Abstract
Acute pulpitis (AP), one of the most common diseases in the endodontics, usually causes severe pain to the patients, which makes the search for therapeutic target of AP essential in clinic. Toll-like receptor 4 (TLR4) signaling is widely involved in the mechanism of pulp inflammation, while melatonin has been reported to have an inhibition for a various kinds of inflammation. We hereby studied whether melatonin can regulate the expression of TLR4/NF-ĸB signaling in the pulp tissue of AP and in human dental pulp cells (HDPCs). Two left dental pulps of the adult rat were drilled open to establish the AP model, and the serum levels of melatonin and pro-inflammatory cytokines, including interleukin 1β (IL-1β), interleukin 18 (IL-18) and tumor necrosis factor α (TNF-α), were assessed at 1, 3 and 5 d post injury. At the same time points, the expression of TLR4 signaling in the pulp was explored by quantitative real-time PCR and immunohistochemistry. The AP rats were administered an abdominal injection of melatonin to assess whether melatonin rescued AP and TLR4/NF-ĸB signaling. Dental pulp injury led to an approximately five-day period acute pulp inflammation and necrosis in the pulp and a significant up-regulation of IL-1β, IL-18 and TNF-α in the serum. ELISA results showed that the level of melatonin in the serum decreased due to AP, while an abdominal injection of melatonin suppressed the increase in serum cytokines and the percentage of necrosis at the 5 d of the injured pulp. Consistent with the inflammation in AP rats, TLR4, NF-ĸB, TNF-α and IL-1β in the pulp were increased post AP compared with the baseline expression. And melatonin showed an inhibition on TLR4/NF-ĸB signaling as well as IL-1β and TNF-α production in the pulp of AP rats. Furthermore, melatonin could also regulate the expression of TLR4/NF-ĸB signaling in LPS-stimulated HDPCs. These data suggested that dental pulp injury induced AP and reduced the serum level of melatonin and that supplementation with melatonin may have a protective effect on AP by modulating TLR4/NF-ĸB signaling in the pulp and in pulp cells.
Keywords: Acute pulpitis (AP), dental pulp injury, Toll-like receptor 4 (TLR4), melatonin, rat
Introduction
Various causes may lead to pulp exposure and injury, but dental decalcification and mechanical injury is perceived as the most common one. Pulp exposure and injury leads to pulpitis and induces severe inflammation, which frequently leads to persistent pain and referred pain [1-3]. The mechanism of acute pulpitis is complex and involved with repetitive trauma, inflammation, bacterial invasion, stimulation of the afferent nerve, secondary hyperalgesia, and in rare cases periodontitis. Without any effective treatment, the outcome is always root canal treatment. Therefore, the identification of a new therapeutic target is of significantly important for the treating pulpitis.
TLR4 is an important transmembrane pattern-recognition receptor of the innate immune system, which is widely expressed in dental pulp cells to sense exogenous pathogen-associated molecular patterns (PAMPs) and endogenous danger-associated molecular patterns (DAMPs) that are released after tissue injury or cellular stress [4,5]. TLR4 has been extensively documented in a variety of inflammatory conditions such as pneumonia, hepatitis, sepsis and so on. In addition, stimulation of TLR4 initiates a series of signaling cascades that result in the activation of NF-ĸB and mitogen-activated protein kinases (MAPKs) to induce the release of pro-inflammatory cytokines such as TNF-α and IL-1β [6-8]. The most recent in vitro investigations of pulp cell showed that activation of TLR4 by LPS enhances Wnt5a, TGF-β1 and pro-inflammatory cytokines expression [4,9,10]. Furthermore, TLR4 signaling also contributes to tongue-referred pain associated with tooth pulp inflammation [11]. Therefore, TLR4 signaling is integral to the process of pulp inflammation, and inhibition of TLR4 may have a therapeutic effect.
Recent research has found that melatonin is closely associated with the expression of TLR4 and TLR4-mediated inflammation. Melatonin (N-acetyl-5-methoxytryptamine) is a neuro-endocrine hormone that is predominantly produced by the pineal gland at night as well as by many other organs, including the cerebellum, ovary and so on, independent of the light/dark cycle [12-14]. It is involved in numerous physiological functions such as sleep improvement, antioxidant properties, and endothelial function, among others [15-19]. Moreover, melatonin modulates the TLR4 signaling pathway during inflammation and possesses anti-inflammatory properties in the early and late stages of responses through the MyD88- and TRIF-dependent signaling pathway [20]. Numerous studies have shown that this modulation results in the inhibition of TLR4 expression and decrease of the expression of pro-inflammatory cytokines, including IL-1, IL-6, IL-12, C-reactive protein, and TNF-α, in neuro-inflammation, sleep-deprivation-related diseases and vascular endothelial dysfunction [21-23]. Therefore, we hypothesized that melatonin might also inhibit the oral inflammatory progression of acute pulpitis. However, Mi-Zhen Xia et al reported that pretreatment RAW264.7 cells with melatonin increased TLR4 gene expression compared with the control [20]. Thus, the specific roles of melatonin in the regulation of TLR4/NF-ĸB signaling and the anti-inflammatory activity of melatonin in the AP require further investigation.
To determine the role of melatonin in acute pulpitis, the present studies (1) established an AP model in the rat and tracked the progression of AP by hematoxylin-eosin staining (HE staining) and enzyme-linked immunosorbent assay (ELISA); (2) determined the serum level of melatonin in AP; (3) explored the expression of TLR4/NF-ĸB signaling in the pulp of the AP models; (4) evaluated the rescue effect of melatonin in AP and activation of TLR4/NF-ĸB signaling; (5) established human dental pulp stem cells; and (6) evaluated the rescue effect of melatonin for TLR4/NF-ĸB signaling in LPS-stimulated HDPCs.
Materials and methods
Animals and groups
Adult male Sprague Dawley rats weighing 250 to 350 g used in the present study were provided by the experimental animal center of the Fourth Military Medical University. The animals were maintained in a temperature-controlled room (23°C) with a 12-hours light/dark cycle. Food and water were freely available. All the experimental procedures were approved by the Fourth Military Medical University Committee on Animal Care and Use.
The rats were randomly assigned to one of the following four groups: (1) SHAM group: rats were anesthetized without any treatment; (2) Acute Pulpitis (AP) group: rats were anesthetized and the left upper molars tooth pulps (M1 and M2) were exposed under anesthetization as previously described [24]; (3) AP+M group: melatonin (Sigma, St. Louis, MO) was dissolved in a 5% ethanol solution in saline (vehicle) and administered intraperitoneally (10 mg/kg) once daily for five successive days post AP model establishment; (4) AP+E group: 5% ethanol solution in saline was administered in the abdomen after dental pulp injury once daily for five successive days post AP model establishment.
Establishment of the acute pulpitis model
Rats were lightly anesthetized with 2% isoflurane in oxygen and then deeply anesthetized with an intraperitoneal application of 7% chloral hydrate (30 ml/100 g body weight). Next, the rats were placed on a warm mat (37°C) in the supine position for surgery. The mouths of the rats was gently opened with metal tweezers, and the left maxillary first and second molars were drilled with a high-speed handpiece and 1/4 round bar under water cooling [24]. The exposed pulp cavity was kept open, and the rats are conveyed back.
Hematoxylin-eosin (HE) staining for acute pulpitis
At 1 d, 3 d and 5 d after AP establishment, the rats were decapitated, and the molar tooth specimens were rapidly removed and cut into fragments that were subjected to decalcification in ethylenediaminetetraacetic acid (EDTA; 41.3 g disodium EDTA, 4.4 g NaOH in 1000 ml distilled water) for 30 days. Next, the specimens were embedded in paraffin and frozen at 2-8°C. The frozen specimens were sliced with a rotary microtome to yield slices with a thickness of 4 μm. The slices were mounted onto microscopic glass slides and treated with 50-60°C water. After being tempered overnight in an oven maintained at 37°C, the slices were stained with hematoxylin and eosin, sealed with fat-soluble gel, and examined microscopically.
DAB staining analysis of TLR4 and NF-ĸB expression in the pulp
The maxillary molars were excised and harvested at 1 d, 3 d and 5 d post AP establishment for histological examination. The rats were lightly anesthetized with 2% isoflurane in oxygen and then deeply anesthetized with an intraperitoneal injection of 7% chloral hydrate (30ml/100 g body weight). The chest cavity of each rat was opened to expose the cardiac left ventricle into which a lavage needle was inserted after making a small incision; a second incision was made on the right atrial appendage to allow outflow. PBS containing heparin was delivered through the needle at a low flow rate for approximately 3 minutes to clear the blood, and then perfusion was performed with 4% paraformaldehyde in PBS (pH 7.4) as a fixative for 10 minutes. The specimens were quickly removed and post-fixed overnight at 4°C in a 50-ml tube containing fresh fixative. The dental tissues were then demineralized in EDTA for 6 weeks at 4°C. After demineralization, the dental tissues were dehydrated in a graded ethanol series and embedded in paraffin wax. Sections with a thickness of 3-4 μm were cut according to routine procedures, mounted on silane-coated slides, and finally air-dried.
Prior to the immunohistochemical examination, 3 μm slices from pretreated tissue were placed in a bathing solution of 3% H2O2 and 60% methanol PBS (pH 7.4) for 30 min and then treated with 0.01 mol/L sodium citrate buffer at 95°C in a microwave oven for 13 min (antigen retrieval). The specimens were then treated with 5% normal goat serum and 5% bovine serum albumin in PBS. Before each step, the sections were rinsed three times in PBS buffer. The samples were incubated with anti-TLR4 (1:50; Abcam, Cambridge, MA) or anti-NF-ĸB p65 (1:50; Abcam) primary antibodies in a PBS-based solution of 1% bovine serum albumin for 12 h at 4°C at the recommended dilutions. After rinsing with PBS, the sections were incubated with the corresponding biotinylated goat anti-rabbit secondary antibodies for 1 h at room temperature (1:100; Abcam). A streptavidin/horseradish peroxidase complex was then applied as a detection system for 1 h (1:100; Abcam). Finally, the staining was developed with 3,3’diaminobenzidine tetra-hydrochloride in 0.05 mol/L Tris-HCl buffer and 0.1% H2O2. Negative control sections were incubated without the primary antibody. All of the data were analyzed using “Image Pro Plus” software (Media Cybernetics, Bethesda, MD).
Enzyme-linked immunosorbent assay to evaluate cytokine levels
To detect the cytokine levels, blood samples were collected from rats in sterilized test tubes without anticoagulant. Serum was separated by centrifugation at 1500 r/min for 10 min. The samples were divided in small aliquots and stored at -80°C for future assessment of cytokine levels. Next, the rats were decapitated, and the dental pulp was rapidly removed and quickly frozen on dry ice. The samples were homogenized in PBS buffer via a mechanical trituration method and centrifuged at 20000 r/min for 30 min at 4°C. The protein concentration was determined in the supernatants using the BCA protein assay according to the manufacturer’s instructions (Sigma, Missouri, USA). The concentrations of IL-1β, IL-18, and TNF-α were determined using commercially available ELISA kits according to the manufacturer’s protocol (Sigma, Missouri, USA). Each cytokine sample was assessed in duplicate, and the mean cytokine concentration was calculated. In the cell assays, the cytokine levels in the culture medium were measured directly by ELISA kits.
Dental pulp cell culture and treatments
Cells were prepared from the dental pulp tissues of healthy patients (aged 18-22 years) after obtaining informed consent. The crowns of human molars were dissected, and the pulp was gently removed using a sterile spoon excavator and a dental probe. Confluent cells were detached by incubation with 0.25% trypsin and 0.05% EDTA for 5 minutes, and aliquots of separated cells were subcultured in Minimum Essential Medium Alpha (Gibco, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (Sigma), 50 U/mL penicillin, and 50 mg/mL streptomycin (Sigma). Pulp cell cultures at passage 4 and HDPCs isolated as for subsequent experiments. The HDPCs were grouped to receive the following four treatments separately: (1) incubation with saline; (2) incubation with LPS (2.0 μg/mL); (3) incubation with LPS (2.0 μg/mL) in the presence of melatonin (1.0 mM, dissolved in 0.25% ethanol: saline); and (4) incubation with LPS (2.0 μg/mL) in the presence of 0.25% ethanol solution in saline.
Quantitative real-time PCR to evaluate the TLR4 signaling
Quantitative real-time PCR (qRT-PCR) analyses of mRNA expression were performed using RNA isolated from the pulp tissue of rats and from HDPCs. The RNA was purified by TRIzol reagent according to the manufacturer’s instructions (Takara, Kyoto, Japan). RNA was transcribed to cDNA using the PrimeScript RT reagent Kit (Takara). Next, qRT-PCR was performed using the CFX96TM real-time system (Bio-Rad, Hercules, CA), and the relative gene expression was normalized to the internal control β-actin. Analysis of the melting curves for each amplified PCR product and visualization of the PCR amplicons in 1.5% agarose gels permitted control of the specificity of the amplification. Primer sequences for SYBR Green probes of target genes are listed in Table 1.
Table 1.
Primers for real-time RT-PCR
| Name | Sequences | Size |
|---|---|---|
| TLR4 | Forward: 5’-GGCATCATCTTCATTGTCCTTG-3’ | 180 |
| Reverse: 5’-AGCATTGTCCTCCCACTCG-3’ | ||
| NF-kB | Forward: 5’-CTG AAC CAG GGC ATA CCT GT-3’ | 197 |
| Reverse: 5’-GAG AAG TCC ATG TCC GCA AT-3’ | ||
| IL-1β | Forward: 5’-GCCTCGTGCTGTCGGACCCATAT-3’ | 143 |
| Reverse: 5’-TCCTTTGAGGCCCAAGGCCACA-3’ | ||
| TNF-α | Forward: 5’-CCCTCCTGGCCAACGGCATG-3’ | 109 |
| Reverse: 5’-TCGGGGCAGCCTTGTCCCTT-3’ | ||
| β-actin | Forward: 5’-GGAGATTACTGCCCTGGCTCCTA-3’ | 629 |
| Reverse: 5’-GACTCATCGTACTCCTGCTTGCTG-3’ |
Western blot analysis of the TLR4 signaling
After pretreatment, the cells were washed with ice-cold PBS/phosphatase inhibitors, and the cell pellet was resuspended in hypotonic buffer and maintained on ice for 15 min. The pellet was then collected with a cell scraper and harvested by centrifugation. For nuclear protein extraction, the suspension was mixed with detergent and centrifuged for 30 s at 14,000 g. The nuclear pellet was resuspended in complete lysis buffer in the presence of protease inhibitor cocktail. For the total protein extraction, the cell suspension from different groups was directly lysed by incubation for 30 min with lysis buffer and protease inhibitors on ice (Sigma). Both the nuclear protein lysates and total protein lysates were centrifuged at 12,000 r/min for 10 min to remove insoluble material. The protein concentration was determined using a bicinchoninic acid kit (Sigma). The same amount of protein (80 µg) was used for western blot analysis. The samples were resolved in 10% SDS-PAGE gels and transferred to polyvinylidene fluoride membranes. The immunoblots were probed with anti-TLR4, anti-I-ĸB, anti-NF-ĸB (p65), anti-NF-ĸB (p50) antibodies (1:100, 1:200, 1:100, respectively; Abcam) overnight at 4°C followed by incubation with goat anti-rabbit IgG (1:200; Invitrogen) at room temperature for 1 h. The blots were visualized using ECL-Plus reagent (Millipore, Billerica, MA). For nuclear protein, lamin A/C was used as a loading control. For total protein, β-actin was used as a loading control.
Statistical analysis
Data were expressed as the mean ± SEM. Statistical analyses were performed using Student’s t-test, one-way ANOVA or two-way repeated-measures ANOVA followed by Bonferroni’s multiple comparison test where appropriate. A value of P<0.05 was considered significant.
Results
Melatonin supplementation modulates the serum level of pro-inflammatory cytokines and tissue necrosis in AP subjected to dental pulp injury
The histological features suggested that dental pulp injury caused a severe 5-day period of pulp inflammation in the AP group (Figure 1). Pulp exposed to inflammation displayed a striking increase of inflammatory cells and broad hemorrhagic necrosis, especially at 1 d and 3 d (Figure 1A). And 5 d later, almost all of the tissue was necrotic tissue and few cells could be found. The pulp inflammation led to a change in the expression of serum cytokines. The serum ELISA results indicated that the levels of inflammatory cytokines IL-1β, IL-18 and TNF-α were much higher in the AP group than in the SHAM group, and the content of these inflammatory cytokines were found reaching the peak at 1 d post AP establishment (Figure 1B). In contrast, the serum levels of melatonin were lower in the AP group than in the SHAM group, with the lowest levels detected at 1 d post AP establishment (Figure 1C). When the rats were treated by an abdominal injection of melatonin in the AP+M group, the high levels of inflammatory cytokines (IL-1β, IL-18 and TNF-α) were significantly decreased (mean ± SEM of 22.5430 ± 1.1343, 20.4140 ± 1.0432 and 21.4321 ± 2.3410, respectively) compared to the AP group (Figure 1D). Furthermore, the histological damage was ameliorated by melatonin. The percentage of necrosis at 5 d in the AP+M group decreased to 74.800% ± 2.869% compared with the AP or AP+E group (Figure 1E).
Figure 1.
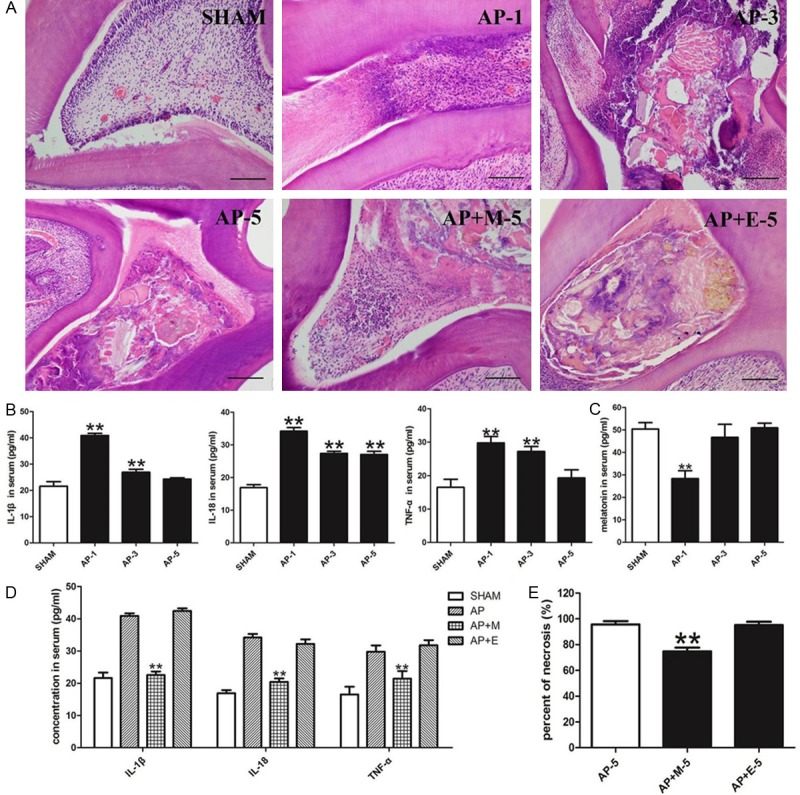
Melatonin inhibited inflammation of acute pulpitis and pro-inflammatory cytokine levels in the serum. A, E. HE-staining of pulp from the SHAM group, AP group and 5 d of AP+M and AP+E groups. Dental pulp injury induced a 5 day period of inflammation. Compared with HE-staining of the pulp at 5 d of AP group or AP+E group, AP+M displayed a relatively low percentage of necrosis at 5 d (bar=50 μm; n=8). B, C. Increased pro-inflammatory cytokine levels and decreased melatonin in the serum were observed at different time points of the AP group. D. Abdominal injection of melatonin ameliorated the increased level of IL-1β, IL-18 and TNF-α in the serum of AP rats. SHAM: results in the SHAM group; AP-1: results in the AP group at 1 d; AP-3: results in the AP group at 3 d; AP-5: results in the AP group at 5 d; AP+M-5: results in the AP+M group at 5 d; AP+E-5: results in the AP+M group at 5 d. **P<0.01 vs. SHAM; n=8.
Melatonin inhibits activation of TLR4/NF-ĸB signaling in the AP
The expression of TLR4 and NF-ĸB in the pulp occurred simultaneously with the pulp inflammation. The q-RT PCR results demonstrated that the expression of TLR4 and NF-ĸB were remarkably up-regulated in the pulp during AP, especially at 1 d after AP establishment (Figure 2A). Administration of melatonin prevented the up-regulation of TLR4 and NF-ĸB in AP. However, the gene expression levels of TLR4 and NF-ĸB were significantly lower in the AP+M group than in the AP group or the AP+E group at 1 d post AP establishment (Figure 2B). Immunohistochemistry analysis provided similar results (Figure 2C). There was no TLR4 expression in the central zone of the pulp; however, positive expression was detected in and around blood vessels in the SHAM group. In addition, NF-ĸB expression was diffuse and low in fibroblasts and odontoblasts. However, DAB staining revealed that TLR4 and NF-ĸB were markedly up-regulated in the pulp of AP group compared with the SHAM group. After melatonin treatment, expression levels of TLR4 and NF-ĸB were significantly reduced in the pulp of the AP+M group compared with the AP group.
Figure 2.
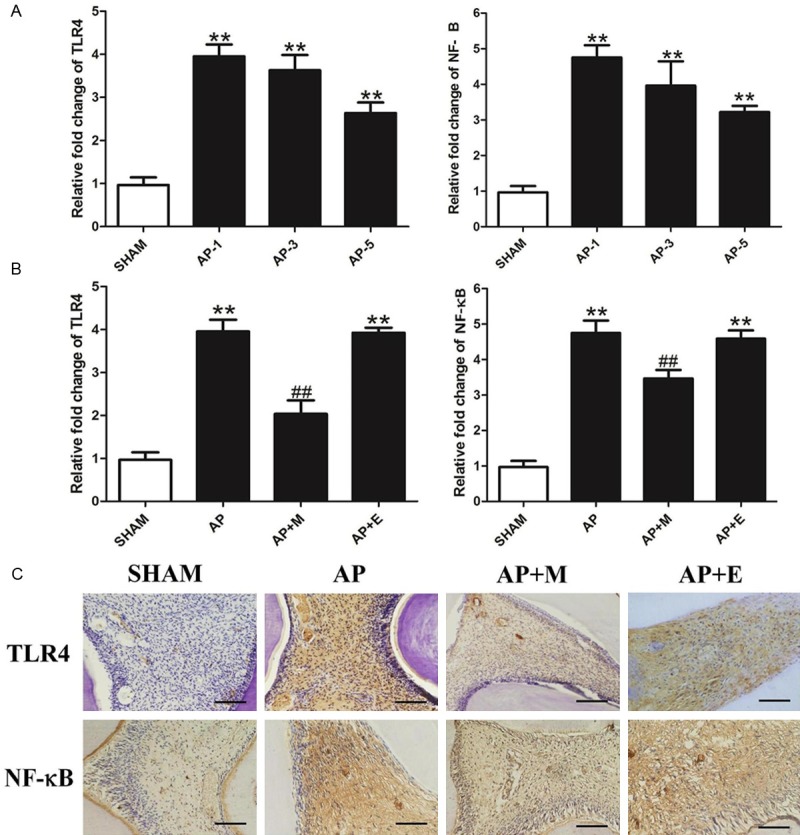
Melatonin inhibited TLR4/NF-ĸB signaling in the pulp of AP. A. AP induced the up-regulation of TLR4 and NF-ĸB expression at the different time points of the AP group. B. Abdominal injection of melatonin inhibited the increased level of TLR4 and NF-ĸB expression at 1 d of AP rats. C. DAB staining of TLR4 and NF-ĸB of AP and AP+M group at 1 d (bar=50 μm;n=8). SHAM: results in the SHAM group; AP-1: results in the AP group at 1 d; AP-3: results in the AP group at 3 d; AP-5: results in the AP group at 5 d. **P<0.01 vs. SHAM; n=8.
Melatonin decreases the release of pro-inflammatory cytokines in AP
The levels of pro-inflammatory cytokines downstream of TLR4/NF-ĸB, IL-1β and TNF-α were also determined in the pulp. The expression levels of IL-1β and TNF-α were up-regulated in the AP group compared with the baseline expression in the SHAM group, with cytokines hitting the peaked at 1 d post AP establishment (Figure 3A). When AP rats were treated with melatonin at 1 d of the AP+M group, the levels of TNF-α and IL-1β were significantly decreased compared to the AP or AP+E group (Figure 3B).
Figure 3.
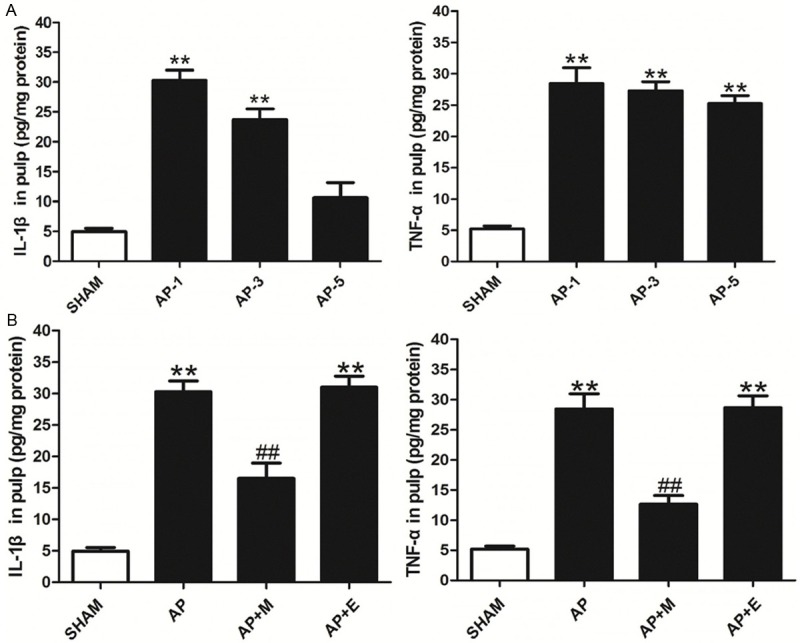
Melatonin inhibited the levels of pro-inflammatory cytokines in the pulp of AP rats. A. Pro-inflammatory cytokines in the pulp at different time points of AP group. B. Melatonin inhibited the levels of pro-inflammatory cytokines at 1 d of the pulp. SHAM: results in the SHAM group; AP: results in the AP group at 1 d; AP+M: results in the AP+M group at 1 d; AP+E: results in the AP+M group at 1 d. **P<0.01 vs. SHAM; ##P<0.01 vs. AP; n=8.
Melatonin decreases the release of pro-inflammatory cytokines in LPS-stimulated HDPCs
To validate the effects of melatonin on TLR4 signaling, we analyzed the effects of melatonin on LPS-induced pro-inflammatory cytokines in HDPCs. The gene expression of pro-inflammatory cytokines, such as IL-1β and TNF-α, were significantly increased at 1 h post LPS-treatment in the LPS group in response to LPS. The expression of these cytokines remained elevated at 6 h post LPS-treatment (Figure 4A). Pretreatment with melatonin significantly attenuated LPS-evoked upregulation of pro-inflammatory cytokine gene expression in the LPS+M group compared with the LPS or the LPS+E group. The results obtained by ELISA provided similar results (Figure 4B). Melatonin significantly relieved the up-regulation of IL-1β and TNF-α at 6 h post LPS-stimulation, while ethanol did not.
Figure 4.
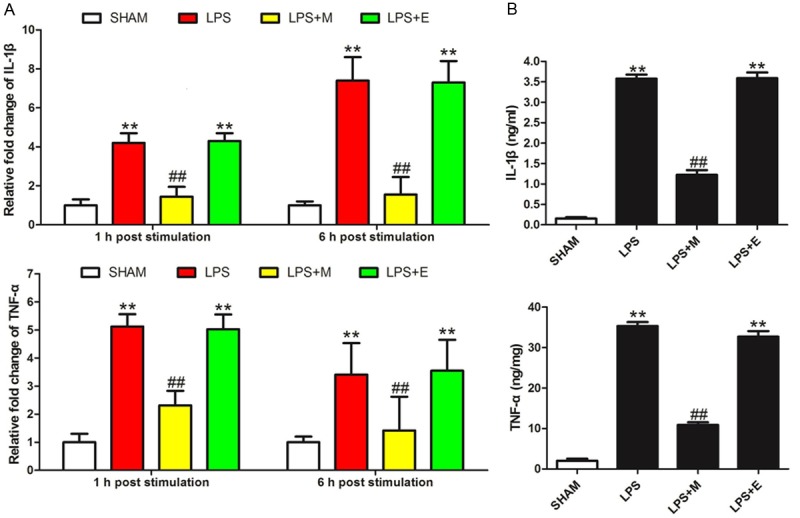
Melatonin inhibited pro-inflammatory cytokine levels in HDPCs. A. Melatonin inhibited the gene expression of pro-inflammatory cytokines at 1 h and 6 h post-LPS stimulation. B. ELISA results of pro-inflammatory cytokines at 6 h of LPS-stimulated HDPCs. SHAM: results in the SHAM group; LPS: results in the LPS group; LPS+M: results in the AP+M group; LPS+E: results in the LPS+E group. **P<0.01 vs. SHAM; ##P<0.01 vs. LPS; n=8.
Melatonin ameliorates LPS-induced NF-ĸB activation in the HDPCs
The effects of melatonin on LPS-induced NF-ĸB activation are presented in Figure 5. As expected, the level of I-ĸB was significantly decreased in LPS-stimulated HDPCs at 1 h post stimulation, whereas the levels of nuclear NF-ĸB p65 and p50 were dramatically increased in LPS-stimulated HDPCs (Figure 5). Melatonin significantly attenuated LPS-induced I-ĸB degradation and inhibited LPS-induced nuclear translocation of NF-ĸB p65 and p50 in HDPCs (Figure 5).
Figure 5.
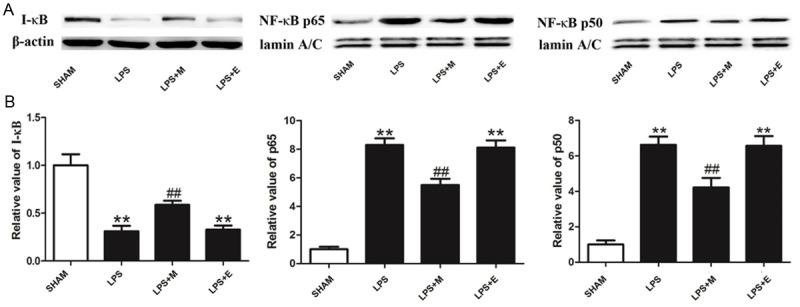
The effects of melatonin on LPS-induced NF-ĸB activation. A. Representative immunoblots of I-ĸB, nuclear NF-ĸB p65 and NF-ĸB p50 in HDPCs subjected to different treatments using β-actin or lamin A/C as an internal standard. B. Quantitative densitometric analysis of I-ĸB, NF-ĸB p65 and NF-ĸB p50 blots. SHAM: results in the SHAM group; LPS: results in the LPS group; LPS+M: results in the AP+M group; LPS+E: results in the LPS+E group. **P<0.01 vs. SHAM; ##P<0.01 vs. LPS; n=8.
Melatonin attenuates the expression of TLR4 in LPS-stimulated HDPCs
Finally, the effects of melatonin on the expression of TLR4 were analyzed in LPS-stimulated HDPCs. As shown in Figure 6, LPS significantly upregulated the gene and protein expression levels of TLR4 at 1 h or more at 6 h post pretreatment in the LPS group. This result is consistent with previous research showing that melatonin significantly attenuated LPS-induced elevation of TLR4 in HDPCs.
Figure 6.
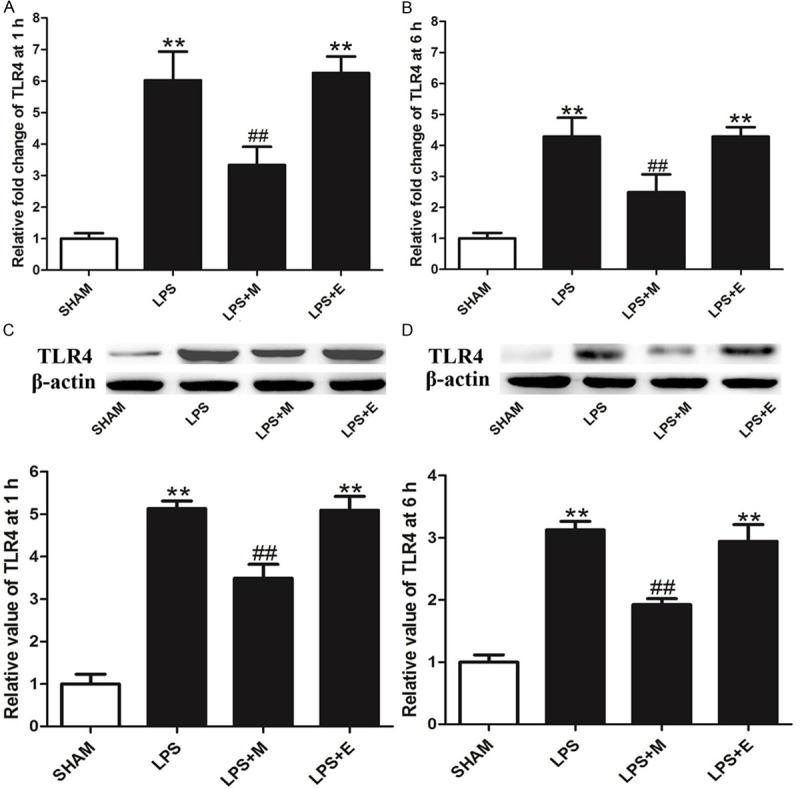
Effects of melatonin on the expression of TLR4 in HDPCs. A and B. The expression of TLR4 mRNA was measured by real-time PCR at 1 h or 6 h after LPS treatment. C and D. The expression of TLR4 protein was measured by western blot analysis at 1 h or 6 h after LPS treatment. SHAM: results in the SHAM group; LPS: results in the LPS group; LPS+M: results in the AP+M group; LPS+E: results in the LPS+E group. **P<0.01 vs. SHAM; ##P<0.01 vs. LPS; n=8.
Discussion
Pulp exposure and injury serves as a stable experimental model for observing the progress of the natural outcome of pulp inflammation, namely AP [24]. The results of HE staining revealed that the pulp, without the protection of enamel, was vulnerable to repetitive trauma and bacterial invasion, which led to necrosis of the pulp. As shown in the experiment, five-day period of pulpitis in rats resulted in infiltration of inflammatory cells at the interface between the necrosis and normal pulp tissue. Additionally, AP induced the up-regulation of the inflammatory cytokines IL-1β, IL-18 and TNF-α in the serum, with a peak level of cytokines observed at 1 d post-surgery. These observations are in accordance with the clinical features of AP and indicate that the most serious inflammation in AP in the rats occurred at 1 d post-surgery.
Meanwhile, as the rats suffered from serious AP-associated inflammation, TLR4 expression was found to peak in the pulp ipsilateral to the injury. TLR4 is a transmembrane protein that contains repeated leucine-rich motifs in its extracellular portions and a cytoplasmic domain that is homologous to the signaling domain of IL-1 receptors [25]. Previous research has demonstrated the extensive expression of TLR4 in the pulp and periodontium, as well as its involvement in many oral pathological processes [5,26]. Activation of TLR4 by LPS treatment of dental pulp fibroblasts activates mitogen-activated protein kinase (MAPK) and up-regulates the expression of cytokines, chemokines, adhesion molecules, prostaglandin E(2) and its key enzyme COX-2 [27-29]. TLR4 also induces the phosphorylation of IκB-α, FAK, AKT, and ERK signaling in odontoblasts to promote adhesion and migration [30]. These research findings all suggest that the effects of TLR4 is crucial in the process of pulpitis, while in our study, direct evidence is proffered to show that AP induced by dental pulp exposure and injury can lead to a significant up-regulation of TLR4 expression in the pulp that is derived from local pulp cells. Moreover, the intensified expression of TLR4 leads to the activation of NF-κB, which is a typical downstream target of TLR4.
The activation of NF-κB resulting in pro-inflammatory cytokine release is well verified [31,32]. Pro-inflammatory cytokines, such as IL-1β and TNF-α, play important roles in many types of inflammation. IL-1β is a potent pro-inflammatory cytokine that promotes a variety of innate immune processes that are associated with infection, inflammation, and autoimmunity [33,34]. It is essential for the host response and resistance to pathogens, and it also exacerbates damage during chronic disease and acute tissue injury [35,36]. TNF-α is a prototype member of the TNF family of ligands that is generated and expressed by immune cells [37]. It mediates numerous inflammatory and immunoregulatory activities that include the following underlying mechanisms. (1) TNF-α induces cell damage and apoptosis caused by the release of oxygen free radicals, proteolytic enzymes, and cytotoxic substances. (2) TNF-α induces the release of other inflammatory mediators and further causes vascular endothelial cell damage, thus increasing vascular permeability and edema. (3) TNF-α induces activation of inflammatory cells and further increases the inflammatory response. (4) TNF-α increases the expression of cell adhesion factor, promoting neutrophil adhesion to endothelial cells and thus amplifying inflammation. (5) TNF-α promotes neutrophil adhesion to the vascular endothelium and small blood vessels occlusion resulting in a no-reflow phenomenon. Therefore, increased levels of the cytokines IL-1β, IL-18 and TNF-α validated the presence of inflammation in the pulp. These cytokines may be the effector molecules of TLR4/NF-ĸB signaling activation that results in necrosis of the pulp.
In contrast to the up-regulated expression of TLR4/NF-κB signaling in the pulp and the increased levels of cytokines in the serum, AP also induces a significant reduction of melatonin at 1 d post AP establishment compared with the baseline. Compared with the five day period of the inflammation in the pulp, the duration of melatonin reduction was relatively short. The trough of this decline was accompanied by the most serious inflammation observed in AP, which suggests that the decline of melatonin may be an indicator of the early stage of inflammation in acute pulpitis. An abdominal injection of melatonin could ameliorate the up-regulation of serum cytokines at 1 d post AP establishment and the necrosis percentage at 5 d post AP establishment, which suggested that melatonin could modulate acute dental pulp inflammation. Furthermore, melatonin could decrease the expression of TLR4 and NF-κB at 1 d post AP establishment. The ELISA results also demonstrated that downstream of TLR4/NF-ĸB, the pro-inflammatory cytokines IL-1β and TNF-α were down-regulated after pretreatment with melatonin at 1 d post AP establishment. These results suggested that low serum levels of melatonin at 1 d of AP might be important for the development of AP, and supplementation with melatonin could inhibit the inflammation and reduce the tissue necrosis by modulating TLR4/NF-ĸB signaling.
Our in vitro results showed that melatonin also modulated TLR4/NF-ĸB signaling in HDPCs. Human dental pulp cells (hDPCs) are composed of ectodermal and mesenchymal components and can specifically generate reparative dentin in response to external stimuli [38]. HDPCs and fibroblasts have a common fibroblast phenotype, and it has been reported that fibroblasts express TLR4 [29]. The pretreatment of HDPCs with the TLR4 agonist LPS significantly activated TLR4/NF-ĸB signaling and up-regulated the expression of cytokines, and these change could be inhibited by melatonin. These results confirm that melatonin can act directly on pulp cells and may have therapeutic effects in tooth diseases.
In conclusion, dental pulp exposure and injury is a suitable model to induce inflammation and necrosis in dental pulp for the study of AP. This procedure leads to the release of cytokines IL-1β, IL-18 and TNF-α and to the down-regulation of melatonin in the serum. TLR4/NF-ĸB signaling is activated in the pulp to induce pro-inflammatory cytokine expression. An abdominal injection of melatonin can significantly inhibit this activation and has a protective effect in AP, which indicates that melatonin may be a promising therapeutic strategy for oral disease.
Acknowledgements
The authors would like to thank all of the volunteers who participated in the present study and indicate that they have no conflicts of interest related to this study. This work was supported by the National Natural Science Foundation of China (NO. 81170946, 81371139), the open project of State Key Laboratory of Oral Medicine, military (No. 2014KB12) and the China Postdoctoral Science Foundation (No. 2014M552697).
Disclosure of conflict of interest
None of the authors report any conflict of interest or financial disclosure.
References
- 1.Ertekin C, Secil Y, Yuceyar N, Aydogdu I. Oropharyngeal dysphagia in polymyositis/dermatomyositis. Clin Neurol Neurosurg. 2004;107:32–37. doi: 10.1016/j.clineuro.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 2.Bender IB. Pulpal pain diagnosis--a review. J Endod. 2000;26:175–179. doi: 10.1097/00004770-200003000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Farella M, Michelotti A, Gargano A, Cimino R, Ramaglia L. Myofascial pain syndrome misdiagnosed as odontogenic pain: a case report. Cranio. 2002;20:307–311. doi: 10.1080/08869634.2002.11746224. [DOI] [PubMed] [Google Scholar]
- 4.Du W, Jia M. Effect of lipopolysaccharide and transforming growth factor-beta 1 on expression and signal pathway of Toll like receptor 4 in dental pulp cells. Hua Xi Kou Qiang Yi Xue Za Zhi. 2012;30:77–81. [PubMed] [Google Scholar]
- 5.Mutoh N, Tani-Ishii N, Tsukinoki K, Chieda K, Watanabe K. Expression of toll-like receptor 2 and 4 in dental pulp. J Endod. 2007;33:1183–6. doi: 10.1016/j.joen.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull. 2012;28:131–144. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calil I, Zarpelon A, Guerrero A, Alves-Filho J, Ferreira S, Cunha F, Cunha T, Verri W. Lipopolysaccharide Induces Inflammatory Hyperalgesia Triggering a TLR4/MyD88-Dependent Cytokine Cascade in the Mice Paw. PLoS One. 2014;9:e90013. doi: 10.1371/journal.pone.0090013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stokes J, Corr M, Yaksh T. Spinal toll-like receptor signaling and nociceptive processing: regulatory balance between TIRAP and TRIF cascades mediated by TNF and IFNβ. Pain. 2013;154:733–742. doi: 10.1016/j.pain.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He W, Qu T, Yu Q, Wang Z, Lv H, Zhang J, Zhao X, Wang P. LPS induces IL-8 expression through TLR4, MyD88, NF-kappaB and MAPK pathways in human dental pulp stem cells. Int Endod J. 2013;46:128–136. doi: 10.1111/j.1365-2591.2012.02096.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Gao Y, Zhan X, Cui L, Xu S, Ma D, Yue J, Wu B, Gao J. TLR4 Activation by Lipopolysaccharide and Streptococcus mutans Induces Differential Regulation of Proliferation and Migration in Human Dental Pulp Stem Cells. J Endod. 2014;40:1375–1381. doi: 10.1016/j.joen.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Ohara K, Shimizu K, Matsuura S, Ogiso B, Omagari D, Asano M, Tsuboi Y, Shinoda M, Iwata K. Toll-like receptor 4 signaling in trigeminal ganglion neurons contributes tongue-referred pain associated with tooth pulp inflammation. J Neuroinflammation. 2013;10:139. doi: 10.1186/1742-2094-10-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lerner AB, Case JD, Takahashi Y. Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J Biol Chem. 1960;235:1992–1997. [PubMed] [Google Scholar]
- 13.Skinner DC, Malpaux B. High melatonin concentrations in third ventricular cerebrospinal fluid are not due to Galen vein blood recirculating through the choroid plexus. Endocrinology. 1999;140:4399–4405. doi: 10.1210/endo.140.10.7074. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura Y, Tamura H, Takayama H, Kato H. Increased endogenous level of melatonin in preovulatory human follicles does not directly influence progesterone production. Fertil Steril. 2003;80:1012–1016. doi: 10.1016/s0015-0282(03)01008-2. [DOI] [PubMed] [Google Scholar]
- 15.Rodella LF, Favero G, Foglio E, Rossini C, Castrezzati S, Lonati C, Rezzani R. Vascular endothelial cells and dysfunctions: role of melatonin. Front Biosci (Elite Ed) 2013;5:119–29. doi: 10.2741/e601. [DOI] [PubMed] [Google Scholar]
- 16.Markus RP, Cecon E, Pires-Lapa MA. Immune-Pineal Axis: Nuclear Factor kappaB (NF-kB) Mediates the Shift in the Melatonin Source from Pinealocytes to Immune Competent Cells. Int J Mol Sci. 2013;14:10979–10997. doi: 10.3390/ijms140610979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyseng-Williamson KA. Melatonin prolonged release: in the treatment of insomnia in patients aged >/=55 years. Drugs Aging. 2012;29:911–923. doi: 10.1007/s40266-012-0018-z. [DOI] [PubMed] [Google Scholar]
- 18.Lax P, Otalora BB, Esquiva G, Rol Mde L, Madrid JA, Cuenca N. Circadian dysfunction in P23H rhodopsin transgenic rats: effects of exogenous melatonin. J Pineal Res. 2011;50:183–191. doi: 10.1111/j.1600-079X.2010.00827.x. [DOI] [PubMed] [Google Scholar]
- 19.Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. 2011;51:1–16. doi: 10.1111/j.1600-079X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 20.Xia MZ, Liang YL, Wang H, Chen X, Huang YY, Zhang ZH, Chen YH, Zhang C, Zhao M, Xu DX, Song LH. Melatonin modulates TLR4-mediated inflammatory genes through MyD88- and TRIF-dependent signaling pathways in lipopolysaccharide-stimulated RAW264.7 cells. J Pineal Res. 2012;53:325–334. doi: 10.1111/j.1600-079X.2012.01002.x. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Wu L, You W, Ji C, Chen G. Melatonin alleviates secondary brain damage and neurobehavioral dysfunction after experimental subarachnoid hemorrhage: possible involvement of TLR4-mediated inflammatory pathway. J Pineal Res. 2013;55:399–408. doi: 10.1111/jpi.12087. [DOI] [PubMed] [Google Scholar]
- 22.Laliena A, San Miguel B, Crespo I, Alvarez M, Gonzalez-Gallego J, Tunon MJ. Melatonin attenuates inflammation and promotes regeneration in rabbits with fulminant hepatitis of viral origin. J Pineal Res. 2012;53:270–278. doi: 10.1111/j.1600-079X.2012.00995.x. [DOI] [PubMed] [Google Scholar]
- 23.Kim JY, Lee YD, Kim BJ, Kim SP, Kim DH, Jo KJ, Lee SK, Lee KH, Baik HW. Melatonin improves inflammatory cytokine profiles in lung inflammation associated with sleep deprivation. Mol Med Rep. 2012;5:1281–1284. doi: 10.3892/mmr.2012.814. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Zhai S, Wang H, Jia Q, Jiang W, Zhang X, Zhang A, Liu J, Ni L. Absent in Melanoma 2 (AIM2) in Rat Dental Pulp Mediates the Inflammatory Response during Pulpitis. J Endod. 2013;39:1390–1394. doi: 10.1016/j.joen.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Alizadeh H, Tripathi T, Abdi M, Smith AD. Pathogenic strains of Acanthamoeba are recognized by TLR4 and initiated inflammatory responses in the cornea. PLoS One. 2014;9:e92375. doi: 10.1371/journal.pone.0092375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang HW, Zhang W, Ren BP, Zeng JF, Ling JQ. Expression of toll like receptor 4 in normal human odontoblasts and dental pulp tissue. J Endod. 2006;32:747–751. doi: 10.1016/j.joen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Botero TM, Son JS, Vodopyanov D, Hasegawa M, Shelburne CE, Nor JE. MAPK signaling is required for LPS-induced VEGF in pulp stem cells. J Dent Res. 2010;89:264–269. doi: 10.1177/0022034509357556. [DOI] [PubMed] [Google Scholar]
- 28.Staquet MJ, Durand SH, Colomb E, Romeas A, Vincent C, Bleicher F, Lebecque S, Farges JC. Different roles of odontoblasts and fibroblasts in immunity. J Dent Res. 2008;87:256–261. doi: 10.1177/154405910808700304. [DOI] [PubMed] [Google Scholar]
- 29.Hirao K, Yumoto H, Takahashi K, Mukai K, Nakanishi T, Matsuo T. Roles of TLR2, TLR4, NOD2, and NOD1 in pulp fibroblasts. J Dent Res. 2009;88:762–767. doi: 10.1177/0022034509341779. [DOI] [PubMed] [Google Scholar]
- 30.Park JH, Kwon SM, Yoon HE, Kim SA, Ahn SG, Yoon JH. Lipopolysaccharide promotes adhesion and migration of murine dental papilla-derived MDPC-23 cells via TLR4. Int J Mol Med. 2011;27:277–281. doi: 10.3892/ijmm.2010.568. [DOI] [PubMed] [Google Scholar]
- 31.Karki R, Igwe O. Toll-like receptor 4-mediated nuclear factor kappa B activation is essential for sensing exogenous oxidants to propagate and maintain oxidative/nitrosative cellular stress. PLoS One. 2013;8:e73840. doi: 10.1371/journal.pone.0073840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin XW, Xu WC, Luo JG, Guo XJ, Sun T, Zhao XL, Fu ZJ. WW domain containing E3 ubiquitin protein ligase 1 (WWP1) negatively regulates TLR4-mediated TNF-α and IL-6 production by proteasomal degradation of TNF receptor associated factor 6 (TRAF6) PLoS One. 2013;8:e67633. doi: 10.1371/journal.pone.0067633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Shi X, Zhang X, Lei H, Long S, Su H, Pei Z, Huang R. Scutellarin attenuates hypertension-induced expression of brain Toll-like receptor 4/nuclear factor kappa B. Mediators Inflamm. 2013;2013:432623. doi: 10.1155/2013/432623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahoo M, Ceballos-Olvera I, del Barrio L, Re F. Role of the inflammasome, IL-1beta, and IL-18 in bacterial infections. ScientificWorldJournal. 2011;11:2037–2050. doi: 10.1100/2011/212680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1beta secretion. Cytokine Growth Factor Rev. 2011;22:189–195. doi: 10.1016/j.cytogfr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong M, Ziring D, Korin Y, Desai S, Kim S, Lin J, Gjertson D, Braun J, Reed E, Singh RR. TNFalpha blockade in human diseases: mechanisms and future directions. Clin Immunol. 2008;126:121–136. doi: 10.1016/j.clim.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sloan AJ, Smith AJ. Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Dis. 2007;13:151–157. doi: 10.1111/j.1601-0825.2006.01346.x. [DOI] [PubMed] [Google Scholar]


