Abstract
The psychiatric care of opioid users receiving agonist therapies is often complicated by high rates of illicit drug use (Brooner et al., 2013). The present study evaluates if illicit drug use (i.e., opioids, cocaine, sedatives) detected at the start of psychiatric care affects treatment response. Methadone maintenance patients (n = 125) with at least one current psychiatric disorder completed a 3-month randomized clinical trial evaluating the efficacy of financial incentives on attendance to on-site integrated substance abuse and psychiatric services (Kidorf et al., 2013). The present study re-analyzes the data set by grouping participants into one of two conditions based on the 4-week baseline observation: 1) no illicit drug use (Baseline Negative; n = 50), or 2) any illicit drug use (Baseline Positive; n = 75). All participants received a similar schedule of psychiatric services, and had good access to prescribed psychiatric medications. The Global Severity Index (GSI) of the Hopkins Symptom Checklist-Revised was administered monthly to evaluate changes in psychiatric distress. Results showed that while both conditions evidenced similar utilization of on-site psychiatric services, Baseline Negative participants remained in treatment somewhat longer (80.7 vs. 74.8 days, p = .04) and demonstrated greater reductions in GSI scores than Baseline Positive participants at Month 3 (p = .004). These results have implications for interpreting previous studies that have shown inconsistent efficacy of pharmacotherapy and other psychiatric treatments, and for providing clinical care for patients with co-occurring substance use and psychiatric disorders.
Keywords: opioid dependence, methadone maintenance, psychiatric treatment, poly-drug use
1.0 Introduction
Opioid-dependent individuals experience much higher rates of co-occurring psychiatric disorders than the general population (Brooner, King, Kidorf, Schmidt, & Bigelow, 1997; Kessler, Chiu, Demler, Merikangas, & Walters, 2005; Strain, 2002). Well over half have at least one co-occurring psychiatric disorder, with major depression and antisocial personality disorder (APD) generally found to be the most prevalent conditions (Brooner et al., 1997; Kidorf et al., 2004; McGovern, Xie, Segal, Siembab, & Drake, 2006). Numerous studies have shown that psychiatric comorbidity in opioid-dependent individuals is associated with considerable psychiatric distress, higher rates of lifetime and current substance use disorder, and often a poorer response to substance abuse treatment (Brooner et al., 1997; Cacciola, Alterman, Rutherford, McKay, & Mulvaney, 2001; Compton, Cottler, Jacobs, Ben-Abdallah, & Spitznagel, 2003; Darke et al., 2007; Kidorf et al., 2004).
Unfortunately, less is known about effective strategies to treat psychiatric comorbidity in people with opioid dependence. Two major categories of studies have evaluated the efficacy of psychiatric treatment in this population. Placebo-controlled medication trials, most often conducted with those experiencing major depression or elevated depression symptom severity, have produced very mixed results. For example, Nunes et al. (1994) showed that 57% of depressed methadone maintenance patients completing at least 6-weeks of an imipramine trial were rated as clinically improved (compared to only 7% of the placebo condition), though few patients demonstrated abstinence in routine urinalysis testing. Other studies show little advantage of pharmacotherapy versus placebo in reducing either psychiatric symptoms or drug use Carpenter, Brooks, Vosburg, & Kleber et al., 1983; Nunes, 2004; Petrakis et al, 1998; see Nunes & Levin, 2004 and Pedrelli et al., 2011, for reviews). A second category of studies evaluating varying models of integrated psychiatric and substance abuse treatment have shown some promise (Brooner et al., 2013), though most studies of integrated care for combinations of opioid users and other substance users have reported little benefit compared to parallel or sequential models of care (see Donald, Dower, & Kavanaugh, 2005, for a review).
It is possible that variation in response to placebo-controlled trials and integrated care in this population may be associated with current substance use. Treatment-seeking opioid users commonly use cocaine and sedatives (Chutuape, Brooner, & Stitzer, 1997; Epstein et al., 2009; Lintzeris & Nielsen, 2010; Peirce et al., 2006). Ongoing substance use might affect adherence and/or response to psychiatric services. The correlation between adherence and psychiatric treatment response was illustrated nicely in a randomized trial of pharmacotherapy and cognitive-behavioral therapy for depressed injection drug users not receiving substance abuse care (Stein et al., 2004). Current substance use might also be associated with more severe psychosocial problems and impoverished environments, thereby reducing the effectiveness of both psychosocial and medication interventions for comorbid psychiatric problems (Carpenter et al., 2004).
While illicit drug use is frequently implicated as a predictor of poorer response to substance abuse treatment in this population (Kidorf, Brooner, King, & Stoller, 1998; Saxon, Wells, Fleming, Jackson, & Calsyn, 1996), it has not been examined as a predictor of response to psychiatric treatment. In addition, study of the impact of substance use on psychiatric treatment response may help explain the inconsistent findings of previous studies, and potentially help establish conditions required for optimal response to psychiatric treatment. For example, one concern related to drug use during episodes of psychiatric care is that many integrated care approaches appear to reduce the amount of time and focus on substance use to make time to address the comorbid psychiatric condition (Donald et al., 2005). Evidence that current substance use reduces response to psychiatric treatment for the comorbid disorder might caution against the development of integrated care approaches that dilute attention to the substance use problem.
We recently completed a 3-month randomized clinical trial evaluating the efficacy of financial incentives on attendance to on-site integrated psychiatric treatment for methadone maintenance patients (Kidorf et al., 2013). The present study re-analyzes this dataset by grouping participants on the absence or presence of illicit drug use in urine samples tested during the one-month study baseline, and evaluating condition differences on psychiatric service utilization and psychiatric treatment response over the observation period. We hypothesized that participants using illicit drugs at baseline would have poorer psychiatric service utilization and response to treatment.
2.0 Methods
2.1 Participants
Study participants were 125 opioid-dependent outpatients enrolled in a community-based opioid-agonist clinic and recruited from 12/15/09 to 4/30/12. Patients were eligible to participate if they reported psychiatric concerns consistent with a current psychiatric disorder to their substance abuse counselor, and expressed interest in receiving psychiatric treatment offered within the program. Exclusion criteria included: 1) pregnancy, 2) experiencing an acute medical or psychiatric problem that required immediate and intense intervention, or 3) having severe cognitive impairment that interfered with understanding study procedures. The Johns Hopkins University Institutional Review Board approved the study.
Patients providing informed written consent to participate in the evaluation (n = 158) were informed of the requirements, risks, and benefits of study participation. Participants were excluded from randomization if they: 1) failed to meet criterion for a current psychiatric disorder on the SCID interview and subsequent clinical reappraisal done by one of the co-investigators (n = 4); 2) left the treatment program prior to randomization (n = 13); 3) failed to complete study assessments (n = 3); 4) exhibited poor cognitive functioning or acute medical concerns (n = 5); 5) reported receiving psychiatric care elsewhere (n = 1). An additional seven participants withdrew from the study for unspecified reasons, leaving a randomized sample pool of 125 participants.
Table 1 (column 1) reports baseline demographic and psychiatric characteristics, methadone dose, and urinalysis results for the sample. Participants were maintained on average of 84.6 (SD = 23.3) mg of methadone. Major Depression and Post-traumatic Stress Disorder (PTSD) were the most prevalent Axis I psychiatric disorders; 42% were diagnosed with Antisocial Personality Disorder (APD). Only one participant was diagnosed with a substanceinduced psychiatric disorder (i.e., psychiatric sympoms developed within a month of a clinically notable change in the frequency or amount of substance use which appeared sustained by the change).
Table 1.
Baseline demographics, psychiatric and substance use disorders1, psychiatric distress, urinalysis results, and prescribed medications2
| Characteristic | Overall (n=125) |
Baseline Negative3 (n=50) |
Baseline Positive3 (n=75) |
χ2 or t-test (df=123) | p-value |
|---|---|---|---|---|---|
| M (SD) or % | M (SD) or % | M (SD) or % | |||
| Demographics | |||||
| Gender (%) Male Female |
46.4% 53.6% |
40.0% 60.0% |
50.7% 49.3% |
χ2=1.37 | 0.24 |
| Race (%) White Minority4 |
64.8% 35.2% |
66.0% 34.0% |
64.0% 36.0% |
χ2=0.05 | 0.81 |
| 35.2% | 34.0% | 36.0% | |||
| Age (years) | 39.1 (10.2) | 40.4 (10.2) | 38.3 (10.2) | t=1.14 | 0.25 |
| Education (highest grade completed) | 11.5 (2.2) | 11.8 (2.2) | 11.3 (2.1) | t= 1.20 | 0.23 |
| Married (%) | 24.8% | 32.0% | 20.0% | χ2=2.31 | 0.12 |
| Employed (%) | 20.0% | 22.0% | 18.7% | χ2=0.20 | 0.64 |
| New admission (< 60 days in treatment) | 40.0% | 32.0% | 45.3% | χ2=2.22 | 0.13 |
| Receiving treatment at ATS ≥ 1 year prior to starting study | 24.8% | 38.0% | 16.0% | χ2=7.78 | 0.005 |
| Psychiatric Disorders1 | |||||
| Major Depression Bipolar I Posttraumatic stress disorder Panic disorder Generalized anxiety disorder |
36.8% 17.6% 30.1% 17.6% 10.4% |
32.0% 14.0% 28.0% 10.0% 6.0% |
40.0% 20.0% 30.6% 22.6% 13.3% |
χ2=0.82 χ2=0.74 χ2=0.08 χ2=3.31 χ2=1.73 |
0.36 0.38 0.76 0.07 0.18 |
| Antisocial personality disorder (APD) Borderline personality disorder (BPD) |
41.6% 27.2% |
34.0% 24.0% |
46.6% 29.3% |
χ2=1.98 χ2=0.43 |
0.15 0.51 |
| > 1 Axis I or Axis II disorder | 80.8% | 72.0% | 86.6% | χ2=4.15 | 0.04 |
| Substance Use Disorders1 | |||||
| Alcohol Sedative Cocaine |
6.4% 15.3% 16.8% |
6.0% 4.0% 4.0% |
6.6% 22.6% 25.3% |
* * * |
* * * |
| Psychiatric Distress | |||||
| Hopkins Symptoms Checklist-R (GSI)5 | 50.9 (11.1) | 49.2 (11.9) | 52.0 (10.5) | t=1.40 | 0.16 |
| Urinalysis Results and Self-report Alcohol Use | |||||
| Opioid-positive (%) Cocaine-positive (%) Sedative-positive (%) Alcohol use (days in past 30) |
16% (0.28) 18% (0.32) 16% (0.29) 0.88 (3.23) |
0% (0.00) 0% (0.00) 0% (0.00) 0.36 (1.22) |
26% (0.32) 30% (0.36) 26% (0.34) 1.24 (4.02) |
t= 5.70 t=5.91 t=5.38 t=1.77 |
< .001 < .001 < .001 0.07 |
| Prescribed Medications | |||||
| Substance abuse Methadone Methadone dose |
100.0% 84.6 (23.3) |
100.0% 88.6 (27.3) |
100.0% 81.9 (20.0) |
-- t=1.58 |
-- 0.11 |
| Psychiatric Any psychiatric medication Heterocyclic antidepressants SSI antidepressants Unspecified antidepressants Mood stabilizers Anti-anxiety medications Typical antipsychotics Atypical antipsychotics |
93.6% 38.4% 52.0% 31.2% 18.4% 16.0% 8.8% 14.4% |
92.0% 32.0% 54.0% 40.0% 18.0% 20.0% 8.0% 8.0% |
94.7% 42.7% 50.7% 25.3% 18.7% 13.3% 9.3% 18.7% |
* χ2=1.44 χ2=0.13 χ2=3.00 χ2=0.00 χ2=0.99 * * |
* 0.22 0.71 0.08 0.92 0.31 * * |
Diagnoses determined using the Structured Clinical Interview for the DSM-IV. With the exception of alcohol use disorder, only Axis I psychiatric disorders prevalent in at least 10% of the sample are included. APD and BPD were the most prevalent Axis II disorders. Participants were often diagnosed with more than one Axis I and II disorder.
Methadone and psychiatric medications. Methadone dose and class of psychiatric medications were assessed throughout the trial. Participants were often prescribed more than one psychiatric medication.
Baseline Negative: 4-week baseline urinalysis tests negative for illicit drugs; Baseline Positive: 4-week baseline urinalysis tests positive at least once for illicit drugs.
Most minority participants (n = 44) were African American (n = 32; 73%) or Hispanic (n = 5; 11%).
Global Severity Index
due to low cell counts, chi square results would be invalid, therefore no significance testing is included
2.2 Assessments
Participants completed the Structured Clinical Interview for the DSM-IV-R (SCID-I and SCID-II; First, Spitzer, Gibbon, & Williams, 1995) during the second week of baseline. The SCID-I is a structured interview that uses a decision-tree approach for determining diagnoses of many DSM-IV Axis I psychiatric disorders; the SCID-II was used for making diagnoses of Axis II personality disorders. Participants receiving a psychiatric diagnosis were clinically reappraised by one of the study investigators, who also evaluated participants for suicidal ideation, thought disorder, delusions, and hallucinations. The Hopkins Symptom Checklist - Revised (SCL-90-R; Derogatis, 1983; Deoragitis & Cleary, 1977) was administered at baseline and monthly to measure self-reported psychiatric distress (using a 0–4 Likert Scale) across 90-items and 9-subscales (e.g., depression, anxiety). The present study used the Global Severity Index (GSI) score, which is the average rating given to all 90 items and correlates highly to the individual scales. Finally, the Self-Report Measure of Medication Adherence (SMMA; Morisky, Green, & Levine, 1986) was administered monthly to assess adherence to prescribed psychiatric medications. The SMMA uses a 4-point Likert Scale, with lower scores indicating better adherence. Interviewers completed a comprehensive and ongoing training protocol to establish and help sustain good inter-rater reliability over the course of the study (see Kidorf et al., 2013).
Participants submitted urine samples for testing once per week using a modified random schedule (Monday, Wednesday, or Friday). Urine samples were obtained under direct observation (through a one-way mirror) and tested at a certified laboratory that employed TLC and EMIT testing for the presence of opioids, cocaine, and benzodiazepines. Alcohol use was measured monthly using self-reported number of days drinking alcohol in the past 30-days. Most participants (78%; n = 97) completed all three monthly assessment follow-ups, though a trend finding showed that Baseline Negative participants completed somewhat more follow-ups than Baseline Positive participants (M=2.76; SD=0.71 vs. M=2.46; SD=0.92; t = 1.90, df = 123, p = .06). Participants were paid $40.00 for completion of the baseline assessment battery, and $15.00 for completing each follow-up assessment.
2.3 Procedure
The present study re-analyzes data from the parent study (Kidorf et al., 2013) by classifying participants into one of two conditions based on the 4-week baseline urinalysis results: 1) no illicit drug (i.e., opioid, cocaine, sedatives) positive urine samples (Baseline Negative; n = 50) or 2) at least one illicit drug-positive urine sample (Baseline Positive: n = 75). The full study procedures of the parent study are detailed elsewhere (Kidorf et al., 2013) and are summarized here. Participants in the parent study were randomly assigned to either an attendance reinforced on-site integrated psychiatric care or a standard on-site integrated care condition that did not include the voucher-based attendance reinforcement. The only difference between these two conditions was that those receiving the attendance reinforcement had the opportunity to earn voucher-based incentives ($25.00 per week) for each week they attended all of their scheduled psychiatric sessions. Participants subsequently exchanged voucher earnings for goods and services in the community.
All participants were offered a psychiatric service schedule that included individual psychiatrist appointments (usually scheduled once every 2 weeks), individual mental health counseling sessions (once per week), and group mental health education and support sessions (once per week). The psychiatrists formulated the initial care plan that routinely included the prescription of psychiatric medications. The study paid for psychiatric medications for those participants without pharmacy coverage. The participant’s primary substance abuse counselor conducted the individual mental health counseling sessions, using a case management approach advocated by the Center for Substance Abuse Treatment (CSAT, 2005). Doctoral staff led the mental health education and support group. Information on counselor training, supervision, and fidelity to the psychiatric treatment schedule is provided in Kidorf et al. (2013). All participants received daily methadone administration within an adaptive stepped care substance abuse counseling schedule, in which individuals using illicit substances are systematically advanced to more substance abuse group sessions and returned to less frequent schedules following two consecutive drug-negative urine specimens (Kidorf, King, & Brooner, 2006). Most of the substance abuse groups were manual-guided and led by senior clinical staff.
2.4 Data analyses
Chi-square and t-tests were used to compare Baseline Negative and Baseline Positive participants on demographic and psychiatric characteristics, methadone dose, study condition and voucher earnings, baseline urinalysis data, psychiatric medication prescribing practices, episode of substance abuse care prior to study start (>1 year), and days of psychiatric care. Analyses of variance were used to compare conditions on number mental health sessions attended (individual, group, psychiatrist modalities), number of substance abuse sessions attended (individual or group), SRMS (medication compliance) scores, and drug-positive urinalysis results (opioids, cocaine, sedatives). Mixed models for between and within subjects effects were used to evaluate monthly psychiatric distress (SCL-90-R GSI) scores over the 3-month observation period; post-hoc testing employed the Tukey-Kramer method for multiple comparisons. These final analyses were repeated using baseline GSI score, methadone dose, mental health session attendance, substance abuse session attendance, > 1 psychiatric diagnosis, parent treatment condition (vouchers vs. no vouchers), length of treatment prior to study start, and number of follow-ups as covariates and yielded similar results that are not reported here. No data were imputed in these analyses.
3.0 Results
3.1 Comparison of baseline characteristics across conditions
As shown in Table 1, the study conditions did not differ on demographic or psychiatric diagnostic characteristics, though Baseline Positive participants were more likely to have more than one psychiatric disorder. It should be noted that both study conditions included a small percent of participants diagnosed with an alcohol use disorder and the most common comorbid substance use disorder across conditions were cocaine and sedatives. Baseline Negative participants were more likely to have been in substance abuse treatment for at least one year prior to beginning the study (61.3% vs. 38.7%; χ2 = 7.78, p = .005).
3.2 Psychiatric treatment engagement
Only two Baseline Negative participants and one Baseline Positive participant failed to initiate psychiatric care. Baseline Negative participants remained in the study for slightly longer (M = 80.7; SD = 12.0 vs. M = 74.8, SD = 18.4; t = 2.03, p = .04); 92% of Baseline Negative and 76% of Baseline Positive participants completed the study. Despite this, Baseline Negative and Baseline Positive participants attended a similar number of individual counseling (Baseline Negative: M = 1.8, SD = 1.5 vs. Baseline Positive: M = 1.7, SD = 1.4; F (1, 123) = 0.07, p = .79), group counseling (Baseline Negative: M = 1.2, SD = 1.5 vs. Baseline Positive: M = 1.3, SD = 1.5; F(1, 123) = 0.16, p = .68), and psychiatrist sessions (Baseline Negative: M = 1.7, SD = 1.2 vs. Baseline Positive: M = 1.8, SD = 1.2; F (1, 123) = 0.88, p = .35) over the three-month period. A trend finding for voucher earnings showed that Baseline Negative participants earned more weeks of voucher incentives (M = 7.8; SD = 3.1) than Baseline Positive participants (M = 6.0, SD = 3.8) for attending psychiatric treatment sessions (t = 1.90, p = .06). Participants in both conditions were prescribed similar classes of psychiatric medications (see Table 1), and reported similar self-reported levels of (SMMA) medication adherence (Baseline Negative: M = 0.6, SD = 0.8 vs. Baseline Positive: M = 0.7, SD = 0.8; F (1, 78) = 0.27, p = .60).
3.3 Psychiatric distress
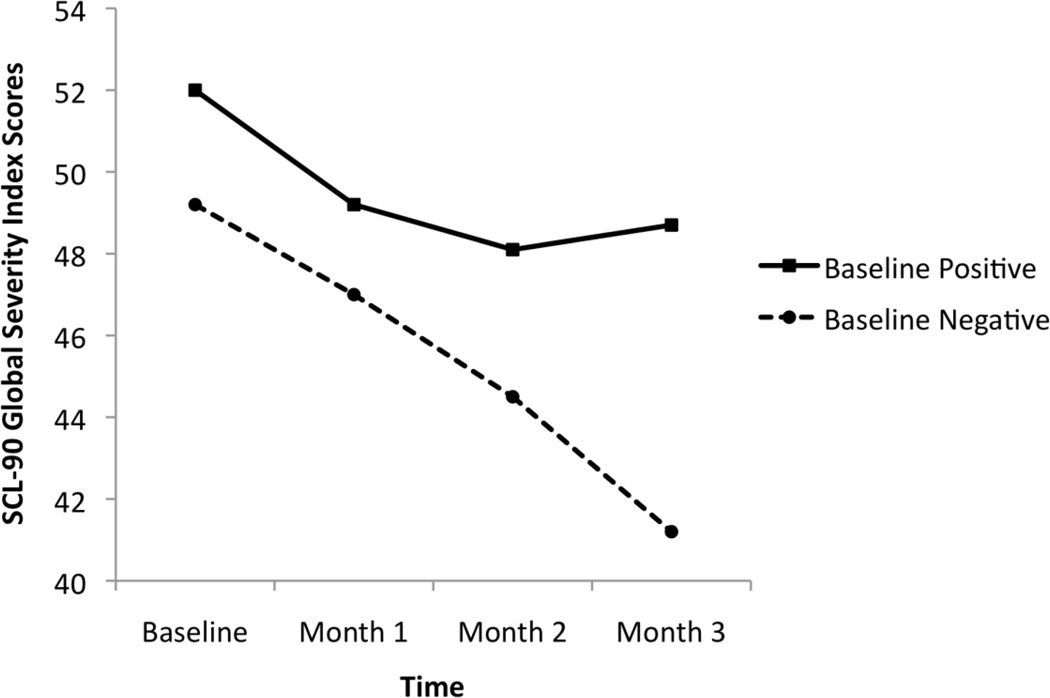
No condition effect was observed (t = 0.63, df = 321, p = .53) in mixed model results. There was a time effect (t = −6.35, df = 321, CI (−3.73, −1.97), p < .001) and a condition × time interaction (t = 2.84, df = 321, CI (0.52, 2.89), p = .004). As shown in Figure 1, while both conditions achieved reductions in psychiatric distress over the first two months, distress levels continued to decrease in Month 3 only in the Baseline Negative condition. Between-group comparisons of GSI psychiatric distress scores at each month showed no differences at month 1 (Baseline Negative: n = 48; M = 47.0; SD = 12.7 vs. Baseline Positive: n = 70; M = 49.2; SD = 10.5) (t = −1.05, ns) and month 2 (Baseline Negative: n = 45; M = 44.5; SD = 13.6 vs. Baseline Positive: n = 61; M = 48.1; SD = 10.7) (t = −1.54, ns), but Baseline Negative participants reported significantly lower GSI scores at the month-3 time point than Baseline Positive participants (Baseline Negative: n = 45; M = 41.2; SD = 14.5 vs. Baseline Positive: n = 54; M = 48.7; SD = 11.3) (t = −2.82; p < .01). Adjusted means (using all covariates) yielded similar findings (Baseline Negative: Ms = 49.1, 46.7, 44.3, 41.7, respectively; Baseline Positive: Ms = 52.0, 49.3, 48.5, 49.0, respectively).
Figure 1.
Mean Global Severity Index (GSI) scores at baseline and months 1–3 across study conditions. Baseline Negative participants demonstrated lower GSI scores at month 3 (p < .01).
To explore if condition differences in GSI scores at Month 3 could be accounted for by changes in rates of drug use, post-hoc t-tests were done to evaluate changes in urinalysis results from Month 2 to Month 3 in Baseline Positive participants. While no within-subjects differences were identified for proportion of opioid-positive (Month 2: M = 0.19; SD = 0.30 vs. Month 3: M = 0.17; SD = 0.28; t = 0.31, p = .75) or sedative-positive (Month 2: M = 0.20; SD = 0.34 vs. Month 3: M = 0.24; SD = 0.35; t = −0.67, p = .50) urine samples, the group had a significant reduction in cocaine-positive specimens (Month 2: M = 0.38; SD = 0.40 vs. Month 3: M = 0.24; SD = 0.33; t = 2.11, p = .04). The reduced rate of cocaine use for the group in Month 3 was still considerably higher than the Baseline Negative group, and the potential benefits of the reduction of cocaine use on psychiatric distress may have been further mitigated by their continuation of higher rates of opioid and sedative use. We further analyzed drug use in the seven participants that reported GSI data in Month 2 but not Month 3 to examine the effects of the missing data on outcomes. Rates of cocaine-positive and sedative-positive urine samples, and GSI scores at Month 2, were similar to the remaining Baseline Positive participants (n=54) that completed the GSI at both time points, although they did have a higher rate of Month 2 opioid-positive urine samples (n=7: M = 0.54; SD = 0.37 vs. n=54: M = 0.14; t = 2.74, p = 0.02).
Exploratory mixed models analyses (with all covariates) were conducted to evaluate changes in GSI scores over time, stratifying on parent treatment assignment (i.e., “reinforced” vs. “non-reinforced” mental health session attendance). A condition × time interaction was found only for “reinforced” participants (t = 3.66; df = 161; CI (1.62, 1.51), p < .001). Baseline Negative participants in the reinforced attendance condition showed large reductions in psychiatric distress from baseline to Month 3 (M = 52.5 vs. 40.9; p < .001), while Baseline Positive participants had no significant reduction in distress (M = 51.1 vs. 48.3, n.s.). For participants in the non-reinforced psychiatric attendance condition, Baseline Negative (M = 47.4 vs. 43.4; p < .01) and Baseline Positive participants (M = 51.5 vs. 48.0; p < .01) had only modest reductions in psychiatric distress from baseline to Month 3.
3.4 Substance use outcomes
As expected, Baseline Positive participants submitted a higher proportion of opioidpositive urine samples (M = 0.20, SD = 0.30 vs. M = 0.04, SD = 0.16; F(1, 105) = 18.05, p < .001), cocaine-positive urine samples (M = 0.30, SD = 0.36 vs. M = 0.02, SD = 0.10; F(1, 105) = 31.08, p < .001), and sedative-positive urine samples (M = 0.21, SD = 0.33 vs. M = 0.05, SD = 0.30; F(1, 105) = 9.94, p = .002) than Baseline Negative participants. Self-reported days of alcohol use was low at baseline across both conditions (see Table 1) and remained consistently low across time (Baseline Negative -- Month 1: M = 0.17 (0.43), Month 2: M = 0.27 (0.86), Month 3: M = 0.53 (3.58); Baseline Positive -- Month 1: M = 1.19 (4.26), Month 2: M = 0.79 (2.21), Month 3: M= 1.20 (4.02). Because participants with drug-positive urine samples were advanced to more intensive substance abuse treatment schedules, Baseline Positive participants were scheduled for and attended more substance abuse counseling services than Baseline Negative participants (M = 7.3, SD = 7.1 vs. M = 3.8, SD = 4.3; F(1, 123) = 24.41, p < .001).
4.0 Discussion
Participants beginning psychiatric treatment following submission of at least one month of illicit drug-free urine samples reported less psychiatric distress at the 3-month observation point than those who submitted at least one illicit drug use-positive sample during baseline. This difference was observed even though a small number of Baseline Negative participants intermittently submitted drug-positive urine samples during the 12-week trial, and 6% of participants in both conditions had an alcohol use disorder. The specific mechanism accounting for this finding is unclear. While Baseline Negative participants were retained somewhat longer in psychiatric treatment, both Baseline Negative and Baseline Positive participants had good and similar exposure to psychiatric pharmacotherapy and the other psychiatric interventions, and participants in both conditions self-reported good adherence to prescribed psychiatric medications.
It is possible that distress associated with ongoing substance use symptoms (e.g., psychosocial problems and conflicts; withdrawal and intoxication symptoms) may have moderated the benefits of concurrent psychiatric care. Even some improvement to baseline levels of anxiety or depressive symptoms attributable to psychiatric medication and counseling services may not be sufficient to reduce the considerable substance-related psychiatric symptoms that routinely accompany persistent sedative and cocaine use (Carpenter et al., 2004; Darke, Swift, Hall, & Ross, 1993; Disney et al., 2005). This hypothesis is supported by the observation that both conditions showed reductions in psychiatric distress over the first 2-months, while only Baseline Negative participants reported sustained distress reduction at Month 3. The large reduction in psychiatric distress in the Baseline Negative participants assigned to the attendance reinforcement condition that was revealed in our exploratory analysis is also relevant and interesting. These participants may have derived more benefit from the psychiatric counseling and the attendance incentives because they were less affected by the cognitive (Verdejo-Garcia & Perez-Garcia, 2007) or delay-discounting (Kirby & Petry, 2004) effects associated with active substance use.
One implication of this study is that illicit drug use by opioid-dependent patients may account at least partially for some of the considerable variability in response to psychiatric treatment reported in placebo-controlled and integrated care clinical trials (Brooner et al., 2013; Donald et al., 2005; Nunes & Levin, 2004; Pedrelli et al., 2011). For example, studies with lower rates of ongoing substance use in their sample might produce psychiatric treatment responses that differ significantly from studies using samples with higher rates of substance use. These findings suggest the importance of stratifying participants on pre-trial drug use and/or analyzing results separately for those with and without drug use during baseline observation.
While the treatment program routinely followed recommendations from the field to intensify the frequency of substance abuse counseling services for patients with continuous periods of drug use despite standard levels of care (e.g., Brooner et al., 2013; Nunes et al. 2010), participants in the Baseline Positive group benefited less from the integrated psychiatric care over the 12-week trial despite their concurrent exposure to more intensive substance abuse counseling schedules. It is possible that the short evaluation period may have been insufficient to observe the amount of change in drug use necessary to achieve expected responses to psychiatric care. While the present study does not illuminate this issue, it does show that exposure to psychiatric pharmacotherapy and counseling over 12-weeks in participants actively taking drugs was not associated with worsening in psychiatric distress. At the very least, these data provide additional support for earlier recommendations to treat comorbid psychiatric disorders in patients with substance use disorder, even in the context of ongoing drug use (Nunes et al., 2010).
The study has important limitations that are consistent with others that pre-select assignment to study conditions. Other baseline characteristics not measured in the study may have independently (or in combination with drug use) accounted for some of the difference in psychiatric distress. Similarly, the presence of one month of drug-free urine samples may reflect other participant skills or motivations that account for a more favorable response to psychiatric care. It is also important to note that the low rates of monthly alcohol use across both conditions were based on self-report and subject to a host of potential reporting biases, including intentional underreporting of use. Higher rates of dropout in the Baseline Positive condition of participants may have also affected study results, though the drop-out rates in both conditions were lower than reported in many treatment trials (Baseline Negative: 8%; Baseline Positive: 24%). The 12-week evaluation period also precluded evaluation of results following withdrawal of psychiatric treatment. Finally, the generalizability of these findings to other substance abuse samples and other opioid-agonist treatment settings, or to integrated psychiatric care approaches that differ from this study is unknown. Despite these limitations, the findings are largely intuitive and provide at least one possible explanation for previous studies showing only mixed support for the efficacy of psychiatric treatment in this population.
HIGHLIGHTS.
we studied methadone-treated outpatients with co-occurring psychiatric disorders]
all participants received on-site substance use and psychiatric services for 3-months
we evaluated the impact of baseline illicit drug use on psychiatric distress
Baseline Negative participants reported less psychiatric distress at Month 3
Acknowledgements
This study was supported by research grant DA028154-02 from the National Institute of Health - National Institute on Drug Abuse (NIH-NIDA). NIH-NIDA had no other role other than financial support. We gratefully acknowledge the research support staff whose effort and diligence were instrumental to both the quality and integrity of the study, especially Kori Kindbom, M.A. (Research Coordinator), Michael Sklar, M.A., Rachel Burns, B.A., Jennifer Mucha, M.A., and Mark Levinson, M.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors have no conflicts of interest to report in connection with this manuscript.
References
- Brooner RK, Kidorf MS, King VL, Peirce J, Neufeld K, Stoller K, Kolodner K. Managing psychiatric comorbidity within versus outside of methadone treatment settings. A randomized and controlled evaluation. Addiction. 2013;108:1942–1951. doi: 10.1111/add.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooner RK, King VL, Kidorf M, Schmidt CW, Bigelow GE. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Archives of General Psychiatry. 1997;54:71–80. doi: 10.1001/archpsyc.1997.01830130077015. [DOI] [PubMed] [Google Scholar]
- Cacciola JS, Alterman AI, Rutherford MJ, McKay JR, Mulvaney FD. The relationship of psychiatric comorbidity to treatment outcomes in methadone maintained patients. Drug and Alcohol Dependence. 2001;61:271–280. doi: 10.1016/s0376-8716(00)00148-4. [DOI] [PubMed] [Google Scholar]
- Carpenter KM, Brooks AC, Vosburg SK, Nunes EV. The effect of sertraline and environmental context on treating depression and illicit substance use among methadone maintained opiate dependent patients: a controlled clinical trial. Drug and Alcohol Dependence. 2004;74:123–134. doi: 10.1016/j.drugalcdep.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Chutuape MA, Brooner RK, Stitzer M. Sedative use disorders in opiatedependent patients: association with psychiatric and other substance use disorders. Journal of Nervous and Mental Disease. 1997;185:289–297. doi: 10.1097/00005053-199705000-00002. [DOI] [PubMed] [Google Scholar]
- Center for Substance Abuse Treatment. Substance abuse treatment for persons with co-occurring disorders. Rockville, MD: 2005. TIP series 42 No. DHHS Publication No. (SMA) 05-3922). [PubMed] [Google Scholar]
- Compton WM, Cottler LB, Jacobs JL, Ben-Abdallah A, Spitznagel EL. The role of psychiatric disorders in predicting drug dependence treatment outcomes. American Journal of Psychiatry. 2003;160:890–895. doi: 10.1176/appi.ajp.160.5.890. [DOI] [PubMed] [Google Scholar]
- Darke S, Swift W, Hall W, Ross M. Drug use, HIV risk-taking and psychosocial correlates of benzodiazepine use among methadone maintenance clients. Drug and Alcohol Dependence. 1993;34:67–70. doi: 10.1016/0376-8716(93)90047-t. [DOI] [PubMed] [Google Scholar]
- Darke S, Ross J, Williamson A, Mills KL, Havard A, Teesson M. Borderline personality disorder and persistently elevated levels of risk in 36-month outcomes for the treatment of heroin dependence. Addiction. 2007;102:1140–1146. doi: 10.1111/j.1360-0443.2007.01876.x. [DOI] [PubMed] [Google Scholar]
- Disney ER, Kidorf MS, King VL, Neufeld K, Kolodner K, Brooner RK. Prevalence and correlates of cocaine physical dependence subtypes using the DSM-IV in outpatients receiving opioid agonist medication. Drug and Alcohol Dependence. 2005;79:23–32. doi: 10.1016/j.drugalcdep.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. SCL-90-R. Administration, scoring, and procedures manual II. 2nd ed. Baltimore, MD: Clinical Psychometric Research; 1983. [Google Scholar]
- Derogatis LR, Cleary P. Confirmation of the dimensional structure of the SCL-90: A study in construct validation. Journal of Clinical Psychology. 1977;33:981–989. [Google Scholar]
- Donald M, Dower J, Kavanaugh D. Integrated versus non-integrated management and care for clients with co-occurring mental health and substance use disorders: A qualitative systematic review of randomized controlled trials. Social Science & Medicine. 2005;60:1371–1383. doi: 10.1016/j.socscimed.2004.06.052. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Schmitter J, Umbricht A, Schroeder JR, Moolchan ET, Preston KL. Promoting abstinence from cocaine and heroin with a methadone dose increase and a novel contingency. Drug and Alcohol Dependence. 2009;101:92–100. doi: 10.1016/j.drugalcdep.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis Disorders - Patient Edition (SCID-I/P, Version 2.0) New York State Psychiatric Institute. 1995 [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidorf M, Brooner RK, Gandotra N, Antoine D, King VL, Peirce J, Ghazarian S. Reinforcing integrated psychiatric service attendance in an opioid-agonist program: A randomized and controlled trial. Drug and Alcohol Dependence. 2013;133:30–36. doi: 10.1016/j.drugalcdep.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidorf M, Brooner RK, King VL, Stoller KB. Predictive validity of cocaine, sedative, and alcohol dependence diagnoses. Journal of Consulting and Clinical Psychology. 1998;66:168–173. doi: 10.1037//0022-006x.66.1.168. [DOI] [PubMed] [Google Scholar]
- Kidorf M, Disney ER, King VL, Neufeld K, Beilenson PL, Brooner RK. Prevalence of psychiatric and substance use disorders in opioid abusers in a community syringe exchange program. Drug and Alcohol Dependence. 2004;74:115–122. doi: 10.1016/j.drugalcdep.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Kidorf M, King VL, Brooner RK. Counseling and psychosocial services. In: Strain EC, Stitzer ML, editors. The treatment of opioid dependence. Johns Hopkins University Press; 2006. pp. 119–150. [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholic s or non-drug-using controls. Addiction. 2004;99:461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Kleber HD, Weissman MM, Rounsaville BJ, Wilber CH, Prusoff BA, Riordan CE. Imipramine as treatment for depression in addicts. Archives of General Psychiatry. 1983;40:649–653. doi: 10.1001/archpsyc.1983.04390010059007. [DOI] [PubMed] [Google Scholar]
- Lintzeris N, Nielsen S. Benzodiazepines, methadone, and burprenorphine: interaction and clinical management. American Journal on the Addictions. 2010;19:59–72. doi: 10.1111/j.1521-0391.2009.00007.x. [DOI] [PubMed] [Google Scholar]
- McGovern MP, Xie H, Segal SR, Siembab L, Drake RE. Addiction treatment services and co-occurring disorders. Journal of Substance Abuse Treatment. 2006;31:267–275. doi: 10.1016/j.jsat.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a selfreported measure of medication adherence. Medical Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence. Journal of the American Medical Association. 2004;291:1887–1896. doi: 10.1001/jama.291.15.1887. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Quitkin FM, Donovan SJ, Deliyannides D, Occpek-Welikson K, Koenig T, Brady R, McGrath PJ, Woody G. Imipramine treatment of opiate21 dependent patients with depressive disorders. Archives of General Psychiatry. 1998;55:153–160. doi: 10.1001/archpsyc.55.2.153. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Selzer J, Levounis P, Davies CA, editors. Substance dependence and co-occurring psychiatric disorders: Best practices for diagnosis and clinical treatment. Civic Research Institute; 2010. [Google Scholar]
- Pedrelli P, Iovieno N, Vitali M, Tedeschini E, Bentley KH, Papakostas GI. Treatment of major depressive disorder and dysthymic disorder with antidepressants in patients with comorbid opiate use disorders enrolled in methadone maintenance therapy. 2011 doi: 10.1097/JCP.0b013e31822c0adf. [DOI] [PubMed] [Google Scholar]
- Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, Schwartz M, Krasnansky J, Perncer E, Silva-Vazquez L, Kirby KC, Royer-Malvestuto C, Roll JM, Cohen A, Copersino ML, Kolodner K, Li R. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: a National Drug abuse Treatment Clinical Trials Network study. Archives of General Psychiatry. 2006;63:201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- Petrakis I, Carroll KM, Nich C, Gordon L, Kosten T, Rounsaville B. Fluoxetine treatment of depressive disorders in methadone-maintained opioid addicts. Drug and Alcohol Dependence. 1998;50:221–226. doi: 10.1016/s0376-8716(98)00032-5. [DOI] [PubMed] [Google Scholar]
- Saxon AJ, Wells EA, Fleming C, Jackson TR, Calsyn DA. Pre-treatment characteristics, program philosophy, and level of ancillary services as predictors of methadone maintenance treatment outcome (1996) Addiction. 1996;91:1197–1209. doi: 10.1046/j.1360-0443.1996.918119711.x. [DOI] [PubMed] [Google Scholar]
- Stein MD, Solomon DA, Herman DS, Anthony JL, Ramsey SE, Anderson BJ, Miller IW. Pharmacotherapy plus psychotherapy for treatment of depression in active injection drug users. Archives of General Psychiatry. 2004;61:152–159. doi: 10.1001/archpsyc.61.2.152. [DOI] [PubMed] [Google Scholar]
- Strain EC. Assessment and treatment of comorbid psychiatric disorders. Clinical Journal of Pain. 2002;18:S14–S27. doi: 10.1097/00002508-200207001-00003. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia, Perez-Garcia Profile of executive deficits in cocaine and heroin polysubstance users: common and differential effects on separate executive components. Psychopharmacology. 2007;190:517–530. doi: 10.1007/s00213-006-0632-8. [DOI] [PubMed] [Google Scholar]