Abstract
Adiponectin, an adipocyte-derived protein, exerts anti-atherosclerotic effects on the vascular endothelium. Recently adiponectin protein has been reported in murine vascular endothelial cells, however, whether adiponectin is present in human vascular endothelial cells remains unexplored. We sought to examine 1) adiponectin protein in vascular endothelial cells collected from older adults free of overt cardiovascular disease; 2) the relation between endothelial cell adiponectin and in vivo vascular endothelial function; and 3) the relation between endothelial cell adiponectin, circulating (plasma) adiponectin and related factors. We measured vascular endothelial function (brachial artery flow-mediated dilation using ultrasonography), vascular endothelial cell adiponectin (biopsy coupled with quantitative immunofluorescence) and circulating adiponectin (Mercodia, ELISA) in older, sedentary, non-smoking, men and women (55 – 79 years). We found that higher endothelial cell adiponectin was related with greater flow-mediated dilation (r=0.43, P<0.05) and greater flow-mediated dilation normalized for shear stress (r=0.56, P<0.01), but was not related with vascular smooth muscle responsiveness to nitric oxide (r=0.04, P=0.9). Vascular endothelial cell adiponectin was not related with circulating adiponectin (r=−0.14, P=0.6). Endothelial cell and circulating adiponectin were differentially associated with adiposity, metabolic and other factors, but both were inversely associated with renal function (r=0.44 to 0.62, P ≤ 0.04). In conclusion, higher endothelial cell adiponectin levels are associated with higher vascular endothelial function, independent of circulating adiponectin levels in older adults.
Keywords: vascular endothelial function, ultrasound, aging, renal function
1. Introduction
Atherosclerosis is a major cause of morbidity and mortality in the Western world. A key early event in the development and progression of atherosclerosis is vascular endothelial dysfunction. Adiponectin, an adipocyte-derived protein, is known to exert anti-atherosclerotic effects on the vascular endothelium and has been found to accumulate in the endothelium of injured vessels (Ouchi et al. 2001). In vascular endothelial cell cultures, adiponectin stimulates nitric oxide (NO) production by increasing phosphorylation of endothelial NO synthase (eNOS) via phosphatidylinositol (PI) 3-kinase-dependent pathways (Cao et al. 2009; Chen et al. 2003; Xi et al. 2005). In isolated vessels, adiponectin has recently been reported to improve NO bioavailability and decrease vascular O2− production/eNOS uncoupling in both human arteries and veins by increasing PI3/Akt-mediated phosphorylation of eNOS and BH4-mediated eNOS coupling (Margaritis et al. 2013). Adiponectin-related production of NO and eNOS phosphorylation are mediated through AdipoR1 and AdipoR2 receptors (Cheng et al. 2007), both of which are expressed in human endothelial cells (Tan et al. 2004). A third adiponectin receptor is T-cadherin, which is highly expressed in endothelial cells (Hug et al. 2004).
Recently, adiponectin protein has been reported in endothelial cells collected from mouse thoracic aorta (Komura et al. 2013) and in murine endothelial cell lines (Rutkowski et al. 2014), but whether adiponectin is present in human vascular endothelial cells remains unexplored. Colombo et al have developed a technique to collect vascular endothelial cells from peripheral blood vessels (arteries or veins) in humans (Colombo et al. 2002) and have validated the use of quantitative immunofluorescence to measure vascular endothelial protein levels. We have recently used this technique to examine protein levels of factors known to influence vascular endothelial function (e.g., eNOS, superoxide dismutase, NADPH oxidase, xanthine oxidase and nuclear factor k B in human vascular endothelial cells) (Hwang et al. 2013; Silver 2007; Silver et al. 2010). We are not aware of any studies examining adiponectin protein in human vascular endothelial cells and its relation to in vivo vascular endothelial function.
There are some reports of a positive relation between circulating (plasma) adiponectin and vascular endothelial function (Ouchi et al. 2003; Saarikoski et al. 2010; Tan et al. 2004). There are also reports of an inverse relation of circulating adiponectin with adiposity and glucose, and a direct relation with HDL cholesterol (Belalcazar et al. 2012; Cnop et al. 2003). Interestingly, higher circulating adiponectin has also been linked with decreased renal function (Kawamoto et al. 2010; Kruger et al. 2011) and markers of anemia (red blood cell count, hematocrit, and hemoglobin) (Aso et al. 2009; Aso et al. 2012; Kawamoto et al. 2011). However, it is not known whether endothelial cell adiponectin is similarly related with these factors and with circulating adiponectin levels. In older adults, vascular endothelial function is impaired, but the underlying mechanisms are incompletely understood. Investigating the influence of endothelial cell adiponectin on vascular function in human aging is important because it can potentially lead to strategies to improve vascular endothelial dysfunction.
Therefore, in the current investigation we sought to examine for the first time: 1) adiponectin in vascular endothelial cells collected from older adults free of overt cardiovascular disease; 2) the relation between vascular endothelial cell adiponectin and vascular endothelial function measured in vivo in response to reactive hyperemia using brachial high resolution ultrasonography (flow-mediated dilation; FMD); and 3) the relation between vascular endothelial cell adiponectin, circulating (plasma) adiponectin, and related factors.
2. Methods
2.1 Subjects
We studied 23 older adults (55–79 years; 11 men and 12 women) who were sedentary and did not smoke or use other tobacco products. Subjects were free of overt cardiovascular and other clinical disease (e.g., diabetes, liver and renal disease) as assessed by medical history, physical examination, resting ECG, urinalysis, blood chemistries and hematological evaluation. All subjects demonstrated normal ECG and blood pressure responses to a graded exercise test, which was performed on a treadmill on a different day than the vascular measures. For the graded exercise test, subjects walked for 6 min at a comfortable speed that corresponded to 70 to 80 % of their age-predicted maximal heart rate to warm-up, followed by 2.5% increases in the treadmill grade every two minutes until volitional exhaustion (Hwang et al. 2013). None of the subjects were taking antihypertensive or vasoactive drugs and subjects who were taking antioxidant supplements completed a 4-week washout prior to enrolling in the study. Women were all postmenopausal, established by absence of menses for at least 2 years and follicle stimulating hormone >40 IU/L. Postmenopausal women were not on hormone replacement therapy for at least 1 year prior to data collection.
The study was carried out in accordance with the Declaration of Helsinki (2008) and was approved by the Institutional Review Boards of the University of Florida, Texas A&M University, and Scott & White Health System. The purpose, nature and risk of all procedures were explained to the subjects and their written informed consent was obtained prior to participation.
2.2 General experimental procedures
All measurements were performed in the morning, after a minimum of 20 minutes of supine rest in a quiet, semi-darkened, temperature-controlled room. Prior to data collection, subjects fasted overnight for 12 hours. They also abstained from caffeine and alcohol for 12 hours and from vigorous physical activity for at least 20 hours.
2.3 Vascular endothelial function (FMD)
Brachial artery FMD was assessed non-invasively using an ultrasound/Doppler system equipped with a 7.5 MHz linear transducer (Aplio XV, Toshiba) following established guidelines (Corretti et al. 2002; Thijssen et al. 2011) as we have previously described (Hwang et al. 2013).
Briefly, a duplex ultrasound image of the brachial artery (i.e., 2D image and spectral Doppler waveforms) was obtained ~7 cm proximal to the antecubital fossa while the subject rested supine with the right arm abducted and fixed in position at heart level. The Doppler angle of insonation for assessing blood velocity was set ≤60 degrees. Following image optimization the vascular transducer was clamped (Flexbar, Flexbar Machine Corporation, Islandia, NY) in place to prevent movement during data collection. Reactive hyperemia was induced by inflating a cuff at the widest part of the forearm (E20 and AG 101, D. E. Hokanson, Bellevue, WA) to 250 mmHg for 5 minutes followed by rapid deflation.
ECG R-gated duplex ultrasound images of the brachial artery were digitally recorded (Vascular Imager, Medical Imaging Applications, LLC, Coralville, IA) for one minute to establish pre-occlusion baseline and for 2 minutes after cuff deflation to assess peak dilatory response (the maximum brachial artery diameter). End-diastolic diameters were analyzed (Brachial Analyzer, Medical Imaging Applications, LLC, Coralville, IA) and individual post-occlusion diameters were averaged (bin: 3 R-gated diameters) to determine the peak diameter. FMD was expressed as absolute change in mm (maximum diameter baseline diameter) and as % change ([(maximum - baseline diameter)/baseline diameter] x 100). To quantify the hyperemic response, blood velocity was calculated by tracing the first 15 post-occlusion spectral Doppler envelopes and 15 baseline spectral Doppler envelopes using the Toshiba ultrasound system software. Blood flow (ml/min) was calculated as mean blood velocity x [(baseline diameter)2/4] x π x 6 x 10−1. Shear stress (dyne/cm2) was calculated as 8 x μ x mean blood velocity/baseline diameter, where μ was blood viscosity, which was assumed to be 0.035 dyne/cm2 (Mitchell et al. 2004).
To assess vascular smooth muscle responsiveness to NO, a measure of endothelium-independent dilation, brachial artery images were acquired at baseline and for 10 minutes following sublingual nitroglycerin (0.4 mg) administration as previously described (Christou et al. 2012). Maximum brachial response to nitroglycerin expressed as % change was calculated as ([(maximum - baseline diameter)/baseline diameter] x 100). Ultrasound images were analyzed by an investigator who was blind to subject identity.
2.4 Vascular endothelial cell collection and adiponectin immunostaining
Endothelial cells were collected from an antecubital vein by sequentially advancing and retracting two sterile J-shaped guidewires (Daig, Inc., Minnetonka, MN) ~ 10 cm through an 18 gauge intravenous catheter as previously described by our group and others (Colombo et al. 2002; Hwang et al. 2013; Shenouda et al. 2011; Silver 2007; Silver et al. 2007; Silver et al. 2010). Cells were recovered from the wires by washing with a dissociation buffer and centrifugation. Cells were fixed with 4% paraformaldehyde (USB corporation, Cleveland, OH), washed thoroughly with PBS, plated on poly-L-lysine coated slides (Sigma Chemical, St. Louis, MO), and stored at −80°C until the immunofluorescence staining was performed.
For immunofluorescence staining, fixed vascular endothelial cells were rehydrated with PBS containing 50 mmol/L glycine. After blocking non-specific sites with 5% donkey serum (Jackson Immunoresearch, West Grove, PA), slides were incubated with a primary antibody for adiponectin (Santa Cruz, Dallas, TX) followed with a secondary antibody with Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen, Carlsbad, CA). To allow identification of endothelial cells, slides were also incubated with a primary antibody for von Willebrand factor (DAKO, Carpinteria, CA) and a secondary antibody with Alexa Fluor 555 donkey anti-rabbit IgG (Invitrogen, Carlsbad, CA). Finally, to determine nuclear integrity, slides were mounted with Vectashield containing the nuclear stain DAPI (Vector Laboratories, Inc., Burlingame, CA). To minimize the potential confound of inter-batch variability in staining, 2 slides of human umbilical venous endothelial cells (HUVEC) were stained in each batch and endothelial cell adiponectin levels are reported relative to average HUVEC intensity in that batch.
For analysis, cells were examined with a fluorescence microscope (Eclipse 80i, Nikon Instruments, Inc., Melville, NY) at x100 magnification using the same exposure time. Images of endothelial cells (identified by the presence of von Willebrand factor staining) with intact nuclei (confirmed by DAPI staining) were digitally captured by a coolSNAP ES2 camera (Photometrics, Tuscon, AR). Endothelial cell adiponectin levels were measured by quantifying Alexa Fluor 488 intensity while correcting for background fluorescence (NIS Elements software (version 3.2), Nikon Instruments, Inc., Melville, NY) and are reported as intensity per HUVEC intensity.
2.5 Plasma adiponectin, renal function, and other blood measures
Plasma adiponectin (hexameric and high-molecular-weight forms) was measured using a commercially-available ELISA kit (Mercodia, Sweden). Standard blood chemistries, hematological evaluation, and renal function including blood urea nitrogen and serum creatinine tests were performed by a clinical laboratory using conventional assays. Creatinine clearance was based on a 24-hour urine collection.
2.6 Height, weight and adiposity measures
Subjects were dressed in light clothing and were barefoot. Height was measured to the nearest mm with a stadiometer. Body weight was measured to the nearest 0.1 kg using an electronic scale (Tanita, Arlington Heights, IL, USA). Body mass index was calculated as weight divided by height squared (kg/m2). Waist circumference was measured with a non-stretchable tape at the smallest horizontal narrowing between the ribs and iliac crest with the subject in the standing position. Three measures were taken to the nearest 1 mm.
2.7 Resting blood pressure
Resting blood pressures were recorded over the brachial artery with a semi-automated device (Dinamap, GE, Salt Lake City, UT).
2.8 Statistics
Statistical analyses were performed using SPSS version 22 (SPSS, Chicago, IL). Statistical significance for all analyses was set at P< 0.05. Pearson product moment correlation coefficients were used to examine the bivariate relations between endothelial cell adiponectin and vascular endothelial function, plasma adiponectin and other factors. Multiple linear regression was used to examine the independent relation between endothelial cell adiponectin and vascular endothelial function while controlling for factors related with both endothelial cell adiponectin and vascular endothelial function. Stepwise linear regression analysis with P<0.05 used for inclusion and P>0.1 for exclusion of variables was performed to evaluate all factors that were related with endothelial cell adiponectin and construct a model with the factors exhibiting the strongest independent relations with endothelial cell adiponectin.
3. Results
Study participants ranged in age from 55 to 79 years (64±7 years; mean±SD). Mean values and ranges for other main subject characteristics are presented in Table 1. Figure 1 demonstrates lack of fluorescence in a negative control endothelial cell stained with the secondary antibody but without the primary antibody for adiponectin.
Table 1.
Subject characteristics
| Mean±SD | Min-Max | |
|---|---|---|
| Body mass index, kg/m2 | 29.4±6.9 | 20.0–44.6 |
| Systolic blood pressure, mm Hg | 125±13 | 102–146 |
| Diastolic blood pressure, mm Hg | 77±8 | 66–93 |
| LDL cholesterol, mg/dL | 109±25 | 69–162 |
| HDL cholesterol, mg/dL | 46±11 | 30–69 |
| Fasting glucose, mg/dL | 95±7 | 84–109 |
| Fasting insulin, mU/mL | 3.0±2.1 | 2–10 |
Figure 1.
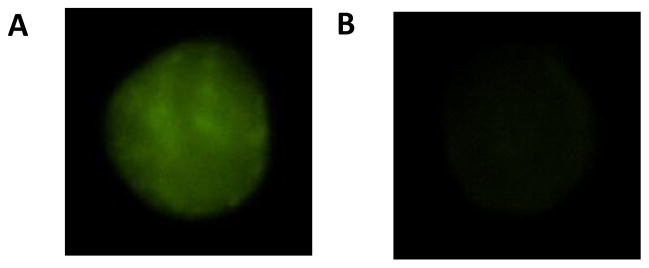
Endothelial cell stained with both the primary antibody for adiponectin and the corresponding secondary antibody conjugated with the fluorescent tag (A). Negative control endothelial cell stained with the secondary antibody, but without the primary antibody for adiponectin (B).
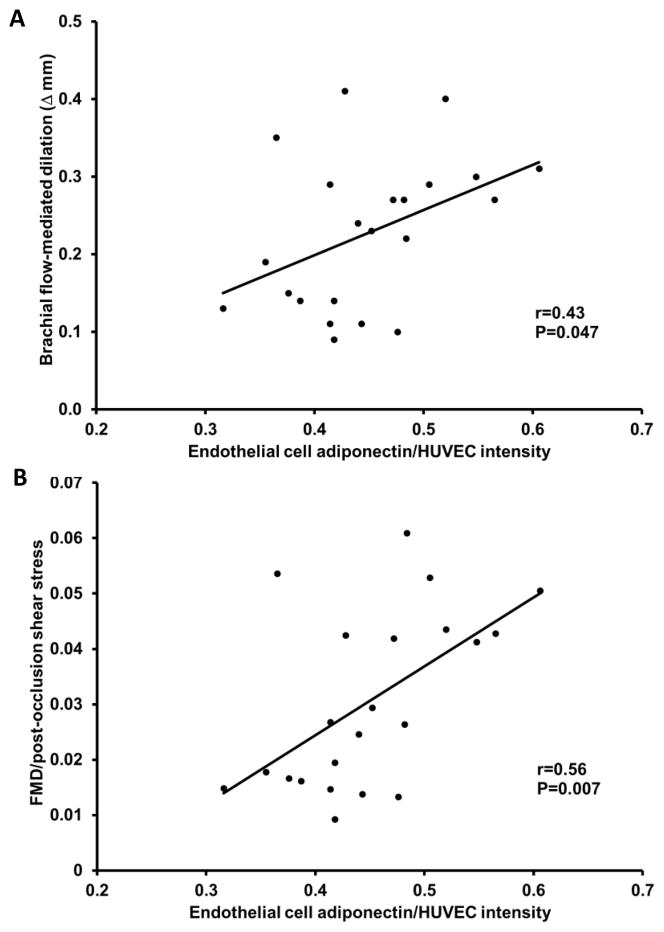
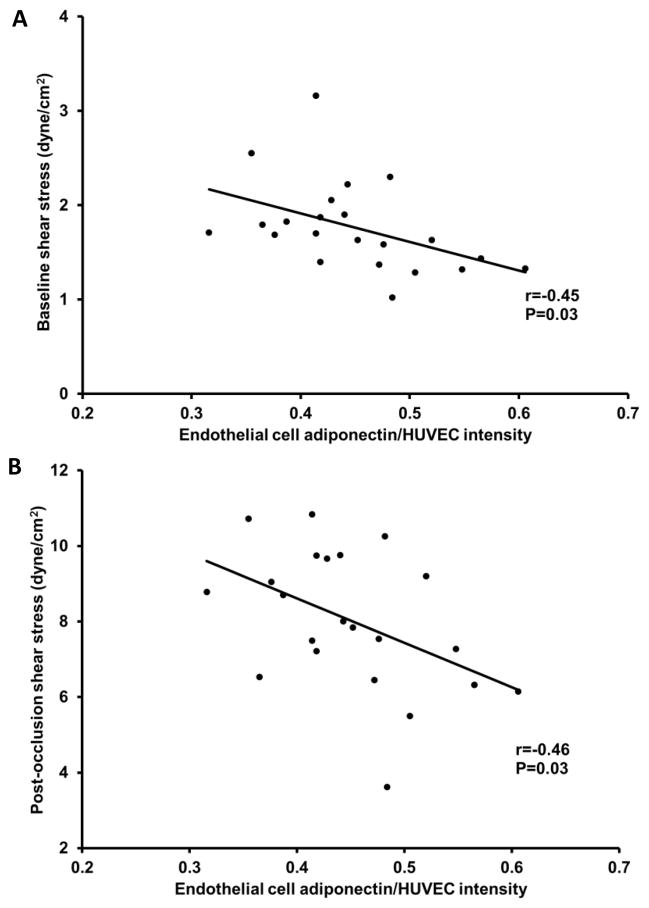
Higher endothelial cell adiponectin levels were related with greater FMD and with greater FMD normalized for post-occlusion shear stress (P=0.047 and P=0.007, respectively; Figure 2A and B). However, there was no relation between endothelial cell adiponectin and nitroglycerin-induced (endothelium-independent) dilation (r=0.04, P=0.9). Higher endothelial cell adiponectin levels were also related with lower baseline shear stress and post-occlusion shear stress (P=0.03 and P=0.03, respectively; Figure 3A and B). Circulating adiponectin was not significantly related with FMD (r=0.36, P>0.05).
Figure 2.
Relation between endothelial cell adiponectin, flow-mediated dilation (FMD) (A) and FMD normalized for post-occlusion shear stress (B).
Figure 3.
Relation between endothelial cell adiponectin, baseline shear stress (A), and post-occlusion shear stress (B).
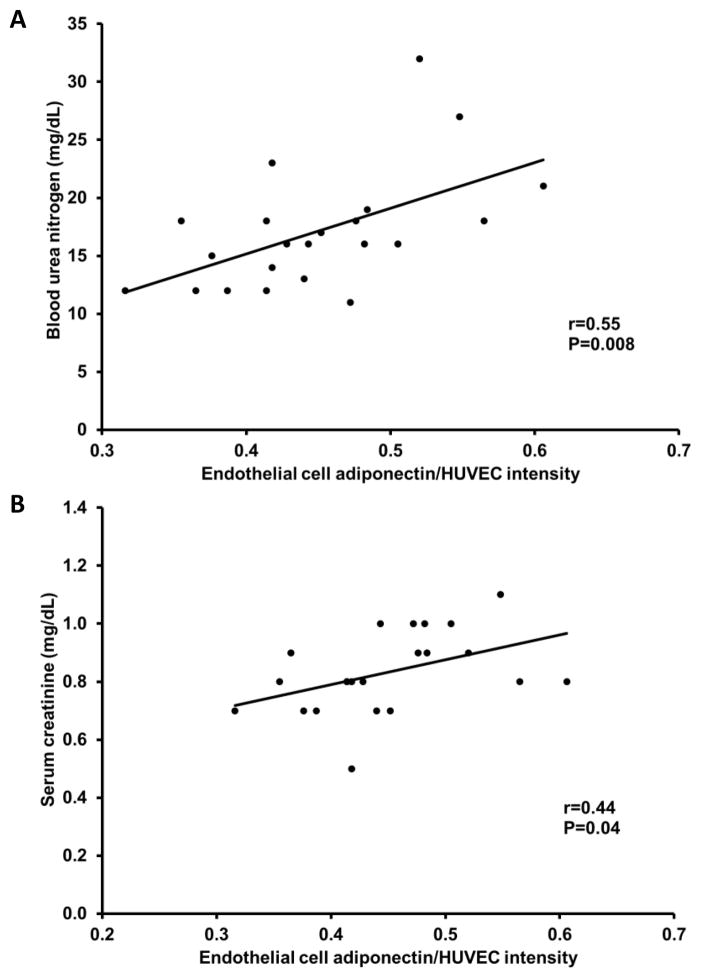
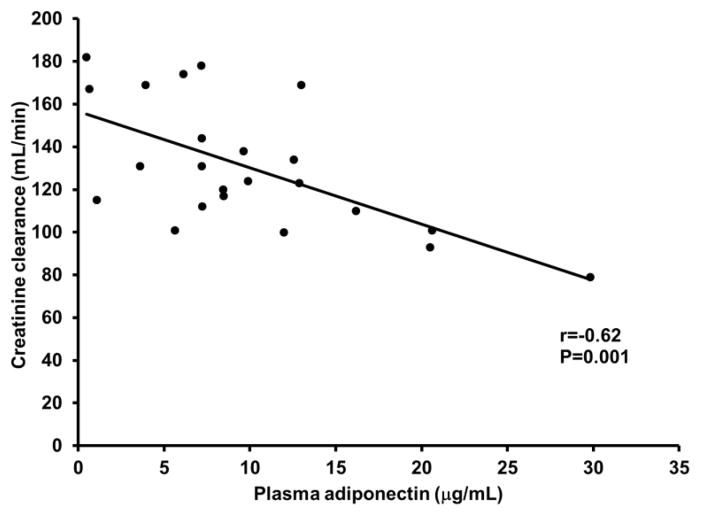
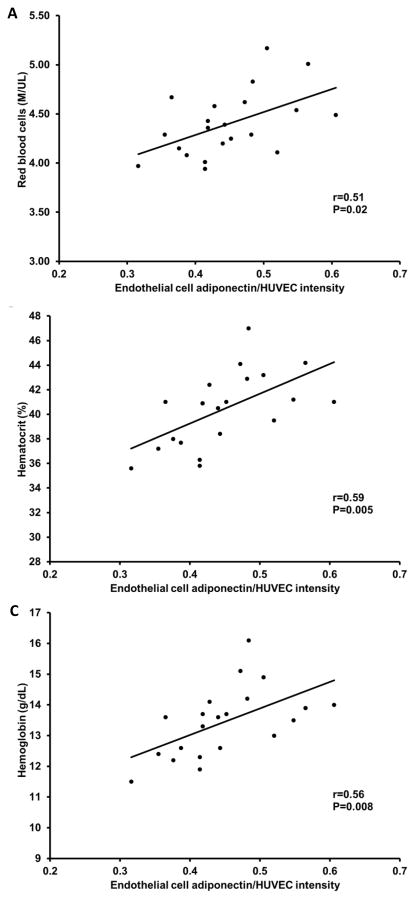
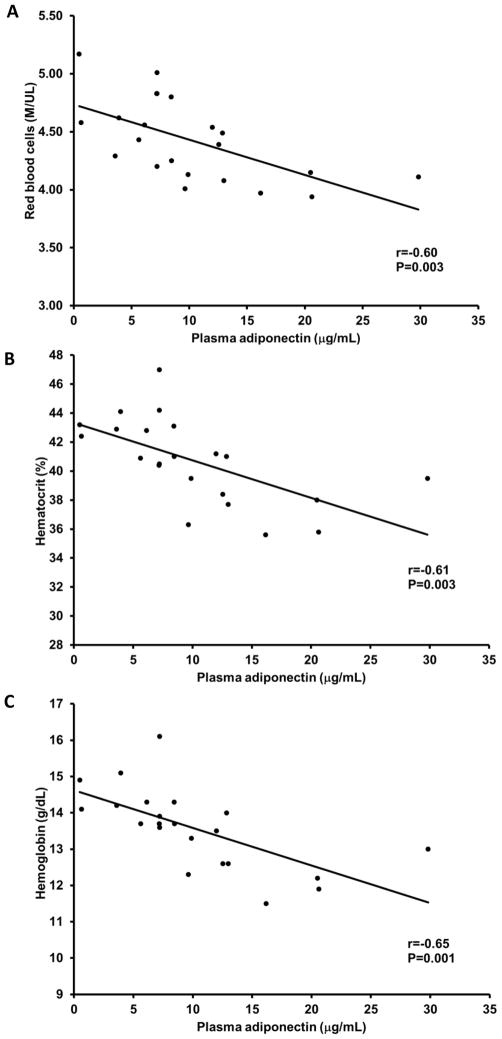
There was no relation between vascular endothelial cell adiponectin and circulating adiponectin (r=−0.14, P=0.6). As expected, higher circulating adiponectin levels were related with lower body mass index, waist circumference and fasting glucose, and higher HDL cholesterol (P<0.05; Table 2). Endothelial cell adiponectin, however, was not related with these factors (P=0.2 to 0.6). Both endothelial cell and circulating adiponectin were related with reduced renal function; endothelial cell adiponectin was positively related with blood urea nitrogen and serum creatinine (P=0.008 and P=0.04, respectively; Figure 4A and B), whereas, circulating adiponectin was inversely related with creatinine clearance (P=0.001; Figure 5). Interestingly, endothelial cell adiponectin was positively related with red blood cell count, hematocrit and hemoglobin concentration (P≤0.02; Figure 6A–C), whereas, circulating adiponectin was negatively related with these factors (P≤0.003; Figure 7A–C).
Table 2.
Correlation coefficients
| Endothelial cell adiponectin | Plasma adiponectin | |
|---|---|---|
| Body mass index | ns | −0.46* |
| Waist circumference | ns | −0.64* |
| Fasting glucose | ns | −0.49* |
| HDL cholesterol | ns | 0.65* |
ns: not significant (P≥0.2);
P<0.05
Figure 4.
Relation between endothelial cell adiponectin, blood urea nitrogen (A) and serum creatinine (B).
Figure 5.
Relation between circulating adiponectin and creatinine clearance.
Figure 6.
Relation between endothelial cell adiponectin, red blood cell count (A), hematocrit (B) and hemoglobin (C).
Figure 7.
Relation between circulating adiponectin red blood cell count (A), hematocrit (B) and hemoglobin (C).
Based on multiple linear regression analyses, the strength of the relation between endothelial cell adiponectin and FMD normalized for post-occlusion shear stress slightly decreased after accounting for baseline shear stress, blood urea nitrogen or serum creatinine (rpart=0.4 to 0.44), but the relation remained significant (P=0.02 to 0.045). When including red blood cells, hematocrit or hemoglobin as independent variables, these factors and FMD normalized for post-occlusion shear stress were not significant predictors of endothelial cell adiponectin in the model. This could be due to the existing correlations between each of these factors and FMD normalized for post-occlusion shear stress (r ≥0.72, P<0.0001). To construct a model with all the factors exhibiting the strongest independent relations with endothelial cell adiponectin, we performed stepwise linear regression model and included endothelial cell adiponectin as the dependent variable and all factors that were related with endothelial cell adiponectin as independent variables in the analysis. Only FMD normalized for shear stress (rpart=0.47, P=0.01) and blood urea nitrogen (rpart=0.40, P=0.02) remained in the final model (R2=0.52, P=0.02).
4. Discussion
We examined the levels of adiponectin in plasma and vascular endothelial cells collected from older adults free of overt cardiovascular disease. Our study demonstrates for the first time that higher levels of endothelial cell adiponectin are associated with higher vascular endothelial function in human aging and this relation is independent of circulating adiponectin levels. Another novel finding is the lack of association between circulating and endothelial cell adiponectin levels. Finally, both circulating and endothelial cell adiponectin are inversely related with renal function, but are differentially related with adiposity, metabolic and other related factors.
To our knowledge, the present data are the first to report adiponectin protein in vascular endothelial cells collected from humans. Our findings are in agreement with two recent investigations reporting adiponectin protein in freshly-isolated murine aortic endothelial cells (Komura et al. 2013) and in murine endothelial cell lines (Rutkowski et al. 2014). Currently, the mechanism of endothelial cell adiponectin uptake is unknown and whether the adiponectin receptors AdipoR1, AdipoR2 and T-Cadherin are involved remains uncertain.
Our finding of a direct relation between adiponectin measured in human endothelial cells and FMD provides new insight in the potential role of endothelial cell adiponectin in modulating nitric oxide bioavailability and vascular endothelial function in vivo. The lack of association with endothelium-independent dilation suggests that endothelial cell adiponectin does not influence vascular smooth muscle responsiveness to NO. We are not aware of other reports of adiponectin protein in human vascular endothelial cells, but previous studies on circulating adiponectin have demonstrated a significant positive association with endothelium-dependent dilation (r=0.1 to 0.3) and no association with endothelium-independent dilation (Margaritis et al. 2013; Ouchi et al. 2003; Saarikoski et al. 2010; Tan et al. 2004). In our data, circulating adiponectin was not significantly related with vascular endothelial function, but the magnitude of the correlation coefficient was similar with the published values reported above. It is possible that the relatively small sample size in our study might have contributed to the test not reaching statistical significance.
Our data demonstrate no association between circulating and endothelial cell adiponectin levels suggesting that the total amount of circulating adiponectin is not the driving factor for endothelial cell adiponectin levels. Furthermore, their discordant relations with other measures including vascular endothelial function suggest that the significant relation of endothelial cell adiponectin to FMD in our study is independent of the effects of circulating adiponectin. Our findings confirm previous investigations reporting that circulating adiponectin is inversely related with adiposity and glucose, and directly related with HDL cholesterol (Belalcazar et al. 2012; Cnop et al. 2003), however endothelial cell adiponectin is not related with these factors in our study. Our data also confirm that higher circulating adiponectin levels are associated with lower red blood cell count, hematocrit and hemoglobin concentration (Aso et al. 2009; Aso et al. 2012; Kawamoto et al. 2011). Surprisingly, in our study higher endothelial cell adiponectin levels are associated with higher levels of these factors, but the underlying mechanisms are unknown.
Our results are in agreement with previous reports demonstrating higher levels of circulating adiponectin are associated with reduced renal function (Kawamoto et al. 2010; Kruger et al. 2011). Consistent with these associations, endothelial cell adiponectin is also inversely associated with renal function. Based on the current incomplete understanding of the physiological functions of adiponectin, it is not possible to explain why endothelial cell adiponectin is inversely related with renal function, while it is positively related with vascular endothelial function. The mechanisms linking adiponectin (circulating or endothelial cell) with renal function are not currently known. With reduced renal function there might be upregulated compensatory production of adiponectin and entry into the endothelial cell (Turer and Scherer 2012), but this will need to be further elucidated by future studies. There is some evidence of elevated circulating adiponectin levels being partly due to low clearance rate by the kidney (Komura et al. 2010), but other studies indicate that adiponectin is not cleared by the kidney except in cases of severe proteinuria (von Eynatten et al. 2009).
Although it is unclear why lower levels of baseline shear stress are associated with higher levels of endothelial cell adiponectin, it is possible that adiponectin levels increase as a compensatory defense mechanism against atherogenesis. In support of this idea, low shear stress has been linked to increased uptake of atherogenic blood particles (Zarins et al. 1983) and atherogenesis (Malek et al. 1999), while adiponectin exerts anti-atherogenic effects (Okamoto et al. 2002) and has been found to localize in the endothelium of human injured vessels (Ouchi et al. 2001). Post-occlusion shear stress was also negatively related with endothelial cell adiponectin, but this association was not significant after controlling for baseline shear stress (r=−0.17, P=0.5). This suggests that the relation between post-occlusion shear stress and endothelial cell adiponectin is modulated by baseline shear stress.
Our study has some potential limitations. First, if one assumes that a positive relation exists between FMD and post-occlusion shear stress, then the positive relation between endothelial cell adiponectin and FMD and the negative association between endothelial cell adiponectin and post-occlusion shear stress appear to be contradictory and complicate interpretation of the results. However, in our data post-occlusion shear stress was not significantly related with FMD (P=0.8). In addition, based on the mathematical relation of post-occlusion shear stress to FMD/post-occlusion shear stress, these two factors are negatively related (r=−0.63, P=0.002); i.e., greater post-occlusion shear stress is associated with lower ratio of FMD/post-occlusion shear stress. Thus, the relations of adiponectin with vascular endothelial function and post-occlusion shear stress are not contradictory. Second, due to the limited number of endothelial cells that can be obtained in vivo from human subjects, it was not possible to use Western blotting to measure protein levels. Therefore, quantitative immunofluorescence was used to measure endothelial cell protein levels, but this approach has been validated against immunoblotting (Colombo et al. 2002; Silver et al. 2007). Despite the use of immunofluorescence in our study, several novel relations were identified. Third, our data are cross sectional, thus they cannot demonstrate a cause and effect relation between endothelial cell adiponectin levels and vascular endothelial function. However, our study provides the first evidence in humans supporting a significant association. Future investigations need to examine the independent effects of increasing or decreasing adiponectin levels on vascular endothelial function. Fourth, our subjects were older, thus our results might not be applicable to young adults. Additional studies are needed to investigate the relation between endothelial cell adiponectin levels and vascular endothelial function in healthy young adults.
Conclusions
Our data demonstrate for the first time that higher levels of adiponectin in endothelial cells are associated with higher in vivo vascular endothelial function independent of circulating adiponectin levels in older adults free from cardiovascular disease. Endothelial cell and circulating adiponectin are not related, and are differentially related to adiposity, metabolic and other blood factors. However, both endothelial cell and circulating adiponectin are inversely associated with renal function. Future studies designed to understand the mechanism of adiponectin uptake by endothelial cells and the specific adiponectin receptors/signaling cascade involved in modulating vascular endothelial function could potentially lead to the development of therapeutic strategies in atherosclerosis.
Highlights.
Endothelial cell adiponectin is positively related with flow-mediated dilation.
Circulating and endothelial cell adiponectin are not related.
Adiposity is related with circulating but not endothelial cell adiponectin.
Acknowledgments
This study was supported by the National Institutes of Health grant AG 032067 to DDC and American Heart Association grant 0865117F to DDC. The authors would like to thank Ms. Sharon Greer, R.N., Mr. Creighton Wilson R.N. and the Division of Cardiology at the Scott and White Clinic at College Station, Texas, for their contributions. The authors would also like to thank Ms. Molly Cernosek for technical assistance and the study participants for their time and efforts.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aso Y, Suganuma R, Wakabayashi S, Hara K, Nakano T, Suetsugu M, Matsumoto S, Nakamachi T, Takebayashi K, Morita K, Inukai T. Anemia is associated with an elevated serum level of high-molecular-weight adiponectin in patients with type 2 diabetes independently of renal dysfunction. Transl Res. 2009;154:175–182. doi: 10.1016/j.trsl.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Aso Y, Wakabayashi S, Terasawa T, Naruse R, Hara K, Takebayashi K, Inukai T. Elevation of serum high molecular weight adiponectin in patients with Type 2 diabetes and orthostatic hypotension: association with arterial stiffness and hypercoagulability. Diabet Med. 2012;29:80–87. doi: 10.1111/j.1464-5491.2011.03364.x. [DOI] [PubMed] [Google Scholar]
- Belalcazar LM, Lang W, Haffner SM, Hoogeveen RC, Pi-Sunyer FX, Schwenke DC, Balasubramanyam A, Tracy RP, Kriska AP, Ballantyne CM. Adiponectin and the mediation of HDL-cholesterol change with improved lifestyle: the Look AHEAD Study. J Lipid Res. 2012;53:2726–2733. doi: 10.1194/jlr.M030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Tao L, Yuan Y, Jiao X, Lau WB, Wang Y, Christopher T, Lopez B, Chan L, Goldstein B, Ma XL. Endothelial dysfunction in adiponectin deficiency and its mechanisms involved. J Mol Cell Cardiol. 2009;46:413–419. doi: 10.1016/j.yjmcc.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, Wong C, Xu A. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56:1387–1394. doi: 10.2337/db06-1580. [DOI] [PubMed] [Google Scholar]
- Christou DD, Pierce GL, Walker AE, Hwang MH, Yoo JK, Luttrell M, Meade TH, English M, Seals DR. Vascular smooth muscle responsiveness to nitric oxide is reduced in healthy adults with increased adiposity. Am J Physiol Heart Circ Physiol. 2012;303:H743–750. doi: 10.1152/ajpheart.00394.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- Colombo PC, Ashton AW, Celaj S, Talreja A, Banchs JE, Dubois NB, Marinaccio M, Malla S, Lachmann J, Ware JA, Le Jemtel TH. Biopsy coupled to quantitative immunofluorescence: a new method to study the human vascular endothelium. J Appl Physiol. 2002;92:1331–1338. doi: 10.1152/japplphysiol.00680.2001. [DOI] [PubMed] [Google Scholar]
- Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R International Brachial Artery Reactivity Task F. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force.[erratum appears in J Am Coll Cardiol 2002 Mar 20;39(6):1082] Journal of the American College of Cardiology. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang MH, Yoo JK, Luttrell M, Kim HK, Meade TH, English M, Segal MS, Christou DD. Mineralocorticoid receptors modulate vascular endothelial function in human obesity. Clin Sci (Lond) 2013;125:513–520. doi: 10.1042/CS20130200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto R, Tabara Y, Kohara K, Miki T, Abe M, Kusunoki T, Katoh T, Ohtsuka N. Serum high molecular weight adiponectin is associated with mild renal dysfunction in Japanese adults. J Atheroscler Thromb. 2010;17:1141–1148. doi: 10.5551/jat.5124. [DOI] [PubMed] [Google Scholar]
- Kawamoto R, Tabara Y, Kohara K, Miki T, Kusunoki T, Takayama S, Abe M. Hemoglobin is associated with serum high molecular weight adiponectin in Japanese community-dwelling persons. J Atheroscler Thromb. 2011;18:182–189. doi: 10.5551/jat.6379. [DOI] [PubMed] [Google Scholar]
- Komura N, Kihara S, Sonoda M, Maeda N, Tochino Y, Funahashi T, Shimomura I. Increment and impairment of adiponectin in renal failure. Cardiovasc Res. 2010;86:471–477. doi: 10.1093/cvr/cvp415. [DOI] [PubMed] [Google Scholar]
- Komura N, Maeda N, Mori T, Kihara S, Nakatsuji H, Hirata A, Tochino Y, Funahashi T, Shimomura I. Adiponectin protein exists in aortic endothelial cells. PLoS One. 2013;8:e71271. doi: 10.1371/journal.pone.0071271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger IM, Huisman HW, Schutte AE. The relationship between adiponectin, ageing and renal function in a bi-ethnic sample. Regul Pept. 2011;169:58–63. doi: 10.1016/j.regpep.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- Margaritis M, Antonopoulos AS, Digby J, Lee R, Reilly S, Coutinho P, Shirodaria C, Sayeed R, Petrou M, De Silva R, Jalilzadeh S, Demosthenous M, Bakogiannis C, Tousoulis D, Stefanadis C, Choudhury RP, Casadei B, Channon KM, Antoniades C. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation. 2013;127:2209–2221. doi: 10.1161/CIRCULATIONAHA.112.001133. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, Hotta K, Muraguchi M, Ohmoto Y, Yamashita S, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H, Kumada M, Ohashi K, Okamoto Y, Nishizawa H, Kishida K, Maeda N, Nagasawa A, Kobayashi H, Hiraoka H, Komai N, Kaibe M, Rakugi H, Ogihara T, Matsuzawa Y. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003;42:231–234. doi: 10.1161/01.HYP.0000083488.67550.B8. [DOI] [PubMed] [Google Scholar]
- Rutkowski JM, Halberg N, Wang QA, Holland WL, Xia JY, Scherer PE. Differential transendothelial transport of adiponectin complexes. Cardiovasc Diabetol. 2014;13:47. doi: 10.1186/1475-2840-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarikoski LA, Huupponen RK, Viikari JS, Marniemi J, Juonala M, Kahonen M, Raitakari OT. Adiponectin is related with carotid artery intima-media thickness and brachial flow-mediated dilatation in young adults--the Cardiovascular Risk in Young Finns Study. Ann Med. 2010;42:603–611. doi: 10.3109/07853890.2010.514284. [DOI] [PubMed] [Google Scholar]
- Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, Hamburg NM, Frame AA, Caiano TL, Kluge MA, Duess MA, Levit A, Kim B, Hartman ML, Joseph L, Shirihai OS, Vita JA. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124:444–453. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver A, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, Gates PE, Seals DR. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H Oxidase-p47phox expression and evidence of endothelial oxidative stress. Circulation. 2007;115 doi: 10.1161/CIRCULATIONAHA.106.657486. [DOI] [PubMed] [Google Scholar]
- Silver AE, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, Gates PE, Seals DR. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47(phox) expression and evidence of endothelial oxidative stress. Circulation. 2007;115:627–637. doi: 10.1161/CIRCULATIONAHA.106.657486. [DOI] [PubMed] [Google Scholar]
- Silver AE, Christou DD, Donato AJ, Beske SD, Moreau KL, Magerko KA, Seals DR. Protein expression in vascular endothelial cells obtained from human peripheral arteries and veins. J Vasc Res. 2010;47:1–8. doi: 10.1159/000231715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KC, Xu A, Chow WS, Lam MC, Ai VH, Tam SC, Lam KS. Hypoadiponectinemia is associated with impaired endothelium-dependent vasodilation. J Clin Endocrinol Metab. 2004;89:765–769. doi: 10.1210/jc.2003-031012. [DOI] [PubMed] [Google Scholar]
- Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300:H2–12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- von Eynatten M, Liu D, Hock C, Oikonomou D, Baumann M, Allolio B, Korosoglou G, Morcos M, Campean V, Amann K, Lutz J, Heemann U, Nawroth PP, Bierhaus A, Humpert PM. Urinary adiponectin excretion: a novel marker for vascular damage in type 2 diabetes. Diabetes. 2009;58:2093–2099. doi: 10.2337/db09-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi W, Satoh H, Kase H, Suzuki K, Hattori Y. Stimulated HSP90 binding to eNOS and activation of the PI3-Akt pathway contribute to globular adiponectin-induced NO production: vasorelaxation in response to globular adiponectin. Biochem Biophys Res Commun. 2005;332:200–205. doi: 10.1016/j.bbrc.2005.04.111. [DOI] [PubMed] [Google Scholar]
- Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53:502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]