Abstract
Oral adenine (0.75% w/w in feed), is an established model for human chronic kidney disease (CKD). Gum acacia (GA) has been shown to be a nephroprotective agent in this model. Here we aimed at developing a new adenine-induced CKD model in rats via a systemic route (intraperitoneal, i.p.) and to test it with GA to obviate the possibility of a physical interaction between GA and adenine in the gut. Adenine was injected i.p. (50 or 100 mg/Kg for four weeks), and GA was given concomitantly in drinking water at a concentration of 15%, w/v. Several plasma and urinary biomarkers of oxidative stress were measured and the renal damage was assessed histopathologically. Adenine, at the two given i.p. doses, significantly reduced body weight, and increased relative kidney weight, water intake and urine output. It dose-dependently increased plasma and urinary inflammatory and oxidative stress biomarkers, and caused morphological and histological damage resembling that which has been reported with oral adenine. Concomitant treatment with GA significantly mitigated almost all the above measured indices. Administration of adenine i.p. induced CKD signs very similar to those induced by oral adenine. Therefore, this new model is quicker, more practical and accurate than the original (oral) model. GA ameliorates the CKD effects caused by adenine given i.p. suggesting that the antioxidant and anti-inflammatory properties possessed by oral GA are the main mechanism for its salutary action in adenine-induced CKD, an action that is independent of its possible interaction with adenine in the gut.
Keywords: Adenine, animal model, chronic kidney disease, rats
Introduction
Chronic kidney disease (CKD), a worldwide health problem, is a slowly progressive disorder that might lead to end-stage renal disease (ESRD). The prevalence of CKD has grown rapidly in both the developed and developing countries [1-3]. This increase gauges the need for more resources and the rising demand on health-care systems already burdened by paucity of resources. In some regions it is estimated that the burden of CKD will rise in parallel with the growing prevalence of type two diabetes mellitus [4,5]. Therefore, there is a strong demand for studies exploring new therapeutic strategies and ameliorating agents, especially for the earlier stages of CKD in order to slow its progression towards ESRD. Among these agents is gum acacia (GA), a dietary fibrous heteropolysaccharide obtained from either Acacia senegal or A. seyal trees. It is widely used in the pharmaceutical, cosmetic and food industries as an emulsifier and stabilizer [6-9]. The addition of GA to the diet has been shown to increase fecal nitrogen excretion and decrease serum urea nitrogen concentration in patients with CKD [10,11]. It also has been shown to be effective in ameliorating the effects of adenine-induced CKD, one of the models employed to induce CKD in rats where adenine is given mixed with the feed at a concentration of 0.75%, w/w, for four weeks [12,13]. Both adenine and its metabolite, 2,8-dihydroxyadenine (DHA), have low solubility and can precipitate in the renal tubules and form crystals [14]. The consumption of oral adenine thus might cause the occlusion of renal tubules which retards the excretion of nitrogenous substances leading to a biochemical and physiological status resembling CKD in humans [7,15]. Although it has been shown by some studies that the basis for the ameliorative effects of GA is via its anti-inflammatory and antioxidant actions [6,16-18] and not only its possible local effect of excreting fecal nitrogen when administered with oral adenine, there still remains a need for further scientific work on the mechanism of action of GA as a nephroprotective agent. Our approach towards a better understanding of the mechanisms of action of GA led us to use the CKD-inducing agent (adenine) by a systemic route (and not the reported oral route), and this is, as far as we are aware, the first time that induction of CKD has been carried out by intraperitoneal injection of adenine to produce a model of CKD. In this work, we also wanted to verify the salutary effects of GA in this novel animal model of CKD.
Methods
This research work was approved by the Institutional Review Board of the Animal Research and Ethics Committee of the Sultan Qaboos University (SQU). The procedures involving animals and their care were conducted in conformity with international laws and policies (EEC Council directives 86/609, OJL 358, 1 December, 12, 1987; NIH Guide for the Care and Use of Laboratory Animals, NIH Publications No. 85-23, 1985).
Animals
Thirty male Wistar rats (9-10 weeks old, weighing initially 216 ± 5 g) were housed in a room at a temperature of 22 ± 2°C, relative humidity of about 60%, with a 12 h light-dark cycle (lights on 6:00), and free access to standard pellet chow diet containing 0.85% phosphorus, 1.12% calcium, 0.35% magnesium, 25.3% crude protein and 2.5 IU/g vitamin D3 (Oman Flour Mills, Muscat, Oman) and water.
Chemicals
GA and adenine were both obtained from Sigma (St. Louis, MO, USA). All other chemicals used were of Analytical Reagent grade.
Experimental design
After an acclimatization period of seven days, rats were randomly divided into five equal groups (n=6 in each group). The first group continued to receive the same diet without treatment and unmedicatd tap water ad libitium and served as control. The second group was given adenine intraperitoneally (i.p.) at a dose of 50 mg/kg daily for 28 days. The third group was also given the same dose as group two and in addition received concomitantly GA in drinking water at a concentration of 15%, w/v, until the end of the experiment. The fourth group was given adenine i.p. 100 mg/kg and the fifth group was given the same dose as the fourth group and received GA in drinking water at a concentration of 15%, w/v, simultaneously until the end of the experiment. The animals were weighed weekly and placed individually in metabolic cages to enable the collection of the urine voided in 24 h. At 24-h after the end of the treatment the animals were anesthetized with an intraperitoneal injection of ketamine (75 mg/kg) and xylazine (5 mg/kg), and blood (5 mL from each rat) was collected from the anterior vena cava into heparinized tubes. The blood and urine collected were centrifuged at 900 g at 4°C for 15 min and then stored frozen at -80°C pending analysis. The two kidneys were excised, cleared of fat, blotted on filter paper, and weighed. A small piece of the right kidney was placed in 10% formalin for subsequent histological processing. The rest of the kidneys were kept frozen at -80°C to await biochemical analysis within a week. The left kidney was homogenized in ice-cold Tris buffer (pH 7.4) to give a 10%, w/v homogenate. This was centrifuged at 1500 g at 4°C for 15 min, and the supernatant obtained was used to measure superoxide dismutase (SOD) activity and reduced glutathione (GSH) concentration.
Biochemical and physiological measurements
The plasma L-γ-glutamyltransferase (GGT) and lactate dehydrogenase (LDH) were measured using kits from Human GmbH (Mannheim, Germany) and Sigma Chemical, St. Louis, MO, USA, respectively. The plasma and urine concentrations of creatinine, as well as the plasma urea concentrations and aspartate transaminase (AST), alanine transaminase (ALT), and creatine kinase (CK) activities were measured using standard laboratory methods by an LX20 multiple automated analyzer (Beckman Coulter, CA, USA). The supernatants of renal homogenates were separated into two aliquots and were used for the measurement of the following parameters, using assay kits according to the manufactures’ instructions: glutathione (GSH) concentration with GSH/GSSG assay kit (Biovision, Mountain View, CA, USA), total antioxidant capacity (TAC) (Randox Laboratories Crumlin, UK), catalase (Cayman Chemical Co., Ann Arbor, MI, USA), and superoxide dismutase (SOD) (Cell Technology Inc., Mountain view, CA, USA). The plasma concentration of indoxyl sulfate (IS) was measured by an HPLC method as previously described [19]. Neutrophil gelatinase-associated lipocalin (NGAL) concentration was measured in plasma by an ELISA method using kits obtained from Bioporto Diagnostics (Gentofte, Denmark). The concentrations of 8-isoprostane and 8-hydroxy-2’-deoxyguanosine (8-OHdG) measured by kits from Statok Kino, Shizuoka, Japan. N-acetyl-beta-D-glucosaminidase (NAG) and cystatin C were measured by commercial kits.
Histopathology
The kidneys were fixed in 10% neutral-buffered formalin, dehydrated in increasing concentrations of ethanol, cleared with xylene and embedded in paraffin. Two micrometer (µm) sections were prepared from kidney paraffin blocks and stained with hematoxylin and eosin, periodic acid-Schiff stain (PAS) and Masson’s trichrome stain.
Statistical analysis
Statistical analysis was carried out using GraphPad Prism 5.0 (GraphPad Software, SanDiego, CA, USA). Data are expressed as means ± SEM. Comparisons between the groups were performed by one-way analysis of variance followed by Tukey’s multiple comparison tests. P<0.05 was considered statistically significant.
Results
General appearance, body weight and kidney weight
The kidneys of the control rats appeared normal. However, the kidneys of adenine-treated animals were pale, and a few adenine crystals were seen, mainly in the cortex area (Figure 1). The kidneys of the groups that had been treated with adenine and GA together visually appeared improved compared with those of the kidneys of groups treated with adenine alone.
Figure 1.
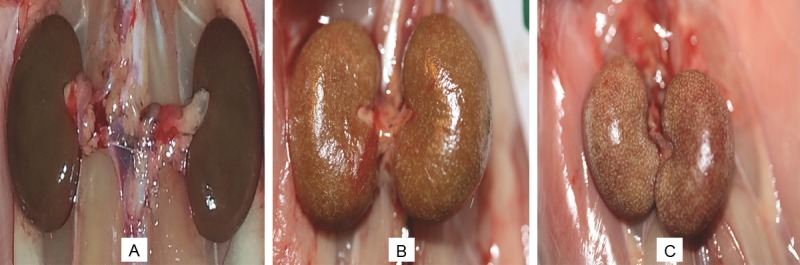
Gross morphology of the kidneys of rats treated for 28 days with: (A) saline (control), (B) adenine injected intraperitoneally, i.p., at a dose of 50 mg/Kg, and (C) adenine i.p. at a dose of 100 mg/Kg.
As shown in Table 1, adenine reduced the final body weight of rats, and the concomitant administration of GA with adenine did not significantly affect the reduction in body weight caused by adenine alone. The weight of the kidneys relative to that of the body was significantly increased by adenine and concomitant administration of GA with adenine significantly abated that increase (P<0.001). Also, adenine significantly (P<0.001) increased the water intake and urine output, and GA mitigated that action.
Table 1.
Effect of treatment with adenine with or without addition of gum acacia (GA) on weight, total relative kidney weight (wt), water intake, urine and fecal output and food intake
| PARAMETERS | CONTROL | ADENINE 50 (mg kg-1) | ADENINE 50 (mg kg-1) + GA | ADENINE 100 (mg kg-1) | ADENINE 100 (mg kg-1) + GA |
|---|---|---|---|---|---|
| Initial weight (gm) | 216 ± 1.67a | 216 ± 7.51a | 217 ± 8.51a | 220 ± 1.63a | 215 ± 4.32a |
| Final weight (gm) | 224 ± 5.14a | 198 ± 7.9b | 218 ± 5.14a | 207 ± 3.12a | 208 ± 4.47a |
| Bwt change % | 3.44 ± 1.89a | -4.7 ± 1.02b | 0.10 ± 1.81a | -5.9 ± 1.91b | -3.4 ± 0.79b |
| Total kidney wt (gm) | 1.27 ± 0.04a | 1.69 ± 0.03a | 1.28 ± 0.03a | 2.5 ± 0.21b,c,d | 1.48 ± 0.09a,c,e |
| Relative kidney wt% | 0.57 ± 0.02a | 1.02 ± 0.06b | 0.59 ± 0.01a,c | 1.24 ± 0.16b,d | 0.71 ± 0.04a,e |
| Water intake (ml) | 25.0 ± 2.71a | 28.6 ± 6.32a | 22.5 ± 1.54a | 46.3 ± 1.35b,c,d | 24.8 ± 1.92a,e |
| Urine output (ml) | 9.50 ± 0.67a | 21.2 ± 4.51b | 14.0 ± 1.54a | 31.8 ± 1.27b,c,d | 18.0 ± 0.81a,e |
| Food intake (gm) | 11.9 ± 0.57a | 15.3 ± 3.28a | 12.2 ± 1.05a | 13.8 ± 0.78a | 11.9 ± 0.36a |
| Feces production (gm) | 2.25 ± 0.19a | 5.13 ± 1.19a | 4.70 ± 0.52a | 5.61 ± 0.61b | 5.51 ± 0.25b |
Values are expressed as means ± SEM (n=6). Values (for the same parameter) with different superscript are significantly different (P<0.05 or less). Adenine was injected intraperitoneally (50 or 100 mg Kg-1 for four weeks). GA was given in the drinking water at a concentration of 15% w/v for four weeks.
Biochemical findings
The concentrations of urea and creatinine increased significantly in the two groups treated with adenine alone. This effect was significantly reversed by GA (Figure 2). The plasma activity of NGAL and urinary activity of NAG were significantly raised in adenine treated groups alone and this rise was significantly ameliorated by the addition of GA (Figure 2). The oxidative markers GSH, catalase, SOD, TAC and 8-isoprostane and uremic toxin IS were all elevated in adenine treated groups and GA significantly reduced their concentrations (Figure 3). The enzymes LDH, AST, ALT, GGT and CK were raised in adenine treated groups and GA decreased these significantly (Table 2). Administration of adenine significantly raised the concentration of cystatin C and 8-OHdG, an effect significantly reduced by addition of GA (P<0.001) (Figure 4).
Figure 2.
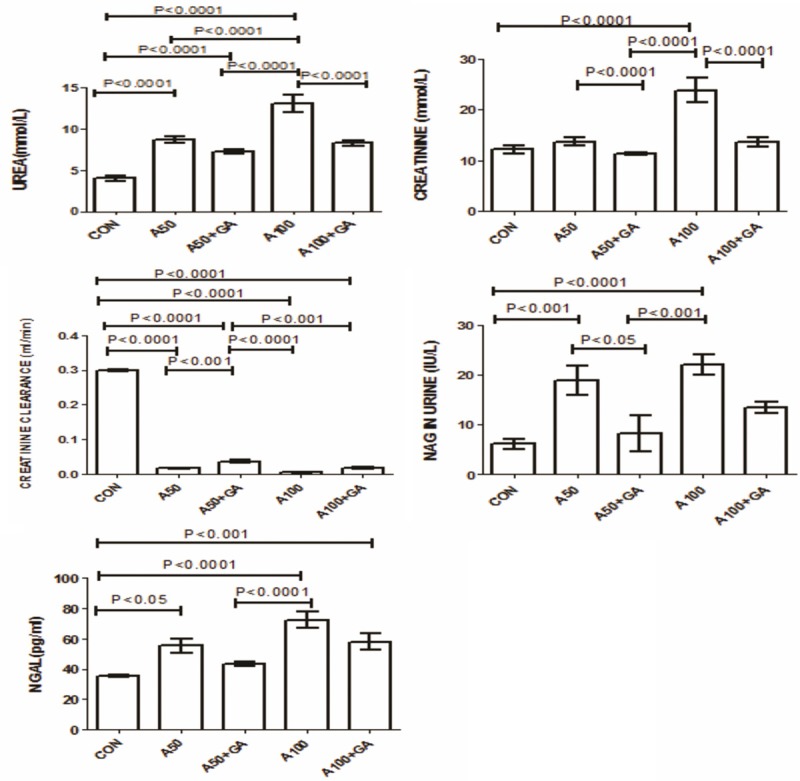
Plasma concentrations of, urea, creatinine, and creatinine clearance and N-acetyl-beta-D-glucosaminidase (NAG) and Neutrophil gelatinase-associated lipocalin (NGAL) in control (CON) rats and in rats treated with adenine (A) 50 mg/Kg, 100 mg/Kg, 50 mg/Kg + gum acacia (GA), 100 mg/Kg + GA. Each column is mean ± SEM (n=six rats). Statistical analysis was done by ANOVA followed by Tukey’s multiple comparison tests.
Figure 3.
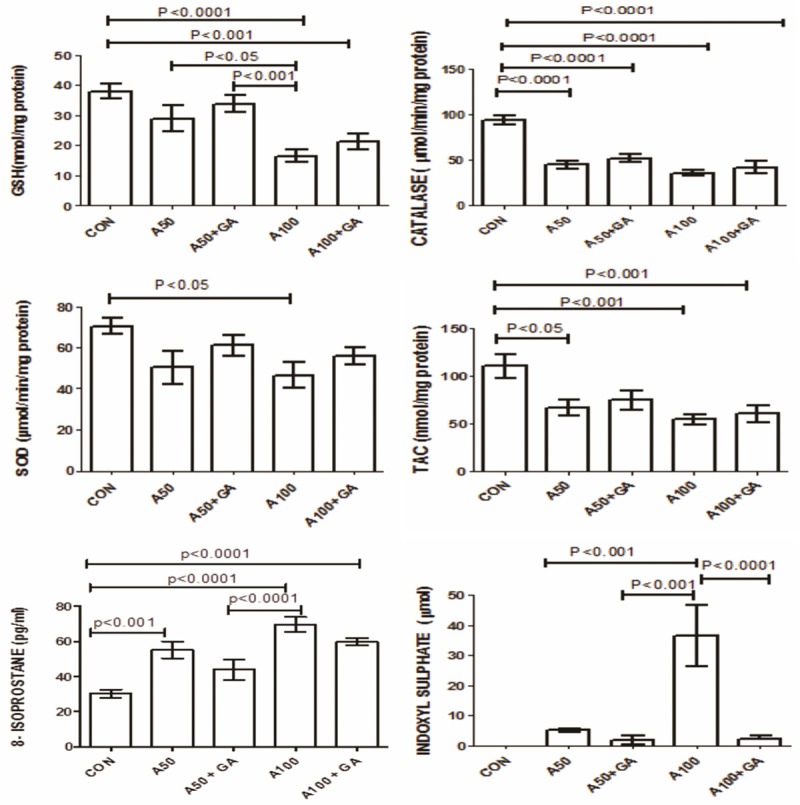
The concentrations of glutathione (GSH), catalase, superoxide dismutase (SOD), antioxidant capacity (TAC), 8-isoprostane and indoxyl sulfate in control (CON) rats and in rats treated with adenine (A) 50 mg/Kg, 100 mg/Kg, 50 mg/Kg + gum acacia (GA), 100 mg/Kg + GA. Each column is mean ± SEM (n=six rats). Statistical analysis by ANOVA followed by Tukey’s multiple comparison tests.
Table 2.
Effect of treatment of rats with two different doses of intraperitoneal adenine with or without addition of gum acacia (GA) on activities of some plasma enzymes
| GROUPS | ALT (IU L-1) | AST(IU L-1) | CK (U L-1) | GGT(U L-1) | LDH (IU L-1) |
|---|---|---|---|---|---|
| Control | 30.2 ± 3.6a | 56.8 ± 3.6a | 107.8 ± 17.9a | 3.4 ± 0.4a | 170.4 ± 29.6a |
| Adenine 50 | 70.8 ± 7.1b | 146.0 ± 23.7a | 317.0 ± 80.7a | 3.6 ± 1.1a | 385.7 ± 63.9a |
| Adenine 50+GA | 54.8 ± 13.5a | 104.3 ± 20.8a | 219.3 ± 40.2a | 3.0 ± 1.1a | 152.0 ± 19.8a |
| Adenine 100 | 78.2 ± 11.3b | 172.2 ± 37.3b | 1373 ± 254.7b,c,d | 5.8 ± 1.4a | 748.5 ± 89.5b,c,d |
| Adenine 100+GA | 57.0 ± 9.4a | 124.3 ± 24.3a | 795 ± 188.8b | 3.2 ± 0.6a | 312.0 ± 39.3a,e |
Values are expressed as mean ± SEM (n=6). Values (for the same parameter) with different superscript are significantly different (P<0.05 or less). ALT: alanine transaminase; AST: aspartate transaminase, CK: creatine kinase; GGT: L-γ-glutamyltransferase; LDH: lactate dehydrogenase.
Figure 4.
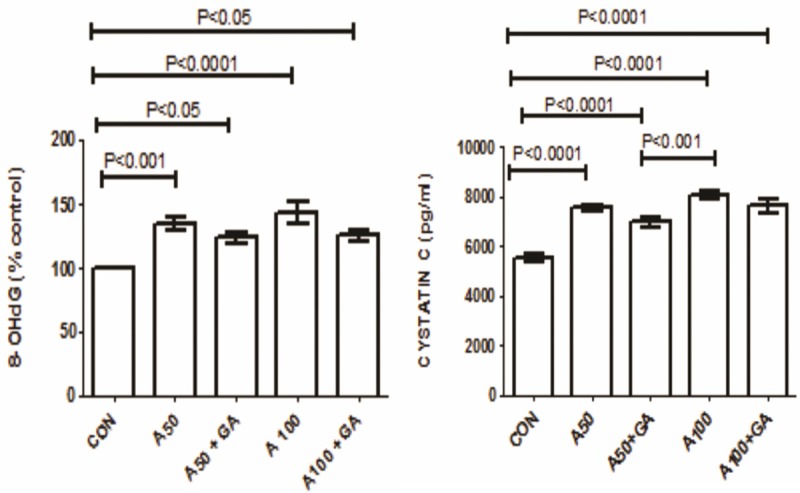
The concentration of 8-hydroxy-2’-deoxyguanosine (8-OHdG) and cystatin C in control (CON) rats and in rats treated with adenine (A) 50 mg/Kg, 100 mg/Kg, 50 mg/Kg + gum acacia (GA), 100 mg/Kg + GA. Each column is mean ± SEM (n=six rats). Statistical analysis by ANOVA followed by Tukey’s multiple comparison tests.
Histopathology
Adenine treatment caused severe changes in the histopathology of the kidneys as shown in the slides stained with three stains, hematoxylin and eosin, PAS and Masson’s trichrome stain. Glomeruli of control rats exhibited normal architecture, while glomeruli of rats treated with adenine (50 mg/kg and 100 mg/Kg) showed an expansion of mesangial matrices, and the glomeruli of rats treated with adenine (100 mg/kg) also showed capillary widening (Figures 5 and 6). Glomeruli of animals treated simultaneously with adenine (50 mg/kg) and GA again showed normal architecture, while glomeruli from animals treated simultaneously with adenine (100 mg/kg) and GA still showed mesangial expansion. Tubulointerstitial injury in animals treated with adenine (100 mg/kg) predominantly was characterized by tubular dilatation and atrophy, which was not observed in the group receiving the lower adenine dose (50 mg/kg) (Figure 5). Animals treated simultaneously with adenine (100 mg/kg) and GA still showed tubular atrophy, but almost no dilatation. Further, in both adenine-treated groups extensive interstitial fibrosis (Figure 6) and inflammation, characterized by infiltration of macrophages (Figure 7), was detected. All these tubular changes were ameliorated to a moderate extent by GA, more effectively in the group receiving simultaneously the lower dose of adenine (50 mg/kg) and GA.
Figure 5.
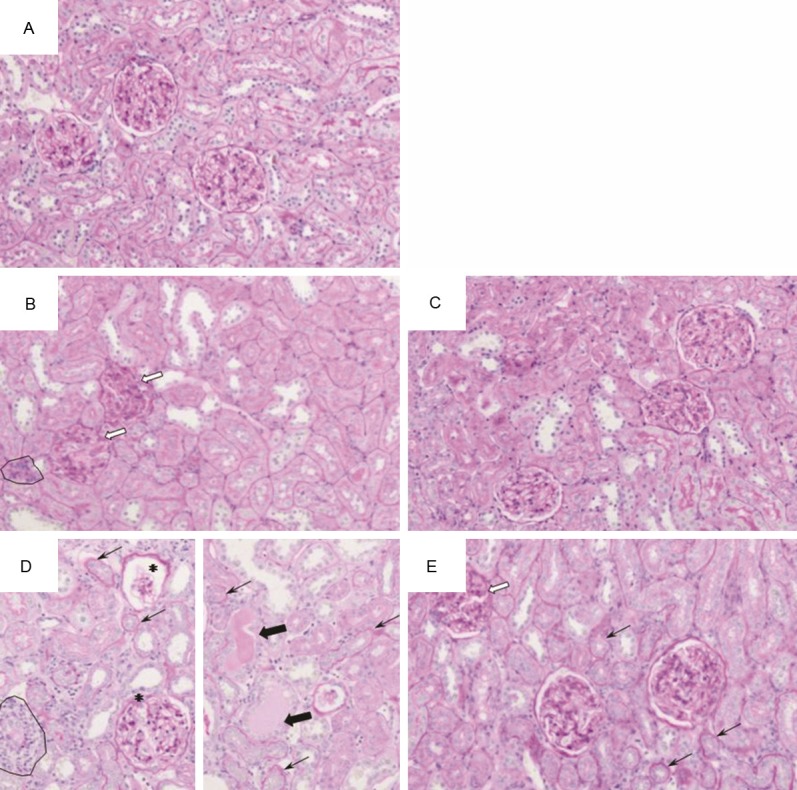
Representative photographs of sections of renal tissue from rats treated for 28 days with: (A) saline (control), (B) adenine injected intraperitoneally, i.p., (50 mg/Kg), (C) adenine i.p. (50 mg/kg) + GA (15% w/v, in drinking water), (D) adenine i.p. (100 mg/Kg), and (E) adenine i.p. (100 mg/kg) + GA as above. The photographs show the presence of tubular dilatation (thick black arrow), glomeruli with mesangial expansion (thick white arrow), inflammation (circles), tubular atrophy (thin arrow), and glomeruli showing capillary widening (asterisk). Staining was carried out with periodic acid-Schiff stain.
Figure 6.
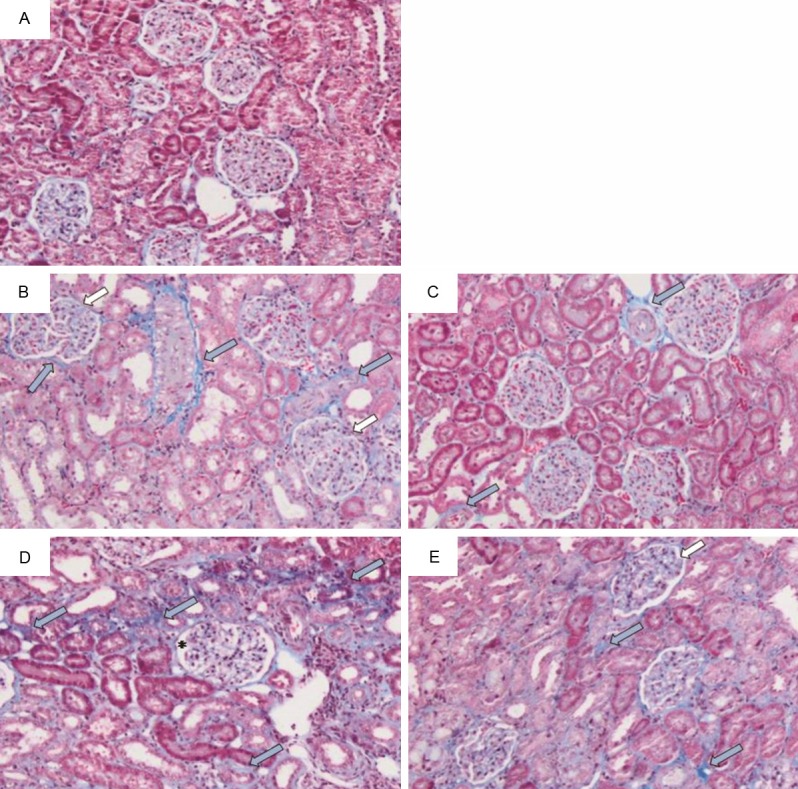
Representative photographs of sections of renal tissue from rats treated for 28 days with: (A) saline (control), (B) adenine injected intraperitoneally, i.p., (50 mg/Kg), (C) adenine i.p. (50 mg/kg) + GA (15% w/v, in drinking water), (D) adenine i.p. (100 mg/Kg), and (E) adenine i.p. (100 mg/kg) + GA as above. The photographs show the presence of fibrosis (thick blue arrow), glomeruli with mesangial expansion (thick white arrow), and golermuli showing capillary widening (asterisk). Staining was carried out with Masson’s trichrome stain.
Figure 7.
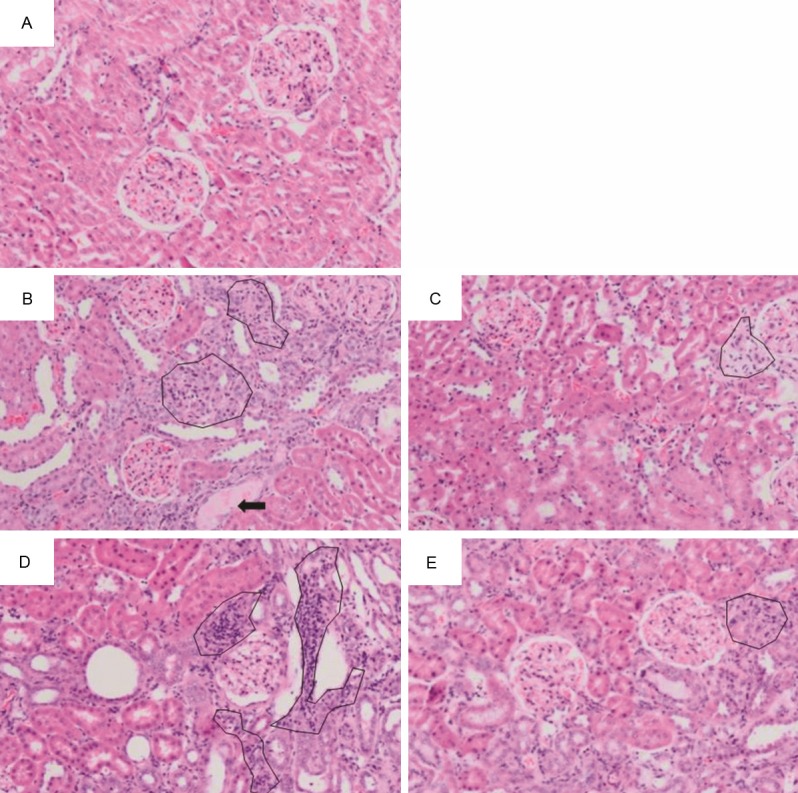
Representative photographs of sections of renal tissue from rats treated for 28 days with: (A) saline (control), (B) adenine injected intraperitoneally, i.p., (50 mg/Kg), (C) adenine i.p. (50 mg/kg) + GA (15% w/v, in drinking water), (D) adenine i.p. (100 mg/Kg), and (E) adenine i.p. (100 mg/kg) + GA as above. The photographs show the presence of tubular dilatation (thick black arrow), and inflammation (circles). Staining was carried out with hematoxylin and eosin.
Discussion
In vivo animal models of uremia are important tools in examining the biochemical, physiological and histopathological mechanisms of CKD and the effect of potential therapeutic intervention on these mechanisms prior to studies on humans [20,21]. Adenine was first reported in 1986 to successfully induce uremia as an alternative method for the highly demanding cost and skill required for the surgical model of uremia [13]. Thereafter, several studies adopted this model whereby adenine is co-administered with feed to rats and mice at 0.75%, w/w, and 0.2%, w/w, respectively to induce CKD [16]. In the present work the intraperitoneal administration of adenine at doses of 50 and 100 mg/kg induced CKD in rats in a similar manner and to the same extent seen in rats fed with adenine [12,15]. This was concluded from the significant increases in water intake and urine and feces production, accumulation of IS, creatinine and urea and the decrease in creatinine clearance as well as in body weight. In addition, the morphological and histopathological alterations in kidneys of adenine administered groups confirm the development of CKD. The administration of adenine i.p. can thus be considered an alternative superior model to oral adenine for the induction of CKD. The benefits of this model are that adenine directly enters the systemic circulation bypassing any possible local (intestinal) direct physical interaction with any enteral ameliorating agent such as GA and charcoal [17]. It also is more practical, convenient and accurate.
The mechanism by which adenine induces CKD is not well elucidated. Adenine and its metabolite, DHA, have low solubility and precipitate in the renal tubules leading to their occlusion and the development of uremia [7,14]. Furthermore, adenine has a tendency to cause several oxidative and inflammatory reactions in renal tissues which might cause an increase in several oxidative and inflammatory markers such as GSH, SOD, catalase and TAC as seen in the present and other studies [12,17,22-24]. The increase in the oxidative derivative of deoxyguanosine, 8-OHdG, one of the major DNA oxidative products, in the adenine treated groups also indicates oxidative stress within the cells. These oxidative biomarkers in the long run, as reported previously, might have systemic toxicity potentially causing damage to several other organs such as liver and heart [25-28]. The liver enzymes LDH, AST, GGT, ALT and CK, significantly raised in the adenine treated groups compared with the control, suggest a degree of liver damage that might be caused by oxidative and inflammatory reaction towards adenine [29,30].
GA, given orally, has been used in the treatment of CKD in several developing countries such as Iraq and Sudan [10,31,32]. GA is thought to act primarily via increasing the fecal nitrogen excretion which in turn lowers serum urea nitrogen concentration. Recently, GA has been found in several studies to have anti-inflammatory and antioxidative properties making it an apoptosis scavenger [12,15]. Furthermore, previous in vitro studies showed that GA dose dependently scavenged generated superoxide radicals [18]. In this study concomitant treatment with GA significantly reduced the inflammatory and oxidative stress induced by the administration of intraperitoneal adenine. These include a reduction in GSH, SOD, catalase and TAC. It also significantly reduced NGAL, NAG and cystatin C, the biomarkers of acute kidney injury [33,34]. In addition, GA abrogated the histopathological findings seen in the adenine administered groups. These beneficial effects suggest that the antioxidative and anti-inflammatory properties possessed by oral GA are the main mechanism for its salutary action in adenine-induced CKD. These findings suggest the need for further in vivo studies on a molecular basis for the systemic effects associated with the use of GA and perhaps suggest the need for a translational move towards a small scale human study.
In conclusion, oral GA significantly ameliorated the effects associated with use of adenine given i.p. This last route of administration can successfully replace the oral model of induction of CKD.
Acknowledgements
This work was supported by The Research Council (TRC) of Oman (RC/MED/PHAR/13/01). Thanks to Ms. P. Manoj for helping with the animal experimentation, and the staff of the Animal House for looking after the rats.
References
- 1.Harambat J, van Stralen KJ, Kim JJ, Tizard EJ. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. 2012;27:363–373. doi: 10.1007/s00467-011-1939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–1270. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- 3.Nugent RA, Fathima SF, Feigl AB, Chyung D. The burden of chronic kidney disease on developing nations: a 21st century challenge in global health. Nephron Clin Pract. 2011;118:c269–277. doi: 10.1159/000321382. [DOI] [PubMed] [Google Scholar]
- 4.Anand S, Khanam MA, Saquib J, Saquib N, Ahmed T, Alam DS, Cullen MR, Barry M, Chertow GM. High prevalence of chronic kidney disease in a community survey of urban Bangladeshis: a cross-sectional study. Global Health. 2014;10:9. doi: 10.1186/1744-8603-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van den Hurk K, Magliano DJ, Alssema M, Schlaich MP, Atkins RC, Reutens AT, Nipels G, Dekker JM, Shaw JE. Type 2 diabetes strengthens the association between pulse pressure and chronic kidney disease: the AusDiab study. J Hypertens. 2011;29:953–960. doi: 10.1097/HJH.0b013e328344d9cf. [DOI] [PubMed] [Google Scholar]
- 6.Ali BH, Ziada A, Blunden G. Biological effects of gum arabic: a review of some recent research. Food Chem Toxicol. 2009;47:1–8. doi: 10.1016/j.fct.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Nasir O, Umbach AT, Rexhepaj R, Ackermann TF, Bhandaru M, Ebrahim A, Artunc F, Kempe DS, Puchchakayala G, Siraskar B, Foller M, Saeed A, Lanf F. Effects of gum arabic (Acacia senegal) on renal function in diabetic mice. Kidney Blood Press Res. 2012;35:365–372. doi: 10.1159/000336359. [DOI] [PubMed] [Google Scholar]
- 8.Phillips GO. Acacia gum (Gum Arabic): a nutritional fibre; metabolism and calorific value. Food Addit Contam. 1998;15:251–264. doi: 10.1080/02652039809374639. [DOI] [PubMed] [Google Scholar]
- 9.Verbeken D, Dierckx S, Dewettinck K. Exudate gums: occurrence, production, and applications. Appl Microbiol Biotechnol. 2003;63:10–21. doi: 10.1007/s00253-003-1354-z. [DOI] [PubMed] [Google Scholar]
- 10.Ali AA, Ali KE, Fadlalla AE, Khalid KE. The effects of gum arabic oral treatment on the metabolic profile of chronic renal failure patients under regular haemodialysis in Central Sudan. Nat Prod Res. 2008;22:12–21. doi: 10.1080/14786410500463544. [DOI] [PubMed] [Google Scholar]
- 11.Bliss DZ, Stein TP, Schleifer CR, Settle RG. Supplementation with gum arabic fiber increases fecal nitrogen excretion and lowers serum urea nitrogen concentration in chronic renal failure patients consuming a low-protein diet. Am J Clin Nutr. 1996;63:392–398. doi: 10.1093/ajcn/63.3.392. [DOI] [PubMed] [Google Scholar]
- 12.Ali BH, Al-Husseni I, Beegam S, Al-Shukaili A, Nemmar A, Schierling S, Queisser N, Schupp N. Effect of gum arabic on oxidative stress and inflammation in adenine-induced chronic renal failure in rats. PLoS One. 2013;8:e55242. doi: 10.1371/journal.pone.0055242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokozawa T, Zheng PD, Oura H, Koizumi F. Animal model of adenine-induced chronic renal failure in rats. Nephron. 1986;44:230–234. doi: 10.1159/000183992. [DOI] [PubMed] [Google Scholar]
- 14.Wyngaarden JB, Dunn JT. 8-Hydroxyadenine as the intermediate in the oxidation of adenine to 2, 8-dihydroxyadenine by xanthine oxidase. Arch Biochem Biophys. 1957;70:150–156. doi: 10.1016/0003-9861(57)90088-7. [DOI] [PubMed] [Google Scholar]
- 15.Ali BH, Al-Salam S, Al Husseni I, Kayed RR, Al-Masroori N, Al-Harthi T, Al Zaabi M, Nemmar A. Effects of Gum Arabic in rats with adenine-induced chronic renal failure. Exp Biol Med (Maywood) 2010;235:373–382. doi: 10.1258/ebm.2009.009214. [DOI] [PubMed] [Google Scholar]
- 16.Ali BH, Al-Salam S, Al Za’abi M, Waly MI, Ramkumar A, Beegam S, Al-Lawati I, Adham SA, Nemmar A. New model for adenine-induced chronic renal failure in mice, and the effect of gum acacia treatment thereon: comparison with rats. J Pharmacol Toxicol Methods. 2013;68:384–393. doi: 10.1016/j.vascn.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Ali BH, Alza’abi M, Ramkumar A, Al-Lawati I, Waly MI, Beegam S, Nemmar A, Brand S, Schupp N. The effect of activated charcoal on adenine-induced chronic renal failure in rats. Food Chem Toxicol. 2014;65:321–328. doi: 10.1016/j.fct.2013.12.038. [DOI] [PubMed] [Google Scholar]
- 18.Gado AM, Aldahmash BA. Antioxidant effect of Arabic gum against mercuric chloride-induced nephrotoxicity. Drug Des Devel Ther. 2013;7:1245–1252. doi: 10.2147/DDDT.S50928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al Za’abi M, Ali B, Al Toubi M. HPLC-fluorescence method for measurement of the uremic toxin indoxyl sulfate in plasma. J Chromatogr Sci. 2013;51:40–43. doi: 10.1093/chromsci/bms103. [DOI] [PubMed] [Google Scholar]
- 20.Becker GJ, Hewitson TD. Animal models of chronic kidney disease: useful but not perfect. Nephro Dial Transplant. 2013;28:2432–8. doi: 10.1093/ndt/gft071. [DOI] [PubMed] [Google Scholar]
- 21.Desrochers TM, Palma E, Kaplan DL. Tissue-engineered kidney disease models. Adv Drug Deliv Rev. 2014;20:67–80. doi: 10.1016/j.addr.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waring WS, Moonie A. Earlier recognition of nephrotoxicity using novel biomarkers of acute kidney injury. Clin Toxicol (Phila) 2011;49:720–728. doi: 10.3109/15563650.2011.615319. [DOI] [PubMed] [Google Scholar]
- 23.James MT, Hemmelgarn BR, Tonelli M. Early recognition and prevention of chronic kidney disease. Lancet. 2010;375:1296–1309. doi: 10.1016/S0140-6736(09)62004-3. [DOI] [PubMed] [Google Scholar]
- 24.Baumgarten M, Gehr T. Chronic kidney disease: detection and evaluation. Am Fam Physician. 2011;84:1138–1148. [PubMed] [Google Scholar]
- 25.Fraga CG, Shigenaga MK, Park JW, Degan P, Ames BN. Oxidative damage to DNA during aging: 8-hydroxy-2’-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci U S A. 1990;87:4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loft S, Fischer-Nielsen A, Jeding IB, Vistisen K, Poulsen HE. 8-Hydroxydeoxyguanosine as a urinary biomarker of oxidative DNA damage. J Toxicol Environ Health. 1993;40:391–404. doi: 10.1080/15287399309531806. [DOI] [PubMed] [Google Scholar]
- 27.Valavanidis A, Vlachogianni T, Fiotakis C. 8-Hydroxy-2’ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 28.Astor BC, Shafi T, Hoogeveen RC, Matsushita K, Ballantyne CM, Inker LA, Coresh J. Novel markers of kidney function as predictors of ESRD, cardiovascular disease, and mortality in the general population. Am J Kidney Dis. 2012;59:653–662. doi: 10.1053/j.ajkd.2011.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amacher DE. A toxicologist’s guide to biomarkers of hepatic response. Hum Exp Toxicol. 2002;21:253–262. doi: 10.1191/0960327102ht247oa. [DOI] [PubMed] [Google Scholar]
- 30.Lacour S, Gautier JC, Pallardy M, Roberts R. Cytokines as potential biomarkers of liver toxicity. Cancer Biomark. 2005;1:29–39. doi: 10.3233/cbm-2005-1105. [DOI] [PubMed] [Google Scholar]
- 31.Al Mosawi AJ. The use of acacia gum in end stage renal failure. J Trop Pediatr. 2007;53:362–365. doi: 10.1093/tropej/fmm033. [DOI] [PubMed] [Google Scholar]
- 32.Al Mosawi AJ. Six-year dialysis freedom in end-stage renal disease. Clin Exp Nephrol. 2009;13:494–500. doi: 10.1007/s10157-009-0181-7. [DOI] [PubMed] [Google Scholar]
- 33.Ghonemy TA, Amro GM. Plasma neutrophil gelatinase-associated lipocalin (NGAL) and plasma cystatin C (CysC) as biomarker of acute kidney injury after cardiac surgery. Saudi J Kidney Dis Transpl. 2014;25:582–588. doi: 10.4103/1319-2442.132194. [DOI] [PubMed] [Google Scholar]
- 34.Ghys L, Paepe D, Smets P, Lefebvre H, Delanghe J, Daminet S. Cystatin C. a new renal marker and its potential use in small animal medicine. J Vet Intern Med. 2014;28:1152–64. doi: 10.1111/jvim.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]


