Abstract
Molecular and cellular mechanisms underlying the peripheral conditioning lesion remain unsolved. We show here that injection of a chemical demyelinating agent, ethidium bromide, into the sciatic nerve induces a similar set of regeneration-associated genes and promotes a 2.7-fold greater extent of sensory axon regeneration in the spinal cord than sciatic nerve crush. We found that more severe peripheral demyelination correlates with more severe functional and electrophysiological deficits, but more robust central regeneration. Ethidium bromide injection does not activate macrophages at the demyelinated sciatic nerve site, as observed after nerve crush, but briefly activates macrophages in the dorsal root ganglion. This study provides a new method for investigating the underlying mechanisms of the conditioning response and suggests that loss of the peripheral myelin may be a major signal to change the intrinsic growth state of adult sensory neurons and promote regeneration.
Introduction
Peripheral conditioning lesion refers to the phenomenon that an initial peripheral nerve injury induces an intrinsic growth state in dorsal root ganglia (DRG) sensory neurons. This growth state can increase the rate of regeneration after a subsequent peripheral injury (McQuarrie and Grafstein, 1973), and, incredibly, drive regeneration of the normally quiescent central branch within the central nervous system (CNS) (Richardson and Issa, 1984). The expression of many genes undergo changes caused by peripheral nerve injury, some of which may be responsible for the acquired capacity for regeneration (Hoffman and Cleveland, 1988; Tsujino et al., 2000; Costigan et al., 2002; Seijffers et al., 2007; Stam et al., 2007). While some identified cues can increase regeneration, none have been shown to fully mimic the growth-promoting effects of the nerve crush (Qiu et al., 2002; Seijffers et al., 2007; Parikh et al., 2011).
Sciatic nerve crush leads to axon damage and loss of myelin (Gupta et al., 2004). Peripheral demyelination results in clearance of myelin debris by macrophages, Schwann cell dedifferentiation and proliferation, followed in time by eventual remyelination of spared or regenerated peripheral axons (Hall, 1973; Riet-Correa et al., 2002). After chemical demyelination, peripheral axons sprout small branches that associate with proliferating Schwann cells (Hall, 1973). We propose that this sprouting is a correlate of axon regeneration and asked whether peripheral injection of demyelinating agents results in regeneration after spinal cord injury. We hypothesize that demyelination may be a major component of the conditioning lesion effect that drives the increase of intrinsic growth potential. We tested this hypothesis utilizing two distinct demyelinating agents, the intercalating agent ethidium bromide (EtBr) and the detergent lysolecithin (lysophosphatidylcholine, LPC) that both result in the breakdown of the myelin sheath through progressive vesiculation (Allt et al., 1988; Riet-Correa et al., 2002). We show here that peripheral injection of EtBr produces a much greater conditioning response than nerve crush, resulting in dramatically increased spinal cord regeneration. Additionally, injection of EtBr or LPC, in the absence of concurrent axotomy, induces gene changes in the DRG characteristic of peripheral conditioning. Unlike nerve crush, EtBr injection does not induce macrophage activity in the demyelinated region of the sciatic nerve over the period of observed peripheral conditioning. Nor does it induce a sustained macrophage activation in the DRG compared to nerve crush. This suggests that either inflammation is an early component of peripheral conditioning lesion, or a divergence in the mechanisms of EtBr-mediated and nerve crush-mediated peripheral conditioning.
Materials and Methods
Surgical procedures
Sciatic nerve crush
All animal work in this research was approved by the University of California, San Diego Institutional Animal Care and Use Committee. Unilateral or bilateral sciatic nerve crush was performed on adult female Fischer 344 rats (120-135g) unresponsive to toe or tail pinch under isoflurane anaesthetic. An area over the hindlimb was shaved and cleaned with povidone-iodine before incision caudal and parallel to the femur. The sciatic nerve was exposed and crushed for 10s with a pair of fine (#55) forceps. After crush, the skin was closed with surgical staples.
Sciatic nerve injection
Unilateral or bilateral sciatic nerve injection was performed on adult female Fischer 344 rats (120-135g) unresponsive to toe or tail pinch under isoflurane anaesthetic. The sciatic nerve was exposed as above and injected with 4μl (2μl/branch) 1%wt/vol LPC in PBS with 10%vol/vol DMSO, 2μl (1μl/branch) of 0.01%, 0.05% or 0.1%wt/vol EtBr in PBS or PBS alone. Injections were made longitudinally towards the DRG, with a 36ga NanoFil needle (World Precision Instruments Inc., Sarasota, FL) at a rate of 2μl/min and the needle was held in place for an additional 10s following injection. After injection, the skin was closed with surgical staples.
C4 dorsal column injury
Animals were deeply anaesthetized with 2ml/kg of ketamine cocktail (25mg/ml ketamine, 1.3mg/ml xylazine and 0.25mg/ml acepromazine). Spinal level C4 was exposed by laminectomy and the dura was punctured over the dorsal horn, approximately 1.2mm lateral to midline. A Scouten wire-knife (David Kopf Instruments, Tujunga, CA) was lowered to a depth of 1mm from the surface of the spinal cord and extruded. The dorsal columns were lesioned with two passes of the wire-knife and the intact dura was pressed against the wire edge of the lifted wire-knife to ensure complete transection of ascending dorsal column sensory axons. The lesion cavity was filled with approximately 200,000 syngeneic bone marrow stromal cells (BMSCs) suspended in PBS [100,000cells/μl], injected using glass micropipettes connected to a picospritzer (General Valve, Fairfield, NJ) as previously described (Hollis II and Zou, 2012). Primary BMSCs were isolated from adult female Fischer 344 rats by flushing cells out with DMEM with 10% FBS and Pen/Strep/Glu, cells were cultured in the same media and passaged at 80% confluence. The dorsal musculature was sutured with 4-0 silk sutures and the skin was closed with surgical staples. Three days prior to sacrifice, animals were injected bilaterally into the sciatic nerve with a 1%wt/vol solution of the transganglionic tracer cholera toxin B (CTB; List Biological Laboratories, Campbell, CA) in dH2O using a 36ga NanoFil syringe. The sciatic nerve was exposed as described above and 1μl was injected into each of the tibial and common peroneal branches bilaterally (4μl total).
Sacrifice and tissue processing
Animals were deeply anaesthetized with ketamine cocktail and transcardially perfused with ice-cold PBS followed by 4%wt/vol paraformaldehyde in PBS. Spinal columns, sciatic nerves, DRGs and tibialis anterior muscles were post-fixed overnight at 4°C in 4%wt/vol paraformaldehyde. Tissue was transferred to 30%wt/vol sucrose in PBS for cryoprotection and sectioned on a cryostat (Leica, Buffalo Grove, IL) at 20μm (DRGs, sciatic nerves) or 30μm (muscle) and mounted directly on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA). Grafted spinal cords were sectioned sagittally at 40μm thick and collected as free-floating sections. Sections were washed three times with PBS, blocked for one hour at room temperature (RT) in PBS with 0.25% triton-X100 (PBST) and 5% donkey serum, then incubated overnight (except where noted below) at 4°C with primary antibodies in PBST plus 5% donkey serum. On the second day of staining, sections were washed three times, incubated with Alexa Fluor conjugated secondary antibodies (Life Technologies, Grand Island, NY; Jackson ImmunoResearch, West Grove, PA) for 2.5hrs at RT, counterstained with DAPI [1 g/ml] (Sigma-Aldrich, St. Louis, MO) and washed three final times in PBS. For Frizzled2 immunohistochemistry, antigen retrieval with ice-cold acetone for 10 minutes prior to staining was utilized. Antibodies used for fluorescent immunohistochemistry were: goat anti-CTB [1:10,000; 3-day incubation] (List Biological Laboratories, Cat# 703, RRID:AB_10013220), monoclonal N52 anti-NF200 [1:500] (Sigma-Aldrich, Cat# N0142, RRID:AB_477257), rat anti-Frizzled2 [1:50] (R&D Systems, Minneapolis, MN, Cat# MAB1307-050, RRID:AB_2109221), rabbit anti-GFAP [1:750] (Dako, Carpinteria, CA, Cat# Z0334, RRID:AB_10013382), rabbit anti-cJun [1:100] (Cat# 9165, RRID:AB_2130165) and rabbit anti-pSmad [1:100] (Cell Signaling, Danvers, MA, Cat# 9516S, RRID:AB_491015), rabbit anti-ATF3 [1:200] (Cat# sc-188, RRID:AB_2258513), goat anti-arginase I [1:200] (Cat# sc-18354, RRID:AB_2227469) and rabbit anti-synaptophysin [1:100] (Santa Cruz Biotechnology, Santa Cruz, CA, Cat# sc-9116, RRID:AB_2199007), rabbit anti-Iba1 [1:1500] (Wako Chemicals USA, Inc., Richmond, VA, Cat# 019-19741, RRID:AB_839504), monoclonal ED1 anti-CD68 [1:500] (AbD Serotec, Raleigh, NC, Cat# MCA341R, RRID:AB_2291300), monoclonal 24F anti-CD86 [1:100] (BD Biosciences, San Jose, CA, Cat# 555016, RRID:AB_395648), Isolectin B4 biotin conjugated [1:100] (Sigma-Aldrich, Cat# L2140), tetramethylrhodamine-conjugated α-bungarotoxin [1:1500] (Life Technologies, Cat# T-1175).
Myelin staining
Myelin staining was performed as described (Schmued, 1990). Briefly, sections washed twice in dH2O then twice in 0.025M PB with 0.9% NaCl (working solution) were incubated for 1hr at RT in 0.2%wt/vol gold (III) chloride solution with 0.0075%vol/vol H2O2 in working solution. Sections were washed twice more in working solution, then incubated for 5min in 5% sodium thiosulfate at room temperature before three final washes in dH2O.
DRG neuron culture
Rats were deeply anaesthetized with ketamine cocktail, L4 and L5 DRGs were surgically removed, and the animals were decapitated. DRGs were collected in ice-cold L15 medium then digested for 1hr at 37°C in collagenase type XI (1:1, DMEM/F-12:0.5% collagenase XI in L15 medium; Worthington Biochemical Corp., Lakewood, NJ) with gentle agitation every 15min. Cells were centrifuged 2min at 3000rpm, collagenase solution was removed and gently replaced with DMEM/F-12 + 10% FBS so as not to disturb the cell pellet. Cells were then washed twice by gently applying DRG culture medium (DMEM/F-12 with B-27 supplement and Pen/Strep/Glu) so as not to disturb the pellet. Cells were resuspended in DRG culture medium, allowed to settle for 45s then the top cell suspension was added to DRG culture medium, leaving behind the sedimented tissue fragments. DRG cell suspension was then plated on poly-D-lysine coated cell culture dishes. 16 hours after plating, cells were fixed with 4% PFA in PBS for 30min at RT, washed 3 times in PBS and stained with monoclonal N52 anti-NF200 as described above for free-floating sections.
Image acquisition and analysis
Images were acquired on an inverted Zeiss LSM510 confocal microscope with LSM acquisition software (Carl Zeiss Microscopy, LLC, Thornwood, NY). Ethidium bromide fluorescence emission at 590nm was imaged on a Zeiss LSM510. An Axiovert 40 CFL with an AxioCam MRm and AxioVision software (Carl Zeiss Microscopy, LLC) was used to image gold (III) chloride myelin staining of sciatic nerves. For neurite length quantification images of NF200-immunoreactive DRG neurons were acquired with PictureFrame software for a MicroFire digital camera (Optronics, Goleta, CA) mounted on an upright fluorescent microscope (Olympus, Center Valley, PA). The NeuronJ plugin for ImageJ (NIH, Bethesda, MD) was used for longest neurite tracing and quantification on acquired images (Meijering et al., 2004). Image density quantification was done on thresholded images using ImageJ (NIH, Bethesda, MD). ImageJ was used to measure myelin density along a 100μm thick tracing of thresholded panoramic images stitched together in Photoshop CS5 (Adobe, San Jose, CA). NF200 ratios were calculated by comparing ImageJ density measurements of thresholded and skeletonized (linearized) NF200 images 1.5mm distal and proximal to the sciatic injury site. For neuromuscular junction (NMJ) innervation, 100 tetramethylrhodamine-conjugated α-bungarotoxin labeled NMJs per animal were examined, in tibialis anterior muscles, for apposition by synaptophysin-labeled pre-synaptic terminals. Statistical tests indicated in main text were performed using JMP 9 software (SAS Institute, Cary, NC). An investigator blinded to the experimental group performed all analyses.
Behavioral testing
Animals were trained on accelerating rotarod (5rpm initial, 45rpm max, 300s max; Stoelting, Wood Dale, IL) and beam crossing (1inch diameter, 60inch span, 3 passages per trial) for two weeks prior to bilateral sciatic injection of 0.1%wt/vol EtBr, 1%wt/vol LPC, or sciatic nerve crush. For toe spread analysis, hind paw plantar surfaces were dipped in povidone-iodine solution and animals walked on paper in a narrow path between two barriers. The mean distance between the first and fifth toes from three prints was averaged bilaterally. Animals were tested prior to sciatic injury and weekly for 16 weeks after spinal cord injury by investigators blind to the experimental groups.
Paw 50% withdrawal threshold (PWT) was assessed bilaterally using a series of calibrated von Frey filaments, (Kom Kare, Middletown, OH, USA) using the up-down method exactly as described elsewhere(Chaplan et al., 1994; Calcutt, 2004). Thermal withdrawal latency was assessed bilaterally using a Hargreaves apparatus (UARDG, Department of Anesthesiology, University of California, San Diego; La Jolla, CA). Prior to testing, rats were acclimated in individual acrylic chambers on a glass surface that was maintained at 30°C. After an initial acclimation measurement, the median of three subsequent measurements tested at a heating rate of 1°C/sec with a maximal cut off at 20 sec was taken as the thermal response latency for that paw, and the mean of values of both paws was used to represent each animal.
Electrophysiology
For measurement of motor nerve conduction velocity (MNCV), rats were anesthetized with isoflurane and nerve temperature maintained at 37°C using a heating lamp and near-nerve thermistor. The sciatic nerve was stimulated (single 5 V, 0.05 ms square wave pulse) by fine needle electrodes placed at the sciatic notch and Achilles tendon and the evoked electromyograms (EMGs) recorded from the interosseus muscles of the ipsilateral foot via two fine needle electrodes. MNCV was calculated by dividing distance between the two stimulation sites by the time difference between their associated M wave peaks.
Results
Peripheral injection of the chemical demyelinating agent ethidium bromide promotes more robust axon regeneration than nerve crush
In this study, we tested the peripheral conditioning effects of two known demyelinating agents that do not result in peripheral axon degeneration (Hall, 1973; Riet-Correa et al., 2002): ethidium bromide (EtBr), and lysophosphatidylcholine (lysolecithin, LPC). Animals received a sham operation, bilateral sciatic nerve crush, bilateral PBS injection, bilateral LPC, or bilateral EtBr injection in the sciatic nerve. One week later, we lesioned the dorsal columns at cervical level 4 (C4) and grafted naïve bone marrow stromal cells (BMSCs). One month later, bilateral sciatic injection of cholera toxin B subunit (CTB) was used to assess regeneration into BMSC grafts. BMSC grafts fill the lesion cavity, preventing cystic cavitation, and allow for clear determination of regenerated axons as those that have grown into the graft. Peripheral nerve crush resulted in a nearly 3.7-fold increase in the number of regenerated axon profiles (axon segments counted in every seventh sagittal section and normalized to total CTB axons below the injury) within BMSC grafts compared to sham operated animals (Figure 1). Demyelination with EtBr augmented axon regeneration much more robustly, with a 7.1-fold increase over PBS injected and 9.9-fold increase over sham operated animals (Figure 1). The regenerative response induced by EtBr was 2.7-fold greater than the response to sciatic nerve crush (ANOVA P<0.0001, Bonferroni corrected post-hoc t-test P<0.0001). The number of regenerated axon profiles in LPC injected animals was not significantly higher than PBS injected or sham controls.
Figure 1. EtBr injection promotes robust regeneration after spinal cord injury.
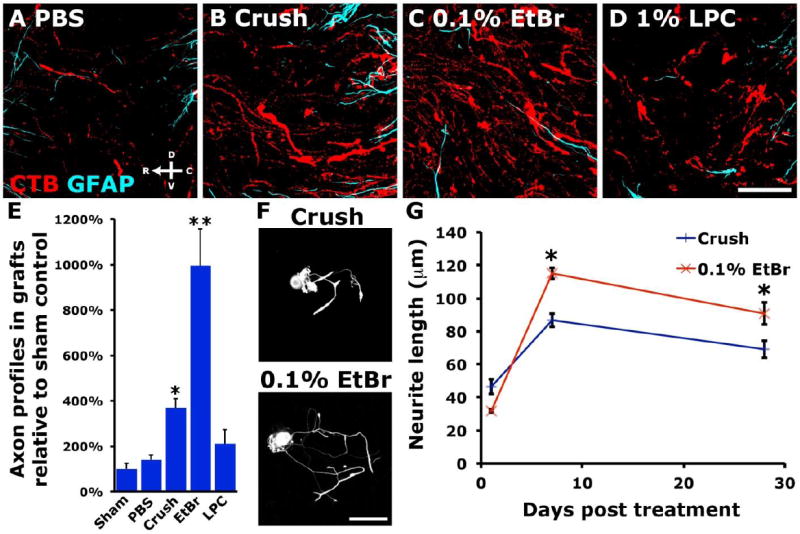
Regeneration of CTB-labeled DRG axons into BMSC grafts one month after C4 lesion. Control, sham operated animals or those injected in the sciatic nerve with PBS (A) show little regeneration of ascending dorsal column sensory axons. The significant regeneration observed after peripheral nerve crush (B) is dwarfed by the regeneration induced by 0.1%wt/vol EtBr injection (C). (D) Injection of 1%wt/vol LPC has a more limited effect than EtBr on sensory axon regeneration. (E) Quantification of CTB-labeled regenerated axon profiles shows a 7.1-fold increase in regeneration of EtBr treated neurons over PBS controls, 2.7-fold more regenerated axons than observed after peripheral nerve crush (ANOVA P<0.0001, Bonferroni corrected post-hoc t-test *P<0.05, **P<0.0001; n=5 (Sham, LPC), 6 (PBS, EtBr, Crush)). (F, G) 0.1%wt/vol EtBr sciatic injection promotes greater neurite outgrowth than contralateral sciatic nerve crush when NF200+ L4 and L5 DRG neurons are dissociated and cultured at 7 or 28 days after injury (paired two-tailed t-test *P<0.05; n=3 animals/time point, >100 neurons per data point). Data presented as mean±s.e.m. Scale bars represent 50μm (A-D) and 100μm (F).
One, 7 and 28 days after sciatic nerves were treated with EtBr, L4 and L5 DRG neurons were dissociated and grown in vitro. Large-diameter, NF200-immunoreactive neurons extended significantly longer neurites when isolated from DRGs ipsilateral to demyelinated sciatic nerve, compared to contralateral crushed sciatic nerve (Figure 1; paired t-test, P<0.05).
The extent of demyelination differs between peripheral injection of LPC or EtBr and nerve crush
Injection of the demyelinating agents LPC [1%wt/vol] or EtBr [0.1%wt/vol] longitudinally along the sciatic nerve reduced myelin staining with gold (III) chloride as did nerve crush (Figure 2). The most severe demyelination occurred proximal to injection of 0.1% EtBr and at the site of nerve crush, however EtBr or LPC disrupted sciatic myelin over significantly longer distances (3.52±0.83mm and 3.41±0.81mm, respectively) than nerve crush (1.52±0.06mm), at 3 days post-injury (ANOVA P<0.001, Bonferroni corrected post-hoc t-test P<0.05). Importantly, nerve crush, and not demyelination, resulted in axotomy marked by the loss of axons 1.5mm distal to the lesion at 3 days (Figure 2). Demyelination continued to progress after EtBr injection with extensive demyelination apparent at two weeks after injection, a time at which LPC injected animals were showing some recovery of myelin (Figure 2).
Figure 2. Demyelination within the sciatic nerve.
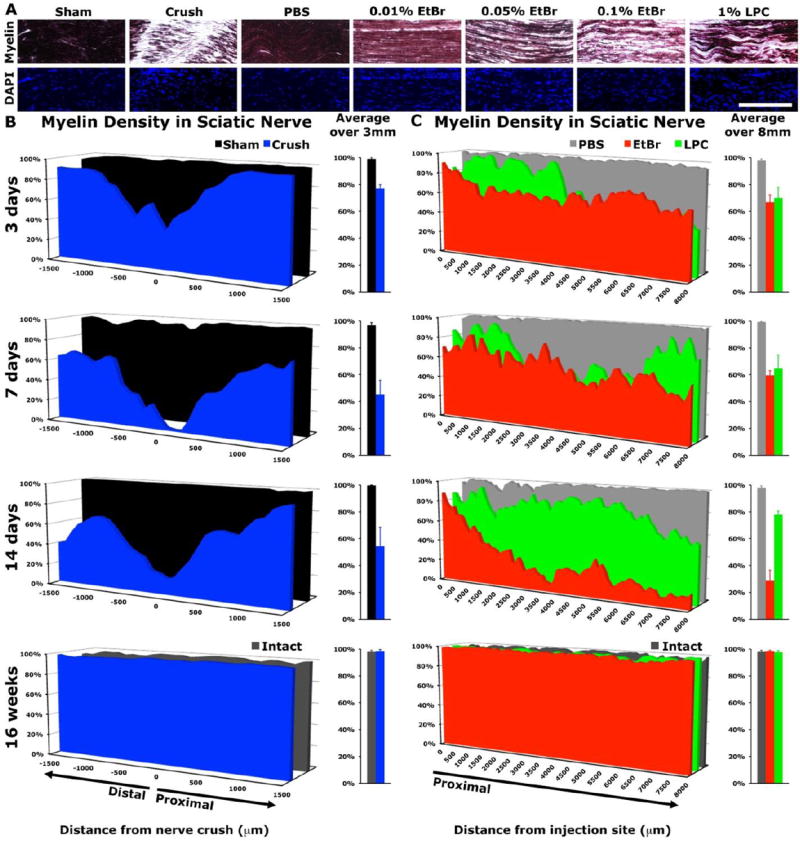
(A) Gold (III) chloride staining of longitudinal sciatic nerve sections demonstrates loss of myelin staining three days after nerve crush or injection of demyelinating agents (dark purple indicates myelin staining) (B) At the lesion epicenter, myelin density is lowest at the site of sciatic nerve crush, with considerable demyelination at 14 days distal to the injury, along the region of Wallerian degeneration (two-tailed t-test: 3 days P<0.005, 7 days P<0.001, 14 days P<0.05). (C) EtBr [0.1%wt/vol] and LPC [1%wt/vol] injected to the sciatic nerve resulted in demyelination over several millimeters proximal to the injection site (ANOVA: 3, 7 days P<0.05, 14days P<0.0001). Myelin density recovered in all groups by 16 weeks post injury. Data presented as mean (n=3/group). Scale bar represents 250μm.
We examined the integrity of the sensory and motor circuitry of the sciatic nerve after nerve crush or demyelinating agent injection. Images of large-diameter, heavy-chain neurofilament (NF200) immunoreactive axons in the sciatic nerve proximal to the injury or demyelinated region and 1.5mm distal to the crush or injection site were skeletonized (linearized) and the ratio of distal to proximal pixel density showed a dramatic reduction in axons 3 days after nerve crush (Figure 3). Additionally, nerve crush resulted in a total loss of innervation of the tibialis anterior at one week after injury, while LPC and EtBr injected animals showed pre-synaptic terminals at every neuromuscular junction (NMJ) examined (Figure 3).
Figure 3. Only nerve crush interrupted innervation distal to sciatic injury.
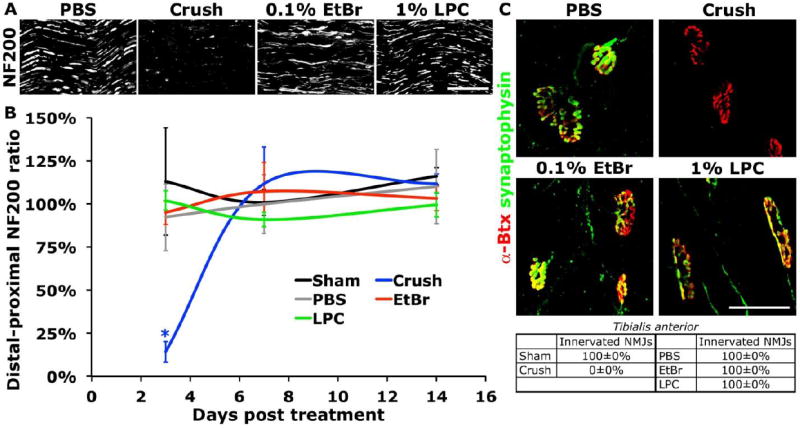
(A) NF200 immunostaining at day 3 demonstrates the disruption of sciatic axons 1.5mm distal to the nerve crush or injection site. (B) The distal:proximal ratio of skeletonized NF200 immunoreactive axons illustrates that axons distal to the injury remained intact following demyelination (ANOVA P<0.05, Dunnett’s t-test *P<0.05). (C) All neuromuscular junctions (stained with tetramethylrhodamine-conjugated α-bungarotoxin, α-Btx) in Tibialis anterior lacked pre-synaptic synaptophysin apposition at 7 days after sciatic nerve crush, but not after demyelination. Data presented as mean±s.e.m. Scale bars represent 100μm (A) and 50μm (C).
Peripheral injection of chemical demyelinating agents induces a similar set of regeneration-associated genes (RAGs) as nerve crush
Peripheral nerve crush and transection result in the induction of many RAGs in sensory neurons, with two of the most robust being ATF-3 and c-Jun (Jenkins and Hunt, 1991; Tsujino et al., 2000; Boeshore et al., 2004; Seijffers et al., 2007; Stam et al., 2007). Additionally, chemical demyelination with LPC induces expression of the RAG ATF-3 in DRG neurons in vitro (Kiryu-Seo et al., 2010). We examined whether RAG induction after injection of peripheral demyelinating agents was similar to RAG induction after nerve crush in vivo. ATF-3 and c-Jun were dramatically induced in DRG neurons three days after unilateral sciatic injection of EtBr (0.05% or 0.1%wt/vol) in adult rats, similar to sciatic nerve crush (Figure 4). A lower concentration of EtBr [0.01%wt/vol] or 1%wt/vol LPC induced expression of c-Jun and ATF-3 at lower levels than nerve crush (Figure 4). ATF-3 and c-Jun were induced in NF200-immunoreactive proprioceptive neurons, the axons of which constitute the ascending dorsal funiculi. c-Jun and ATF-3 expression persisted over two weeks in NF200-immunoreactive neurons in animals after 0.1% EtBr and nerve crush at levels significantly higher than those induced by LPC (Figure 4). Across all treatment groups and time points measured, the levels of c-Jun and ATF-3 induction in NF200-immunoreactive DRG neurons correlated tightly with the extent of remaining myelin (Spearman’s ρ = -0.730 (ATF-3 vs. average myelin density) P<0.0001, Spearman’s ρ = -0.571 (c-Jun vs. average myelin density) P<0.0001, Figure 4). Specifically, in animals injected with different concentrations of EtBr (examined at day 3), the extent of remaining myelin correlated tightly with the induction of c-Jun and ATF-3 (Spearman’s ρ = -0.741 (ATF-3 vs. average myelin density) P<0.01, Spearman’s ρ = -0.641 (c-Jun vs. average myelin density) P<0.05, Figure S1).
Figure 4. Chemical demyelinating agents induced expression of regeneration-associated genes.
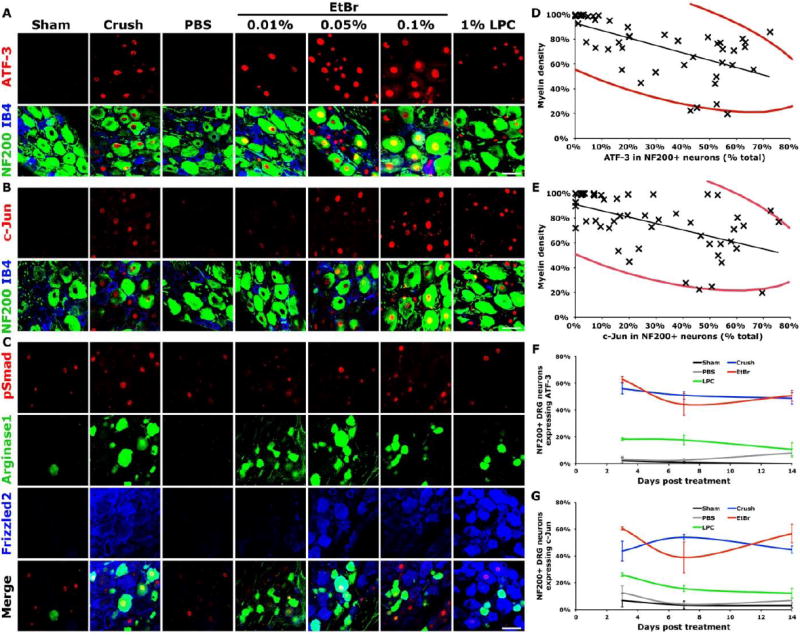
At three days after treatment, numerous RAGs are induced by nerve crush or peripheral injection of either EtBr or LPC. EtBr (0.01%, 0.05% and 0.1%wt/vol in PBS) and 1%wt/vol LPC in PBS with 10%DMSO induce expression of (A) ATF-3, (B) c-Jun, (C) phosphorylated-Smad1/5, arginase-1 and Frizzled-2. (D, E) Decreased myelin density, independent of treatment or time post-injury, correlates strongly with the induction of (D) ATF-3 or (E) c-Jun in NF200+ DRG neurons (ATF-4: Spearman’s ρ = -0.730 P<0.0001, c-Jun: Spearman’s ρ = -0.571 P<0.0001, red ellipses represent 95% bivariate normal density). (F) ATF-3 and (G) c-Jun expression in NF200+ neurons after 0.1%wt/vol EtBr injection was similar to that after sciatic crush (ATF-3: ANOVA at 3, 7 and 14 days P<0.0001, c-Jun: ANOVA at 3, 7 and 14 days P<0.0005). Data presented as mean±s.e.m. Scale bars represent 50μm, n = 3/group.
In addition to ATF-3 and c-Jun, injection of peripheral demyelinating agents induced expression of arginase-1, and components of the morphogen signaling pathways of bone morphogenic protein and Wnt in the DRG, similar to peripheral nerve crush (Figure 4) (Boeshore et al., 2004; Zou et al., 2009; Hollis II and Zou, 2012). We found that the highest concentration of EtBr tested, 0.1%wt/vol in PBS, most closely mimicked sciatic nerve crush in terms of RAG gene expression. It should be noted that EtBr and LPC at these concentrations induce Schwann cell myelin rejection but not Schwann cell death (Hall and Gregson, 1971; Allt et al., 1988; Riet-Correa et al., 2002). EtBr was not detected in axons or DRGs, by confocal microscopy, and is unable to enter the nuclei of living cells (Beletsky and Umansky, 1990). Control injections of PBS affected neither gene expression in the DRG, nor myelin density in the sciatic nerve, similar to sham operated controls.
EtBr induces macrophage activity on a different time scale than nerve crush
Infiltrating non-resident macrophages clear myelin debris in damaged peripheral nerves (Hall and Gregson, 1971; Griffin et al., 1993; Brück, 1997). Additionally, inflammatory agents induce a form of peripheral conditioning (Lu and Richardson, 1991; Steinmetz et al., 2005) while macrophages pre-incubated with injured sciatic nerve have been shown to promote regenerative growth (Lazarov-Spiegler et al., 1996). Within the DRG, Iba1-immunoreactive macrophages are activated following sensory axon injury, surrounding large-diameter NF200 immunoreactive neurons (Ton et al., 2013). Recently, it has been proposed that activation or increase of DRG macrophages is required for conditioning-mediated pro-regenerative effects (Kwon et al., 2013; Niemi et al., 2013).
We tested whether the elevation of RAGs induced by EtBr or LPC injection is correlated with activation of resident or infiltrating macrophages. Following nerve crush, the density of Iba1 immunoreactive macrophages peaked at one week post-crush in both the DRG and the injured sciatic nerve, while ED1 reactivity of phagocytic CD68+ macrophages (Damoiseaux et al., 1994) in the injured sciatic nerve decreased from 3 to 14 days post-crush (Figure 5). In contrast, activation of Iba1 macrophages in the DRG was highest at 3 days post-injection of EtBr before dropping precipitously to control levels by 7 days (Figure 5). Additionally, despite clear differences in RAG induction, nerve crush, control PBS, or LPC injection mildly increased macrophage staining at 3 days, though not significantly compared to sham control (Dunnett’s test: crush P=0.78, PBS P=0.31, LPC P=0.15; Figure 5). High levels of DRG macrophage activation were present in both EtBr and nerve crushed animals, however macrophage activation after nerve crush peaked at 7 days, later than the 3 days after EtBr injection.
Figure 5. The inflammatory response after demyelination differs from that after nerve crush.
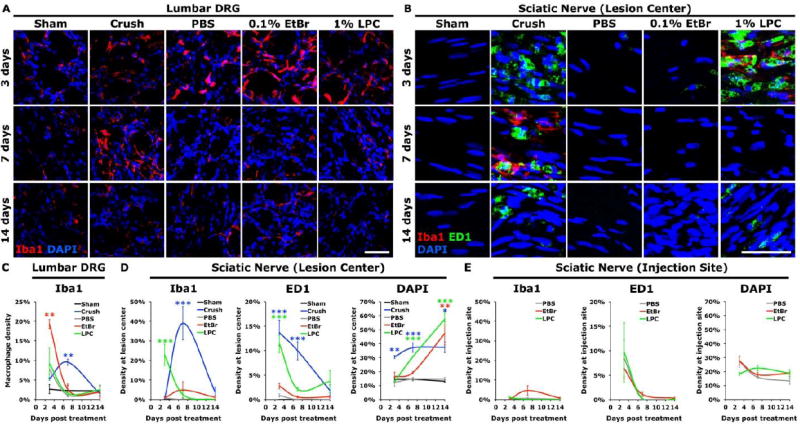
(A, C) Macrophage (Iba1) activation in DRGs was induced by nerve crush and by injection of EtBr into the sciatic nerve (ANOVA at 3 and 7d P<0.005). (B) Longitudinal sections of sciatic nerve show CD68-immunoreactive (ED1) macrophages were present at the site of nerve crush and LPC-mediated demyelination, but not significantly elevated at the epicenter of EtBr intoxication. (D) Quantification of macrophage density at the lesion epicenter show that macrophage activity was limited in EtBr injected animals relative to animals after nerve crush or LPC injection (ANOVA: Iba1 at 3, 7days P<0.0005; ED1 at 3, 7days P<0.0001; DAPI 3days P<0.001, 7, 14days P<0.0001). (E) Inflammation at the injection site was similar in LPC, EtBr and control PBS injected sciatic nerves. Data presented as mean±s.e.m. Scale bar represents 50μm. Dunnett’s test *P<0.05, **P<0.005, ***P<0.0005.
In the center of the demyelinated sciatic nerve, the density of phagocytic ED1 stained macrophages was elevated over the first 14 days after LPC injection, but was noticeably lower after EtBr injection (Figure 5). Only limited ED1 staining was present after EtBr injection, and only at 3 days post-injection (Dunnett’s P=0.22). As expected there was observable inflammation along the needle tracts at the site of injection and epineuria disruption where PBS, EtBr and LPC resulted in similarly elevated levels of phagocytic macrophage infiltration shortly after injection (Figure 5). Crushed sciatic nerves showed robust elevation in glial fibrillary acid protein (GFAP) positive reactive Schwann cells, and DAPI stained nuclei by 3 days post injury (ANOVA P<0.0001 DAPI, P<0.05 GFAP, Bonferroni corrected post-hoc t-tests, Figure S2). Chemical demyelination resulted in the elevation of GFAP and DAPI staining over a much longer time course, with elevation through 14 days post-injection, by which time EtBr-damaged myelin had been cleared from the sciatic nerve (Figure S2). While only limited phagocytic macrophages stained with ED1 were detected during the clearance of EtBr-damaged myelin, Schwann cells may be able to drive the early clearance of myelin after peripheral injury in the absence of infiltrating macrophages (Perry et al., 1995). The alternatively-activated M2 macrophage marker arginase-1 was found to be substantially elevated only in crushed sciatic nerves (Figure S2) (Kigerl et al., 2009).
Within the injured sciatic nerve, we observed no correlation between activation of macrophages, or GFAP induction by reactive Schwann cells and the expression of RAGs (Figures 6, 7). In the DRG, both nerve crush and EtBr activated Iba1-immunoreactive macrophages robustly, but on disparate time courses (Figure 5).
Figure 6. EtBr injection dramatically impairs recovery of behavioral performance.
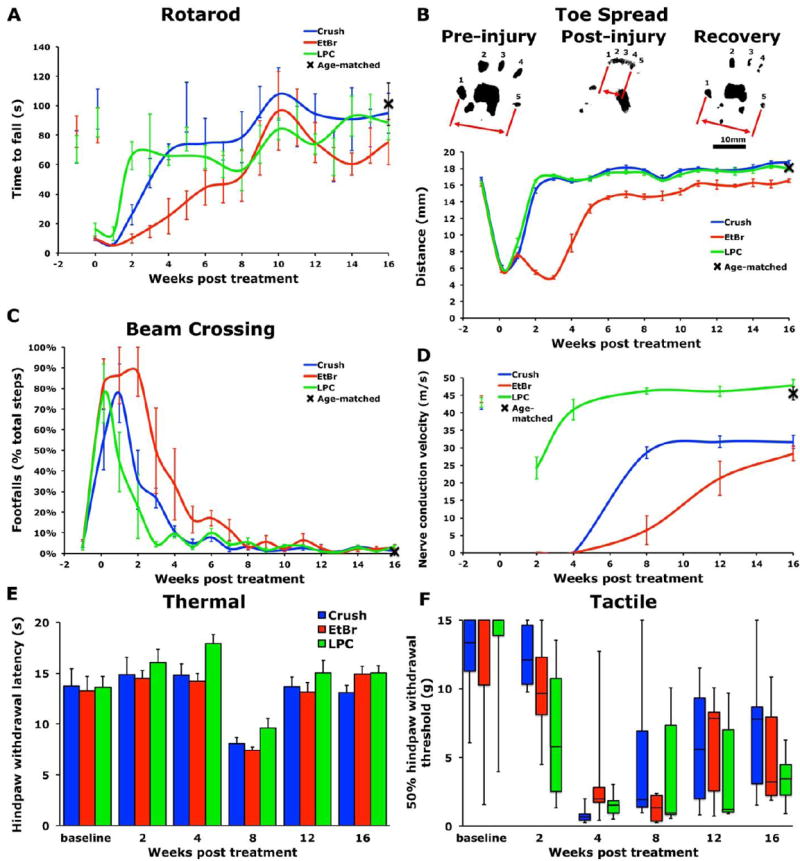
0.1%wt/vol EtBr treated animals exhibit slower recovery on (A) accelerating rotarod (repeated measures ANOVA P<0.05) and (C) beam crossing (repeated measures ANOVA P<0.005). (B) EtBr treatment resulted in reduced recovery of hindpaw toe spread (repeated measures ANOVA P<0.0001). (D) EtBr treatment and nerve crush reduced recovery of sciatic nerve conduction velocity (repeated measures ANOVA P<0.0001). No differences were seen in thermal responses (E) with hindpaw withdrawal latencies measured by Hargreaves apparatus or mechanical responses (F) measured with Von Frey filaments. Data presented as mean±s.e.m., except tactile data presented as interquartile-range. n=6/group
EtBr causes more severe functional deficits than LPC or nerve crush
In the study of regeneration, the recovery of behavioral function is a key outcome measure after injury. After LPC injection, it has been shown that remyelination and recovery of electrophysiological function is completed between 2 weeks and 2 months post injection (Hall, 1973). Due to the remarkable increase in regeneration found following EtBr injection, we sought to determine the consequences of EtBr-induced demyelination on sciatic nerve function and whether it was significantly different from LPC-induced demyelination. Animals were trained to perform behavioral tasks for two weeks prior to bilateral sciatic injection with LPC or EtBr, or bilateral sciatic nerve crush. Recovery of sensory and motor function was then assessed weekly on an accelerating rotarod, beam crossing, and hindlimb toe spread tasks. Animals injected with EtBr exhibited the slowest recovery of function on the rotarod (repeated measures ANOVA P<0.05) and beam crossing task (repeated measures ANOVA P<0.005) and also failed to completely recover hindlimb toe spread (repeated measures ANOVA P<0.0001; Figure 6). Animals subject to bilateral nerve crush or LPC injection recovered to pre-lesion and age-matched control levels on rotarod, beam crossing, and hindlimb toe spread tasks. In addition to the recovery of positive behavioral function, we tested animals for adverse reactions characterized by mechanical allodynia and thermal hyperalgesia as peripheral nerve crush has been demonstrated to induce neuropathic pain-like states, characterized by mechanical allodynia and increased temperature sensitivity (Lancelotta et al., 2003; Vogelaar et al., 2004). Von Frey filaments were used to test mechanical sensation and thermal sensitivity was tested using a Hargreaves apparatus. No treatment group exhibited more severe adverse reactions than any other (Figure 6).
Nerve conduction velocity (NCV) was measured at five time points after sciatic injury, starting at two weeks. The nerve was stimulated at the sciatic notch and Achilles tendon and the evoked electromyogram recorded from the ipsilateral interosseous muscles. Animals injected with LPC exhibited full recovery of electrophysiological function within 2 to 8 weeks (Figure 6). After nerve crush, recovery of NCV peaked between 8 and 12 weeks at significantly reduced levels relative to pre-injury measurements or age-matched controls. Consistent with observed behavioral deficits, EtBr injected animals exhibited a delayed and attenuated recovery of NCV (repeated measures P<0.0001, EtBr vs. Crush P<0.005; Figure 6).
Discussion
We report here the novel finding that peripheral injection of the demyelinating agent EtBr induces a more robust axon regeneration response than peripheral nerve crush and provides a second method of peripheral conditioning to increase the intrinsic growth state of sensory neurons. Our key finding is that axotomy is not a required component of the peripheral conditioning effect and that injection of a chemical demyelinating agent alone can promote regeneration of the central branch in the spinal cord. This alternative peripheral conditioning model will provide a new tool for studying the mechanisms underlying the induction of regeneration by peripheral conditioning.
We also observed that the more severe treatments resulting in a longer period to recover function caused better regeneration of the central branch. While EtBr-injected animals were delayed in their peripheral functional recovery, both EtBr-injected and nerve crushed animals recovered to similar levels on most behavioral measures, with the only exception being the measure of total toe spread, a component of the sciatic functional index (de Medinaceli et al., 1982). The induction of mechanical allodynia by the injection of chemical demyelinating agents followed a similar time course as after sciatic nerve crush, peaking at 4 weeks, similar to previous work on nerve crush (Vogelaar et al., 2004).
After sciatic nerve crush, clearance of distal myelin has been proposed to be required for normal peripheral regeneration (Brown et al., 1994). This is based on observations in the slow Wallerian degeneration mouse (Wlds), in which recruitment of hematogenous macrophages is limited, de-afferented axons remain, demyelination proceeds slowly, and peripheral conditioning by nerve crush is attenuated (Brown et al., 1994; Niemi et al., 2013). It has been shown that induced immunological demyelination distal to a crush site significantly enhances the rate of peripheral regeneration after nerve crush (Kosins et al., 2011). These studies are consistent with our finding that removal of myelin correlates with enhanced regeneration. In our studies, EtBr augmented the growth potential of large diameter DRG neurons over nerve-crushed neurons at individual time points (Figure 1), not just over a longer period; consistent with the idea that peripheral myelin actively inhibits axon regeneration (Brown et al., 1994). Therefore, lack of peripheral myelin per se could be a major signal for a conditioning lesion effect. Postmortem analysis of patients with the demyelinating disease multiple sclerosis has shown increased expression of another RAG, growth-associated protein-43 (GAP43) (Schirmer et al., 2012). This suggests that myelin loss in the central nervous system may also be a growth-promoting signal for axons in the central nervous system.
Following the primary insult to axons and myelin, inflammation occurs through the activation of resident microglia or macrophages and the infiltration of blood-derived macrophages. Macrophage infiltration of the CNS correlates with secondary degeneration of neurons and glia after injury, as depletion of peripheral macrophages results in fewer infiltrating macrophages and reduced secondary damage after spinal cord contusion injury (Popovich et al., 1999). Macrophage infiltration in the injured sciatic that occurs along with Schwann cell proliferation has been proposed to promote regeneration (Hall, 1989). These pro-regenerative effects may be due in part to macrophage clearance of debris, but could potentially be through anti-inflammatory pathways similar to the alternatively-activated M2 macrophages found after CNS injury (Kigerl et al., 2009). In contrast to sciatic nerve crush, we did not observe a correlation between macrophage activity, alternative or inflammatory, in the sciatic nerve with the induction of peripheral conditioning through the injection of chemical demyelinating agents. Instead, we did observe strong macrophage activation in the DRG following both nerve crush and EtBr injection, indicating that macrophage activity proximal to the cell body may have a role in mediating the conditioning response to injury, consistent with previous studies (Kwon et al., 2013; Niemi et al., 2013). However, we found that EtBr induced a robust macrophage response in the DRG at an earlier time point than nerve crush and that unlike in nerve transection experiments (Kwon et al., 2013), the macrophage response to EtBr and nerve crush was transient and reduced within a few days. The difference in the timing of the macrophage response to sciatic crush compared to transection may be relevant in light of the distinct differences in initiation and rate of regeneration after peripheral injury. Animals that undergo nerve crush injury show faster onset and rate of regeneration than animals after nerve transection (Gutmann et al., 1942; Forman et al., 1979). It is perhaps more likely then that macrophage activation is an early event in peripheral conditioning and not necessary for the maintenance of the conditioning response. Alternatively, nerve crush and EtBr could promote RAG expression through different mechanisms.
In addition to its role as a demyelinating agent, chronic EtBr exposure over multiple weeks in vitro can inhibit mitochondrial RNA synthesis and cell growth in some eukaryotic cells (von Wurmb-Schwark et al., 2006; Kao et al., 2012); however, it should be noted that it is unlikely that EtBr will have such chronic effects in our experiments. EtBr was cleared from Schwann cell myelin quickly (Figure S2), completely undetectable by confocal microscopy by day 14, and we observed extensive proliferation or infiltration of cells in the sciatic nerve after demyelination with either LPC or EtBr (Figure 5). This potential proliferation is consistent with previous reports of Schwann cell proliferation following experimental demyelination of the sciatic by LPC, EtBr or anti-galactocerebroside (Hall, 1973; Saida and Saida, 1986; Griffin et al., 1990; Riet-Correa et al., 2002).
We observed no evidence of sciatic axon disruption by injection of the chemical demyelinating agents LPC or EtBr, indicating that axotomy was not present in our novel model of peripheral conditioning. This is consistent with previous reports of treatment of the sciatic nerve as well as the caudal cerebellar peduncle that found no demonstrable induction of axonal damage by demyelinating agents (Hall and Gregson, 1971; Woodruff and Franklin, 1999; Riet-Correa et al., 2002). It is possible that EtBr injection affected other aspects of axonal morphology and function not evident from analysis of neurofilament staining, despite evidence that LPC-mediated demyelination showed no evidence of morphological changes of sciatic axons by electron microscopy (Hall and Gregson, 1971). Functionally, however, chemical demyelination of sensory neuron cultures has demonstrated an increase in the rate of mitochondrial transport (Kiryu-Seo et al., 2010).
We hypothesize that the abnormal distribution, trafficking and function of mitochondria caused by demyelination is a candidate signal for triggering the conditioning growth state. 1) Both chemical demyelination (with LPC) and sciatic nerve transection increase mitochondrial transport in injured sensory neurons (Kiryu-Seo et al., 2010; Mar et al., 2014). Chemical demyelination, both in vitro and in vivo (with either LPC or EtBr, respectively), results in increased axonal mitochondrial content and mitochondrial hypertrophy (Kiryu-Seo et al., 2010; Zambonin et al., 2011), similar to mitochondrial hypertrophy seen after axotomy (Dimova and Markov, 1976), or to the hypertrophy observed in axons with impaired Node of Ranvier formation (Einheber et al., 2006). This may be due to a higher energy demand for nerve conduction in incompletely remyelinated or dysmyelinated axons (Liu et al., 2001). 2) Knocking down the RAG ATF-3 in sensory neuron cultures blocks chemical demyelination-induced increases in mitochondrial transport velocity and increases a marker of oxidative stress in the axon (Kiryu-Seo et al., 2010). 3) Activity of axonal mitochondria is required for local protein synthesis and branch formation (Spillane et al., 2013). Therefore, local sprouting of axon branches associated with supernumerary Schwann cells after chemical demyelination (Hall, 1973) may be indicative of the convergence on a mitochondrial response to stress pathways induced by either nerve crush or chemical demyelination.
In summary, we found that axotomy is not required for peripheral conditioning. Our results suggest that loss of myelin in the periphery can trigger a growth state of adult dorsal root ganglion axons in the central nervous system. This new model of peripheral conditioning provides new opportunities to study the mechanisms underlying the growth promoting effects. By determining the common underlying mechanisms between these two models, we can hope to gain a fundamental understanding of how to reprogram the adult nervous system to respond differently to injury.
Highlights.
A novel model of preconditioning lesion without axon transection: demyelination of the peripheral branch.
Demyelination of sciatic nerve generates more robust regeneration of central branch in the spinal cord than nerve crush.
Demyelination causes similar inflammation in the DRG to nerve crush but on different time scales.
Acknowledgments
This work was supported by NINDS RO1 NS47484, the Wings for Life Foundation and the Roman Reed foundation to Y.Z., a Craig H. Neilsen Foundation Fellowship to E. H., and NIDDK R01 DK57629 to N.A.C. Y.Z., E.H. and N.A.C. designed experiments, analyzed data, and wrote and edited the manuscript. E.H., N.I., E.D. K.T. and M.J.R. performed experiments and analyzed data. Y.Z. supervised the study and coordinated collaborations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allt G, Ghabriel MN, Sikri K. Lysophosphatidyl choline-induced demyelination. Acta Neuropathol. 1988;75:456–464. doi: 10.1007/BF00687132. [DOI] [PubMed] [Google Scholar]
- Beletsky IP, Umansky SR. A new assay for cell death. J Immunological Methods. 1990;134:201–205. doi: 10.1016/0022-1759(90)90381-5. [DOI] [PubMed] [Google Scholar]
- Boeshore KL, Schreiber RC, Vaccariello SA, Sachs HH, Salazar R, Lee J, Ratan RR, Leahy P, Zigmond RE. Novel changes in gene expression following axotomy of a sympathetic ganglion: A microarray analysis. J Neurobiol. 2004;59:216–235. doi: 10.1002/neu.10308. [DOI] [PubMed] [Google Scholar]
- Brown MC, Perry VH, Hunt SP, Lapper SR. Further studies on motor and sensory nerve regeneration in mice with delayed wallerian degeneration. Eur J Neurosci. 1994;6:420–428. doi: 10.1111/j.1460-9568.1994.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Brück W. The role of macrophages in wallerian degeneration. Brain Pathology. 1997;7:741–752. doi: 10.1111/j.1750-3639.1997.tb01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcutt NA. Modeling diabetic sensory neuropathy in rats. In: Luo ZD, editor. Pain Research. Humana Press; 2004. pp. 55–65. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Costigan M, Befort K, Karchewski L, Griffin RS, D’Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JG, Dopp EA, Calame W, Chao D, MacPherson GG, Dijkstra CD. Rat macrophage lysosomal membrane antigen recognized by monoclonal antibody ED1. Immunology. 1994;83:140–147. [PMC free article] [PubMed] [Google Scholar]
- de Medinaceli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol. 1982;77:634–643. doi: 10.1016/0014-4886(82)90234-5. [DOI] [PubMed] [Google Scholar]
- Dimova R, Markov D. Changes in the mitochondria in the initial part of the axon during regeneration. Acta Neuropathol. 1976;36:235–242. doi: 10.1007/BF00685367. [DOI] [PubMed] [Google Scholar]
- Einheber S, Bhat MA, Salzer JL. Disrupted axo-glial junctions result in accumulation of abnormal mitochondria at nodes of ranvier. Neuron glia biology. 2006;2:165–174. doi: 10.1017/S1740925X06000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman DS, Wood DK, DeSilva S. Rate of regeneration of sensory axons in transected rat sciatic nerve repaired with epineurial sutures. J Neurological Sci. 1979;44:55–59. doi: 10.1016/0022-510x(79)90222-3. [DOI] [PubMed] [Google Scholar]
- Griffin JW, George R, Ho T. Macrophage systems in peripheral nerves. A review J Neuropath Exp Neurol. 1993;52:553–560. doi: 10.1097/00005072-199311000-00001. [DOI] [PubMed] [Google Scholar]
- Griffin JW, Stocks EA, Fahnestock K, Praagh A, Trapp BD. Schwann cell proliferation following lysolecithin-induced demyelination. J Neurocytol. 1990;19:367–384. doi: 10.1007/BF01188405. [DOI] [PubMed] [Google Scholar]
- Gupta R, Rowshan K, Chao T, Mozaffar T, Steward O. Chronic nerve compression induces local demyelination and remyelination in a rat model of carpal tunnel syndrome. Exp Neurol. 2004;187:500–508. doi: 10.1016/j.expneurol.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Gutmann E, Guttmann L, Medawar PB, Young JZ. The rate of regeneration of nerve. J Exp Biol. 1942;19:14–44. [Google Scholar]
- Hall SM. Some aspects of remyelination after demyelination produced by the intraneural injection of lysophosphatidyl choline. J Cell Sci. 1973;13:461–477. doi: 10.1242/jcs.13.2.461. [DOI] [PubMed] [Google Scholar]
- Hall SM. Regeneration in the peripheral nervous system. Neuropathology and Applied Neurobiology. 1989;15:513–529. doi: 10.1111/j.1365-2990.1989.tb01251.x. [DOI] [PubMed] [Google Scholar]
- Hall SM, Gregson NA. The in vivo and ultrastructural effects of injection of lysophosphatidyl choline into myelinated peripheral nerve fibres of the adult mouse. J Cell Sci. 1971;9:769–789. doi: 10.1242/jcs.9.3.769. [DOI] [PubMed] [Google Scholar]
- Hoffman PN, Cleveland DW. Neurofilament and tubulin expression recapitulates the developmental program during axonal regeneration: induction of a specific beta-tubulin isotype. Proceedings of the National Academy of Sciences. 1988;85:4530–4533. doi: 10.1073/pnas.85.12.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis ER, II, Zou Y. Reinduced Wnt signaling limits regenerative potential of sensory axons in the spinal cord following conditioning lesion. Proceedings of the National Academy of Sciences. 2012;109:14663–14668. doi: 10.1073/pnas.1206218109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R, Hunt SP. Long-term increase in the levels of c-jun mRNA and jun protein-like immunoreactivity in motor and sensory neurons following axon damage. Neurosci Lett. 1991;129:107–110. doi: 10.1016/0304-3940(91)90731-8. [DOI] [PubMed] [Google Scholar]
- Kao L-P, Ovchinnikov D, Wolvetang E. The effect of ethidium bromide and chloramphenicol on mitochondrial biogenesis in primary human fibroblasts. Toxicology and Applied Pharmacology. 2012;261:42–49. doi: 10.1016/j.taap.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiryu-Seo S, Ohno N, Kidd GJ, Komuro H, Trapp BD. Demyelination increases axonal stationary mitochondrial size and the speed of axonal mitochondrial transport. J Neurosci. 2010;30:6658–6666. doi: 10.1523/JNEUROSCI.5265-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosins AM, Scholz T, Mendoza C, Lin P, Shepard B, Evans GR, Keirstead HS. Improvement of peripheral nerve regeneration following immunological demyelination in vivo. Plast Reconstr Surg. 2011;127:1813–1819. doi: 10.1097/PRS.0b013e31820cf2b0. [DOI] [PubMed] [Google Scholar]
- Kwon MJ, Kim J, Shin H, Jeong SR, Kang YM, Choi JY, Hwang DH, Kim BG. Contribution of macrophages to enhanced regenerative capacity of dorsal root ganglia sensory neurons by conditioning injury. J Neurosci. 2013;33:15095–15108. doi: 10.1523/JNEUROSCI.0278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancelotta MP, Sheth RN, Meyer RA, Belzberg AJ, Griffin JW, Campbell JN. Severity and duration of hyperalgesia in rat varies with type of nerve lesion. Neurosurgery. 2003;53:1200–1209. doi: 10.1227/01.neu.0000089482.80879.9a. [DOI] [PubMed] [Google Scholar]
- Lazarov-Spiegler O, Solomon AS, Zeev-Brann AB, Hirschberg DL, Lavie V, Schwartz M. Transplantation of activated macrophages overcomes central nervous system regrowth failure. FASEB J. 1996;10:1296–1302. doi: 10.1096/fasebj.10.11.8836043. [DOI] [PubMed] [Google Scholar]
- Liu YY, Wong-Riley MT, Liu HL, Jia Y, Jiao XY, Wang CT, You SW, Ju G. Increase in cytochrome oxidase activity in regenerating nerve fibers of hemitransected spinal cord in the rat. Neuroreport. 2001;12:3239–3242. doi: 10.1097/00001756-200110290-00019. [DOI] [PubMed] [Google Scholar]
- Lu X, Richardson PM. Inflammation near the nerve cell body enhances axonal regeneration. J Neurosci. 1991;11:972–978. doi: 10.1523/JNEUROSCI.11-04-00972.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar FM, Simões AR, Leite S, Morgado MM, Santos TE, Rodrigo IS, Teixeira CA, Misgeld T, Sousa MM. CNS axons globally increase axonal transport after peripheral conditioning. J Neurosci. 2014;34:5965–5970. doi: 10.1523/JNEUROSCI.4680-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuarrie IG, Grafstein B. Axon outgrowth enhanced by a previous nerve injury. Archives of Neurology. 1973;29:53–55. doi: 10.1001/archneur.1973.00490250071008. [DOI] [PubMed] [Google Scholar]
- Meijering E, Jacob M, Sarria JC, Steiner P, Hirling H, Unser M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A. 2004;58:167–176. doi: 10.1002/cyto.a.20022. [DOI] [PubMed] [Google Scholar]
- Niemi JP, DeFrancesco-Lisowitz A, Roldán-Hernández L, Lindborg JA, Mandell D, Zigmond RE. A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve tegeneration. J Neurosci. 2013;33:16236–16248. doi: 10.1523/JNEUROSCI.3319-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh P, Hao Y, Hosseinkhani M, Patil SB, Huntley GW, Tessier-Lavigne M, Zou H. Regeneration of axons in injured spinal cord by activation of bone morphogenetic protein/Smad1 signaling pathway in adult neurons. Proceedings of the National Academy of Sciences. 2011;108:E99–E107. doi: 10.1073/pnas.1100426108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Tsao JW, Feam S, Brown MC. Radiation-induced reductions in macrophage recruitment have only slight effects on myelin degeneration in sectioned peripheral nerves of mice. Eur J Neurosci. 1995;7:271–280. doi: 10.1111/j.1460-9568.1995.tb01063.x. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT. Spinal Axon Regeneration Induced by Elevation of Cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- Richardson PM, Issa VM. Peripheral injury enhances central regeneration of primary sensory neurones. Nature. 1984;309:791–793. doi: 10.1038/309791a0. [DOI] [PubMed] [Google Scholar]
- Riet-Correa G, Fernandes CG, Pereira LAV, Graça DL. Ethidium bromide-induced demyelination of the sciatic nerve of adult Wistar rats. Brazilian Journal of Medical and Biological Research. 2002;35:99–104. doi: 10.1590/s0100-879x2002000100014. [DOI] [PubMed] [Google Scholar]
- Saida K, Saida T. Proliferation of Schwann cells in demyelinated rat sciatic nerve. Acta Neuropathol. 1986;71:251–258. doi: 10.1007/BF00688047. [DOI] [PubMed] [Google Scholar]
- Schirmer L, Merkler D, König FB, Brück W, Stadelmann C. Neuroaxonal regeneration is more pronounced in early multiple sclerosis than in traumatic brain injury lesions. Brain Pathology. 2012;23:2–12. doi: 10.1111/j.1750-3639.2012.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC. A rapid, sensitive histochemical stain for myelin in frozen brain sections. J Histochem Cytochem. 1990;38:717–720. doi: 10.1177/38.5.1692056. [DOI] [PubMed] [Google Scholar]
- Seijffers R, Mills CD, Woolf CJ. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J Neurosci. 2007;27:7911–7920. doi: 10.1523/JNEUROSCI.5313-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Merianda T, Twiss J, Gallo G. Mitochondria coordinate sites of axon branching through localized intra-axonal protein synthesis. Cell Reports. 2013;5:1564–1575. doi: 10.1016/j.celrep.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam FJ, MacGillavry HD, Armstrong NJ, de Gunst MC, Zhang Y, van Kesteren RE, Smit AB, Verhaagen J. Identification of candidate transcriptional modulators involved in successful regeneration after nerve injury. Eur J Neurosci. 2007;25:3629–3637. doi: 10.1111/j.1460-9568.2007.05597.x. [DOI] [PubMed] [Google Scholar]
- Steinmetz MP, Horn KP, Tom VJ, Miller JH, Busch SA, Nair D, Silver DJ, Silver J. Chronic enhancement of the intrinsic growth capacity of sensory neurons combined with the degradation of inhibitory proteoglycans allows functional regeneration of sensory axons through the dorsal root entry zone in the mammalian spinal cord. J Neurosci. 2005;25:8066–8076. doi: 10.1523/JNEUROSCI.2111-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton B-HT, Chen Q, Gaina G, Tucureanu C, Georgescu A, Strungaru C, Flonta M-L, Sah D, Ristoiu V. Activation profile of dorsal root ganglia Iba-1 (+) macrophages varies with the type of lesion in rats. Acta Histochemica. 2013;115:840–850. doi: 10.1016/j.acthis.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;15:170–182. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]
- Vogelaar CF, Vrinten DH, Hoekman MFM, Brakkee JH, Burbach JPH, Hamers FPT. Sciatic nerve regeneration in mice and rats: recovery of sensory innervation is followed by a slowly retreating neuropathic pain-like syndrome. Brain Res. 2004;1027:67–72. doi: 10.1016/j.brainres.2004.08.036. [DOI] [PubMed] [Google Scholar]
- von Wurmb-Schwark N, Cavelier L, Cortopassi GA. A low dose of ethidium bromide leads to an increase of total mitochondrial DNA while higher concentrations induce the mtDNA 4997 deletion in a human neuronal cell line. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2006;596:57–63. doi: 10.1016/j.mrfmmm.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Woodruff RH, Franklin RJM. Demyelination and remyelination of the caudal cerebellar peduncle of adult rats following stereotaxic injections of lysolecithin, ethidium bromide, and complement/anti-galactocerebroside: A comparative study. Glia. 1999;25:216–228. doi: 10.1002/(sici)1098-1136(19990201)25:3<216::aid-glia2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Zambonin JL, Zhao C, Ohno N, Campbell GR, Engeham S, Ziabreva I, Schwarz N, Lee SE, Frischer JM, Turnbull DM, Trapp BD, Lassmann H, Franklin RJM, Mahad DJ. Increased mitochondrial content in remyelinated axons: implications for multiple sclerosis. Brain. 2011;134:1901–1913. doi: 10.1093/brain/awr110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Ho C, Wong K, Tessier-Lavigne M. Axotomy-induced Smad1 activation promotes axonal growth in adult sensory neurons. J Neurosci. 2009;29:7116–7123. doi: 10.1523/JNEUROSCI.5397-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


