Abstract
Bistable perception occurs when a stimulus is ambiguous and has two distinct interpretations that spontaneously alternate in observers’ consciousness. Studies using functional magnetic resonance imaging, electroencephalography (EEG), and transcranial magnetic stimulation (TMS) in healthy subjects and patient studies point towards a right fronto-parietal network regulating the balance between percept stabilization and the arising of alternative interpretations. However, the causal role of the interaction between parietal and prefrontal areas is not clearly understood. Using intermittent presentations of bistable images, we confirmed that maintaining or switching percepts had neural correlates identifiable on EEG. Single-pulse TMS applied over the right anterior intraparietal sulcus (IPS) 70 ms before image presentation interfered with evoked potentials and destabilized the percept. However, with paired-pulse TMS applied over right IPS and dorsolateral prefrontal cortex (DLPFC) 70 and 60 ms before image presentation, both perceptual and neurophysiological effects were cancelled. Thus, TMS over IPS and DLPFC interacted with each other and influenced upcoming percepts. We suggest that when the visual world is ambiguous, IPS plays a stabilizing role, whereas DLPFC is important for triggering perceptual switches or for modulating parietal activity. The balance between maintaining and switching visual conscious percepts relies on the dynamic interaction between IPS and DLPFC.
Keywords: Conscious perception, Dorsolateral Prefrontal Cortex, Intraparietal Sulcus, Stabilization, Switching
Introduction
Bistable perception occurs when a stimulus is ambiguous and can have two distinct, exclusive and plausible interpretations. Such distinct percepts randomly and spontaneously alternate in observers’ consciousness. Such a phenomenon might be functionally relevant: in case of ambiguity in the world, we need to alternate between a percept stabilization phase allowing us to gather information, and short periods of percept destabilization and reinterpretation allowing the emergence of an alternative interpretation. However, the mechanisms of the brain responsible for such antagonist mechanisms are not fully understood.
Studies with patients (Bonneh, Pavlovskaya, Ring, & Soroker, 2004; Meenan & Miller, 1994; Ricci & Blundo, 1990), with functional magnetic resonance imaging (fMRI) (Brouwer, Tong, Hagoort, & van Ee, 2009; Kleinschmidt, Buchel, Zeki, & Frackowiak, 1998; Lumer, Friston, & Rees, 1998; Preston, Kourtzi, & Welchman, 2009; Raz, Lamar, Buhle, Kane, & Peterson, 2007; Slotnick & Yantis, 2005) and with electro-encephalography (EEG) (Britz, Landis, & Michel, 2009; Kornmeier & Bach, 2005; Mathes, Struber, Stadler, & Basar-Eroglu, 2006; Muller et al., 2005; Nakatani & van Leeuwen, 2006) point towards a major involvement of a right fronto-parietal attentional network. More recently, several studies, using repetitive transcranial magnetic stimulation (TMS) to decrease the excitability of several areas within the right intraparietal sulcus (IPS), conclude on the existence of a functional fractionation of the IPS, with an anterior part playing a stabilizing role and a posterior part enabling switches to occur (Carmel, Walsh, Lavie, & Rees, 2010; Kanai, Bahrami, & Rees, 2010; Kanai, Carmel, Bahrami, & Rees, 2011). Another study showed different results with online rTMS (Zaretskaya, Thielscher, Logothetis, & Bartels, 2010); however, if the offline rTMS protocols used by former studies are expected to induce a decrease of excitability in the stimulated area, the effects on cortical excitability of the online rTMS protocol used by the latter study are unknown. Concerning the prefrontal cortex, a study using a similar offline method failed to demonstrate a causal role of the dorsolateral prefrontal cortex (DLPFC) in spontaneous switching; only voluntary control of switches was impaired with TMS over the DLPFC (de Graaf, de Jong, Goebel, van Ee, & Sack, 2011). The negative result for spontaneous switching was surprising given the difficulties of patients with prefrontal lesions or excisions to initiate switches of percept towards an alternative interpretation of an ambiguous stimulus (Meenan & Miller, 1994; Ricci & Blundo, 1990). More recently, however, a study examining both spontaneous and voluntary switches in patients with prefrontal lesions showed that only voluntary switches, i.e., when the patients tried to speed-up the switching rate, were impaired (Windmann, Wehrmann, Calabrese, & Gunturkun, 2006). Nevertheless, patients or rTMS studies neither allow exploring the time window during which IPS or DLPFC are involved, nor the role of interactions between these two areas.
In this study, we wanted to explore the causal interaction between IPS and the DLPFC in bistable perception. For this, we used a bifocal (twin-coils, dual-coils, or paired-pulse) TMS procedure combined with a perceptual task and EEG. The timings of stimulation over both areas (70–60 ms before the onset of the image) were chosen to be slightly earlier than the time window (~ 50 ms) during which right parietal activity was shown to be predictive of upcoming percepts (Britz et al., 2009). In addition, in absence of direct measures estimating the latency of spreading activation towards the IPS or DLPFC after TMS over the DLPFC or IPS, we used a short time delay between the two pulses (10 ms) consistent with known times of spreading activation across ipsilateral areas following a single-pulse of TMS over the primary motor cortex (Ilmoniemi et al., 1997; Litvak et al., 2007). Using a short time delay also maximizes the chance that the effects of the two TMS pulses interfere with each other at both stimulated areas. As we were interested in real life 3D ambiguity that can be perceived in concave/convex objects, we chose to study the perception of the Mach Card, used in previous studies (Preston et al., 2009), that can be perceived either convex (like viewing the covers of a book) or concave (like viewing the internal pages of a book, see Fig. 3B). Behavioral results (in terms of change of perceptual stability) were related to neurophysiological results (in terms of evoked-potentials in the EEG) and also to neuropsychological results (to account for TMS effects variability). We expected that a single-pulse over the IPS would interfere with visual potentials and decrease perceptual stability, consistent with a stabilizing role of the anterior part of the IPS (Carmel et al., 2010; Kanai et al., 2011). Moreover, we expected that a second pulse applied over the DLPFC would further modulate visual potentials and reverse the perceptual effects, reducing or cancelling the destabilizing effect of the first pulse or even inducing an increased perceptual stability, consistent with a triggering role of the DLPFC in bistable perception (Meenan & Miller, 1994; Ricci & Blundo, 1990).
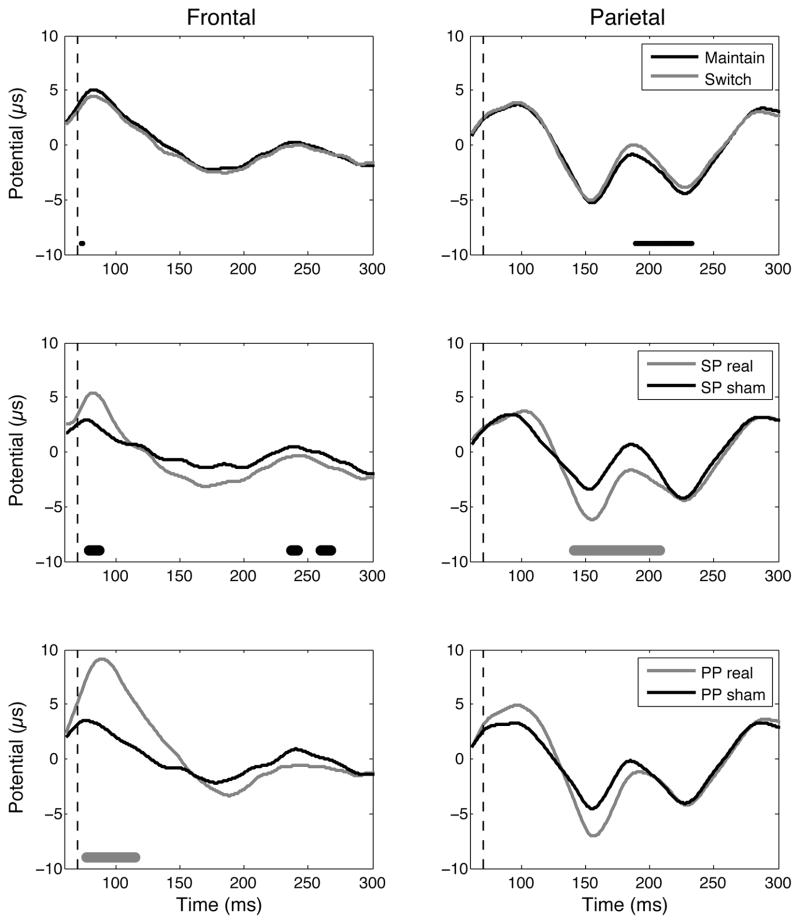
Figure 3. EEG results.
EEG responses at the electrodes of interest over the right frontal (F4) and right parietal (P4) areas. Top: Maintain versus switch trials (data pooled from SP sham and PP sham conditions); Middle: SP real versus SP sham; Bottom: PP real versus PP sham. Below the potentials, the thin black line, thick black line, and thick grey line represent the time points for which the two recorded potentials are significantly different according to the t-test, single threshold permutation test and suprathreshold cluster permutation test, respectively.
Materials and Methods
Participants
A total of 21 adult subjects were included in the study. Among them, 7 did not finish the experiment. One of them was not able to see the two different percepts when visualizing the bistable image and was excluded at the very beginning of the screening visit, 1 was excluded because he got sick before his second visit, without any causal link to the experiment, and 5 participants withdrew, 3 for scheduling incompatibility, and 2 because they felt uncomfortable receiving TMS (one dropped at the end of the screening visit and one at the end of the second visit which tested the single-pulse condition (see below). The 14 remaining subjects (6 females; 1 left-handed, age range 21–35 years old; mean 23, SD: 4) completed the 3 visits of the experiment. All subjects gave written informed consent prior to participation. The study was in accordance with the declaration of Helsinki and was approved by the Institutional Review Board of the Beth Israel Deaconess Medical Center (Boston MA, USA).
Visits
During the screening visit, a battery of questionnaires was performed to ensure that the TMS and MRI procedures were safe for the participants. Participants were also tested for their ability to see the bistable stimulus and trained with the tasks. The participants underwent four computerized neuropsychological tests from the Cantab battery (Cambridge Cognition) to test their attention and executive functions (see below). Due to technical problems, two participants did not perform the neuropsychological tests. A T1-weighted anatomical MRI (3T GE scanner) was acquired to guide stimulation. At the end of the visit we measured the resting motor threshold (RMT) for each stimulator/coil. This visit lasted about 3 hours. The second and third visits were separated by at least 2 days, lasting about 4–5 hours. In both visits, the subjects participated in two experiments. This article reports the results of the first experiment only, which was always performed first. Two conditions were tested: single-pulse (SP) or paired-pulse (PP) TMS, one visit for each condition, in a randomized order. Eight subjects performed the SP condition first while 6 subjects performed the PP condition first.
TMS and EEG
Neuronavigated TMS was delivered to the DLPFC and the IPS through two figure-of-eight coils (a 70 mm-diameter Magstim air-cooled coil and a 40 mm-diameter custom-made Magstim coil, respectively) attached to two stimulators (a Magstim Rapid 2 and a Magstim SuperRapid with only one booster turned on in order to reduce recharge artifacts in the EEG (Veniero, Bortoletto, & Miniussi, 2009), respectively). Such material was chosen based on the necessity to use at least one small coil in order to ensure that the two coils would fit on all participants’ head. The focality and intensity of the induced current depend on numerous parameters including coil shape and size, individual coil-to-brain distance, etc. (Deng, Lisanby, & Peterchev, 2013). We decided to use stimulation intensities that were “functionally-equivalent” when applied to the primary motor cortex, i.e., based on RMT (see below). One should remember, however, that the TMS-measured excitability of one area poorly predicts the TMS-measured excitability of another area (Kahkonen, Komssi, Wilenius, & Ilmoniemi, 2005; Stewart, Walsh, & Rothwell, 2001). Therefore, one of our targeted areas might have received a stronger interference than the other. At the screening visit, the RMT was determined independently for the two stimulator-coil combinations as the lowest intensity needed to induce a motor-evoked potential (Powerlab and Scope software) in the first dorsal interosseus muscle of at least 50 μV in 5 out of 10 consecutive pulses. Although subjects’ RMT was not necessarily the same for the two stimulator-coil combinations tested, the RMT was on average 62 % (SD: 9 %) of maximum stimulator output for both. The stimulation intensity used for the remaining study visits was then adjusted to 120% of RMT. The participants wore earplugs at all times.
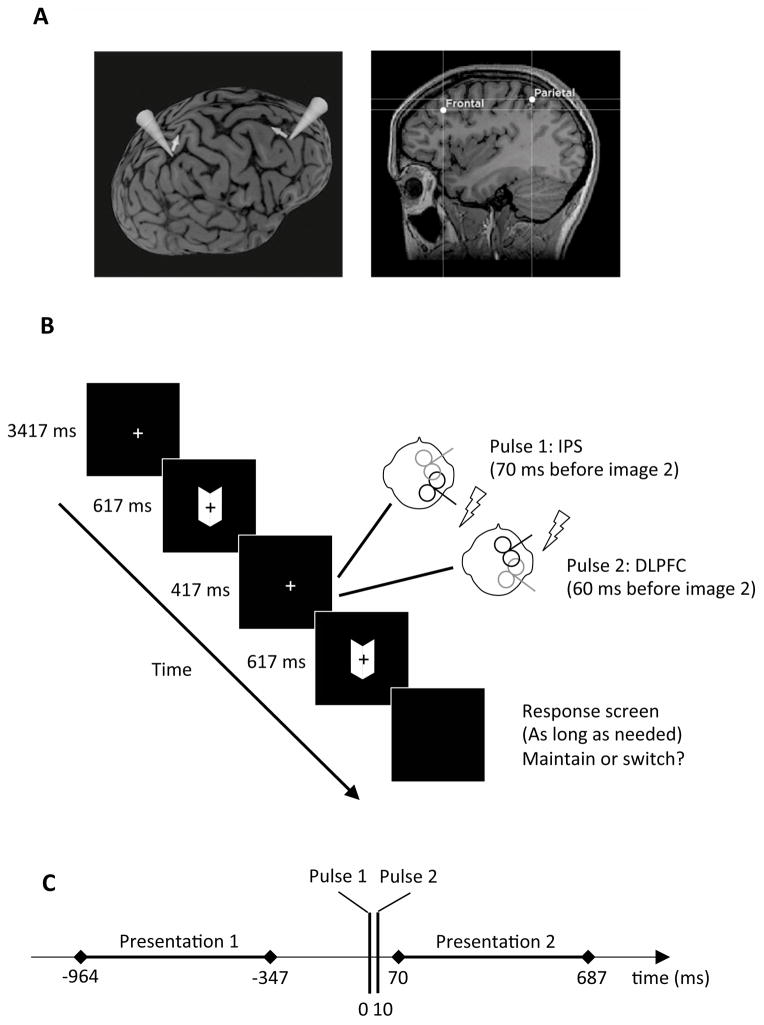
After normalization of each MRI (Brainsight), two sites of the right hemisphere were selected for stimulation based on previous studies. The target within the DLPFC was chosen at the Talairach coordinates [x, y, z] = [25, 27, 43] (de Graaf et al., 2011) whereas the target within the IPS was chosen at the MNI coordinates [x, y, z] = [35.84, −46.39, 53.69] (Zaretskaya et al., 2010), such coordinates being within 3 mm of the stimulation site used by Carmel et al. (2010) corresponding to the anterior part of the IPS (Fig. 1A).
Figure 1. Experimental procedures.
A. Targeted areas within the frontal and parietal cortex for one subject. The cones symbolize the axis perpendicular to the coils and the arrows symbolize the direction of the current induced in brain tissues. B. For each trial, two presentations of the Mach Card were separated by a black screen, during which TMS was performed. For the SP condition, a TMS pulse was applied over the IPS target 70 ms before the onset of the second presentation of the Mach Card. For the PP condition, this TMS pulse over the IPS was followed by a second TMS pulse over the DLPFC 60 ms before the onset of the second presentation of the Mach Card. At the end of the trial, the subject pressed a key to indicate if the percept was the same for the two presentations or if it had changed. Note that in this figure: (i) the size of the fixation cross has been increased compared to the real experiment; (ii) although during real PP stimulation the two coils could be in contact, they were not overlapping as they were tangential to the head at different scalp locations and consequently not parallel to each other. During sham stimulation, the intensity was the same but the coil’s surface was perpendicular to the head, thus preventing the magnetic field from reaching the brain. C. Time line of a trial. Time 0 corresponds to the TMS pulse over the IPS.
EEG was continuously acquired from 30 electrodes with the reference at Cz and the ground at AFz using a TMS-compatible system (BrainAmp MR+ and BrainVision Recording Software, BrainProducts GmbH). EOG was recorded with two additional electrodes. Skin/electrode impedance was maintained below 5 kOhm. The signal was digitized at a sampling rate of 1 kHz.
Experimental task
The experimental task was presented with MATLAB and the Psychtoolbox on a MacBook situated at 40 cm from the eyes of the subject (distance maintained with a back and head rest). The stimulus was a Mach Card, i.e., two adjacent parallelograms (size: 6.5 x 2.8 cm; angle: 125° and 55°) that can be perceived either convex (e.g., the cover of a book) or concave (e.g., the interior of a book). During each trial, after a fixation screen lasting 3417 ms, the Mach Card was presented twice (presentation time: 617 ms, inter-stimulus interval: 417 ms). The participants had to report via keypress whether the percept of the second presentation was similar to the first presentation (maintain) or opposite (switch). The subject could respond whenever she/he wanted and the next trial started automatically after the keypress. In case of a perceptual switch, this type of intermittent presentation allows attributing the timing of switch occurrence to the onset of the second presentation (Kornmeier & Bach, 2005). The inter-stimulus interval was chosen to maximize the number of switches in such intermittent presentation design and it was suggested that such perceptual reversals were similar to the ones obtained during continuous observation of bistable images (Kornmeier & Bach, 2012). Moreover, the presentation of the images was long enough to allow for the development of evoked-potentials but short enough to minimize the probability that a switch would occur later during image presentation (Kornmeier & Bach, 2012).
One visit tested the SP condition where a TMS pulse was applied over IPS 70 ms before the onset of the second stimulus presentation. The other visit tested the PP condition, where the same pulse over the IPS was followed 10 ms later by a TMS pulse over DLPFC (Fig. 1B). For each condition, both real stimulation and sham stimulation were performed in separate blocks. Sham stimulation was performed by tilting the coil 90° such that it was resting on the edge of one wing. Each condition (SP real, SP sham, PP real and PP sham) contained 200 trials. Participants were never told the type of condition that was applied and the order of conditions was randomized.
Neuropsychological testing
During the screening visits, 12 of the participants underwent 4 computerized neuropsychological tests from the Cantab battery (Cambridge Cognition) to test their attention and executive functions. The tests selected were the Match to Sample Visual Search (MTS, a matching test with a speed/accuracy trade-off), the Rapid Visual Information Processing (RVP, a test calling for a detection of sequences within a succession of elements), the Stop-Signal Task (SST, a test measuring the trade-off between the speed of response to a GO signal and the ability to inhibit the movement when a STOP signals follows), and the Intra/Extradimensional Set Shift (IED, a test of rule acquisition and reversal). MTS and RVP are mainly designed to test spatial distribution of attention and sustained attention, respectively; SST tests response inhibition in addition to sustained attention, and IED assesses switching ability. For each test, several measures are automatically calculated to quantify performance. The primary outcome measures for these tests were: (i) for MTS, percentage of correct responses, latencies of correct responses for different number of distracters, and increase of latencies with increasing number of distracters; (ii) for RVP, parameters of the signal detection theory (sensitivity, bias) and mean latency of response; (iii) for SST, latency on go trials (mean, median, maximum, minimum), stop signal delay, and estimate of the delay between go signal and stop signal for which 50 % of responses are successfully stopped; (iv) for IED, errors and number of completed stages. The neuropsychological results were then related to the behavioral results from the bistable task using an exploratory analysis with t-tests followed by Pearson correlations (see details in Results).
Data analysis
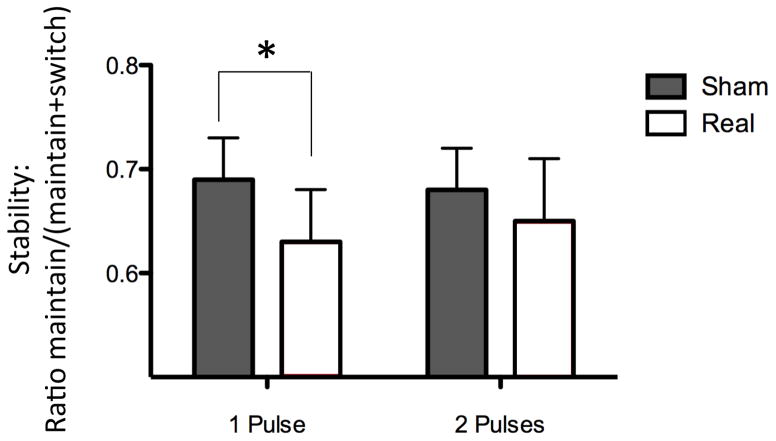
Behavioral parameters were extracted from the perceptual reports, i.e., the stability of the percept (maintain trials/(maintain trials + switch trials)). The results (see Fig. 2) were then expressed, for SP or PP condition, as a stability ratio: [(stability in real-stability in sham)/stability in sham]. With SP and PP conditions being tested on different days, the effects of real TMS compared to sham TMS were tested separately for each condition. In coherence with previous offline studies that used an inhibitory form of rTMS on IPS and showed a decrease of stability (Carmel et al., 2010; Kanai et al., 2011), we expected that our online disturbing single-pulse approach over the IPS would also decrease stability. Thus, the statistical significance of this change was assessed with a paired one-tailed t-test comparing sham and real TMS. For the PP condition, the prediction was less straightforward as we expected that disturbing the IPS and disturbing the DLPFC would have opposite effects. Thus, the statistical significance of this change was assessed with a paired two-tailed t-test. However, to allow for direct comparisons, we also ran a paired two-tailed t-test for the SP condition and a paired one-tailed t-test for the PP condition.
Figure 2. Behavioral responses.
Real SP TMS over IPS significantly decreased percept stability compared to sham SP TMS. When the TMS pulse over IPS was followed by a second pulse over DLPFC, the stability decrease was not significant anymore. Indicated values correspond to mean ± standard error.
EEG signals were analyzed with MATLAB and EEGLAB (Delorme & Makeig, 2004). EEG data were epoched around the first TMS pulse ([−2 s, +3 s]) and a baseline was removed ([−100 ms, −10 ms]). The infinite impulse response (IIR) Butterworth filter (1–45 Hz) of second order was used with a forward-backward filtering to maintain a zero phase shift. To discard any TMS-induced electromagnetic artifacts from the EEG, the filter was not applied to the time window containing the TMS pulse(s) ([−20 ms +60 ms]). Thus, our analysis started at t=60 ms, i.e., 10 ms before the onset of the second image. Noisy channels (maximum 3 per subject and never the channel of interest, see below) were rejected. Epochs contaminated by blinks or other artifacts were also discarded. On average, 140 (SD: 24) trials / 200 survived this cleaning. Analyses were then performed on two electrodes of interest, chosen for being close to the right IPS and DLPFC: P4 and F4.
The statistical analysis was performed on the recorded EEG potentials (see Fig. 3). For the two electrodes of interest, we performed the following comparisons: (i) maintain versus switch trials, pooled from SP and PP sham conditions; (ii) SP real versus SP sham; (iii) PP real versus PP sham. We used paired two-tailed t-test (p<0.05). To correct for multiple comparison, we then used 1000 random permutations to assess at each time point whether the t-test was significant by chance or not (single threshold test, alpha = 0.05) and then 1000 random permutations to assess whether each cluster of points that are significant according to the single threshold test was obtained by chance or not (suprathreshold cluster test, alpha = 0.05).
Finally, Pearson correlations between individual TMS-induced effects on EEG and individual TMS-induced effects on behavior were tested. The EEG markers chosen for these correlation analyses were peak-to-peak amplitudes of the signal measured during time-windows for which the potential was significantly modulated by TMS at the group-level (see details in Results).
Dealing with non-specific TMS effects
Unfortunately, sham TMS poorly mimics the auditory and somato-sensory stimulation of real TMS; the use of sham conditions in TMS studies is therefore controversial with regard to behavioral outcomes and, to a greater extent, EEG data (Thut, Ives, Kampmann, Pastor, & Pascual-Leone, 2005). We nevertheless expected to find (i) modulations of EEG potentials consistent with modulations of perception and (ii) different effects across different TMS conditions. Such findings would increase the probability that the results are related to genuine stimulation of the targeted areas rather than to general TMS effects associated with auditory and somato-sensory stimulation. Also, we cannot exclude the possibility that the experience of receiving PP is different from receiving SP. Moreover, stimulation of prefrontal areas has been associated with facial twitches and blinks, which can be unpleasant, may startle subjects and can make it hard for the subject to concentrate on a concurrent cognitive task. To minimize the potential impact of such effects, participants were familiarized specifically with the TMS condition to be employed in the present experiment. Moreover, all the subjects had relatively low motor thresholds and thus received low TMS intensity; for this reason the likelihood of induction of facial muscle twitches and blinks was low. Finally, none of the subjects who completed the study reported being more uncomfortable or more distracted with one or the other stimulation condition.
Results
Perceptual stabilization and switches occurred with characteristic neurophysiological responses
The experimental design with short presentations of a bistable stimulus led to the induction of a minor but non-negligible percentage, around 30% in the sham conditions (Fig. 2), of cases for which the second percept was different from the first one. The maintain or switch behaviors were associated with a distinct magnitude of potentials recorded over the right parietal cortex starting from about 120 ms after the onset of the second presentation, corresponding to 190 ms after the TMS pulse over the IPS (t-test significant for 189–233 ms, Fig 3, top right). This significant modulation of potentials did not survive the correction for multiple comparisons (single threshold test). However, the weakness of this effect can be related to the low number of trials (see discussion).
TMS influences both upcoming perception and associated EEG responses
TMS was able to manipulate both perception and physiological responses. A first TMS pulse over the IPS 70 ms before the onset of the second presentation decreased percept stability (one-tailed p < 0.029 according to our strong a priori hypothesis; two-tailed p = 0.059 also close to statistical significance; Fig. 2, left), suggesting that the anterior part of the right IPS plays a role in stabilizing the percept. In line with this behavioral evidence, this pulse also significantly modulated the potential recorded over the right parietal cortex, during a time window (suprathreshold cluster test significant for 141–208 ms; Fig. 3, middle right) that overlaps with the time window with significant differences between maintain and switch trials (189–233 ms, Fig. 3, top right). However, when this first pulse was followed 10 ms later by a second pulse over the right DLPFC, the perceptual destabilization was not significant anymore (two-tailed p = 0.59 according to our lack of a strong a priori hypothesis; one-tailed p = 0.30 also far from statistical significance; Fig. 2, right), suggesting that the DLPFC might play a role in triggering a switch or in modulating parietal activity. In line with this behavioral evidence, the potential recorded over the right IPS was no longer significantly modulated by the stimulation (p > 0.05; Fig 3, bottom right). On the contrary, the double-pulse stimulation modulated an earlier potential recorded over the DLPFC (suprathreshold cluster test significant for 77–115 ms; Fig. 3, bottom left). The behavioral and neurophysiological data thus suggest that the second pulse over the DLPFC blocked the effect of the first pulse over the IPS.
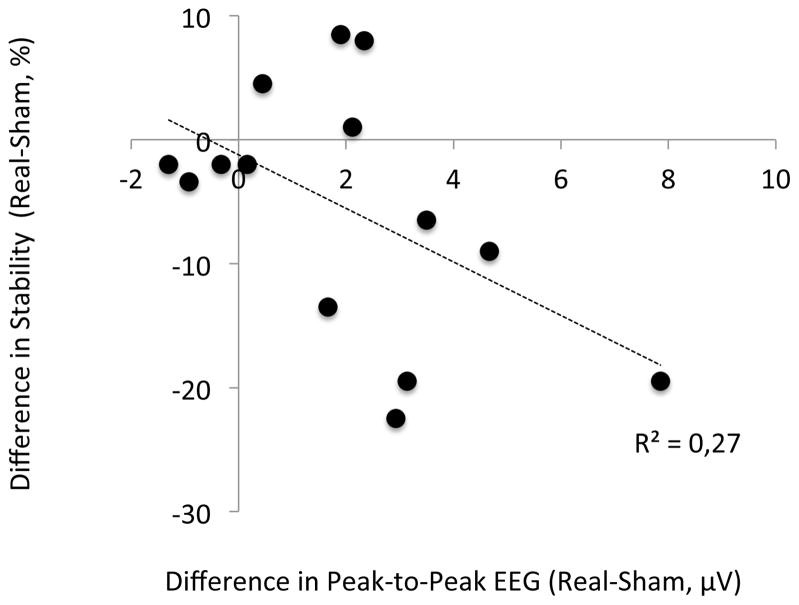
At the individual level, for the SP condition, the correlation between TMS-induced EEG changes over the IPS (peak-to-peak amplitude changes searched for in the 141–208 ms time-window according to group results and Fig. 3) and TMS-induced behavior changes trends towards significance (R2 = 0.27, p = 0.056, Fig. 4). This result further supports the existence of a relationship between TMS-induced modulations of EEG potentials and TMS-induced modulations of behavior. For the PP condition, the correlation between TMS-induced EEG changes over the DLPFC (peak-to-peak amplitude changes, with the maximum searched for in the 77–115 ms time-window and the minimum searched for in the 125–225 ms time-window, according to group results and Fig. 3) and TMS-induced behavior changes was not significant (p = 0.65). This result is compatible with the finding of an interaction between stimulation of the IPS and the DLPFC leading to a null behavioral result.
Figure 4. Relationship between EEG and behavioral results.
Pearson’s correlation between TMS-induced changes in EEG and TMS-induced changes in behavior for the SP condition.
Neuropsychological findings might account for some of the variability of response to TMS
At the group level, real SP TMS significantly decreased the stability of percept compared to sham SP TMS. However, an examination of individual behavioral results showed that 4 out of 14 subjects showed the opposite tendency, i.e., real SP TMS increased the stability compared to sham SP TMS. In order to look for a possible origin of such variability, we related these results to results of the neuropsychological tests. We divided the group of subjects into expected responders versus opposite responders following their decrease versus increase of stability after SP TMS over the IPS (compared to sham stimulation). According to this classification, 10 subjects were expected responders, among which 8 performed the neuropsychological tests; 4 were opposite responders who all performed the neuropsychological tests.
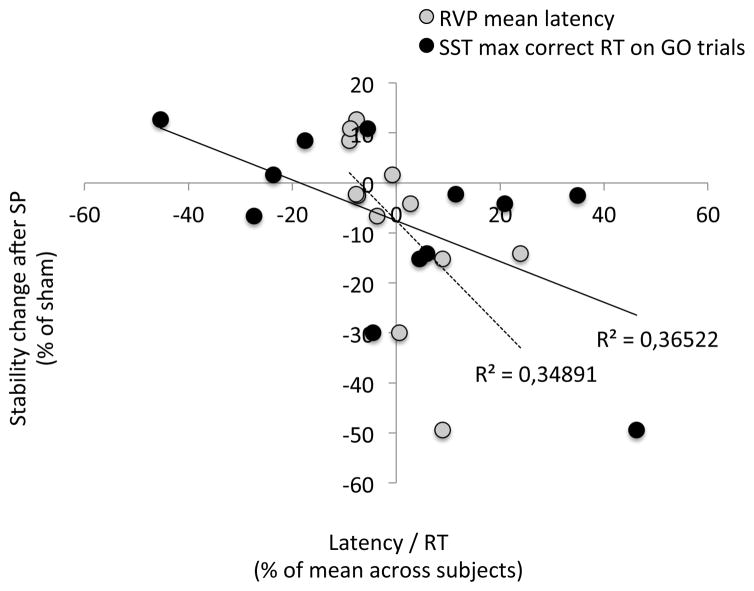
Among all the metrics listed in the methods section, there were significant differences between expected (8 subjects in this analysis) versus opposite responders (4 subjects) for the RVP and SST tests (p< 0.05; two-tailed, unpaired, unequal variances, uncorrected t-tests) in all metrics of latency/reaction time, i.e., mean latency for RVP (p = 0.021) and mean (p = 0.034), median (p = 0.050), maximum (p = 0.009), minimum (p = 0.050) latency on GO trials in SST. In addition, in SST, the stop signal delay was significantly different for expected versus opposite responders (p = 0.016). Among these measures, two metrics also show a significant Pearson correlation (12 subjects, Fig. 5) with the change of stability after SP TMS over the IPS: mean latency for RVP (p = 0.043) and maximum latency on GO trials in SST (p = 0.037). However, only the mean latency for RVP still maintains a reasonable tendency of correlation (p < 0.08) after correction for outliers. Thus, we found weak but interesting evidence suggesting that the size of the behavioral response in the expected direction to SP TMS over the IPS was inversely correlated with latencies of correct responses. In other words, the slower subjects’ reaction times were, the more likely the subjects showed the expected response to TMS.
Figure 5. Behavioral and neuropsychological results (12 subjects).
Pearson’s correlation between the change in stability after SP TMS and latency/reaction time metrics of RVP and SST.
Discussion
Top-down and bottom-up mechanisms
Several studies on bistable perception focused on understanding the mechanisms that allow switching between percepts or, on the contrary, stabilizing percepts. Distinct, yet complementary, hypotheses have been proposed. On the one hand, bottom-up mechanisms suggest that neural fatigue and reciprocal inhibition between the neural representations of the two percepts result in oscillation between the two percepts. On the other hand, top-down mechanisms suggest that perceptual switches are initiated by high-level brain areas exerting control over low-level brain areas. Recent evidence showed that both top-down and bottom-up influences are dramatically enhanced during bistable perception (Wang, Arteaga, & He, 2013).
Our results bring new insights concerning this perspective. First, we found a significant (although not resistant to correction for multiple comparisons) difference between maintain and switch trials on the potentials recorded over the parietal electrode around 120 ms after the onset of the second presentation of the bistable stimulus. Such timing corresponds to the “reversal positivity” described by Kornmeier and Bach (2005) over occipital and parietal electrodes. The small reversal positivity typically has an amplitude of around or below 1 μV and takes at least 100–120 trials to be detected with robustness (Kornmeier & Bach, 2012). Thus, the small number of only 140 (SD: 24) trials for both maintain and switch trials might explain the weakness of our result. Therefore, we confirm the existence of this potential modulated by conscious perception with a different type of bistable stimulus and a different experimental set-up. Such an early posterior potential could suggest that the mechanisms of perceptual reversals occur early in visual processing, i.e., following the activation of bottom-up mechanisms (Kornmeier & Bach, 2005) and could reflect a decision conflict when perceiving ambiguous stimuli (Kornmeier & Bach, 2012). However, its modulation by TMS over the right IPS suggests that it could also be related to top-down processes.
Indeed, our study also demonstrates that interfering with parietal activity 70 ms before the onset of the image has an influence on how it will be perceived. Although it is known that TMS provokes a cascade of excitation and inhibition within the stimulated area and interconnected areas, we assumed in this study that the primary effects of TMS occurred at the time of stimulation, which does not exclude delayed neurophysiological and behavioral effects (Amassian et al., 1989; Preston et al., 2009; Thut et al., 2003). Our use of TMS to disturb ongoing parietal activity in a precise time window and influence future perceptual states suggests that top-down influences on bistable perception probably start even before image onset.
Causal role of attentional areas
Another approach considers that attention might balance the weight of bottom-up and top-down mechanisms. Exogenous bottom-up capture of attention and endogenous top-down control of attention might both influence the percept (Raz et al., 2007), and voluntary switch of perception and voluntary switch of attention share a largely overlapping fronto-parietal network (Slotnick & Yantis, 2005). Two pioneering fMRI studies demonstrated right fronto-parietal activations during bistable perception. In the first study, brain activations recorded during perceptual changes in a binocular rivalry condition were compared to activations during a replay condition where conscious percepts (similar to those spontaneously generated during the binocular rivalry condition) were imposed by the visual stimulation (Lumer et al., 1998). In the second study, activations during perceptual transitions while observing ambiguous figures were compared to activations obtained during periods of perceptual stability (Kleinschmidt et al., 1998). Taken together, these studies showed that the right fronto-parietal network is involved in endogenously triggered changes of percept while observing ambiguous or rivalry stimuli. However, it has recently been suggested that fronto-parietal activation was more related to the response to perceptual transitions during binocular rivalry than to their cause (Knapen, Brascamp, Pearson, van Ee, & Blake, 2011). On the contrary, a recent fMRI study using a dynamic causal modeling approach indicated that during bistable switches, fronto-parietal activation was best modeled by a modulation of top-down connectivity from the right inferior frontal gyrus to visual areas (Weilnhammer, Ludwig, Hesselmann, & Sterzer, 2013). Indeed, one of the main challenges when studying conscious perception is to disentangle neural prerequisites such as attention, neural substrates per se and neural consequences (de Graaf, Hsieh, & Sack, 2012). Studies with patients or with brain stimulation techniques are thus essential in order to provide causal evidence.
Indeed, patients with right parietal lesions may have abnormal alternation rates in binocular rivalry (Bonneh et al., 2004), and patients with right frontal lesions experience difficulty in perceptual switching (Meenan & Miller, 1994; Ricci & Blundo, 1990), although a more recent study reported that this difficulty might be limited to voluntary switching (Windmann et al., 2006). TMS studies also explored the role of parietal and frontal areas in bistable perception in healthy subjects. In brief, three studies altogether, applying offline repetitive TMS protocols over the right superior parietal lobe (SPL) to decrease excitability, suggested a fractionation of the parietal cortex (Carmel et al., 2010; Kanai et al., 2010; Kanai et al., 2011). An anterior part of the SPL would have a stabilizing role, whereas a posterior part of the SPL would allow switching. On the contrary, Zaretskaya et al. (2010) showed different results with online repetitive TMS; however, online rTMS effects are much less explored than offline rTMS effects and some studies suggest that such effects can be different. For instance, performing a task related to the stimulated area during the stimulation might abolish or even reverse the rTMS effects (Huang, Rothwell, Edwards, & Chen, 2008). Finally, offline 1 Hz stimulation over the DLPFC succeeded in impairing voluntary control of bistable perception, showing for the first time a causal role of the frontal areas for bistable perception in healthy subjects (de Graaf et al., 2011). However, the DLPFC stimulation had no effect on spontaneous switching rate.
Our results are consistent with the major role played by the right anterior part of the parietal cortex and right prefrontal cortex in bistable perception. Whereas TMS can have both facilitation or suppression effects within the stimulated area depending on the current brain states according to recent models (Miniussi, Harris, & Ruzzoli, 2013; Miniussi, Ruzzoli, & Walsh, 2010; Perini, Cattaneo, Carrasco, & Schwarzbach, 2012; Ruzzoli, Marzi, & Miniussi, 2010), our results are most compatible with existing literature if we assume that TMS disturbs the stimulated areas. On the one hand, we confirmed previous TMS studies on the stabilizing role of the anterior IPS (Carmel et al., 2010; Kanai et al., 2011) with a different bistable stimulus and, more importantly, with a single-pulse TMS paradigm instead of an offline rTMS paradigm. Our use of intermittent presentations of a bistable stimulus to investigate the frequency of switches also complements a previous study showing that such intermittent presentation tends to strongly stabilize percepts (Leopold, Wilke, Maier, & Logothetis, 2002). Although our timings of image presentation and intermission were much shorter than the ones investigated in the study of Leopold et al. (2002) and chosen to maximize the number of switches (Kornmeier & Bach, 2012), our study brings evidence that such stabilization may depend on top-down control mediated by right parietal cortex. On the other hand, we showed for the first time the causal role of the DLPFC in spontaneous bistable perception in healthy subjects. The effects were indirect: stimulating DLPFC cancelled the effects of a preceding IPS stimulation. Without a condition with single-pulse TMS over the DLPFC, we cannot draw conclusions about the isolated role of the DLPFC. Future studies are warranted to further explore the role of DLPFC in spontaneous and voluntary switching. However, our study strongly suggests that the role of DLPFC might be coordinated with the role of IPS. Passive bistable perception might depend strongly on parieto-frontal circuits (present study) whereas voluntarily controlled bistable perception might be more concerned with prefrontal activity (de Graaf et al., 2011). Another interpretation is that TMS modulates perceptual outcomes and early evoked-potentials similarly to the modulation induced by attentional top-down control processes (Pitts, Gavin, & Nerger, 2008). However, whereas the early potentials modulated in our present study reflect early markers of conflict detection, attention, awareness or even memory processes still need to be explored as it has been done for later evoked-potentials (Intaite, Koivisto, Ruksenas, & Revonsuo, 2010).
Network perspective
Previous studies suggested that communication between parietal and frontal areas are at the origin of perceptual switches. Indeed, a cascade of fronto-parietal gamma synchronizations precedes a perceptual switch, showing that several transitions between distinct states of the brain are needed for the occurrence of a perceptual switch (Nakatani & van Leeuwen, 2006). These evidences are in line with recent theories of cognitive control according to which the fronto-parietal network is a flexible hub that alters its functional connectivity with other neural networks based on the specific task (Zanto & Gazzaley, 2013). Thus, stabilizing and reinterpreting the percept are complementary tasks between which the fronto-parietal network would allow alternation.
To examine the joint roles of parietal and prefrontal cortices, we used this novel single-pulse / paired-pulse design and showed that a TMS pulse over the DLPFC could cancel the neurophysiological and behavioral effects of an earlier TMS pulse over the IPS. Due to time limitation, we did not perform other interesting conditions, for example, exploring the effect of a single TMS pulse over the DLPFC, or different timings between the two pulses. Nevertheless, this paired-pulse methodology combined with EEG revealed the interaction between these areas of the attentional network, which is important for preparing and triggering a perceptual switch.
Variability of responses
The size of the behavioral response to SP TMS over the IPS in the expected direction is inversely correlated with latencies of correct responses in neuropsychological tests. In other words, the slower subjects’ reaction times, the more likely they had the expected response to TMS. There are at least two possible, non-mutually exclusive, interpretations for this result. First, the faster participants might have better sustained attention abilities and would respond differently to disturbance of the parietal cortex. Second, the stimulation might have not occurred in the optimal time window to disturb the perceptual stability of the fastest participants. Although beyond the scope of this study, it would have been interesting to test the effect of TMS applied over the IPS, over the DLPFC and over control areas on these types of neuropsychological tasks to strengthen our interpretations and rule out unspecific TMS effects affecting attention and arousal.
Several factors might explain the variability of TMS outcomes, including genetic factors, age, brain connectivity, and recent or ongoing brain activity. These factors might be gathered within a single theoretical framework according to which TMS effects are state-dependent (Silvanto & Pascual-Leone, 2008). For instance, variability in the timing of perceptual perturbation induced by V1 stimulation during binocular rivalry reflects inter-individual differences in alternation frequencies (Pearson, Tadin, & Blake, 2007). Such differences in alternating frequencies might be related to GABAergic concentration within the visual cortex (van Loon et al., 2013). In the present study, although our analysis was mainly explanatory, we showed that one of the potential sources of inter-individual variability could be found using neuropsychological tests. Such analyses suggest that ultimately, the parameters of stimulation (e.g., timing) may need to be individually adjusted to the findings of such neuropsychological testing.
Conclusion
Our study shows that a single TMS pulse over the right IPS modulates evoked potentials and destabilizes the percept during observation of a bistable image. However, a second TMS pulse over the right DLPFC cancels the behavioral and neurophysiological effects of the first pulse. Our interpretation is the following: when the visual world is ambiguous, the anterior part of the right IPS allows an endogenous stabilization of the conscious percept. However, the role of the parietal cortex is not isolated from the prefrontal cortex. In order to enable a switch, the IPS has to decrease its stabilizing role and the DLPFC has to send a triggering signal. Alternatively, the frontal lobe could be involved in top-down control of the activity within the parietal cortex. In conclusion, the stabilization/reinterpretation strategy of our brain for visual conscious perception relies on the activity within a distributed network that is modulated spontaneously, voluntarily, or else by neural stimulation.
Acknowledgments
This project was supported by the Fyssen foundation (France) through a fellowship attributed to MV and internal funds from the Berenson-Allen Center for Noninvasive Brain Stimulation. APL serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neosync and Novavision, and is a listed inventor on issued and pending patents on the real-time integration of transcranial magnetic stimulation (TMS) with electroencephalography (EEG) and magnetic resonance imaging (MRI). AKB was supported by the Young Academics Support and the Stiefel-Zangger Foundation of the University of Zurich, Switzerland, and the Swiss National Foundation (PBZHP1_147196). FF was supported by the Canadian Institute of Health Research (CIHR) fellowship award (201102MFE-246635-181538). We thank Shahid Bashir, Charles J. Beck, Christa Villari and Qinghua (Rocky) Luo for their participation during data acquisition and Andrea Vatulas, Ann Connor and Jennifer Perez for their administrative support.
Footnotes
Conflict of Interest
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amassian VE, Cracco RQ, Maccabee PJ, Cracco JB, Rudell A, Eberle L. Suppression of visual perception by magnetic coil stimulation of human occipital cortex. Electroencephalography and clinical neurophysiology. 1989;74(6):458–462. doi: 10.1016/0168-5597(89)90036-1. [DOI] [PubMed] [Google Scholar]
- Bonneh YS, Pavlovskaya M, Ring H, Soroker N. Abnormal binocular rivalry in unilateral neglect: evidence for a non-spatial mechanism of extinction. Neuroreport. 2004;15(3):473–477. doi: 10.1097/00001756-200403010-00018. [DOI] [PubMed] [Google Scholar]
- Britz J, Landis T, Michel CM. Right parietal brain activity precedes perceptual alternation of bistable stimuli. Cereb Cortex. 2009;19(1):55–65. doi: 10.1093/cercor/bhn056. [DOI] [PubMed] [Google Scholar]
- Brouwer GJ, Tong F, Hagoort P, van Ee R. Perceptual incongruence influences bistability and cortical activation. PLoS One. 2009;4(3):e5056. doi: 10.1371/journal.pone.0005056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel D, Walsh V, Lavie N, Rees G. Right parietal TMS shortens dominance durations in binocular rivalry. Curr Biol. 2010;20(18):R799–800. doi: 10.1016/j.cub.2010.07.036. [DOI] [PubMed] [Google Scholar]
- de Graaf TA, de Jong MC, Goebel R, van Ee R, Sack AT. On the Functional Relevance of Frontal Cortex for Passive and Voluntarily Controlled Bistable Vision. Cereb Cortex. 2011;21(10):2322–2331. doi: 10.1093/cercor/bhr015. [DOI] [PubMed] [Google Scholar]
- de Graaf TA, Hsieh PJ, Sack AT. The 'correlates' in neural correlates of consciousness. Neuroscience and biobehavioral reviews. 2012;36(1):191–197. doi: 10.1016/j.neubiorev.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Deng ZD, Lisanby SH, Peterchev AV. Electric field depth-focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul. 2013;6(1):1–13. doi: 10.1016/j.brs.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Edwards MJ, Chen RS. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex. 2008;18(3):563–570. doi: 10.1093/cercor/bhm087. [DOI] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Virtanen J, Ruohonen J, Karhu J, Aronen HJ, Naatanen R. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport. 1997;8(16):3537–3540. doi: 10.1097/00001756-199711100-00024. [DOI] [PubMed] [Google Scholar]
- Intaite M, Koivisto M, Ruksenas O, Revonsuo A. Reversal negativity and bistable stimuli: Attention, awareness, or something else? Brain and cognition. 2010;74(1):24–34. doi: 10.1016/j.bandc.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Kahkonen S, Komssi S, Wilenius J, Ilmoniemi RJ. Prefrontal TMS produces smaller EEG responses than motor-cortex TMS: implications for rTMS treatment in depression. Psychopharmacology. 2005;181(1):16–20. doi: 10.1007/s00213-005-2197-3. [DOI] [PubMed] [Google Scholar]
- Kanai R, Bahrami B, Rees G. Human parietal cortex structure predicts individual differences in perceptual rivalry. Curr Biol. 2010;20(18):1626–1630. doi: 10.1016/j.cub.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Carmel D, Bahrami B, Rees G. Structural and functional fractionation of right superior parietal cortex in bistable perception. Curr Biol. 2011;21(3):R106–107. doi: 10.1016/j.cub.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt A, Buchel C, Zeki S, Frackowiak RS. Human brain activity during spontaneously reversing perception of ambiguous figures. Proc Biol Sci. 1998;265(1413):2427–2433. doi: 10.1098/rspb.1998.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapen T, Brascamp J, Pearson J, van Ee R, Blake R. The role of frontal and parietal brain areas in bistable perception. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(28):10293–10301. doi: 10.1523/JNEUROSCI.1727-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmeier J, Bach M. The Necker cube--an ambiguous figure disambiguated in early visual processing. Vision Res. 2005;45(8):955–960. doi: 10.1016/j.visres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kornmeier J, Bach M. Ambiguous figures - what happens in the brain when perception changes but not the stimulus. Frontiers in human neuroscience. 2012;6:51. doi: 10.3389/fnhum.2012.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold DA, Wilke M, Maier A, Logothetis NK. Stable perception of visually ambiguous patterns. Nat Neurosci. 2002;5(6):605–609. doi: 10.1038/nn0602-851. [DOI] [PubMed] [Google Scholar]
- Litvak V, Komssi S, Scherg M, Hoechstetter K, Classen J, Zaaroor M. Artifact correction and source analysis of early electroencephalographic responses evoked by transcranial magnetic stimulation over primary motor cortex. Neuroimage. 2007;37(1):56–70. doi: 10.1016/j.neuroimage.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Lumer ED, Friston KJ, Rees G. Neural correlates of perceptual rivalry in the human brain. Science. 1998;280(5371):1930–1934. doi: 10.1126/science.280.5371.1930. [DOI] [PubMed] [Google Scholar]
- Mathes B, Struber D, Stadler MA, Basar-Eroglu C. Voluntary control of Necker cube reversals modulates the EEG delta- and gamma-band response. Neurosci Lett. 2006;402(1–2):145–149. doi: 10.1016/j.neulet.2006.03.063. [DOI] [PubMed] [Google Scholar]
- Meenan JP, Miller LA. Perceptual flexibility after frontal or temporal lobectomy. Neuropsychologia. 1994;32(9):1145–1149. doi: 10.1016/0028-3932(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Miniussi C, Harris JA, Ruzzoli M. Modelling non-invasive brain stimulation in cognitive neuroscience. Neuroscience and biobehavioral reviews. 2013;37(8):1702–1712. doi: 10.1016/j.neubiorev.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Miniussi C, Ruzzoli M, Walsh V. The mechanism of transcranial magnetic stimulation in cognition. Cortex. 2010;46(1):128–130. doi: 10.1016/j.cortex.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Muller TJ, Federspiel A, Horn H, Lovblad K, Lehmann C, Dierks T. The neurophysiological time pattern of illusionary visual perceptual transitions: a simultaneous EEG and fMRI study. Int J Psychophysiol. 2005;55(3):299–312. doi: 10.1016/j.ijpsycho.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Nakatani H, van Leeuwen C. Transient synchrony of distant brain areas and perceptual switching in ambiguous figures. Biol Cybern. 2006;94(6):445–457. doi: 10.1007/s00422-006-0057-9. [DOI] [PubMed] [Google Scholar]
- Pearson J, Tadin D, Blake R. The effects of transcranial magnetic stimulation on visual rivalry. Journal of vision. 2007;7(7):2, 1–11. doi: 10.1167/7.7.2. [DOI] [PubMed] [Google Scholar]
- Perini F, Cattaneo L, Carrasco M, Schwarzbach JV. Occipital transcranial magnetic stimulation has an activity-dependent suppressive effect. J Neurosci. 2012;32(36):12361–12365. doi: 10.1523/JNEUROSCI.5864-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts MA, Gavin WJ, Nerger JL. Early top-down influences on bistable perception revealed by event-related potentials. Brain and cognition. 2008;67(1):11–24. doi: 10.1016/j.bandc.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Preston TJ, Kourtzi Z, Welchman AE. Adaptive estimation of threedimensional structure in the human brain. J Neurosci. 2009;29(6):1688–1698. doi: 10.1523/JNEUROSCI.5021-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A, Lamar M, Buhle JT, Kane MJ, Peterson BS. Selective biasing of a specific bistable-figure percept involves fMRI signal changes in frontostriatal circuits: a step toward unlocking the neural correlates of top-down control and selfregulation. Am J Clin Hypn. 2007;50(2):137–156. doi: 10.1080/00029157.2007.10401611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci C, Blundo C. Perception of ambiguous figures after focal brain lesions. Neuropsychologia. 1990;28(11):1163–1173. doi: 10.1016/0028-3932(90)90052-p. [DOI] [PubMed] [Google Scholar]
- Ruzzoli M, Marzi CA, Miniussi C. The neural mechanisms of the effects of transcranial magnetic stimulation on perception. J Neurophysiol. 2010;103(6):2982–2989. doi: 10.1152/jn.01096.2009. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Pascual-Leone A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 2008;21(1):1–10. doi: 10.1007/s10548-008-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD, Yantis S. Common neural substrates for the control and effects of visual attention and perceptual bistability. Brain Res Cogn Brain Res. 2005;24(1):97–108. doi: 10.1016/j.cogbrainres.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Stewart LM, Walsh V, Rothwell JC. Motor and phosphene thresholds: a transcranial magnetic stimulation correlation study. Neuropsychologia. 2001;39(4):415–419. doi: 10.1016/s0028-3932(00)00130-5. [DOI] [PubMed] [Google Scholar]
- Thut G, Ives JR, Kampmann F, Pastor MA, Pascual-Leone A. A new device and protocol for combining TMS and online recordings of EEG and evoked potentials. J Neurosci Methods. 2005;141(2):207–217. doi: 10.1016/j.jneumeth.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Thut G, Northoff G, Ives JR, Kamitani Y, Pfennig A, Kampmann F. Effects of single-pulse transcranial magnetic stimulation (TMS) on functional brain activity: a combined event-related TMS and evoked potential study. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2003;114(11):2071–2080. doi: 10.1016/s1388-2457(03)00205-0. [DOI] [PubMed] [Google Scholar]
- van Loon AM, Knapen T, Scholte HS, St John-Saaltink E, Donner TH, Lamme VA. GABA shapes the dynamics of bistable perception. Current biology : CB. 2013;23(9):823–827. doi: 10.1016/j.cub.2013.03.067. [DOI] [PubMed] [Google Scholar]
- Veniero D, Bortoletto M, Miniussi C. TMS-EEG co-registration: on TMS-induced artifact. Clin Neurophysiol. 2009;120(7):1392–1399. doi: 10.1016/j.clinph.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Wang M, Arteaga D, He BJ. Brain mechanisms for simple perception and bistable perception. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(35):E3350–3359. doi: 10.1073/pnas.1221945110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilnhammer VA, Ludwig K, Hesselmann G, Sterzer P. Frontoparietal cortex mediates perceptual transitions in bistable perception. J Neurosci. 2013;33(40):16009–16015. doi: 10.1523/JNEUROSCI.1418-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windmann S, Wehrmann M, Calabrese P, Gunturkun O. Role of the prefrontal cortex in attentional control over bistable vision. Journal of cognitive neuroscience. 2006;18(3):456–471. doi: 10.1162/089892906775990570. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Gazzaley A. Fronto-parietal network: flexible hub of cognitive control. Trends Cogn Sci. 2013 doi: 10.1016/j.tics.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretskaya N, Thielscher A, Logothetis NK, Bartels A. Disrupting parietal function prolongs dominance durations in binocular rivalry. Curr Biol. 2010;20(23):2106–2111. doi: 10.1016/j.cub.2010.10.046. [DOI] [PubMed] [Google Scholar]