Abstract
The goal of this study was to evaluate whether using an aseptic free-hand approach for ultrasound-guided injection facilitates injection into the thymic gland in mice. We used this interventional radiology technique in young, aged, and immunodeficient mice and found that the thymus was visible in all cases. The mean injection period was 8 s in young mice and 19 s in aged or immunodeficient mice. Injection accuracy was confirmed by intrathymic location of an injected dye, or by in vivo bioluminescence imaging of injected luciferase-expressing cells. Accurate intrathymic injection was confirmed in 97% of cases. No major complications were observed. We conclude that an aseptic free-hand technique for ultrasound-guided intrathymic injection is safe, accurate, and reduces the time required for intrathymic injections. This method facilitates large-scale experiments, injection of individual thymic lobes, and is clinically relevant.
Keywords: Thymus, Preclinical ultrasound, Interventional radiology, Intrathymic injection
Introduction
As the main site for T cell development, the thymus plays a fundamental role in immunity. Age-related thymic involution, genetic disorders, infections, and cancer treatments may all cause T cell deficiency, leading to significant morbidity and mortality (Haeryfar and Berczi 2001; Gruver and Sempowski 2008; Chinn et al. 2012). Hence immunologists have spent decades studying this organ, primarily in mouse models, to better understand its role in immune function. Mouse models are preferred because of economics and availability. Moreover, mouse thymic anatomy is similar to humans. Intrathymic injection has been central to some studies of thymic biology where it has been used in several genetic mouse models (Vukmanovic et al. 1992; Schwarz and Bhandoola 2004; Adjali et al. 2005a; Adjali et al. 2005b; Marodon et al. 2006; Zlotoff et al. 2010). A substantial barrier to large-scale research, however, has been the difficulty of selectively injecting this often sub-centimeter organ. Intrathymic injections are traditionally performed either by thoracotomy and intrathymic injection under direct visualization (Marodon et al. 2006), or by “blind” injection into the mediastinum (Adjali et al. 2005b). The surgical approach increases the risk of infection and death and introduces a high level of stress that impacts immune studies (Hogan et al. 2011) while “blind” thymic injection is less accurate.
Recently, ultrasound-guided intrathymic injection in mice was introduced as an alternate, minimally invasive and less stressful approach (Blair-Handon et al. 2010). As currently implemented, this method relies on use of a rail system with mounted attachments including an imaging platform, needle guide, and a boom to accommodate the ultrasound probe. Using a series of knobs, the operator controls the movement of each component to achieve an acceptable position for image-guided needle insertion. We hypothesized that a rapid and accurate alternative solution could be achieved by an interventional radiologist using an aseptic free-hand approach. To test this hypothesis, we performed a two-part study with ex vivo and in vivo confirmation of injection accuracy.
Materials and Methods
Mice and cell preparation
Female C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). C57BL/6 mice were either 5-weeks or 10-months old. Male NOD/scid/IL2Rγ(null) (NSG) mice were purchased from The Jackson Laboratory.
Prior to intrathymic injection and while under anesthesia, fur over the ventral thorax of the mice was removed by applying a depilatory, Nair Hair Remover (Church & Dwight Co., Princeton, NJ). After a waiting period of 1-3 minutes, the thoracic region was wiped with water resulting in midline epilation from base of neck to mid-chest.
Luciferase-expressing T cell precursors were generated in vitro by culturing hematopoietic progenitor cells from C57BL/6.Luc+ mice (obtained from Robert S. Negrin, Stanford University) with OP9-DL1 cells, as described previously (Zakrzewski et al. 2006).
Imaging Procedure
Mice were anesthetized using continuous administration of 1–4% isoflurane anesthesia via a nose cone and precision calibrated vaporizer. After induction of anesthesia, mice were positioned supine on the heated (37°C) animal platform of the Vevo Imaging Station (Visualsonics, Toronto, Canada). Mice were secured to the stage by applying transparent hypoallergenic tape (Covidien, Mansfield, MA) to the hind and fore limbs. The previously epilated skin of the upper thorax was sterilely prepared using Chloraprep One-Step (CareFusion, Leawood, KS), a chlorhexidine gluconate wash. For each imaging subject, the transducer was sheathed with a sterile latex cover (Sheathing Technologies, Morgan Hill, CA), and then sterile Aquasonic 100 gel (Parker Laboratories, Fairfield, NJ) was applied to the probe to produce several millimeters of stand-off during imaging.
Preclinical ultrasound imaging was performed using the Vevo 2100 preclinical scanner (Visualsonics, Toronto, Ontario, Canada) that comes equipped with two linear array multifrequency transducers, the M250, which ranges from 16 MHz to 21 MHz, and the MS550, which ranges from 32 MHz to 40 MHz. The M250 transducer produced poor resolution images of the thymus and intrathymic injection image guidance was hindered significantly. Moreover, there was no difference in resolution quality between the minimum and maximum frequency ranges for this probe. Using the MS550S transducer resulted in high-resolution images of the thymus proper, enabling for accurate and fast image guidance. Therefore, the MS550 transducer was utilized for the intrathymic injection procedure. Mice were imaged at 40 MHz frequency with base line 100 percent power and General Imaging or Superficial preset. Imaging depth ranged from 6-8mm, with frame rate of 24, line density set to High, dynamic range of 65dB and display map at G5.
Ultrasound-guided intrathymic injection technique
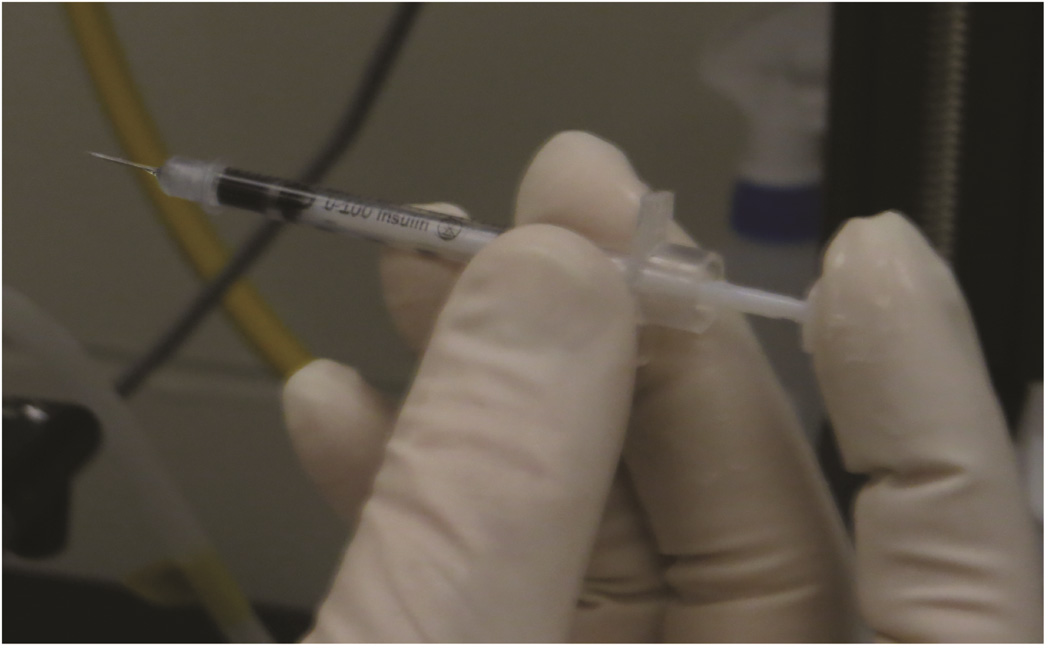
While sterilely gloved, the interventional radiologist (RT) held the ultrasound transducer in the left hand and a 30-gauge × 5/16 inch insulin needle/syringe (BD, Franklin Lakes, NJ) containing 10 μl of injectate in the right hand, as shown in Figure 1. The needle was moved in the stand-off gel under the transducer until adequately visualized at the skin surface, and then inserted into the thymus gland via a percutaneous trajectory away from blood vessels. The needle approach was longitudinal, perpendicular to the long axis of the animal. Once the needle tip was within the thymus, syringe contents were injected during sonographic visualization, with the ultrasound probe in transverse position over the thymus gland. The needle was then removed. The time from the needle insertion into the skin to injection was defined as the injection period.
Figure 1. Free-hand ultrasound guided injection technique.
Free-hand ultrasound guided injection technique. A: Positioning for free-hand injection. The fourth and fifth fingers and palm are applied to the edge of the platform. B: To stabilize the syringe for insertion and injection, the syringe is held between the thumb and third finger, and the index finger controls the syringe plunger
Study design
All procedures were performed in accordance with Institutional Animal Care and Use Committee (IACUC) standards on protocol. In part one of the experiment, ten 5-week-old mice underwent intrathymic injection with 10 μl of Trypan Blue (Fisher Scientific, Waltham, MA); from a left side approach, the right lobe was injected on 5 occasions and the left lobe was injected on 5 occasions. Mice were immediately sacrificed for ex vivo confirmation of intrathymic location of the dye. In part two of the experiment, ten 5-week-old and ten 10-month-old mice underwent intrathymic injection of 10 μl luciferase-expressing cell suspension in phosphate-buffered saline, followed by in vivo bioluminescent imaging. Thymic anterior-posterior diameter perpendicular to the needle trajectory was measured and the injection period was recorded. All injection-related complications were recorded.
In vivo bioluminescent imaging
Bioluminescent signal intensity of injected cells was determined six hours after intrathymic injection. Fifteen minutes after intraperitoneal injection of 3 mg/mouse D-Luciferin (Goldbio, St. Louis, MO) mice were anesthetized and placed into the light tight chamber of an IVIS 200 bioluminescence imaging system (PerkinElmer, Melville, NY). Grayscale photographic images of the mice were acquired first and then a low-level bioluminescent signal was recorded. Pseudo-color images showing the whole body distribution of bioluminescent signal intensity (photons/s) were superimposed on the grayscale photographs.
Results
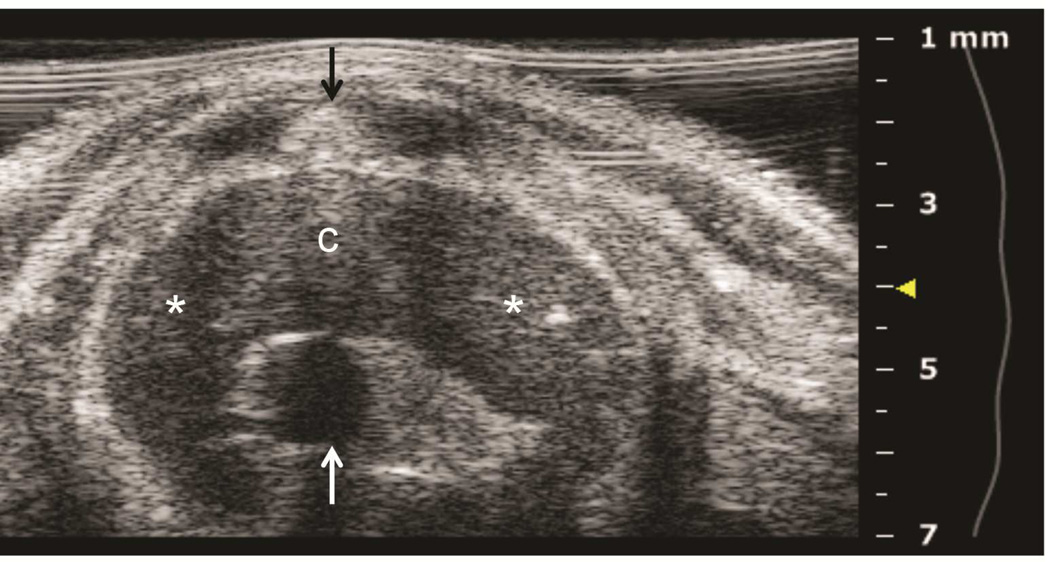
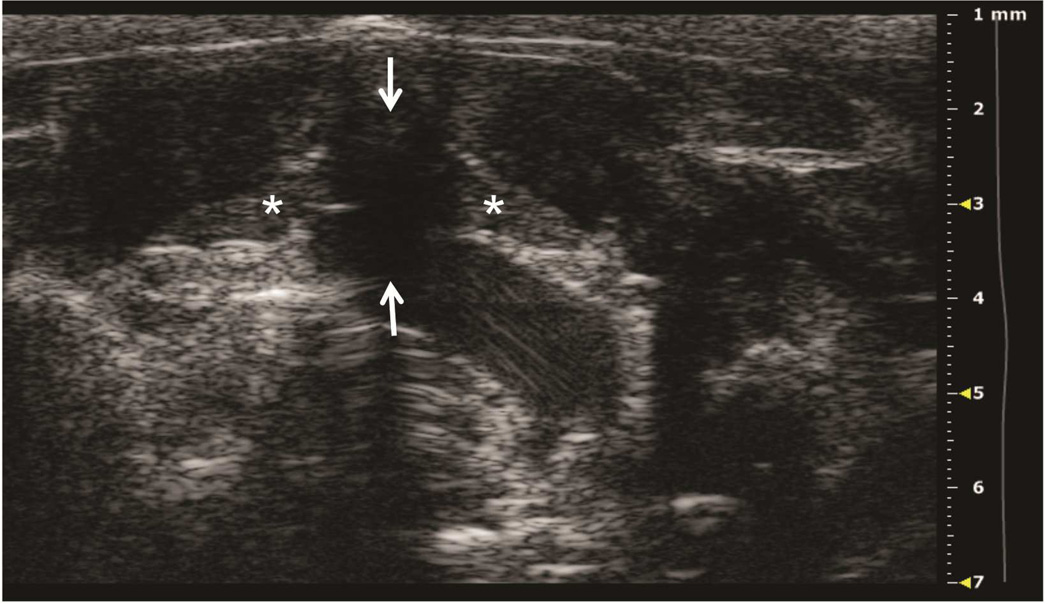
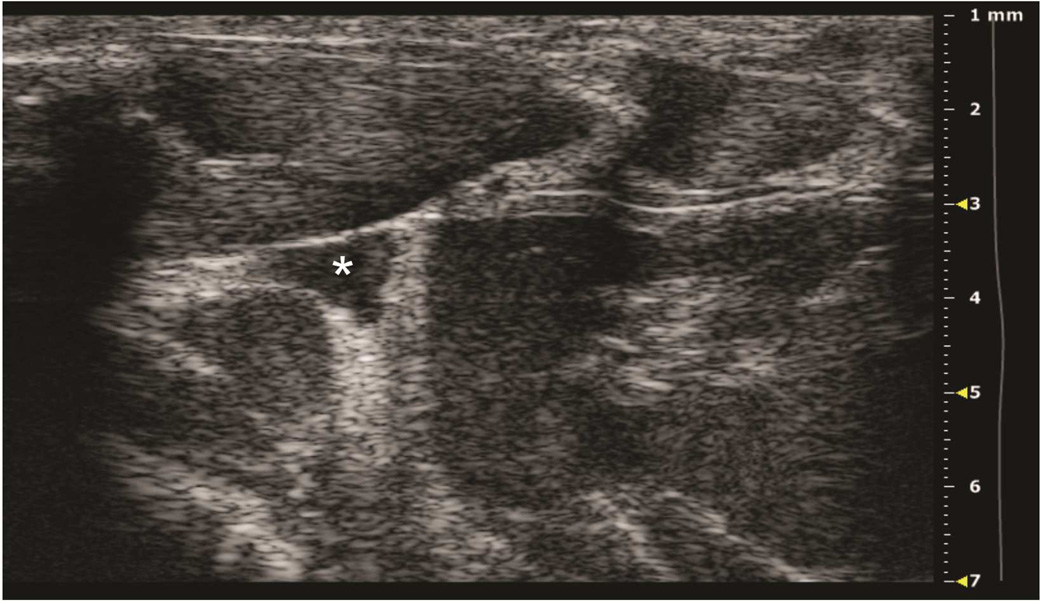
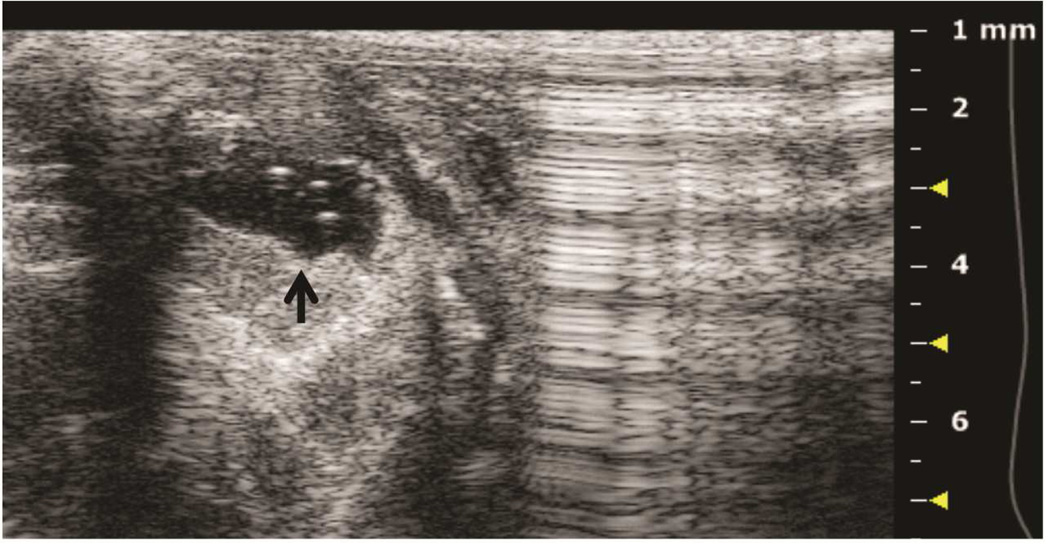
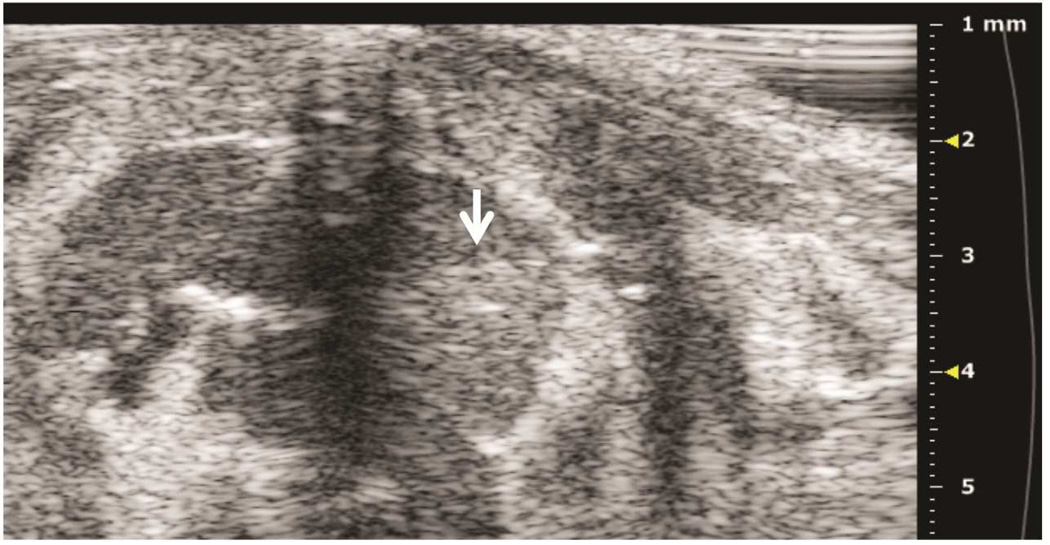
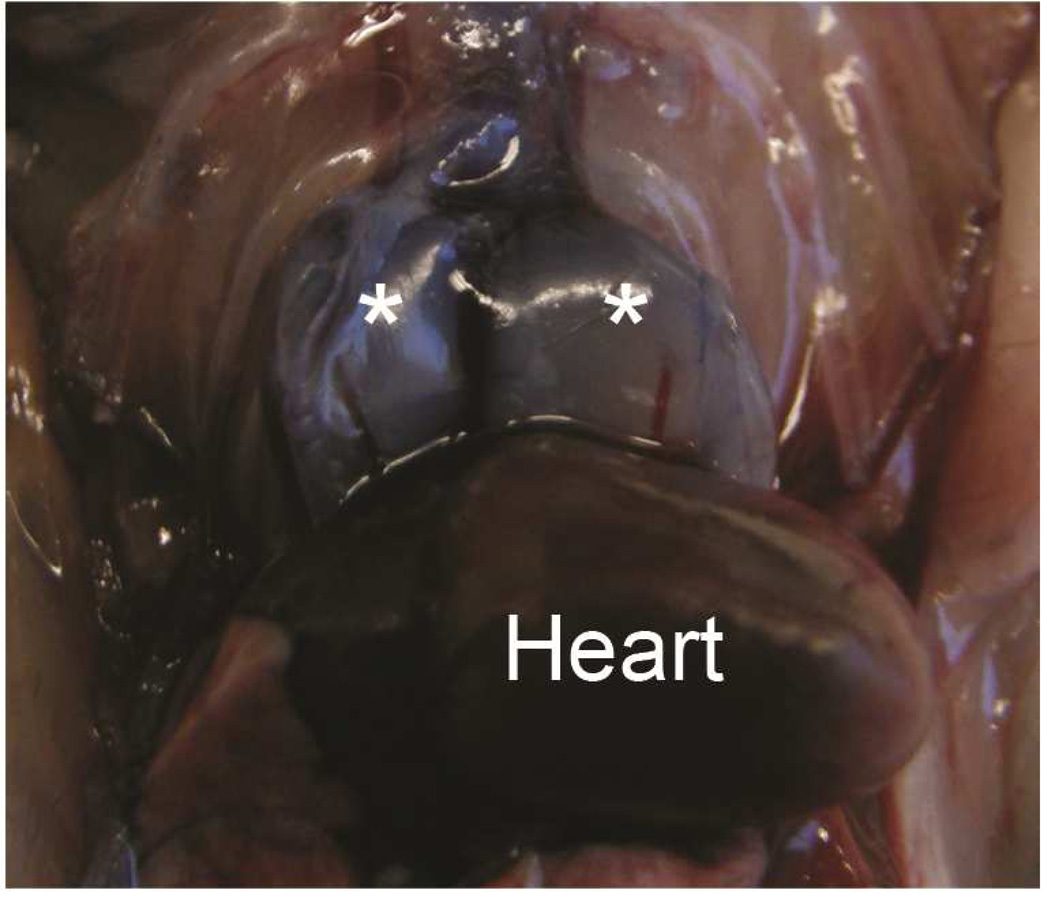
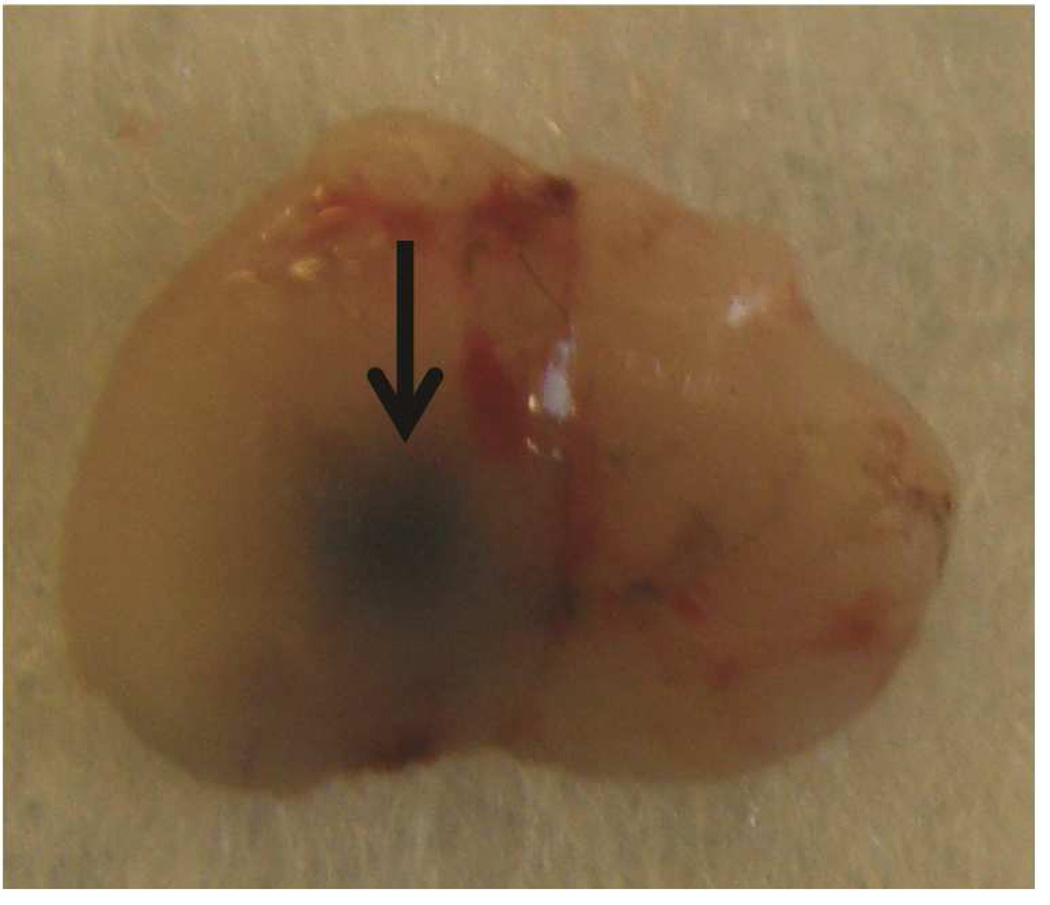
Preclinical ultrasound equipment is required for high-resolution imaging of small animals (Moran et al. 2011). Using the Vevo 2100 preclinical scanner, the thymus of young C57BL/6 healthy mice could easily be visualized by ultrasound in all cases as a bi-lobed structure in the anterior mediastinum interposed between the sternum and heart/great vessels (Figure 2). Even in mice with a much smaller thymus – either due to severe immunodeficiency (NSG mice are animals with a genetic defect equivalent to severe combined immunodeficiency [SCID]) (Figure 3), or due to age-related thymic involution (Figure 4b) – the thymus was clearly sonographically visible. Color Doppler imaging of the thymic region in NSG mice confirmed the presence of large blood vessels but that no blood flow was found in areas that were thought to be thymic lobes (Figure 3c). Utilizing ultrasound guidance to perform free-hand injections of the individual thymic lobes in both young and aged healthy mice, as well as in NSG mice was feasible (Figures 3 and 4). Overall, we have successfully performed intrathymic injections in 400 young mice, 50 aged (9 to 12-month-old) mice (Tuckett et al. 2014) and 20 NSG mice. In 5-week-old young mice, the mean anterior-posterior thymic diameter was 2.0 mm, the mean transverse diameter was 4.7 mm and the mean injection period was 8 s (range 4-25 s). In 10-month-old aged mice, the mean anterior-posterior thymic diameter was 0.6 mm, the mean transverse diameter was 3.0 mm, and the mean injection period was 19 s (range 16-21 s). In NSG mice, the mean anterior-posterior thymic diameter was 1.25 mm, the mean transverse diameter was 3.62 mm, and the mean injection was in the same range as for aged C57BL/6 mice. Two different patterns were observed sonographically following injection into the central portion of a thymic lobe. In some cases, the injectate appeared to pass through the gland and accumulate in the subcapsular space, contained by the thymic capsule (Figure 5a); in the majority of cases, the injectate appeared to be contained within the gland (Figure 5b). However, it is unclear if the different injectate trajectories have any significance since we did not observe any differences in injection outcomes. Confirmation of accurately localized intrathymic injection was confirmed ex vivo in 10 of 10 cases in young mice (Figure 6 and data not shown). Intrathymic bioluminescence of intrathymically injected bioluminescent T cell precursors was confirmed in 19 of 20 cases including both young and aged mice (Figure 7 and data not shown).
Figure 2. Ultrasound imaging of thymic anatomy in a 5-week-old mouse.
A: Axial plane image showing left and right thymic lobes (asterisks), the thymic interlobar connective tissue (c) interposed between the sternum (black arrow) and the ascending aorta (white arrow).
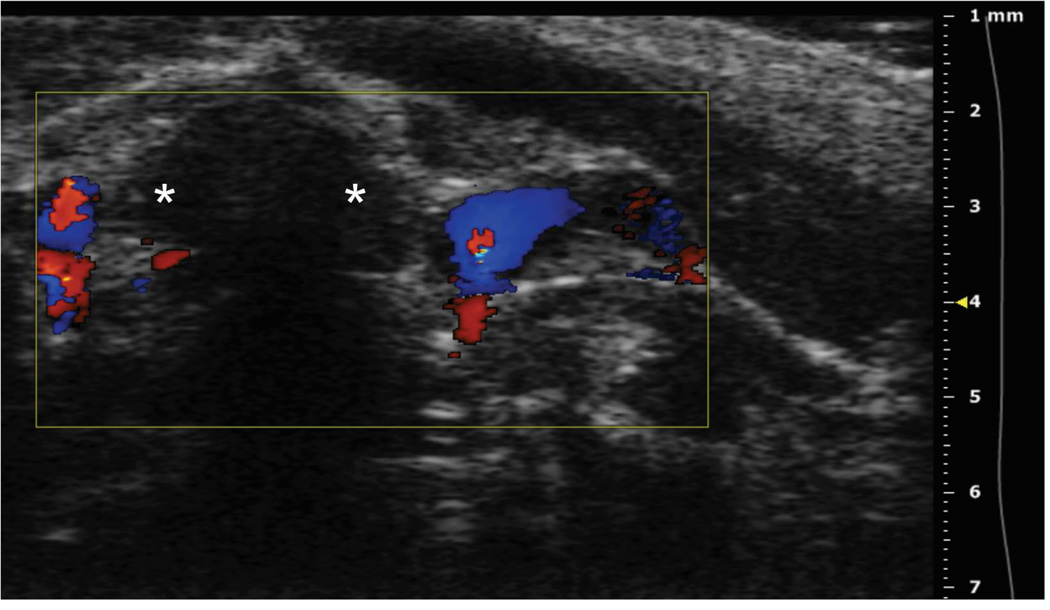
Figure 3. Ultrasound imaging of a thymus in a mouse model of severe combined immunodeficiency.
A: Axial plane image showing left and right thymic lobes (asterisks) between the sternum (upper arrow) and ascending aorta (lower arrow). B: Sagittal plane showing left thymic lobe (asterisk). C: Color Doppler of axial view showing blood flow sparing the left and right thymic lobes (asterisks). D: Axial plane showing the tip of the needle in the right thymic lobe (asterisk).
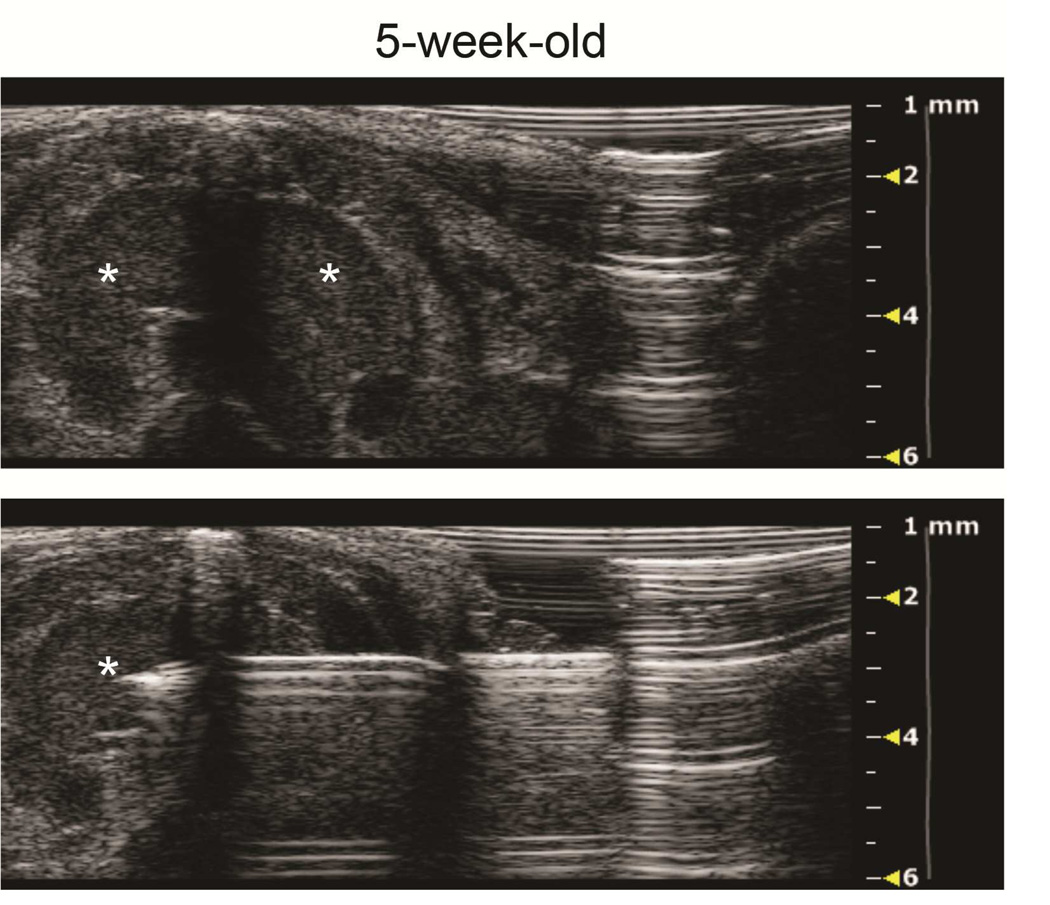
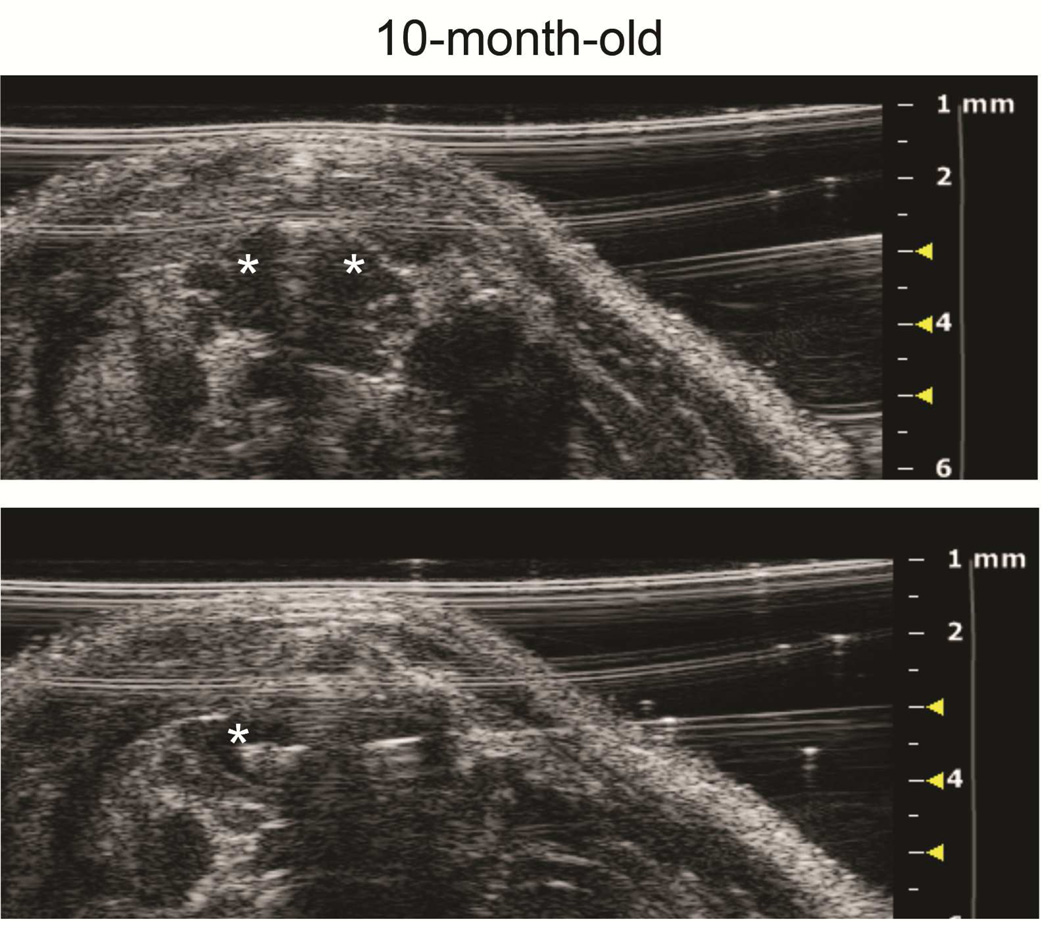
Figure 4. Ultrasound imaging of intrathymic injection in a young and an old mouse.
A: Top panel: axial view of a 5-week-old thymus, left and right lobes (asterisks), pre-injection. Bottom panel: The needle has been advanced from left to right across the midline septum for right thymic lobe injection. B: Top panel: axial view of 10-month-old mouse thymus, left and right lobes (asterisks), pre-injection. Bottom panel: Tip of needle (asterisk) is positioned for right lobe injection.
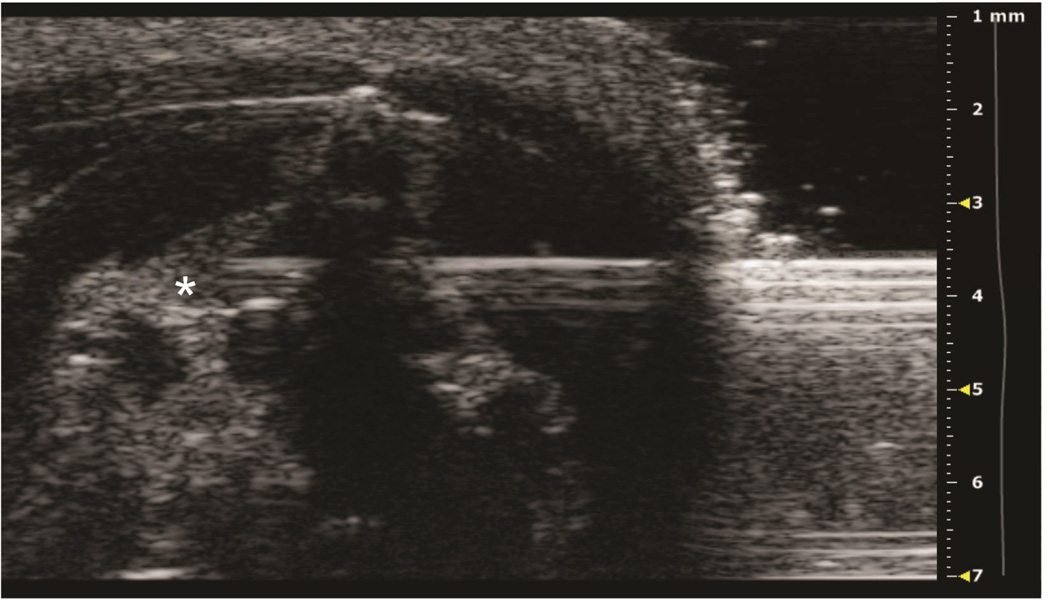
Figure 5. Two distinct patterns of intrathymic injections.
A: Axial view of thymus, post-injection of left thymic lobe. Subcapsular accumulation of injectate above the left lobe (black arrow) is shown. B: Axial view of thymus, post-injection of left thymic lobe. Diffuse intrathymic accumulation of injectate within the left lobe (white arrow) is shown.
Figure 6. Verification of accuracy of intrathymic injection.
The thymus of 5-week-old mice was injected with Trypan Blue and the accuracy of injections was confirmed by necropsy. A: Trypan Blue stained left and right thymic lobes (asterisks), in situ following injection of both lobes. Dorsal surface is shown. B: Trypan Blue stained thymus ex situ after injection of a single (left) lobe. Ventral surface is shown.
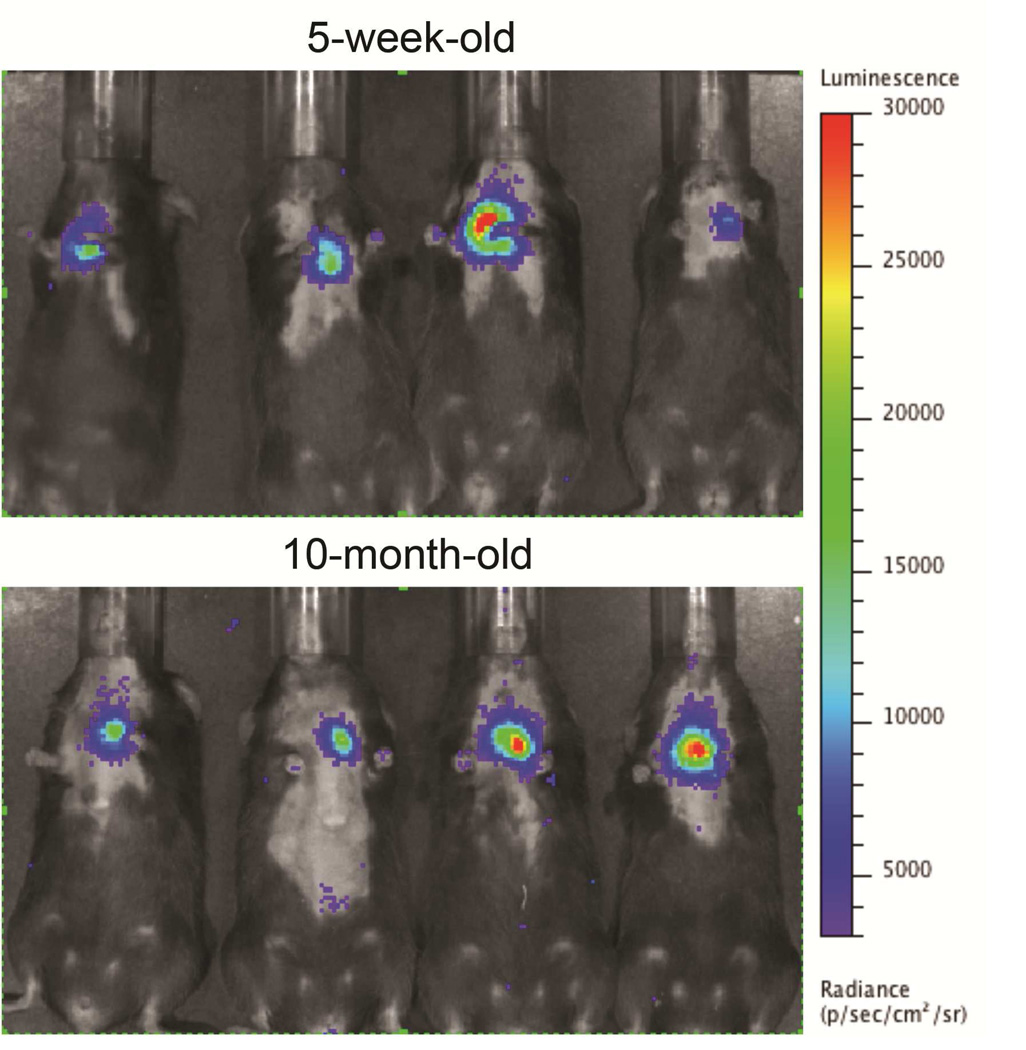
Figure 7. Intravital imaging of intrathymically injected cells.
5-week-old and 10-month-old C57BL/6 mice received an intrathymic injection with 7×105 luciferase-expressing T cell precursors. Luciferin was injected intraperitoneally 6 hours later and the whole-body distribution of bioluminescent cells was monitored using in vivo bioluminescent imaging. Pseudocolor images superimposed on conventional photographs are presented, verifying that the bioluminescent signal is emitted from the thymic region.
Discussion
In comparison to thoracotomy or blind injection, the usual methods for intrathymic injections in mice, free-hand ultrasound-guided injection appears to be a safe, fast and highly accurate alternative. In our past experience, ultrasound guided mouse intrathymic injections using the rail platform required about 10 minutes per injection, whereas the interventional radiologist was able to accomplish the injection in about 10 s for young mice and about 20 s for older or immunodeficient mice. Another advantage of this free-hand approach is that it also avoids the limitations associated with rigidity of the ultrasound imaging station, enabling the radiologist to not only significantly reduce the injection period but also to accurately inject either left or right lobes (or both). Injection of individual lobes has the potential to reduce the number of individual experimental subjects since in each mouse one lobe could be subjected to placebo injection while the other lobe could undergo an experimental injection. Importantly, crosstalk between thymic lobes does not occur (Matsuzaki et al. 1993). To perform injections in both thymic lobes using the imaging station, the needle injection attachment would have to be dismounted, transferred to the opposite side, and remounted, resulting in considerably increased anesthesia time for each injection.
The ability to accurately inject the small, involuted thymus of older mice introduces the potential for a wide range of studies concerning the utility of intrathymic injection to assist immune reconstitution in adult diseases. It should, however, be noted that in humans, in contrast to mice, thymic involution is associated with replacement of thymic tissue by interdigitating adipose tissue (Chinn et al. 2012), making identification of bioactive thymic tissue more challenging.
Our method for intrathymic injection may even have potential for clinical translation; amongst others, progenitor T cell injection may be a promising candidate for intrathymic injection-based therapies. Mouse progenitor T cells leave the bone marrow and home to the thymus where they develop into mature, naïve T cells. These progenitor cells travel to the thymus during a responsive period based upon specific chemokine signaling (Foss et al. 2001; Donskoy et al. 2003). During a refractory period lasting for 3 out of every 4 weeks, progenitor T cells cannot home to the thymus, and thymic T cell production is significantly reduced (Foss et al. 2001). It is hypothesized that direct injection of progenitor T cells into the thymus may facilitate continuous T cell production, even during refractory periods and without reliance on chemokines. Of note, our findings in NSG mice suggest that the thymuses of these mice can be targeted by ultrasound-guided injections, indicating that our technique could considerably facilitate intrathymic injection-based studies in mouse models of SCID. Several previous studies demonstrated that delivery of healthy bone marrow cells to the thymus of mice with defective T cell development can establish long-lasting T cell generation, underscoring the clinical relevance of this approach (Adjali et al. 2005b; Vicente et al. 2010).
Finally, no major complications were observed during this study; there were no procedure-related deaths and only two symptomatic complications in the course of over 400 subsequent thymus injections that were performed for this and other studies (Tuckett et al. 2014) – both were instances of hind-leg paralysis, presumably related to air embolism from the injection procedure. In one other case, a mediastinal hematoma was observed immediately after injection, but there was no clinical correlate to this imaging finding.
In conclusion, our observations provide evidence that using the free-hand technique, an experienced interventional radiologist can inject both or individual lobes of a mouse thymus safely and accurately. This technique will therefore not only be invaluable to researchers targeting the thymus for large-scale preclinical studies, but it may even hold promise for clinical interventions.
Acknowledgements
This research was supported by National Institutes of Health award numbers R01-HL069929 (M.v.d.B.) and 1K08CA160659-01 (J.L.Z.). Technical services provided by the MSKCC Small-Animal Imaging Core Facility, supported in part by NIH Cancer Center Support Grant No 2 P30 CA008748-48, are gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adjali O, Marodon G, Steinberg M, Mongellaz C, Thomas-Vaslin V, Jacquet C, Taylor N, Klatzmann D. In vivo correction of ZAP-70 immunodeficiency by intrathymic gene transfer. J Clin Invest. 2005a;115:2287–2295. doi: 10.1172/JCI23966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjali O, Vicente RR, Ferrand C, Jacquet C, Mongellaz C, Tiberghien P, Chebli K, Zimmermann VS, Taylor N. Intrathymic administration of hematopoietic progenitor cells enhances T cell reconstitution in ZAP-70 severe combined immunodeficiency. Proceedings of the National Academy of Sciences of the United States of America. 2005b;102:13586–13591. doi: 10.1073/pnas.0504268102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair-Handon R, Mueller K, Hoogstraten-Miller S. An alternative method for intrathymic injections in mice. Lab animal. 2010;39:248–252. doi: 10.1038/laban0810-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinn IK, Blackburn CC, Manley NR, Sempowski GD. Changes in primary lymphoid organs with aging. Semin Immunol. 2012;24:309–320. doi: 10.1016/j.smim.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donskoy E, Foss D, Goldschneider I. Gated importation of prothymocytes by adult mouse thymus is coordinated with their periodic mobilization from bone marrow. J Immunol. 2003;171:3568–3575. doi: 10.4049/jimmunol.171.7.3568. [DOI] [PubMed] [Google Scholar]
- Foss DL, Donskoy E, Goldschneider I. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice. J Exp Med. 2001;193:365–374. doi: 10.1084/jem.193.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruver AL, Sempowski GD. Cytokines, leptin, and stress-induced thymic atrophy. Journal of leukocyte biology. 2008;84:915–923. doi: 10.1189/jlb.0108025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeryfar SM, Berczi I. The thymus and the acute phase response. Cellular and molecular biology (Noisy-le-Grand, France) 2001;47:145–156. [PubMed] [Google Scholar]
- Hogan BV, Peter MB, Shenoy HG, Horgan K, Hughes TA. Surgery induced immunosuppression. The surgeon : journal of the Royal Colleges of Surgeons of Edinburgh and Ireland. 2011;9:38–43. doi: 10.1016/j.surge.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Marodon G, Fisson S, Levacher B, Fabre M, Salomon BL, Klatzmann D. Induction of antigen-specific tolerance by intrathymic injection of lentiviral vectors. Blood. 2006;108:2972–2978. doi: 10.1182/blood-2006-03-010900. [DOI] [PubMed] [Google Scholar]
- Matsuzaki Y, Gyotoku J, Ogawa M, Nishikawa S, Katsura Y, Gachelin G, Nakauchi H. Characterization of c-kit positive intrathymic stem cells that are restricted to lymphoid differentiation. Journal of Experimental Medicine. 1993;178:1283–1292. doi: 10.1084/jem.178.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran CM, Pye SD, Ellis W, Janeczko A, Morris KD, McNeilly AS, Fraser HM. A comparison of the imaging performance of high resolution ultrasound scanners for preclinical imaging. Ultrasound in Medicine and Biology. 2011;37:493–501. doi: 10.1016/j.ultrasmedbio.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz BA, Bhandoola A. Circulating hematopoietic progenitors with T lineage potential. Nature immunology. 2004;5:953–960. doi: 10.1038/ni1101. [DOI] [PubMed] [Google Scholar]
- Tuckett AZ, Thornton RH, Shono Y, Smith OM, Levy ER, Kreines FM, van den Brink MR, Zakrzewski JL. Image-guided intrathymic injection of multipotent stem cells supports life-long T cell immunity and facilitates targeted immunotherapy. Blood. 2014 doi: 10.1182/blood-2013-10-535401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente R, Adjali O, Jacquet C, Zimmermann VS, Taylor N. Intrathymic transplantation of bone marrow-derived progenitors provides long-term thymopoiesis. Blood. 2010;115:1913–1920. doi: 10.1182/blood-2009-06-229724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukmanovic S, Grandea AG, 3rd, Faas SJ, Knowles BB, Bevan MJ. Positive selection of T-lymphocytes induced by intrathymic injection of a thymic epithelial cell line. Nature. 1992;359:729–732. doi: 10.1038/359729a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewski JL, Kochman AA, Lu SX, et al. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med. 2006;12:1039–1047. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- Zlotoff DA, Sambandam A, Logan TD, Bell JJ, Schwarz BA, Bhandoola A. CCR7 and CCR9 together recruit hematopoietic progenitors to the adult thymus. Blood. 2010;115:1897–1905. doi: 10.1182/blood-2009-08-237784. [DOI] [PMC free article] [PubMed] [Google Scholar]