Abstract
Background
Observational studies have linked vitamin D status and infectious disease. This association is supported by the presence of the vitamin D receptor and CYP27B1 in immune cells. This review aims to consolidate data from clinical trials that used vitamin D for the treatment or prevention of infectious disease.
Methods
We searched the term “(vitamin D OR ergocalciferol OR cholecalciferol OR vitamin D2 OR vitamin D3 OR calcitriol) AND (infection OR tuberculosis OR sepsis OR pneumonia)” with limits preset to manuscripts published in English and with human subjects. We identified controlled trials that measured infectious outcomes (e.g. incidence and severity of disease, time to disease resolution or recurrence, measures of clinical improvement, mortality). Studies were excluded that: used analog, topical, or micronutrient formulations of vitamin D, assessed only vitamin D status, or lacked a comparison group. The references from eligible manuscripts and from 2 recent reviews were scanned for additional manuscripts.
Results
1284 manuscripts were identified with our search terms, with 60 papers still eligible after review of the title and abstract. Full review of these papers, their references and 2 related reviews yielded 38 manuscripts.
Conclusion
Though some prospective studies show positive results regarding vitamin D on infectious disease, several robust studies are negative. Factors such as high variability between studies, the difference in individual responsiveness to vitamin D, and study designs that do not primarily investigate infectious outcomes may mask the effects of vitamin D on infections.
Keywords: vitamin D, cholecalciferol, infection, tuberculosis, respiratory tract infection
Introduction
Although best studied in bone disease [1, 2], studies suggest that vitamin D plays a role in various infectious processes (e.g. tuberculosis (TB) [3-5], respiratory tract infections (RTI) and influenza [6-11], chronic obstructive pulmonary disease (COPD) exacerbations [12, 13], cystic fibrosis (CF) [14, 15], sepsis [16] and human immunodeficiency virus (HIV) [17]). Furthermore, vitamin D deficiency is associated with an increased risk for all-cause and infection-related mortality in the general population [18, 19]. Although mechanistic evidence linking vitamin D with immunity is strong, randomized controlled trials have yielded inconsistent results.
The active form of vitamin D is 1,25-dihydroxycholecalciferol (1,25(OH)2D3, or calcitriol), which is formed by two sequential hydroxylation reactions. The first reaction occurs in the liver and forms 25-hydroxyvitamin D (25(OH)D), the levels of which indicate vitamin D status [20]. The second reaction forms calcitriol viaCYP27B1, an enzyme located in the kidneys and target organs [21]. Calcitriol acts through the vitamin D receptor (VDR), a polymorphic nuclear receptor that modulates the expression of genes involved in immune function and cytokine production [22-26]. The VDR and CYP27B1are present in immune cells [27, 28] and bronchial and pulmonary epithelial cells [29, 30], among others, and is up-regulated following the ligation of specific toll-like receptors by extracellular pathogens [31], implicating vitamin D in innate immunity [32]. By binding the VDR, calcitriol induces several endogenous antimicrobial peptides (AMP) in human monocytes, neutrophils and epithelial cells (e.g. cathelicidin LL-37 [31, 33-35], α-defensin [35], β defensin [33] and neutrophil gelatinase-associated lipocalin [33]) and up-regulates nitric oxide (NO) synthase [36]. AMPs inhibit infection by bacteria, viruses and fungi [37-39], while NO synthase augments bacterial killing by up-regulating the oxidative burst in activated macrophages [40]. Vitamin D may also induce a T helper 2 (Th2)-based response [41], characterized by high Immunoglobulin IgE and eosinophilia [42, 43], to combat extracellular infections by parasites, protozoa and fungi [44, 45].
In addition to its role in innate immunity, calcitriol suppresses pro-inflammatory cytokines in vitro [46] and in vivo [47, 48], and up-regulates anti-inflammatory cytokines, such as IL-10 [47, 49]. Since the inflammatory response associated with infections such influenza, pneumonia and sepsis increases both clinical severity and mortality [50-53], the ability to reduce inflammation may allow vitamin D to decrease mortality and disease burden in certain infections.
This review focuses on controlled, intervention studies that use vitamin D in the treatment and prevention of infectious disease. We aimed to identify existing trials, assess comparisons between dosing strategies and populations previously tested, and identify sources of variability that may contribute to the inconsistencies between trials.
Materials and Methods
We performed a systematic review of controlled, intervention trials in which vitamin D was used for the treatment or prevention of infectious disease. We evaluated the following outcomes related to infection: incidence and severity of infection, time to infection resolution or recurrence, mortality, and measures of clinical improvement, such as weight gain and improvements on imaging studies. We searched PubMed through the date (5/21/2014) using the search term: “(vitamin D OR ergocalciferol OR cholecalciferol OR vitamin D2 OR vitamin D3 OR calcitriol) AND (infection OR sepsis OR pneumonia OR tuberculosis).” Limits were present to manuscripts published in the English language and involving human subjects. The titles and abstracts of search results were reviewed for: prospective, controlled, intervention trials using vitamin D2, D3 or calcitriol. Exclusion criteria were: 1) review articles, 2) retrospective studies, 3) studies lacking a comparison group, 4) studies that used vitamin D analogs, 5) studies that used vitamin D as part of micronutrient or topical formulas, or 6) studies that evaluated vitamin D status as the only endpoint. Manuscripts not excluded by information in the abstract and title were examined in their entirety and their references reviewed for additional eligible manuscripts. The references of relevant review articles identified in our search were also scanned for eligible manuscripts. Two independent reviewers (J.A., M.K.) identified manuscripts using these criteria and a third reviewer (V.T.) settled disagreements. Data was collected and paper quality assessed in duplicate by 2 independent reviewers (M.K., N.S.). The quality of each paper was scored on 2 rating scales (the 5-point Maryland Scientific Methods Scale [54] and the 17-point Methodological Quality Rating Scale (MQRS)[55]), and a final quality score agreed upon by both reviewers. In both scales, higher numbers represent a more rigorous study design [54, 55].
PUBMED search results
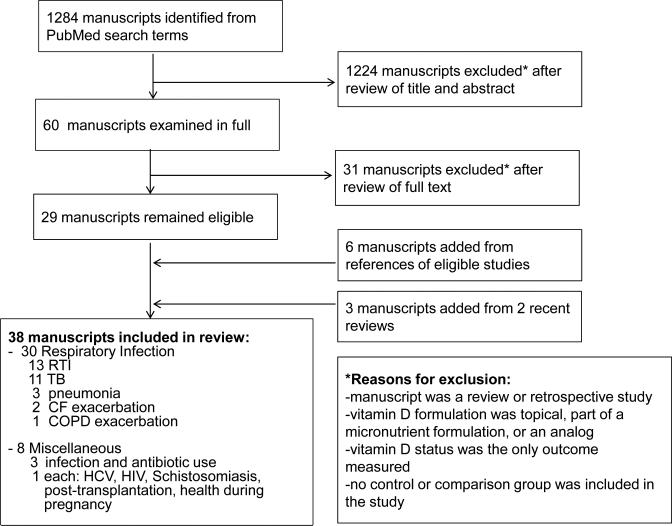
After evaluating the titles and abstracts of 1284 manuscripts identified by our search terms (Figure 1), 60 manuscripts were examined in their entirety and 29 manuscripts remained eligible. Six additional manuscripts were added following review of the references of these papers. The references of 2 recent reviews identified by our search terms [56, 57] were also evaluated, yielding 3 additional manuscripts. A total of 38 papers were included in this review.
Figure 1.
Flow diagram of studies identified for review.
Results
Study design
The 38 prospective, controlled trials included in this paper were published after 1994 and evaluated populations receiving vitamin D for treatment, an adjunct to treatment, or prevention of infectious disease. A majority of these trials studied respiratory disease [58-87], such as: RTI (Table 1), pneumonia and exacerbations linked to RTI (Table 2), and TB (Table 3). Of the remaining trials (Table 4), 3 evaluated infection rates and antibiotic use in adults [88-90] and single trials studied populations with: hepatitis C virus (HCV)[91], heart transplants[92], HIV [93], schistosomiasis [94], and infection in pregnancy [95]. Of these manuscripts, 2 combined multiple randomized, controlled data sets [86, 95] and 8 provided a secondary analysis of previously published data [64, 73, 78, 84, 86, 89, 90, 95]. All studies were double blinded except for 2 that used a single blind model [85, 94] and 6 that were non-blinded [58, 61, 65, 71, 87, 91]. Two trials did not randomize participants [65, 87]. All studies contained placebo groups except for 3 that used an alternative vitamin D dose for comparison [85, 88, 95] and 4 that used no treatment for comparison [61, 75, 87, 91]. Paper quality scores ranged from 5 to 16 on the MQRS scale, with 36% of included studies having ‘excellent methodology’ indicated by a score ≥14 [55].
Table 1.
Vitamin D supplementation in respiratory tract infections
| Author, year, country, latitudea | Study Design | Subjects: number, ageb, baseline VitD sufficiency → VitD status following supplementationc | Intervention: dosed, formulation, intervals of administration, total study length | Total VitD dosed (IU) | Infectious outcome | Study-related AE | Scoree |
|---|---|---|---|---|---|---|---|
|
Positive Studies | |||||||
| Aloia 2007 [84] Long Island, NY, USA 40.8° | placebo, randomized, double blind, single center 2 °analysis [119] | 280 postmenopausal women VitD n=104, 59.9(6.2) yr Placebo n= 104, 61.2(6.3) yr VitD deficient → (NP) |
800 D3 PO daily for 2 yr and 2000 D3 PO for 1 yr, followed to 3 yr | 1,314,000 | ↓ cold and influenza symptoms, ↓ self-reported RTI | none | 5, 14 |
| Laaksi 2010 [79] Pori, Finland 61.5 ° | placebo, randomized, double blind, single center | 164 healthy adults VitD n=80 Placebo n=84 Age 18-28 yr VitD sufficient → sufficient |
400 D3 PO daily for 6 m | 73,200 | ↔ # of days absent from duty due to RTI, ↓ RTI reported over 6 m, ↔ 25D over winter in intervention group, ↑ 25D compared to placebo after winter | nausea (n=1), diarrhea (n=1) | 5, 11 |
| Urashima 2010 [83] Tokyo, Japan 35.7 ° | placebo, randomized, double blind, multicenter | 430 healthy children VitD n=217, 10.0(2.2) yr Placebo n=213, 10.4(2.4) yr VitD sufficiency (NP) |
1,200 D3 PO daily for 3 m | 108,000 | ↓ influenza A incidence, ↓ asthma attacks in children w/asthma | none | 5, 13 |
| Majak 2011 [76] Lodz, Poland 51.8 ° | placebo, randomized, double blind, single center | 48 children w/ new asthma VitD n=24, 10.8(3.2) yr Placebo n=24, 11.1(3.3) yr VitD sufficient →sufficient |
500 D3 PO daily for 6 m | 152,500 | ↓ symptom score, # of asthma exacerbations due to RTI, ↑ lung function, ↔ 25D | none | 5, 14 |
| Bergman 2012 [81] Huddinge, Sweden 59.2 ° | placebo, randomized, double blind, single center | 140 w/ antibody deficiency or >4 RTI/yr VitD n=70, 55.4 yr Placebo n=70, 50.8 yr VitD deficient/ insufficient → sufficient |
4000 D3 PO daily for 12 m | 1,460,000 | ↓ rate of infection, ↓ antibiotic use, ↓ # of days on antibiotics, ↑ 25D | none | 5, 15 |
| Camargo 2012 [78] Ulaanbaatar, Mongolia 48.0 ° | placebo, randomized, double blind, multicenter, 2 ° analysis [120] | 247 children VitD n=144, 10.1(0.9) yr Placebo n=103, 9.8(1) yr VitD deficient → insufficient |
300 D3 PO daily for 3 m | 27,000 | ↓ RTI, ↑ 25D | none | 5, 12 |
| Kalra 2012 [85] Lucknow, India 26.8 ° | comparison, randomized, single blind, single center | 97 pregnant women randomized to Group 1 or 2. 43 controls,
received usual care VitD Group 1 n=48, 26.3(4.2) yr VitD Group 2 n=49, 27.0 (3.8) yr Placebo n=43, 26.2(3.7) yr VitD deficient → insufficient |
Group 1: 60,0000 D3 PO in 2nd trimester, Group 2: 120,000 D3 PO in both 2nd and third 3rd, followed to 9 m in infants | 60,000 or 240,000 | ↑ growth characteristics continuing past 9 m, ↔ RTI, ↑ 25D in Group 2 compared to Group 1 and placebo | none | 4, 11 |
| Negative Studies | |||||||
|---|---|---|---|---|---|---|---|
| Rehman 1994 [87] Kerala, India 8.5° | comparison, non-randomized, single center | 27 children prone to RTI, 20 age/sex-matched
controls VitD n=27 Comparison n=20 Age 3-12 yr VitD sufficiency (NP) |
60,000 PO weekly for 6 wk, followed to 24 wk, formulation (NP) | 360,000 | ↔ observed infections | none | 4, 5 |
| Kriesel 1999 [82] UT, USA 30.5 ° | placebo, randomized, double blind, single center | 175 healthy adults VitD n=87, 32(8) yr Placebo n=88, 32(8) yr VitD sufficiency (NP) |
40 calcitriol IM w/ flu shot, followed to 3 m | 40 | ↔ HAI titers against H1N1, H3N2, influenza B | ↑ pain at injection site | 5, 13 |
| Li-Ng 2009 [80] Long Island, NY, USA 40.8 ° | placebo, randomized, double blind, single center | 148 healthy adults VitD n=84, 59.3(13) yr Placebo n=78, 58.1(13.4) yr VitD insufficient→sufficient |
2000 D3 PO daily for 4 m | 168,000 | ↔ incidence or severity of RTI during winter, ↑ 25D | none | 5, 10 |
| Jorde 2012 [86] 6 centers, varying countries | combined analysis of 10 placebo, Randomized, double blind, multicenter, 2 ° analysis of 10 trials (NP) [121] | 569 healthy adults VitD n=289 Placebo n=280 Age 63 (32–84)g yrs VitD sufficiency (NP) |
1111-6800 PO daily, followed to at least 12 wk between September and April, formulation (NP) | Varied | ↔ length or incidence of influenza-like illness | none | 5, 6 |
| Murdoch 2012 [77] Christchurch, New Zealand 43.0 ° | placebo, randomized, double blind, multicenter | 322 healthy adults VitD n=161, 47(10) yr Placebo n=161, 48(10) yr VitD insufficient → sufficient |
200,000 D3 PO baseline and 1 m both groups, 100,000 D3 PO monthly for 18 m | 2,000,000 or 400,000 | ↔ incidence or severity of RTI, ↑ 25D | none | 5, 16 |
| Goldring 2013 [75] London, England 51.5 ° | comparison, randomized, double blind, single center | 180 pregnant women at 27 wk gestation Daily VitD2 n= 60, 37.1(36.5-38.8)f yr Bolus VitD3 n= 60, 37.9(36.9-39.9)f yr No VitD n=60, 37.4(36.5-39.5)f yr VitD sufficiency (NP) |
Daily 800 D2 PO until birth or 200,000 D3 PO at baseline, followed to 3 years | 200,000 or 800 per day (# of days varied between subjects) | ↔ wheezing, 2 ° OC ↔ RTI, atopy, eczema risk, lung function, exhaled NO during first 3 years | none | 5, 16 |
Latitudes approximated using cities identified as locations of study recruitment
Age listed as mean (SD) unless otherwise noted: fmedian (IQR); gmedian(range)
Vitamin D deficiency is described by 25(OH)D concentration: Sufficient >30 ng/ml, insufficient 20-30 ng/ml, deficient <20 ng/ml.
All doses are listed as international units (IU).
Paper quality measures listed as: Maryland Scientific Methods Scale (scored 1-5) [54], Methodological Quality Rating Scale (scored 1-17) [55]. In both scales, a higher number indicates better quality methods. Scores were determined independently by two reviewers, with differences reconciled to decide on a quality score for each paper.
Abbreviations: 2 ° OC, infectious disease was a secondary outcome in study; 25D, 25-hydroxyvitamin D; AE, study-related adverse events; AMP, antimicrobial peptide; CCl5, chemokine ligand 5; CD4, cluster of differentiation 4; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; d, days; DM, diabetes mellitus; ECP, eosinophil cationic protein; FEV1, forced expiratory volume; HAI, hemagglutination inhibition; HIV, human immunodeficiency virus; hyperCa2+, hypercalcemia (mild hypercalcemia, 10.8-11.6 mg/dl); IFN γ, interferon γ; IL, interleukin; IM, intramuscular; IV, intravenous; LL-37, cathelicidin; m, months; MMP-9, matrix metalloproteinase 9; MTB, mycoplasma tuberculosis; NGAL, neutrophil gelatinase associated lipocalin; NO, nitric oxide; (NP), information not published; (NS), not significant; PB, phenylbutyrate; PO, per os; QOL, quality of life; RTI, respiratory tract infection; TB, tuberculosis; TNF-α, Tumor necrosis factor- α; TST, tuberculin skin test; VDR, vitamin D receptor; VitD, vitamin D; wk, weeks; yr, years;
Table 2.
Vitamin D supplementation in pneumonia and conditions related to respiratory tract infections
| Author, year, country, latitudea | Study Design | Subjects: number, ageb, baseline VitD sufficiency → VitD status following supplementationc | Intervention: dosed, formulation, intervals of administration, total study length | Total VitD dosed (IU) | Infectious outcome | Study-related AE | Scoree |
|---|---|---|---|---|---|---|---|
|
Positive Studies | |||||||
| Cystic Fibrosis | |||||||
| Grossman 2012 [72] Atlanta, GA, USA 33.9° | placebo, randomized, double blind, single center | 30 hospitalized for CF exacerbations VitD n=15, 24.9(16.01) yr Placebo n=15, 28.2(30.89) yr VitD sufficient → sufficient |
250,000 D3 PO baseline, followed to 12 m | 250,000 | ↑ 1-year survival and hospital free days, ↑ (NS) IV antibiotic-free days, ↑ (NS) >95% pre-admission FEV1, ↑ 25D | none | 5, 15 |
| Grossman 2012 [73] Atlanta, GA, USA, 33.9° | 2 °analysis [72] | As in [72] | As in [72] | 250,000 | ↓ TNF-α at 12 weeks, ↓ (NS) IL-6, ↔ IL-1β, IL-8, IL-10, IL-18BP, NGAL | none | 5, 13 |
| COPD exacerbations | |||||||
| Lehouck 2012 [74] Leuven, Belgium 50.9° | placebo, randomized, double blind, single center | 182 severe COPD and history of recent infections VitD n=91, 68(9) yr Placebo n=91, 68(8) yr VitD insufficient → sufficient |
100,000 D3 PO monthly, followed to 12 m | 1,200,000 | ↔ time to first exacerbation, rate of exacerbation, FEV1, QOL, or mortality, ↓ exacerbations in subset w/ 25D <10 ng/mL, ↑ 25D | mild hyperCa2+ (n=4) | 5, 15 |
| Pneumonia Prevention | |||||||
| Manaseki-Holland 2010 [70] Kabul, Afghanistan 33.5° | placebo, randomized, double blind, single center | 453 children w/ severe pneumonia VitD n=224, 13.18(9.1) m Placebo n=229, 13.19(9.2) m VitD sufficiency (NP) |
100,000 IU PO D3 at once, followed to 3 m | 100,000 | ↔ time to recovery of pneumonia, ↓ repeat episode of pneumonia in 90 d after admission | none | 5, 12 |
| Negative studies | |||||||
|---|---|---|---|---|---|---|---|
| Pneumonia Treatment | |||||||
| Choudhary 2011 [69] India 21.0 ° | placebo, randomized, double blind, single center | 200 children w/ severe pneumonia VitD n=100, 14.1(12.2) m Placebo n=100, 13.8(14.1) m VitD sufficiency (NP) |
1,000-2,000 IU PO, given up to 5 days, formulation (NP) | 5,000 to 10,000 | ↔ duration of pneumonia, hospitalization, or symptoms | vomiting (n=1), diarrhea (n=1) | 5, 10 |
| Pneumonia Prevention | |||||||
| Manaseki-Holland 2012 [71] Kabul, Afghanistan 33.5° | placebo, randomized, single center | 3046 children w/ pneumonia VitD n=1524 Placebo n=1522 Age 1-11 m VitD sufficiency (NP) |
100,000 D3 PO every 3 m, followed to 18 m | 600,000 | ↔ incidence or severity of pneumonia | toxic 25D level (150 ng/ml, n=1) | 5, 14 |
Latitudes approximated using cities identified as locations of study recruitment
Age listed as mean (SD) unless otherwise noted: fmedian (IQR); gmedian(range)
Vitamin D deficiency is described by 25(OH)D concentration: Sufficient >30 ng/ml, insufficient 20-30 ng/ml, deficient <20 ng/ml.
All doses are listed as international units (IU).
Paper quality measures listed as: Maryland Scientific Methods Scale (scored 1-5) [54], Methodological Quality Rating Scale (scored 1-17) [55]. In both scales, a higher number indicates better quality methods. Scores were determined independently by two reviewers, with differences reconciled to decide on a quality score for each paper.
Abbreviations: 2 ° OC, infectious disease was a secondary outcome in study; 25D, 25-hydroxyvitamin D; AE, study-related adverse events; AMP, antimicrobial peptide; CCl5, chemokine ligand 5; CD4, cluster of differentiation 4; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; d, days; DM, diabetes mellitus; ECP, eosinophil cationic protein; FEV1, forced expiratory volume; HAI, hemagglutination inhibition; HIV, human immunodeficiency virus; hyperCa2+, hypercalcemia (mild hypercalcemia, 10.8-11.6 mg/dl); IFN γ, interferon γ; IL, interleukin; IM, intramuscular; IV, intravenous; LL-37, cathelicidin; m, months; MMP-9, matrix metalloproteinase 9; MTB, mycoplasma tuberculosis; NGAL, neutrophil gelatinase associated lipocalin; NO, nitric oxide; (NP), information not published; (NS), not significant; PB, phenylbutyrate; PO, per os; QOL, quality of life; RTI, respiratory tract infection; TB, tuberculosis; TNF-α, Tumor necrosis factor- α; TST, tuberculin skin test; VDR, vitamin D receptor; VitD, vitamin D; wk, weeks; yr, years;
Table 3.
Vitamin D supplementation in tuberculosis
| Author, year, country, latitudea | Study Design | Subjects: number, ageb, baseline VitD sufficiency → VitD status following supplementation | Intervention: dosed, formulation, intervals of administration, total study length | Total VitD dosed (IU) | Infectious outcome | Study-related AE | Scoree |
|---|---|---|---|---|---|---|---|
|
Positive Studies | |||||||
| TB treatment | |||||||
| Macros 1998 [58] Cairo, Egypt 30.1 ° | placebo, randomized, single center | 24 children w/ TB VitD n=12 Placebo n=12 Age 1.5-13 yr VitD Insufficient → insufficient |
1,000 PO daily for 8 wk, formulation (NP) | 56,000 | ↑ clinical improvement on sonography, ↔ x-ray, ↑ weight, ↔ 25D | none | 5, 10 |
| Nursyam 2006 [59] Jakarta, Indonesia 6.2 ° | placebo, randomized, double blind, single center | 67 adults w/ TB VitD n=34, 29.85(11.08) yr Placebo n=33, 32.55(11.6) yr VitD sufficiency (NP) |
10,000 PO daily, for 6 wk, followed to 12 wk, formulation (NP) | 420,000 | ↑ sputum smear conversion, ↑ (NS) radiologic improvement | none | 5, 9 |
| Martineau 2011 [62] London, England 51.5 ° | placebo, randomized, double blind, multicenter | 126 adults w/ TB VitD n=62, 30.7(24.5-41.5)f yr Placebo n=64, 30.5(24.8-38.4)f yr VitD insufficient → sufficient |
100,000 D3 PO every 2 wk for 6 wk, followed to 8 wk | 400,000 | ↔ sputum culture conversion overall, ↑ in tt genotype of Taq1 VDR, ↑ 25D | enlarging abscess (n=2), mild hyperCa2+ (n=2) | 5, 14 |
| Coussens 2012 [64] London, England 51.5 ° | 2 °analysis [62] | selected subsets from [62] | as reported in [62] | 400,000 | ↑ sputum smear conversion, ↑ treatment-induced resolution of lymphopenia and monocytosis, ↓ IL-4, CCl5, IFNγ, AMP, MMP-9 | as reported in [62] | 5, 11 |
| Mily 2013 [65] Bangladesh 23.7 ° | placebo, multicenter | 15 healthy adults Vit D + PB n=12 Placebo n=3 Age 18-55 VitD deficient→ deficient |
5000 D3 PO daily for 4d | 20,000 | 500 mg PB w/ VitD ↑ LL-37 peptide and transcript expression, ↑ MTB killing by macrophages, ↔ MTB killing by lymphocytes, ↔ 25D | none | 4, 7 |
| Salahuddin 2013 [63] Nairobi, Kenya 1.3 ° | Placebo, randomized, double blind, multicenter | 259 adults w/ TB VitD n=132, 27.8(13.2) yr Placebo n=127, 28.3(14.1) yr VitD insufficient → (NP) |
600,000 D3 IM baseline and 1 m, followed to 3 m | 1,200,000 | ↑ weight gain, ↓ disease on chest x-ray (decreased cavity size and fewer lobes involved), ↑ IFN-γ secretion in deficient subgroup, ↔ TB score | none | 5, 14 |
| TB Prevention | |||||||
| Martineau 2007 [68] London, England 51.5 ° | placebo, randomized, double blind, multicenter | 192 healthy adults w/ TB contacts VitD n=96, 30.1(25.1-44.1)f Placebo n=96, 37.5(29.8-45.2)f VitD deficient → insufficient |
100,000 PO D2 baseline, followed to 6 wk | 100,000 | ↑ whole blood restriction BCG-lux luminescence in vitro, ↔ IFN-γ response, ↑ 25D | none | 5, 13 |
| Ganmaa 2012 [67] Ulaanbaatar, Mongolia 47.9 ° | placebo, randomized, double blind, single center | 120 healthy children VitD n=61, 13.1(1.5) yr Placebo n=59, 13.0(1.1) yr VitD deficient → insufficient |
800 PO D3 daily for 6 m | 146,400 | ↑ growth, ↓ (59%, NS) reduction in TST conversion rate, ↑ 25D | none | 5, 13 |
| Negative Studies | |||||||
|---|---|---|---|---|---|---|---|
| TB treatment | |||||||
| Wejse 2009 [60] Guinea-Bissau, West Africa 12.0 ° | placebo, randomized, double blind, multicenter | 365 adults w/ TB VitD n=187, 37(13) yr Placebo n=180, 38(14) yr VitD sufficient → sufficient |
100,000 D3 PO at baseline, 5 m, 8 m, followed to 12 m | 300,000 | ↔ TB clinical severity score, sputum smear conversion rate, mortality, weight gain, 25D | none | 5, 15 |
| Kota 2011 [61] Hyderabad, India 17.4 ° | comparison, randomized, controlled, single center | 35 adults w/ type 2 DM and TB, VitD n=15, 38.4(19.6) yr No VitD n=15, 40.2(17.7) yr VitD deficient → insufficient |
60,000 D3 PO weekly for 12 wk | 720,000 | ↔ sputum smear conversion, ↑ 25D | none | 5, 12 |
| Ralph 2013 [66] Timika, Indonesia 4.5 ° | placebo, randomized, double blind, factorial, single center | 200 adults w/ smear positive TB VitD (some w/ arginine) n=101 Placebo n=99 Age 28(15-73)g VitD sufficiency (NP) |
50,000 PO D3 baseline and 4 wk, followed to 24 wk | 100,000 | ↔ sputum culture conversion rate or chest x-ray findings | none | 5, 12 |
Table 4.
Vitamin D supplementation in miscellaneous conditions
| Author, year, country, latitudea | Study Design | Subjects: number, ageb, baseline VitD sufficiency → VitD status following supplementation | Intervention: dosed, formulation, intervals of administration, total study length | Total VitD dosed (IU) | Infectious outcome | Study-related AE | Scoree |
|---|---|---|---|---|---|---|---|
|
Positive Studies | |||||||
| Infection and antibiotic use | |||||||
| Bischoff-Ferrari [88] Zurich, Switzerland 47.4 ° | comparison, randomized, double blind, single center | 173 elderly w/ acute hip fracture 2000 VitD n=86, 84.1(7.0) yr 800 VitD n=87, 84.4(6.8) yr VitD deficient → sufficient in both groups |
800 or 1200 D3 PO D3 daily for 12 m | 292,000 or 730,000 | ↓ hospital readmission in 1200 group, ↓ infection rate, ↑ 25D | mild hyperCa2+ (n=3) at 7-10 d and 6 m |
5, 15 |
| Tran 2014 [90] Australia and Tasmania 23.0-48.4 ° | placebo, randomized, double blind, multicenter, 2 °analysis [121] | 644 elderly adults 60,000 VitD n=205 30,000 VitD n=210 Placebo n=205 Age 60-84 yr VitD deficient → insufficient 30,000 group, sufficient in 60,000 group |
30,000 or 60,000 D3 PO monthly for 12 m | 360,000 or 720,000 | ↓ (28%, NS) antibiotic prescription overall, ↓ antibiotic use in subjects > 70 yr, ↑ 25D | hyperCa2+ (n=1) | 5, 16 |
| Pregnancy Outcomes | |||||||
| Wagner 2013 [95] SC and NC, USA 34.0 °, 35.5 ° | combined analysis of 2 randomized, comparison, double blind, 2 °analysis [123, 124] | 758 pregnant women in 2nd trimester 4000 VitD n= 295, 27(18-41)g yr 2000 VitD n=297, 26(16-41)g yr 400 VitD n=166, 27(18-41)g yr VitD insufficient → sufficient in mothers, insufficient in neonate |
400, 2000 or 4000 D3 PO daily from 2nd trimester to birth, followed to 12 m | varied | ↑ 25D in mothers and cord blood in 2000 and 4000 IU groups. 2 ° OC ↓ odds infection, preterm birth, preeclampsia, and gestational DM combined with every 10 ng/ml ↑ in 25D. | none | 5, 13 |
| Schistosomiasis | |||||||
| Snyman 1997 [94] Eastern S. Africa 25.0 ° | placebo, randomized, single blind, # of centers (NP) | 59 males w/ schistosomiasis VitD n=30 Placebo n=29 Ages 14-18 yr VitD sufficiency (NP) |
40 IU calcitriol daily for 5 d, followed to 3 wk | 200 | ↑ circulating lymphocytes, % eosinophil vacuolization, specific antibody formation, ↓ ECP levels | none | 5, 9 |
| HCV Treatment | |||||||
| Abu-Mouch 2011 [91] Hadera, Israel 32.5 ° | comparison, randomized, multicenter | 72 w/ chronic HCV VitD n=36, 47(11) yr Placebo n=36, 49(7) yr VitD deficient/ insufficient → sufficient |
2000 D3 PO daily for 48 wk | 672,000 | ↑ virologic response at 4, 12 and 24 wk, ↓ non-response rate in VitD group, ↑ 25D | none | 5, 13 |
| Negative studies | |||||||
|---|---|---|---|---|---|---|---|
| Infection and antibiotic use | |||||||
| Avenell 2007 [89] England and Scotland 51.5, 56.0 ° | placebo, randomized, double blind, factorial, multicenter, 2 °analysis [122] | 5292 adults VitD n=2643, 77(6) yr Placebo n=2649, 77(6) yr VitD sufficiency (NP) |
800 D3 PO daily for 24-64 m | 134,400 to 358,400 | ↓ (10-15%, NS) self-reported infections | none | 5, 12 |
| HIV | |||||||
| Arpadi 2009 [93] NY, USA 40.7 ° | placebo, randomized, double blind, multicenter | 56 children w/ HIV VitD n=29, 10.2(2.9) yr Placebo n=27, 10.6(2.4) yr VitD insufficient → sufficient |
100,000 D3 PO every 2 m for 12 m | 600,000 | ↑ 25D, 2 ° OC: ↔ CD4 count, CD4%, viral load at 12 m | none | 5, 15 |
| Post-Transplantation | |||||||
| Briffa 2013 [92] Sydney, Australia 33.9 ° | placebo, randomized, double blind, single center, some control from other cohort study | 99 heart transplant recipients VitD n=52 Placebo n=29 Control (cohort) n=18 Age (NP) VitD sufficiency (NP) |
20 daily for 6 m, 20-40 daily for 12 m, or 20-40 daily for 24 m | 3,660 to 29,280 | ↓ oral cyclosporine requirement 2 ° OC : ↔ in infection, rejection, or mortality rates | none | 5, 10 |
Respiratory Tract Infections
Thirteen papers assessed vitamin D in the prevention of RTI [75-87, 90], while others studied conditions associated with RTI, including exacerbations of COPD [74] and CF [72, 73]. RTI prevention with vitamin D yielded mixed results in healthy adults. Two positive studies found fewer RTI [79] and decreased cold and influenza incidences [84] with daily vitamin D3 doses between 400 IU and 2000 IU for 6 months and 36 months, respectively. However, the remaining trials found no change in the incidence or severity of RTI or influenza symptoms following doses of vitamin D3 given daily (2,000 IU doses for 4 months [80] or 1,111-6,800 IU doses for 3 months during influenza season [86]) or monthly (100,000 IU doses for 18 months)[77].
Positive studies in healthy, school-aged children found daily doses between 300 and 1,200 IU of vitamin D3 given over periods between 3 and 6 months to significantly decrease the risk of RTI [78], influenza A [83], and asthma exacerbations [76, 83]. However, no change in wheezing, RTI, or other atopic illness was seen in the offspring of pregnant women who received either 800 IU of vitamin D2 daily until delivery or 200,000 IU of vitamin D3 given once [75]. There also was no change in RTI incidence in the offspring of women who received between 60,000 IU and 240,000 IU of vitamin D3 during the second and third trimesters of pregnancy [85].
Several papers evaluated RTI-prone populations. In children with a high incidence of RTI [87], 60,000 IU of vitamin D3 given weekly for 6 weeks showed no significant change in observed RTI. However, 4,000 IU of vitamin D3 given daily for a year in RTI-prone adults reduced overall rates of infections and the number of days on antibiotics by 50% [81]. Similarly, a population of CF patients who received a single, 250,000 IU dose of vitamin D3 while hospitalized experienced a significant increase in hospital-free days during the year following the dose [72] and 50.4% decrease in TNF-α concentrations up to 12 weeks following the dose [73]. In this population, however, there were no differences between the vitamin D and placebo groups in the number of antibiotic free days, recovery of function [72], or change in IL-6 [73]. In patients with moderate to very severe COPD, 100,000 IU of vitamin D3 at monthly intervals resulted in improvement in lung function, rates of exacerbation, and morbidity and mortality [74], although positive results were limited to participants who were vitamin D deficient at baseline.
In summary, only a few studies suggest that vitamin D may have some benefit in reducing the risk of RTI in young healthy adults and adolescents whereas the efficacy of vitamin D in adults with chronic lung disease is less clear. Vitamin D intervention during pregnancy did not appear to reduce the risk of URTI or asthmatic symptoms in the offspring.
Pneumonia
Three studies evaluated the impact of vitamin D on pneumonia [69-71], yielding largely negative results. Hospitalized children received no benefit on the duration of illness, hospitalization or symptoms with 1,000 - 2,000 IU doses of vitamin D3 given for up to 5 days [69]. Two studies by Manaseki-Holland et al [70, 71] found 100,000 IU doses of vitamin D3 given once [70] and at 3 month intervals for 18 months [71] to show no change in the incidence, severity or time to recovery from pneumonia. Although the initial trial showed a 13% decreased risk of repeat pneumonia compared to placebo in the 90 days following the dose [70], this result was not reproduced in the second trial, which had a large sample size [71].
Tuberculosis
Vitamin D has been extensively studied in the prevention [67, 68] and treatment [58-66] of TB. Studies that assessed TB prevention showed a single dose of 100,000 IU of vitamin D2 to significantly increase whole blood restriction of BCG-lux luminescence (an in vitro indicator of TB growth restriction) in adults [68], and for daily doses of 800 IU of vitamin D3 to cause a significant increase in anthropometric measurements and a 59% (non-significant) reduction in tuberculin skin test (TST) conversion rates when given to children for 6 weeks [67].
The remaining studies assessed vitamin D both as an adjunct to antibiotic treatment for TB [58-64, 66] and in its ability to kill mycobacterium tuberculosis (MTB) [65]. Vitamin D supplementation led to clinical improvements in four studies, including: weight gains (in adults receiving two 600,000 IU doses of vitamin D3[63] and children receiving 1000 IU doses daily for 8 weeks [58]),less tissue involvement on sonography (after 1,000 IU daily for 2 months [58]) and chest x-ray (after 5,000 IU of vitamin D3 daily for 3 months [63]). Conversely, no improvement on x-ray was seen in children receiving daily doses of vitamin D (1,000 IU over 2 months [58]) or adults receiving daily (10,000 IU for 6 weeks [59]) or monthly (50,000 IU given twice [66]) doses of vitamin D3. In Wejse et al [60], 100,000 IU doses of vitamin D3 given to adults with TB at baseline, 5, and 8 months also did not impact clinical severity scores or weight gain, among other outcomes.
Conversion of sputum smear or sputum culture was used to measure response to treatment in several studies, though only sputum culture conversion is independently linked to long-term risk of treatment failure and relapse [96]. Nurasyam et al [59] found 10,000 IU of vitamin D3 given daily for 6 weeks to significantly increase sputum smear conversion (100% in the treatment group vs. 76.7% in the placebo group, p=0.002). However, 2 studies showed no acceleration of sputum smear conversion with: 3 doses of 100,000 IU of vitamin D3 given over 8 months [60] or 6 weekly doses of 60,000 IU of vitamin D3[61]. Two studies [62, 66] also showed negative results when testing the time to sputum culture conversion using vitamin D3 given as two 50,000 IU doses 1 month apart [66] and four 100,000 IU doses of at 2 week intervals [62]. In spite of negative results in the study overall, Martineau et al [62], however, did find in subgroup analysis based on the genotype of the Taq1 vitamin D receptor polymorphism that those with a tt genotype experienced a significantly accelerated conversion compared to those with the Tt or TT genotype.
Several studies tracked the impact of vitamin D on cytokines that promote anti-MTB activity and the resolution of infection. Suppression of antigen-stimulated pro-inflammatory cytokines, attenuation of anti-inflammatory cytokines, and a more rapid treatment-induced resolution of lymphopenia and monocytosis associated with TB infection occurred following 100,000 IU doses of vitamin D3 given monthly for 4 months [64]. IFN-γ levels were impacted variably; 2 doses of 600,000 IU of vitamin D3 increased IFN-γ expression [63], while a single 100,000 IU dose of vitamin D2 showed no change [68]. Two studies evaluated vitamin D in combination with another chemical thought to modulate activity against MTB; 5,000 IU of vitamin D3 given for 4 days alone or in combination with phenylbutyrate (PB) induced both circulating levels and transcript expression of the anti-microbial peptide LL-37 [65], while 50,000 IU doses with or without l-arginine showed no significant change in sputum culture conversion rate or x-ray involvement [66].
In summary, vitamin D given largely as an adjunctive therapy with traditional anti-TB regimens has little impact on clearance of MTB from sputum in larger randomized controlled trials of patients with active TB infection. However, certain sub-populations may demonstrate benefit, such as patients with: different polymorphisms of the vitamin D receptor, severely low vitamin D status, or infection with different strains of TB. There may be other benefits of vitamin D besides on the clearance of MTB from sputum, such as dampening the inflammatory response or anthropometric changes that may help TB patients recover in the long term.
Miscellaneous
Eight papers evaluated non-respiratory illness, with variable results. Negative findings were present for HIV patients, who experienced no improvements in viral load and CD4 count [93], heart transplant recipients, who experienced no change in infection rates [92], and for a population of elderly adults who experienced no decrease in self-reported infections [89]. However, several studies yielded positive results. In elderly adults,1200 IU of vitamin D3 given daily reduced infection rates [88] while monthly, 60,000 IU doses of vitamin D3 caused a significant reduction in the amount of antibiotics used [90]. A population of pregnant women experienced a significant decrease in the combined odds of infection, preterm birth, preeclampsia, and gestational diabetes associated with each 10 ng/mL increase in 25(OH)D concentration following supplementation, though the impact of vitamin D on rates of infection alone was not significant [95]. A 2000 IU daily dose of vitamin D3 given in addition to standard HCV treatment increased the response to treatment at 4, 12 and 24 weeks (p<0.01 at all points)[91], and calcitriol augmented the Th2 response necessary for eradication of protozoan infections in patients with schistosomiasis [94].
Adverse effects
A majority of studies reported no difference in adverse events between intervention and control groups, nor observed events deemed related to vitamin D [58-61, 63, 65-68, 70, 72, 75-78, 80, 81, 83-87, 89, 91-95]. Mild hypercalcemia in the intervention group was reported in 4 studies [62, 74, 88, 90] and a toxic level of 25(OH)D [71] was reported in 1 study, though no subsequent complications were reported. Two participants developed enlarging abscesses thought to be potentially due to the intervention in 1 study [62], and symptoms such as vomiting and diarrhea [69, 79] were identified in a small number of participants (n=4). Calcitriol administered with the influenza vaccination caused increased pain at the injection site [82].
Discussion
Although studies have consistently demonstrated an epidemiologic association between vitamin D deficiency and infectious processes [3-17], the evidence from controlled, interventional trials has been inconsistent. Vitamin D administration yielded at least 1 positive endpoint in: 10 of 16 trials evaluating RTI and RTI-associated clinical conditions, 1 of 3 trials evaluating pneumonia, 9 of 11 trials measuring TB treatment and prevention, and 1 of 3 trials evaluating infections and antibiotic use. However, these positive endpoints were not necessarily the primary endpoints of their respective trials and were, at times, restricted to particular subgroups within the cohort. Ultimately, there was a high degree of variability between the results of the studies, making it difficult to draw a unifying conclusion on the impact of vitamin D on infectious outcomes. While the association between vitamin D deficiency and infection does not necessarily imply causation, it is possible that a causal relationship may be masked by several variables inherent to the current trials that contribute to the inconsistencies of results between studies.
Unlike vitamin D as treatment for bone health, the dose and duration of treatment necessary to optimize infectious outcomes is currently unknown. This lack of standardization has led to a variety of dosing strategies in the studies included in this review. For one, vitamin D was administered at intervals ranging from days to months. Although vitamin D administered daily, weekly, or monthly can sustain the same circulating concentrations of 25(OH)D over an equivalent period of time [97], the high number of trials with daily dosing (more than 50% of studies)[58, 59, 65, 67, 69, 75, 76, 78-81, 83, 84, 86, 88, 89, 91, 92, 94, 95] raises concerns over compliance; several large clinical trials have reported low adherence to daily doses of vitamin D [98, 99]. The doses of vitamin D3 also varied (300 [78] to 10,000 IU [59] for daily doses, 1,400 [87] to 60,000 IU [61, 87] for weekly doses and 30,000 [90] to 600,000 IU [63] for single or monthly doses), as did the formulation. Although most trials used oral vitamin D3, 2 trials used vitamin D2[68, 75], which is less effective than vitamin D3 at increasing 25(OH)D concentration when used at similar doses[100, 101]. Ultimately, some dosing strategies may have been inadequate.
The wide variation in individual response to vitamin D supplementation [102, 103] may have also led to some of the inconsistencies observed. While many physiologic characteristics impact response to vitamin D, including body composition [104] and genetic variations of the vitamin D binding protein [105], the factors most relevant to these studies are: baseline 25(OH)D concentration [106] and VDR polymorphisms [107]. Though many studies did not measure baseline 25(OH)D [59, 66, 69-71, 75, 82, 83, 86, 87, 89, 92, 94], multiple studies observed results dependent on the baseline 25(OH)D concentration of participants. Improvements in severe COPD [74] and increases in IFN- γ production in TB patients [63] were only observed in subsets that were vitamin D deficient at baseline, and the greatest reduction in influenza A incidence was observed in children naïve to vitamin D supplements [83]. Conversely, studies in several vitamin D sufficient [60] and nearly sufficient [77, 93] populations had negative results. The effectiveness of vitamin D in deficient individuals may be, in part, related to the inverse relationship between baseline 25(OH)D concentration and response to vitamin D administration [101, 108]. Individuals who are vitamin D sufficient at baseline achieve a lesser increase in 25(OH)D concentration compared to deficient individuals receiving supplementation[106], likely because larger vitamin D doses are required to raise 25(OH)D concentrations even higher above the range 25(OH)D range considered already sufficient (>30 ng/mL) [109]. Another potential reason for negative results in more sufficient populations is suggested by Heaney [110], who found that in order to assess the physiologic response to supplementation of a nutrient, a study must measure an increase in the concentration of that nutrient through the range at which the desired physiologic effects would occur. Thus, studies with subjects whose vitamin D status falls outside the range where the effects on infectious outcomes are seen may not demonstrate an improvement following supplementation. Sufficient populations that had improvements in infectious outcomes included CF patients [72] and children with previously untreated asthma [76]. These populations, who are at high risk for infection and inflammation at baseline, may benefit from smaller increases in 25(OH)D concentration than other populations. Positive results in similarly susceptible populations, including adults with a high-prevalence of Ig deficiencies [81] and an elderly subset of a trial performed on the general population [90], support this observation.
However, negative results were also seen in several studies performed in vitamin D deficient and insufficient individuals [61, 62, 80], indicating that factors besides baseline vitamin D status likely contribute to the inconsistencies between study results. Large, genome-wide association studies have identified variants of the VDR, in addition to other enzymes and genetic determinants that impact response to vitamin D supplementation [111, 112]. While evaluating genetic factors is not necessarily feasible at a population level, Martineau et al [62] demonstrated that a particular subgroup analyzed based on genotype (TB patients with the tt genotype of the TaqI vitamin D receptor) had a significant acceleration in sputum culture conversion not seen in the population as a whole. Furthermore, a recent study has suggested that polymorphisms of the vitamin D binding protein, the major carrier protein for 25(OH)D in circulation, may influence the amount of bioavailable serum 25(OH)D and thus be more reflective of true vitamin D status than total serum 25(OH)D [113]. It is possible that these and other genetic variations may also hide the effects of vitamin D in certain populations.
In spite of inconsistencies between study results, vitamin D consistently improved anthropometric measures. Improvements in growth and weight gain were seen in children and adults with, and at risk for, TB [58, 63, 67] and children whose mothers received high-dose supplementation [85]. Similar improvements were seen in another trial in which low-birth weight infants received vitamin D supplementation [114]. Only Wejse et al [60], which measured weight gain in vitamin D sufficient children with TB, showed no improvement. Weight gain is a marker of clinical improvement in CF [115, 116], TB [117, 118] and HIV [119], among other infectious processes, and may be a valuable outcome measure in future studies.
Strengths of this review include its broad scope and inclusivity; prior reviews on vitamin D in infectious disease use more restrictions for paper selection or are more limited in their scope. This paper aimed to consolidate the varied information across numerous sources. In addition to limitations previously described regarding the high variability between included studies, the quality of some studies may be limiting. Although 36% of papers had an ‘excellent methodology’ according to the scoring system we applied, many trials in this review measured infectious disease as secondary outcomes [75, 88, 92, 93, 95] or in a post-hoc analysis [64, 73, 78, 84, 86, 89, 90, 95]. These studies may not have been optimal for measurement of infectious outcomes. Furthermore, most trials are limited to only evaluating vitamin D as an adjunctive therapy to standard antibiotic treatment, as it would be unethical to withhold established antibiotic therapy in patients with infection in any randomized controlled trial.
Conclusion
Though vitamin D supplementation has promising effects on several infectious outcomes, inconsistencies between study results make it difficult to draw definitive, unifying conclusions. Positive results seem to occur more frequently in vitamin D deficient populations, in addition to those at high risk for infection and inflammation at baseline. This observation suggests that the effect of vitamin D may be masked in trials in the general population, where the variability in response to vitamin D supplementation between individuals may make it difficult to detect less robust improvements. Furthermore, the lack of an established dosing strategy for infectious outcomes leads to the use of many different doses and dosing intervals, which may have been inadequate in some studies. Overall, future studies must take note of previously effective dosing strategies and account for factors inherent in an individual, such as vitamin D status.
Acknowledgments
Source of Funding: This work was supported in part by National Institutes of Health grants K01 DK102851 (JAA), and UL1 TR000454 (VT).
Abbreviations
- 2 ° OC
infectious disease was a secondary outcome in study
- 25D
25-hydroxyvitamin D
- AE
study-related adverse events
- AMP
antimicrobial peptide
- CCl5
chemokine ligand 5
- CD4
cluster of differentiation 4
- CF
cystic fibrosis
- COPD
chronic obstructive pulmonary disease
- d
days
- DM
diabetes mellitus
- ECP
eosinophil cationic protein
- FEV1
forced expiratory volume
- HAI
hemagglutination inhibition
- HIV
human immunodeficiency virus
- hyperCa2+
hypercalcemia (mild hypercalcemia, 10.8-11.6 mg/dl)
- IFN γ
interferon γ
- IL
interleukin
- IM
intramuscular
- IV
intravenous
- LL-37
cathelicidin
- m
months
- MMP-9
matrix metalloproteinase 9
- MTB
mycoplasma tuberculosis
- NGAL
neutrophil gelatinase associated lipocalin
- NO
nitric oxide
- (NP)
information not published
- (NS)
not significant
- PB
phenylbutyrate
- PO
per os
- QOL
quality of life
- RTI
respiratory tract infection
- TB
tuberculosis
- TNF-α
Tumor necrosis factor- α
- TST
tuberculin skin test
- VDR
vitamin D receptor
- VitD
vitamin D
- wk
weeks
- yr
years
Footnotes
Conflicts of Interests The authors have no other financial conflicts of interest to report.
References
- 1.Boonen S, Vanderschueren D, Haentjens P, et al. Calcium and vitamin D in the prevention and treatment of osteoporosis - a clinical update. J Intern Med. 2006;259(6):539–52. doi: 10.1111/j.1365-2796.2006.01655.x. [DOI] [PubMed] [Google Scholar]
- 2.Fraser DR. Vitamin D. Lancet. 1995;345(8942):104–7. doi: 10.1016/s0140-6736(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353–73. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari M, Schenk K, Papadopoulou C, et al. Serum 25-hydroxy vitamin D and exercise capacity in COPD. Thorax. 2011;66(6):544–5. doi: 10.1136/thx.2010.152785. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson RJ, Llewelyn M, Toossi Z, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000;355(9204):618–21. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 6.Laaksi I, Ruohola JP, Tuohimaa P, et al. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86(3):714–7. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
- 7.Roth DE, Shah R, Black RE, et al. Vitamin D status and acute lower respiratory infection in early childhood in Sylhet, Bangladesh. Acta Paediatr. 2010;99(3):389–93. doi: 10.1111/j.1651-2227.2009.01594.x. [DOI] [PubMed] [Google Scholar]
- 8.Karatekin G, Kaya A, Salihoglu O, et al. Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. Eur J Clin Nutr. 2009;63(4):473–7. doi: 10.1038/sj.ejcn.1602960. [DOI] [PubMed] [Google Scholar]
- 9.Ginde AA, Mansbach JM, Camargo CA., Jr. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169(4):384–90. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant WB. Variations in vitamin D production could possibly explain the seasonality of childhood respiratory infections in Hawaii. Pediatr Infect Dis J. 2008;27(9):853. doi: 10.1097/INF.0b013e3181817bc1. [DOI] [PubMed] [Google Scholar]
- 11.Cannell JJ, Vieth R, Umhau JC, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134(6):1129–40. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donaldson GC, Seemungal TA, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–52. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janssens W, Bouillon R, Claes B, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65(3):215–20. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 14.Wolfenden LL, Judd SE, Shah R, et al. Vitamin D and bone health in adults with cystic fibrosis. Clin Endocrinol (Oxf) 2008;69(3):374–81. doi: 10.1111/j.1365-2265.2008.03216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pincikova T, Nilsson K, Moen IE, et al. Inverse relation between vitamin D and serum total immunoglobulin G in the Scandinavian Cystic Fibrosis Nutritional Study. Eur J Clin Nutr. 2011;65(1):102–9. doi: 10.1038/ejcn.2010.194. [DOI] [PubMed] [Google Scholar]
- 16.Danai PA, Sinha S, Moss M, et al. Seasonal variation in the epidemiology of sepsis. Crit Care Med. 2007;35(2):410–5. doi: 10.1097/01.CCM.0000253405.17038.43. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez M, Daniels B, Gunawardene S, et al. High frequency of vitamin D deficiency in ambulatory HIV-Positive patients. AIDS Res Hum Retroviruses. 2009;25(1):9–14. doi: 10.1089/aid.2008.0183. [DOI] [PubMed] [Google Scholar]
- 18.Ginde AA, Scragg R, Schwartz RS, et al. Prospective study of serum 25- hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J Am Geriatr Soc. 2009;57(9):1595–603. doi: 10.1111/j.1532-5415.2009.02359.x. [DOI] [PubMed] [Google Scholar]
- 19.Melamed ML, Michos ED, Post W, et al. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629–37. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22(4):477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 21.Holick MF, Uskokovic M, Henley JW, et al. The photoproduction of 1 alpha,25- dihydroxyvitamin D3 in skin: an approach to the therapy of vitamin-D-resistant syndromes. N Engl J Med. 1980;303(7):349–54. doi: 10.1056/NEJM198008143030701. [DOI] [PubMed] [Google Scholar]
- 22.Srviastava MD, DeLuca H, Ambrus JL. Inhibition of IL-6 and IL-8 production in human fibroblast cell lines by 1,25 (OH)2 vitamin D3 and two of its analogs with lower calcemic activity. Res Commun Chem Pathol Pharmacol. 1994;83(2):145–50. [PubMed] [Google Scholar]
- 23.Manolagas SC, Yu XP, Girasole G, et al. Vitamin D and the hematolymphopoietic tissue: a 1994 update. Semin Nephrol. 1994;14(2):129–43. [PubMed] [Google Scholar]
- 24.Lemire JM. Immunomodulatory role of 1,25-dihydroxyvitamin D3. J Cell Biochem. 1992;49(1):26–31. doi: 10.1002/jcb.240490106. [DOI] [PubMed] [Google Scholar]
- 25.Reichel H, Koeffler HP, Tobler A, et al. 1 alpha,25-Dihydroxyvitamin D3 inhibits gamma-interferon synthesis by normal human peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1987;84(10):3385–9. doi: 10.1073/pnas.84.10.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rachez C, Freedman LP. Mechanisms of gene regulation by vitamin D(3) receptor: a network of coactivator interactions. Gene. 2000;246(1-2):9–21. doi: 10.1016/s0378-1119(00)00052-4. [DOI] [PubMed] [Google Scholar]
- 27.Bhalla AK, Amento EP, Clemens TL, et al. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57(6):1308–10. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 28.Stumpf WE, Sar M, Reid FA, et al. Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary, and parathyroid. Science. 1979;206(4423):1188–90. doi: 10.1126/science.505004. [DOI] [PubMed] [Google Scholar]
- 29.Yim S, Dhawan P, Ragunath C, et al. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3). J Cyst Fibros. 2007;6(6):403–10. doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansdottir S, Monick MM, Hinde SL, et al. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181(10):7090–9. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 32.White JH. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect Immun. 2008;76(9):3837–43. doi: 10.1128/IAI.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173(5):2909–12. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 34.Ciornei CD, Sigurdardottir T, Schmidtchen A, et al. Antimicrobial and chemoattractant activity, lipopolysaccharide neutralization, cytotoxicity, and inhibition by serum of analogs of human cathelicidin LL-37. Antimicrob Agents Chemother. 2005;49(7):2845–50. doi: 10.1128/AAC.49.7.2845-2850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thijssen HH, Janssen CA, Drittij-Reijnders MJ. The effect of S-warfarin administration on vitamin K 2,3-epoxide reductase activity in liver, kidney and testis of the rat. Biochem Pharmacol. 1986;35(19):3277–82. doi: 10.1016/0006-2952(86)90424-7. [DOI] [PubMed] [Google Scholar]
- 36.Rockett KA, Brookes R, Udalova I, et al. 1,25-Dihydroxyvitamin D3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect Immun. 1998;66(11):5314–21. doi: 10.1128/iai.66.11.5314-5321.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramanathan B, Davis EG, Ross CR, et al. Cathelicidins: microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes Infect. 2002;4(3):361–72. doi: 10.1016/s1286-4579(02)01549-6. [DOI] [PubMed] [Google Scholar]
- 38.Gropp R, Frye M, Wagner TO, et al. Epithelial defensins impair adenoviral infection: implication for adenovirus-mediated gene therapy. Hum Gene Ther. 1999;10(6):957–64. doi: 10.1089/10430349950018355. [DOI] [PubMed] [Google Scholar]
- 39.Klotman ME, Chang TL. Defensins in innate antiviral immunity. Nat Rev Immunol. 2006;6(6):447–56. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- 40.Sly LM, Lopez M, Nauseef WM, et al. 1alpha,25-Dihydroxyvitamin D3-induced monocyte antimycobacterial activity is regulated by phosphatidylinositol 3-kinase and mediated by the NADPH-dependent phagocyte oxidase. J Biol Chem. 2001;276(38):35482–93. doi: 10.1074/jbc.M102876200. [DOI] [PubMed] [Google Scholar]
- 41.Rook GA, Hernandez-Pando R, Lightman SL. Hormones, peripherally activated prohormones and regulation of the Th1/Th2 balance. Immunol Today. 1994;15(7):301–3. doi: 10.1016/0167-5699(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 42.Cox FE, Liew FY. T-cell subsets and cytokines in parasitic infections. Immunol Today. 1992;13(11):445–8. doi: 10.1016/0167-5699(92)90072-F. [DOI] [PubMed] [Google Scholar]
- 43.Thorne KJ, Mazza G. Eosinophilia, activated eosinophils and human schistosomiasis. J Cell Sci. 1991;98(Pt 3):265–70. doi: 10.1242/jcs.98.3.265. [DOI] [PubMed] [Google Scholar]
- 44.Diaz A, Allen JE. Mapping immune response profiles: the emerging scenario from helminth immunology. Eur J Immunol. 2007;37(12):3319–26. doi: 10.1002/eji.200737765. [DOI] [PubMed] [Google Scholar]
- 45.Romani L. Cell mediated immunity to fungi: a reassessment. Med Mycol. 2008;46(6):515–29. doi: 10.1080/13693780801971450. [DOI] [PubMed] [Google Scholar]
- 46.Martineau AR, Wilkinson KA, Newton SM, et al. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. 2007;178(11):7190–8. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 47.Schleithoff SS, Zittermann A, Tenderich G, et al. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83(4):754–9. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 48.Zittermann A, Frisch S, Berthold HK, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89(5):1321–7. doi: 10.3945/ajcn.2008.27004. [DOI] [PubMed] [Google Scholar]
- 49.Coussens A, Timms PM, Boucher BJ, et al. 1alpha,25-dihydroxyvitamin D3 inhibits matrix metalloproteinases induced by Mycobacterium tuberculosis infection. Immunology. 2009;127(4):539–48. doi: 10.1111/j.1365-2567.2008.03024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobasa D, Takada A, Shinya K, et al. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature. 2004;431(7009):703–7. doi: 10.1038/nature02951. [DOI] [PubMed] [Google Scholar]
- 51.Cheung CY, Poon LL, Lau AS, et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360(9348):1831–7. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- 52.Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167(15):1655–63. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yende S, D'Angelo G, Kellum JA, et al. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med. 2008;177(11):1242–7. doi: 10.1164/rccm.200712-1777OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sherman LW. National Institute of Justice (U.S.), and University of Maryland at College Park. Department of Criminology and Criminal Justice., Preventing crime : what works, what doesn't, what's promising : a report to the United States Congress. Research report. U.S. Dept. of Justice, Office of Justice Programs; Washington, DC: 1997. [Google Scholar]
- 55.Miller WR, Wilbourne PL. Mesa Grande: a methodological analysis of clinical trials of treatments for alcohol use disorders. Addiction. 2002;97(3):265–77. doi: 10.1046/j.1360-0443.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- 56.Jolliffe DA, Griffiths CJ, Martineau AR. Vitamin D in the prevention of acute respiratory infection: systematic review of clinical studies. J Steroid Biochem Mol Biol. 2013;136:321–9. doi: 10.1016/j.jsbmb.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 57.Yamshchikov AV, Desai NS, Blumberg HM, et al. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr Pract. 2009;15(5):438–49. doi: 10.4158/EP09101.ORR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morcos MM, Gabr AA, Samuel S, et al. Vitamin D administration to tuberculous children and its value. Boll Chim Farm. 1998;137(5):157–64. [PubMed] [Google Scholar]
- 59.Nursyam EW, Amin Z, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones. 2006;38(1):3–5. [PubMed] [Google Scholar]
- 60.Wejse C, Gomes VF, Rabna P, et al. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2009;179(9):843–50. doi: 10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- 61.Kota SK, Jammula S, Kota SK, et al. Effect of vitamin D supplementation in type 2 diabetes patients with pulmonary tuberculosis. Diabetes Metab Syndr. 2011;5(2):85–9. doi: 10.1016/j.dsx.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 62.Martineau AR, Timms PM, Bothamley GH, et al. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011;377(9761):242–50. doi: 10.1016/S0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salahuddin N, Ali F, Hasan Z, et al. Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis'. BMC Infect Dis. 2013;13:22. doi: 10.1186/1471-2334-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coussens AK, Wilkinson RJ, Hanifa Y, et al. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc Natl Acad Sci U S A. 2012;109(38):15449–54. doi: 10.1073/pnas.1200072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mily A, Rekha RS, Kamal SM, et al. Oral intake of phenylbutyrate with or without vitamin D3 upregulates the cathelicidin LL-37 in human macrophages: a dose finding study for treatment of tuberculosis. BMC Pulm Med. 2013;13:23. doi: 10.1186/1471-2466-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ralph AP, Waramori G, Pontororing GJ, et al. L-arginine and vitamin D adjunctive therapies in pulmonary tuberculosis: a randomised, double-blind, placebo-controlled trial. PLoS One. 2013;8(8):e70032. doi: 10.1371/journal.pone.0070032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ganmaa D, Giovannucci E, Bloom BR, et al. Vitamin D, tuberculin skin test conversion, and latent tuberculosis in Mongolian school-age children: a randomized, double-blind, placebo-controlled feasibility trial. American Journal of Clinical Nutrition. 2012;96(2):391–396. doi: 10.3945/ajcn.112.034967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martineau AR, Wilkinson RJ, Wilkinson KA, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176(2):208–13. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 69.Choudhary N, Gupta P. Vitamin D supplementation for severe pneumonia--a randomized controlled trial. Indian Pediatr. 2012;49(6):449–54. doi: 10.1007/s13312-012-0073-x. [DOI] [PubMed] [Google Scholar]
- 70.Manaseki-Holland S, Qader G, Isaq Masher M, et al. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Trop Med Int Health. 2010;15(10):1148–55. doi: 10.1111/j.1365-3156.2010.02578.x. [DOI] [PubMed] [Google Scholar]
- 71.Manaseki-Holland S, Maroof Z, Bruce J, et al. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet. 2012;379(9824):1419–27. doi: 10.1016/S0140-6736(11)61650-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grossmann RE, Zughaier SM, Kumari M, et al. Pilot study of vitamin D supplementation in adults with cystic fibrosis pulmonary exacerbation: A randomized, controlled trial. Dermatoendocrinol. 2012;4(2):191–7. doi: 10.4161/derm.20332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grossmann RE, Zughaier SM, Liu S, et al. Impact of vitamin D supplementation on markers of inflammation in adults with cystic fibrosis hospitalized for a pulmonary exacerbation. Eur J Clin Nutr. 2012;66(9):1072–4. doi: 10.1038/ejcn.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lehouck A, Mathieu C, Carremans C, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2012;156(2):105–14. doi: 10.7326/0003-4819-156-2-201201170-00004. [DOI] [PubMed] [Google Scholar]
- 75.Goldring ST, Griffiths CJ, Martineau AR, et al. Prenatal vitamin d supplementation and child respiratory health: a randomised controlled trial. PLoS One. 2013;8(6):e66627. doi: 10.1371/journal.pone.0066627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Majak P, Olszowiec-Chlebna M, Smejda K, et al. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol. 2011;127(5):1294–6. doi: 10.1016/j.jaci.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 77.Murdoch DR, Slow S, Chambers ST, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308(13):1333–9. doi: 10.1001/jama.2012.12505. [DOI] [PubMed] [Google Scholar]
- 78.Camargo CA, Jr., Ganmaa D, Frazier AL, et al. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics. 2012;130(3):e561–7. doi: 10.1542/peds.2011-3029. [DOI] [PubMed] [Google Scholar]
- 79.Laaksi I, Ruohola JP, Mattila V, et al. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blinded trial among young Finnish men. J Infect Dis. 2010;202(5):809–14. doi: 10.1086/654881. [DOI] [PubMed] [Google Scholar]
- 80.Li-Ng M, Aloia JF, Pollack S, et al. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect. 2009;137(10):1396–404. doi: 10.1017/S0950268809002404. [DOI] [PubMed] [Google Scholar]
- 81.Bergman P, Norlin AC, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2(6) doi: 10.1136/bmjopen-2012-001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kriesel JD, Spruance J. Calcitriol (1,25-dihydroxy-vitamin D3) coadministered with influenza vaccine does not enhance humoral immunity in human volunteers. Vaccine. 1999;17(15-16):1883–8. doi: 10.1016/s0264-410x(98)00476-9. [DOI] [PubMed] [Google Scholar]
- 83.Urashima M, Segawa T, Okazaki M, et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91(5):1255–60. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 84.Aloia JF, Li-Ng M. Re: epidemic influenza and vitamin D. Epidemiol Infect. 2007;135(7):1095–6. doi: 10.1017/S0950268807008308. author reply 1097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kalra P, Das V, Agarwal A, et al. Effect of vitamin D supplementation during pregnancy on neonatal mineral homeostasis and anthropometry of the newborn and infant. Br J Nutr. 2012;108(6):1052–8. doi: 10.1017/S0007114511006246. [DOI] [PubMed] [Google Scholar]
- 86.Jorde R, Witham M, Janssens W, et al. Vitamin D supplementation did not prevent influenza-like illness as diagnosed retrospectively by questionnaires in subjects participating in randomized clinical trials. Scand J Infect Dis. 2012;44(2):126–32. doi: 10.3109/00365548.2011.621446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rehman PKM. Subclinical Rickets and Recurrent Infection. Journal of Tropical Pediatrics. 1994;40(1):58–58. doi: 10.1093/tropej/40.1.58. [DOI] [PubMed] [Google Scholar]
- 88.Bischoff-Ferrari HA, Dawson-Hughes B, Platz A, et al. Effect of High-Dosage Cholecalciferol and Extended Physiotherapy on Complications After Hip Fracture A Randomized Controlled Trial. Archives of Internal Medicine. 2010;170(9):813–820. doi: 10.1001/archinternmed.2010.67. [DOI] [PubMed] [Google Scholar]
- 89.Avenell A, Cook JA, Maclennan GS, et al. Vitamin D supplementation to prevent infections: a sub-study of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438). Age Ageing. 2007;36(5):574–7. doi: 10.1093/ageing/afm091. [DOI] [PubMed] [Google Scholar]
- 90.Tran B, Armstrong BK, Ebeling PR, et al. Effect of vitamin D supplementation on antibiotic use: a randomized controlled trial. Am J Clin Nutr. 2014;99(1):156–61. doi: 10.3945/ajcn.113.063271. [DOI] [PubMed] [Google Scholar]
- 91.Abu-Mouch S, Fireman Z, Jarchovsky J, et al. Vitamin D supplementation improves sustained virologic response in chronic hepatitis C (genotype 1)-naive patients. World J Gastroenterol. 2011;17(47):5184–90. doi: 10.3748/wjg.v17.i47.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Briffa NK, Keogh AM, Sambrook PN, et al. Reduction of immunosuppressant therapy requirement in heart transplantation by calcitriol. Transplantation. 2003;75(12):2133–4. doi: 10.1097/01.TP.0000065179.06731.99. [DOI] [PubMed] [Google Scholar]
- 93.Arpadi SM, McMahon D, Abrams EJ, et al. Effect of bimonthly supplementation with oral cholecalciferol on serum 25-hydroxyvitamin D concentrations in HIV-infected children and adolescents. Pediatrics. 2009;123(1):e121–6. doi: 10.1542/peds.2008-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Snyman JR, de Sommers K, Steinmann MA, et al. Effects of calcitriol on eosinophil activity and antibody responses in patients with schistosomiasis. Eur J Clin Pharmacol. 1997;52(4):277–80. doi: 10.1007/s002280050289. [DOI] [PubMed] [Google Scholar]
- 95.Wagner CL, McNeil RB, Johnson DD, et al. Health characteristics and outcomes of two randomized vitamin D supplementation trials during pregnancy: a combined analysis. J Steroid Biochem Mol Biol. 2013;136:313–20. doi: 10.1016/j.jsbmb.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Benator D, Bhattacharya M, Bozeman L, et al. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet. 2002;360(9332):528–34. doi: 10.1016/s0140-6736(02)09742-8. [DOI] [PubMed] [Google Scholar]
- 97.Ish-Shalom S, Segal E, Salganik T, et al. Comparison of daily, weekly, and monthly vitamin D3 in ethanol dosing protocols for two months in elderly hip fracture patients. J Clin Endocrinol Metab. 2008;93(9):3430–5. doi: 10.1210/jc.2008-0241. [DOI] [PubMed] [Google Scholar]
- 98.Unson CG, Litt M, Reisine S, et al. Adherence to calcium/vitamin D and estrogen protocols among diverse older participants enrolled in a clinical trial. Contemp Clin Trials. 2006;27(3):215–26. doi: 10.1016/j.cct.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 99.Sanfelix-Genoves J, Gil-Guillen VF, Orozco-Beltran D, et al. Determinant factors of osteoporosis patients' reported therapeutic adherence to calcium and/or vitamin D supplements: a cross-sectional, observational study of postmenopausal women. Drugs Aging. 2009;26(10):861–9. doi: 10.2165/11317070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 100.Kearns MD, Alvarez JA, Tangpricha V. Large, Single-Dose, Oral Vitamin D Supplementation in Adult Populations: A Systematic Review. Endocr Pract. 2013:1–36. doi: 10.4158/EP13265.RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Trang HM, Cole DE, Rubin LA, et al. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr. 1998;68(4):854–8. doi: 10.1093/ajcn/68.4.854. [DOI] [PubMed] [Google Scholar]
- 102.Binkley N, Novotny R, Krueger D, et al. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab. 2007;92(6):2130–5. doi: 10.1210/jc.2006-2250. [DOI] [PubMed] [Google Scholar]
- 103.Aloia JF, Patel M, Dimaano R, et al. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am J Clin Nutr. 2008;87(6):1952–8. doi: 10.1093/ajcn/87.6.1952. [DOI] [PubMed] [Google Scholar]
- 104.Blum M, Dallal GE, Dawson-Hughes B. Body size and serum 25 hydroxy vitamin D response to oral supplements in healthy older adults. J Am Coll Nutr. 2008;27(2):274–9. doi: 10.1080/07315724.2008.10719700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fu L, Yun F, Oczak M, et al. Common genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvitamin D [25(OH)D] to vitamin D supplementation. Clin Biochem. 2009;42(10-11):1174–7. doi: 10.1016/j.clinbiochem.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 106.Zhao LJ, Zhou Y, Bu F, et al. Factors predicting vitamin D response variation in non-Hispanic white postmenopausal women. J Clin Endocrinol Metab. 2012;97(8):2699–705. doi: 10.1210/jc.2011-3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Uitterlinden AG, Fang Y, Van Meurs JB, et al. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338(2):143–56. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 108.Nelson ML, Blum JM, Hollis BW, et al. Supplements of 20 microg/d cholecalciferol optimized serum 25-hydroxyvitamin D concentrations in 80% of premenopausal women in winter. J Nutr. 2009;139(3):540–6. doi: 10.3945/jn.108.096180. [DOI] [PubMed] [Google Scholar]
- 109.Heaney RP, Armas LA, Shary JR, et al. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr. 2008;87(6):1738–42. doi: 10.1093/ajcn/87.6.1738. [DOI] [PubMed] [Google Scholar]
- 110.Heaney RP. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr Rev. 2014;72(1):48–54. doi: 10.1111/nure.12090. [DOI] [PubMed] [Google Scholar]
- 111.Ahn J, Yu K, Stolzenberg-Solomon R, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19(13):2739–45. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–8. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Powe CE, Karumanchi SA, Thadhani R. Vitamin D-binding protein and vitamin D in blacks and whites. N Engl J Med. 2014;370(9):880–1. doi: 10.1056/NEJMc1315850. [DOI] [PubMed] [Google Scholar]
- 114.Kumar GT, Sachdev HS, Chellani H, et al. Effect of weekly vitamin D supplements on mortality, morbidity, and growth of low birthweight term infants in India up to age 6 months: randomised controlled trial. BMJ. 2011;342:d2975. doi: 10.1136/bmj.d2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kraemer R, Rudeberg A, Hadorn B, et al. Relative underweight in cystic fibrosis and its prognostic value. Acta Paediatr Scand. 1978;67(1):33–7. doi: 10.1111/j.1651-2227.1978.tb16273.x. [DOI] [PubMed] [Google Scholar]
- 116.Beker LT, Russek-Cohen E, Fink RJ. Stature as a prognostic factor in cystic fibrosis survival. J Am Diet Assoc. 2001;101(4):438–42. doi: 10.1016/S0002-8223(01)00113-4. [DOI] [PubMed] [Google Scholar]
- 117.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis. 2004;8(3):286–98. [PubMed] [Google Scholar]
- 118.van Lettow M, Harries AD, Kumwenda JJ, et al. Micronutrient malnutrition and wasting in adults with pulmonary tuberculosis with and without HIV co-infection in Malawi. BMC Infect Dis. 2004;4(1):61. doi: 10.1186/1471-2334-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.van der Sande MA, Schim van der Loeff MF, Aveika AA, et al. Body mass index at time of HIV diagnosis: a strong and independent predictor of survival. J Acquir Immune Defic Syndr. 2004;37(2):1288–94. doi: 10.1097/01.qai.0000122708.59121.03. [DOI] [PubMed] [Google Scholar]