Abstract
Chronic rotator cuff (RC) tears affect a large portion of the population and result in substantial upper extremity impairment, shoulder weakness, pain and limited range of motion. Regardless of surgical or conservative treatment, persistent atrophic muscle changes limit functional restoration and may contribute to surgical failure. We hypothesized that deficits in the skeletal muscle progenitor (SMP) cell pool could contribute to poor muscle recovery following tendon repair. Biopsies were obtained from patients undergoing arthroscopic RC surgery. The SMP population was quantified, isolated and assayed in culture for its ability to proliferate and fuse in-vitro and in-vivo. The SMP population was larger in muscles from cuffs with partial tears compared with no tears or full thickness tears. However, SMPs from muscles in the partial tear group also exhibited reduced proliferative ability. Cells from all cuff states were able to fuse robustly in culture and engraft when injected into injured mouse muscle, suggesting that when given the correct signals, SMPs are capable of contributing to muscle hypertrophy and regeneration regardless of tear severity. The fact that this does not appear to happen in-vivo helps focus future therapeutic targets for promoting muscle recovery following rotator cuff repairs and may help improve clinical outcomes.
Keywords: Muscle, Progenitor, Rotator Cuff, Shoulder, Regeneration
Introduction
Rotator cuff (RC) injuries are common causes of shoulder weakness, pain and limited mobility in humans. It has been estimated that 30% of the population over 60 years of age has a full thickness tear of at least one rotator cuff tendon1. Frequently these injuries are chronic and remain untreated for years, which complicates surgical repair of the tear site. The success of surgical intervention is highly variable with re-tear rates ranging from 20 to 90% depending on tear size and chronicity 2,3.
One reason for the large outcome range may be the maladaptation of RC musculature in response to chronic tears, including atrophy, fibrosis and fatty degeneration4-6. In cases of chronic tendon tears with retraction, muscle volume may be nearly entirely replaced by fat 6,7. Furthermore, MRI-based muscle cross-sectional areas may overestimate the more functionally relevant value of physiological cross-sectional area (PCSA). PCSA is a calculated value that estimates the total muscle cross sectional area from muscle volume, fiber length, and pennation angle. Increased in intramuscular fat and connective tissue, increased pennation angle, and sarcomere shortening from muscle retraction would all lead to reduced PCSA, but these changes would not be captured by MRI-based measurements of muscle area. This poses a significant challenge for surgical repair as the degree of atrophy and fatty degeneration is highly correlated with negative clinical outcomes and high re-tear rates 4,7,8. Furthermore, fatty degeneration and muscle atrophy are observed to persist following repair 4,7. This is surprising since muscular atrophy due to unloading is completely reversible in rodents and humans following limb casting 9,10. Similar chronic ruptures to the achilles or the distal biceps tendon with muscle retraction (which remain untreated for > 1 year) can be surgically repaired with good recovery of strength, muscle volume and low re-tear rates 11,12. This evidence suggests that there may be a number of possible defects in the recovery of rotator cuff muscles following tendon tear.
In response to sudden load increase, skeletal muscle will typically undergo rapid growth to increase fiber size and number, utilizing progenitors that surround the muscle fiber13,14. These skeletal muscle progenitor (SMP) or “satellite” cells respond to signals for muscle growth or repair by activating, proliferating and fusing with each other or with existing myofibers15. In this way, muscle is able to adapt to loading changes for optimal use. Experimental interventions in mice have demonstrated that later stages of hypertrophy, muscle repair and the formation of nascent muscle fibers are all severely blunted if SMPs are unable to proliferate or fuse13,14,16. Thus, we hypothesized that poor rotator cuff muscle recovery following restoration of functional loading could be due to SMP population defects.
To test this hypothesis, muscle biopsies were obtained from patients undergoing arthroscopic rotator cuff surgery and the population of skeletal muscle progenitor cells was quantified and isolated. Interestingly, both the size and the proliferation rates of these populations varied as a function of cuff tear state. However, the ability to fuse and contribute to new myotubes in-vitro and myofibers in-vivo was unchanged. These findings provide further insight into a cellular pathology that may underlie muscle changes seen following tears to the rotator cuff and suggest SMPs as a therapeutic target.
Methods
Muscle biopsies were obtained from the distal third of the supraspinatus (SS) and infraspinatus (IS) muscles from 17 patients undergoing arthroscopic rotator cuff (RC) surgery (Table 1). Biopsies of the deep surface of the deltoid muscle were also obtained in a subset of 10 of these patients as a non-rotator cuff (NRC) control. RC muscles were classified as having no tear (NT), a partial thickness tear (PT) or a full thickness tear (FT) by the operating surgeon. Patients classified as PT had torn one or more tendons partially but not completely through the sagittal plane of the tendon. Conversely patients classified as FT had completely torn through the sagittal plane of the tendon but do not have muscle retraction. The study was approved by UC San Diego's IRB and signed written consent obtained from all participants. See Supplemental Methods for a detailed description of all techniques used.
Table 1. Patient Demographic Information.
The symbol used for each patient in the Figures is listed in the Demarcation column (square ■, diamond ◆, down triangle ▼, up triangle ▲, star ★, circle ●). The classification used for each patient is listed in the column labeled Class (NT no tear, PT partial thickness tear, FT full thickness tear). The sex (M male, F female), age at surgery (years), and body mass index (BMI m2/kg) are also provided in similarly labeled columns. SS (supraspinatus) and IS (infraspinatus) tears are classified in SS Class and IS Class columns (0 = no tear, 1 = partial thickness tear, 2 = full thickness tear). Finally, surgical notes on the health and shoulder status of each patient are provided in the column labeled Notes.
| Patient Number | Demarcation | Class | Sex | Age | BMI | SS Class | IS Class | Notes |
|---|---|---|---|---|---|---|---|---|
| 21 | square | NT | M | 39 | 45.8 | 0 | 0 | Bursitis |
| 22 | diamond | NT | M | 42 | 24.4 | 0 | 0 | Prior RCR with adhesions and immobile arthrofibrosis |
| 34 | down triangle | NT | M | 38 | 28.9 | 0 | 0 | Small labra tear (no repair needed) |
| 37 | up trianlge | NT | F | 54 | 26.8 | 0 | 0 | Impingement with bursitis and biceps tenosynovitis |
| 62 | star | NT | M | 56 | 28.5 | 0 | 0 | Biceps rupture, tenodesis (meds: Lipitor) |
| 63 | circle | NT | F | 53 | 23.9 | 0 | 0 | Adhesions |
| 27 | circle | PT | M | 59 | 33.0 | 1 | 0 | Partial thickness SS tear with adhesions, Smoker |
| 32 | square | PT | F | 54 | 26.6 | 1 | 0 | Partial thickness SS tear, type I labral tear |
| 57 | up triangle | PT | M | 57 | 27.4 | 1 | 0 | Partial thickness SS tear, bursitis, Smoker |
| 82 | down triangle | PT | M | 60 | 31.6 | 1 | 0 | Partial thickness SS tear, bursitis, Hypertension |
| 86 | diamond | PT | M | 56 | 31.0 | 1 | 0 | Partial thickness SS tear, intact IS (meds: Omeprazole, Tramado) |
| 33 | circle | FT | F | 62 | 34.7 | 2 | 2 | 2 cm full thickness SS and IS tears with some retraction, (meds: sulfasalazine and steroids) |
| 47 | diamond | FT | F | 57 | 24.6 | 2 | 0 | Large full thickness SS tear with retraction to humeral head |
| 64 | star | FT | M | 43 | 29.8 | 2 | 0 | Full thickness SS 2cm tear, superior labral tear |
| 68 | up triangle | FT | M | 53 | 38.0 | 2 | 2 | Full thickness bursal sided tear of SS and IS with retraction, bursitis, labral tear, (meds: Lipitor) |
| 80 | down triangle | FT | M | 62 | 22.8 | 2 | 2 | Full thickness SS and IS tears, partial subscapularis tear, bursitis |
| 83 | square | FT | F | 59 | 23.0 | 2 | 1 | 8 mm full thickness SS tear, partial IS tears, subscapularis fraying, (meds: hormone replacement) |
Cell Isolation and Quantification
Muscle biopsies of approximately 15-25 mg were obtained using an arthroscopic rongeur. Tissue was digested and cells isolated as described in the Supplemental Methods.
Myogenic Hydrogel Preparation and Chemical Induction
Polyacrylamide matrices of muscle-mimicking stiffness were prepared as described in the Supplemental Methods. To chemically induce differentiation, cells were exposed to a myogenic induction media. Cultures were allowed to differentiate for 4 days, then cells were either prepared for RNA isolation or immunostaining.
Cell Proliferation
Cell proliferation was measured using a Click-iT EdU assay (Life Technologies) with an 18 hour pulse. EdU positive nuclei were identified using a detection algorithm designed in Matlab, and the percentage of dividing cells was calculated as EdU positive nuclei divided by total nuclei.
Quantification of Myogenic Gene Expression
RNA was isolated from confluent cultures using Trizol reagent (Life Technologies) according to the manufacturer's protocol. cDNA was generated and gene expression quantified as described in the Supplemental Methods.
Immunostaining
Fixed and permeabilized cultures were stained with anti-Myosin Heavy Chain and counterstained with DAPI. Nuclei inside myosin heavy chain positive myotubes were counted using a custom algorithm designed in Matlab.
Cell Transplantation
Tibialis anterior (TA) muscles of Rag1tm/Mom were injected bilaterally with 10 μL notexin to induce muscle degeneration. 24 hours following notexin injection, either 100,000 SMPs, suspended in saline, or saline alone was injected into individual TA muscles. All procedures were performed in accordance with NIH's Guide for the Use and Care of Laboratory Animals and approved by UC San Diego.
Histology
One week following injection, mice were euthanized and TA muscles were dissected bilaterally. Muscles were flash frozen in liquid nitrogen cooled isopentane, embedded in OCT and 10 μm sections were cut through the cross section of the muscle midbelly. Sections were stained with anti-human lamin A and anti-laminin b2 and co-stained with DAPI.
Statistical Analysis
For comparisons across tear states, a one-way ANOVA with repeated measures was performed with a significance level (α) set at 0.05. Where appropriate, a multiple corrections Bonferroni post-hoc test was done. Significance levels were also assessed by Mann-Withney tests as indicated. Whenever possible, data were averaged from experimental technical duplicates across passages 3 and 4. Results in the text are presented as mean ± standard error.
Results
Seventeen patients were recruited; six patients with no tear (NT), five with a partial thickness (PT) tear, and six with a full thickness (FT) tear of at least one tendon, respectively (Table 1). All three groups contained a mixture of male and female patients with no significant difference in age (NT: 47.0 ± 3.3 years, PT: 57.2 ± 1.1 years, FT: 56.0 ± 3.2 years) or body mass index (NT: 29.7 ± 3.3 m2/kg, PT: 29.9 ± 1.2 m2/kg, FT: 28.8 ± 2.9 m2/kg) between groups as assessed by non-parametric analysis. All patients in the PT group had a partial tear to the supraspinatus tendon only while patients in the FT group had either a full thickness tear to the supraspinatus tendon only or to both the supraspinatus and infraspinatus tendons.
RC tear state influences cell population dynamics
Skeletal muscle progenitor (SMP) cells were identified by their unique expression of the surface protein NCAM 17 and lack of expression of the hematopoietic marker CD45 and endothelial marker CD31. Contour plots of fluorescence intensity show a distinct population of higher fluorescence intensity on the NCAM axis in biopsies from all tears states (Figure 1A), but that population was a significantly larger percentage of cells in PT biopsies compared with NT or FT groups (Figure 1B). Interestingly, NCAM populations in both the supraspinatus (Figure 1B, closed symbols) and infraspinatus (Figure 1B, open symbols) biopsies were elevated in this group despite the partial tear occurring in the supraspinatus tendon; these data suggest that local tears may induce remodeling in neighboring muscles. Endothelial cells and inflammatory cells, indicated by expression of CD31 and CD45, respectively (Figure 1A), showed no significant differences in expression as a function of tear state (Figure 1C and D). There were no significant correlations between any of the cell populations and the patient age or BMI.
Figure 1. Quantification of three cell populations in rotator cuff biopsies.
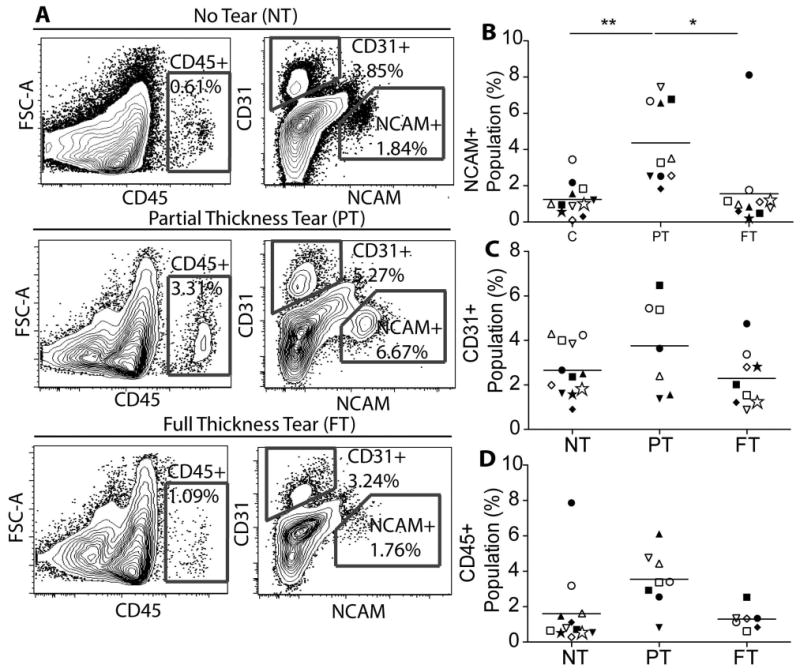
A) Representative flow cytometry contour plots of isolated cells labeled with three population specific fluorescent antibodies: NCAM (skeletal muscle progenitor cells), CD31 (endothelial cells), and CD45 (inflammatory cells). B) Quantification of the percentage of the NCAM+ (skeletal muscle progenitor) cell population relative to all single cells. Quantification is based on the gray gates shown in panel A and in Supplementary Figure 1A. Within each category, individual patient data are indicated by different symbols. Closed symbols indicate supraspinatus data and open symbols indicate infraspinatus data. ** p < 0.01.
To control for quantification differences driven by other patient-specific, non-cuff state related variables, we compared rotator cuff (RC) quantification to a non-rotator cuff (NRC), patient-matched control biopsy of the deltoid muscle. Even when normalized to NRC samples, biopsies from PT cuffs had elevated NCAM positive populations compared to the NT and FT groups (Figure 2A). To ensure the accuracy of the NCAM positive population identification and persistence of the population expression profile over multiple passages, sorted and cultured SMPs were re-evaluated for NCAM expression. Cultures from each RC state were >95% positive for NCAM, indicating a highly pure population of skeletal muscle progenitors (SMPs) (Supplementary Figure 1). These data suggest that the NCAM positive SMP population expands in response to a partial but not full tendon tear.
Figure 2. Ratiometric comparison of patient-matched cell populations.
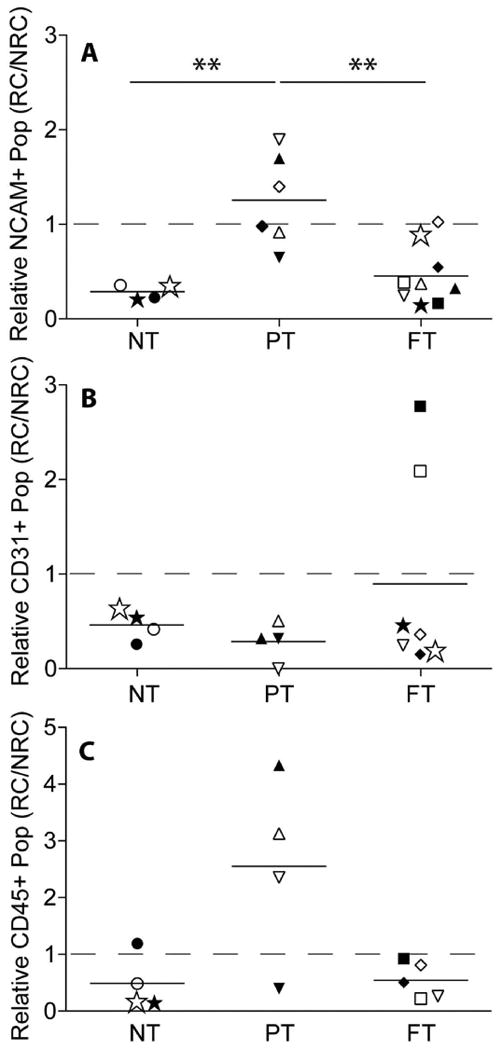
Ratio of patient-matched rotator cuff (RC) to non-rotator cuff (NRC) muscles for the NCAM+ (A), CD31 (B) or CD45 (C) populations in the no tear (NT), partial thickness (PT) tear and full thickness (FT) tear groups. ** p < 0.01.
SMPs from cuffs with partial thickness tears have impaired proliferation
Upon injury, SMPs must expand in-vivo 18. To assess their proliferative capacity in-vitro, SMPs were grown on muscle stiffness-mimicking hydrogels and exposed to a division-marking label (EdU). Fewer SMPs from PT biopsies divided in the experimental time-frame, as indicated by the deficit of red EdU+ nuclei in the partial tear compared to the NT cultures (Figure 3A). EdU signal quantification showed a nearly two-fold reduction in the percentage of EdU positive cells in the PT cultures compared with the NT cultures. SMPs from FT cultures also exhibited slower proliferation, albeit not as reduced as from PT patients (Figure 3B). Interestingly when these values were normalized to the patient-matched non-rotator cuff (NRC) cultures, there were no significant differences in the relative EdU positive fraction between groups (Figure 3C). This implies that there is a deficit in proliferation in the NRC muscles from PT cuffs as well.
Figure 3. Proliferation rates of skeletal muscle progenitor cell populations, quantified using an EdU assay.
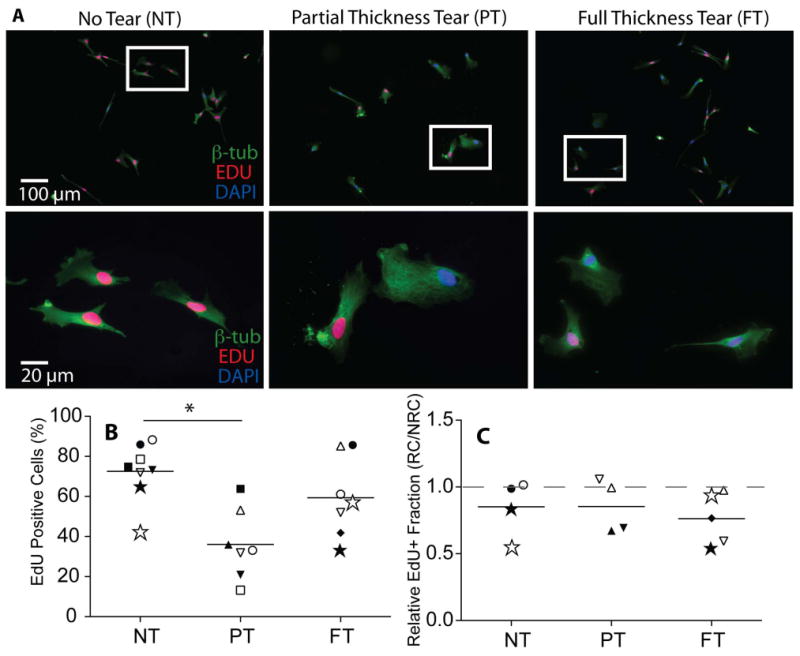
A) Immunofluorescence images of EdU pulsed cultures counter-stained with DAPI (blue) and β tubulin (green). Cells that have divided during the 18 hour pulse can be identified by the red EdU tag incorporated into the nucleus. B) Quantification of the percentage of EdU positive cells as a function of tear state. C) Quantification of the percentage of EdU positive populations from rotator cuff (RC) muscles normalized to a patient-matched non-rotator cuff (NRC) Deltoid control. * p < 0.05.
SMPs are able to fuse robustly in culture regardless of cuff state
SMPs must also be able to robustly fuse in-vivo as a precursor to mature muscle fiber development 19,20. Myotube formation can be identified in SMP cultures by expression and striated organization of the muscle specific isoform of myosin heavy chain (MHC). Following fusion induction, many myotubes containing a large number of nuclei and striated MHC were visible in SMP cultures from each tear state (Figure 4A, green). Quantification of the percentage of the culture that participated in fusion events, i.e. the percentage of total nuclei contained within MHC positive structures, was not significantly different between groups (Figure 4B). Additionally, overall MHC expression was not significantly different between cultures from the different cuff states (Figure 4C). When these data were normalized to the patient-matched non-rotator cuff (NRC) cultures, there was still no difference detected between groups (Figure 4D and E). Similarly, expression of two earlier stage indicators of myogenesis, MyoD and MEF2C, were unchanged between groups (Supplementary Figure 2). These data suggest that despite proliferative differences, cells are equally capable of contributing to myotubes independent of cuff state.
Figure 4. Quantification of fusion rates in skeletal muscle progenitor cell populations as a function of tear state.
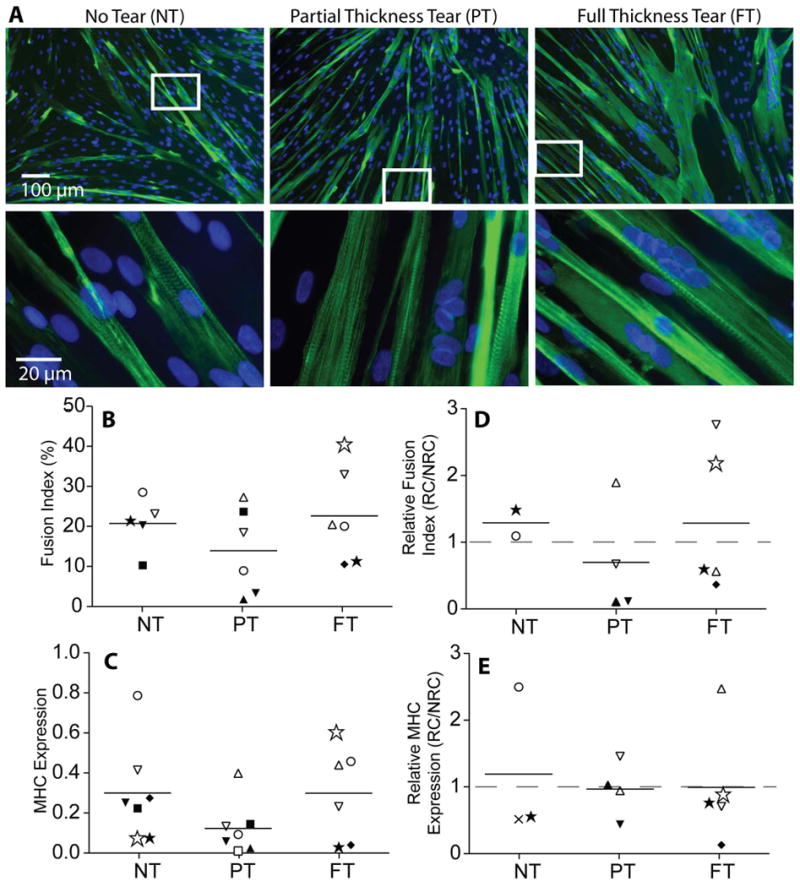
A) Immunofluorescence images of myotubes formed from the fusion of SMPs identified by myosin heavy chain (MHC, green). Striations of MHC are visible in higher magnification images. B) Quantification of the fusion index (% nuclei in myotubes) as a function of tear state. C) MHC gene expression quantified by qPCR as a function of tear state. D) Fusion index from rotator cuff (RC) muscles normalized to a patient-matched non-rotator cuff (NRC) Deltoid control. E) MHC expression from rotator cuff (RC) muscles normalized to a patient-matched non-rotator cuff (NRC) deltoid control.
SMPs from all cuff states are able to fuse with regenerating fibers in-vivo
While SMPs appear to have equal fusion capacity in-vitro regardless of cuff state, their ability to fuse in-vivo with host muscle is unclear. SMPs from each cuff state were injected into notexin injured mouse tibialis anterior muscles to determine if they were capable of participating in host regeneration. Engrafted human cells derived from each cuff state were identified histologically by positive staining for a human-specific isoform of Lamin A (red), a nuclear protein, which co-localized with DAPI (blue) in the center of a laminin (green) outlined fiber (Figure 5). Examples are marked with white boxes and shown at higher magnification in the right column of Figure 5. These data show that SMPs are capable of differentiation and fusion in an in-vivo model of regeneration as well as in-vitro independent of cuff state.
Figure 5. Engraftment of rotator cuff muscle progenitor cells into regenerating mouse muscle.
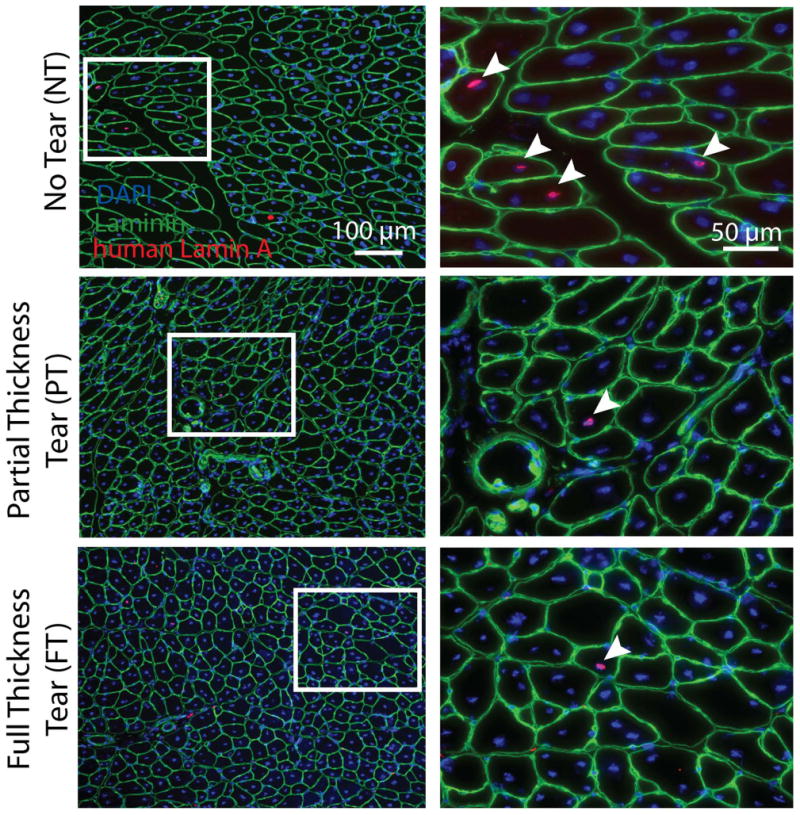
Images in the left-hand column show histological sections based on tear state. Nuclei derived from human SMPs are indentified by a human-specific lamin A antibody (red). Counterstaining with laminin (green) and DAPI (blue) demonstrate human nuclei centrally located in muscle fibers indicating that they were incorporated during regeneration (arrows). White box (left) indicates where images were magnified in right-hand column.
Discussion
Chronic rotator cuff tears are frequently characterized by persistent muscle atrophy and fatty degeneration4-6, which is thought to contribute to poor clinical outcomes4,8. Much of this failure has been attributed to the inability of RC muscle to properly recover in response to restored functional loading 4,7. Muscle regeneration and hypertrophy should normally rely on skeletal muscle progenitor (SMP) cells to add fiber volume and sarcomeres, but limited RC muscle recovery following repair suggests a potential defect with SMPs. We thus hypothesized that the insensitivity of these muscles to growth cues, e.g. increased loading and fiber damage 21, could be due to negative and persistent changes to the progenitor cells following chronic RC tears. This alteration could arise from a depletion of SMPs, a defect in their ability to proliferate or differentiate in response to stimuli or a combination thereof.
Impaired SMP population size and proliferation but not fusion may contribute to poor RC repair outcomes
First, if chronic RC tears result in a depletion of the muscle progenitor pool, the reduced SMP population could be unable to meet the muscle's recovery needs. Animal studies have shown that disease, unloading and aging all decrease the progenitor cell population and negatively effect the ability of the muscle to regenerate and hypertrophy22,23. For RC muscle, in addition to atrophying, chronically retracted muscles subtract sarcomeres24. During surgical repair, the shortened muscle is stretched to its original length, potentially forcing sarcomeres to operate at distances and velocities that exceed normal function and potentially inducing muscle injury21. The sudden increase in loading coupled with the increased potential for injury following repair, suggests that RC muscles may need to activate their resident SMPs.
Contrary to clinical outcomes that suggest a potential problem with this cell population, we found no decrease in the SMP population from the muscles of the fully torn rotator cuff compared with cuffs without tears, which suggests that SMP population size may not be the limiting factor. In line with the recent histological findings of Lundgreen et al., we found an elevated SMP population in muscles from cuffs with partial thickness tears25, compared with full thickness tears. The increase in SMP population in PT tears may be a consequence of activation and proliferation in response to dynamic changes in loading at the fiber level, though other factors such as inflammation and altered kinematics cannot be excluded and should be investigated in future studies. However, rotator cuff biopsies generally had a lower percentage of SMPs than the patient matched non-rotator cuff deltoid control or other human muscles with SMP fractions between 9 and 11%17,26. Thus it is possible that rotator cuff muscles simply have an inherently smaller number of progenitor cells and an associated lower regenerative potential regardless of tear state.
Secondly, regeneration could be blunted by defective SMP proliferation. Animal studies have demonstrated that muscle injury, inflammation and atrophy negatively effect progenitor cells' ability to proliferate10,18,27, which along with reduced cell activation lead to poor muscle regeneration16. We found significant reduction in SMP proliferation from partially but not from fully torn cuffs relative to untorn cuffs. Tear-dependent differences between expansion in-vivo and in-vitro suggest that the altered loading among other changes in the SMP niche in PT tears may pre-condition cells to respond to mechanically active environments; conversely, the niche change for untorn RCs and FT tears must similarly activate signals within SMPs that regulate the cell cycle in-vitro when passive but not active loading is present. Overall these data suggest that although there are more SMPs in the PT condition, they may be less able to activate, proliferate and meet the regenerative need. However when normalized to a non-rotator cuff control, this effect disappeared indicating that deltoid SMP proliferation was similarly reduced in partial thickness tears. It has been suggested that tears to one rotator cuff tendon more globally alter loading and affect adjacent muscles28; our data appear consistent with this suggestion.
Finally, impaired muscle regeneration could result from poor SMPs differentiation and fusion into new or hypertrophying muscle fibers. While there is not yet consensus about whether progenitor cells are required for muscle hypertrophy, there is general agreement that SMPs are necessary for injury repair and the formation of new fibers13. We found that cells from all cuff states were able to differentiate and fuse in culture and could contribute to muscle regeneration in a mouse injury model in-vivo. Though this model is limited in representing a torn RC, i.e. chemically induced injury vs. mechanical reloading, it does show that RC SMPs are capable of fusing into fibers should they get the appropriate signals.
It is worth noting that SMPs are not the only muscle-resident cell population potentially contributing to muscle hypertrophy and repair, including PW1+ interstitial cells, muscle-derived stem cells (MDSCs), mesangioblasts and pericytes15,29. Though SMPs are required for muscle regeneration, these other populations could supplement the response when SMPs are unable to fully meet the regenerative need. Future studies should investigate these population dynamics as well.
These experiments have several important limitations that warrant discussion. First although patients in the NT group did not have a cuff tear, they did have another RC pathology (bursitis, tendonitis etc.). This means that their cuff muscles likely had confounding factors such as inflammation and decreased use, limiting their power as true “controls.” Future studies might use biopsies from patients with instability, though loading might be altered in this cohort as well. Second, though there was no difference in age between groups in this study, the patient population as a whole was relatively older. As the SMP population has been shown to decline in both numbers and proliferative capacity with increasing age30,31, muscles from all cuff states could have impaired regenerative capacity at this age. Finally, biopsy size was a limiting factor in this study as relatively few SMPs (on the order of 1,000) could be isolated per patient requiring significant expansion in culture and limiting the number of experiments that could be run. Future studies will benefit from elimination of confounding factors known to influence SMP function such as age and gender 31,32, larger biopsies, NRC controls distant from the shoulder and a NT group without shoulder dysfunction.
Our data suggests that rehabilitating RC muscles will likely require the (re)activation of SMPs, and to date, our poor understanding of how SMPs are affected during chronic tears has created a critical knowledge gap. This study suggests that poor clinical outcomes in full thickness RC repairs are potentially due to the microenvironment rather than SMP's ability to differentiate or participate in fusion; in the absence of pro-myogenic cues, this niche could influence SMPs (or other vessel derived myogenic populations) to contribute to the disease state through transdifferentiation into myofibroblasts33. Yet these data at least suggest that in addition to surgical repair, activation strategies (i.e. pharmacological or mechanical) for SMPs to push them into the regenerative cycle may be required so they can remodel and strengthen the muscle after tendon repair.
Supplementary Material
Acknowledgments
The authors would like to thank Drs. Meagan McCarthy and Morgan Silldorff for assistance with sample acquisition, Jeremy Tuler for assistance with cell injections in the mouse model, and Dr. Simon Schenk for helpful discussions. The authors acknowledge the National Institutes of Health (F32AR063588 to G.A.M., R01HD073180 to S.R.W., and DP02OD006460 to A.J.E.) and Muscular Dystrophy Association (241665 to A.J.E.) from funding support
References
- 1.Tempelhof S, Rupp S, Seil R. Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg. 1999;8(4):296–299. doi: 10.1016/s1058-2746(99)90148-9. [DOI] [PubMed] [Google Scholar]
- 2.Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A(2):219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Bishop J, Klepps S, Lo IK, et al. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. 2006;15(3):290–299. doi: 10.1016/j.jse.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Gerber C, Schneeberger AG, Hoppeler H, Meyer DC. Correlation of atrophy and fatty infiltration on strength and integrity of rotator cuff repairs: a study in thirteen patients. J Shoulder Elbow Surg. 2007;16(6):691–696. doi: 10.1016/j.jse.2007.02.122. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs B, Weishaupt D, Zanetti M, et al. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8(6):599–605. doi: 10.1016/s1058-2746(99)90097-6. [DOI] [PubMed] [Google Scholar]
- 6.Goutallier D, Postel JM, Bernageau J, et al. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;(304):78–83. [PubMed] [Google Scholar]
- 7.Gladstone JN, Bishop JY, Lo IKY, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 8.Harryman DT, Mack LA, Wang KY, et al. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982–989. [PubMed] [Google Scholar]
- 9.Jones SW, Hill RJ, Krasney PA, et al. Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass. FASEB J. 2004;18(9):1025–1027. doi: 10.1096/fj.03-1228fje. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell PO, Pavlath GK. Skeletal muscle atrophy leads to loss and dysfunction of muscle precursor cells. Am J Physiol, Cell Physiol. 2004;287(6):C1753–62. doi: 10.1152/ajpcell.00292.2004. [DOI] [PubMed] [Google Scholar]
- 11.Wapner KL, Pavlock GS, Hecht PJ, et al. Repair of chronic Achilles tendon rupture with flexor hallucis longus tendon transfer. Foot Ankle. 1993;14(8):443–449. doi: 10.1177/107110079301400803. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Sotelo J, Morrey BF, Adams RA, O'Driscoll SW. Reconstruction of chronic ruptures of the distal biceps tendon with use of an achilles tendon allograft. J Bone Joint Surg Am. 2002;84-A(6):999–1005. doi: 10.2106/00004623-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Fry CS, Lee JD, Jackson JR, et al. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J. 2014;28(4):1654–1665. doi: 10.1096/fj.13-239426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salleo A, La Spada G, Falzea G, et al. Response of satellite cells and muscle fibers to long-term compensatory hypertrophy. J Submicrosc Cytol. 1983;15(4):929–940. [PubMed] [Google Scholar]
- 15.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93(1):23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruffell D, Mourkioti F, Gambardella A, et al. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci U S A. 2009;106(41):17475–17480. doi: 10.1073/pnas.0908641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dellavalle A, Sampaolesi M, Tonlorenzi R, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9(3):255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 18.Schultz E, Jaryszak DL, Valliere CR. Response of satellite cells to focal skeletal muscle injury. Muscle Nerve. 1985;8(3):217–222. doi: 10.1002/mus.880080307. [DOI] [PubMed] [Google Scholar]
- 19.Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats.Anat. Rec. 1971;170(4):421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- 20.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138(17):3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward SRS, Sarver JJJ, Eng CMC, et al. Plasticity of muscle architecture after supraspinatus tears. J Orthop Sports Phys Ther. 2010;40(11):729–735. doi: 10.2519/jospt.2010.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jejurikar SS, Kuzon WM. Satellite cell depletion in degenerative skeletal muscle. Apoptosis. 2003;8(6):573–578. doi: 10.1023/A:1026127307457. [DOI] [PubMed] [Google Scholar]
- 23.Mozdziak PE, Pulvermacher PM, Schultz E. Unloading of juvenile muscle results in a reduced muscle size 9 wk after reloading. J Appl Physiol. 2000;88(1):158–164. doi: 10.1152/jappl.2000.88.1.158. [DOI] [PubMed] [Google Scholar]
- 24.Tomioka T, Minagawa H, Kijima H, et al. Sarcomere length of torn rotator cuff muscle. J Shoulder Elbow Surg. 2009;18(6):955–959. doi: 10.1016/j.jse.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Lundgreen K, Lian OB, Engebretsen L, Scott A. Lower muscle regenerative potential in full-thickness supraspinatus tears compared to partial-thickness tears. Acta Orthop. 2013;84(6):565–570. doi: 10.3109/17453674.2013.858289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith LR, Chambers HG, Lieber RL. Reduced satellite cell population may lead to contractures in children with cerebral palsy. Dev Med Child Neurol. 2013;55(3):264–270. doi: 10.1111/dmcn.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langen RC, Schols AM, Kelders MC, et al. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-kappaB. FASEB J. 2001;15(7):1169–1180. doi: 10.1096/fj.00-0463. [DOI] [PubMed] [Google Scholar]
- 28.Cheung S, Dillon E, Tham SC, et al. The Presence of Fatty Infiltration in the Infraspinatus: Its Relation With the Condition of the Supraspinatus Tendon. Arthroscopy. 2011;27(4):8–8. doi: 10.1016/j.arthro.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Chen CW, Corselli M, Péault B, Huard J. Human blood-vessel-derived stem cells for tissue repair and regeneration. J Biomed Biotechnol. 2012;2012:597439. doi: 10.1155/2012/597439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Renault V, Thornell LE, Eriksson PO, et al. Regenerative potential of human skeletal muscle during aging. Aging Cell. 2002;1(2):132–139. doi: 10.1046/j.1474-9728.2002.00017.x. [DOI] [PubMed] [Google Scholar]
- 31.Corbu A, Scaramozza A, Badiali-DeGiorgi L, et al. Satellite cell characterization from aging human muscle. Neurol Res. 2010;32(1):63–72. doi: 10.1179/174313209X385725. [DOI] [PubMed] [Google Scholar]
- 32.Deasy BM, Schugar RC, Huard J. Sex differences in muscle-derived stem cells and skeletal muscle. Crit Rev Eukaryot Gene Expr. 2008;18(2):173–188. doi: 10.1615/critreveukargeneexpr.v18.i2.60. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Huard J. Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. Am J Pathol. 2002;161(3):895–907. doi: 10.1016/S0002-9440(10)64250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


