Abstract
Membrane type 1 matrix metalloproteinase (MT1-MMP) has been demonstrated to play an important role in tumor progression. The aim of the present study was to analyze the expression of MT1-MMP in breast cancer and its correlation with clinicopathologic characteristics, including the survival of breast cancer patients. In our results, MT-MMP1 was up-expressed in breast cancer tissues compared with ductal hyperplasia tissues in microarray data (GSE2429). MT1-MMP mRNA and protein expression was markedly higher in breast cancer tissues than in normal breast tissues (P=0.005 and P=0.037, respectively). Using immunohistochemistry, high levels of MT1-MMP protein were positively correlated with the status of clinical stage (I-II vs. III-IV; P=0.043), lymph node metastasis (absence vs. presence; P=0.024), and distant metastasis (No vs. Yes; P=0.017) of breast cancer patients. Patients with higher MT1-MMP expression had a significantly shorter overall survival time than did patients with low MT1-MMP expression. Multivariate analysis indicated that the level of MT1-MMP expression was an independent prognostic indicator (P<0.001) for the survival of patients with breast cancer. In conclusions, MT1-MMP plays an important role on breast cancer aggressiveness and prognosis and may act as a promising target for prognostic prediction.
Keywords: Breast cancer, membrane type 1 matrix metalloproteinase, prognosis, immunohistochemistry
Introduction
Breast cancer is the most common cancer in females, and the second leading cause of cancer related mortality in women, accounting for approximately 29% of all new cancer cases among women and 14% cancer related mortality, representing a serious health threat to women worldwide [1,2]. The prognosis of breast cancer is encouraging because of the advances in diagnosis and systemic therapy, including surgery, chemotherapy, hormone therapy, and radiation. However, the clinical outcome of breast cancer patients remains unsatisfactory. Similar to many other solid tumors, distant metastases account for more than 90% of breast cancer-related death [3]. This is largely because of a lack of effective and specific biomarkers that predict the risk of metastases in patients with breast cancer. Thus, new prognostic markers enabling oncologists to effectively distinguish high-risk patients with unfavorable prognosis and choose appropriate treatment strategies for breast cancer patients are urgently needed.
The matrix metalloproteinase (MMP) family has twenty-three members that differ in their substrate specificities toward varied components of the extracellular matrix. Structurally, the MMPs usually include a highly conserved propeptide domain, a zinc-binding catalytic domain, and a hemopexin-like domain [4]. MMPs are involved in many phases of cancer progression, including tumor invasion, metastasis, and angiogenesis [5,6]. Membrane-type 1 MMP (MT1-MMP), which is a member of the MMPs family, has been implicated in multiple biological processes for its extracellular matrix degrading and accelerating angiogenesis [7]. Generally, MMPs are produced by tissues as inactive zymogens and require further activation. However, MT1-MMP does not require additional activation, due to its capacity to be presented on the cell membrane in its active form [8]. Furthermore, present studies indicated that the expression of MT1-MMP was increased in tumor cells and overexpression of MT1-MMP directly correlate with accelerated cell migration [9].
The aim of this study was to identify the pathological roles of MT1-MMP in breast cancer. This study suggested that MT1-MMP was increased expression in breast cancer tissue and positively associated with clinical stage, lymph node metastasis, and distant metastasis. Furthermore, we found that overexpression of MT1-MMP was a significant predictor of poor prognosis for breast cancer patients.
Materials and methods
Analysis of microarray data
Microarray data set (GEO accession number: GSE2429) from four ductal hyperplasia tissues and four breast cancer tissues submitted by Poola was retrieved from the GEO database. Those differentially expressed genes were screened and identified by Real-time PCR for the following study.
Patients and specimens
One hundred and twenty six paraffin-embedded breast cancer samples and twenty one non-cancerous breast samples were retrieved from Xijing Hospital. Fifteen fresh breast cancer samples and fifteen non-cancerous fresh breast samples were collected from Xijing Hospital. All fresh samples were immediately preserved in liquid nitrogen. No patients had received any form of relevant tumor therapy before diagnosis. Before the use of these clinical samples, prior consents from the patients and approval from the Institutional Ethics Committee of the Xijing Hospital were obtained. The histopathological diagnosis of all samples was respectively diagnosed by two pathologists. The clinical staging was based on the 7th AJCC Cancer Staging Manual. Overall survival (OS) was defined as the interval from the date of diagnosis to breast cancer related death. In the 126 breast cancer cases, the age ranged from 23 to 79 years (median, 57.18 years). The clinical follow-up time of patients ranged from 6 to 96 months.
Real-time PCR
The isolated total RNA was reversely transcribed using the PrimeScript RT Master Kit (Takara). qPCR was performed using SYBR Premix Ex TaqTM II (Takara) on a LightCycler (Roche). The sequence-specific forward and reverse primers sequences for MT1-MMP mRNA were 5’-AAGAGGAGAAGAGCAAACAG-3’ and 5’-CGGTAGGCACTGAACTTG-3’ respectively. Forward and reverse primers sequences for ARF5 mRNA were 5’-ATCTGTTTCACAGTCTGGGACG-3’ and 5’-CCTGCTTGTTGGCAAATACC-3’ respectively. Relative quantification of mRNA expression was calculated by using the 2-ΔΔct method. The raw data were presented as the relative quantity of MT1-MMP mRNA, normalized with ARF5, and relative to a calibrator sample. All qRT-PCR reactions were performed in triplicate.
Immunohistochemistry
Paraffin sections from normal and breast cancer specimens were deparaffinized in xylene and rehydrated in a descending ethanol series (100%, 90%, 80%, 70% ethanol) and double distilled water according to standard protocols. Heat-induced antigen retrieval was performed in citrate buffer and boiled for 10 min. After antigen retrieval, sections were treated with 3% hydrogen peroxide and 1% bovine serum albumin to block the endogenous peroxidase activity and non-specific binding. The sections were incubated with MT1-MMP antibody (Chemicon, clone 114-6G6, dilution 1:100) overnight at 4°C. After phosphate buffered saline (PBS) washing, the tissue sections were incubated with the biotinylated secondary antibody and streptavidin-horseradish peroxidase complex, each for 20 min at room temperature. Diaminobenzidine (DAB) was used as the chromogen, and tissue sections were counterstained with hematoxylin and then viewed under a bright-field microscope.
Evaluation of staining
The tissue sections stained immunohistochemically for MT1-MMP were reviewed, and scored separately by two pathologists blinded to the clinical parameters. Any disagreements were arbitrated by the third pathologists. Staining intensity was graded (0, negative; 1, weak; 2, moderate; 3, strong), and percentage of positive-staining cells was counted (0, 10%; 1, 11-50%; 2, 51-75%; 3, >76%).The final score was determined by the combined staining score and proportion score (intensity score × proportion score) [10]. The final staining score ranged from 0 to 9. For statistical analysis, a final staining scores of 0-4 and 6-9 were respectively considered to be low and high expression.
Statistical analysis
All statistical analyses were performed using SPSS version 17.0 software. Data were presented as mean ± SD. The unpaired T test was applied to test the differential mRNA expression of MT1-MMP in breast cancer compared to non-cancerous breast tissues. The Chi-square test was used to examine the differences of MT1-MMP protein expression between breast cancer and non-cancerous breast tissues. The Chi-square test was applied to the examination of relationship between MT1-MMP expression levels and clinicopathologic characteristics. Survival curves were plotted using the Kaplan-Meier method and compared using the log-rank test. The significance of survival variables was analysed using the Cox multivariate proportional hazards model. A P-value of less than 0.05 was considered statistically significant.
Results
MT1-MMP mRNA and protein was overexpressed in breast tissue
From Poola et al.’s microarray data (GSE2429), MT-MMP1 was up-expressed in breast cancer tissues compared with ductal hyperplasia tissues by an average of 1.30-folds (Figure 1).
Figure 1.
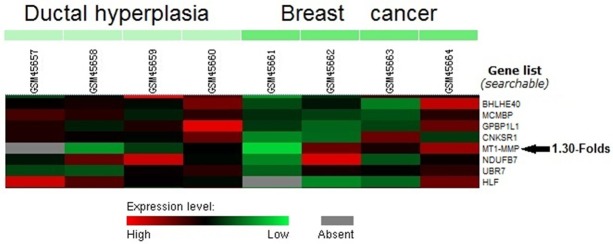
Increased MT1-MMP expression was shown in breast cancer by microarray data analysis of GSE2429 data set retrieved from the GEO database.
In order to verify the role of MT1-MMP in breast cancer, we performed real-time PCR to measure the expression of MT1-MMP mRNA transcripts in fifteen fresh breast cancer tissues and fifteen fresh normal breast tissues. This expression pattern was similar to the microarray data. Compared with normal non-cancerous breast tissues, the levels of MT1-MMP mRNA was overexpressed with an average increase of 2.49-fold (P=0.005) (Figure 2).
Figure 2.
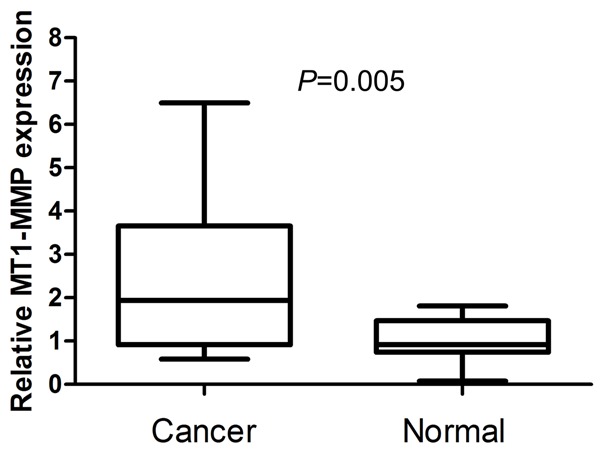
Expression of MT1-MMP mRNA is increased in breast cancer tissues compared with normal breast tissues by real-time PCR.
We detected the expression levels of MT1-MMP protein in 126 archived paraffin-embedded breast cancer samples and 21 non-cancerous breast samples using immunohistochemical staining (Figure 3A-D). We observed that MT1-MMP protein was overexpressed in 53.17% (67/126) of breast cancer samples. In comparison, only 28.57% of normal breast samples had highly expressed MT1-MMP protein, significantly lower than that in the breast cancer samples (P=0.037) (Table 1).
Figure 3.
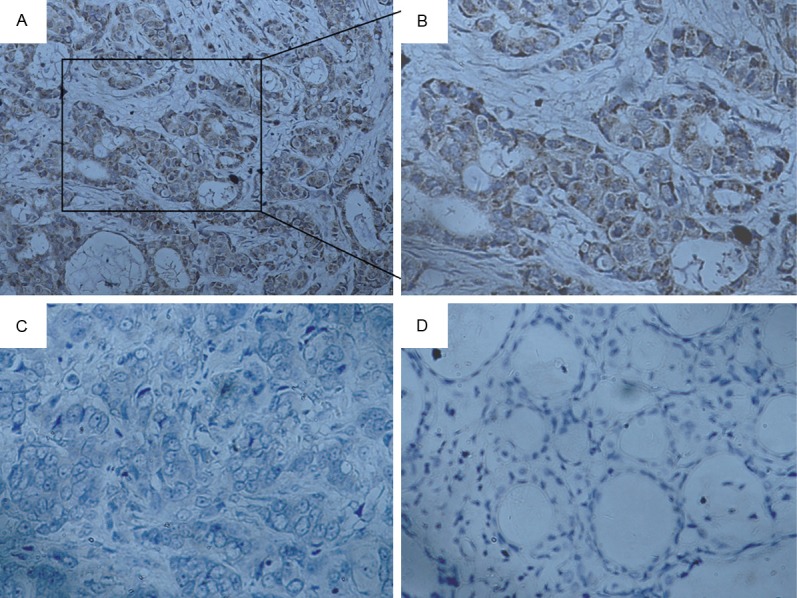
Immunohistochemical staining of MT1-MMP in breast cancer tissues. (A, B) Strong staining of MT1-MMP in breast cancer (A, original magnification ×200; B, original magnification ×400). (C) Negative expression of MT1-MMP in breast cancer (original magnification ×400). (D) Negative expression of MT1-MMP in normal breast tissues (original magnification ×400).
Table 1.
Expression of MT1-MMP protein between breast cancer and non-cancerous samples
| Group | cases | MT1-MMP | p | |
|---|---|---|---|---|
|
| ||||
| High expression | Low expression | |||
| cancer tissue | 126 | 67 | 59 | 0.037 |
| non-cancerous tissues | 21 | 6 | 15 | |
Correlation between MT1-MMP and clinicopathological characteristics in breast cancer patients
We next analyzed the correlation between the expression of MT1-MMP protein and clinicopathological characteristics of breast cancer. As summarized in Table 2, there were no significant correlations between MT1-MMP expression and age (P=0.213), histological grade (P=0.067), tumor size (P=0.100) or triple-negative breast cancer (P=0.080). However, MT1-MMP was associated significantly with clinical stage (I-II vs. III-IV; P=0.043), lymph node metastasis (absence vs. presence; P=0.024), distant metastasis (No vs. Yes; P=0.017).
Table 2.
Correlations between MT-1MMP protein expression and clinicopathological characteristics in breast cancer
| Characteristics | n | High expression | Low expression | P |
|---|---|---|---|---|
| Age (y) | ||||
| <50 | 50 | 30 | 20 | 0.213 |
| ≥50 | 76 | 37 | 39 | |
| Histological grade | ||||
| I | 43 | 18 | 25 | 0.067 |
| II-III | 83 | 49 | 34 | |
| Clinical stage | ||||
| I-II | 46 | 19 | 27 | 0.043 |
| III-IV | 80 | 48 | 32 | |
| Tumor size | ||||
| <5 cm | 78 | 37 | 41 | 0.100 |
| ≥5 cm | 48 | 30 | 18 | |
| Lymph node metastasis | ||||
| absence | 57 | 24 | 33 | 0.024 |
| presence | 69 | 43 | 26 | |
| Distant metastasis | ||||
| No | 118 | 59 | 59 | 0.017 |
| Yes | 8 | 8 | 0 | |
| Triple-negative breast cancer | ||||
| No | 108 | 54 | 54 | 0.080 |
| Yes | 18 | 13 | 5 |
Survival analysis
To explore the prognostic value of MT1-MMP expression in patients with breast cancer, we measured the association between the levels of MT1-MMP expression and patients’ survival using Kaplan-Meier analysis with the log-rank test. In 126 breast cancer patients with prognosis information, we found that the level of MT1-MMP protein expression was significantly associated with the overall survival of breast cancer patients, as patients with higher levels of MT1-MMP expression had poorer survival than those with lower levels of MT1-MMP expression (P<0.001) (Figure 4). Furthermore, we also found that increased expression of MT1-MMP showed poor prognosis in breast cancer patients, regardless of clinical stage, tumor size, lymph node metastasis and distant metastasis. Multivariate analysis showed that increased MT1-MMP expression was a poor independent prognostic factor for breast cancer patients (Table 3).
Figure 4.
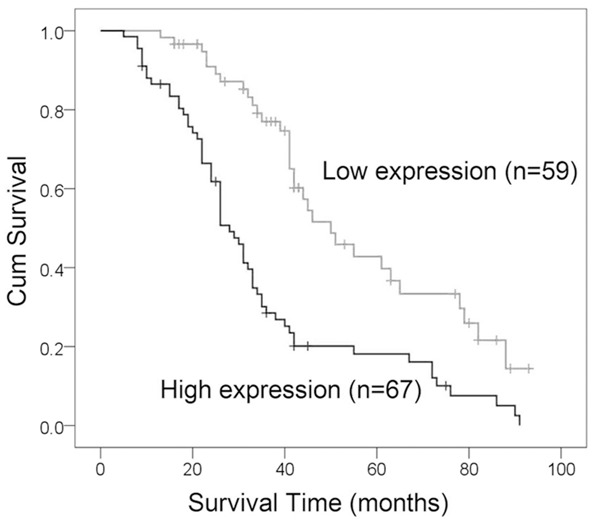
Increased MT1-MMP protein expression predicts an unfavorable prognosis. The association between patient survival and MT1-MMP expression was estimated using the Kaplan-Meier method and the log-rank test (P<0.001).
Table 3.
Univariate and multivariate Cox regression analyses of overall survival in 126 breast cancer
| Parameter | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 0.913 | 0.605-1.377 | 0.663 | |||
| (<50 vs. years ≥50) | ||||||
| Histological grade | 1.234 | 0.794-1.919 | 0.349 | |||
| I vs. II-III | ||||||
| Clinical stage | 4.505 | 2.652-7.652 | 0.000 | 1.928 | 0.785-4.739 | 0.152 |
| I-II vs. III-IV | ||||||
| Tumor size | 2.592 | 1.681-3.997 | 0.000 | 2.473 | 1.555-3.934 | 0.000 |
| (<5 cm vs. ≥5 cm) | ||||||
| Lymph node metastasis | 4.992 | 2.855-8.727 | 0.000 | 2.694 | 1.103-6.582 | 0.030 |
| (absence vs. presence) | ||||||
| Distant metastasis | 10.716 | 3.392-25.564 | 0.000 | 3.187 | 1.294-7.845 | 0.012 |
| (No vs. Yes) | ||||||
| Triple-negative breast cancer | 1.681 | 0.990-2.857 | 0.055 | |||
| (No vs. Yes) | ||||||
| MT1-MMP | 2.643 | 1.714-4.077 | 0.000 | 1.988 | 1.270-3.112 | 0.003 |
| (Low vs. High) | ||||||
HR, hazard ratio; 95% CI, 95% confidence interval
Discussion
MT1-MMP, which is a member of the membrane type MMPs, has been implicated in many biological processes [11]. MT1-MMP activity is not restricted to extracellular matrix degradation and is necessary for several processes involved in tumorigenesis, including angiogenesis, regulation of growth factor and induction of the epithelial-mesenchymal transition [12]. Generally, MMPs are produced by tissues as inactive zymogens and require further activation. However, MT1-MMP does not require additional activation, due to its capacity to be presented on the cell membrane in its active form [8]. Furthermore, present studies demonstrated that MT1-MMP played a significant role in tumor progression. MT11-MMP was originally known as a tumour specific activator of MMP2 [13] and is now identified to activate MMP13 and degrade a variety of ECM components, including fibronectin, collagens, and laminins [14]. Moreover, MT1-MMP also processes and interacts with membrane proteins such as integrins [15], CD44 [16], syndecan-1 [17] and BRCA2 [18].
MT1-MMP is highly expressed in almost all types of human cancer, such as lung cancer [10,19], breast cancer [20], colon cancer [20], cervical cancer [21], prostate cancer [22], and glioblastomas [23]. In a recent microarray analysis, we found significantly high levels of MT1-MMP mRNA in squamous cell lung cancer compared to normal lung tissues [24]. In addition, we also found increased level of MT1-MMP in human pleural mesotheliomas compared with normal pleural specimens in a microarray analysis for 39,000 human transcripts [25]. Similar to a microarray analysis performed by Poola et al. (GSE2429) [26], we found MT1-MMT was higher level in breast cancer samples than in ductal hyperplasia. Then, we verified the mRNA and protein expression of MT1-MMP in breast cancer and normal breast samples through Real-time PCR and immunohistochemistry. This expression pattern was similar to the microarray data.
In order to further identify the role of MT1-MMP in the development and progression of breast cancer. We analyzed the expression of MT1-MMP in 126 breast patients and found MT1-MMP overexpression was significantly associated with clinical stage, lymph node metastasis, and distant metastasis. Our study may suggest that MT1-MMP plays significant roles in breast cancer progression, including tumor invasion and metastasis. Similarly, Têtu et al. also showed that overexpressed MT1-MMP was positively correlated with involved lymph nodes of breast cancer [27]. Furthermore, the expression of MT1-MMP also showed a significant positive correlation with the expression of MMP1, MMP2, MMP3, MMP9, and MMP11 in breast cancer [28]. Inhibition of MT1-MMP significantly suppressed breast cancer cell migration [29]. These studies consistently indicated that overexpressed MT1-MMP may play an unfavorable role in breast cancer pathogenesis. However, the correlation between MT1-MMP expression and the survival of breast cancer patients has been seldom reported.
MT1-MMP overexpression in tumor cells has been shown to be an independent unfavorable prognostic factor in many types of tumors [30]. In gastric cancer, He et al. [31] and Peng et al. [32] observed that a high MT1-MMP protein level correlated with an unfavorable prognosis, which was consistent with Wang et al’s. [10] and Zhou et al’s. [19] reports in non-small cell lung cancer. Furthermore, a meta-analysis, which included 1,918 cases from 11 studies, showed that MT1-MMP is a potential prognostic factor in human cancers, and the pooled hazard ratio (HR) and corresponding 95% confidence interval (CI) was 2.46 (95% CI: 1.75-3.47) [30].
In this study, we first presented that MT1-MMP expression in breast cancer was inversely correlated with patient’s overall survival in protein level. The patients with higher expression of MT1-MMP protein had shorter survival time. According to multivariate analyses, overexpression of MT1-MMP protein was a significant predictor of unfavorable prognosis for breast cancer patients. These results were consistent with Têtu et al’s and McGowan et al’s reports. Têtu et al. [27] and McGowan et al. [28] found that high MT1-MMP mRNA predicted a significantly shorter overall survival for patients with breast cancer. Thus, the MT1-MMP mRNA and protein expression has been shown to play an important role in prognosis of breast cancer patients.
In conclusions, Our study indicated that the mRNA and protein level of MT1-MMP is significantly increased in breast cancer and correlated with clinical stage, lymph node metastasis, and distant metastasis of breast cancer patients. Moreover, our results suggested that MT1-MMP was a significant prognostic factor for breast cancer.
Acknowledgements
This study was supported by grants from the Technology Development Foundation of Pudong District (PKJ2013-Y67) and the Experimental Animal Special Purpose Foundation of Science and Technology Commission of Shanghai Municipality (13140902901) and the National Natural Science Foundation of China (No. 81072179 and No. 31100774).
Disclosure of conflict of interest
None.
References
- 1.Bombonati A, Sgroi DC. The molecular pathology of breast cancer progression. J Pathol. 2011;223:307–317. doi: 10.1002/path.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 4.Murphy G, Nagase H. Progress in matrix metalloproteinase research. Mol Aspects Med. 2008;29:290–308. doi: 10.1016/j.mam.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadler-Olsen E, Winberg JO, Uhlin-Hansen L. Matrix metalloproteinases in cancer: their value as diagnostic and prognostic markers and therapeutic targets. Tumour Biol. 2013;34:2041–2051. doi: 10.1007/s13277-013-0842-8. [DOI] [PubMed] [Google Scholar]
- 6.Shuman Moss LA, Jensen-Taubman S, Stetler-Stevenson WG. Matrix metalloproteinases: changing roles in tumor progression and metastasis. Am J Pathol. 2012;181:1895–1899. doi: 10.1016/j.ajpath.2012.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pahwa S, Stawikowski MJ, Fields GB. Monitoring and Inhibiting MT1-MMP during Cancer Initiation and Progression. Cancers (Basel) 2014;6:416–435. doi: 10.3390/cancers6010416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol. 2006;206:1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 9.Strongin AY. Proteolytic and non-proteolytic roles of membrane type-1 matrix metalloproteinase in malignancy. Biochim Biophys Acta. 2010;1803:133–141. doi: 10.1016/j.bbamcr.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang YZ, Wu KP, Wu AB, Yang ZC, Li JM, Mo YL, Xu M, Wu B, Yang ZX. MMP-14 overexpression correlates with poor prognosis in non-small cell lung cancer. Tumour Biol. 2014;35:9815–21. doi: 10.1007/s13277-014-2237-x. [DOI] [PubMed] [Google Scholar]
- 11.Ulasov I, Yi R, Guo D, Sarvaiya P, Cobbs C. The emerging role of MMP14 in brain tumorigenesis and future therapeutics. Biochim Biophys Acta. 2014;1846:113–120. doi: 10.1016/j.bbcan.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Poincloux R, Lizarraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci. 2009;122:3015–3024. doi: 10.1242/jcs.034561. [DOI] [PubMed] [Google Scholar]
- 13.Sato H, Takino T, Miyamori H. Roles of membrane-type matrix metalloproteinase-1 in tumor invasion and metastasis. Cancer Sci. 2005;96:212–217. doi: 10.1111/j.1349-7006.2005.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takino T, Guo L, Domoto T, Sato H. MT1-MMP prevents growth inhibition by three dimensional fibronectin matrix. Biochem Biophys Res Commun. 2013;436:503–508. doi: 10.1016/j.bbrc.2013.05.134. [DOI] [PubMed] [Google Scholar]
- 15.Deryugina EI, Ratnikov BI, Postnova TI, Rozanov DV, Strongin AY. Processing of integrin alpha(v) subunit by membrane type 1 matrix metalloproteinase stimulates migration of breast carcinoma cells on vitronectin and enhances tyrosine phosphorylation of focal adhesion kinase. J Biol Chem. 2002;277:9749–9756. doi: 10.1074/jbc.M110269200. [DOI] [PubMed] [Google Scholar]
- 16.Kajita M, Itoh Y, Chiba T, Mori H, Okada A, Kinoh H, Seiki M. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol. 2001;153:893–904. doi: 10.1083/jcb.153.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endo K, Takino T, Miyamori H, Kinsen H, Yoshizaki T, Furukawa M, Sato H. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J Biol Chem. 2003;278:40764–40770. doi: 10.1074/jbc.M306736200. [DOI] [PubMed] [Google Scholar]
- 18.Wali N, Hosokawa K, Malik S, Saito H, Miyaguchi K, Imajoh-Ohmi S, Miki Y, Nakanishi A. Centrosomal BRCA2 is a target protein of membrane type-1 matrix metalloproteinase (MT1-MMP) Biochem Biophys Res Commun. 2014;443:1148–1154. doi: 10.1016/j.bbrc.2013.12.103. [DOI] [PubMed] [Google Scholar]
- 19.Zhou H, Wu A, Fu W, Lv Z, Zhang Z. Significance of semaphorin-3A and MMP-14 protein expression in non-small cell lung cancer. Oncol Lett. 2014;7:1395–1400. doi: 10.3892/ol.2014.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okada A, Bellocq JP, Rouyer N, Chenard MP, Rio MC, Chambon P, Basset P. Membrane-type matrix metalloproteinase (MT-MMP) gene is expressed in stromal cells of human colon, breast, and head and neck carcinomas. Proc Natl Acad Sci U S A. 1995;92:2730–2734. doi: 10.1073/pnas.92.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Zhang X, Huang L, Li J, Qu S, Pan F. Matrix Metalloproteinase-14 Expression and Its Prognostic Value in Cervical Carcinoma. Cell Biochem Biophys. 2014;70:729–34. doi: 10.1007/s12013-014-9974-8. [DOI] [PubMed] [Google Scholar]
- 22.Udayakumar TS, Chen ML, Bair EL, Von Bredow DC, Cress AE, Nagle RB, Bowden GT. Membrane type-1-matrix metalloproteinase expressed by prostate carcinoma cells cleaves human laminin-5 beta3 chain and induces cell migration. Cancer Res. 2003;63:2292–2299. [PubMed] [Google Scholar]
- 23.Munaut C, Noel A, Hougrand O, Foidart JM, Boniver J, Deprez M. Vascular endothelial growth factor expression correlates with matrix metalloproteinases MT1-MMP, MMP-2 and MMP-9 in human glioblastomas. Int J Cancer. 2003;106:848–855. doi: 10.1002/ijc.11313. [DOI] [PubMed] [Google Scholar]
- 24.Kettunen E, Anttila S, Seppanen JK, Karjalainen A, Edgren H, Lindstrom I, Salovaara R, Nissen AM, Salo J, Mattson K, Hollmen J, Knuutila S, Wikman H. Differentially expressed genes in nonsmall cell lung cancer: expression profiling of cancer-related genes in squamous cell lung cancer. Cancer Genet Cytogenet. 2004;149:98–106. doi: 10.1016/S0165-4608(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 25.Crispi S, Calogero RA, Santini M, Mellone P, Vincenzi B, Citro G, Vicidomini G, Fasano S, Meccariello R, Cobellis G, Menegozzo S, Pierantoni R, Facciolo F, Baldi A, Menegozzo M. Global gene expression profiling of human pleural mesotheliomas: identification of matrix metalloproteinase 14 (MMP-14) as potential tumour target. PLoS One. 2009;4:e7016. doi: 10.1371/journal.pone.0007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poola I, DeWitty RL, Marshalleck JJ, Bhatnagar R, Abraham J, Leffall LD. Identification of MMP-1 as a putative breast cancer predictive marker by global gene expression analysis. Nat Med. 2005;11:481–483. doi: 10.1038/nm1243. [DOI] [PubMed] [Google Scholar]
- 27.Tetu B, Brisson J, Wang CS, Lapointe H, Beaudry G, Blanchette C, Trudel D. The influence of MMP-14, TIMP-2 and MMP-2 expression on breast cancer prognosis. Breast Cancer Res. 2006;8:R28. doi: 10.1186/bcr1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGowan PM, Duffy MJ. Matrix metalloproteinase expression and outcome in patients with breast cancer: analysis of a published database. Ann Oncol. 2008;19:1566–1572. doi: 10.1093/annonc/mdn180. [DOI] [PubMed] [Google Scholar]
- 29.Zarrabi K, Dufour A, Li J, Kuscu C, Pulkoski-Gross A, Zhi J, Hu Y, Sampson NS, Zucker S, Cao J. Inhibition of matrix metalloproteinase 14 (MMP-14)-mediated cancer cell migration. J Biol Chem. 2011;286:33167–33177. doi: 10.1074/jbc.M111.256644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu AW, Xu GW, Wang HY, Ji JF, Tang JL. WITHDRAWN: Neoadjuvant chemotherapy versus none for resectable gastric cancer. Cochrane Database Syst Rev. 2007:CD005047. doi: 10.1002/14651858.CD005047.pub2. [DOI] [PubMed] [Google Scholar]
- 31.He L, Chu D, Li X, Zheng J, Liu S, Li J, Zhao Q, Ji G. Matrix metalloproteinase-14 is a negative prognostic marker for patients with gastric cancer. Dig Dis Sci. 2013;58:1264–1270. doi: 10.1007/s10620-012-2513-9. [DOI] [PubMed] [Google Scholar]
- 32.Peng CW, Wang LW, Fang M, Yang GF, Li Y, Pang DW. Combined features based on MT1-MMP expression, CD11b + immunocytes density and LNR predict clinical outcomes of gastric cancer. J Transl Med. 2013;11:153. doi: 10.1186/1479-5876-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]


