Abstract
Our recent study indicates that the lesions of the prefrontal cortex in rats result in depressive-like behavior in forced swim test and REM sleep alterations, two well-established biomarkers of depression disorder. We hypothesized that antidepressants may target the PFC to reverse depression. Systemic injections of antidepressants: the tricyclic antidepressant desipramine (DMI), the selective serotonin reuptake inhibitor fluoxetine, and the NMDA-antagonist ketamine selectively increased cFos expression (a marker of neuronal activity) in the deep layers of the ventromedial PFC (vmPFC) in rats. Of the vmPFC's limbic system targets, only the nucleus accumbens (NAc) was also activated by DMI. Using a retrograde tracer and a neuronal toxin, we also found that DMI-activated vmPFC neurons project to the NAc and that NAc activation by DMI was lost following vmPFC lesion. These results suggest that the vmPFC may be an essential target of antidepressant drugs, its projections to the NAc may be a key circuit regulating antidepressant action, and dysfunction of this pathway may contribute to depression.
Keywords: desipramine, fluoxetine, ketamine, nucleus accumbens, depression, cFos
Introduction
Monoamine oxidase inhibitors, tricyclic antidepressants (TCAs), and selective serotonin reuptake inhibitors (SSRIs) have all been prescribed over the past several decades to treat Major Depressive Disorder (MDD), yet we lack a complete understanding of the neural mechanisms of these drugs. Although these antidepressant medications are taken systemically, they have different effects on different regions of the brain (Conti et al, 2006). It is unclear, however, if these different classes of antidepressants share a common site of action in the brain and, if so, where that site is.
MDD likely involves multiple brain regions, but there is mounting evidence supporting the notion that the prefrontal cortex (PFC) plays an important role. Volume decreases have been reported in the orbitofrontal and subgenual cingulate cortices of depressed patients (Bremner et al, 2002; Drevets, 1998; Drevets et al, 1992; Ongür et al, 1998), and post-mortem analyses of the brains of depressed individuals show a significantly reduced number of glial cells (Drevets, 1998; Rajkowska et al, 1999) as well as neuronal size reduction (Chana et al, 2003). Depressed patients demonstrate increased activity per volume in the subgenual and ventrolateral PFC compared to non-depressed individuals (Drevets et al, 1992, 1997), and these changes are reversed in patients in remission (Drevets et al, 1992; Mayberg et al, 2000). In addition, there is evidence that early-life stress diminishes the ability of the vmPFC to sustain activity in response to repeated stressors (Wang et al, 2013).
Animal models of depression also suggest a role for the PFC in depression and its amelioration by antidepressants. Chronic restraint stress leads to dendritic atrophy in the rat medial PFC (mPFC), including reduced length and total branch numbers of apical dendrites in pyramidal cells (Brown, 2005; Cook and Wellman, 2004; Izquierdo et al, 2006; Radley et al, 2004), while antidepressant treatment increases markers of neuroplasticity (Sairanen et al, 2007) and changes in cell morphology in the PFC (Benes and Vincent, 1991; Li et al, 2010). Our recent study shows that the lesioning the vmPFC reduces REM sleep latency, increases REM sleep amounts, and increases immobile time in forced swim test (FST) in rats (Chang et al, 2014). These changes of REM sleep and hopelessness are consistent in behaviors of unipolar and bipolar depression in human. Ketamine is an NMDA-antagonist typically used as an anesthetic agent but has recently been found to rapidly alleviate depression in humans and rodents models of depression (Berman et al, 2000; Engin et al, 2009; Garcia et al, 2008; Li et al, 2010). Infusion of rapamycin – which blocks an important signaling pathway involved in cell growth and survival – into the rat mPFC prevents this antidepressant response (Li et al, 2010). Therefore, the PFC may be an important region for investigating both the underlying pathophysiology of MDD and the mechanism of antidepressants.
The evidence for a relationship between sleep, depression, and antidepressant medications was motivation to investigate how the medications may be acting on the sleep circuitries. We thus analyzed sleep-wake behavior of animals that were administered an antidepressant agent. In addition, relatively few studies have investigated the effects these drugs have on the PFC and its downstream targets. The rat mPFC, like the human PFC, projects to many limbic areas implicated in the control of affect, including the lateral septum, basolateral amygdala, insular cortex, and nucleus accumbens (Chiba et al, 2001; Uylings et al, 2003; Uylings and Van Eden, 1990). We hypothesized that antidepressants would directly alter neuronal activity in the mPFC and its circuitry. To test this hypothesis, we acutely administered the TCA desipramine, the SSRI fluoxetine, and ketamine to rats and examined patterns of the neuronal activity marker cFos in the mPFC and its downstream limbic targets. Furthermore, to verify the pathway of downstream neural activation, we repeated a subset of the experiments with animals that had neuronal lesions in the ventromedial PFC.
Materials and Methods
Animals
All animals used were pathogen-free adult male Sprague-Dawley rats (300-350g) purchased from Taconic (Hudson, NY). They were housed in rat-specific holding rooms controlled for temperature (22±1°C) and humidity in pairs until surgery, and subsequently separated into individual cages (7-9 days before sleep tests were performed). Food and water were available ad libitum, and lights were automatically switched on and off according to a 12:12 L:D cycle (lights on 8:00am – 8:00pm). The animals were cared for in accordance with National Institutes of Health standards, and all procedures were pre-approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee.
Animal lesion surgery
Prior to surgery, animals (n = 37) were anesthetized with ketamine-xylazine (i.p, 800 mg/kg ketamine, 80 mg/kg xylazine, Med-Vet, Mettawa, IL) and then placed in a stereotaxic frame so that their head was fixed. Injections of ibotenic acid (n = 11, IBO, Tocris, Ellisville, MO), 0.9% saline (n = 26, Med-Vet, Mettawa, IL) or cholera toxin subunit B (n = 4, CTB, List Biological, Campbell, CA) were administered directly into the brain using a fine glass pipette (1 mm glass stock, tapering slowly to a 10-20um tip) connected to an air compression system. A series of 20-40psi puffs of air were used to deliver the compounds into the brain at the following coordinates and volumes: vmPFC: AP+3.0mm DV-3.4mm RL+/−0.6mm, 66-99nL 5% IBO, 16.5nL 1.0% CTB; NAc: AP+2.0mm DV-6.8mm RL+/−1.0mm, 23.1nL 1% CTB (Paxinos and Watson, 2007). Incisions were closed with wound clips. Upon completion of the procedure, the animal was given a subcutaneous injection of the analgesic meloxicam (1.0 mg/kg, Med-Vet, Mettawa, IL) and allowed to recover on a warm plate until awakened from anesthesia.
On the same day six animals received four EEG screw electrodes (Plastics One, Roanoke, VA) that were screwed into skull, and two flexible EMG wire electrodes were also placed on the left and right nuchal muscles. The free ends of the leads were placed in a plastic electrode pedestal that was cemented onto the skull using Jet Denture Repair Powder and Jet Liquid (Henry Schein, Melville, NY). Any animals that did not receive electrodes had their incision closed with wound clips.
Sleep Recordings and Analysis
After at least a week of post-surgical recovery, animals undergoing sleep recordings (n = 6) were placed in isolated recording chambers. Flexible cables that were mounted to fixed commutators were attached to the electrode pedestals, and the cages were placed such that the animals could move freely. As before, food and water were available ad libitum, ambient temperature was controlled, and the light:dark cycle was 12:12. Video cameras were placed to capture movement in the entire cage, and the animals were habituated without disturbance for at least two days and then recorded for 72h using VitalRecorder (Kissei Comtec Co., Nagano, Japan). Animals were injected 2 hours after lights on (9:00am) with drug (n = 3) (or saline, n = 3) on the second recording day, and with saline (or drug) on the third day. Upon completion of the recordings, animals were detached from the cables and returned to the holding room.
The EEG/EMG recordings were analyzed using SleepSign (Kissei Comtec Co., Nagano, Japan). The recordings were divided into 12s epochs and each epoch scored manually as wake, REM, or NREM sleep. Wake was identified by high frequency, desynchronized EEG accompanied by frequent EMG activity and observed behaviors on the video playback. NREM sleep was identified by the dominant presence of high-amplitude, low frequency (<4 Hz) EEG activity and little muscle tone on the EMG recording. REM sleep was identified by theta waves (4-7 Hz) of consistent low amplitude on the EEG recording accompanied by very low EMG activity. REM sleep latency was defined as the interval of time between sleep onset and REM sleep onset averaged over 24 hours. Sleep-wake percentages, bout numbers, bout durations and REM latency were analyzed using unpaired t-test and adjusted using Bonferroni's correction, using a significance threshold p<0.05.
Antidepressant Drug Treatments
To study neuronal activation patterns upon acute drug treatment, and the effect of prefrontal cortical lesions on the activation patterns, lesioned (n = 11) and sham-lesioned (n = 22) animals were placed in isolated chambers for at least two days. At 10am after habituation, animals were gently handled and weighed. At 10am the following day, animals were injected i.p. with desipramine hydrochloride (n = 11, 10 mg/kg in saline, Sigma, St. Louis, MO), fluoxetine hydrochloride (n = 7, Sigma, St. Louis, MO; 20 mg/kg in saline), ketamine (n = 5, 10 mg/kg in saline, Med-Vet, Mettawa, IL), or sterile saline (n = 10, 0.9%, Fisher Scientific, Pittsburgh, PA). This dose of desipramine has been shown to be effective in previous rodent models of depression (Borsini et al, 1981; Detke et al, 1995; Pulvirenti and Samanin, 1986), and the doses of fluoxetine and ketamine were chosen based on their effectiveness in decreasing immobility in the forced swim test rodent model of depression (Detke et al, 1995; Li et al, 2010) while not causing arousal. We believe this latter feature was key to minimize confounding effects on neuronal activation due to wakefulness. The animals were then placed back in their cages and in their chambers for two hours, after which they were sacrificed via perfusion and fixation (see below) and their brains stained for cFos immunohistochemistry. We chose these conditions (including performing injections in the morning) to maximize the likelihood that the animals would fall sleep following the injection.
We chose to implement a single, acute injection of antidepressant drugs because we wanted to first understand how and where the drugs are acting in the brain. Although antidepressant drugs generally improve symptoms after several weeks of regular ingestion, we believe it is important to understand what a single dose does as we attempt to unwrap its chronic effects. Therefore in this work we begin with acute injections to discover what areas of the brain are the drugs’ main targets. In addition, for these reasons we did not test a wide range of drug concentrations. The purpose of our investigation was to discover the action of the antidepressant drugs at their biologically relevant concentrations.
Perfusion and fixation
Animals were anesthetized with 7% chloral hydrate (i.p. 500 mg/kg, Sigma, St. Louis, MO). The body cavity was opened using surgical scissors and a 16G needle was inserted into the left ventricle of the heart. The top of the right atrium was cut to allow blood to be drained. About 100 mL of saline was flushed through the vascular system using an intravenous line, followed by 500mL of 10% buffered formalin (Fisher Scientific, Pittsburgh, PA). Upon fixation of the tissue the brain was removed from the skull and stored in 10% formalin for 4-5 hours. The brains were then moved to 20% sucrose and 0.02% azide solution overnight.
Histology and Immunohistochemistry
Brains were sliced into four series of 40um sections using a freezing microtome. The sections were stored in PBS-0.02% azide in 20°C. For immunohistochemical staining, tissue sections were rinsed in PBS three times, 3-5 min each, then incubated for 30 min in 0.3% H2O2 (Sigma, St. Louis, MO) in PBT (phosphate buffer with Triton X-100; Sigma, St. Louis, MO) to oxidize any remaining blood. The sections were again rinsed in PBS and then incubated in primary antibody diluted in PBT-Azide for 1-2 nights, depending on the antibody (cFos Ab-5, PC38, rabbit polyclonal, 1:30,000, Calbiochem, Billerica, MA; Chemicon, Billerica, MA; CTB, 127H4810, goat polyclonal, 1:50,000, Sigma, St. Louis, MO). Tissue were then rinsed in PBS three times, and incubated in secondary antibody (1:1000, biotin SP-conjugated against appropriate species IgG, Jackson ImmunoResearch Laboratories, West Grove, PA) for 60-90 min. Sections were again rinsed in PBS and placed in ABC solution (1:1000 each Vectastain solutions A and B, Vector Laboratories, Burlingame, CA) for 60-90 min. Sections were rinsed in PBS and stained for 5 min in a solution consisting of: 1% DAB, 0.3% H2O2 (and 0.01% Ni, 0.005% CoCl2 if desired a black stain). Tissue were then rinsed and mounted on microscope slides in gelatin.
Sections were Nissl stained by placing microscope slides of mounted tissue in ddH2O for 5 min, followed by 10-30 sec in 0.1% thionin staining solution (Sigma, St. Louis, MO). The slides were dehydrated step-wise by incubating in 50%, 70%, 95%, and 100% EtOH for 2 min each. Slides were then placed in xylene for at least three hours before covering with glass coverslips. The delineation of the vmPFC and dmPFC was based on Paxinos and Watson's Rat Atlas (Paxinos and Watson, 2007) and the cytoarchitectural characteristics as described in Hurley et al. (Hurley et al, 1991).
Cell Counting
All cFos-stained cells were counted manually using a guide grid at 25x magnification. (While automated cell counting was attempted, variance in background staining – not unexpected despite identical treatment of the sections – warranted more careful analyses.) For the ACC and PLC, three sections were counted and summed (rostral: Bregma +4.2mm, middle: Bregma +3.2mm, caudal: Bregma +2.5mm) and for the ILC two sections were counted (middle: Bregma +3.2mm, caudal: Bregma +2.5mm). Counting boxes used were 400μm × 400μm. For the ACC, two boxes were placed corner to corner at the dorsomedial edge of the ACC, and for the ventral regions one box each was placed in the deep and superficial layers. Three sections were counted of the rostral NAc (Bregma +2.0 to +2.5mm): 400μm × 400μm counting boxes were placed immediately ventral to the anterior commissure (core) and lateral to the core along the medial edge of the NAc (shell). Three sections in the basolateral amygdala (Bregma −3.0mm to −3.3mm) were counted; two sections of the lateral septum (Bregma +1.7mm to +1.5mm) with counting box aligned to lateral ventricle; and two sections of the insular cortex (Bregma +3.0mm and +2.5mm) with counting box placed along the cortical edge. Counting box used for these structures was also 400μm × 400μm. The anterior-posterior levels of each of the limbic structures counted were chosen based on the specific projection patterns of the vmPFC (Vertes, 2003). All cell counting data were corrected using Abercrombie's correction (Guillery, 2002).
Statistical Analyses
All of the cell count data were analyzed using the unpaired t-test, except in the case of the nucleus accumbens cFos comparisons in animals with and without vmPFC lesions when a 2-way ANOVA was used. In the comparison between fluoxetine, ketamine and saline injections, p-values were adjusted with Bonferroni's adjustment. p<0.05 was used as the threshold for significance.
Results
Desipramine activates deep layers of the rat vmPFC
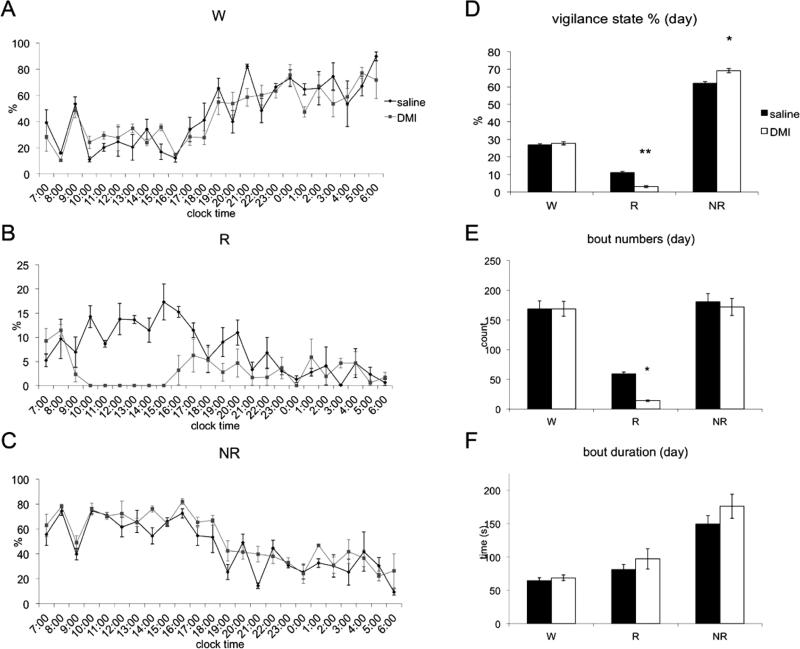
To investigate whether antidepressant drugs target the prefrontal cortex, we administered desipramine (DMI) at 10:00 am to adult male Sprague-Dawley rats. Saline was injected into animals under identical conditions as a control. Both groups (n=5) showed sleeping postures in the interval between injection and perfusion, which was verified by EEG/EMG recording in four rats that received DMI (Fig. 1). REM sleep was completely suppressed by the DMI for about 6 hours, with a compensatory increase in NREM sleep.
Figure 1.
Sleep-wake analysis of animals administered saline or DMI on consecutive days (n=4). A, B, and C show hourly wake, REM sleep, and NREM sleep percentages over 24h. D, E, and F show vigilance state percentages, bout numbers, and average bout durations during the 12h light period. Injections were given at 9:00am.
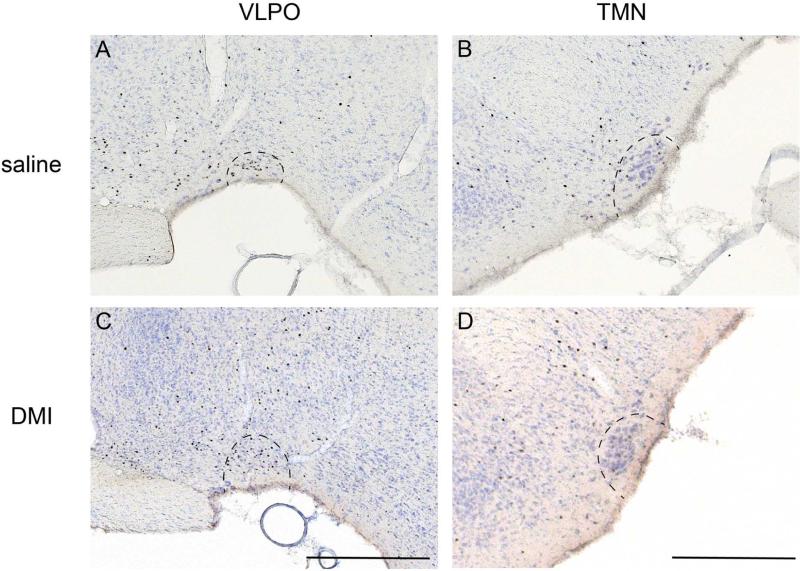
Animals of both groups showed a cFos activation pattern typical of sleeping animals two hours after DMI and saline injections, including high cFos expression in the sleep-active neurons of the ventrolateral preoptic nucleus (VLPO) and low expression in the arousal regions such as the tuberomammillary nucleus (Fig. 2), thalamus, and cortex. However, compared to controls, animals administered DMI exhibited a significant and distinct increase in cFos expression in the ventral mPFC (the prelimbic and infralimbic cortices; average cell counts, saline v. DMI: 54.1±13.5 v. 120.1±22.4; t(8)=2.52 p=0.036), but not the dorsal mPFC (anterior cingulate cortex; saline v. DMI: 4.4±1.8 v. 10.5±3.5; t(8)=1.54, p>0.05; Fig. 3) as well as other cortical regions.
Figure 2.
cFos expression in the sleep-active ventrolateral preoptic nucleus (A and C) and wake-active tuberomammillary nucleus (B and D) following saline (A and B) or DMI (C and D) injections indicate that animals of both conditions were sleeping during the interval between injection and sacrifice. Scale bars indicate 0.5mm.
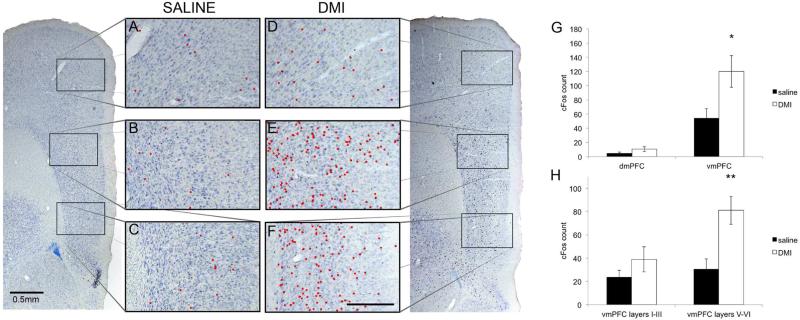
Figure 3.
Animals were injected with saline (A-C) or DMI (D-F) (i.p., 10 mg/kg) at 10:00am and sacrificed two hours later. Cells in the mPFC expressing cFos are indicated by red dots in the anterior cingulate (A and D), prelimbic (B and E), and infralimbic (C and F) cortices. The prelimbic and infralimbic cortices, collectively called the vmPFC below, show a dramatic increase in cFos expression following drug administration whereas the anterior cingulate (dmPFC) is relatively quiet. Scale bar in F indicates 0.25mm. G The vmPFC, but not the dmPFC, was significantly activated upon DMI injection (i.p. 10 mg/kg, n=5) compared to saline-injected animals (n=5). H Within the vmPFC, there was a clear distinction in the number of cFos-stained cells between the deep layers (V-VI) and superficial layers (I-III).
The mPFC consists of five cortical cell layers (I,II,III,V,VI), and we divided the vmPFC into superficial (I-III) and deep layers (V-VI) and counted the number of cFos-stained cells in each. We found that the statistical increase in cFos expression in the vmPFC due to DMI could be attributed to the deep layers (Fig. 3H; saline v. DMI, layers V-VI: 30.5±8.9 v. 81.1±12.1,t(8)=3.37, p=0.0099). The number of cFos-stained cells in the superficial layers of DMI-injected animals was not statistically different from that of saline-injected animals (saline v. DMI, layers I-III: 23.6±5.9 v. 38.9±10.8, t(8)=1.25, p>0.05).
Fluoxetine and ketamine also selectively activate vmPFC
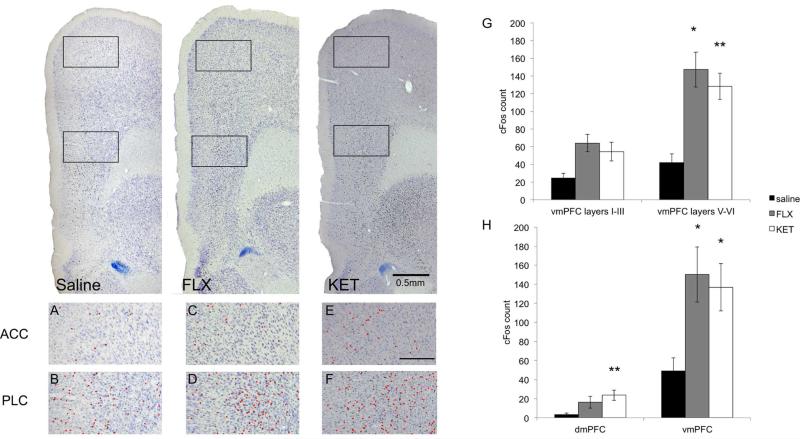
To investigate whether antidepressant classes other than TCAs produce a similar pattern of neuronal activation in the vmPFC, we treated animals with fluoxetine (FLX, 20 mg/kg, n=5) or ketamine (KET, 10 mg/kg, n=5; Fig. 4). Like DMI, FLX induced an increase in cFos expression in the vmPFC compared to animals that received saline (saline v. FLX: 49.1±13.6 v. 150.3±29.0, t(10)=2.47, p=0.033) without significantly affecting cFos expression in the dmPFC (saline v. FLX: 3.2±1.8 v. 16.3±6.1, t(10)=1.75, p>0.05; Fig. 4G). In the mPFC, KET increased cFos expression in both the dmPFC and vmPFC (dmPFC: saline v. KET: 3.2±1.8 v. 23.7±5.4, t(8)=3.26, p=0.0098; vmPFC: saline v. KET: 49.1±13.6 v. 136.8±24.9, t(8)=2.75, p=0.023). Furthermore, the increased vmPFC expression in both FLX and KET groups was exclusive to the deep cortical layers (layers V-VI: saline v. FLX: 31.0±9.8 v. 104.8±19.8, t(10)=2.68, p=0.023; saline v. KET: 31.0±9.8 v. 96.0±14.9, t(8)=3.33, p=0.0088; layers I-III: saline v. FLX: 18.1±5.3 v. 45.5±9.8, t(10)=1.79, p>0.05; saline v. KET: 18.1±5.3 v. 40.8±10.5, t(8)=1.43, p>0.05; Fig. 4H). Animals that received FLX or KET at these doses showed typical sleeping posture, and cFos expression in regions involved in sleep and arousal like the active VLPO and inactive TMN was comparable to sleep control animals, indicating that these doses of antidepressants do not significantly alter sleep behavior during the inactive period.
Figure 4.
Animals were injected i.p. with saline, FLX (20 mg/kg), or KET (10 mg/kg) at 10:00am and sacrificed two hours later. The prelimbic cortex (D and F) demonstrated increased cFos expression following administration of both drugs compared to saline (B), whereas the anterior cingulate cortex did not (A, C and E). Scale bar in panel E indicates 0.25mm. G-H. Animals were administered saline (n=6), FLX (20 mg/kg, n=5) or KET (10 mg/kg, n=5) at 10:00am and sacrificed two hours later. FLX and KET selectively activate the vmPFC, and the deep layers of this region, similar to DMI. ‘*’, p<0.05; ‘**’, p<0.01.
DMI activates the vmPFC- NAc circuit
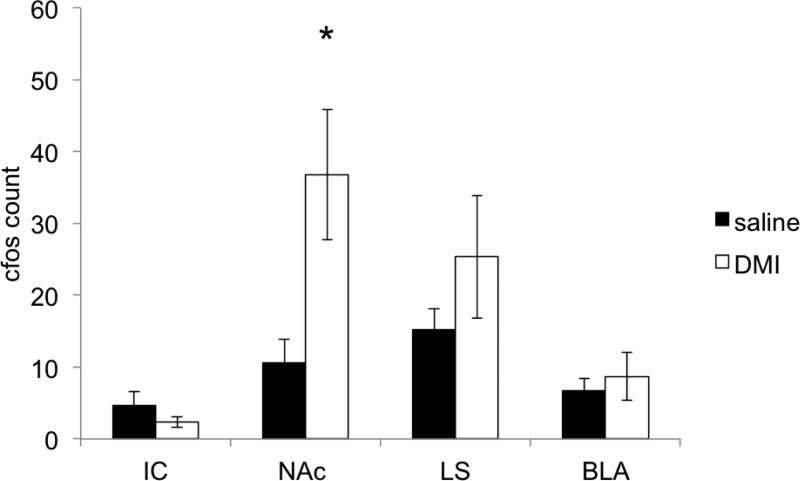
As cFos expression patterns in response to antidepressants were heaviest in the deep, projecting layers of vmPFC, we tested whether limbic structures that receive projections from these layers are also activated by DMI (Hurley et al, 1991; Vertes, 2003). We used DMI to examine the downstream projections of the vmPFC as DMI induced more specific and selective cFos expression in this region compared to FLX or KET. We examined cFos expression in three known limbic structures innervated by the vmPFC: the lateral septum, basolateral amydgala and nucleus accumbens.
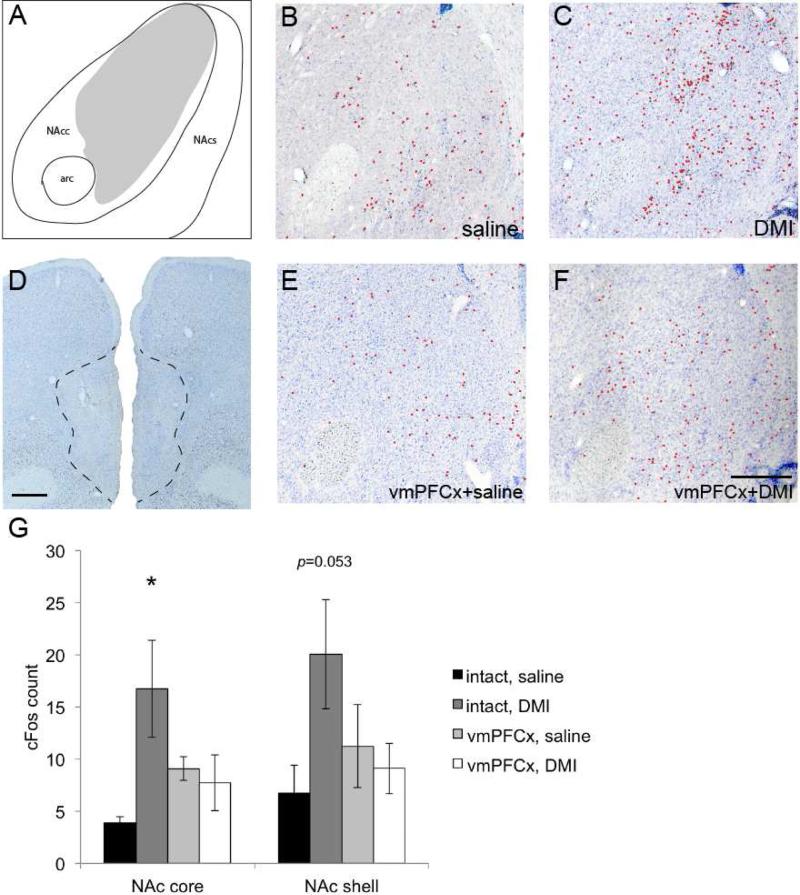
Of the limbic sites projected from the vmPFC, DMI (n=5) did not significantly activate the lateral septum (saline v. DMI: 15.2±2.3 v. 25.3±8.5, t(8)=1.12, p>0.05), insular cortex (saline v. DMI: 4.6±2.0 v. 2.3±0.7, t(8)=1.08, p>0.05), nor basolateral amygdala (saline v. DMI: 6.7±1.7 v. 8.7±3.3, t(8)=0.5, p>0.05) compared to saline (n=5) injections (Fig. 5). On the other hand, the NAc exhibited significantly increased cFos expression in response to DMI (saline v. DMI: 10.6±3.2 v. 36.8±9.1, t(8)=2.72, p=0.026;). As the NAc core and shell are believed to subserve separate behavioral functions (Bassareo et al, 2002), we analyzed the anatomic specificity of the vmPFC-dependent activation of the NAc by DMI (Fig. 6). We found that DMI significantly increased the number of cFos-stained neurons in the NAc core compared to that of controls (saline v. DMI: 3.9±0.6 v. 16.8±4.7, t(8)=2.73, p=0.026). The NAc shell had ~190% more cFos positive neurons after DMI, but this difference did not reach statistical significance (saline v. DMI: 6.7±2.7 v. 20.0±5.2, t(8)=2.27, p=0.053). These results suggest that the vmPFC-NAc projection may be particularly important for DMI's action on the limbic structures.
Figure 5.
cFos-labeled cells were counted in the insular cortex (IC), nucleus accumbens (NAc), lateral septum (LS), and basolateral amygdala (BLA) in animals given i.p. saline (n=5) or DMI (10 mg/kg, n=5). Among these structures, only the NAc was highly activated by the drug. ‘*’, p<0.05.
Figure 6.
Cells in the NAc are also activated by DMI (10 mg/kg) (C) compared to animals that received saline (B). However, cFos staining was reduced when DMI was injected into animals with neuronal lesions in the vmPFC (A, E). mPFC: Bregma +3.5mm; NAc: Bregma +2.0mm. Scale bars in A and E are 0.5mm. G. Sham- and vmPFC-lesioned animals were administered saline or DMI (10 mg/kg) i.p. (10:00am) and sacrificed two hours later. In intact animals, the number of cells that were stained by cFos in the NAc was significantly greater in animals that received DMI (n=5) compared to those that received saline (n=5). However, the levels of cFos expression in the core and shell were reduced by vmPFC lesions (n=6). n=6 for lesion group that received saline injection. ‘*’, p<0.05.
To determine if the vmPFC is necessary for the effect of DMI on the NAc, we lesioned the vmPFC using ibotenic acid (n=6), administered DMI, and examined cFos in the NAc. The vmPFC lesions led to decreases in cFos-stained neurons in both the NAc core and shell (DMI, intact v. lesion, NAc core: 16.8±4.7 v. 7.7±2.7; NAc shell: 20.0±5.2 v. 9.1±2.4), whereas vmPFC lesions did not affect NAc expression when saline was administered as a control (n=6; intact v. lesion: NAc core: 3.9±0.6 v. 9.1±1.1; NAc shell: 6.7±2.7 v. 11.2±4.0). A 2x2 ANOVA confirmed that the combination of lesion and drug injection was necessary to reduce cFos expression in the NAc (p=0.023), for drug injection alone (p or lesion alone did not cause a significant difference in the group means. Note that although some lesions included dorsal peduncular cortex (DPC, as in Fig. 6), a tracing study (Montaron et al, 1996, which we confirmed using CTB) showed that few cells in this structure project to the NAc ipsilaterally, whereas the vmPFC heavily projects to NAc from both sides. Therefore inclusion of the DPC in vmPFC lesions is unlikely to play a major role in the cFos counts in the NAc. We thus hypothesized that excitatory (presumably glutamatergic) cells in the vmPFC were being activated by DMI – indirectly or directly – and then stimulating cells in the NAc.
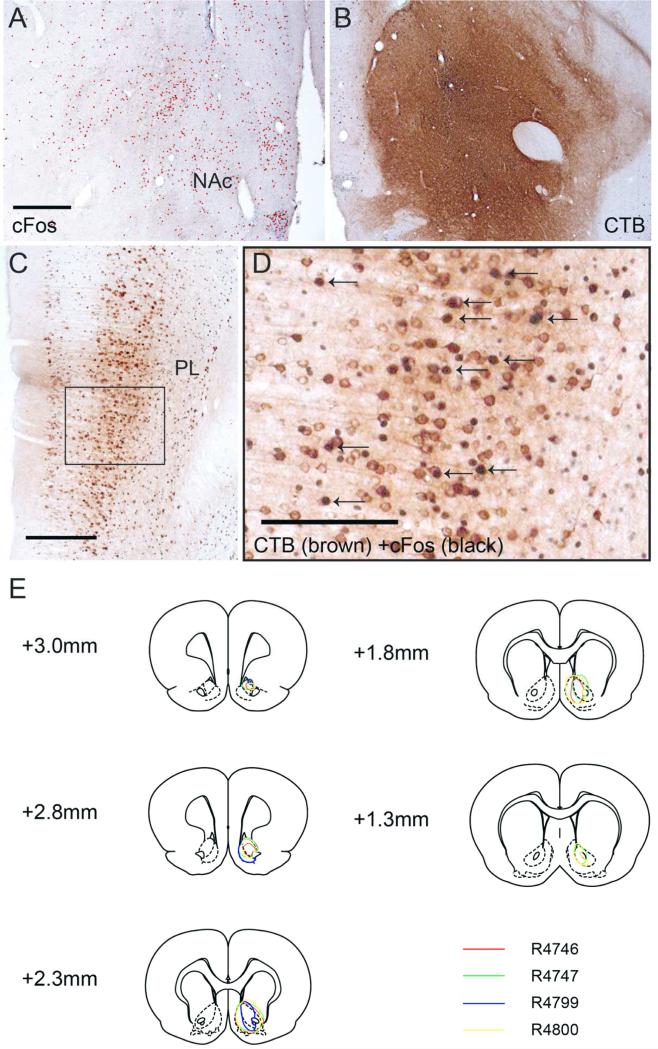
To investigate whether the vmPFC neurons activated by DMI project to the NAc, we injected the retrograde tracer cholera toxin subunit B (CTB) unilaterally into the NAc of the animals (n=4; Fig. 7). After surgical recovery, we administered DMI as before and stained brain tissue for both CTB and cFos. We specifically looked for cells in the vmPFC that had cytoplasmic staining for CTB (brown, DAB) and nuclear cFos stain (black, DAB with Ni and Co), which would indicate that they were activated by DMI and project to the NAc. As before, DMI injection resulted in increased cFos staining in the NAc (Fig. 7A) and vmPFC (Fig. 7C). Of the cell bodies in the vmPFC stained with CTB, 12.6±2.1% had cFos-labeled nuclei on the ipsilateral side (Fig. 7D). These results support our hypothesis that DMI activates the vmPFC-NAc pathway, which may thus be involved in the regulation of mood and antidepressive effects of antidepressant drugs.
Figure 7.
B Retrograde tracer CTB was unilaterally injected into the NAc (Bregma + 2.0mm), and upon postsurgical recovery the animal was injected with DMI (10:00am, 10 mg/kg) and sacrificed (12:00pm). A indicates opposite side that did not receive injection. C Many cell bodies were stained brown in the vmPFC, particularly layer V (Bregma +3.2mm). The black arrows in D point to CTB-labeled neurons that are also cFos positive, indicating neurons activated by DMI that project to the NAc. Scale bars in A, C and D indicate 0.5mm, 0.5mm and 0.25mm. E. Retrograde tracer (CTB) injections into the NAc of four animals. Labels indicate distance from Bregma.
Discussion
Although antidepressant medications have been used for decades, how and where they work in the brain is unclear. In this study, we found that acute administration of three different types of antidepressant drugs, the tricyclic antidepressant DMI, selective serotonin reuptake inhibitor FLX, and NMDA-antagonist KET selectively activate a common set of structures in the rat prefrontal cortex, specifically the ventromedial prefrontal cortex (vmPFC, prelimbic and infralimbic cortex), and in particular the vmPFC deep layers. These deep layer putative glutamatergic neurons project to the nucleus accumbens, basolateral amygdala and lateral septum, suggesting that specific neurons within the vmPFC may be important in mediating antidepressant effects via these sites. Of these potential sites, we found that the NAc receives inputs from the DMI-activated neurons in the vmPFC, and that NAc activation by DMI is dependent on the vmPFC.
The rodent vmPFC is a growing focus of depression research because of a possible homology (based on similar projection patterns) with the human subgenual prefrontal cortex or Broadmann area 25 (Gabbott et al, 2003; Hamani et al, 2010), which has been implicated in depression (Chiba et al, 2001; Vertes, 2003). Chronic social defeat stress resulted in decreased levels of Zif268 transcripts in the rat infralimbic cortex (Covington et al, 2005), suggesting that the vmPFC may be impaired in this animal model of depression. More recently, deep brain stimulation (DBS) in rats (Hamani et al, 2010) and optogenetic stimulation in mice (Covington et al, 2010) have been performed that target the vmPFC. DBS decreased FST immobility, anxiety, and the effects of footshock stress. Vialou and colleagues demonstrate that CCK likely released by GABAergic interneurons produces low neuronal activity in the vmPFC by social defeat (Vialou et al., 2014). Our recent study (Chang et al, 2014) shows that lesions of the vmPFC but not the dmPFC reduce REM sleep latency and increase REM sleep amounts and immobile time in forced swim test (FST) in rats. These REM sleep changes are in line with human MDD. Altogether, these findings suggest that the vmPFC may be important in both the expression of depression-like behaviors and the mechanism of antidepressant therapies.
In contrast to our conclusions, Hamani et al propose that DBS had an inhibitory effect on the vmPFC to result in these behavioral outcomes. The disparity may stem from the difficulty in parsing the effect of DBS in the brain, as the technique does not distinguish between acting on excitatory and inhibitory cells, in contrast to our technique of excitoxic neuron-specific lesions as referenced below. In addition, although Slattery et al and Scopiho et al performed studies demonstrating that inhibition of the infralimbic or prelimbic cortices have antidepressant-like effects, respectively, they either do not provide visual histological verification of their targets(Slattery et al, 2011) or they provide images suggesting structural damage to the mPFC (including the dorsal regions) upon cannula insertion (Scopinho et al, 2010). It is therefore difficult to attribute their results exclusively to the administration of inhibitory agents.
The vmPFC, as opposed to the dmPFC, has many connections to limbic areas of the brain that are involved in the control of emotion and mood (Sesack et al, 1989). Specifically, the deep layers of the vmPFC project to subcortical limbic regions (Felleman and Van Essen, 1991), and activation of the deep-layer cells may increase activation of the downstream limbic structures. Of these vmPFC-projecting limbic sites, the NAc was the only one that showed significant activation with DMI compared to saline-injected controls. The NAc normally plays a role in reward and motivated behavior to both conditioned and unconditioned stimuli, and NAc dysfunction is thought to be involved in the MDD symptom of anhedonia (Nestler and Carlezon, 2006). Our finding that DMI activates the NAc via the vmPFC indicates that this circuit may be particularly important in antidepressant action. In contrast, we did not detect drug-induced changes in cFos expression in the insular cortex, lateral septum or basolateral amygdala that receive projections from the vmPFC. However, vmPFC lesions did not eliminate the known effect of DMI on complete suppression of REM sleep (Chang and Lu, unpublished), suggesting that there are other sites involved in REM sleep effects of antidepressants.
DMI is a reuptake inhibitor of norepinephrine (NE), while FLX is a serotonin (5-HT) reuptake inhibitor, and both NE and 5-HT may play a role in activating deep-layer vmPFC. DMI may act through presynaptic alpha-2 receptors, moderating NE release and altering activity directly (Garcia et al, 2004) or indirectly via cortical GABAergic interneurons (Andrews and Lavin, 2005), resulting in disinhibition of vmPFC pyramidal neurons. FLX may act directly on the 80% of PFC pyramidal cells containing both 5-HT1A and 5-HT2C receptors (Puig and Gulledge, 2011) or indirectly via inhibition of 5-HT1A expression fast-spiking interneurons (Puig et al, 2010). Similarly, as ketamine blocks excitation by NMDA glutamate receptors, the effects of ketamine on vmPFC activity may result from disinhibition of pyramidal neurons by inhibitory interneurons (Homayoun and Moghaddam, 2007). Acting on fast NMDA receptors may explain why ketamine produces fast anti-depressive effects, compared to slow antidepressive effects of DMI and FLX acting slow transmission of NE and 5-HT. The localization of receptor subtypes may explain why layers V-VI pyramidal neurons in this region are selectively activated following administration of the drugs, while the pyramidal cells in layers II-III are not. 5-HT2C receptor mRNA expression is light in the superficial layers of the mPFC (Pompeiano et al, 1994), and there appears to be increased expression of 5-HT1A receptor mRNA in the vmPFC layer VI (Wright et al, 1995).
There are only two studies examining cFos patterns in response to single injection of antidepressants. In the first, Beck reported that DMI and FLX increased cFos expression in many structures of the cortex, striatum, hypothalamus and central amygdala as well as the locus coeruleus (Beck, 1995). These cFos expression patterns of arousal state are different from ours. It is possible that other factors, such as prior wakefulness, may contribute to and mask the effects of drugs. In another recent paper by Miyata and colleagues (Miyata et al, 2005), cFos expression was observed following FLX administration (5 mg/kg and 10 mg/kg) in the NAc shell and core, but only increased cFos expression in the NAc shell reaches statistical significance. They also reported high cFos expression in the cingulate cortex. However, the location of the cingulate cortex in their study is in fact the prelimbic cortex (see Fig. 1). In addition, it is unclear, based on the counting boxes used in these studies if cFos was induced in the same deep layers of vmPFC area as in our study. Nevertheless, the Miyata et al study is mostly consistent with our results.
In summary, we demonstrate that three different classes of antidepressants all convergently and selectively target a common brain region, the vmPFC, which has been implicated in human and rodent studies in the pathophysiology of MDD. Our study suggests that the vmPFC-NAc pathway is a key circuit for the mechanism of action for DMI, which works similarly to multiple classes of major antidepressants. In the future, it will be imperative to investigate how an acute response in neuronal activity to antidepressant administration develops into a chronic behavioral response, and whether this antidepressant effect is dependent on neuroplasticity in the vmPFC and/or NAc.
Acknowledgements
The authors thank Quan Ha and Xi Chen for their technical assistance in preparing this work.
Funding
This research was supported by NIH Training Grant HL007901, NS062727 and NS061841.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors report no financial interests or potential conflicts of interest.
References
- Andrews GD, Lavin A. Methylphenidate Increases Cortical Excitability via Activation of Alpha-2 Noradrenergic Receptors. Neuropsychopharmacology. 2005;31:594–601. doi: 10.1038/sj.npp.1300818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, Luca MA De, Chiara G Di. Differential Expression of Motivational Stimulus Properties by Dopamine in Nucleus Accumbens Shell versus Core and Prefrontal Cortex. Journal of Neuroscience. 2002;22:4709–4719. doi: 10.1523/JNEUROSCI.22-11-04709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CH. Acute treatment with antidepressant drugs selectively increases the expression of c-fos in the rat brain. Journal of Psychiatry & Neuroscience. 1995;20:25–32. [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Vincent SL. Changes in dendritic spine morphology in response to increased availability of monoamines in rat medial prefrontal cortex. Synapse. 1991;9:235–237. doi: 10.1002/syn.890090311. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. BPS. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Borsini F, Bendotti C, Velkov V, Rech R, Samanin R. Immobility test: effects of 5-hydroxytryptaminergic drugs and role of catecholamines in the activity of some antidepressants. J Pharm Pharmacol. 1981;33:33–37. doi: 10.1111/j.2042-7158.1981.tb13697.x. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- Brown SM. Mild, Short-term Stress Alters Dendritic Morphology in Rat Medial Prefrontal Cortex. Cerebral Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Chana G, Landau S, Beasley C, Everall IP, Cotter D. Two-dimensional assessment of cytoarchitecture in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia: evidence for decreased neuronal somal size and increased neuronal density. Biol Psychiatry. 2003;53:1086–1098. doi: 10.1016/s0006-3223(03)00114-8. [DOI] [PubMed] [Google Scholar]
- Chang CH, Chen MC, Qiu MH, Lu J. Ventromedial prefrontal cortex regulates depressive-like behavior and rapid eye movement sleep in the rat. Neuropharmacology. 2014;86C:125–132. doi: 10.1016/j.neuropharm.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T, Kayahara T, Nakano K. Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Research. 2001;888:83–101. doi: 10.1016/s0006-8993(00)03013-4. [DOI] [PubMed] [Google Scholar]
- Conti B, Maier R, Barr AM, Morale MC, Lu X, Sanna PP, et al. Region-specific transcriptional changes following the three antidepressant treatments electro convulsive therapy, sleep deprivation and fluoxetine. Molecular Psychiatry. 2006;12:167–189. doi: 10.1038/sj.mp.4001897. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. Journal of Neurobiology. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Covington HE, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Covington HE, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, et al. Antidepressant Effect of Optogenetic Stimulation of the Medial Prefrontal Cortex. Journal of Neuroscience. 2010;30:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology. 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Functional neuroimaging studies of depression: the anatomy of melancholia. Annual review of medicine. 1998;49:341–361. doi: 10.1146/annurev.med.49.1.341. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin E, Treit D, Dickson CT. Anxiolytic- and antidepressant-like properties of ketamine in behavioral and neurophysiological animal models. Neuroscience. 2009;161:359–369. doi: 10.1016/j.neuroscience.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Essen DC Van. Distributed hierarchical processing in the primate cerebral cortex. Cerebral cortex (New York, NY: 1991) 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Warner TA, Jays PRL, Bacon SJ. Areal and synaptic interconnectivity of prelimbic (area 32), infralimbic (area 25) and insular cortices in the rat. Brain Research. 2003;993:59–71. doi: 10.1016/j.brainres.2003.08.056. [DOI] [PubMed] [Google Scholar]
- Garcia AS, Barrera G, Burke TF, Ma S, Hensler JG, Morilak DA. Autoreceptor-mediated inhibition of norepinephrine release in rat medial prefrontal cortex is maintained after chronic desipramine treatment. Journal of neurochemistry. 2004;91:683–693. doi: 10.1111/j.1471-4159.2004.02748.x. [DOI] [PubMed] [Google Scholar]
- Garcia LSB, Comim CM, Valvassori SS, Réus GZ, Barbosa LM, Andreazza AC, et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Progress in neuropsychopharmacology & biological psychiatry. 2008;32:140–144. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Guillery RW. On counting and counting errors. J Comp Neurol. 2002;447:1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- Hamani C, Diwan M, Macedo CE, Brandão ML, Shumake J, Gonzalez-Lima F, et al. Antidepressant-Like Effects of Medial Prefrontal Cortex Deep Brain Stimulation in Rats. BPS. 2010;67:117–124. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA Receptor Hypofunction Produces Opposite Effects on Prefrontal Cortex Interneurons and Pyramidal Neurons. Journal of Neuroscience. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. The Journal of Comparative Neurology. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-Dependent Synapse Formation Underlies the Rapid Antidepressant Effects of NMDA Antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng LY, Shors TJ. The stressed female brain: neuronal activity in the prelimbic but not infralimbic region of the medial prefrontal cortex suppresses learning after acute stress. Front Neural Circuits. 2013;7:198. doi: 10.3389/fncir.2013.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, et al. Regional metabolic effects of fluoxetine in major depression: Serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Miyata S, Hamamura T, Lee Y, Miki M, Habara T, Oka T, et al. Contrasting Fos expression induced by acute reboxetine and fluoxetine in the rat forebrain: neuroanatomical substrates for the antidepressant effect. Psychopharmacology. 2005;177:289–295. doi: 10.1007/s00213-004-2072-7. [DOI] [PubMed] [Google Scholar]
- Montaron MF, Deniau JM, Menetrey A, Glowinski J, Thierry AM. Prefrontal cortex inputs of the nucleus accumbens-nigro-thalamic circuit. Neuroscience. 1996;71:371–382. doi: 10.1016/0306-4522(95)00455-6. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. BPS. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Waltham, MA: 2007. [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Molecular brain research. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Puig MV, Gulledge AT. Serotonin and Prefrontal Cortex Function: Neurons, Networks, and Circuits. Molecular Neurobiology. 2011;44:449–464. doi: 10.1007/s12035-011-8214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig MV, Watakabe A, Ushimaru M, Yamamori T, Kawaguchi Y. Serotonin Modulates Fast-Spiking Interneuron and Synchronous Activity in the Rat Prefrontal Cortex through 5-HT1A and 5-HT2A Receptors. Journal of Neuroscience. 2010;30:2211–2222. doi: 10.1523/JNEUROSCI.3335-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvirenti L, Samanin R. Antagonism by dopamine, but not noradrenaline receptor blockers of the anti-immobility activity of desipramine after different treatment schedules in the rat. Pharmacological Research Communications. 1986;18:73–80. doi: 10.1016/0031-6989(86)90160-8. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo J, Wei J, Dilley G, Pittman S, Meltzer H, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. BPS. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Sairanen M, O'Leary OF, Knuuttila JE, Castrén E. Chronic antidepressant treatment selectively increases expression of plasticity-related proteins in the hippocampus and medial prefrontal cortex of the rat. Neuroscience. 2007;144:368–374. doi: 10.1016/j.neuroscience.2006.08.069. [DOI] [PubMed] [Google Scholar]
- Scopinho AA, Scopinho M, Lisboa SF, Aguiar Correa FM de, Guimarães FS, Joca SRL. Acute reversible inactivation of the ventral medial prefrontal cortex induces antidepressant-like effects in rats. Behavioural Brain Research. 2010;214:437–442. doi: 10.1016/j.bbr.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. The Journal of Comparative Neurology. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID, Cryan JF. Transient inactivation of the infralimbic cortex induces antidepressant-like effects in the rat. Journal of Psychopharmacology. 2011;25:1295–1303. doi: 10.1177/0269881110368873. [DOI] [PubMed] [Google Scholar]
- Uylings HBM, Eden CG Van. The prefrontal cortex: its structure, function, and pathology: Proceedings of the 16th International Summer School of Brain Research. 1990;85 [Google Scholar]
- Uylings HBM, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behavioural Brain Research. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2003;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Wang L, Paul N, Stanton SJ, Greeson JM, Smoski MJ. Loss of sustained activity in the ventromedial prefrontal cortex in response to repeated stress in individuals with early-life emotional abuse: implications for depression vulnerability. Front Psychol. 2013;4:320. doi: 10.3389/fpsyg.2013.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. The Journal of Comparative Neurology. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]