking the expression ratio of the gene of interest to β-Actin.
Table 1. Primer Description.
Primer description and sequences.
| Gene | Accession # | Primer Sequence 5’ → 3’ |
Function |
|---|---|---|---|
| β-Actin | NM_031144 | F: TTCCTTCCTGGGTATGGAAT R: GAGGAGCAATGATCTTGATC |
Cytoskeletal protein (Housekeeping gene) |
| CD163 | NM_001107887 | F: GTAGTAGTCATTCAACCCTCAC R: CGGCTTACAGTTTCCTCAAG |
Macrophage antigen not expressed by microglia |
| IL-1β | NM_031512 | F: CCTTGTGCAAGTGTCTGAAG R: GGGCTTGGAAGCAATCCTTA |
Pro-inflammatory cytokine |
| IL-6 | NM_012589 | F: AGAAAAGAGTTGTGCAATGGCA R: GGCAAATTTCCTGGTTATATCC |
Pro-inflammatory cytokine |
| TNFα | NM_012675 | F: CAAGGAGGAGAAGTTCCCA R: TTGGTGGTTTGCTACGACG |
Pro-inflammatory cytokine |
| GILZ | NM_031345 | F: CAGGCCATGGATCTAGTGAA R: AGCGTCTTCAGGAGGGTATT |
Glucocorticoid immunomodulation |
| Iba-1 | NM_017196 | F: GGCAATGGAGATATCGATAT R: AGAATCATTCTCAAGATGGC |
Microglia/Macrophage antigen |
| MHCII | NM_001008847 | F: AGCACTGGGAGTTTGAAGAG R: AAGCCATCACCTCCTGGTAT |
Microglia/Macrophage antigen |
Abbreviations: IL, interleukin; Iba-1, ionized calcium-binding adaptor molecule-1; MHCII, Major histocompatibility complex II; GILZ, glucocorticoid-induced leucine zipper; TNFα, tumor necrosis factor-α;
2.11 Immune challenge
To examine the effects of antagonizing hippocampal GRs prior to a bacterial infection on hippocampal memory formation, rats received an intraperitoneal (i.p.) injection of either live Escherichia coli (E. coli), or vehicle 24 h after the second mifepristone injection and then given 4 days to recover prior to commencing the fear conditioning protocol (see below). One day prior to administering the infection, stock E. coli cultures (ATCC #15746; American Type Culture Collection, Manassas, VA) were thawed and cultured overnight (15–20 hr) in 40 mL of brain-heart infusion (BHI; DIFCO Laboratories, Detroit, MI) in an incubator (37°C). The number of bacteria in cultures was quantified by extrapolating from previously determined growth curves. Cultures were then centrifuged for 15 min at 4°C, 3000 RPM, supernatants discarded, and bacteria resuspended in sterile phosphate buffered saline (PBS). Bacteria were resuspended with a volume of PBS to achieve a concentration of 1.0 × 1010 colony forming units (CFU). A volume of 250 µL was injected i.p. Vehicle-treated rats received an injection of sterile PBS of an equal volume (250 µL).
2.12 Context pre-exposure fear conditioning
A context pre-exposure fear conditioning paradigm was employed to measure memory performance, as we have previously reported this paradigm to be highly sensitive to disruptions to the hippocampus (Barrientos, et al., 2006, Barrientos, et al., 2002, Rudy, et al., 2002). Contextual fear conditioning depends on two processes: the construction of a conjunctive representation of the conditioning context, and the association of that representation with shock. We have established that acquiring a conjunctive representation depends on an intact hippocampus. Because in this paradigm the two processes are presented independently (on separate days) it allows us to more readily detect impairments selective to the hippocampus. The conditioning context was one of two identical Igloo ice chests (54 L × 30 W × 27 H, cm) with white interiors. A speaker and an activated 24-V DC light bulb were mounted on the ceiling of each chest. The conditioning chambers (26 L × 21 W × 24 H, cm), placed inside each chest, were made of clear plastic and had window screen tops. A 2 sec, 1.5 mA shock was delivered through a removable floor of stainless steel rods 1.5 mm in diameter, spaced 1.2 cm center to center. Each rod was wired to a shock generator and scrambler (Coulbourn Instruments, Allentown, PA). Chambers were cleaned with water before each animal was conditioned or tested. Rats were taken two at a time from their home cage and transported to conditioning context in a black ice bucket with the lid on so that the rats could not see where they were being taken. Rats were placed in the context and allowed to freely explore and then were transported back to their home cage where they remained approximately 40 sec before the next exposure. This procedure was repeated 6 times. Rats remained in the novel context for 5 min on the first exposure and for 40 sec on the 5 subsequent exposures. The rats were transported in the black bucket each time that they were returned to their home cage, but with the lid off, so that they could discern whether they were headed to the context or to their home cage. The purpose of these multiple exposures was to establish the features of the black bucket as retrieval cues that could activate the representation of the context (for further detail see (Rudy, et al., 2002)). Forty-eight h later, each animal was taken from its home cage and transported to the conditioning context in the black bucket. There, they received one 2 s shock immediately after being placed in the context. They were then quickly taken out of the context and transported back to their home cage. The rats’ time in the conditioning context never exceeded 5 sec. To establish a baseline of generalized fear, 24 h later, freezing behavior was assessed over a 6 min period in a novel, neutral, control context. The control context was a circular animal enclosure with a solid plastic floor and ceiling, and wire walls. Attached to the wall of each enclosure was a small plastic running wheel (the size of which is suitable for a hamster or mouse, but not a rat) to serve only as a visual or manual distraction. To assess the rats’ memory for the conditioning context, freezing behavior while in the conditioning context was scored over a 6 min period. The order of these two tests was counterbalanced across the groups so that half of each group was tested in the control context first, and in the conditioning context several hours later, and the other half was tested in the conditioning context first, and in the control context several hours later. After placing the rat into the respective context, every 10 s each rat was judged as either freezing or active at the instant the sample was taken. Freezing, the rat’s dominant defensive fear response, is a complete suppression of behavior that is accompanied by immobility, shallow breathing, and a variety of other autonomic changes including an increase in heart rate and pilo-erection (Kim and Fanselow, 1992). Freezing in these experiments was defined as the absence of all visible movement, except for respiration. Scoring began approximately 10 sec after the animal was placed into the chamber. Scoring was carried out by observers blind to experimental treatment and inter-rater reliability exceeded 97% for all experiments.
2.13 Voluntary wheel running
To assess whether voluntary exercise reduces microglial sensitization by decreasing CORT or reducing GR expression, aged rats (Runners) were housed in a cage with a running wheel (circumference 1.08 m). A bicycle computer was attached to each cage/wheel to monitor running. Another group of rats was housed in a cage with a running wheel that was locked in place (Locked) to control for having the novel wheel in their cage, but these rats were unable to run. Rats were maintained in these conditions for a total of 6–7 wk. At the end of the 5th wk, rats were stereotaxically implanted with a microdialysis guide cannula into the hippocampus as described earlier, and allowed to recover for 2 days in a standard cage prior to returning to their assigned running wheel cage. At the 6th or 7th wk, rats were dialyzed over 24 h as described earlier. Rats did not remain in their running wheel cages during microdialysis, but did return to them immediately afterward for several days prior to being euthanized to collect hippocampal tissue for western blot.
2.14 Statistical analyses
Statistical analyses were conducted using StatView v.5.0 and GraphPad Prism v.5.0 software. Two-way ANOVAs were employed in most cases. In a few cases, where only two groups being compared, unpaired Student’s t-tests were used. All measures using samples collected throughout the diurnal cycle (plasma CORT and CBG, and hippocampal CORT) were analyzed using two-way repeated measures ANOVAs and area under the curve (AUC) analysis. Separate analyses were run for the 12 h of the inactive phase, and the 12 h of the active phase. Where appropriate (when the interaction term was significant), Scheffe’s post-hoc tests were conducted to reveal pairwise differences between groups. Statistical significance for all tests was set at alpha = 0.05.
3. Results
3.1 Diurnal rhythms of plasma CORT in young and aged rats
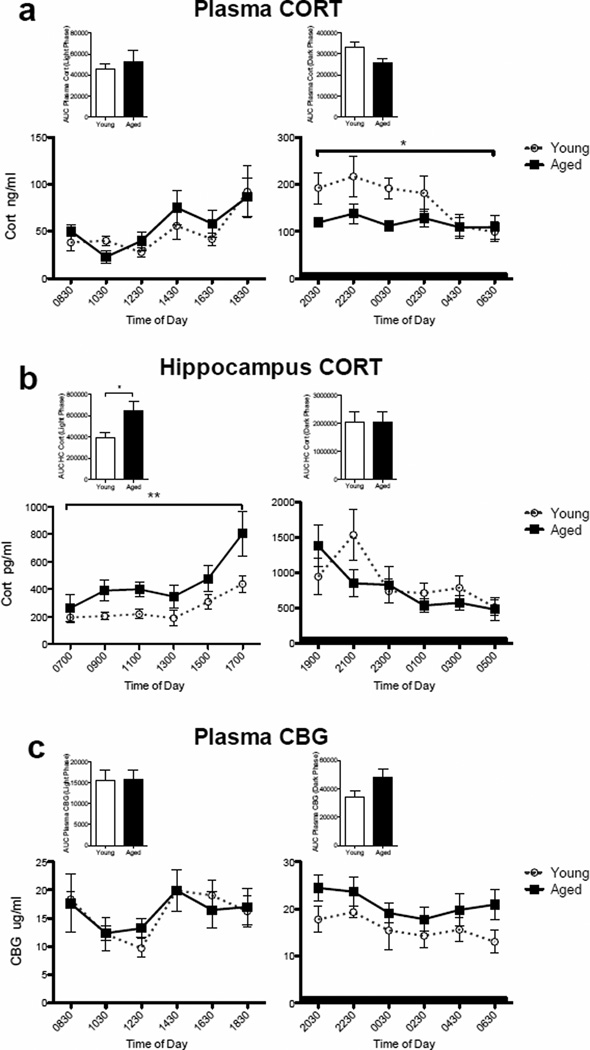
We employed an automatic blood sampling system to measure circulating levels of CORT in young adult (n=4) and aged (n=4) subjects. This system allowed the collection of blood samples throughout the circadian cycle without handling of the subjects. This procedure has previously produced clear and reliable CORT measurements (Lowry, et al., 2009). Fig. 1a shows plasma levels across the circadian cycle. Age had no effect during the inactive phase of the cycle (lights on), but during the active phase blood levels were lower in the aging subjects. A two-way repeated measures ANOVA (age × time of day) for the inactive phase revealed no main effect of age (F(1,6) = 0.274, P = 0.62), a main effect of time of day (F(1,5) = 6.57, P = 0.0003), and no interaction (F(1,30) = 0.717, P = 0.62). A two-way repeated measures ANOVA (age × time of day) for the active phase revealed a main effect of age (F(1,6) = 8.60, P = 0.03) with young rats on average expressing higher concentrations of CORT than aged rats, a main effect of time of day (F(1,5) = 2.60, P = 0.04), but no interaction (F(1,30) = 1.27, P = 0.30). Area under the curve (AUC) analysis produced no differences between the two age groups at either phase (light phase: t=0.59, p > 0.05; dark phase: t=2.395, p > 0.05) (Fig. 1a inset). These data are consistent with those of others showing in humans and rodents (Colucci, et al., 1975, Hauger, et al., 1994, Milcu, et al., 1978), that circulating CORT levels are blunted in aged compared to young adult subjects, especially in the active phase.
Figure 1.
CORT and CBG levels between young and aged rats. (a) Plasma CORT levels between young and aged rats across the diurnal cycle. Age had no effect during the inactive phase of the cycle (lights on), but during the active phase blood levels were lower in the aged subjects (p < 0.05). Inset: area under the curve for inactive and active phases. (b) Hippocampal CORT levels between young and aged rats across the diurnal cycle. Aged rats exhibited significantly elevated levels of CORT during the light phase compared to young adult rats (p < 0.01), but not during the active phase. Inset: area under the curve for inactive and active phases. (c) Plasma CBG levels between young and aged rats across the diurnal cycle. Age did not have a significant effect on circulating CBG at either phase of the cycle. Inset: area under the curve for inactive and active phases. Dark bar represents active phase hours. Error bars represent S.E.M. *p<0.05; **p<0.01.
3.2 Diurnal rhythms of hippocampal CORT in young and aged rats
Brain levels of CORT do not always reflect blood levels (Droste, et al., 2008, Garrido, et al., 2012). Thus, even though aging did not increase plasma CORT levels, we next examined CORT levels specifically in the hippocampal region of the brain in the two age groups, using in vivo microdialysis. Samples were collected automatically throughout the diurnal cycle with minimal handling of the rats using a refrigerated automated fraction collector. Again, we analyzed the diurnal data separately (inactive phase and active phase) to better capture potentially important differences between age groups (young n=8; aged n=8; Fig 1b). A two-way repeated measures ANOVA (age × time of day) indicated that aged rats exhibited significantly elevated levels of CORT during the light phase compared to young adult rats (F(1,13) = 8.74, P = 0.01). There was a significant main effect of time of day (F(1,5) = 7.01, P < 0.0001), but no interaction between these two factors (F(1,65) = 0.831, P = 0.53). The same analysis for the active phase indicated no main effect of age (F(1,12) = 0.30, P = 0.59), a main effect of time of day (F(1,5) = 7.48, P < 0.0001), and no interaction (F(1,60) = 2.26, P = 0.06). AUC analysis demonstrated significantly elevated levels of hippocampal CORT in aged rats during the light phase (t=2.50, p < 0.05), but not during the dark phase (t=0.012 p > 0.05) (Fig. 1b inset). These data demonstrate that aged rats had elevated basal concentrations of CORT in the hippocampus throughout the inactive phase, but were no different from young adults during the active phase. Additionally, and importantly, these data illustrate that relative CORT levels in the hippocampus do no necessarily follow blood levels, perhaps especially in the aged rat. Taken together, these data suggest that chronically elevated hippocampal CORT in aged rats is specific to brain and to the inactive phase of the light-dark cycle.
3.3 Diurnal rhythms of corticosteroid binding globulin (CBG) in young and aged rats
Given that ~95% of basal circulating CORT is bound by corticosteroid binding globulin (CBG), only ~5% of the CORT measured peripherally is free or unbound and able to exert a biological effect or cross the blood-brain barrier. CBG levels are altered by a variety of factors such as time of day (Hsu and Kuhn, 1988), body temperature (Chan, et al., 2013, Henley and Lightman, 2011), and age (van Eekelen, et al., 1992). Therefore, measuring CBG levels here is important for understanding whether measured levels of total CORT are representative of free CORT. Therefore, in the same blood samples as above, we measured CBG (Fig 1c). Age did not have a pronounced effect on circulating CBG. Although two-way repeated measures ANOVA (age × time of day) indicated that there was a main effect of time of day for both the inactive (F(1,5) = 4.71, P = 0.003) and active phase (F(1,5) = 2.75, P = 0.04), there was no main effect of age or an age by time of day interaction for either the inactive or active phase. AUC analysis also produced no differences between the two age groups at either phase (light phase: t=0.07, p > 0.05; dark phase: t=1.91, p > 0.05) (Fig. 1c inset). Clearly, the difference in brain and blood CORT cannot be explained by aging-induced alterations in plasma CBG.
3.4 Elevations in 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) in the aged brain
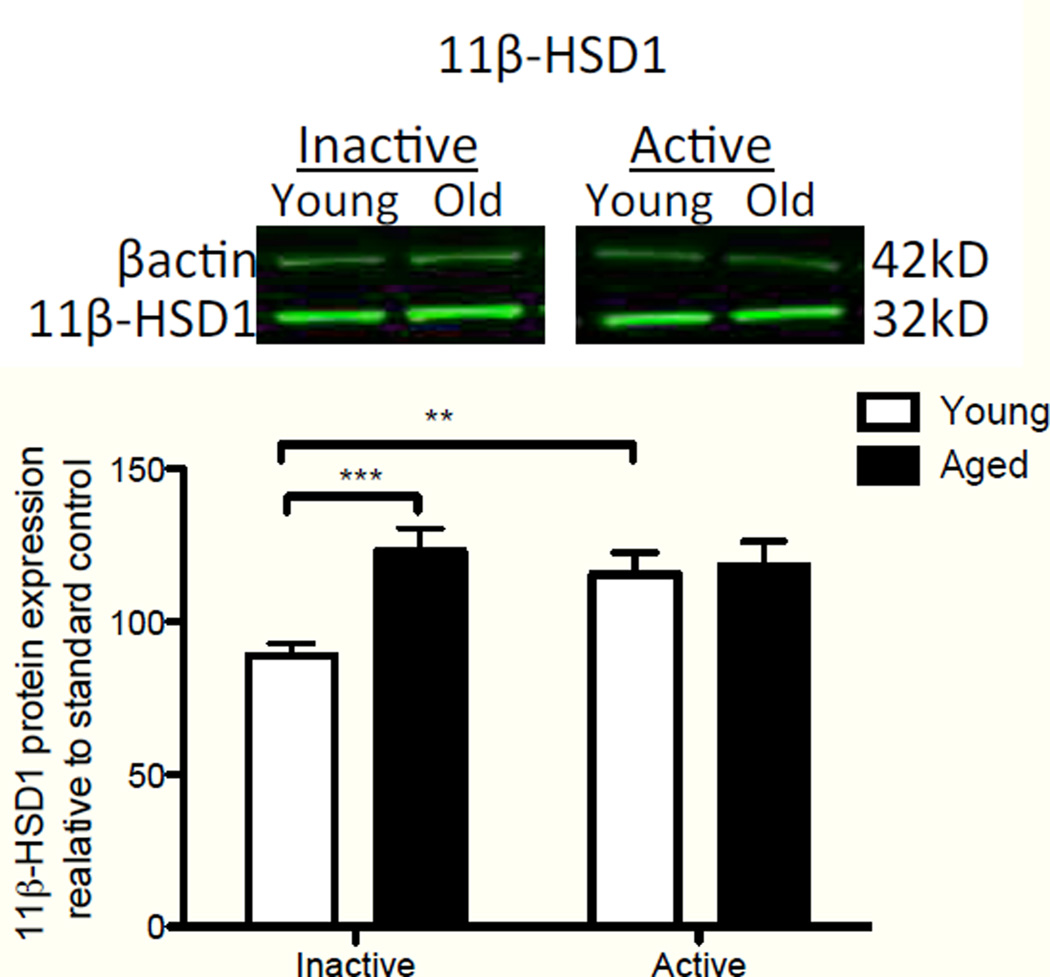
The principal factor that dictates CORT levels in the hippocampus are the relative systemic plasma CORT levels. However, there are a number of mechanisms that could be responsible for age-related differences in hippocampal levels of CORT in the absence of differences in blood levels. One centers on the hippocampal expression of the CORT modulator 11β-hydroxysteroid dehydrogenase type 1 (11β- HSD1). 11β-HSD1 has reductase activity, converting the inactive derivative of CORT (11-dehydrocorticosterone) into CORT, enabling the steroid to bind to its receptor and exert biological action (Wyrwoll, et al., 2011). Higher levels of this enzyme result in greater cellular levels of the active hormone. The isozyme of 11β-HSD1, 11β-HSD2, has the opposite effect. As a dehydrogenase, it catalyzes the conversion of active CORT into the inactive metabolite, ultimately reducing the available levels of biologically active hormone. Importantly, 11β-HSD1 is highly expressed in liver, adipose tissue, and brain (Wyrwoll, et al., 2011). However, 11β-HSD2 is not expressed in the adult brain (Wyrwoll, et al., 2011). Thus, 11β-HSD1 activity in the hippocampus can modulate the relative levels of CORT within the hippocampus. We measured 11β-HSD1 protein levels in the hippocampus of young and aged rats from tissue harvested at lights on (active phase, n=11 young, n=12 aged), and lights off (inactive phase; n=7 young, n=8 aged) using western blot (Fig. 2). Interestingly, aged rats, relative to young rats, expressed elevated levels of 11β-HSD1 protein in the inactive phase. A two-way ANOVA (age × time of day) resulted in a main effect of age (F(1,34) = 6.76, P = 0.01), no main effect of time of day (F(1,34) = 2.43, P = 0.13), and a significant interaction (F(1,34) = 5.01, P = 0.03). Scheffe post-hoc tests revealed that hippocampal 11β-HSD1 protein expression in aged rats during the inactive phase was significantly elevated compared to that of young rats (P = 0.0009), but expression levels during the active phase did not differ between age groups (P=0.82). Additionally, within young rats, 11β-HSD1 was significantly decreased during the inactive phase compared to the active phase (P = 0.003). These data suggest a mechanism that could mediate elevations of CORT in the aged brain, selectively in the inactive phase. Aging-associated increases in 11β-HSD1 mRNA have been reported previously (Holmes, et al., 2010), though here we demonstrate, for the first time, that these increases also occur in protein, and fluctuate with time of day in the hippocampus of young rats.
Figure 2.
Hippocampal expression of 11β-HSD1 protein between young and aged rats during the inactive and active phases of the dirunal cycle. Aged rats expressed elevated levels of 11β-HSD1 protein, relative to young rats, in the inactive phase (p<0.001). Young rats demonstrated a significant increase in expression in the active phase over the inactive phase (p<0.01), but there were no significant differences in expression of 11β-HSD1 in the active phase between the two ages. Error bars represent S.E.M. **p<0.01; ***p<0.001.
3.5 Glucocorticoid receptor activation
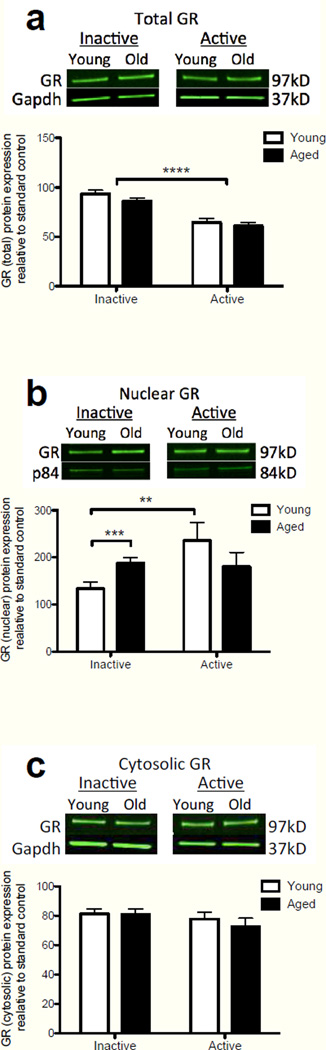
To determine whether the observed elevations in basal levels of CORT in the hippocampus during the inactive phase have functional significance, we examined the protein levels of the glucocorticoid receptor (GR) using western blot. GR are expressed in most cells throughout the brain. In the absence of ligand, GR are predominantly localized in the cytoplasm of the cell as a component of a multimeric protein complex. After CORT binds to GR, the activated receptor dissociates from some elements of the protein complex, thereby exposing a nuclear localization domain. The CORT-GR complex then translocates to the nucleus and modulates gene transcription (Guiochon-Mantel, et al., 1996, McKay and Cidlowski, 1999). Thus, greater nuclear GR localization represents greater receptor activation (Spencer, et al., 2000). In this experiment, we examined total GR protein expression in whole hippocampal sonicates (Fig 3a), as well as nuclear (Fig. 3b) and cytosolic fractions (Fig. 3c) from tissue harvested at lights on (active phase; n=11 young, n=12 aged), and lights off (inactive phase; n=7 young, n=8 aged). Though there were no age differences in total GR expression, nuclear-specific expression of GR during the inactive phase was significantly higher in aged rats. A two-way ANOVA of total GR expression (age × time of day) yielded no main effect of age (F(1,34) = 2.00, P = 0.17), a significant main effect of time of day (F(1,34) = 55.36, P < 0.0001), and no significant interaction (F(1,34) = 0.27, P = 0.60). These data indicate that total GR expression was diminished during the active phase of the light-dark cycle in both age groups, but that there were no differences within time of day between the age groups. A two-way ANOVA of nuclear GR expression (age × time of day) yielded no main effect of age (F(1,34) = 0.001, P = 0.97), a significant main effect of time of day (F(1,34) = 4.23, P = 0.047), and a significant interaction (F(1,34) = 5.70, P = 0.02). Scheffe post-hoc tests revealed that nuclear GR expression of aged rats during the inactive phase was significantly elevated compared to that of young rats (P = 0.009), but expression levels during the active phase did not differ between age groups (P=0.28). Additionally, within young rats, expression was significantly increased during the active phase compared to the inactive phase (P = 0.01). Within the aged group, nuclear GR expression was not different between times of day. These data strongly suggest that elevated CORT levels observed in aged rats during the inactive phase result in greater GR activation at that time of day. A two-way ANOVA of cytosolic GR expression (age × time of day) yielded no main effect of age (F(1,34) = 0.30, P = 0.59), no main effect of time of day (F(1,34) = 1.49, P = 0.23), and no significant interaction (F(1,34) = 0.44, P = 0.51), perhaps reflecting small changes in the large pool of unactivated GR at both times of day under basal conditions. Taken together, these data show a pattern of GR nuclear localization that is consistent with higher hippocampal 11β-HSD1 expression and CORT levels in aged rats during the inactive period, and support the notion that microglia in the aged hippocampus are exposed to greater GR-dependent actions throughout the daytime.
Figure 3.
Hippocampal expression of GR protein between young and aged rats during the inactive and active phases of the circadian cycle. (a) Total GR expression from whole hippocampal lysates. There was a main effect of time of day resulting in a significant reduction of GR expression during the active phase (p<0.0001). However, there were no age differences in total GR expression at either timepoint. (b) GR expression from nuclear fraction of hippocampal lysates. GR expression was significantly elevated in aged rats compared to that of young rats during the inactive phase (p<0.001). Young rats demonstrated a significant increase in expression in the active phase over the inactive phase (p<0.01), but there were no significant differences in expression of GR in the active phase between the two ages. (c) GR expression from cytosolic fraction of hippocampal lysates. There were no significant age nor time of day differences in GR expression in this fraction. Error bars represent S.E.M. **p<0.01; ***p<0.001; ****p<0.0001.
3.6 Central mifepristone administration attenuates hippocampal microglial phenotype and inflammatory responses to LPS
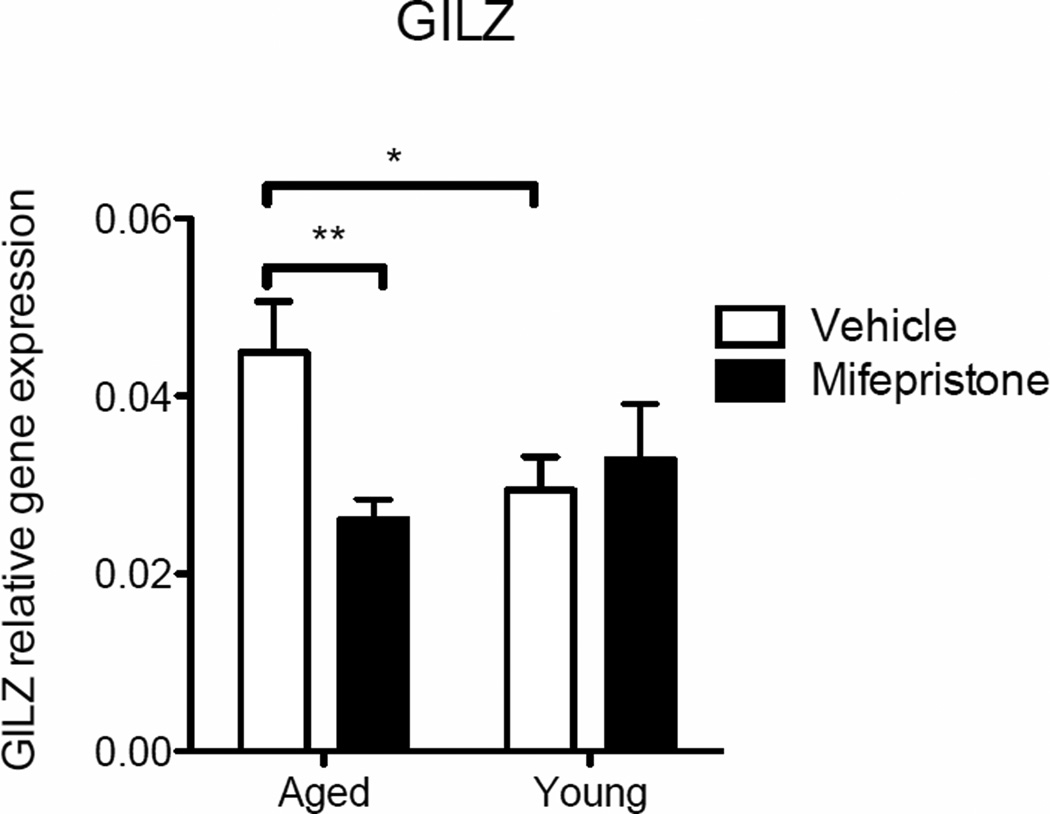
To more directly test the hypothesis that greater hippocampal GR activation in aged rats contributes to elevated microglial sensitization, a low dose of the GR antagonist mifepristone, or vehicle, was administered in aged and young adult rats (n=8 in each group), once a day over 2 days, via the cisterna magna. Twenty-four hours after the second administration rats were sacrificed, hippocampi were dissected, and microglia from these tissues were isolated and cultured for 4 h in the presence of increasing doses of the potent immunogen lipopolysaccharide (LPS) ex vivo, to elicit an inflammatory response, as we have done previously (Frank, et al., 2010a). To confirm that mifepristone treatment had in fact inhibited GR action, cell lysates from media only samples were analyzed using real-time PCR for expression of glucocorticoid-induced leucine zipper (GILZ), an important and reliable mediator of glucocorticoid action (Ayroldi and Riccardi, 2009). A two-way (age × drug) ANOVA revealed a significant interaction between the groups (F(1, 28) = 5.45, P < 0.05). Post-hoc analyses confirmed a significant reduction of GILZ expression in cells taken from mifepristone-treated aged rats (p<0.01) and vehicle-treated young rats (p<0.05) compared to vehicle-treated aged rats (Fig. 4).
Figure 4.
GILZ gene expression measured in hippocampal microglial cell lysates isolated from young and aged rats that were treated with either vehicle or mifepristone and incubated ex vivo with media for 4 hr. GILZ expression was significantly reduced in cells taken from mifepristone-treated aged rats (p<0.01) and vehicle-treated young rats (p<0.05) compared to vehicle-treated aged rats. Error bars represent S.E.M. *p<0.05; **p<0.01.
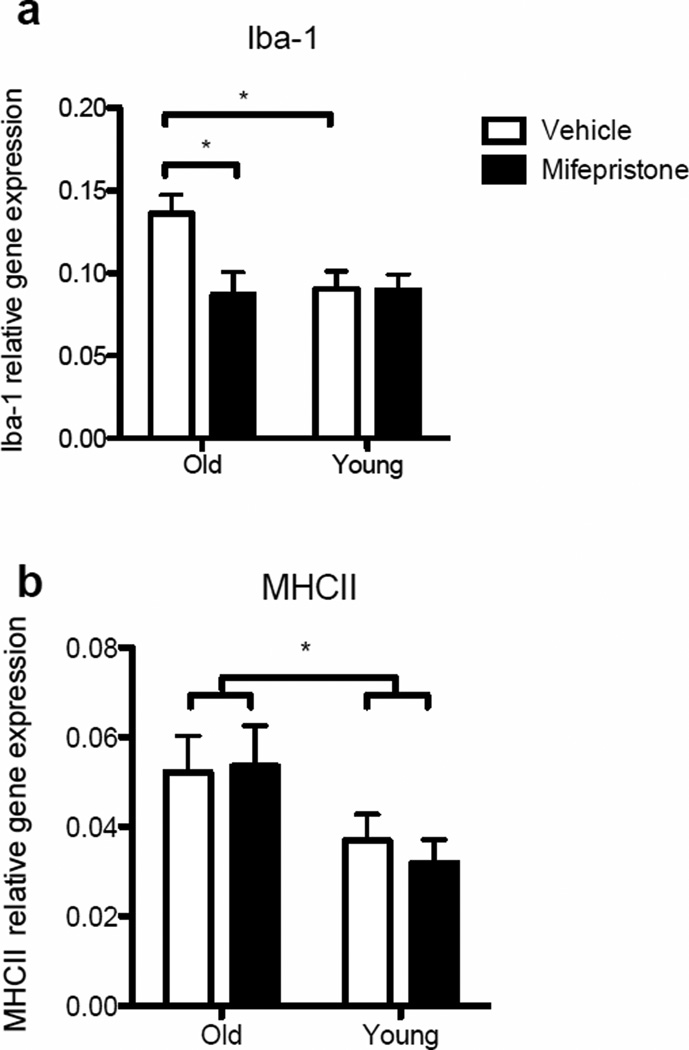
To determine whether mifepristone treatment altered the immunophenotype of aged hippocampal microglia, cell lysates from the four groups were analyzed for the expression of Iba-1 and MHCII mRNA. A two-way ANOVA determined a significant interaction in Iba-1 expression between the groups (F(1, 27) = 4.45, P < 0.05). Post-hoc analyses confirmed a significant reduction of Iba-1 expression in cells taken from mifepristone-treated aged rats (p<0.05) and vehicle-treated young rats (p<0.05) compared to vehicle-treated aged rats (Fig. 5a). With regard to MHCII expression, a significant aging-related increase in gene expression was evident (F(1, 28) = 6.49, P < 0.05; Fig 5b). However, there was no effect of the drug on MHCII expression (F(1, 28) = 0.06, P > 0.05).
Figure 5.
Gene expression of microglial activation markers (a) Iba-1 and (b) MHCII measured in hippocampal microglial cell lysates isolated from young and aged rats that were treated with either vehicle or mifepristone and incubated ex vivo with media for 4 hr. Iba-1 expression was significantly reduced in cells taken from mifepristone-treated aged rats (p<0.05) and vehicle-treated young rats (p<0.05) compared to vehicle-treated aged rats. A significant aging-related increase in MHCII expression was evident (p<0.05), but there was no effect of the drug. Error bars represent S.E.M. *p<0.05.
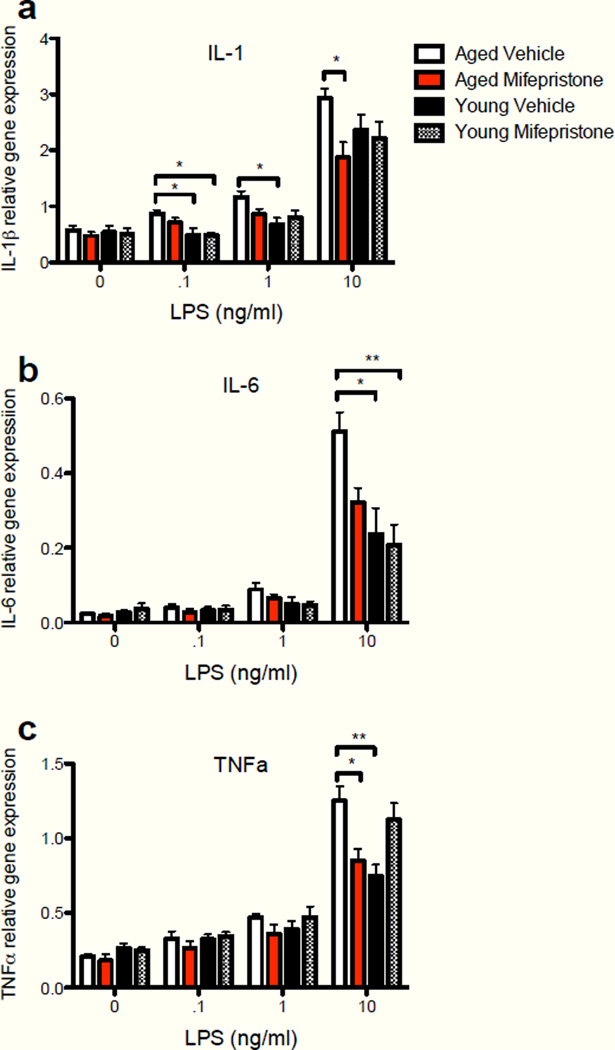
To examine the effectiveness of mifepristone to normalize the sensitized proinflammatory response of aged hippocampal microglia, cell lysates were analyzed for mRNA expression of IL-1β (Fig. 6a), IL-6 (Fig. 6b), and TNF-α (Fig. 6c). Mifepristone pretreatment in vivo was effective at reducing the age-related enhanced expression of all 3 pro-inflammatory cytokines following LPS stimulation ex vivo. A two-way ANOVA (treatment group × LPS dose) revealed a significant interaction between treatment groups and LPS dose for IL-1β (F(9,84) = 2.28, P < 0.05), IL-6 (F(9,84) = 5.29, P < 0.0001), and TNFα (F(9,84) = 4.33, P < 0.0001). Post-hoc tests revealed that mifepristone treatment significantly reduced gene expression (IL-1β: p < 0.05; TNFα: p < 0.05) in aged rats to levels comparable to expression levels of vehicle-treated young adult rats at the 10 ng LPS dose. Mifepristone treatment in young rats did not alter cytokine gene expression compared to age-matched vehicle-treated rats. These data strongly suggest that greater GR activation contributes to microglial sensitization, as blocking GR action with a low dose antagonist ameliorated the microglial immunophenotype as measured by Iba-1 and normalized LPS-induced proinflammatory cytokine expression to levels comparable to those of young adults. It should be noted that though mifepristone is a highly effective GR antagonist, it also antagonizes the progesterone receptor (Sarkar, 2002). Microglia express both GR and MR, however microglia do not express the progesterone receptor (Sierra, et al., 2008), suggesting that the effects of mifepristone observed here were mediated predominantly through the GR.
Figure 6.
Cytokine gene expression measured in hippocampal microglial cell lysates isolated from young and aged rats that were treated in vivo with either vehicle or mifepristone and incubated ex vivo with escalating doses of LPS for 4 hr. (a) Mifepristone treatment significantly reduced IL-1β gene expression in cell lysates of aged rats at the 10ng/mL LPS dose (p<0.05). (b) Mifepristone treatment significantly reduced IL-6 gene expression in cell lysates at the 10ng/mL LPS dose (p<0.05). (c) Mifepristone treatment significantly reduced TNFα gene expression in cell lysates of aged rats at the 10ng/mL LPS dose (p<0.05). Error bars represent S.E.M. *p<0.05; **p<0.01.
3.7 Central mifepristone administration prevents E. coli-induced hippocampal memory impairments
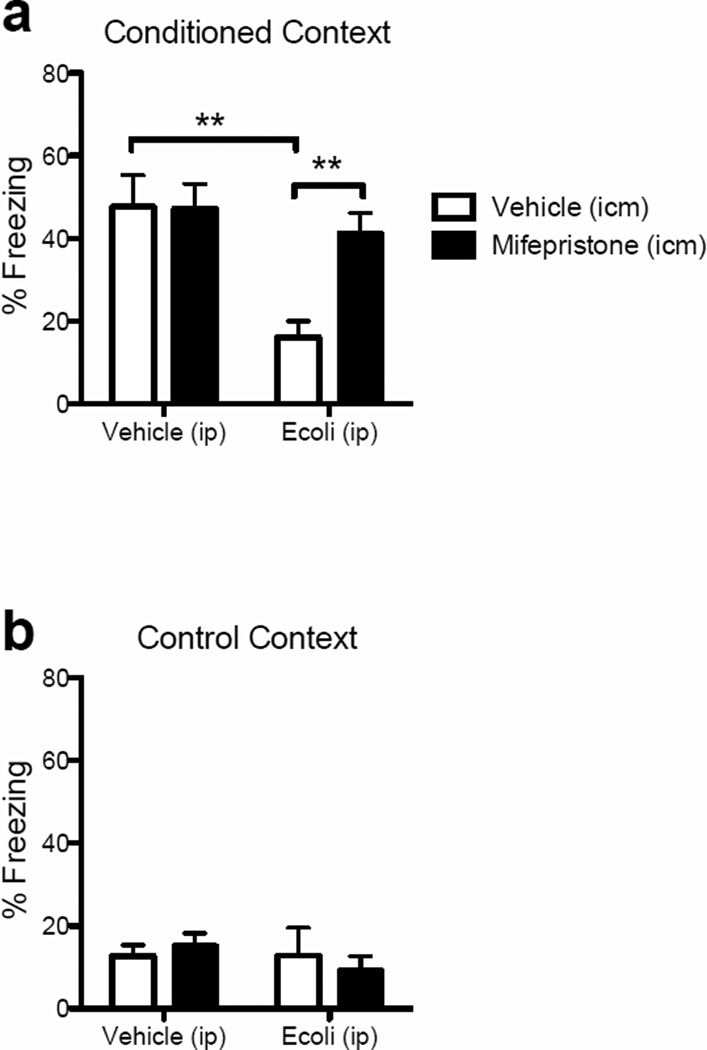
To determine whether the findings of the previous ex vivo experiment would extend to hippocampal-based memory susceptibility to E. coli infection in aged subjects, we treated rats exactly as we did above, with either mifepristone (n=14) or vehicle (n=11) prior to infecting half of each group with E. coli or vehicle. Four days following administration of E. coli (or vehicle), when sickness responses have abated (Barrientos, et al., 2009b), all rats were fear-conditioned to a context, as described in Methods. Two days later, hippocampal memory of that context was assessed. Because young adult rats do not exhibit memory impairments following E. coli infection (Barrientos, et al., 2006), only aged rats were examined here. As is typical, E. coli interfered with fear context memory. Mifepristone pretreatment did not alter fear context memory in uninfected subjects, but importantly, mifepristone was robustly effective at preventing the E. coli-induced memory impairment. A two-way ANOVA (peripheral challenge × central treatment) confirmed a main effect of peripheral challenge (F(1,21) = 9.78, P = 0.005) and of central treatment (F(1,21) = 4.23, P = 0.05; Fig 7a) on contextual fear behavior (freezing). A significant interaction effect (F(1,21) = 4.56, P = 0.04) followed by Scheffe post-hoc tests revealed that mifepristone significantly prevented the E. coli-induced reduced freezing (P < 0.01). A similar analysis of freezing behavior in a control context (to control for generalized fear) produced no main effect of peripheral challenge (F(1,21) = 0.40, P = 0.54), central treatment (F(1,21) = 0.11, P = 0.74), nor an interaction effect (F(1,21) = 0.75, P = 0.40; Fig 7b). These data further support the conclusion that greater GR-dependent sensitization of hippocampal microglial contributes to hippocampal-dependent memory deficits produced by an E. coli infection.
Figure 7.
Percent freezing in a contextual fear conditioning task. (a) Among rats pre-treated with vehicle (icm), E. coli (ip) infection significantly reduced freezing to the conditioning context compared to vehicle (ip)-treated rats (p<0.01). Among rats infected with E. coli (ip), percent freezing was significantly increased in mifepristone (icm)-treated rats compared vehicle (icm)-treated rats (p<0.01). There was no difference in freezing percentage between vehicle/mifepristone-treated rats and E. coli/mifepristone-treated rats. (b) Percent freezing to a control (neutral) context was not different between any of the groups. Error bars represent S.E.M. **p<0.01.
3.8 Voluntary exercise effects on hippocampal CORT, 11β-HSD1, and total GR expression
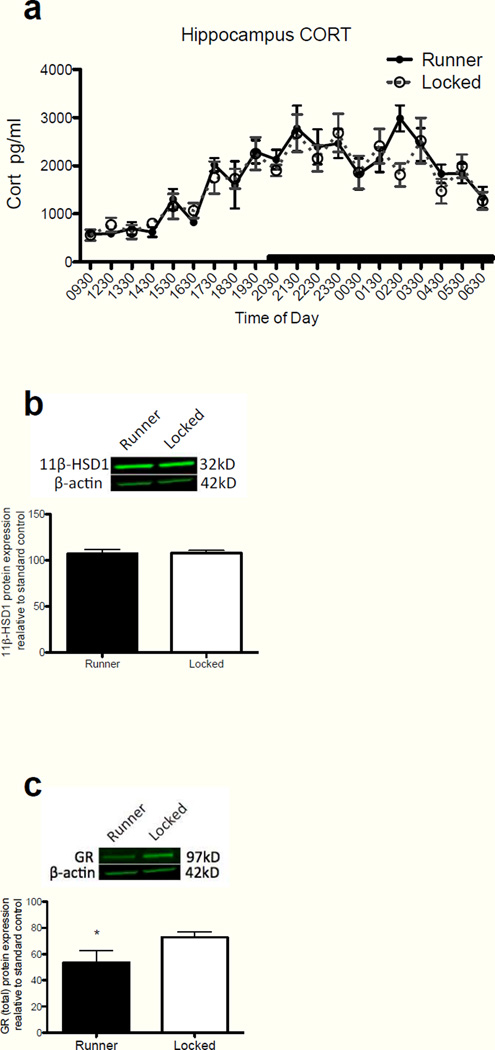
Previous findings from our laboratory demonstrated that a small amount of exercise in aged rats is effective at preventing E. coli-induced memory deficits, and reducing the hippocampal microglial response to LPS (Barrientos, et al., 2011). Here we explored the possibility that exercise exerts these effects via a reduction in hippocampal CORT. Again, because young adult rats do not display E. coli-induced memory impairments (Barrientos, et al., 2006), do not exhibit sensitized microglia (Frank, et al., 2010a), nor do they exhibit elevated levels of hippocampal CORT, they were not included in this experiment. Microdialysis samples taken over a 24 h period from rats allowed to run (n=7) or not allowed to run (n=10), were assayed for CORT as described in Methods. Unfortunately, several samples from the 1030 and 1130 timepoints were compromised prior to assay. Therefore, those timepoints were eliminated from the statistics and figure. CORT levels from both groups were indistinguishable. A two-way repeated measures ANOVA (exercise group × time of day) for the inactive phase revealed no main effect of exercise group (F(1,12) = 0.256, P = 0.62), a main effect of time of day (F(1,7) = 14.129, P < 0.0001), and no interaction (F(1,84) = 1.64, P = 0.14). The active phase yielded similar insignificant results. No main effect of exercise group (F(1,15) = 0.201, P = 0.66), a main effect of time of day (F(1,11) = 4.69, P < 0.0001), and no interaction (F(1,165) = 1.03, P = 0.43; Fig 8a). Whole cell sonicates from hippocampal tissue collected during the inactive phase from these rats were analyzed for 11β-HSD1 and total GR protein expression, as before. Hippocampal tissue in the hemisphere contralateral to the microdialysis probe was used for this analysis and resulted in insufficient hippocampal tissue to allow for parallel GR measure in cytosolic and nuclear tissue fractions. Therefore, we only examined these proteins in a whole cell preparation. Although voluntary exercise had no effect on 11β-HSD1 expression in the hippocampus, exercise produced a significant reduction in total GR expression. A Student’s t-test comparing 11β-HSD1 expression levels between runners and non-runners yielded no significant difference between the groups (t=0.19, df=14, P = 0.85; Fig 8b). Given that CORT levels were not altered with exercise, it was not surprising that 11β-HSD1 was also not altered, as we had demonstrated that these two products in brain tended to fluctuate in synchrony. Strikingly, a Student’s t-test revealed a significant exercise-induced reduction of total GR expression in the hippocampus of runners compared to non-runners (t=2.18, df=14, P = 0.046; Fig 8c). These findings suggest that exercise may reduce microglial sensitization through a reduction in total GRs, thus reducing the impact of elevated hippocampal CORT.
Figure 8.
(a) Hippocampal CORT levels between runners and non-runners across the circadian cycle. There were no significant differences between the groups at any timepoint across the circadian cycle. (b) There was no significant difference in hippocampal expression of 11β-HSD1 protein between runners and non-runners during the inactive phase of the circadian cycle. (c) Runners compared to non-runners exhibited a significant decrease in total GR expression from whole hippocampal lysates during the inactive phase of the circadian cycle (*p<0.05). Error bars represent S.E.M.
4. Discussion
The link between protracted CORT exposure and aging was first described over 50 years ago in several papers documenting the marked similarities between the pathologies of stress and aging (Findley, 1949, Solez, 1952). Many years later Landfield and colleagues went on to describe a hypothesis by which alterations in neuroendocrine control mechanisms would lead to a “runaway” positive feedback loop with the resulting accumulation of glucocorticoids possibly accelerating the aging process (Landfield, et al., 1978). They demonstrated a significant correlation between plasma CORT levels and glial reactivity in the aged hippocampus, suggesting for the first time that aging was not only associated with elevated plasma CORT, but that this hormonal increase was highly correlated with a marker of neuroinflammation. Furthermore, these findings suggested that aging was a vulnerability factor for this runaway positive feedback loop. They further showed that adrenalectomy in the aged rodent reduced hippocampal gliosis (Landfield, et al., 1981). Soon thereafter, Sapolsky and colleagues reported an elevation in basal plasma CORT of aged rats and an impaired capacity for their stress-induced levels of CORT to return to basal levels (Sapolsky, et al., 1983). Others have since reported similar findings as well (Meaney, et al., 1992).
Our present findings are generally consistent with these prior studies, and go further to suggest that protracted basal elevations in hippocampal CORT cause potentiated inflammatory responses to a peripheral immune challenge due to a CORT-mediated sensitization of hippocampal microglia, as suggested recently (Frank, et al., 2014, Sorrells, et al., 2013). Although we did not observe elevations in plasma CORT in aged rats, we demonstrated modest, but significant, elevations in hippocampal CORT, lasting only throughout the inactive phase. Plasma CORT levels were comparable between young and aged rats throughout the inactive phase, while levels were blunted throughout the active phase in the aged rats, as has been established previously in aged humans and rodents (Colucci, et al., 1975, Hauger, et al., 1994, Milcu, et al., 1978). The discrepancy between the plasma CORT data reported here and that of some, but not other studies (Sapolsky, 1992) may be explained by the unique use of an automated serial blood sampling technique here, whereby rats were not handled at all during a sampling period that extended across the entire day.
The elevated hippocampal CORT levels that we observed in aged rats likely resulted from increases in hippocampal 11β-HSD1 protein, the enzyme that catalyzes the conversion of inactive 11-dehydrocorticosterone to active CORT. Consistent with hippocampal CORT levels, 11β-HSD1 expression in aged rats compared to young rats was heightened only during the inactive phase. These data point to a mechanism likely playing a key role in the observed elevated CORT levels in the aged brain. Robust aging-induced increases in hippocampal 11β-HSD1 mRNA have been reported previously (Holmes, et al., 2010), and so the present data support those findings and extend them to demonstrate that these increases also occur in protein, and track with fluctuations in CORT levels. It is also noteworthy that aged 11β-HSD1 knock-out mice lack the elevated hippocampal CORT levels that are present in aged wildtype mice, despite no difference in plasma CORT levels (Yau, et al., 2011). These are potentially important findings, as inhibiting 11β-HSD1 at specific times of day, in the hippocampus, may prove to be a useful therapeutic target.
While total GR expression did not differ between the age groups at either time point, nuclear GR expression, a measure of GR activation, was significantly increased in aged rats during the inactive phase, compared to that of young rats. These findings confirm that the observed hippocampal CORT levels in aged rats are indeed functionally significant, as the GR must be activated before GR-related modulation of gene transcription can occur. Consistent with hippocampal CORT observations, nuclear GR expression levels during the active phase did not differ between age groups. As expected, in young rats nuclear GR expression was significantly increased during the active phase compared to the inactive phase. In aged rats a proportionate increase in GR activation during the active phase over the inactive phase was not observed. It is unclear why, though close examination of the hippocampal CORT data suggest that the circadian peak may be shifted in aged rats, occurring earlier than in young rats. Thus, it is possible that the time of sacrifice for the GR experiment may have coincided with the young rats’ CORT peak, but after the aged rats’ levels had started to drop. Taken together, these data might suggest that over a 24 h period, overall hippocampal GR activation levels are fairly comparable between young and aged rats. However, there is evidence to suggest that variations in circadian CORT rhythm (that is, increases or decreases at the “wrong” time of day) dysregulates cellular clock gene expression and produces longlasting myeloid cell activation (Nguyen, et al., 2013, O'Callaghan, et al., 2012). This scenario would be consistent with the general hypothesis that aberrantly elevated CORT sensitizes microglia. It should be noted that the nuclear GR findings in the present paper are at odds with a similar study that reported nuclear GR expression levels to be significantly reduced rather than increased in aged rats (Murphy, et al., 2002). A possible explanation for this discrepancy is that aged rats in that study may not have been maintained under truly basal conditions as those aged rats had substantially higher plasma CORT levels than we observed in this study.
Even though we observed age-related, time-dependent, differences in nuclear GR, we did not detect diurnal differences in cytosolic GR levels of either young or aged rats. This lack of an age-related difference in cytosolic GR is consistent with previous findings (Murphy, et al., 2002) and might be attributable to the much greater level of GR protein present in the cytosolic than the nuclear tissue fraction under basal (non-stress) conditions throughout the day. Thus, a movement of a small percentage of cytosolic GR into the nucleus could produce a relatively large increase in the nuclear pool of GR that we were able to detect, without registering a significant decline in the cytosolic pool of GR.
The blockade of central GR action with a low dose GR antagonist produced a significant reduction in GILZ gene expression, as expected. This antagonist treatment also robustly reduced gene expression of the microglial activation marker Iba-1 in aged rats, suggesting that mifepristone treatment altered the microglial immunophenotype to resemble that of a young adult rat. Somewhat surprisingly, no differences in MHCII gene expression were detected across the groups. Given the lower expression levels of MHCII compared to Iba-1, and the small number of cultured cells used in this study, it is possible that expression levels of MHCII were too low to detect reliable changes. Furthermore, mifepristone treatment successfully reduced microglial sensitization, as measured by decreased mRNA expression of the proinflammatory cytokines IL-1β, IL-6, and TNFα in response to subsequent ex vivo challenge of isolated microglia with LPS. It should be noted that mifepristone treatment did not alter gene expression of any of the genes analyzed here in young adult rats, suggesting that this therapeutic treatment is specifically decreasing aging-induced microglial priming, and not just decreasing LPS response generally. These data strongly support a growing literature suggesting that greater GR activation drives microglial sensitization, causing an amplified proinflammatory response to immune challenge (Frank, et al., 2014, Sorrells, et al., 2013, Weber, et al., 2013). Strikingly, this GR antagonist approach also successfully prevented the hippocampal-dependent memory deficits produced by an in vivo E. coli infection in aged rats. The fact that 2 daily administrations of mifepristone were sufficient to normalize both age-related microglial sensitization and infection-induced memory decline points to a clinically useful therapeutic approach to ameliorate these effects, though it is unclear how long these beneficial effects may persist. The current findings strongly suggest that excessive diurnal CORT in the aged hippocampus provides an elevated activational priming signal, possibly increasing NFkB activation, which interacts with aging-related phenotypic changes in microglia, resulting in potentiated proinflammatory responses to an immune challenge. Accordingly, blocking the elevated GR signal rapidly reduced microglia sensitization and the consequent memory decline. Recent evidence demonstrating that acute glucocorticoids are capable of sensitizing proinflammatory responses supports this notion (Busillo, et al., 2011, Frank, et al., 2014). Indeed, these studies have shown that exogenous glucocorticoids sensitize macrophages and microglia by upregulating NLRP3 (Nucleotide-binding domain, Leucine-Rich Repeat, Pyrin domain containing protein), a component of the NLRP3 inflammasome (Busillo, et al., 2011). The NLRP3 inflammasome, expressed in microglia (Hanamsagar, et al., 2012) regulates the activation of caspase-1 and the subsequent maturation and release of IL-1β and IL-18 (Martinon, et al., 2002). Thus, it is plausible that the acute diurnal CORT elevation exhibited in the aged hippocampus induces increases in NLRP3 expression, resulting in sensitized hippocampal microglia. This is the subject of a future study.
The findings from the present exercise study are also consistent with the GR signaling hypothesis, albeit in an unexpected manner. Physical activity did not reduce hippocampal levels of CORT, bit it did reduce levels of GR expression. Thus, exercise may be neuroprotective in aged rats not because it reduces CORT in the hippocampus, but because it reduces GR. Such a reduction would reduce the potential for GR activation, gene transcription, and other downstream effects.
Advanced age is associated with sensitized hippocampal microglia that when activated as a consequence of an inflammatory stimulus, mediate protracted neuroinflammatory responses. This amplified neuroinflammatory response is known to impair memory processes. Here we present converging evidence for increased GR activation, evident in the hippocampus of aged rats during the inactive phase of the diurnal cycle, resulting in microglial sensitization. Furthermore, reductions in GR activation significantly reduced the LPS-induced microglial inflammatory response and prevented E. coli-induced memory impairments in aged rats. These novel findings point to a clinically useful therapeutic approach to reduce the primed microglial phenotype associated with advanced age and restore cognitive functions that otherwise are impaired following an immune challenge.
Highlights.
Basal CORT is elevated in hippocampus of aged rats during the light phase.
Aged rats exhibit greater GR activation in hippocampus basally during the light phase.
Reducing GR activation normalized microglial phenotype and inflammatory response.
Reducing GR activation prevented E. coli-induced memory impairments in aged rats.
Voluntary exercise reduces GR expression in the aged hippocampus
Acknowledgments
This work was supported by grants from the National Institute on Aging R01AG028271 to R.M.B., M.G.F., L.R.W., & S.F.M., and NSF Grant No. 0921969 to C.A.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayroldi E, Riccardi C. Glucocorticoid-induced leucine zipper (GILZ): a new important mediator of glucocorticoid action. FASEB J. 2009;23(11):3649–3658. doi: 10.1096/fj.09-134684. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HE, Campeau S, Watkins LR, Patterson SL, Maier SF. Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J Neurosci. 2011;31(32):11578–11586. doi: 10.1523/JNEUROSCI.2266-11.2011. doi:31/32/11578 [pii] 10.1523/JNEUROSCI.2266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun. 2009a;23(1):46–54. doi: 10.1016/j.bbi.2008.07.002. doi:S0889-1591(08)00305-X [pii] 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27(5):723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. doi:S0197-4580(05)00104-1 [pii] 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, O'Reilly RC, Rudy JW. Memory for context is impaired by injecting anisomycin into dorsal hippocampus following context exploration. Behav Brain Res. 2002;134(1–2):299–306. doi: 10.1016/s0166-4328(02)00045-1. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Watkins LR, Rudy JW, Maier SF. Characterization of the sickness response in young and aging rats following E. coli infection. Brain Behav Immun. 2009b;23(4):450–454. doi: 10.1016/j.bbi.2009.01.016. doi:S0889-1591(09)00043-9 [pii] 10.1016/j.bbi.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busillo JM, Azzam KM, Cidlowski JA. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J Biol Chem. 2011;286(44):38703–38713. doi: 10.1074/jbc.M111.275370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WL, Carrell RW, Zhou A, Read RJ. How changes in affinity of corticosteroid-binding globulin modulate free cortisol concentration. J Clin Endocrinol Metab. 2013;98(8):3315–3322. doi: 10.1210/jc.2012-4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman TR, Barrientos RM, Ahrendsen JT, Hoover JM, Maier SF, Patterson SL. Aging and infection reduce expression of specific brain-derived neurotrophic factor mRNAs in hippocampus. Neurobiol Aging. 2012;33(4):832, e1–e14. doi: 10.1016/j.neurobiolaging.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman TR, Barrientos RM, Ahrendsen JT, Maier SF, Patterson SL. Synaptic correlates of increased cognitive vulnerability with aging: peripheral immune challenge and aging interact to disrupt theta-burst late-phase long-term potentiation in hippocampal area CA1. J Neurosci. 2010;30(22):7598–7603. doi: 10.1523/JNEUROSCI.5172-09.2010. doi:30/22/7598 [pii] 10.1523/JNEUROSCI.5172-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci CF, D'Alessandro B, Bellastella A, Montalbetti N. Circadian rhythm of plasma cortisol in the aged (Cosinor method) Gerontol Clin (Basel) 1975;17(2):89–95. doi: 10.1159/000245562. [DOI] [PubMed] [Google Scholar]
- Cortese GP, Barrientos RM, Maier SF, Patterson SL. Aging and a Peripheral Immune Challenge Interact to Reduce Mature Brain-Derived Neurotrophic Factor and Activation of TrkB, PLC{gamma}1, and ERK in Hippocampal Synaptoneurosomes. J Neurosci. 2011;31(11):4274–4279. doi: 10.1523/JNEUROSCI.5818-10.2011. doi:31/11/4274 [pii] 10.1523/JNEUROSCI.5818-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25(40):9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pablos RM, Villaran RF, Arguelles S, Herrera AJ, Venero JL, Ayala A, Cano J, Machado A. Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neurosci. 2006;26(21):5709–5719. doi: 10.1523/JNEUROSCI.0802-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkel K, MacPherson A, Sapolsky RM. Novel glucocorticoid effects on acute inflammation in the CNS. J Neurochem. 2003;84(4):705–716. doi: 10.1046/j.1471-4159.2003.01604.x. [DOI] [PubMed] [Google Scholar]
- Droste SK, de Groote L, Atkinson HC, Lightman SL, Reul JM, Linthorst AC. Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology. 2008;149(7):3244–3253. doi: 10.1210/en.2008-0103. doi:en.2008-0103 [pii] 10.1210/en.2008-0103. [DOI] [PubMed] [Google Scholar]
- Findley T. Role of the neurohypophysis in the pathogenesis of hypertension and some allied disorders associated with aging. Am J Med. 1949;7(1):70–84. doi: 10.1016/0002-9343(49)90484-2. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006a;27(5):717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Watkins LR, Maier SF. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. J Neuroimmunol. 2010a;226(1–2):181–184. doi: 10.1016/j.jneuroim.2010.05.022. doi:S0165-5728(10)00206-7 [pii] 10.1016/j.jneuroim.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Hershman SA, Weber MD, Watkins LR, Maier SF. Chronic exposure to exogenous glucocorticoids primes microglia to pro-inflammatory stimuli and induces NLRP3 mRNA in the hippocampus. Psychoneuroendocrinology. 2014;40:191–200. doi: 10.1016/j.psyneuen.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses, to E. coli lipopolysaccharide. Brain Behav Immun. 2010b;24(1):19–30. doi: 10.1016/j.bbi.2009.07.008. doi:S0889-1591(09)00386-9 [pii] 10.1016/j.bbi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics. J Neurosci Methods. 2006b;151(2):121–130. doi: 10.1016/j.jneumeth.2005.06.026. doi:S0165-0270(05)00226-8 [pii] 10.1016/j.jneumeth.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia L, Harbuz MS, Manzanares J, Lightman SL, Fuentes JA. RU-486 blocks stress-induced enhancement of proenkephalin gene expression in the paraventricular nucleus of rat hypothalamus. Brain Res. 1998;786(1–2):215–218. doi: 10.1016/s0006-8993(97)01416-9. [DOI] [PubMed] [Google Scholar]
- Garrido P, de Blas M, Del Arco A, Segovia G, Mora F. Aging increases basal but not stress-induced levels of corticosterone in the brain of the awake rat. Neurobiol Aging. 2012;33(2):375–382. doi: 10.1016/j.neurobiolaging.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19(10):1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Guiochon-Mantel A, Delabre K, Lescop P, Milgrom E. The Ernst Schering Poster Award. Intracellular traffic of steroid hormone receptors. J Steroid Biochem Mol Biol. 1996;56(1–6 Spec No):3–9. doi: 10.1016/0960-0760(95)00268-5. [DOI] [PubMed] [Google Scholar]
- Hanamsagar R, Hanke ML, Kielian T. Toll-like receptor (TLR) and inflammasome actions in the central nervous system. Trends Immunol. 2012;33(7):333–342. doi: 10.1016/j.it.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Thrivikraman KV, Plotsky PM. Age-related alterations of hypothalamic-pituitary-adrenal axis function in male Fischer 344 rats. Endocrinology. 1994;134(3):1528–1536. doi: 10.1210/endo.134.3.8119195. [DOI] [PubMed] [Google Scholar]
- Henley DE, Lightman SL. New insights into corticosteroid-binding globulin and glucocorticoid delivery. Neuroscience. 2011;180:1–8. doi: 10.1016/j.neuroscience.2011.02.053. doi:S0306-4522(11)00223-5 [pii] 10.1016/j.neuroscience.2011.02.053. [DOI] [PubMed] [Google Scholar]
- Holmes MC, Carter RN, Noble J, Chitnis S, Dutia A, Paterson JM, Mullins JJ, Seckl JR, Yau JL. 11beta-hydroxysteroid dehydrogenase type 1 expression is increased in the aged mouse hippocampus and parietal cortex and causes memory impairments. J Neurosci. 2010;30(20):6916–6920. doi: 10.1523/JNEUROSCI.0731-10.2010. doi:30/20/6916 [pii] 10.1523/JNEUROSCI.0731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu BR, Kuhn RW. The role of the adrenal in generating the diurnal variation in circulating levels of corticosteroid-binding globulin in the rat. Endocrinology. 1988;122(2):421–426. doi: 10.1210/endo-122-2-421. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16(4):461–476. doi: 10.1006/brbi.2001.0638. doi:S0889159101906385 [pii]. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Baskin RK, Pitler TA. Brain aging correlates: retardation by hormonal-pharmacological treatments. Science. 1981;214(4520):581–584. doi: 10.1126/science.6270791. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Waymire JC, Lynch G. Hippocampal aging and adrenocorticoids: quantitative correlations. Science. 1978;202(4372):1098–1102. doi: 10.1126/science.715460. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Plant A, Windle RJ, Shanks N, Wood SA, Ingram CD, Renner KJ, Lightman SL, Summers CH. Fluoxetine inhibits corticotropin-releasing factor (CRF)-induced behavioural responses in rats. Stress. 2009;12(3):225–239. doi: 10.1080/10253890802309861. [DOI] [PubMed] [Google Scholar]
- MacPherson A, Dinkel K, Sapolsky R. Glucocorticoids worsen excitotoxin-induced expression of pro-inflammatory cytokines in hippocampal cultures. Exp Neurol. 2005;194(2):376–383. doi: 10.1016/j.expneurol.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20(4):435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Sharma S, Viau V. Basal ACTH, corticosterone and corticosterone-binding globulin levels over the diurnal cycle, and age-related changes in hippocampal type I and type II corticosteroid receptor binding capacity in young and aged, handled and nonhandled rats. Neuroendocrinology. 1992;55(2):204–213. doi: 10.1159/000126116. [DOI] [PubMed] [Google Scholar]
- Milcu SM, Bogdan C, Nicolau GY, Cristea A. Cortisol circadian rhythm in 70--100-year-old subjects. Endocrinologie. 1978;16(1):29–39. [PubMed] [Google Scholar]
- Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, Lima Lde S, Avellar MC, Sapolsky RM, Scavone C. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci. 2006;26(14):3813–3820. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy EK, Spencer RL, Sipe KJ, Herman JP. Decrements in nuclear glucocorticoid receptor (GR) protein levels and DNA binding in aged rat hippocampus. Endocrinology. 2002;143(4):1362–1370. doi: 10.1210/endo.143.4.8740. [DOI] [PubMed] [Google Scholar]
- Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341(6153):1483–1488. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Godbout JP. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol. 2013;39(1):19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan EK, Anderson ST, Moynagh PN, Coogan AN. Long-lasting effects of sepsis on circadian rhythms in the mouse. PLoS One. 2012;7(10):e47087. doi: 10.1371/journal.pone.0047087. doi:10.1371/journal.pone.0047087 PONE-D-12-18752 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O'Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci. 2002;116(4):530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Do glucocorticoid concentrations rise with age in the rat? Neurobiol Aging. 1992;13(1):171–174. doi: 10.1016/0197-4580(92)90025-s. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp Gerontol. 1999;34(6):721–732. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The adrenocortical stress-response in the aged male rat: impairment of recovery from stress. Exp Gerontol. 1983;18(1):55–64. doi: 10.1016/0531-5565(83)90051-7. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Pulsinelli WA. Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science. 1985;229(4720):1397–1400. doi: 10.1126/science.4035356. [DOI] [PubMed] [Google Scholar]
- Sarkar NN. Mifepristone: bioavailability, pharmacokinetics and use-effectiveness. Eur J Obstet Gynecol Reprod Biol. 2002;101(2):113–120. doi: 10.1016/s0301-2115(01)00522-x. doi:S030121150100522X [pii]. [DOI] [PubMed] [Google Scholar]
- Selye H. Stress and disease. Science. 1955;122(3171):625–631. doi: 10.1126/science.122.3171.625. [DOI] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K. Steroid hormone receptor expression and function in microglia. Glia. 2008;56(6):659–674. doi: 10.1002/glia.20644. [DOI] [PubMed] [Google Scholar]
- Solez C. Aging and adrenal cortical hormones. Geriatrics. 1952;7(4):241–245. contd. [PubMed] [Google Scholar]
- Song C, Phillips AG, Leonard BE, Horrobin DF. Ethyl-eicosapentaenoic acid ingestion prevents corticosterone-mediated memory impairment induced by central administration of interleukin-1beta in rats. Mol Psychiatry. 2004;9(6):630–638. doi: 10.1038/sj.mp.4001462. [DOI] [PubMed] [Google Scholar]
- Sorrells SF, Caso JR, Munhoz CD, Hu CK, Tran KV, Miguel ZD, Chien BY, Sapolsky RM. Glucocorticoid signaling in myeloid cells worsens acute CNS injury and inflammation. J Neurosci. 2013;33(18):7877–7889. doi: 10.1523/JNEUROSCI.4705-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64(1):33–39. doi: 10.1016/j.neuron.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Sapolsky RM. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav Immun. 2007;21(3):259–272. doi: 10.1016/j.bbi.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RL, Kalman BA, Cotter CS, Deak T. Discrimination between changes in glucocorticoid receptor expression and activation in rat brain using western blot analysis. Brain Res. 2000;868(2):275–286. doi: 10.1016/s0006-8993(00)02341-6. [DOI] [PubMed] [Google Scholar]
- van Eekelen JA, Rots NY, Sutanto W, de Kloet ER. The effect of aging on stress responsiveness and central corticosteroid receptors in the brown Norway rat. Neurobiol Aging. 1992;13(1):159–170. doi: 10.1016/0197-4580(92)90024-r. [DOI] [PubMed] [Google Scholar]
- Weber MD, Frank MG, Sobesky JL, Watkins LR, Maier SF. Blocking toll-like receptor 2 and 4 signaling during a stressor prevents stress-induced priming of neuroinflammatory responses to a subsequent immune challenge. Brain Behav Immun. 2013;32:112–121. doi: 10.1016/j.bbi.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrwoll CS, Holmes MC, Seckl JR. 11beta-hydroxysteroid dehydrogenases and the brain: from zero to hero, a decade of progress. Front Neuroendocrinol. 2011;32(3):265–286. doi: 10.1016/j.yfrne.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JL, Noble J, Seckl JR. 11beta-hydroxysteroid dehydrogenase type 1 deficiency prevents memory deficits with aging by switching from glucocorticoid receptor to mineralocorticoid receptor-mediated cognitive control. J Neurosci. 2011;31(11):4188–4193. doi: 10.1523/JNEUROSCI.6145-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]