Abstract
Hyperbaric oxygen preconditioning (HBO-PC) has been demonstrated to attenuate hemorrhagic transformation (HT) after middle cerebral artery occlusion (MCAO) in hyperglycemic rats. However, the mechanisms remain to be illustrated. Recently, HBO-PC has been shown to activate peroxisome proliferator-activated receptor-gamma (PPARγ) by increasing 15d-PGJ2 in primary cultured neurons. We hypothesize that HBO-PC reduces HT by suppressing inflammation through increasing 15d-PGJ2 and activating PPARγ in hyperglycemic MCAO rats. HBO (2.5 ATA) was administrated for 1 hour daily for 5 consecutive days. The PPARγ inhibitor GW9662 was administrated intraperitoneally to designated animals. Infarction volume, hemorrhage volume, neurological scores and mortality were analyzed. The levels of 15d-PGJ2, PPARγ, TNF-α and IL-1β, tight junction proteins as well as the activity of MMP-2 and MMP-9 were evaluated 24 hours after MCAO. HBO-PC reduced HT, improved neurological function, down-regulated inflammatory molecules and inhibited the activation of MMP-9 by increasing 15d-PGJ2 and PPARγ at 24 hours after MCAO. The results suggested HBO-PC might be an alternative measure to decrease HT in ischemic stroke.
Keywords: Hyperbaric oxygen preconditioning, PPARγ, hemorrhagic transformation, MCAO
Introduction
Hemorrhagic transformation (HT) is one of the major side effects of tissue plasminogen activator (tPA) treatment and occurs in 5% to 10% of stroke patients (Donnan et al., 2011). Emerging data show that pre-ischemic hyperglycemia dramatically aggravated brain infarct and HT in middle cerebral artery occlusion (MCAO) rat model (Cipolla and Godfrey, 2010; Paciaroni et al., 2009; Xing et al., 2011). The development of HT in hyperglycemic rats may be associated with increased oxidative stress and inflammatory activity (Fabian and Kent, 2013), which causes blood brain barrier (BBB) disruption (Chiu et al., 2013; Paciaroni et al., 2009; Fagan et al., 2013). Preventing inflammation and stabilizing BBB against breakdown may reduce tPA side effects and improve patient outcomes (Hafez et al., 2014).
Preconditioning has been reported to be a promising therapeutic strategy against the ischemia/reperfusion injury in experiment animals (Dezfulian et al., 2013; Koch, 2012). Previously we have demonstrated that hyperbaric oxygen preconditioning (HBO-PC) strongly suppressed HT and improved neurological deficits in hyperglycemic MCAO rats (Soejima et al., 2013; Soejima et al., 2012); however, the mechanisms remain to be illustrated. Peroxisome proliferator activated receptor-γ (PPARγ) is a member of the nuclear hormone receptor superfamily, and plays a central role in the regulation of apoptosis (Chang and Szabo, 2000), oxidative stress and inflammation (Lehrke and Lazar, 2005) as well as affords significant neuroprotection against cerebral ischemia reperfusion injury (Wu et al., 2009). 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) is an endogenous ligand with a high affinity for PPARγ. Activation of PPARγ has been reported to preserve the integrity of the BBB in type 2 diabetic mice (Min et al., 2012). Recently, Zeng and his colleagues found that HBO-PC significantly increased the levels of 15d-PGJ2, PPARγ mRNA and protein in primary cultured cortical neurons with OGD exposure (Zeng et al., 2012). Therefore, in the present study, we will investigate whether HBO-PC attenuates hyperglycemia-induced HT in MCAO rats and protect BBB through increasing 15d-PGJ2 and activating PPARγ signal pathway.
Materials and Methods
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All protocols were approved by the Institutional Animal Care and Use Committee of Loma Linda University.
Animal Groups and Interventions
Two hundred and eighty-six male Sprague-Dawley rats were purchased from Harlan Laboratories (Indianapolis, IN). HBO at 2.5 ATA was administrated for 1 hour daily for 5 consecutive days. 50% dextrose (DX, 6 ml/kg) was injected intraperitoneally 30 minutes before MCAO to induce acute hyperglycemia. PPARγ inhibitor GW9662 was administrated intraperitoneally to designated animals for intervention. Animals were divided into six groups: DX+sham (n=20), DX+MCAO (n=54), DX+HBO+MCAO (n=50), DX+NBO+MCAO (n=50), DX+HBO+MCAO+GW9662 (n=50), and DX+HBO+MCAO+DMSO (n=48). Infarction volume, hemorrhage volume, neurological scores and mortality were measured for outcome study. The protein level of PPARγ, TNF-α and IL-1β, as well as the activity of MMP-2 and MMP-9 were evaluated at 24 hours after MCAO. Two additional groups of animals (Naïve and Naïve+HBO-PC, n=6 for each group) were added to measure the effects of HBO-PC on the level of 15d-PGJ2 in naïve rats.
Hyperglycemia Induction and MCAO
All rats received 50% dextrose (6 ml/kg) intraperitoneally 30 minutes before MCAO to induce acute hyperglycemia. Anesthesia was induced with ketamine and xylazine (80 mg/kg and 10 mg/kg respectively, intraperitoneally), followed by atropine at a dose of 0.1 mg/kg subcutaneously. During the surgery and postoperative period, rectal temperature was maintained at 37.0°C by using a feedback-controlled heating pad. MCAO was performed as previously reported (Hu et al., 2011). Briefly, the right external carotid artery was isolated and coagulated. A 4-0 nylon suture with a round tip was inserted into the internal carotid artery through the external carotid artery stump and advanced to occlude the origin of middle cerebral artery. The suture was removed at 2 hours after occlusion. Sham operated rats underwent the same surgical procedures without insertion of the suture.
HBO-PC Regimen
Due to its potential toxic effects, HBO is currently restricted to short sessions (less than 2 hours), at pressures below the threshold of central nervous system toxicity (0.3 MPa) (Tibbles and Edelsberg, 1996). In our previous studies and preliminary experiments, we tested HBO-PC at 1, 1.5, 2, 2.5, and 3 ATA and found 2.5–3 ATA produced more pronounced protective results compared to 1–2 ATA. Therefore in this study, we used HBO at 2.5 ATA for 1 hour.
Rats were pressurized in a research hyperbaric chamber (1300B; Sechrist) at 2.5 atmospheres absolutes with 100% oxygen (flow of 22 L/min). Compression and decompression were maintained at a rate of 5 psi/min. HBO was administered for 1 hour daily for 5 consecutive days and the last session was performed 24 hours before MCAO. In the intervention groups, PPAR inhibitor GW9662 (4mg/kg) or 10% dimethyl sulfoxide (DMSO, vehicle) was injected intraperitoneally 1 hour before MCAO.
2,3,5-triphenyltetrazolium Chloride (TTC) Staining
TTC staining was performed to determine the infarct volume at 24 hours after MCAO as previously reported (Hu et al., 2011). The possible interference of brain edema with infarct volume was corrected by standard methods (whole contralateral hemisphere volume – nonischemic ipsilateral hemisphere volume) and the infracted volume was expressed as a percentage of the whole contralateral hemisphere.
Spectrophotometric Assay of Hemoglobin
Hemorrhagic volume was quantified with spectrophotometric assay of brain hemoglobin content at 24 hours after MCAO. Cerebral hemorrhage was quantified using a previously described spectrophotometric assay (Hu et al., 2011). A standard curve was obtained using a “virtual” model of hemorrhage. Incremental volumes of homologous blood (0, 2, 4, 8, 16, 32 μl) were added to the perfused brain tissue. The hemispheric brain was then homogenized in distilled water followed by 30-minute centrifugation (13,000 g). Drabkin reagent (1.6 ml; Sigma) was added to 0.4 ml supernatant aliquots and optical density was measured at 540 nm via spectrophotometer (Spectronix 3000; Milton-Roy). Hemoglobin measurements were performed and compared with the standard curve to obtain data in terms of hemorrhage volume. The total hemispheric hemoglobin content was expressed as μl of blood per hemisphere.
Neurological Scores
A neurological examination was performed by a blinded investigator as previously described with modifications (Garcia et al., 1995) at 24 hours after MCAO. The scores given to each rat at completion of the evaluation was the summation of 7 individual test scores (spontaneous activity, symmetry in the movement of four limbs, forepaw outstretching, climbing, body proprioception, response to vibrissae touch, and beam walking). The neurological scoring ranged from 3 (most severe deficit) to 21 (maximum).
Enzyme-linked Immunosorbent Assay (ELISA) for 15d-PGJ2
15d-PGJ2 brain levels were determined using ELISA kit (MyBioSource, Inc, Canada). Rats were fatally anesthetized with isoflurane (≥ %5) followed by cardiovascular perfusion with ice-cold 0.01M phosphate buffered saline. Brains were flash frozen in liquid nitrogen immediately following removal and kept in −80°C. Right hemisphere of the brain tissues were homogenized by sonication in an ice-cold 0.01M phosphate buffered saline and centrifuged for 15 minutes at 1500×g. The supernatants were collected and Aliquots were stored at −80°C until tested. Enzyme immunoassay isolation and prostaglandin quantification were carried out following manufacturer’s instructions. Protein concentration of each sample was determined using a Bio-Rad protein assay kit.
Western Blot Analysis
Animals were anesthetized and underwent transcardiac perfusion using 0.01 M PBS until colorless perfusion fluid was obtained from the right atrium. Tissue samples of the ipsilateral hemisphere were obtained and immersed in 0.5 ml of the Western blot sample buffer and then sonicated for Western blot analysis. Protein concentration of each sample was determined using a Bio-Rad protein assay kit. The Western blot analysis was performed as previously described(Hu et al., 2011). Primary antibodies used were: PPARγ (sc-271392, Santa Cruz Biotechnology), TNF-α (sc-243, Santa Cruz Biotechnology), IL-1β (sc-554859, Santa Cruz Biotechnology), ZO-1 (sc-10804, Santa Cruz Biotechnology), Occludin (sc-5562, Santa Cruz Biotechnology) and β-actin (sc-1616, Santa Cruz Biotechnology).
MMPs Zymography
Similarly prepared protein samples as for Western blot were subjected to gelatin zymography. Approximately 50 μg of each sample was loaded per lane into the well of precast gels (10% polyacrylamide minigels containing 0.1% gelatin; Invetrogen) with SDS running buffer (1:1; Novex). Electrophoresis was performed with a Tris-glycine running buffer at 125 V constant voltage for 1.5–2 h. The gel was removed and incubated for 1 hour at room temperature in 100 ml of 2.7% Triton X-100 (renature buffer, Invetrogen) on a rotary shaker, then washed with distilled water 10 times. Each gel was incubated with 100 ml of development buffer (50 mM Tris base, 40 mM HCl, 200 mM NaCl, 5 mM CaCl2, and 0.2% Brij 35; Invetrogen) at 37°C for 14 hours on a rotary shaker. Staining was performed with 100 ml of 0.5% Coomassie blue G-250 in 30% methanol and 10% acetic acid for at least 1 hour, and gels were then destained with three changes of solutions. Gelatinolytic activity was demonstrated as clear zones or bands at the appropriate molecular weights. Human MMP-9 and human MMP-2 (from Chemicon Temecula, CA) were used as standards. The activity of MMP-2 and MMP-9 was quantified in ImageJ software.
Statistical Analyses
Data were expressed as the mean ± SEM. Statistical differences among groups were analyzed by using ANOVA followed by the Tukey method. Mortality was evaluated by Fisher’s exact test. P < 0.05 was considered statistically significant.
Results
HBO-PC attenuated HT after MCAO in hyperglycemic rats dependent on activation of PPARγ
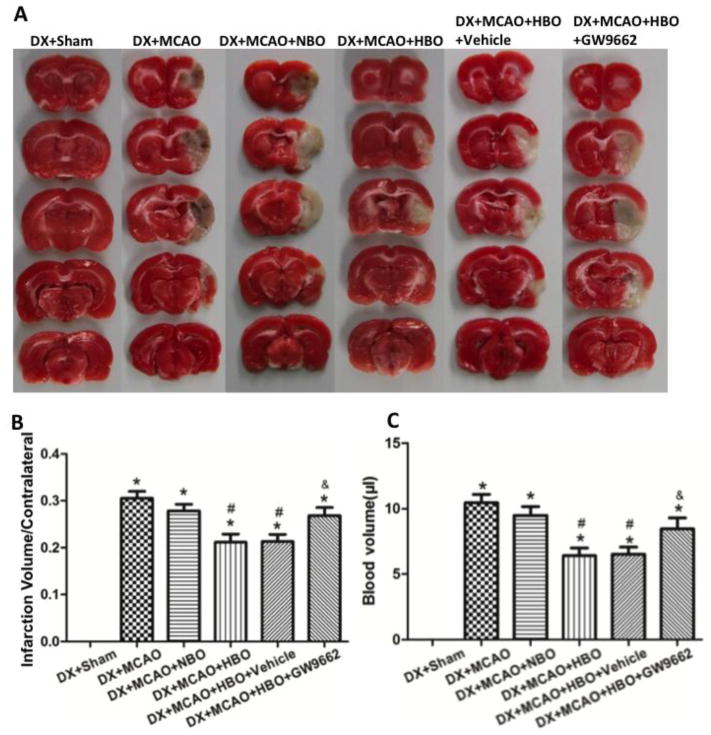
Hyperglycemia induced extensive HT in ischemic territories at 24 hours after MCAO (Figures 1A and 1B). HBO-PC reduced infarct volume (Figures 1A and 1B, p<0.05 vs. DX+MCAO) and hemorrhage volume (Figure 1C, p<0.05 vs. DX+MCAO) significantly. After MCAO, animals showed obvious neurological deficits (Figure 2A, p<0.05 vs. DX+Sham) and exhibited serious brain edema (Figure 2B, p<0.05 vs. DX+Sham); HBO-PC significantly improved neurological functions (Figure 2A, p<0.05 vs. DX+MCAO) and attenuated brain edema (Figure 2B, p<0.05 vs. DX+MCAO) at 24 hours after MCAO. Blocking PPARγ by its inhibitor GW9662 after each HBO-PC abolished the beneficial effects of HBO-PC (Figure 1 and 2, p<0.05 vs. DX+MCAO +HBO). NBO showed the tendency to ameliorate the outcomes, but no significant difference (Figure 1 and 2, p>0.05 vs. DX+MCAO).
Figure 1.
HBO-PC attenuated HT after MCAO in hyperglycemic rats dependent on activation of PPARγ. (A) Representative pictures for TTC staining. (B) HBO-PC effectively reduced the infarct volume and PPARγ inhibitor GW9662 abolished the effects at 24 hours after MCAO in hyperglycemic rats. (C) HBO-PC attenuated the hemorrhage volume significantly and GW9662 reversed the results of HBO-PC. *p<0.05 vs. DX+Sham; #p<0.05 vs. DX+MCAO; &p<0.05 vs. DX+MCAO+HBO. N=6 for each group.
Figure 2.
HBO-PC decreased brain water content (A), improved neurological deficits (B) and showed tendency to reduce mortality (C) at 24 hours after MCAO. HBO-PC had no effects on blood glucose level (D). *p<0.05 vs. DX+Sham; #p<0.05 vs. DX+MCAO. N=6 in each group in (A) and (B); n=12 in each group in (D).
The mortality at 24 hour after surgery in each group is: Sham+DX, 0 (0/20); DX+MCAO, 0.3333 (18/54); DX+MCAO+NBO, 0.2800 (14/50); DX+MCAO+HBO, 0.2400 (12/50); DX+MCAO+HBO+vehicle, 0.2500 (12/48); DX+MCAO+HBO+GW9662, 0.3077 (16/52). HBO-PC showed the tendency to decrease mortality, but there was no significant difference compared with DX+MCAO group (Figure 2C, p>0.05 vs. DX+MCAO).
The blood glucose levels at 2 hours after injection of dextrose in all groups were significantly higher than the baseline and high glucose level lasted until 6 hours after injection. HBO-PC had no effects on the blood glucose level (Figure 2D).
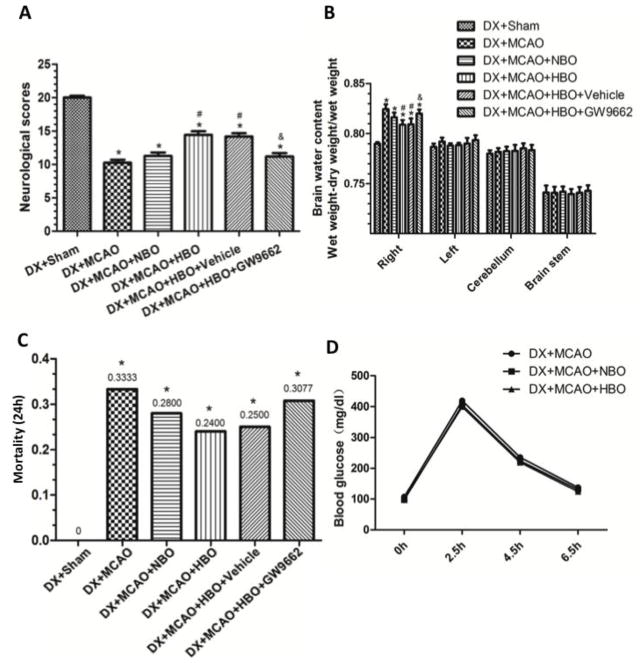
HBO-PC increased the level of 15d-PGJ2 and PPARγ at 24 hour after MCAO
We measured the levels of PPARγ ligand 15d-PGJ2 in brain tissue by ELISA and evaluated the expression of PPARγ by Western blots. HBO-PC significantly increased the level of 15d-PGJ2 in naïve rats 24 hours after the last HBO exposure. (Figure 3A, p<0.05 vs. Naïve). The level of 15d-PGJ2 in sham and MCAO animals were relatively low, and HBO-PC significantly increased the level of 15d-PGJ2 at 24 hours after MCAO (Figure 3B, p<0.05 vs. DX+MCAO). PPARγ was seldom expressed in sham animals, and mildly increased 24 hours after MCAO (Figure 3C, p>0.05 vs. DX+Sham). HBO-PC greatly up-regulated the expression of PPARγ (Figure 3C, p<0.05 vs. DX+MCAO).
Figure 3.
HBO-PC increased 15d-PGJ2 and PPARγ at 24 hours after MCAO. (A) HBO-PC increased the level of 15d-PGJ2 in naïve rats 24 hours after the last HBO exposure. (B) The level of 15d-PGJ2 in sham and MCAO animals were relatively low, and HBO-PC significantly increased the level of 15d-PGJ2 at 24 hours after MCAO. (C) PPARγ mildly increased 24 hours after MCAO and HBO-PC greatly upregulated the expression of PPARγ. @P<0.05 versus Naïve; *p<0.05 vs. DX+Sham; #p<0.05 vs. DX+MCAO. Naïve, Naïve+HBO-PC, and Sham, n=6; other groups, n=12.
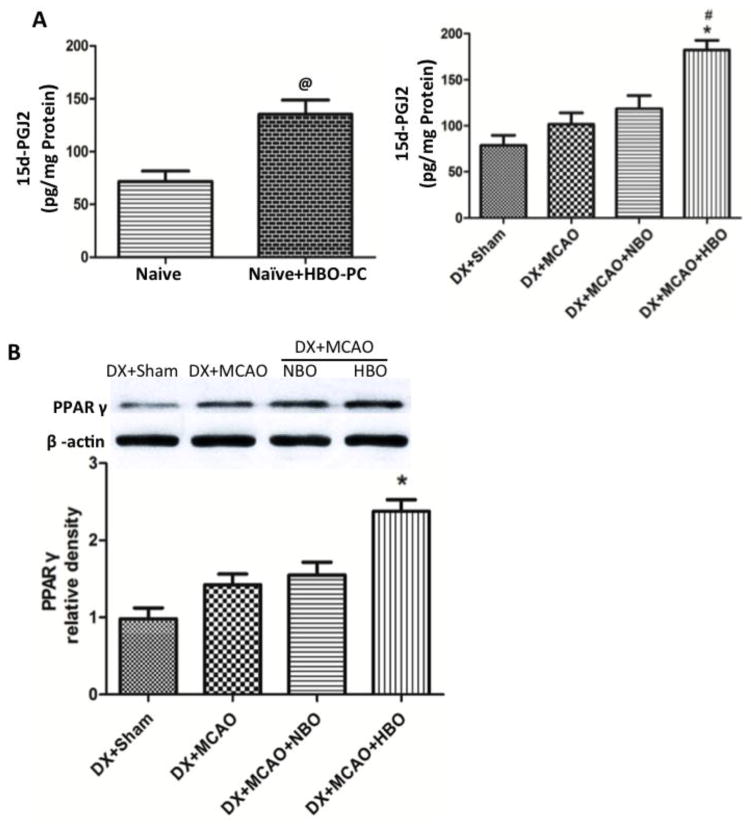
HBO-PC suppressed inflammation and attenuated BBB disruption by decreasing MMP-9 at 24 hour after MCAO
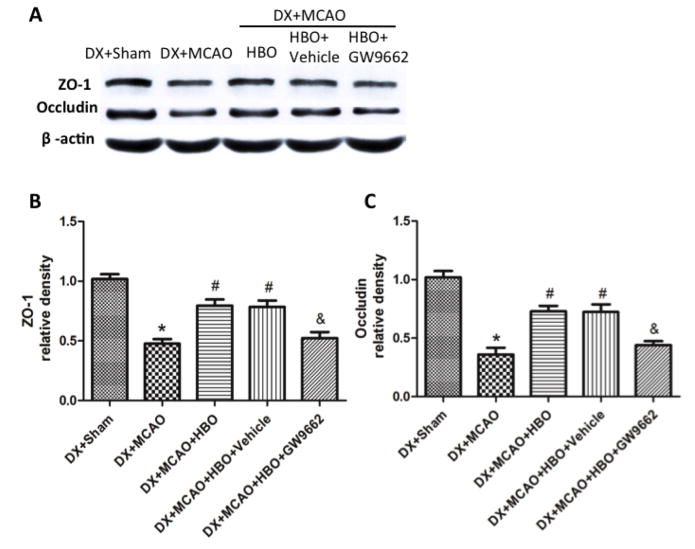
Twenty-four hours after MCAO, the expression of inflammatory molecules TNF-α and IL-1β increased, and HBO-PC effectively suppressed the increase of TNF-α and IL-1β (Figure 4, p<0.05 vs. DX+Sham). Administration of GW9662 before each HBO-PC removed the effects of HBO-PC (Figure 4, p<0.05 vs. DX+MCAO+HBO-PC). The protein level of tight junction proteins ZO-1 and Occludin dramatically decreased after MCAO, and increased by HBO-PC (Figure 4, p<0.05 vs. DX+MCAO).
Figure 4.
HBO-PC suppressed inflammation by increasing PPARγ at 24 hours after MCAO. (A) Representative Western blots of TNF-α and IL-1β. (B) and (C) HBO-PC effectively suppressed the increase of TNF-α and IL-1β, and administration of GW9662 before each HBO-PC removed the effects of HBO-PC. *p<0.05 vs. DX+Sham; #p<0.05 vs. DX+MCAO; &p<0.05 vs. DX+MCAO+HBO. Sham, n=6; other groups, n=12.
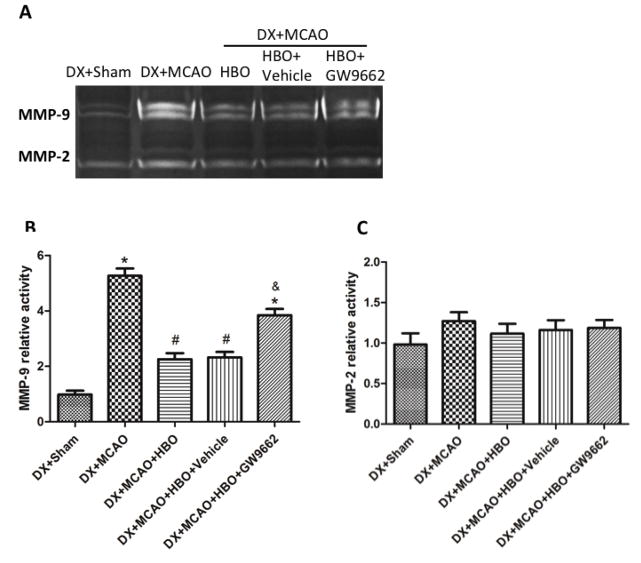
In hyperglycemic rats, the activity of MMP-9 was greatly increased at 24 hours after MCAO (p<0.05 vs. DX+Sham, Figure 5A, 5B), HBO-PC remarkably decreased the activity of MMP-9 (p<0.05 vs. DX+MCAO, Figure 5A, 5B); PPARγ inhibitor GW9662 reversed the results of HBO-PC and increased the activity of MMP-9 (p<0.05 vs. DX+MCAO+HBO-PC, Figure 5A, 5B). HBO-PC had no remarkable effect on the activity of MMP-2 (p>0.05 vs. DX+MCAO, Figure 5A, 5C).
Figure 5.
HBO-PC increased ZO-1 and Occludin through activating PPARγ. (A) Representative Western blots of ZO-1 and Occludin. (B) and (C) HBO-PC significantly increased ZO-1 and Occludin, and administration of GW9662 ablished the effects of HBO-PC.*P<0.05 versus DX+Sham, #P<0.05 versus DX+MCAO, &P<0.05 versus DX+MCAO+HBO. Sham, n=6; other groups, n=12.
Discussion
In the present study, we observed that HBO-PC ameliorated the hyperglycemia-enhanced HT and improved neurologic deficits in rats subjected to MCAO. Administration of PPARγ inhibitor GW9662 abolished the beneficial effects of HBO-PC. HBO-PC prevented HT by down-regulation of inflammatory molecules TNF-α and IL-1β and suppressing the activity of MMP-9, which are mediated by up-regulating 15d-PGJ2 and activating PPARγ. Our results strongly suggested that HBO-PC might turn out to be an attractive strategy for HT in ischemic stroke patients.
15d-PGJ2 is one of the prostaglandins of the J series (PGJs), which are cyclopentenones synthesized from arachidonic acid via enzymatic conversion by cyclooxygenases (COXs, COX-1 and COX-2, respectively) and PGD synthase followed by non-enzymatic dehydration from PGD2 to a series of PGJs (Nosjean and Boutin, 2002). Acting as a physiologic agonist for PPARγ, 15d-PGJ2 has been considered as a potential endogenous protectant counteracting deleterious actions of the pro-inflammatory response in various stressful conditions. An analysis of 552 patients with an acute stroke admitted within 24 hours after symptom onset showed that increased plasma 15d-PGJ2 concentration is associated with good early and late neurologic outcome and smaller infarct volume, suggesting beneficial role of 15d-PGJ2 for stroke patients (Blanco et al., 2005). In experiment stroke models, 15d-PGJ2 has been proven to be neuroprotective through anti-oxidation (Lin et al., 2006) and anti-inflammation (Zhao et al., 2006). HBO-PC protected primary cultured cortical neurons against oxygen-glucose deprivation by releasing 15d-PGJ2 through activation of COX-2 (Zeng et al., 2012). In agreement with these studies, we observed an improvement of neurological function and attenuation of HT in hyperglycemic MCAO rats after HBO-PC, which is associated with suppressing inflammation and preserving tight junction proteins by the increasing 15d-PGJ2. The rate-limiting step in the prostaglandin biosynthesis is the COX-catalyzed conversion of arachidonic acid mobilized from the membrane phospholipid to prostaglandin H2 (PGH2) (Surh et al., 2011). In the mouse model of surgical brain injury or rat model of transient global cerebral ischemia, HBO-PC alone significantly increased COX-2 levels (Cheng et al., 2011; Jadhav et al., 2009). Oxygen has been showed to promote prostacyclin synthesis in intact intrapulmonary arteries, which may be mediated by increasing the activity of COX-2 (Shaul et al., 1992; Shaul et al., 1993).
After activated by 15d-PGJ2, PPARγ forms a heterodimer with another nuclear receptor PPAR-retinoid X receptor (RXR)-α and bind to PPAR response elements in the promoter of their target genes. Recent data have shown that PPARγ acts as a regulator of CNS inflammation and is a powerful pharmacological target for CNS disease (Gillespie et al., 2011). After stroke, the activation of PPARγ can antagonize the harmful effects of oxidative stress and inflammation, indicating a promising and neuroprotective role for PPARγ agonists in stroke (Culman et al., 2007). Activation of PPARγ attenuated the expression of ICAM-1, matrix metalloproteinase (MMP)-9 and various inflammatory cytokines in ischemic brain tissue (Luo et al., 2006; Pereira et al., 2005; Sundararajan et al., 2005). In agreement with these studies, HBO-PC activated PPARγ signaling pathway and significantly decreased inflammation and suppressed the activity of MMP-9 in the present study. HBO-PC attenuated the BBB disruption and therefor reduced HT in the hyperglycemic MCAO rats.
In conclusion, we demonstrated that hyperglycemia enhanced HT after MCAO; HBO-PC attenuated HT and improved neurological function in MCAO rats. HBO-PC preserved the integrity of BBB by suppressing inflammation through activating PPARγ pathway and decreased inflammation. Our results suggested that HBO-PC might be a promising approach to reduce HT in ischemic stroke.
Figure 6.
HBO-PC inhibited the activity of MMP-9 by activation of PPARγ at 24 hours after MCAO. (A) Representative bands for zymography. (B) and (C) Statistical analysis for MMP-9 and MMP-2. HBO-PC remarkably decreased the activity of MMP-9, and had no effects on the activity of MMP-2; PPARγ inhibitor GW9662 increased the activity of MMP-9 after HBO-PC. *p<0.05 vs. DX+Sham; #p<0.05 vs. DX+MCAO; &p<0.05 vs. DX+MCAO+HBO. Sham, n=6; other groups, n=12.
Highlights.
HBO-PC improved neurological deficits and reduced hemorrhagic transformation after MCAO.
HBO-PC increased the level of 15d-PGJ2 and PPARγ.
HBO-PC decreased inflammation and suppressed the activity of MMP-9.
PPARγ inhibitor GW9662 removed the effects of HBO-PC.
HBO-PC has potentials to reduce hemorrhagic transformation for ischemic stroke patients
Acknowledgments
This research was conducted with funding by a grant from NIH NS043338 to J.H. Zhang and the National Natural Science Foundation of China (81301012) to Hetao Bian.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blanco M, Moro MA, Davalos A, Leira R, Castellanos M, Serena J, Vivancos J, Rodriguez-Yanez M, Lizasoain I, Castillo J. Increased plasma levels of 15-deoxyDelta prostaglandin J2 are associated with good outcome in acute atherothrombotic ischemic stroke. Stroke. 2005;36:1189–1194. doi: 10.1161/01.STR.0000166054.55993.e5. [DOI] [PubMed] [Google Scholar]
- Chang TH, Szabo E. Induction of differentiation and apoptosis by ligands of peroxisome proliferator-activated receptor gamma in non-small cell lung cancer. Cancer Res. 2000;60:1129–1138. [PubMed] [Google Scholar]
- Cheng O, Ostrowski RP, Wu B, Liu W, Chen C, Zhang JH. Cyclooxygenase-2 mediates hyperbaric oxygen preconditioning in the rat model of transient global cerebral ischemia. Stroke. 2011;42:484–490. doi: 10.1161/STROKEAHA.110.604421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CD, Chen CC, Shen CC, Chin LT, Ma HI, Chuang HY, Cho DY, Chu CH, Chang C. Hyperglycemia exacerbates intracerebral hemorrhage via the downregulation of aquaporin-4: temporal assessment with magnetic resonance imaging. Stroke. 2013;44:1682–1689. doi: 10.1161/STROKEAHA.113.675983. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Godfrey JA. Effect of hyperglycemia on brain penetrating arterioles and cerebral blood flow before and after ischemia/reperfusion. Transl Stroke Res. 2010;1:127–134. doi: 10.1007/s12975-010-0014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culman J, Zhao Y, Gohlke P, Herdegen T. PPAR-gamma: therapeutic target for ischemic stroke. Trends Pharmacol Sci. 2007;28:244–249. doi: 10.1016/j.tips.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Donnan GA, Davis SM, Parsons MW, Ma H, Dewey HM, Howells DW. How to make better use of thrombolytic therapy in acute ischemic stroke. Nat Rev Neurol. 2011;7:400–409. doi: 10.1038/nrneurol.2011.89. [DOI] [PubMed] [Google Scholar]
- Dezfulian C, Garrett M, Gonzalez NR. Clinical application of preconditioning and postconditioning to achieve neuroprotection. Transl Stroke Res. 2013;4:19–24. doi: 10.1007/s12975-012-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian RH, Kent TA. Hyperglycemia accentuates persistent “functional uncoupling” of cerebral microvascular nitric oxide and superoxide following focal ischemia/reperfusion in rats. Transl Stroke Res. 2012;3:482–490. doi: 10.1007/s12975-012-0210-9. [DOI] [PubMed] [Google Scholar]
- Fagan SC, Lapchak PA, Liebeskind DS, Ishrat T, Ergul A. Recommendations for preclinical research in hemorrhagic transformation. Transl Stroke Res. 2013;4:322–327. doi: 10.1007/s12975-012-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. discussion 635. [DOI] [PubMed] [Google Scholar]
- Gillespie W, Tyagi N, Tyagi SC. Role of PPARgamma, a nuclear hormone receptor in neuroprotection. Indian J Biochem Biophys. 2011;48:73–81. [PubMed] [Google Scholar]
- Hafez S, Coucha M, Bruno A, Fagan SC, Ergul A. Hyperglycemia, acute ischemic stroke, and thrombolytic therapy. Transl Stroke Res. 2014;5:442–453. doi: 10.1007/s12975-014-0336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Ma Q, Zhan Y, He Z, Tang J, Zhou C, Zhang J. Isoflurane enhanced hemorrhagic transformation by impairing antioxidant enzymes in hyperglycemic rats with middle cerebral artery occlusion. Stroke. 2011;42:1750–1756. doi: 10.1161/STROKEAHA.110.603142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav V, Ostrowski RP, Tong W, Matus B, Jesunathadas R, Zhang JH. Cyclo-oxygenase-2 mediates hyperbaric oxygen preconditioning-induced neuroprotection in the mouse model of surgical brain injury. Stroke. 2009;40:3139–3142. doi: 10.1161/STROKEAHA.109.549774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S. Moving towards preconditioning for neurological disorders: are we ready for clinical trials? Transl Stroke Res. 2013;4:15–18. doi: 10.1007/s12975-012-0220-7. [DOI] [PubMed] [Google Scholar]
- Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Lin TN, Cheung WM, Wu JS, Chen JJ, Lin H, Chen JJ, Liou JY, Shyue SK, Wu KK. 15d-prostaglandin J2 protects brain from ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:481–487. doi: 10.1161/01.ATV.0000201933.53964.5b. [DOI] [PubMed] [Google Scholar]
- Luo Y, Yin W, Signore AP, Zhang F, Hong Z, Wang S, Graham SH, Chen J. Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. J Neurochem. 2006;97:435–448. doi: 10.1111/j.1471-4159.2006.03758.x. [DOI] [PubMed] [Google Scholar]
- Min LJ, Mogi M, Shudou M, Jing F, Tsukuda K, Ohshima K, Iwanami J, Horiuchi M. Peroxisome proliferator-activated receptor-gamma activation with angiotensin II type 1 receptor blockade is pivotal for the prevention of blood-brain barrier impairment and cognitive decline in type 2 diabetic mice. Hypertension. 2012;59:1079–1088. doi: 10.1161/HYPERTENSIONAHA.112.192401. [DOI] [PubMed] [Google Scholar]
- Nosjean O, Boutin JA. Natural ligands of PPARgamma: are prostaglandin J(2) derivatives really playing the part? Cellular signal. 2002;14:573–583. doi: 10.1016/s0898-6568(01)00281-9. [DOI] [PubMed] [Google Scholar]
- Paciaroni M, Agnelli G, Caso V, Corea F, Ageno W, Alberti A, Lanari A, Micheli S, Bertolani L, Venti M, Palmerini F, Billeci AM, Comi G, Previdi P, Silvestrelli G. Acute hyperglycemia and early hemorrhagic transformation in ischemic stroke. Cerebrovasc Dis. 2009;28:119–123. doi: 10.1159/000223436. [DOI] [PubMed] [Google Scholar]
- Pereira MP, Hurtado O, Cardenas A, Alonso-Escolano D, Bosca L, Vivancos J, Nombela F, Leza JC, Lorenzo P, Lizasoain I, Moro MA. The nonthiazolidinedione PPARgamma agonist L-796,449 is neuroprotective in experimental stroke. J Neuropathol Exp Neurol. 2005;64:797–805. doi: 10.1097/01.jnen.0000178852.83680.3c. [DOI] [PubMed] [Google Scholar]
- Shaul PW, Campbell WB, Farrar MA, Magness RR. Oxygen modulates prostacyclin synthesis in ovine fetal pulmonary arteries by an effect on cyclooxygenase. J Clin Invest. 1992;90:2147–2155. doi: 10.1172/JCI116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul PW, Farrar MA, Magness RR. Oxygen modulation of pulmonary arterial prostacyclin synthesis is developmentally regulated. Am J Physiol. 1993;265:H621–628. doi: 10.1152/ajpheart.1993.265.2.H621. [DOI] [PubMed] [Google Scholar]
- Soejima Y, Hu Q, Krafft PR, Fujii M, Tang J, Zhang JH. Hyperbaric oxygen preconditioning attenuates hyperglycemia-enhanced hemorrhagic transformation by inhibiting matrix metalloproteinases in focal cerebral ischemia in rats. Exp Neurol. 2013;247:737–743. doi: 10.1016/j.expneurol.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soejima Y, Ostrowski RP, Manaenko A, Fujii M, Tang J, Zhang JH. Hyperbaric oxygen preconditioning attenuates hyperglycemia enhanced hemorrhagic transformation after transient MCAO in rats. Med Gas Res. 2012;2:9. doi: 10.1186/2045-9912-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005;130:685–696. doi: 10.1016/j.neuroscience.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Na HK, Park JM, Lee HN, Kim W, Yoon IS, Kim DD. 15-Deoxy-Delta(1)(2),(1)(4)-prostaglandin J(2), an electrophilic lipid mediator of anti-inflammatory and pro-resolving signaling. Biochem Pharmacol. 2011;82:1335–1351. doi: 10.1016/j.bcp.2011.07.100. [DOI] [PubMed] [Google Scholar]
- Tibbles PM, Edelsberg JS. Hyperbaric-oxygen therapy. N Engl J Med. 1996;334:1642–1648. doi: 10.1056/NEJM199606203342506. [DOI] [PubMed] [Google Scholar]
- Wu JS, Cheung WM, Tsai YS, Chen YT, Fong WH, Tsai HD, Chen YC, Liou JY, Shyue SK, Chen JJ, Chen YE, Maeda N, Wu KK, Lin TN. Ligand-activated peroxisome proliferator-activated receptor-gamma protects against ischemic cerebral infarction and neuronal apoptosis by 14-3-3 epsilon upregulation. Circulation. 2009;119:1124–1134. doi: 10.1161/CIRCULATIONAHA.108.812537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Jiang X, Yang Y, Xi G. Hemorrhagic transformation induced by acute hyperglycemia in a rat model of transient focal ischemia. Acta Neurochir Suppl. 2011;111:49–54. doi: 10.1007/978-3-7091-0693-8_9. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Xie K, Dong H, Zhang H, Wang F, Li Y, Xiong L. Hyperbaric oxygen preconditioning protects cortical neurons against oxygen-glucose deprivation injury: role of peroxisome proliferator-activated receptor-gamma. Brain Res. 2012;1452:140–150. doi: 10.1016/j.brainres.2012.02.063. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhang Y, Strong R, Grotta JC, Aronowski J. 15d-Prostaglandin J2 activates peroxisome proliferator-activated receptor-gamma, promotes expression of catalase, and reduces inflammation, behavioral dysfunction, and neuronal loss after intracerebral hemorrhage in rats. J Cereb Blood Flow Metab. 2006;26:811–820. doi: 10.1038/sj.jcbfm.9600233. [DOI] [PubMed] [Google Scholar]