Abstract
Infectious mononucleosis is a clinical entity characterized by pharyngitis, cervical lymph node enlargement, fatigue and fever, which results most often from a primary Epstein–Barr virus (EBV) infection. EBV, a lymphocrytovirus and a member of the γ-herpesvirus family, infects at least 90% of the population worldwide, the majority of whom have no recognizable illness. The virus is spread by intimate oral contact among adolescents, but how preadolescents acquire the virus is not known. During the incubation period of approximately 6 weeks, viral replication first occurs in the oropharynx followed by viremia as early as 2 weeks before onset of illness. The acute illness is marked by high viral loads in both the oral cavity and blood accompanied by the production of immunoglobulin M antibodies against EBV viral capsid antigen and an extraordinary expansion of CD8+ T lymphocytes directed against EBV-infected B cells. During convalescence, CD8+ T cells return to normal levels and antibodies develop against EBV nuclear antigen-1. A typical clinical picture in an adolescent or young adult with a positive heterophile test is usually sufficient to make the diagnosis of infectious mononucleosis, but heterophile antibodies are not specific and do not develop in some patients especially young children. EBV-specific antibody profiles are the best choice for staging EBV infection. In addition to causing acute illness, long-term consequences are linked to infectious mononucleosis, especially Hodgkin lymphoma and multiple sclerosis. There is no licensed vaccine for prevention and no specific approved treatment. Future research goals are development of an EBV vaccine, understanding the risk factors for severity of the acute illness and likelihood of developing cancer or autoimmune diseases, and discovering anti-EBV drugs to treat infectious mononucleosis and other EBV-spurred diseases.
Infectious mononucleosis is the name coined by Sprunt and Evans in 19201 for an acute infectious disease consisting of fever, cervical lymphadenopathy and pharyngitis accompanied by atypical large peripheral blood lymphocytes. Its major cause is Epstein–Barr virus (EBV). We now know that the characteristic atypical lymphocytes, carefully described morphologically by Downey and McKinlay,2 are actually activated CD8+ T cells,3 which are responding to EBV-infected B cells.4 Infectious mononucleosis represents a significant health risk because of the severity and duration of the acute illness and also because of the potential for long-term complications in the form of certain cancers and autoimmune diseases.
Identification of EBV as the cause of infectious mononucleosis
Infectious mononucleosis was recognized as a unique disease in the 1880s by Nil Filatov, a Russian pediatrician, who called the syndrome ‘idiopathic adenitis.5 Indeed, its etiology remained a mystery until 1967 when a serendipitous event established the causal relationship between infectious mononucleosis and EBV.
EBV was discovered by Epstein et al.6 in 1964 using electron microscopy to detect the virus in cultured Burkitt lymphoma cells. Epstein believed that another laboratory should repeat his finding, but British virologists were not interested in collaborating.7 ‘As a last resort,' Epstein sent the Burkitt cells to Klaus Hummeler in Philadelphia, who had just spent a sabbatical with Epstein.8 As Hummeler's laboratory had been recently dismantled because of lack of funds, he brought the cells to the Henle laboratory, which was also in Philadelphia, where Epstein's discovery of a new herpesvirus was quickly confirmed,9 and additional studies launched to further characterize this virus.
Now comes a truly ‘once-upon-a-time' story. A technologist working in the Henle laboratory who lacked antibodies against EBV regularly donated lymphocytes for EBV transmission/transformation experiments but her cells never survived in culture.8, 10 She became ill in August 1967 and missed 5 days of work. Her physician's clinical impression was rubella versus infectious mononucleosis. Her rubella antibodies were negative but her heterophile antibody test, which had been established as the laboratory method of choice to diagnose infectious mononucleosis,11 was positive. Her lymphocytes now grew continuously in culture and were positive for EBV antigens. She also had acquired EBV-specific antibodies, which was the crucial clue that EBV was responsible for a common acute infectious disease. Additional serum samples were obtained. Especially valuable were those from researchers at Yale, who had amassed a prospective serum bank from sick students and thus had pre- and post-illness samples. These were ideal reagents to prove conclusively that primary EBV infection caused infectious mononucleosis.12
Epidemiology of infectious mononucleosis
EBV infection among adolescents and young adults is spread primarily by deep kissing as documented by Hoagland's clinical observations,13 and confirmed many years later by a prospective study among undergraduate university students.14 Sexual intercourse has been purported to enhance transmission,15 but our University of Minnesota study found that subjects who reported kissing with or without penetrative sexual intercourse had the same higher risk of primary EBV infection throughout the undergraduate years as compared with subjects who reported no kissing and no sex.14
In unusual circumstances, primary EBV infection can also be transmitted by blood transfusion,16 solid organ transplantation17 or hematopoietic cell transplantation.18 For instance, Alfieri et al.19 used polymorphisms in the EBV BAMHI-K fragment length and EBV nuclear antigen (EBNA) -1, -2 and -3 proteins to identify the specific blood donor who transmitted EBV to a 16-year-old liver transplant recipient. That recipient subsequently developed infectious mononucleosis.
How preadolescent children contract EBV is unknown. It could be that they are infected by their parents or siblings who shed EBV periodically into their oral secretions.20 A graphic illustration of this is the acquisition of EBV by Melanesian infants whose multiple caregivers chew the food themselves before giving it to the baby.21
The incubation period of infectious mononucleosis has been observed to be between 32 and 49 days.13 A well-documented Swedish case was reported in which the kissing event occurred 38 days before onset of symptoms.22 Behavioral, virologic and immunologic data collected during prospective studies in university undergraduates point to a modal incubation period of 42 days (Balfour et al., unpublished observations).
Clinical manifestations of the acute illness
Infectious mononucleosis is a clinical entity characterized by pharyngitis, cervical lymph node enlargement, fatigue and fever. The disease occurs worldwide with no seasonal predilection. It is recognized most frequently in adolescents and young adults from developed countries for reasons that are not completely understood. Part of the explanation is lack of recognition of the syndrome in preadolescents. The heterophile antibody test is often unreliable in young children, particularly those under 4 years of age. Thus, assays specific for EBV must be performed in these cases, lest the diagnosis of infectious mononucleosis be missed.23 Infectious mononucleosis in preadolescents is not rare. As a pediatrician, one of us (HHB) has seen numerous cases in children younger than 12 years old. Indeed, infectious mononucleosis was first described by a Russian pediatrician.5
A second reason could be that deep kissing transmits a large amount of infectious virus. In contrast, young children probably acquire the virus from asymptomatic parents or siblings who shed low levels of EBV in their oral secretions and transmit a smaller infectious inoculum. Parents of young children (<6 years of age) have EBV in their oral secretions about 30% of the time but the median quantity is only 4900 copies ml−1 (Cederberg et al., unpublished observations). In contrast, during the acute and convalescent stages of primary EBV infection, young adults shed a median of 63 100 copies ml−1.14
A third possibility is that infectious mononucleosis in adolescents may reflect the response of cross-reactive memory CD8+ T cells. For example, influenza-specific CD8+ T cells may cross-react with EBV.24 As adolescents are presumably more likely to have high numbers of influenza-specific CD8+ T cells as compared with young children who have seen relatively few different influenza types, the adolescents would react more strongly against EBV. However, we did not find any evidence of influenza–EBV dual specific CD8+ T cells in our cohort25 and thus it remains questionable whether preexisting (cross-reactive) CD8+ T-cell immunity to EBV would influence the severity of primary EBV infection.
Recent data implicate certain classes of natural killer (NK) cells as important factors in the early control of EBV. Azzi et al.26 and colleagues detected significantly higher levels of CD56dim NKG2A+ killer-cell immunoglobulin-like receptors (KIR)− NK cells in the peripheral blood of children as compared with either adolescents or adults. These findings suggest that differences in this preexisting NK cell population may affect the course of subsequent infection, and may provide an explanation for why infectious mononucleosis occurs more frequently in adolescents and adults than in children.
A final point is that infectious mononucleosis is more commonly seen in developed countries because the age at acquisition of primary EBV infection is older than it is in the developing world.27
Most young adults develop infectious mononucleosis after primary EBV infection.14 There are two typical clinical presentations. One is the sudden onset of sore throat (Figure 1). Patients also complain of a swollen neck that reflects cervical lymph node enlargement. Another typical presentation is the slow development of malaise, myalgia and fatigue. The most frequent signs and symptoms are: sore throat (95%), cervical lymphadenopathy (80%), fatigue (70%), upper respiratory symptoms (65%), headache (50%), decreased appetite (50%), fever (47%) and myalgia (45%).14 Most findings last 10 days or less but fatigue and cervical lymphadenopathy often persist for at least 3 weeks. Other clinical findings, seen in the minority of cases, include abdominal pain, hepatomegaly, splenomegaly, nausea, vomiting, palatal petechiae, periorbital and eyelid edema. Hepatitis occurs in 75% of patients but is usually subclinical (elevation of alanine aminotransferase levels without jaundice or abdominal pain). Rash is not usually seen except in patients given penicillin derivatives, in which case it results from transient penicillin hypersensitivity.28 As an apt example, the technologist in the Henle laboratory who provided the major clue that EBV caused infectious mononucleosis had been given ampicillin. Her rash prompted clinicians to think of rubella as well her correct diagnosis, infectious mononucleosis.10
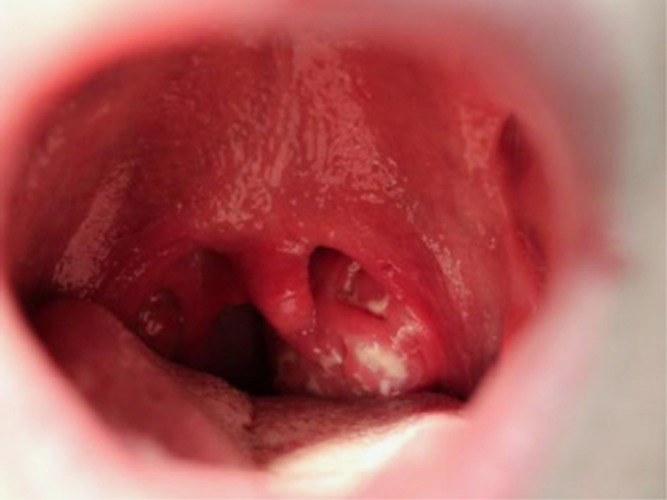
Figure 1.
Pharyngitis demonstrating exudative tonsillitis and an enlarged uvula in a 19-year-old undergraduate university student 5 days after onset of infectious mononucleosis. In addition to pharyngitis, he felt febrile, had cervical lymphadenopathy, fatigue and loss of appetite. His sore throat lasted for 9 days and he was fatigued for 29 days.
Complications of the acute illness
Serious complications during the acute phase of primary EBV infection are rare. Complications that occur in at least 1% of patients are: airway obstruction because of oropharyngeal inflammation, streptococcal pharyngitis, meningoencephalitis, hemolytic anemia and thrombocytopenia.29, 30, 31 Splenic rupture occurs in <1% in patients but is the most feared complication, which sometimes keeps athletes out of competition for weeks.32 A reasonable recommendation is that athletes may resume contact sports after 3 weeks of illness as long as they have no ongoing signs or symptoms of acute EBV infection.33
Dynamics of the infection and immune response
During the 6-week incubation period of primary EBV infection, viral replication is first detected in the oral cavity.14 There EBV infects both B cells and tonsillar epithelial cells.34 Interestingly, the infection efficiency of EBV for these cell types varies depending on the cell type supporting viral replication. In vitro studies have shown that virus derived from epithelial cells is better able to infect B cells and vice versa.35 Therefore, EBV infection in the oral cavity is likely affected by the cyclic pattern of this switch tropism.
The virus transitions from the oral cavity to the peripheral blood at some point during the incubation period. How and when this transition takes place is not well understood, although copies of the EBV genome can be detected in peripheral blood up to 2 weeks before onset of symptoms (Dunmire et al., unpublished observations). In addition, gene expression profiling has revealed that 2 weeks before symptom onset a systemic type I interferon response can be detected in some individuals who subsequently present with infectious mononucleosis.36
The onset of the acute illness is marked by high viral loads in both the oral cavity and blood. This is accompanied by the production of immunoglobulin M (IgM) antibodies against EBV viral capsid antigen (VCA) and an extraordinary expansion of CD8+ T lymphocytes.14 The response of these CD8+ T cells is of particular interest because these cells are important for controlling EBV, a role supported by the fulminant disease that occurs in patients with defects in the function of their T cells, such as the ability to interact and kill EBV-infected B cells.37, 38
Acute infectious mononucleosis is characterized by abnormally high numbers of circulating CD8+ T cells. Of these cells, many are specific for EBV antigens derived from the immediate early and early stages of lytic infection with a marked bias toward the immediate early stage. Late lytic antigens also generate a specific CD8+ T-cell response as revealed by comparing T-cell clones from infectious mononucleosis patients with those of long-term carriers.39 In addition to proteins encoded by EBV in the lytic phase, CD8+ T cells respond to latent antigens, especially EBNA-2 and EBNA-3.40, 41 Thus, the T-cell response is directed at both lytic and latent infections in carriers.
Although it has been established in the literature that numbers of CD4+ T cells are not substantially increased during infectious mononucleosis, data exist to support the concept that CD4+ T cells are important contributors to the control of EBV. Indeed, CD4+ T cells have been shown to recognize several lytic antigens through use of major histocompatibility complex (MHC) II tetramers. These cells are not only present during acute infection, but are maintained in the peripheral blood, albeit at low levels.42
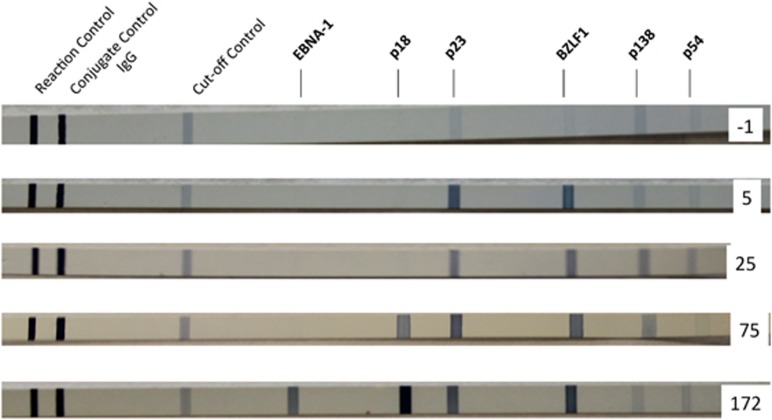
The kinetics of the IgG antibody responses to various EBV proteins are quite distinct as illustrated by the line blot assay (Mikrogen, Neuried, Germany) in Figure 2. This assay contains 6 EBV antigens, 2 of which are components of the VCA structural protein: p23 (BLRF2) and p18 (BFRF3).43 IgG antibodies against EBV VCA are usually detectable after the first week of illness and persist for life. The immune response to the p23 component of VCA develops sooner than the response to p18. Three of the EBV antigens belong to the temporal classes of lytic gene products: immediate early, early, and late. Antibodies directed against the immediate EA BZLF1 also appear quickly and remain. Antibody responses to the EAs p138 and p54 are more variable. Although they can be found early after infection, they often become undetectable after convalescence. In contrast, antibodies against EBNA-1, which is a latent gene product, are slow to develop and are usually not detected until 3 months or longer after onset of illness.44 However, once they are found they remain present for life. The delayed EBNA-1 antibody response has been shown to correlate with a delayed CD4+ T-cell response to EBNA-1.42
Figure 2.
Line immunoblots demonstrating IgG antibody responses to six EBV proteins at five timepoints from the day before onset of illness (day −1) to 172 days postonset. Band intensities equal to or greater than the cutoff control are considered to indicate a specific antibody response. The first antibodies to appear are directed against BZLF1 (immediate early), and p23 (VCA). Antibodies to p138 and p54 (EA) developed next, followed by p18 (VCA) antibodies. Antibodies against EBNA-1 were seen only on the serum sample collected 172 days after onset of illness.
Although CD8+ T cells are recognized as vital factors in the control of EBV infection, NK cells are increasingly being acknowledged as important during infectious mononucleosis, as evidenced by the severe EBV-related outcomes that occur in several immunodeficiencies involving T and NK cells and/or their cytolysis pathways.45, 46 Other data also support a role for NK cells during infectious mononucleosis, such as the observation that NK cells in vitro preferentially kill EBV-infected cells when the virus transitions into the lytic phase.47
Humanized mouse models have more recently allowed for examination of interactions between NK cells and EBV in vivo. One such model, the non-obese diabetic scid gamma mouse is created by reconstituting immune compartments with CD34+ lin− hematopoietic stem cells.48, 49 These mice are then infected with the B95.8 strain of EBV and monitored for signs of infectious mononucleosis-like disease such as CD8 lymphocytosis and EBV viremia. Animals depleted of NK cells were found to have more severe signs of EBV-related disease.50 In regard to this study, it is of particular interest that depletion of NK cells following initial establishment of EBV infection in non-obese diabetic scid gamma mice did not have a significant effect. Given differences in the response to EBV that may be observed between NK cells derived from the tonsil and NK cells derived from the peripheral blood, it seems likely that NK cells have a more prominent role in controlling early infection in the oropharynx than they do in the peripheral blood during the viremic phase.51
The importance of peripheral blood NK cells in humans during infectious mononucleosis remains a point of contention as studies have yielded conflicting results. In one such study, an inverse correlation was found between blood virus and the number of NK cells detected in the periphery,52 while another larger study found a positive correlation.14 Thus, the relevance of NK cells to levels of peripheral blood virus at the viremic stage of infection requires additional investigation.
It is probable, however, that total NK cell numbers may not accurately represent the contribution of this cell type to combating infection. Deeper probing into individual NK subsets in other infections has shown that certain types of NK cells have a more potent effect than others. For example, during primary cytomegalovirus infection, NKG2C+ NK cells become expanded and have been demonstrated to respond specifically to that virus.53 Although NKG2C+ NK cells numbers are not affected by primary EBV infection, NKG2A+ NK cells can be detected at greater frequency in the peripheral blood of infectious mononucleosis patients.54 Larger numbers of NKG2A+ CD54+ NK cells are also found in the tonsils of EBV carriers than in non-carriers.55 Furthermore, CD56dim NKG2A+ KIR− NK cells have been shown to preferentially proliferate in response to EBV-infected cells, as reported in a study of pediatric infectious mononucleosis.26
The importance of NK cells is also highlighted by the fact that the virus has a mechanism to hinder NK cell activation during viral replication. The protein product of the EBV open reading frame BZLF1 encodes a peptide sequence that can bind the non-classical MHC-I molecule human leukocyte antigen (HLA)-E. In turn, HLA-E can then engage the inhibitory receptor NKG2A.56 Interestingly, an interference mechanism targeted toward adaptive immune cells may have evolved to avoid NK cell surveillance. BILF1 downregulates expression of MHC class I molecules, namely HLA-A and HLA-B, but interestingly does not affect HLA-C, which is inhibitory to NK cells.57
In addition, there exists evidence that the transformation of B cells by EBV is limited by the presence of certain types of NK cells. Experiments performed in vitro showed that CD56bright CD16− NK cells were preferentially primed by dendritic cells matured by exposure to EBV. Incubation of these NK cells with B cells in the presence of virus resulted in lower B-cell transformation in an interferon-γ-dependent manner. It is worth mentioning that NK cells derived from tonsillar tissue were much more efficient than those isolated from peripheral blood,51, 58 which is especially relevant given that numbers of CD56bright CD16− NK cells are drastically reduced in the peripheral blood during infectious mononucleosis.54 Thus, NK cells may serve to help control EBV infection in two ways: through direct cytolysis of infected cells and through blockade of transformation via interferon-γ.
During convalescence (3 to 6 months after onset of infectious mononucleosis), CD8+ T-cell and NK cell numbers return to normal levels.14 It was previously proposed that herpesvirus infection during childhood conveys an advantage to the host by ‘priming' the immune system to better combat subsequent threats. These suppositions were supported by data showing that mice infected with the murine gamma herpesvirus-68 could subsequently more rapidly clear a bacterial challenge,59 although these effects were later demonstrated to be transient.60 A study in human subjects provided corroborating evidence in the form of gene expression data from peripheral blood mononuclear cells. No long-term gene expression changes were found after primary EBV acquisition, suggesting that the immunological steady state, at least in the periphery, is not appreciably altered following herpesvirus infection.36
Diagnosis of infectious mononucleosis because of primary EBV infection
The cause of infectious mononucleosis cannot be determined on clinical grounds alone. There is a practical way and a precise way to diagnose infectious mononucleosis because of primary EBV infection. The practical way is to obtain laboratory confirmation using a heterophile antibody test. This assay has been used as the standard point-of-care laboratory method ever since its discovery by Paul and Bunnell in 1932.11 Paul and Bunnell defined heterophile antibodies as ‘having the capacity to react to certain antigens, which are quite different from, and phylogenetically unrelated to the one instrumental in producing the antibody response.' Heterophile tests use mammalian erythrocytes from various species to detect IgM class antibodies against them, which are raised during the generalized immune upregulation that accompanies acute primary EBV infection.
Heterophile tests are a practical method for confirming the clinical diagnosis. However, they do have drawbacks. Approximately 40% of children 4 years of age or younger do not develop heterophile antibodies following a primary EBV infection.23 If the heterophile is the only test ordered, the diagnosis will be missed. Second, heterophile antibodies by definition are not specific and may be present in infections caused by other pathogens, malignancies and autoimmune diseases.61, 62 Finally, heterophile antibodies can persist for a year or more and therefore are not always diagnostic of an acute EBV infection.63
The most useful specific antibody tests are VCA IgM, VCA IgG and EBNA-1 IgG usually measured using an enzyme immunoassay platform. VCA IgM antibodies are present in 75% of patients during the acute illness.14 However, false-positive results have been reported especially with cytomegalovirus infection.64 All patients with infectious mononucleosis develop IgG antibodies to VCA,14 so this is the best laboratory test to document a previous EBV infection. Antibodies against EBNA-1 develop slowly and usually are not detectable until 90 days or longer after onset of illness. Therefore, the presence of EBNA-1 antibodies during an acute illness rules out acute primary EBV infection. In general, the vast majority of EBV infections can be staged by measuring VCA IgM, VCA IgG and EBNA-1 IgG serum antibodies as shown in Table 1. Early antigen (EA) IgG antibodies are not diagnostic of primary EBV infection because only 60–80% of patients are positive during the acute illness and these antibodies can be found in 20% of healthy individuals.65
Table 1. Staging EBV infection by enzyme immunoassay antibody results.
| Stage of infection | Time after onset of illness | VCA IgM | VCA IgG | EBNA-1 IgG |
|---|---|---|---|---|
| EBV naive | — | Negative | Negative | Negative |
| Acute primary infection | 0–3 Weeks | Positive | Negative or positive | Negative |
| Subacute infection | 4 Weeks–3 months | Positive | Positive | Negative |
| Convalescent infection | 4–6 Months | Negative or positive | Positive | Negative or positive |
| Past infection | >6 Months | Negative | Positive | Positive |
Abbreviations: EBNA, EBV nuclear antigen; EBV, Epstein–Barr virus; IgM, immunoglobulin M; VCA, VCA, viral capsid antigen.
IgG avidity assays may be useful in cases where staging is unclear after the above tests have been performed.66, 67 The principle is that IgG antibodies during the acute phase of infection do not bind to their target as tightly as antibodies produced during convalescence. Low avidity antibodies can be dissociated from their target by exposure to urea or another chaotropic reagent. Antibodies remaining after chaotropic reagent treatment are high avidity antibodies representative of late-stage infection.
Influence of genetics on susceptibility to and severity of infectious mononucleosis
Hwang et al.,68 utilizing the California Twin Program registry, reported that concordance for infectious mononucleosis in monozygotic twins was twice that of dizygotic twins. They interpreted their results as ‘compatible with a heritable contribution to the risk of infectious mononucleosis.' Rostgaard et al.69 extended these findings by tracking familial clustering of hospitalized cases of infectious mononucleosis. Using very large Danish national databases, these investigators reported that same-sex twins had a rate ratio of 9.3 for infectious mononucleosis as compared with 2.3 for first-degree relatives (opposite-sex twins, siblings and parents), 1.4 for second-degree relatives (half-siblings, grandparents, uncles and aunts) and 1.0 for third-degree relatives (first cousins). The 95% confidence intervals for those four classes of relationships did not overlap, supporting the conclusion that degree of relatedness increased the likelihood of contracting the disease.
Twins, unless they are separated at birth, share the same environment and probably have similar behavior, which cannot be ruled out as risk factors. However, the significantly greater number of cases in monozygotic or same-sex twins versus dizygotic or opposite-sex twins in both the California and the Danish studies is compelling evidence that susceptibility to infectious mononucleosis has a genetic component.
Sequelae: Hodgkin lymphoma and multiple sclerosis
EBV infection (either symptomatic or asymptomatic) has been associated with a farrago of neoplastic and autoimmune conditions as reviewed by Odumade et al.70 In terms of symptomatic EBV infection, a history of infectious mononucleosis is a strong risk factor for Hodgkin lymphoma,71 as well as for multiple sclerosis.72 The reason why these diseases and symptomatic primary EBV infection are linked is not known. A plausible explanation well worth exploring is that host genetic and/or environmental factors for severity of primary EBV infection and Hodgkin lymphoma or multiple sclerosis are the same.
Prevention and treatment of infectious mononucleosis
Development of a prophylactic EBV vaccine has been a priority for researchers in the field ever since the idea was suggested by Epstein and Achong in 1973.73 Progress has been painfully slow. The first phase 1 trial for a prophylactic EBV vaccine was not reported until 1995,74 and results of the first phase 2 study were not published until 2007.75 To date, two prophylactic vaccine constructs have been tested in humans: subunit gp350 and an EBNA-3A peptide.75, 76 EBV vaccines have been recently reviewed in this journal.77
There is no approved treatment for infectious mononucleosis. Several nucleoside analogs have in vitro activity against EBV,78 but a clinical benefit has not yet been proven for any of them. Valacyclovir is worth mentioning because it is generic and has very few side effects. We compared valacyclovir (3 g day−1 for 2 weeks) with no antiviral therapy in a group of 20 university undergraduate students with acute infectious mononucleosis. The proportion of valacyclovir recipients versus control subjects who had ⩾2 log10 decrease in EBV copies was significantly greater for both the oral wash fluid-derived cell pellet (P=0.03) and supernatant (P=0.001) samples. At the end of the treatment period, the number of reported symptoms (P=0.03) and the severity illness (P=0.05) were significantly reduced among valacyclovir recipients as compared with controls. As our study contained few subjects and was not placebo controlled, these results must be confirmed in a larger, placebo-controlled trial.79
Corticosteroids are often prescribed to treat inflammatory complications such as airway obstruction or autoimmune phenomena such as anemia and thrombocytopenia, but the value of these drugs is controversial and they may impair viral clearance.80
Future research goals
The highest priority in our opinion is development of an EBV vaccine. EBV vaccine has the potential to prevent or modify the severity of infectious mononucleosis, multiple sclerosis, EBV-positive Hodgkin lymphoma, endemic Burkitt lymphoma and nasopharyngeal carcinoma among other entities.81, 82
A second goal is to define the genetic, immunologic and/or environmental factors that affect disease severity and propensity to develop EBV-spurred cancer or autoimmune disease. As part of this endeavor, studies should be directed at the question, ‘Why is primary EBV infection more likely to cause infectious mononucleosis in adolescents and young adults?'
A third goal is to discover specific anti-EBV drugs to treat infectious mononucleosis. In this regard, the field of anti-cytomegalovirus drug development is much further along than that of EBV.83
The authors declare no conflict of interest.
References
- Sprunt TP, Evans FA. Mononuclear leucocytosis in reaction to acute infections (‘infectious mononucleosis') Johns Hopkins Hosp Bull. 1920;31:410–417. [Google Scholar]
- Downey H, McKinlay CA. Acute lymphadenosis compared with acute lymphatic leukemia. Arch Intern Med. 1923;32:82–112. [Google Scholar]
- Callan MF, Steven N, Krausa P, Wilson JD, Moss PA, Gillespie GM, et al. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat Med. 1996;2:906–911. doi: 10.1038/nm0896-906. [DOI] [PubMed] [Google Scholar]
- Callan MF, Tan L, Annels N, Ogg GS, Wilson JD, O'Callaghan CA, et al. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus In vivo. J Exp Med. 1998;187:1395–1402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatov N, Earle FB. Semeiology and Diagnosis of Diseases of Children: Together with a Therapeutic Index. Vol. 2. Cleveland Press: Chicago; 1904. p. 596. [Google Scholar]
- Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1:702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- Epstein MA.The origins of EBV research: discovery and characterization of the virusIn: Robertson ES (ed). Epstein-Barr virus Caister Academic Press: Norfolk, England; 20051–14. [Google Scholar]
- Henle W, Henle G.Epstein-Barr virus: past, present, and futureIn: Levine PH, Ablashi DV, Pearson GR, Kottaridis SD (eds). Epstein-Barr Virus and Associated Diseases: Proceedings of the First International Symposium on Epstein-Barr Virus-Associated Malignant Diseases, Loutraki, Greece, 24–28 September 1984 Martnus Nijhoff Publishing: Boston; 1985677–686. [Google Scholar]
- Epstein MA, Henle G, Achong BG, Barr YM. Morphological and biological studies on a virus in cultured lymphoblasts from Burkitt's lymphoma. J Exp Med. 1965;121:761–770. doi: 10.1084/jem.121.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H.The early days of Epstein-Barr virus research: the Henle yearsIn: Robertson ES (ed). Epstein-Barr Virus Caister Academic Press: Norfolk, England; 200515–22. [Google Scholar]
- Paul JR, Bunnell WW. The presence of heterophile antibodies in infectious mononucleosis. Am J Med Sci. 1932;183:90–104. doi: 10.1097/00000441-197403000-00005. [DOI] [PubMed] [Google Scholar]
- Henle G, Henle W, Diehl V. Relation of Burkitt's tumor-associated herpes-type virus to infectious mononucleosis. Proc Natl Acad Sci USA. 1968;59:94–101. doi: 10.1073/pnas.59.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland RJ. The transmission of infectious mononucleosis. Am J Med Sci. 1955;229:262–272. doi: 10.1097/00000441-195503000-00003. [DOI] [PubMed] [Google Scholar]
- Balfour HH, Jr, Odumade OA, Schmeling DO, Mullan BD, Ed JA, Knight JA, et al. Behavioral, virologic, and immunologic factors associated with acquisition and severity of primary Epstein-Barr virus infection in university students. J Infect Dis. 2013;207:80–88. doi: 10.1093/infdis/jis646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DH, Macsween KF, Higgins CD, Thomas R, McAulay K, Williams H, et al. A cohort study among university students: identification of risk factors for Epstein-Barr virus seroconversion and infectious mononucleosis. Clin Infect Dis. 2006;43:276–282. doi: 10.1086/505400. [DOI] [PubMed] [Google Scholar]
- Gerber P, Walsh JH, Rosenblum EN, Purcell RH. Association of EB-virus infection with the post-perfusion syndrome. Lancet. 1969;1:593–595. doi: 10.1016/s0140-6736(69)91532-3. [DOI] [PubMed] [Google Scholar]
- Hanto DW, Frizzera G, Purtilo DT, Sakamoto K, Sullivan JL, Saemundsen AK, et al. Clinical spectrum of lymphoproliferative disorders in renal transplant recipients and evidence for the role of Epstein-Barr virus. Cancer Res. 1981;41:4253–4261. [PubMed] [Google Scholar]
- Shapiro RS, McClain K, Frizzera G, Gajl-Peczalska KJ, Kersey JH, Blazar BR, et al. Epstein-Barr virus associated B cell lymphoproliferative disorders following bone marrow transplantation. Blood. 1988;71:1234–1243. [PubMed] [Google Scholar]
- Alfieri C, Tanner J, Carpentier L, Perpete C, Savoie A, Paradis K, et al. Epstein-Barr virus transmission from a blood donor to an organ transplant recipient with recovery of the same virus strain from the recipient's blood and oropharynx. Blood. 1996;87:812–817. [PubMed] [Google Scholar]
- Sumaya CV, Ench Y. Epstein-Barr virus infections in families: the role of children with infectious mononucleosis. J Infect Dis. 1986;154:842–850. doi: 10.1093/infdis/154.5.842. [DOI] [PubMed] [Google Scholar]
- Lang DJ, Garruto RM, Gajdusek DC. Early acquisition of cytomegalovirus and Epstein-Barr virus antibody in several isolated Melanesian populations. Am J Epidemiol. 1977;105:480–487. doi: 10.1093/oxfordjournals.aje.a112407. [DOI] [PubMed] [Google Scholar]
- Svedmyr E, Ernberg I, Seeley J, Weiland O, Masucci G, Tsukuda K, et al. Virologic, immunologic, and clinical observations on a patient during the incubation, acute, and convalescent phases of infectious mononucleosis. Clin Immunol Immunopathol. 1984;30:437–450. doi: 10.1016/0090-1229(84)90029-1. [DOI] [PubMed] [Google Scholar]
- Horwitz CA, Henle W, Henle G, Goldfarb M, Kubic P, Gehrz RC, et al. Clinical and laboratory evaluation of infants and children with Epstein-Barr virus-induced infectious mononucleosis: report of 32 patients (aged 10-48 months) Blood. 1981;57:933–938. [PubMed] [Google Scholar]
- Clute SC, Watkin LB, Cornberg M, Naumov YN, Sullivan JL, Luzuriaga K, et al. Cross-reactive influenza virus-specific CD8+ T cells contribute to lymphoproliferation in Epstein-Barr virus-associated infectious mononucleosis. J Clin Invest. 2005;115:3602–3612. doi: 10.1172/JCI25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odumade OA, Knight JA, Schmeling DO, Masopust D, Balfour HH, Jr, Hogquist KA. Primary Epstein-Barr virus infection does not erode preexisting CD8 T cell memory in humans. J Exp Med. 2012;209:471–478. doi: 10.1084/jem.20112401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi T, Lunemann A, Murer A, Ueda S, Beziat V, Malmberg KJ, et al. Role for early-differentiated natural killer cells in infectious mononucleosis. Blood. 2014;124:2533–2543. doi: 10.1182/blood-2014-01-553024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalgrim H, Friborg J, Melbye M, et al. The epidemiology of EBV and its association with malignant diseaseIn: Arvin A, Campadelli-Fiume G, Mocarski E (eds). Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis Cambridge University Press: Cambridge, UK; 2007929–959. [PubMed] [Google Scholar]
- Balfour HH, Jr, Holman CJ, Hokanson KM, Lelonek MM, Giesbrecht JE, White DR, et al. A prospective clinical study of Epstein-Barr virus and host interactions during acute infectious mononucleosis. J Infect Dis. 2005;192:1505–1512. doi: 10.1086/491740. [DOI] [PubMed] [Google Scholar]
- White LR, Karofsky PS. Review of the clinical manifestations, laboratory findings, and complications of infectious mononucleosis. Wis Med J. 1985;84:19–25. [PubMed] [Google Scholar]
- Connelly KP, DeWitt LD. Neurologic complications of infectious mononucleosis. Pediatr Neurol. 1994;10:181–184. doi: 10.1016/0887-8994(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Jenson HB. Acute complications of Epstein-Barr virus infectious mononucleosis. Curr Opin Pediatr. 2000;12:263–268. doi: 10.1097/00008480-200006000-00016. [DOI] [PubMed] [Google Scholar]
- Hoagland RJ, Henson HM. Splenic rupture in infectious mononucleosis. Ann Intern Med. 1957;46:1184–1191. doi: 10.7326/0003-4819-46-6-1184. [DOI] [PubMed] [Google Scholar]
- Putukian M, O'Connor FG, Stricker P, McGrew C, Hosey RG, Gordon SM, et al. Mononucleosis and athletic participation: an evidence-based subject review. Clin J Sport Med. 2008;18:309–315. doi: 10.1097/JSM.0b013e31817e34f8. [DOI] [PubMed] [Google Scholar]
- Wang X, Kenyon WJ, Li Q, Mullberg J, Hutt-Fletcher LM. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J Virol. 1998;72:5552–5558. doi: 10.1128/jvi.72.7.5552-5558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borza CM, Hutt-Fletcher LM. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat Med. 2002;8:594–599. doi: 10.1038/nm0602-594. [DOI] [PubMed] [Google Scholar]
- Dunmire SK, Odumade OA, Porter JL, Reyes-Genere J, Schmeling DO, Bilgic H, et al. Primary EBV infection induces an expression profile distinct from other viruses but similar to hemophagocytic syndromes. PLoS ONE. 2014;9:e85422. doi: 10.1371/journal.pone.0085422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud S, Fondaneche MC, Lambert N, Pasquier B, Mateo V, Soulas P, et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444:110–114. doi: 10.1038/nature05257. [DOI] [PubMed] [Google Scholar]
- Palendira U, Low C, Chan A, Hislop AD, Ho E, Phan TG, et al. Molecular pathogenesis of EBV susceptibility in XLP as revealed by analysis of female carriers with heterozygous expression of SAP. PLoS Biol. 2011;9:e1001187. doi: 10.1371/journal.pbio.1001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott RJ, Quinn LL, Leese AM, Scholes HM, Pachnio A, Rickinson AB. CD8+ T cell responses to lytic EBV infection: late antigen specificities as subdominant components of the total response. J Immunol. 2013;191:5398–5409. doi: 10.4049/jimmunol.1301629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake N, Haigh T, Shaka'a G, Croom-Carter D, Rickinson A. The importance of exogenous antigen in priming the human CD8+ T cell response: lessons from the EBV nuclear antigen EBNA1. J Immunol. 2000;165:7078–7087. doi: 10.4049/jimmunol.165.12.7078. [DOI] [PubMed] [Google Scholar]
- Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol. 2007;25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- Long HM, Chagoury OL, Leese AM, Ryan GB, James E, Morton LT, et al. MHC II tetramers visualize human CD4+ T cell responses to Epstein-Barr virus infection and demonstrate atypical kinetics of the nuclear antigen EBNA1 response. J Exp Med. 2013;210:933–949. doi: 10.1084/jem.20121437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinderer W, Lang D, Rothe M, Vornhagen R, Sonneborn HH, Wolf H. Serodiagnosis of Epstein-Barr virus infection by using recombinant viral capsid antigen fragments and autologous gene fusion. J Clin Micrbiol. 1999;37:3239–3244. doi: 10.1128/jcm.37.10.3239-3244.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W, Henle G, Andersson J, Ernberg I, Klein G, Horwitz CA, et al. Antibody responses to Epstein-Barr virus-determined nuclear antigen (EBNA)-1 and EBNA-2 in acute and chronic Epstein-Barr virus infection. Proc Natl Acad Sci USA. 1987;84:570–574. doi: 10.1073/pnas.84.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasche G, Feldmann J, Fischer A, de Saint Basile G. Primary hemophagocytic syndromes point to a direct link between lymphocyte cytotoxicity and homeostasis. Immunol Rev. 2005;203:165–179. doi: 10.1111/j.0105-2896.2005.00224.x. [DOI] [PubMed] [Google Scholar]
- Parvaneh N, Filipovich AH, Borkhardt A. Primary immunodeficiencies predisposed to Epstein-Barr virus-driven haematological diseases. Br J Haematol. 2013;162:573–586. doi: 10.1111/bjh.12422. [DOI] [PubMed] [Google Scholar]
- Pappworth IY, Wang EC, Rowe M. The switch from latent to productive infection in epstein-barr virus-infected B cells is associated with sensitization to NK cell killing. J Virol. 2007;81:474–482. doi: 10.1128/JVI.01777-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T, Chijioke O, Carrega P, Arrey F, Meixlsperger S, Ramer PC, et al. Human NK cells of mice with reconstituted human immune system components require preactivation to acquire functional competence. Blood. 2010;116:4158–4167. doi: 10.1182/blood-2010-02-270678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer PC, Chijioke O, Meixlsperger S, Leung CS, Munz C. Mice with human immune system components as in vivo models for infections with human pathogens. Immunol Cell Biol. 2011;89:408–416. doi: 10.1038/icb.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chijioke O, Muller A, Feederle R, Barros MH, Krieg C, Emmel V, et al. Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein-Barr virus infection. Cell Rep. 2013;5:1489–1498. doi: 10.1016/j.celrep.2013.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T, Brilot F, Arrey F, Bougras G, Thomas D, Muller WA, et al. Tonsilar NK cells restrict B cell transformation by the Epstein-Barr virus via IFN-gamma. PLoS Pathog. 2008;4:e27. doi: 10.1371/journal.ppat.0040027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H, McAulay K, Macsween KF, Gallacher NJ, Higgins CD, Harrison N, et al. The immune response to primary EBV infection: a role for natural killer cells. Br J Haematol. 2005;129:266–274. doi: 10.1111/j.1365-2141.2005.05452.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci USA. 2011;108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks DW, Balfour HH, Jr., Dunmire SK, Schmeling DO, Hogquist KA, Lanier LL. Cutting edge: NKG2C(hi)CD57+ NK cells respond specifically to acute infection with cytomegalovirus and not Epstein-Barr virus. J Immunol. 2014;192:4492–4496. doi: 10.4049/jimmunol.1303211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunemann A, Vanoaica LD, Azzi T, Nadal D, Munz C. A distinct subpopulation of human NK cells restricts B cell transformation by EBV. J Immunol. 2013;191:4989–4995. doi: 10.4049/jimmunol.1301046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrego F, Ulbrecht M, Weiss EH, Coligan JE, Brooks AG. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence-derived peptides by CD94/NKG2 confers protection from natural killer cell-mediated lysis. J Exp Med. 1998;187:813–818. doi: 10.1084/jem.187.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin BD, Gram AM, Mulder A, Van Leeuwen D, Claas FH, Wang F, et al. EBV BILF1 evolved to downregulate cell surface display of a wide range of HLA class I molecules through their cytoplasmic tail. J Immunol. 2013;190:1672–1684. doi: 10.4049/jimmunol.1102462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlazzo G, Pack M, Thomas D, Paludan C, Schmid D, Strowig T, et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci USA. 2004;101:16606–16611. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, et al. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447:326–329. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- Yager EJ, Szaba FM, Kummer LW, Lanzer KG, Burkum CE, Smiley ST, et al. Gamma-herpesvirus-induced protection against bacterial infection is transient. Viral Immunol. 2009;22:67–72. doi: 10.1089/vim.2008.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher BA, Bhalara S. False-positive result provided by rapid heterophile antibody test in a case of acute infection with hepatitis E virus. J Clin Microbiol. 2004;42:4411. doi: 10.1128/JCM.42.9.4411.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz CA, Henle W, Henle G, Penn G, Hoffman N, Ward PC. Persistent falsely positive rapid tests for infectious mononucleosis. Report of five cases with four—six-year follow-up data. Am J Clin Pathol. 1979;72:807–811. doi: 10.1093/ajcp/72.5.807. [DOI] [PubMed] [Google Scholar]
- Blake JM, Edwards JM, Fletcher W, McSwiggan DA, Pereira MS. Measurement of heterophil antibody and antibodies to EB viral capsid antigen IgG and IgM in suspected cases of infectious mononucleosis. J Clin Pathol. 1976;29:841–847. doi: 10.1136/jcp.29.9.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Ramos A, Patel M, Kadakia K, Haque T. Performance of the architect EBV antibody panel for determination of Epstein-Barr virus infection stage in immunocompetent adolescents and young adults with clinical suspicion of infectious mononucleosis. Clin Vaccine Immunol. 2014;21:817–823. doi: 10.1128/CVI.00754-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RD. Routine Epstein-Barr virus diagnostics from the laboratory perspective: still challenging after 35 years. J Clin Microbiol. 2004;42:3381–3387. doi: 10.1128/JCM.42.8.3381-3387.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert J, Zens W, Weissbrich B. Comparative evaluation of the use of immunoblots and of IgG avidity assays as confirmatory tests for the diagnosis of acute EBV infections. J Clin Virol. 1998;11:161–172. doi: 10.1016/s0928-0197(98)00061-0. [DOI] [PubMed] [Google Scholar]
- Nystad TW, Myrmel H. Prevalence of primary versus reactivated Epstein-Barr virus infection in patients with VCA IgG-, VCA IgM- and EBNA-1-antibodies and suspected infectious mononucleosis. J Clin Virol. 2007;38:292–297. doi: 10.1016/j.jcv.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Hwang AE, Hamilton AS, Cockburn MG, Ambinder R, Zadnick J, Brown EE, et al. Evidence of genetic susceptibility to infectious mononucleosis: a twin study. Epidemiol Infect. 2012;140:2089–2095. doi: 10.1017/S0950268811002457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostgaard K, Wohlfahrt J, Hjalgrim H. A genetic basis for infectious mononucleosis: evidence from a family study of hospitalized cases in Denmark. Clin Infect Dis. 2014;58:1684–1689. doi: 10.1093/cid/ciu204. [DOI] [PubMed] [Google Scholar]
- Odumade OA, Hogquist KA, Balfour HH., Jr. Progress and problems in understanding and managing primary Epstein-Barr virus infections. Clin Microbiol Rev. 2011;24:193–209. doi: 10.1128/CMR.00044-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalgrim H, Askling J, Rostgaard K, Hamilton-Dutoit S, Frisch M, Zhang JS, et al. Characteristics of Hodgkin's lymphoma after infectious mononucleosis. N Engl J Med. 2003;349:1324–1332. doi: 10.1056/NEJMoa023141. [DOI] [PubMed] [Google Scholar]
- Handel AE, Williamson AJ, Disanto G, Handunnetthi L, Giovannoni G, Ramagopalan SV. An updated meta-analysis of risk of multiple sclerosis following infectious mononucleosis. PLoS ONE. 2010;5:e12496. doi: 10.1371/journal.pone.0012496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein MA, Achong BG. The EB virus. Annu Rev Microbiol. 1973;27:413–436. doi: 10.1146/annurev.mi.27.100173.002213. [DOI] [PubMed] [Google Scholar]
- Gu SY, Huang TM, Ruan L, Miao YH, Lu H, Chu CM, et al. First EBV vaccine trial in humans using recombinant vaccinia virus expressing the major membrane antigen. Dev Biol Stand. 1995;84:171–177. [PubMed] [Google Scholar]
- Sokal EM, Hoppenbrouwers K, Vandermeulen C, Moutschen M, Leonard P, Moreels A, et al. Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein-Barr virus vaccine in healthy young adults. J Infect Dis. 2007;196:1749–1753. doi: 10.1086/523813. [DOI] [PubMed] [Google Scholar]
- Elliott SL, Suhrbier A, Miles JJ, Lawrence G, Pye SJ, Le TT, et al. Phase I trial of a CD8+ T-cell peptide epitope-based vaccine for infectious mononucleosis. J Virol. 2008;82:1448–1457. doi: 10.1128/JVI.01409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI. Epstein-Barr virus vaccines. Clin Trans Immunol. 2015;4:e32. doi: 10.1038/cti.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romain CA, Balfour HH, Jr, Vezina HE, Holman CJ. A method for evaluating antiviral drug susceptibility of Epstein-Barr virus. Virus Adapt Treat. 2010;2:1–7. [Google Scholar]
- Balfour HH, Jr, Hokanson KM, Schacherer RM, Fietzer CM, Schmeling DO, Holman CJ, et al. A virologic pilot study of valacyclovir for infectious mononucleosis. J Clin Virol. 2007;39:16–21. doi: 10.1016/j.jcv.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010;362:1993–2000. doi: 10.1056/NEJMcp1001116. [DOI] [PubMed] [Google Scholar]
- Balfour HH., Jr. Epstein-Barr virus vaccine for the prevention of infectious mononucleosis—and what else. J Infect Dis. 2007;196:1724–1726. doi: 10.1086/523815. [DOI] [PubMed] [Google Scholar]
- Cohen JI, Fauci AS, Varmus H, Nabel GJ. Epstein-Barr virus: an important vaccine target for cancer prevention. Science Transl Med. 2011;3:107fs107. doi: 10.1126/scitranslmed.3002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E. Selective anti-herpesvirus agents. Antivir Chem Chemother. 2013;23:93–101. doi: 10.3851/IMP2533. [DOI] [PubMed] [Google Scholar]