Abstract
In this work, we investigated a cytocompatible particulate leaching method for the fabrication of cell-laden macroporous hydrogels. We used dehydrated and uncrosslinked gelatin microspheres as leachable porogens to create macroporous oligo(poly(ethylene glycol) fumarate) hydrogels. Varying gelatin content and size resulted in a wide range of porosities and pore sizes, respectively. Encapsulated mesenchymal stem cells (MSCs) exhibited high viability immediately following the fabrication process, and culture of cell-laden hydrogels revealed improved cell viability with increasing porosity. Additionally, the osteogenic potential of the encapsulated MSCs was evaluated over 16 days. Overall, this study presents a robust method for the preparation of cell-laden macroporous hydrogels with desired porosity and pore size for tissue engineering applications.
Introduction
Hydrogels are a class of biomaterials formed by crosslinked hydrophilic polymers swollen in aqueous solutions. The high water content of hydrogels makes them attractive for a number of biomedical applications, particularly tissue engineering, due to their similarities with extracellular matrix and the ability to deliver a variety of cell types and bioactive molecules to promote the growth of neo-tissue within a three-dimensional (3D) architecture.1–3 The permeability of hydrogels also allows for the diffusion of nutrients from surrounding medium while simultaneously providing an outlet for waste products or biomolecules secreted by cells within the hydrogel. As a result, the porosity of hydrogels is an important parameter to consider in directing tissue formation and function for tissue engineering applications, particularly in the absence of a vascular system.4,5
In particular, the average pore size of a hydrogel can greatly affect cell infiltration, proliferation, and migration within the scaffold and different optimum pore sizes have been demonstrated for different tissue repair applications.4,6,7 A number of fabrication techniques have been developed to generate pores within hydrogels for tissue engineering applications, including electrospinning,8,9 gas foaming,10–13 freeze–thaw,14,15 phase separation,16–19 and salt leaching.20–22 However, the majority of these techniques for generating macroporous hydrogels often involve cytotoxic procedures or chemicals that undermine a key advantage hydrogels have over other traditional tissue engineering scaffolds: the ability to encapsulate viable cells with a homogeneous distribution within the 3D scaffold during fabrication.4,23 Subsequent cell seeding in such scaffolds may lead to low seeding efficiency and a heterogeneous cell distribution, particularly without proper pore interconnectivity.24 Therefore, the development of a cytocompatible process to simultaneously fabricate macroporous hydrogels and encapsulate cells with a homogeneous distribution could provide tremendous advantages for tissue engineering applications.
The present study examines the use of gelatin as a leachable porogen to fabricate macroporous oligo(poly(ethylene glycol) fumarate) (OPF)-based hydrogels. OPF is a macromer with alternating poly(ethylene glycol) (PEG) chains and fumarate groups which allow for crosslinking and degradation through hydrolysis.25–27 Gelatin is a natural material derived from denatured type I collagen and can be dissolved in water at temperatures above the physiological temperature (37°C), while forming a gel at 23°C. Thus, uncrosslinked gelatin microspheres are incorporated within a scaffold at room temperature to maintain their spherical shape, and the gelatin can be leached out in an aqueous solution at 37°C to generate a macroporous structure.28 OPF hydrogels have been developed in our laboratory for the controlled delivery of growth factors and encapsulated cells for a variety of tissue engineering applications.29–36 By adding mesenchymal stem cells (MSCs) to the hydrogel precursor solution before gelation, a homogeneous distribution of cells can be created with minimal cytotoxicity.33,34,36 However, OPF hydrogels have previously been fabricated using thermal radical initiators at 37°C to crosslink the polymer network. To apply uncrosslinked gelatin microspheres as porogens to create macroporous cell-laden OPF-based hydrogels, it would be necessary to fabricate the constructs at room temperature while maintaining the viability of encapsulated cells before leaching the gelatin porogen.
Therefore, we hypothesize that cell-laden macroporous OPF hydrogels can be fabricated through a two-step process: the crosslinking of OPF in the presence of uncrosslinked gelatin microspheres and MSCs at 23°C, followed by leaching of the gelatin at 37°C in an aqueous solution. We also demonstrate the ability to tune the average pore size of the macroporous hydrogels through the use of dehydrated gelatin microspheres as porogens with a wide range of diameters. Accordingly, the specific objectives are (1) to investigate the tunability of pore size and porosity of OPF hydrogels by varying the size and content of gelatin microspheres, (2) to create a homogeneous distribution of cells encapsulated within macroporous OPF hydrogels with high viability, and (3) to examine the effect of hydrogel porosity on the viability as well as the osteogenic differentiation of rat MSCs encapsulated within OPF hydrogels over 16 days.
Materials and Methods
Macroporous hydrogel fabrication
Gelatin microspheres were fabricated using basic gelatin with an isoelectric point of 9.0 and an oil-in-water emulsification process according to established methods.33 Briefly, 45 mL of a 10 wt% gelatin solution in deionized water at 60°C was added in a drop-wise manner to 250 mL olive oil on ice while stirring at 500 rpm for 30 min. Following the emulsion step, 100 mL of chilled acetone (4°C) were added. After 60 min, the resulting microspheres were collected by vacuum filtration, washed with acetone, dried in a fume hood overnight, and finally sieved to different size ranges.
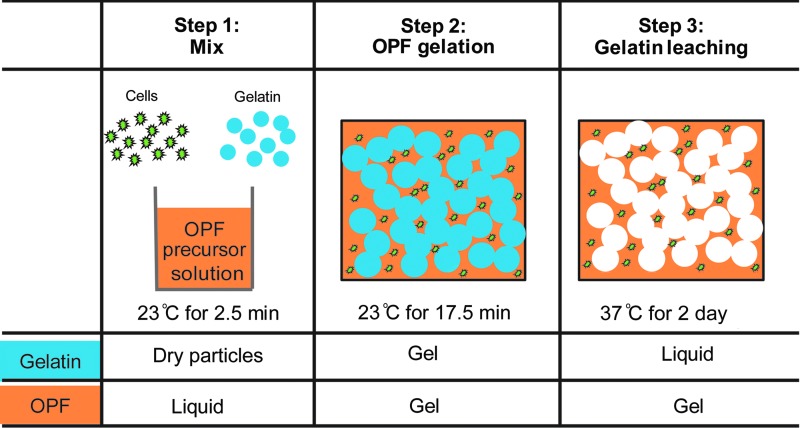
OPF macromers were synthesized as previously described,32 and PEG (Sigma, St. Louis, MO) with a nominal molecular weight of 3000 g/mol was used for the synthesis. Macroporous OPF hydrogels were then fabricated as schematically illustrated in Figure 1. Specifically, to prepare the OPF hydrogel precursor solution, 0.16 g of OPF and 0.08 g of PEG-diacrylate (PEG-DA, 3400 g/mol; Glycosan BioSystems, Alameda, CA) were dissolved in 380 μL of phosphate buffered saline (PBS) and incubated at 23°C for 45 min to eliminate air bubbles. 74.9 μL of 300 mM N,N,N′,N′-tetramethylethylenediamine (TEMED) solution (Sigma) and 74.9 μL of 300 mM ammonium persulfate (APS) solution (Sigma) were then added, and this solution was mixed using a spatula for 1.5 min. Subsequently, 100 μL of PBS or cell suspension in culture medium were added to the hydrogel precursor solution and mixed for 0.5 min. Following the addition of PBS or cell suspension, dry gelatin microspheres were added and mixed for 0.5 min. Finally, the mixture was injected using a positive displacement pipette into Teflon molds with a diameter of 5.1 mm and a depth of 2.0 mm, and was incubated for gelation at 23°C for 17.5 min.
FIG. 1.
A schematic outlining of the three steps involved in fabricating cell-laden macroporous OPF hydrogels. The hydrogel precursor solution was first mixed with cells and dehydrated gelatin microspheres at 23°C. The mixture was then incubated at 23°C to allow for OPF gelation and the gel was finally incubated at 37°C for gelatin leaching and hydrogel swelling. OPF, oligo(poly(ethylene glycol) fumarate).
Four different porogen contents and three porogen size ranges were examined for the characterization of acellular macroporous hydrogel physical properties, including pore morphology, porosity, and water content. The four porogen contents were 11.25 g gelatin/mL gel×100 (m/v), 22.50 m/v, 33.75 m/v, and 45.00 m/v and the three porogen sizes were 0–100, 100–300, and 300–500 μm, resulting in 12 porogen-containing formulations. One group without porogen, 0.00 m/v, was included as a control group. After OPF gelation, all samples in each group were incubated in 50 mL of PBS at 37°C for 2 days on an orbital shaker at 90 rpm to allow the uncrosslinked gelatin to dissolve and diffuse out of the formed macroporous hydrogels. PBS was changed every 12 h.
Gelatin staining by Coomassie Blue reagent
To monitor gelatin leaching, a group containing 30.00 m/v gelatin of porogen size 0–100 μm was stained by an EZBlue Coomassie Blue reagent (Sigma), which stains protein materials blue. Samples from before gelatin leaching and immediately after gelatin leaching were each fixed in 10 mL of 10% phosphate buffered formalin at 4°C overnight. Following fixation, the formalin solution was replaced with 10 mL of the staining reagent at 4°C overnight, and finally washed in PBS three times for light microscopy.
Scanning electron microscopy
Scanning electron microscopy (SEM) was used to observe hydrogel structure in a dehydrated state. Samples were first immersed in liquid nitrogen for 5 min and then lyophilized for 2 days. The lyophilized samples were cut along a radial direction with a sharp surgical blade and were sputter coated with gold. SEM observation was achieved using a Quanta 400 FEG microscope (FEI, Hillsboro, OR).
Confocal microscopy and porosity measurement
Confocal microscopy was used to visualize hydrogel morphology in a hydrated state. Hydrated samples (n=3) were immersed in PBS with 2 mg/mL tetramethylrhodamine-conjugated dextran (2000 kDa; Invitrogen, Grand Island, NY) for 1 day at room temperature on an orbital shaker. The hydrated samples were cut along a radial direction with a surgical blade and the cross-sectional area was then imaged with wavelengths of 555/580 nm (Ex/Em). For each sample, images were generated every 5 μm for 100 μm by optical slicing along the z-direction from the cross-sectional area. The image processing software, ImageJ (http://rsb.info.nih.gov/ij/), was used to reconstruct the images and determine porosity. The 3D projection of the serial images was reconstructed using a projection method of brightest point provided by the software. For porosity determination, two-dimensional image slices were thresholded using an Otus algorithm.37 The areas of the porous phase and the whole image were then measured using ImageJ. Porosity was determined from the ratio of the porous phase area to the total image area.
Equilibrium water content
After the 2-day leaching period, excess water was blotted with weighing paper from the hydrogel surface and the weight of swollen hydrogels (n=3) was measured. Hydrogel samples were then lyophilized for 2 days and the weights of the dehydrated samples were recorded. The equilibrium water content (EWC) was calculated as
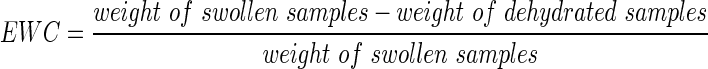 |
Differential scanning calorimetry
Differential scanning calorimetry (DSC) was used to examine the phase transition of water absorbed by hydrogels (TA Instruments 2920, New Castle, DE). The water in the hydrogel can be classified as frozen water or nonfrozen water. Frozen water has a phase transition temperature close to the freezing point of pure water, whereas nonfrozen water has no detectable phase transition due to its strong bonding to polymers. Hydrogel samples (n=3) were first frozen and then heated at a specific rate of 1°C/min. During the process, enthalpy was recorded. Specifically, the hydrogel was blotted with weighing paper, a small piece of ∼10 mg was cut off, and the actual weight of the sample was measured. Samples were then hermetically sealed in aluminum pans for DSC measurement. Temperature was decreased from room temperature to −45°C and was maintained for 5 min. Temperature was then ramped to 23°C at a heating rate of 1°C/min. Frozen water content (Qf) was determined by the ratio of the melting enthalpy of the sample to the melting enthalpy of water. Nonfrozen water content (Qnf) was determined by the difference of EWC and Qf.
Osteogenic differentiation of rat MSCs in hydrogels
Rat bone marrow was pooled from both the tibiae and femora of four male Fischer 344 rats (150–175 g) as previously described.38 Rat MSCs were cultured in complete osteogenic medium containing Minimum Essential Medium Alpha Medium (α-MEM; Invitrogen), 10% fetal bovine serum, 10 mM β-glycerol-2-phosphate (Sigma), 10 nM dexamethasone (Sigma), 50 μg/mL ascorbic acid (Sigma), 50 μg/mL gentamicin, 100 μg/mL ampicillin, and 0.5 μg/mL fungizone for 7 days before cell encapsulation. The medium was changed every 2 days. Three cell-laden hydrogel formulations, including 0.00, 15.00, and 30.00 m/v gelatin with a diameter range from 50–100 μm were prepared. Rat MSCs were encapsulated at a density of 4 million cells per mL of hydrogel precursor solution, and cultured for 16 days in complete osteogenic medium. Blank hydrogels without cells were also cultured as a control group.
Cell viability
At days 0 and 16, cell viability was visualized using a Live/Dead Viability/Cytotoxicity Kit (Invitrogen). Samples were first washed in PBS three times at 10 min intervals, and then incubated for 30 min at 37°C with 4 μM calcein-AM and 4 μM ethidium homodimer in PBS. Viable cells fluoresce green through the interaction of calcein-AM with intracellular esterases, whereas ethidium homodimer enters cells with damaged membranes and fluoresces red upon binding to nucleic acids. After staining, the samples were washed three times in PBS at 10 min intervals before confocal microscopy.
Biochemical analysis
At days 0, 8, and 16, samples were snap frozen in liquid nitrogen and stored at −80°C before biochemical analyses, including DNA, alkaline phosphatase (ALP) activity, and calcium assays (n=4). All frozen samples were pulverized in a BioPulverizer (Bartlesville, OK) and passed through a 27-gauge needle with 1 mL of ultrapure water. Samples were further processed with three freeze–thaw cycles and an 8-second sonication period using a probe sonicator. Following sonication, samples were centrifuged at 10,000 g for 3 min to obtain the supernatant for DNA and ALP activity assays. DNA content was determined using a PicoGreen kit (Invitrogen) following the manufacturer's instructions. ALP activity was measured by a colorimetric assay. A range of p-nitrophenol solutions (Sigma) from 0 to 480 μM was prepared as standards. Briefly, 30 μL of samples or standards were mixed with 20 μL of 2.67 mg/mL phosphate substrate (Sigma) and 150 μL of 1.5 M alkaline buffer (Sigma) in 96-well plates. The mixture was then incubated at 37°C for 1 h. The reaction was stopped by adding 50 μL of 1 M NaOH and absorbance was read at 405 nm. Following ALP and DNA assays, samples were digested in a volume of 1 M acetic acid equal to the remaining volume overnight at 23°C for calcium assays. In brief, 300 μL of calcium Arsenazo III stable liquid reagent (Sekisui Diagnostics, Framingham, MA) was mixed with 20 μL of samples or standards in 96-well plates. CaCl2 solutions from 0–50 μg/mL were prepared as standards. Absorbance was read at 650 nm.
Statistical analysis
All data were analyzed by two-way analysis of variance with interaction. Tukey's Honestly Significant Difference (HSD) post hoc tests were used to determine statistical differences between groups. Statistical tests were conducted using the statistical software of SPSS 12.0.1. A statistical threshold of p<0.05 was used to evaluate whether statistical differences were significant.
Results
Gelatin dissolution and diffusion
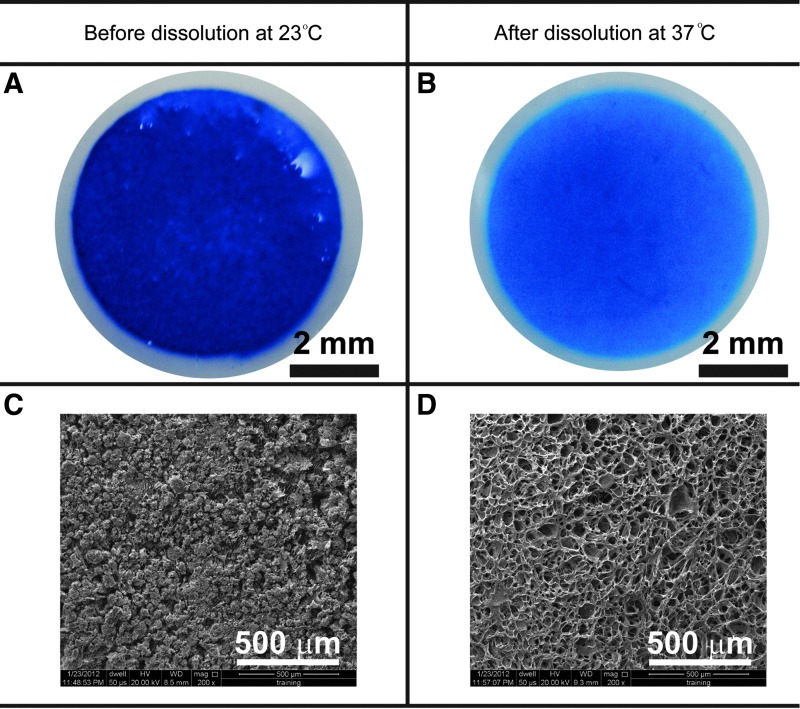
After OPF gelation at 23°C in Step 2 (Fig. 1), the hydrogel containing 30.00 m/v gelatin appeared intensely blue as revealed by Coomassie Blue protein staining (Fig. 2A), indicating the presence of gelatin in the hydrogel. The SEM images of hydrogel cross-sections further revealed that hydrogels were densely packed with gelatin microspheres (Fig. 2C). After gelatin leaching at 37°C for 2 days in Step 3 (Fig. 1), the hydrogel presented a noticeably weaker blue staining than that observed before leaching (Fig. 2B). A highly macroporous structure was observed without visible gelatin microspheres in SEM images obtained after gelatin dissolution (Fig. 2D).
FIG. 2.
Gelatin microspheres (<100 μm in diameter) dispersed in the hydrogel were stained blue (A) and were visible in SEM images (C) before dissolution. After a 2-day incubation at 37°C, most of the gelatin microspheres dissolved and diffused out from the hydrogel (B), resulting in a porous structure (D). SEM, scanning electron microscopy.
Hydrogel structure by SEM and confocal microscopy
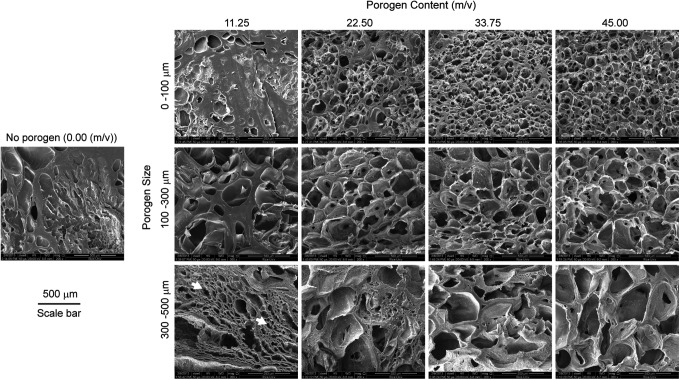
SEM was used to visualize the hydrogel microstructure in a dehydrated state (Fig. 3). Five porogen contents were varied from 0.00 to 45.00 m/v, and three porogen sizes were chosen from 0–100 to 300–500 μm. With the increase of porogen content for each porogen size range, the hydrogel morphology progressed gradually from a mostly solid to a highly porous interior (Fig. 3). The increase of porogen size led to larger pore sizes after gelatin leaching. For instance, the 45.00 m/v group using the porogen size range of 300–500 μm appeared to have remarkably larger pores than the group using a porogen size range of 0–100 μm with the same porogen content.
FIG. 3.
SEM images of OPF hydrogels after leaching of a variety of gelatin microsphere sizes and contents. The increase in porogen content and porogen size results in an increase in porosity and pore size, respectively. White arrows indicate the artificial pores produced by the sample preparation procedure for SEM. Scale bar is 500 μm.
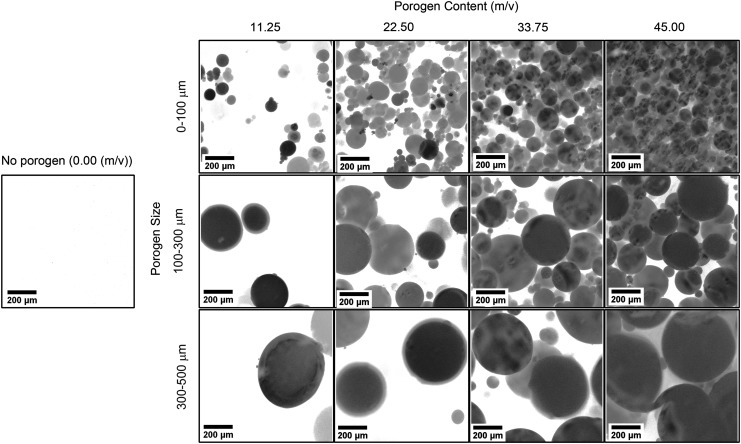
In contrast to SEM images for dehydrated samples, confocal microscopy was used to examine pore morphology in a hydrated state (Fig. 4). Overall, confocal images presented a similar representation of pore area and pore size to the ones obtained by SEM. Pore area appeared to increase with increasing porogen content, whereas pore size tended to increase with increasing porogen size. However, the confocal images of hydrated samples revealed a different hydrogel morphology than the SEM images of dehydrated samples. The dehydration process often produced small artificial pores that were obvious in the 11.25 m/v group with the 300–500 μm pore size (Fig. 3). In addition, hydrated samples had spherical pores in comparison to irregularly shaped pores in the dehydrated samples.
FIG. 4.
Confocal microscopy images of OPF hydrogels with three different porogen sizes and four levels of porogen content. High porogen content and large porogen size led to a high porosity and large pore size, respectively. Scale bar is 200 μm.
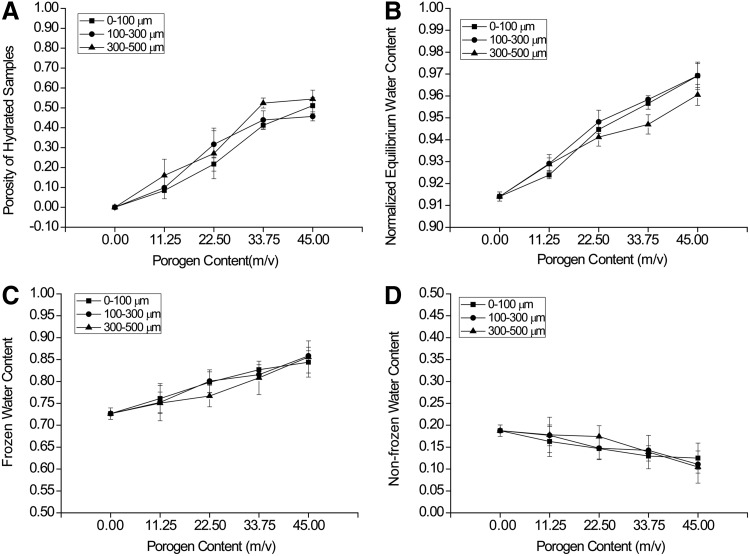
Porosity quantification
Porosity of hydrated samples was determined through image analysis of a series of confocal images (Fig. 5A). It was seen that porogen content had significant effects on porosity. Specifically, porosity increased with increasing porogen content from 0.00 to 33.75 m/v gelatin and was maintained with 33.75 to 45.00 m/v gelatin incorporation, regardless of porogen size. The maximum porosity was∼50% when either 33.75 or 45.00 m/v porogen content was added. Increasing porogen content to more than 45.00 m/v failed to form mechanically stable hydrogels that could be manipulated with a spatula. Porogen size had minimal effects on porosity. There was no difference in porosity between the size ranges of 0–100 and 100–300 μm, whereas the groups with a size range of 300–500 μm had a higher porosity than that of the 0–100 μm groups (p=0.0047), regardless of porogen content. There was no statistical interaction between the two factors of porogen content and porogen size (p=0.0535).
FIG. 5.
Porosity of hydrated samples as measured by confocal microscopy (A), equilibrium water content (EWC) of the macroporous OPF hydrogels as measured by the percentage of water within a swollen macroporous hydrogel (B), and frozen (C) and nonfrozen water (D) content in the macroporous hydrogels as measured by differential scanning calorimetry. Increasing porogen content had a significant increase on the porosity, EWC, and frozen water content and a decrease in the nonfrozen water content of macroporous OPF hydrogels. Porogen size had no effect on both frozen and nonfrozen water content within the macroporous hydrogels.
Equilibrium water content
Both porogen content and size range affected EWC (Fig. 5B). Higher porogen content resulted in a greater EWC in the hydrogels (45.00 m/v>33.75 m/v>22.50 m/v>11.25 m/v>0.00 m/v), regardless of porogen size. The groups containing the 300–500 μm gelatin microspheres had a lower EWC than the other two groups, whereas no difference was observed between the 0–100 and 100–300 μm groups. Moreover, at each level of porogen content, there was no difference in EWC between any two porogen size ranges, except at the level of 33.75 m/v. Specifically, the group containing the 300–500 μm gelatin microspheres had less water than the other two groups (p=0.0049).
Frozen water and nonfrozen water content
Varying porogen content had a significant impact on both frozen and nonfrozen water contents (Fig. 5C, D). A high porogen content resulted in increased frozen water content (Fig. 5C) and reduced nonfrozen water content (Fig. 5D), regardless of porogen size. Porogen size did not affect either the frozen or nonfrozen water contents, regardless of porogen content.
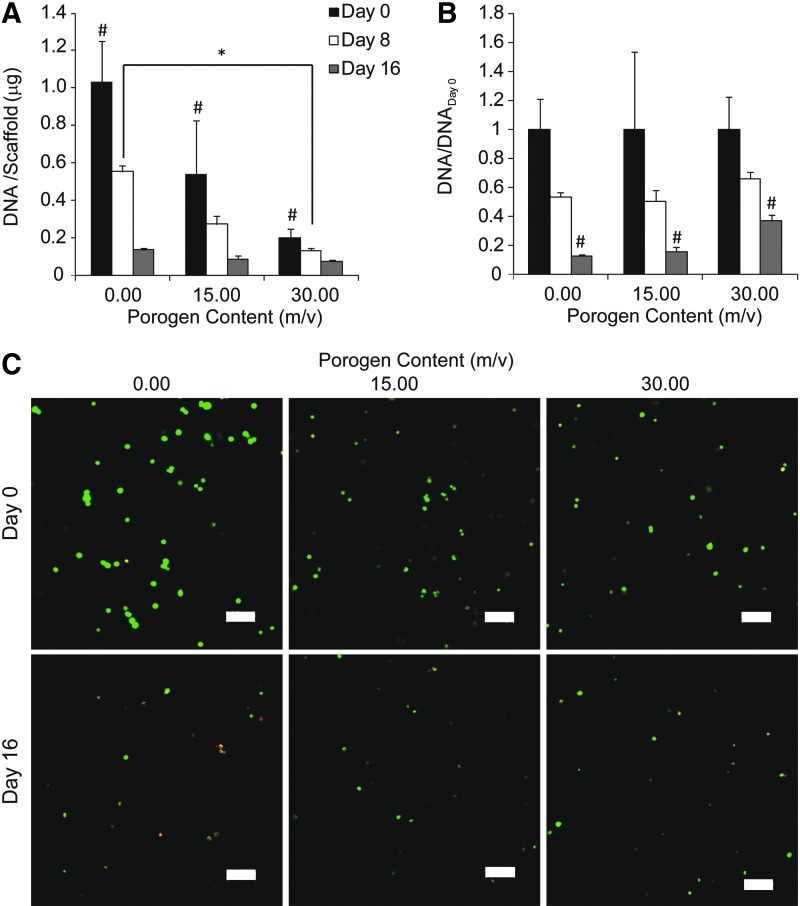
DNA content and cell viability during osteogenic differentiation
Overall, DNA content per scaffold decreased over time during the 16-day culture period (Fig. 6A). After cell encapsulation at day 0, the groups containing 0.00, 15.00, and 30.00 m/v gelatin showed the highest to lowest DNA content per scaffold, respectively, with significant differences among the groups (Fig. 6A). At day 8, the group without gelatin had higher DNA content per scaffold than the 30.00 m/v gelatin group, whereas having no difference from the 15.00 m/v gelatin group. At day 16, all the groups showed similar DNA content. DNA content was further normalized to the DNA content at day 0 within each group to account for the difference in total cells encapsulated between groups with differing porogen content (Fig. 6B). There was no difference in normalized DNA content between groups at both days 0 and 8. However, at day 16, the 30.00 m/v gelatin group had significantly more normalized DNA content than the groups without gelatin and with 15.00 m/v gelatin (37.4%±4.2% vs. 13.0%±1.2% and 16.0%±3.1%). The decreasing trend of DNA content with culture time was further confirmed by cell viability staining (Fig. 6C). Almost all of the stained cells were green at day 0 in all groups, indicating high cell viability during fabrication. At day 16, there appeared to be less cells stained green in all the groups relative to day 0, and more cells stained red were observed in the group without gelatin than the 15.00 and 30.00 m/v gelatin groups.
FIG. 6.
DNA content in all the groups decreased during the 16-day period (A), while the 30.00 m/v gelatin group had the highest ratio of DNA/DNAday 0 at day 16 compared to the other two groups (B). Confocal imaging shows live and dead cells, which fluoresce green and red, respectively (C). The vast majority of cells were alive at day 0 in all the groups and all the groups appeared to have less cells stained green at day 16 relative to day 0. Scale bar is 100 μm. * indicates a statistical difference between groups (p<0.05). # indicates a statistical difference from the other two groups at a specific time point (p<0.05).
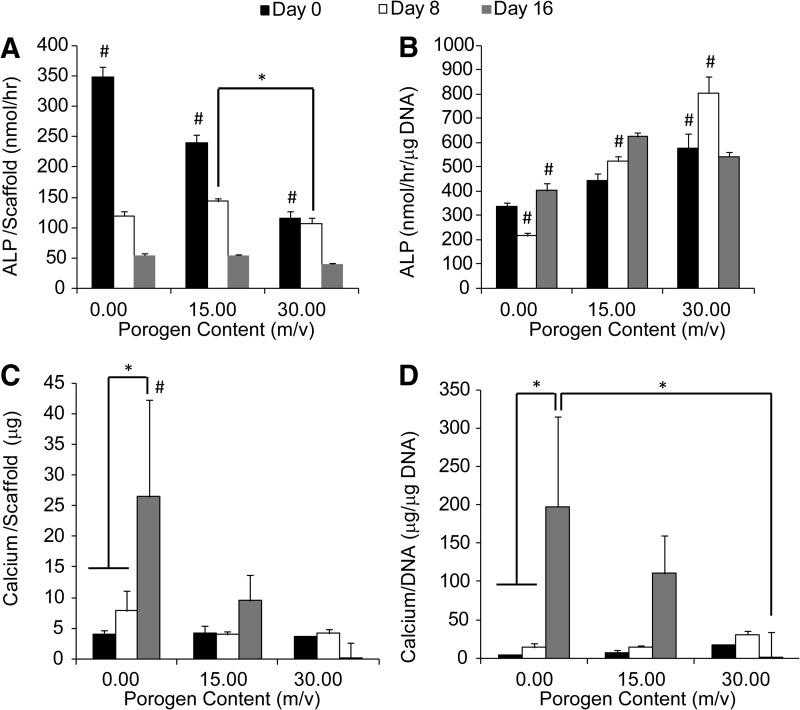
ALP activity and calcium content during osteogenic differentiation
ALP activity per scaffold generally decreased over time (Fig. 7A), whereas ALP activity per unit DNA (Fig. 7B) increased during the 16-day culture period. Specifically, at day 0, ALP activity per scaffold decreased with the increasing gelatin content (0.00 m/v>15.00 m/v>30.00 m/v). At days 8, the 15.00 m/v gelatin group had higher ALP activity per scaffold than the 30.00 m/v gelatin group. At day 16, there was no difference in ALP activity per scaffold between groups. In contrast to ALP activity per scaffold, ALP activity per unit DNA was higher in the 30.00 m/v gelatin group than the other two groups at day 0. At day 8, the groups containing more gelatin microspheres showed higher ALP activity per unit DNA than the ones with less gelatin microspheres (30.00 m/v>15.00 m/v>0.00 m/v). At day 16, both the 15.00 and 30.00 m/v gelatin groups showed higher ALP activity per unit DNA than the pure OPF group, whereas no difference was observed between the 15.00 and 30.00 m/v groups.
FIG. 7.
In all the groups, ALP activity per scaffold (A) decreased over time, while the ALP activity per unit DNA (B) increased during the 16-day period for groups with 0.00 and 15.00 m/v porogen content. For the 30.00 m/v group, ALP activity peaked at day 8. The pure OPF group had both higher calcium content per scaffold and per unit DNA than the 30.00 m/v gelatin group at day 16 (C, D). * indicates a statistical difference between groups (p<0.05). # indicates a statistical difference from the other two groups at a specific time point (p<0.05). ALP, alkaline phosphatase.
Regarding calcium deposition in the hydrogels, the group without gelatin had more calcium content than the other two groups containing gelatin, whereas there was no difference in calcium content between the 15.00 and 30.00 m/v gelatin groups, regardless of the time point. Specifically, the group without gelatin had higher calcium content per scaffold than both the groups containing gelatin at day 16 (Fig. 7C). Calcium content per scaffold increased from days 0 to 16 within the group without gelatin (Fig. 7C). Calcium content per unit DNA showed a similar trend to calcium per scaffold, except that there was no difference between the 0.00 and 15.00 m/v gelatin groups at day 16 (Fig. 7D).
Discussion
This study demonstrated the ability to simultaneously form macroporous OPF hydrogels while encapsulating cells with high viability and homogeneous distribution. Specifically, uncrosslinked gelatin microspheres of varying sizes were incorporated into the OPF hydrogel precursor solution at varying concentrations to elucidate the individual as well as cross effects of pore size and porogen content on the final hydrogel macroporosity. Subsequently, macroporous hydrogels of increasing porogen content encapsulating rat MSCs were fabricated to evaluate the effects of macroporosity on the cellularity and the osteogenic differentiation of the cells over 16 days in vitro.
Since pore size has a big effect on tissue regeneration for different tissue types,4,39,40 the primary goal of the present study was to demonstrate the ability to tune the porosity and pore size of the macroporous OPF hydrogels through varying the porogen content and size, respectively, during fabrication. Indeed, we were able to fabricate macroporous OPF hydrogels by leveraging the sol–gel reversibility of uncrosslinked gelatin at physiological temperature (Fig. 2) to have a wide range of pore sizes and porosities (Fig. 3). While previous studies have utilized uncrosslinked gelatin microspheres to create macroporous hydrogels, these hydrogels were fabricated with hydrated microspheres.24,28,41,42 Fully hydrated gelatin microspheres have a wider range of sizes compared to dehydrated microspheres due to equilibrium swelling,24 and can result in a reduction in control over pore size. In contrast, the present study uses dehydrated gelatin microspheres with a set range in porogen diameter to achieve macroporous hydrogels with controlled pore size.
ImageJ analysis of the confocal images showed that when the porogen content was increased from 0.00 to 45.00 m/v gelatin, the porosity of the resultant hydrogels was increased up to a maximum of 50% (when 45.00 m/v gelatin was added). However, increases in porogen content beyond 45.00 m/v gelatin led to the formation of hydrogels that were not mechanically stable enough to be handled with transferring tools (e.g., spatulas). Overall, the introduction of macropores into the OPF hydrogels had a significant impact on hydrogel swelling. This was evidenced by an increase in EWC from 91% to 97% when the porogen content was increased from 0.00 gelatin to 45.00 m/v gelatin, reflecting an overall decrease in polymer content and an increase in water content as shown by DSC analysis. Given the higher amount of total water present in the macropores of more porous hydrogels, it is, therefore, expected that there would be an increase in the frozen water content with increasing porosity. As shown in both SEM and confocal images (Figs. 3 and 4), improved pore interconnectivity was also achieved with increasing porogen content.
Given that traditional approaches for fabricating macroporous hydrogels generally necessitate cell seeding after fabrication, small pore sizes and poor pore interconnectivity can lead to heterogeneous cell distribution with an abundance of cells located at the peripheral areas of the constructs. In contrast, the macroporous hydrogels presented in the current study were fabricated from a cell-laden hydrogel precursor solution, and thus resulted in a homogeneous cell distribution throughout the hydrogel constructs (Fig. 6C). Additionally, the fabrication process presented in the current study had minimal toxicity on the MSCs as evidenced by the cells staining positive for calcein at day 0 for all groups (Fig. 6C). The subsequent diffusion of uncrosslinked gelatin from the hydrogel composites was not expected to negatively affect cell viability nor cell growth. Instead, any residual gelatin in the hydrogel constructs may actually promote cell adhesion and proliferation since gelatin contains peptide sequences amenable to cell attachment.43,44 Moreover, previous studies using similar OPF-based systems for cell encapsulation have also shown low cytotoxicity with the same concentrations of OPF, PEG-DA, APS, and TEMED.29,34,36
Analysis of the DNA content revealed high cellularities for all groups initially, followed by lower cell numbers over time as indicated by the decrease in DNA content. Such DNA decreases in cell-laden hydrogels are consistent with several previous osteogenic studies employing similar OPF hydrogels36 as well as other systems, including PEG-DA45,46 and chitosan.47 These hydrogel constructs share a common characteristic: the absence of cell-attachment sites. Since MSCs are anchorage-dependent progenitor cells, they likely experience cell death due to the lack of extracellular attachment.48 While all groups displayed high cellularities at day 0, the group with 0.00 m/v gelatin had the greatest DNA content/construct. This could be explained by the absence of porogens in the hydrogel precursor, which leads to an overall higher volume of the cell-laden hydrogel phase in the final construct. As a result, hydrogels from the 0.00 m/v gelatin group would contain more cells as seen in Figure 6C. Indeed, it should be noted that increasing porogen content led to a decrease in the volume of the hydrogel phase as indicated by a decrease in nonfrozen water content (Fig. 5D) with increasing porosity. Hence, the DNA at each time point was normalized to the DNA content at day 0 for each group (Fig. 6B). From the normalized data, the 30.00 m/v gelatin group retained more DNA at the end of the culture period relative to its initial DNA amount at day 0 when compared to the other two groups. This suggested that the highly macroporous structure of constructs from the 30.00 m/v gelatin group may prolong cell viability, which is likely due to enhanced nutrient transfer. However, changes in the diffusional properties of OPF hydrogels as influenced by macroporosity warrant further investigation.
The osteogenic differentiation of encapsulated rat MSCs was also evaluated through the analysis of ALP activity and calcium content per construct. ALP activity, an early osteogenic marker, is characterized by an increase followed by a decline in activity during the early stages of osteogenic differentiation.49 When normalized to the DNA content, the higher ALP activity exhibited by the 30.00 m/v gelatin group at days 0 and 8 when compared to the other groups suggests that greater porosity enhances osteogenic differentiation (Fig. 7B). Additionally, the decrease in normalized ALP activity from day 8 to 16 as observed for the 30.00 m/v gelatin group suggests a change in the osteogenesis of the MSCs from an early to a more mature stage.50 However, an analysis of the calcium content, which is an indication of late-stage osteogenic differentiation, showed significantly higher calcium deposition in the 0.00 m/v gelatin group when compared to the 15.00 and 30.00 m/v gelatin groups. Given that both the 0.00 and 15.00 m/v gelatin groups did not display much normalized ALP activity, it is possible that some of the calcium detected may have resulted from the calcification generally associated with cell death.51–53 Indeed, these two groups displayed the lowest DNA content at day 16 when compared to the 30.00 m/v gelatin group. Further study involving longer time points is warranted to investigate mismatch between ALP activity and calcium content in OPF hydrogels.
Conclusion
Dehydrated, uncrosslinked gelatin microspheres were incorporated into OPF hydrogels at room temperature and leached out in an aqueous solution at physiological temperature to fabricate macroporous hydrogels. The porogen size and content were varied to create macroporous hydrogels with tunable pore sizes and porosities. Additionally, a homogeneous distribution of MSCs was encapsulated in the hydrogels during the fabrication process with high initial viability. The group with the greatest porosity, 30.00 m/v gelatin, depicted the highest normalized DNA content at day 16 as well as the highest normalized ALP activity, indicating osteogenic differentiation of the encapsulated cells. Overall, the use of gelatin as a leachable porogen provides an attractive technique for the fabrication of tunable, macroporous cell-laden hydrogels with potential utility in various tissue engineering applications.
Acknowledgment
Research toward the development of biomaterials for bone tissue engineering was supported by the National Institutes of Health (R01 AR048756 and R01 AR057083) and the Armed Forces Institute of Regenerative Medicine (W81XWH-08-2-0032).
Disclosure Statement
No competing financial interests exist.
References
- 1.Drury J.L., and Mooney D.J.Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24,4337, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Lee K.Y., and Mooney D.J.Hydrogels for tissue engineering. Chem Rev 101,1869, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Nicodemus G.D., and Bryant S.J.Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev 14,149, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annabi N., Nichol J.W., Zhong X., Ji C., Koshy S., Khademhosseini A., et al. . Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng Part B Rev 16,371, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peppas N.A., Hilt J.Z., Khademhosseini A., and Langer R.Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv Mater 18,1345, 2006 [Google Scholar]
- 6.Murphy C.M., Haugh M.G., and O'Brien F.J.The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 31,461, 2010 [DOI] [PubMed] [Google Scholar]
- 7.O'Brien F.J., Harley B.A., Yannas I.V., and Gibson L.J.The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 26,433, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Kim T.G., Chung H.J., and Park T.G.Macroporous and nanofibrous hyaluronic acid/collagen hybrid scaffold fabricated by concurrent electrospinning and deposition/leaching of salt particles. Acta Biomater 4,1611, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Li L., and Hsieh Y.L.Ultra-fine polyelectrolyte hydrogel fibres from poly(acrylic acid)/poly(vinyl alcohol). Nanotechnology 16,2852, 2005 [Google Scholar]
- 10.Behravesh E., Jo S., Zygourakis K., and Mikos A.G.Synthesis of in situ cross-linkable macroporous biodegradable poly(propylene fumarate-co-ethylene glycol) hydrogels. Biomacromolecules 3,374, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Behravesh E., and Mikos A.G.Three-dimensional culture of differentiating marrow stromal osteoblasts in biomimetic poly(propylene fumarate-co-ethylene glycol)-based macroporous hydrogels. J Biomed Mater Res A 66A,698, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Henke M., Baumer J., Blunk T., and Tessmar J.Foamed oligo(poly(ethylene glycol)fumarate) hydrogels as versatile prefabricated scaffolds for tissue engineering. J Tissue Eng Regen Med 8,248, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Keskar V., Marion N.W., Mao J.J., and Gemeinhart R.A.In vitro evaluation of macroporous hydrogels to facilitate stem cell infiltration, growth, and mineralization. Tissue Eng Part A 15,1695, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang Y.S., Sangaj N., and Varghese S.Interconnected macroporous poly(ethylene glycol) cryogels as a cell scaffold for cartilage tissue engineering. Tissue Eng Part A 16,3033, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Phadke A., Hwang Y., Kim S.H., Kim S.H., Yamaguchi T., Masuda K., et al. . Effect of scaffold microarchitecture on osteogenic differentiation of human mesenchymal stem cells. Eur Cell Mater 25,114, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoo S.P., Loh Q.L., Yue Z.L., Fu J., Tan T.T.Y., Choong C., et al. . Preparation of a soft and interconnected macroporous hydroxypropyl cellulose methacrylate scaffold for adipose tissue engineering. J Mater Chem B 1,3107, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Kim H.J., Kim U.J., Vunjak-Novakovic G., Min B.H., and Kaplan D.L.Influence of macroporous protein scaffolds on bone tissue engineering from bone marrow stem cells. Biomaterials 26,4442, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Levesque S.G., Lim R.M., and Shoichet M.S.Macroporous interconnected dextran scaffolds of controlled porosity for tissue-engineering applications. Biomaterials 26,7436, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Gerecht S., Townsend S.A., Pressler H., Zhu H., Nijst C.L.E., Bruggeman J.P., et al. . A porous photocurable elastomer for cell encapsulation and culture. Biomaterials 28,4826, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Betz M.W., Yeatts A.B., Richbourg W.J., Caccamese J.F., Coletti D.P., Falco E.E., et al. . Macroporous hydrogels upregulate osteogenic signal expression and promote bone regeneration. Biomacromolecules 11,1160, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Chen C.W., Betz M.W., Fisher J.P., Paek A., and Chen Y.Macroporous hydrogel scaffolds and their characterization by optical coherence tomography. Tissue Eng Part C Methods 17,101, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Chiu Y.C., Larson J.C., Isom A., and Brey E.M.Generation of porous poly(ethylene glycol) hydrogels by salt leaching. Tissue Eng Part C Methods 16,905, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Desai E.S., Tang M.Y., Ross A.E., and Gemeinhart R.A.Critical factors affecting cell encapsulation in superporous hydrogels. Biomed Mater 7,024108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shepard J.A., Virani F.R., Goodman A.G., Gossett T.D., Shin S.J., and Shea L.D.Hydrogel macroporosity and the prolongation of transgene expression and the enhancement of angiogenesis. Biomaterials 33,7412, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinard L.A., Kasper F.K., and Mikos A.G.Synthesis of oligo(poly(ethylene glycol) fumarate). Nat Protoc 7,1219, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam J., Kim K., Lu S., Tabata Y., Scott D.W., Mikos A.G., et al. . A factorial analysis of the combined effects of hydrogel fabrication parameters on the in vitro swelling and degradation of oligo(poly(ethylene glycol) fumarate) hydrogels. J Biomed Mater Res A 102,3477, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin H., Quinten Ruhe P., Mikos A.G., and Jansen J.A.In vivo bone and soft tissue response to injectable, biodegradable oligo(poly(ethylene glycol) fumarate) hydrogels. Biomaterials 24,3201, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Hwang C.M., Sant S., Masaeli M., Kachouie N.N., Zamanian B., Lee S.H., et al. . Fabrication of three-dimensional porous cell-laden hydrogel for tissue engineering. Biofabrication 2,035003, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo X., Park H., Liu G., Liu W., Cao Y., Tabata Y., et al. . In vitro generation of an osteochondral construct using injectable hydrogel composites encapsulating rabbit marrow mesenchymal stem cells. Biomaterials 30,2741, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holland T.A., Bodde E.W., Baggett L.S., Tabata Y., Mikos A.G., and Jansen J.A.Osteochondral repair in the rabbit model utilizing bilayered, degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds. J Biomed Mater Res A 75,156, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Holland T.A., Tabata Y., and Mikos A.G.Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J Control Release 101,111, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Jo S., Shin H., Shung A.K., Fisher J.P., and Mikos A.G.Synthesis and characterization of oligo(poly(ethylene glycol) fumarate) macromer. Macromolecules 34,2839, 2001 [Google Scholar]
- 33.Park H., Temenoff J.S., Holland T.A., Tabata Y., and Mikos A.G.Delivery of TGF-beta1 and chondrocytes via injectable, biodegradable hydrogels for cartilage tissue engineering applications. Biomaterials 26,7095, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Park H., Temenoff J.S., Tabata Y., Caplan A.I., and Mikos A.G.Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials 28,3217, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Temenoff J.S., Park H., Jabbari E., Conway D.E., Sheffield T.L., Ambrose C.G., et al. . Thermally cross-linked oligo(poly(ethylene glycol) fumarate) hydrogels support osteogenic differentiation of encapsulated marrow stromal cells in vitro. Biomacromolecules 5,5, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Temenoff J.S., Park H., Jabbari E., Sheffield T.L., LeBaron R.G., Ambrose C.G., et al. . In vitro osteogenic differentiation of marrow stromal cells encapsulated in biodegradable hydrogels. J Biomed Mater Res A 70,235, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Rajagopalan S., Lu L., Yaszemski M.J., and Robb R.A.Optimal segmentation of microcomputed tomographic images of porous tissue-engineering scaffolds. J Biomed Mater Res A 75,877, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Maniatopoulos C., Sodek J., and Melcher A.H.Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res 254,317, 1988 [DOI] [PubMed] [Google Scholar]
- 39.Bryant S.J., Cuy J.L., Hauch K.D., and Ratner B.D.Photo-patterning of porous hydrogels for tissue engineering. Biomaterials 28,2978, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whang K., Healy K.E., Elenz D.R., Nam E.K., Tsai D.C., Thomas C.H., et al. . Engineering bone regeneration with bioabsorbable scaffolds with novel microarchitecture. Tissue Eng 5,35, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Sun J., Wei D., Zhu Y., Zhong M., Zuo Y., Fan H., et al. . A spatial patternable macroporous hydrogel with cell-affinity domains to enhance cell spreading and differentiation. Biomaterials 35,4759, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Kim J., Yaszemski M.J., and Lu L.Three-dimensional porous biodegradable polymeric scaffolds fabricated with biodegradable hydrogel porogens. Tissue Eng Part C Methods 15,583, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakai S., Hashimoto I., and Kawakami K.Agarose-gelatin conjugate membrane enhances proliferation of adherent cells enclosed in hollow-core microcapsules. J Biomater Sci Polym Ed 19,937, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Schagemann J.C., Mrosek E.H., Landers R., Kurz H., and Erggelet C.Morphology and function of ovine articular cartilage chondrocytes in 3-d hydrogel culture. Cells Tissues Organs 182,89, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Nuttelman C.R., Benoit D.S., Tripodi M.C., and Anseth K.S.The effect of ethylene glycol methacrylate phosphate in PEG hydrogels on mineralization and viability of encapsulated hMSCs. Biomaterials 27,1377, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Nuttelman C.R., Tripodi M.C., and Anseth K.S.In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in PEG hydrogels. J Biomed Mater Res A 68,773, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Wang L., and Stegemann J.P.Thermogelling chitosan and collagen composite hydrogels initiated with beta-glycerophosphate for bone tissue engineering. Biomaterials 31,3976, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilmore A.P.Anoikis. Cell Death Differ 12,1473, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Birmingham E., Niebur G.L., McHugh P.E., Shaw G., Barry F.P., and McNamara L.M.Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur Cell Mater 23,13, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Lian J.B., and Stein G.S.Concepts of osteoblast growth and differentiation: basis for modulation of bone cell development and tissue formation. Crit Rev Oral Biol Med 3,269, 1992 [DOI] [PubMed] [Google Scholar]
- 51.Kim K.M.Apoptosis and calcification. Scanning Microsc 9,1137, 1995 [PubMed] [Google Scholar]
- 52.Mavroidis M., and Capetanaki Y.Extensive induction of important mediators of fibrosis and dystrophic calcification in desmin-deficient cardiomyopathy. Am J Pathol 160,943, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trump B.F., Berezesky I.K., Sato T., Laiho K.U., Phelps P.C., and DeClaris N.Cell calcium, cell injury and cell death. Environ Health Perspect 57,281, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]