Abstract
We have previously reported that neonatal lipopolysaccharide (LPS) exposure resulted in an increase in interleukin-1β (IL-1β) content, injury to the hippocampus, and cognitive deficits in juvenile male and female rats, as well as female adult rats. The present study aimed to determine whether an antiinflammatory cytokine, interleukin-1 receptor antagonist (IL-1ra), protects against the neonatal LPS exposure-induced inflammatory responses, hippocampal injury, and long-lasting learning deficits in adult rats. LPS (1 mg/kg) or LPS plus IL-1ra (0.1 mg/kg) was injected intracerebrally to Sprague-Dawley male rat pups at postnatal day 5 (P5). Neurobehavioral tests were carried out on P21, P49, and P70, while neuropathological studies were conducted on P71. Our results showed that neonatal LPS exposure resulted in learning deficits in rats at both developmental and adult ages, as demonstrated by a significantly impaired performance in the passive avoidance task (P21, P49, and P70), reduced hippocampal volume, and reduced number of Nissl+ cells in the CA1 region of the middle dorsal hippocampus of P71 rat brain. Those neuropathological and neurobehavioral alterations by LPS exposure were associated with a sustained inflammatory response in the P71 rat hippocampus, indicated by increased number of activated microglia as well as elevated levels of IL-1β. Neonatal administration of IL-1ra significantly attenuated LPS-induced long-lasting learning deficits, hippocampal injury, and sustained inflammatory responses in P71 rats. Our study demonstrates that neonatal LPS exposure leads to a persistent injury to the hippocampus, resulting in long-lasting learning disabilities related to chronic inflammation in rats, and these effects can be attenuated with an IL-1 receptor antagonist.
Keywords: Lipopolysaccharide, Interleukin-1β, Interleukin-1 receptor antagonist, Hippocampus, Microglia
1. Introduction
It has been suggested that early life central nervous system inflammation during early neonatal period may cause long-lasting neurological disabilities and lead to development of cognitive disturbances later in life (Hagberg et al., 2012; Volpe, 2003). Our recent studies have shown that intracerebral injection of lipopolysaccharide (LPS), a component of the cell wall of gram-negative bacteria (Raetz and Whitfield, 2002), into postnatal day 5 (P5) rats induced a sustained neuroinflammation and hippocampal injury, as well as neurobehavioral deficits in neonatal rats (Fan et al., 2005, 2008). The latter manifests as learning and memory deficits in the passive avoidance task, and as less-anxious (anxiolytic-like) responses in the elevated plus-maze task in the juvenile male and female rats (P21) (Fan et al., 2005). Although the mechanisms underlying learning and memory deficits remain unclear, it has been proposed that infection/inflammation-induced chronic cytokine up-regulation by activated microglia plays an important role (Wang et al., 2013; Williamson et al., 2011).
In a preliminary experiment, we found that neonatal LPS exposure induced inflammatory responses and elevation of inter-leukin-1β (IL-1β) content in the hippocampus of juvenile rats (P21). Although physiological levels of IL-1b are necessary for normal memory, pathological high levels of IL-1β can impair cognition and are associated with Alzheimer's disease and other neurodegenerative diseases (Rubio-Perez and Morillas-Ruiz, 2012; Williamson et al., 2011). It has been reported that anti-inflammatory cytokines such as the IL-1 receptor antagonist (IL-1ra) may protect the brain by suppressing IL-1β production and blocking IL-1 signaling (Rubio-Perez and Morillas-Ruiz, 2012). Our previous study showed that IL-1ra attenuated neonatal LPS-induced long-lasting hyper-algesia in adult rats (Wang et al., 2011). In addition, LPS-induced impairment of contextual fear in rats can be prevented by IL-1ra (Pugh et al.,1998). These studies suggest that blocking IL-1β activity by IL-1ra may be an effective approach to attenuate LPS-induced neurological disabilities.
Our previous studies have shown that neonatal LPS exposure induced neurological impairments are long-lasting, since neuro-behavioral deficits and hippocampal injury are also observed in adult rats (Wang et al., 2013). In the current study, we further tested whether IL-1ra could protect against neonatal LPS exposure-induced long-lasting cognitive deficits and hippocampal injury, by disrupting interactions between microglia and neurons in which IL-1b is believed to be a critical mediator in adult rats.
2. Methods and materials
2.1. Chemicals
Unless otherwise stated, all chemicals used in this study were purchased from Sigma (St. Louis, MO, USA). Recombinant rat IL-1ra was purchased from R&D Systems (Minneapolis, MN, USA). Polyclonal rabbit antibodies against ionized calcium binding adapter molecule 1 (Iba1) was obtained from Wako Chemicals USA (Irvine, CA, USA). ELISA kits for immunoassay of rat IL-1β (RLB00), interleukin-6 (IL-6) (R6000B) and tumor necrosis factor-α (TNFα) (RTA00) were purchased from R&D Systems (Minneapolis, MN, USA).
2.2. Animals
Pregnant Sprague-Dawley rats arrived in the laboratory at day 19 of gestation. Animals were maintained in an animal room on a 12-h light/dark cycle at constant temperature (22±2°C). The day of birth was defined as postnatal day 0 (P0). After birth, the litter size was adjusted to 12 pups per litter to minimize the effect of litter size on body weight and brain size. All litters were weaned at P21 and male rats were housed in groups of 4 animals per cage. The female rats were used for our other study. All procedures for animal care were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee at either the University of Mississippi Medical Center or the Fu Jen Catholic University of Taiwan. Every effort was made to minimize the number of animals used and their suffering.
2.3. Surgical procedures and animal treatment
Intracerebral injection of LPS, or LPS in combination with IL-1ra in 5-day-old male Sprague-Dawley rat pups was performed as previously described (Cai et al., 2003; Fan et al., 2011a; Wang et al., 2011, 2013). Under light anesthesia with isoflurane (1.5–5%), LPS (1 mg/kg from Escherichia coli, serotype 055:B5), IL-1ra (0.1 mg/kg), or LPS (1 mg/kg) plus IL-1ra (0.1 mg/kg) in sterile saline containing 0.1% bovine serum albumin (BSA, total volume 2 ml) was administered into the rat brain (1.0 mm posterior to and 1.0 mm left of bregma, and 2.0 mm deep from the skull surface) by using a stereotaxic apparatus with a neonatal rat adapter. The control rats were injected with the same volume of sterile saline containing 0.1% BSA. The dose of LPS used here was based on our previous procedures (Fan et al., 2011a; Wang et al., 2011, 2013). Our previous studies have shown that neonatal LPS (1 mg/kg) exposure induces long-lasting learning impairment, less anxiety-like response and hippocampal injury in adult rats (Wang et al., 2013). The dose of IL-1ra was chosen based on data on peak concentrations of IL-1β achieved in the rat pup brain following LPS administration, as reported previously (Fan et al., 2005; Holmin and Mathiesen, 2000; Pang et al., 2003). The injection site was located in the area just above the left cingulum. All animals survived the intracerebral injection procedure.
Each dam had the same litter size (12 pups including 6 males and 6 females) and equal numbers of saline, saline plus IL-1ra, LPS, and LPS plus IL-1ra-treated rat pups were included in a litter. The pups were weaned at P21 and rats were housed four per cage after weaning (one cage each for saline, saline plus IL-1ra, LPS, and LPS plus IL-1ra-treated rats). The female rats were used for our other study. Ninety-six rats (twenty-four rats from each group) from 16 litters were used in the present study. One day after injection (P6), six rats from 6 litters for each group were sacrificed by a decapitation to collect fresh brain tissues for determination of the levels of cytokines in the hippocampus. Equal numbers of rat pups (6 pups from 6 litters) were included in saline, saline plus IL-1ra, LPS, or LPS plus IL-1ra injection groups for these three sets experiments (P21, P49, and P70). Sixty-six days after the injection (P71), rats were sacrificed by transcardiac perfusion with normal saline followed by 4% paraformaldehyde for brain section preparation. Six rats from each group were used for preparation of free-floating coronal brain sections of 40 mm thickness in a sliding microtome (Leica, SM 2000R, Wetzlar, Germany) for immunohistochemical staining and stereological estimates of the size of cerebrum, ventricle, white matter, striatum, and hippocampus. For determination of the levels of cytokines in the hippocampus, six P71 rats from each group were sacrificed by a decapitation to collect fresh brain tissues. The rest of twenty-four rats were used for our other studies.
2.4. Behavioral testing
The behavioral tests were performed as described previously (Fan et al., 2005, 2008, 2011b; Wang et al., 2013), with modifications. Three groups were included in the present study. Group 1: sixteen days after the injection (P21), rats (6 rats in each group) were performed by open field, elevated plus-maze, and passive avoidance (learning trial) tests. The memory trials were tested on P22, P50, and P71. Group 2: forty-four days after the injection (P49), rats (6 rats in each group) were performed by open field, elevated plus-maze, and passive avoidance (learning trial) tests. The memory trials were tested on P50 and P71. Group 3: sixty-five days after the injection (P70), rats (6 rats in each group) were performed by open field, elevated plus-maze, and passive avoidance (learning trial) tests. The memory trials were tested on the next day (P71).
2.4.1. Open-field test
The open field test measures the activity and habituation responses of animals upon placement in a novel environment (Hermans et al., 1992). Locomotor activity was measured at P21, P49, or P70, using the Any-Maze™ Video Tracking System (Stoelting Co., Wood Dale, IL, USA). Pups were placed in the activity chamber (42 × cm 25 cm × 40 cm) in a quiet room with dimmed light. The total distance traveled by the animal was recorded during a 10-min testing period (Fan et al., 2008; Tien et al., 2011; Wang et al., 2013).
2.4.2. Passive avoidance test
Passive avoidance involves the learned inhibition of a natural response and gives information about learning and memory capabilities (Hermans et al., 1992; Olton, 1973). The passive avoidance procedure consisted of two sessions (Fan et al., 2008). In the first session (P21, P49, or P70), rats were trained in a step-down type of passive avoidance apparatus. The experimental chamber (30 × cm 30 × cm 40 cm) was made of plexiglass. The oor of the chamber was made of parallel, 2-mm-caliber stainless steel rods spaced 1 cm apart and connected to an electric shock generator. The safe part of the camber was provided by a piece of wood board (8 × cm 25 cm × 2.5 cm) placed in a corner above the metal rods. Each animal was placed initially on the safe platform. When the rat stepped down onto the floor, a foot shock (stimulus amplitude: 80 V,1 s duration) was administered using an Isolated Square Wave Stimulator (#7092-611, Phipps and Bird, Inc., Richmond, VA, USA). Although the rats repeatedly stepped up and down, they eventually remained on the board. The number of shocks required to retain an individual animal on the board for 2 min was recorded as a measure of acquisition of passive avoidance. The second session was carried out 1, 29, or 50 days after the first session (P22, P50, or P71). The rat was placed on the safe board but the steel rods were not connected to the electric shock generator. The retention latency, i.e., the time that elapsed before the rat stepped down to the grid floor, was recorded as a measure of the retention of passive avoidance. If the rat did not step down to the grid floor within 2 min, a ceiling score of 2 min was assigned.
2.4.3. Elevated plus-maze test
The elevated plus-maze test is well established to assess anxiety behavior (Agmo and Belzung, 1998; Schmitt et al., 2002) and performed described previously (Wang et al., 2013). The plus-maze consists of two open arms (50 cm × 10 cm × 1.5 cm walls) and two enclosed arms (50 cm × 10 cm × 40 cm high walls) emanating from a common central platform (10 cm 10 cm) to form a plus shape. The entire apparatus was elevated to a height of 50 cm above the oor. A charge-coupled device (CCD) camera and illumination-lamps were mounted on the ceiling. The anxiety-related behaviors of each animal were recorded at P21, P49, or P71, fora period of 5 min, using a CCD camera-coupled recording system.
2.5. Estimation of the volumes of cerebrum and hippocampus
Brain injury was estimated based on the results of Nissl staining on consecutive brain sections prepared from rats sacrificed 66 days (P71) after the intracerebral injection. The stereological estimates of the total volume of cerebrum, ventricles, white matter, striatum, and hippocampus were determined using methods described previously (Gundersen and Jensen, 1987; Wang et al., 2013). The fifty-six equally spaced thick (40 μm) sections that were to be used in the analysis came from a one-in-six series. Nissl stained sections were scanned by a densitometer (Bio-Rad Hercules, CA, USA) and the areas of the ventricles, white matter, striatum, and hippocampus as well as that of the whole brain section (cerebrum) were outlined and determined using NIH image software in each of the fifty-six sections (Fan et al., 2011a,b,b; Wang et al., 2013). The Cavalieri principle (Gundersen and Jensen, 1987) was used to estimate the reference volumes, est V(ref).
2.6. Immunohistochemical studies
For immunohistochemical staining, primary antibodies were used in the following dilutions: anti-Iba1, 1:500. Microglia were detected using Iba1 immunostaining, which recognizes both the resting and the activated microglia. Brain sections were incubated with primary antibodies at 4°C overnight and subsequently incubated with secondary antibodies conjugated with uorescent dyes (Alexa Flour 555, 1:500; Invitrogen, Carlsbad, CA, USA), for 1 h in the dark at the room temperature. 4°, 6-diamidine-2-phenylindole (DAPI, 100 ng/ml) was used simultaneously to stain nuclei, to aid their identification during the final visualization. Sections incubated in the absence of primary antibody were used as negative controls.
2.7. Quantification of immunostaining data
Iba1-stained sections were obtained from the hippocampal area of the diencephalon sections at levels 1/2 and 2/3 rostral from the lambda to the bregma. Most of the immunostaining data were quantified by counting positively stained cells. When the cellular boundary was not clearly separated, numbers of DAPI-stained nuclei from the superimposed images were counted as the cell number. In the present study, CA1 neuronal changes were primarily observed in the hippocampus of the diencephalon following LPS exposure. Therefore, unless otherwise stated, three digital microscopic images were randomly captured in the CA1 region of the hippocampus. The number of positively stained cells in the three images was averaged. Three sections at each of the two section levels were examined by an observer blind to treatment, and mean value of cell counting was used to represent one single brain. For convenience in comparing results among different treatment groups, results were standardized as the average number of Nissl+ cells/mm (CA1) or the average number of Iba1+ cells/mm2 (CA1). In response to LPS challenge, the number of Iba1+ microglia increased and cells became bigger. We therefore developed a method of quantitatively measuring these changes that involved using computer software to determine the percentage of the entire area of the captured image that contained Iba1-positive staining (Fan et al., 2011b; Wang et al., 2013).
2.8. Determination of IL-1β, IL-6, and TNFα expression by ELISA
The levels of three major cytokines, IL-1β, IL-6, and TNFa, were determined by ELISA (Rat IL-1β Immunoassay, #RLB00; Rat IL-6 Immunoassay, #R6000B; Rat TNF Immunoassay, #RTA00; R&D Systems Inc., Minneapolis, MN, USA) as previously described (Fan et al., 2011b; Wang et al., 2013). Briefly, rats were desacribed by a decapitation and the fresh hippocampal tissues from each rat were collected 1 day (P6) or 66 days (P71) after LPS injection. Tissues were homogenized by sonication in 1 ml ice-cold PBS (pH 7.2) and centrifuged at 12,000 g for 20 min at 4°C. The supernatant was collected and the protein concentration was determined by the Bradford method (Hammond and Kruger, 1988). ELISA was performed following the manufacturer's instructions and data were acquired using a 96-well plate reader (Bio-Tek Instruments, Inc., VT, USA). The cytokine contents were expressed as pg cytokines/mg protein.
2.9. Statistical analysis
The behavioral data from open field test and passive avoidance test were presented as the mean±stand error (SD) and analyzed by the two-way repeated measures analysis of variance (ANOVA), which is suitable for data from tests conducted continuously at different postnatal days, followed by the Student–Newman–Keuls test. Data from other behavioral tests and quantitative analyses were presented as mean±SD and analyzed by the two-way ANOVA, followed by the Student–Newman–Keuls test. Results with a p < 0.05 were considered statistically significant.
3. Results
3.1. Neonatal LPS exposure did not affect locomotor activity
There were no significant differences in the total crossing distance of an individual rat during a 10-min period in an open field at P21 (~900 cm), P49 (~1200 cm) or P70 (~1300 cm) (Data not shown).
3.2. IL-1ra improved learning and memory deficits in rats exposure to LPS
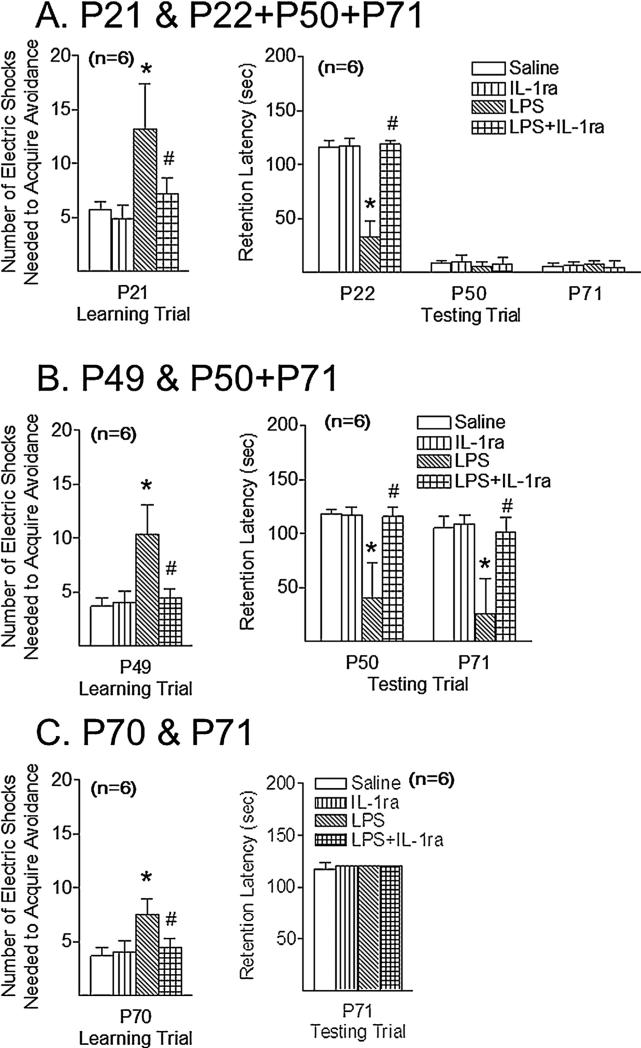
LPS treatment significantly increased the number of electric foot shocks required to retain the rat on the safe board at P21 (p < 0.05) (Fig. 1A, left panel), P49 (p < 0.05) (Fig. 1B, left panel), and P70 (p < 0.05) (Fig. 1C, left panel) compared with the control group. Co-administration IL-1ra protected against LPS-induced learning deficits at P21 (p < 0.05) (Fig. 1A, left panel), P49 (p < 0.05) (Fig. 1B, left panel), and P70 (p < 0.05) (Fig. 1C, left panel).
Fig. 1.
IL-1 receptor antagonist attenuated the LPS exposure-induced learning and memory deficit, as determined by passive avoidance, 16 (P21) (A), 44 (P49) days (B), and 65 days (P70) (C) after the injection. Three group of experiments were performed: (A) learning trial at P21 juvenile rats; (B) learning trial at P49 adolescent rats; (C) learning trial at P70 adult rats. The results are shown as the number of electric foot shocks required to retain the rat on the safe board (left panel) and the retention latency to step down from the board on the next day or longer (right panel). The results are expressed as the mean±SD of six animals in each group and analyzed by the two-way ANOVA (learning, left panel) or the two-way repeated measures ANOVA for data from tests conducted continuously at different postnatal days (memory, right panel), followed by the Student– Newman–Keuls test. *p < 0.05 represents significant difference for the LPS group as compared with the saline group on the same postnatal day. p < 0.05 represents significant difference for the LPS + IL-1ra group as compared with the LPS group on the same postnatal day.
In group 1 experiment (learning trail at P21 juvenile rats), neonatal LPS treatment significantly reduced the retention latency to step down from the board the next day at P22 (p < 0.05) (Fig. 1A, right panel) as compared with the control group. IL-1ra protected against LPS-induced memory deficits at P22 (p < 0.05) (Fig. 1A, right panel). However, all these rats in the control and treatment groups reduced the retention latency to step down from the board at P50 and P71 (Fig. 1A, right panel).
In group 2 experiment (learning trail at P49 adolescent rats), neonatal LPStreatmentsignificantly reducedthe retentionlatency to stepdown from the board the next dayat P50 (p < 0.05) (Fig.1B, right panel) as compared with the control group. LPS-induced memory deficits were also observed at P71 (p < 0.05) (Fig.1B, right panel). IL-1ra protected against LPS-induced memory deficits at P50 (p < 0.05) (Fig. 1A, right panel) and P71 (p < 0.05) (Fig. 1B, right panel).
However, in group 3 experiment (learning trial at P70 adult rats), no significant differences in the memory (passive avoidance test at P71, Fig. 1C, right panel) were observed between LPS and the control group.
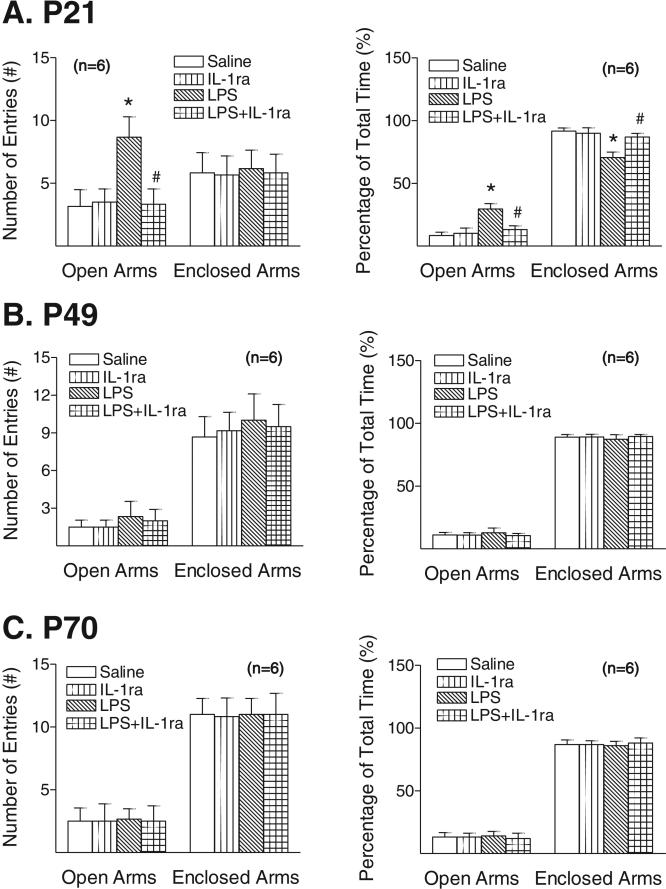
3.3. IL-1ra ameliorated LPS-induced less anxiety-like behaviors
A higher number of entries into the open arm were observed in the LPS-injected group as compared with the control group at P21 (p < 0.05) (Fig. 2A, left panel). LPS administration increased the step-through time spent in the open arm at P21 (p < 0.05) (Fig. 2A, right panel), while decreasing the step-through time spent in the enclosed arm at P21 (p < 0.05) (Fig. 2A, right panel). Those changes, however, were not observed at later developmental stages such as P49 (Fig. 2B) and P70 (Fig. 2C). Therefore, neonatal LPS exposure-induced a less anxiety-like behavior only detectable at early developmental ages (i.e., in P21) as detected in the elevated plus-maze test, which could be significantly ameliorated by IL-1ra treatment (p < 0.05) (Fig. 1A).
Fig. 2.
IL-1 receptor antagonist attenuated the LPS exposure-induced less anxiety-like behavioral, as determined by the elevated plus-maze test, 16 days (P21) (A), 44 days (P49) (B), and 65 days (P70) (C) after the injection. The results are shown as the numbers of open arm or enclosed arm entries (left panel) and the percentage of time spent in open arms or enclosed arms (time spent in open arms or enclosed arms divided by the sum of time spent in either arm) (right panel). The results are expressed as the mean±SD of six animals in each group and analyzed by the two-way ANOVA, followed by the Student–Newman–Keuls test. *p < 0.05 represents significant difference for the LPS group as # compared with the saline group. p < 0.05 represents significant difference for the LPS + IL-1ra group as compared with the LPS group.
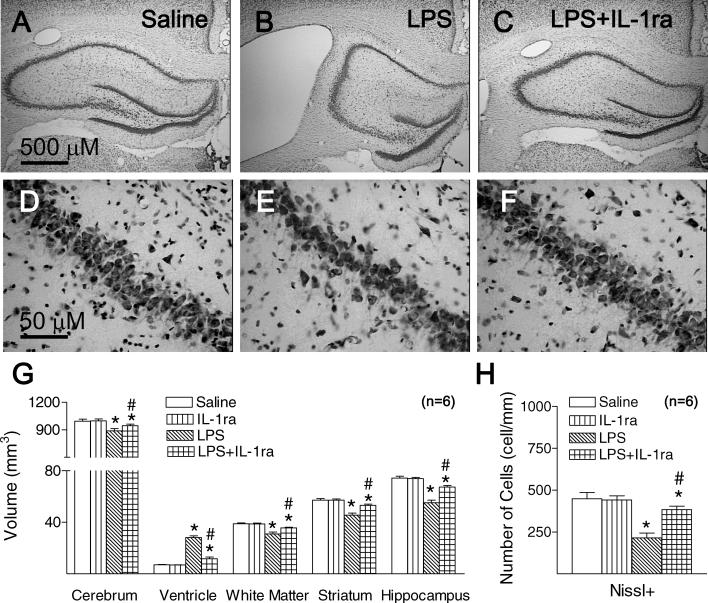
3.4. IL-1ra attenuated LPS-induced reduction of hippocampal volume and loss of hippocampal CA1 neurons
Nissl staining showed that neonatal LPS exposure caused persistent dilatation of bilateral ventricles (p < 0.05) (Fig. 3B and G). LPS exposure decreased the volume of cerebrum (p < 0.05) (Fig. 3G), white matter (p < 0.05) (Fig. 3G), striatum (p < 0.05) (Fig. 3G), and hippocampus (p < 0.05) (Fig. 3B and G) at P71, as compared with the control group (Fig. 3A, and G). Co-administration of LPS with IL-1ra attenuated LPS exposure-induced dilatation of lateral ventricles (p < 0.05) (Fig. 3C and G), and LPS-induced decreases in the volumes of cerebrum (p < 0.05) (Fig. 3G), white matter (p < 0.05) (Fig. 3G), striatum (p < 0.05) (Fig. 3G), and hippocampus (p < 0.05) (Fig. 3C and G) at P71. LPS exposure induced a decrease in the number of Nissl-stained neurons in the CA1 region at the middle dorsal hippocampus (p < 0.05) (Fig. 3E and H), as compared to that of the control group (Fig. 3D and H). Co-administration of LPS with IL-1ra attenuated the LPS-induced reduction in the number of hippocampal CA1 neurons (p < 0.05) (Fig. 3F and H).
Fig. 3.
Representative photomicrographs of Nissl staining in the rat brain 66 days (P71) after LPS injection. The diencephalon sections including hippocampus at a level 1/2 rostral from the lambda to the bregma (A–C) were used. (A) Nissl stained brain sections show normal morphology from the control group. (B) LPS exposure resulted in enlarged ventricles and a decreases size of hippocampus in LPS group. (C) Co-administration of LPS with IL-1ra attenuated LPS exposure-induced enlarged ventricles and a decreases size of hippocampus in LPS + IL-1ra group. (G), stereological estimates of the volume of cerebrum and different regions were performed as described in Section 2. LPS exposure induced a decrease in number of Nissl stained neurons in the hippocampal CA1 region at the middle dorsal hippocampus level (E), as compared with that in the control rat brain (D). Co-administration of LPS with IL-1ra attenuated LPS exposure-induced decreases in the number of Nissl+ cells in the hippocampal CA1 region in LPS + IL-1ra group (F). (H) Quantitation of Nissl+ cells, was performed as described in Section 2. The results are expressed as the mean±SD of six animals in each group and analyzed by the two-way ANOVA, followed by the Student–Newman–Keuls test. *p < 0.05 represents significant difference for the LPS group or LPS + IL-1ra group as compared with the saline group. #p < 0.05 represents significant difference for the LPS + IL-1ra group as compared with the LPS group. The scale bars shown in (A) and (D) represent 500 μm and 50 μm, respectively.
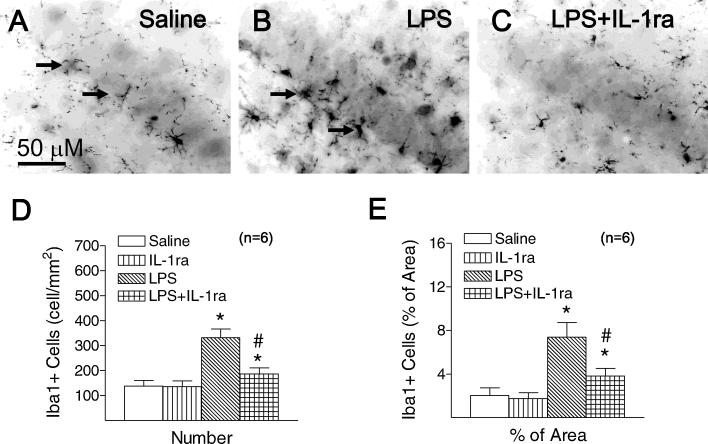
3.5. IL-1ra significantly suppressed LPS-induced chronic microglial activation
Neonatal LPS exposure resulted in a sustained increase in microglial activation in the P71 rat brain, as indicated by the number and morphology of the Iba1+ cells (Fan et al., 2011a). In the control rat brain, most of the Iba1+ microglia were in a resting state, as shown by their small rod-shaped soma and ramified processes (indicated by arrows in Fig. 4A). A significantly higher number of Iba1+ cells were found in the hippocampal CA1 region of the neonatal LPS-exposed rat brain (p < 0.05) (Fig. 4B and D). Many of these Iba1+ cells showed typical features of activated microglia, e.g., bright staining of an elongated or round cell body with blunt or no processes (indicated by arrows in Fig. 4B) (Kreutzberg, 1996). Higher percentage of Iba1+ immunostaining area was observed in the hippocampal CA1 region of the neonatal LPS-exposed rat brain (p < 0.05) (Fig. 4B and E). IL-1ra significantly attenuated LPS-induced increases in the number of Iba1+ cells (p < 0.05) (Fig. 4C and D) and the percentage area that contains Iba1 immunostaining in the hippocampal CA1 region (p < 0.05) (Fig. 4C and E).
Fig. 4.
Representative photomicrographs of microglia (A–C) in the rat brain 66 days (p71) after LPS injection. As shown by Iba1 immunostaining (A) in CA1 region at the middle dorsal hippocampus, a few microglia at the resting status with a small rod shaped soma and ramified processes were found in the hippocampal CA1 region of the control rat brain (the arrow shown in (A)). Numerous activated microglia (the arrow shown in (B)) with a round or elongate shaped cell body and blunt processes were observed in the hippocampal CA1 region of the rat brain with neonatal LPS exposure. Co-administration of LPS with IL-1ra attenuated LPS-induced increases in the number of activated microglia in the hippocampal CA1 region in LPS + IL-1ra group (C). Quantitation of the number of Iba1+ cells (D) and the percentage area of image that contained Iba1 staining in the hippocampal CA1 region (E) were performed as described in Section 2. The results are expressed as the mean SD of six animals in each group and analyzed by the two-way ANOVA, followed by the Student–Newman–Keuls test. *p < 0.05 represents significant difference for the LPS group or LPS + IL-1ra group as compared with the group. #p < 0.05 represents significant difference for the LPS + IL-1ra group as compared with the LPS group. The scale bar in (A) represents 50 μm.
3.6. IL-1ra significantly suppressed LPS-induced expression of inflammatory cytokines
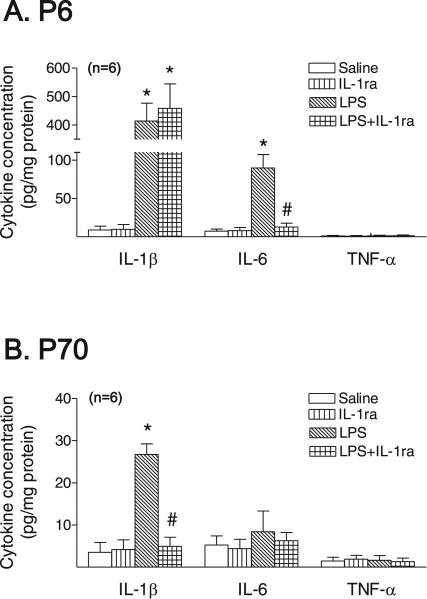
Neonatal exposure to LPS resulted in inflammatory responses in the rat hippocampus, as evidenced by the elevation of proin-flammatory cytokine levels (Fig. 5A). IL-1β and IL-6 concentrations were significantly increased in the hippocampus of the rat brain 1 day after LPS injection (p < 0.05) (Fig. 5A), and IL-1β was still significantly increased in the hippocampus of the rat brain 66 days after LPS injection (p < 0.05) (Fig. 5B). However, the concentrations of IL-6 and TNFa were not elevated in the hippocampus of the LPS-exposed rat brain at P71 (Fig. 5B). Co-administration of LPS with IL-1ra reduced LPS exposure-induced elevation of IL-6 levels at P6 (p < 0.05) (Fig. 5A), and the elevation of IL-1β levels in the hippocampal region at P71 (p < 0.05) (Fig. 5B).
Fig. 5.
IL-1 receptor antagonist attenuated the LPS exposure-induced increases in cytokines in the rat brain 1 day (A) and 66 days (B) after LPS injection. The results are expressed as the mean SD of six animals in each group and analyzed by the two way ANOVA, followed by the Student–Newman–Keuls test. *p < 0.05 represents significant difference for the LPS group or LPS + IL-1ra group as compared with the saline group. #p < 0.05 represents significant difference for the LPS + IL-1ra group as compared with the LPS group.
4. Discussion
Neonatal infection/inflammation has long-term adverse effects on neurodevelopment, which is associated with the pathogenesis of numerous neurological disorders in later life (Hagberg et al., 2002, 2012; Volpe, 2003). In the present study, we found that a single neonatal LPS exposure resulted in chronic neuroinflamma-tory responses (Figs. 4 and 5), which was associated with hippocampal injury in adult male rats (p70) as well as learning deficits in rats at both developing and adult ages (p21, P49 and P70) (Fig. 1). As the hippocampus plays important roles in the process of learning, memory, and anxiety (Spolidorio et al., 2007; Zarrindast et al., 2012), the hippocampal injury observed in LPS-treated rats might contribute to their poor performance in the passive avoidance and elevated plus-maze tasks. It has been shown that in rat, maternal exposure to LPS led to impaired learning and psychotic-like behavior in offspring, which were associated with aberrant forms of synaptic plasticity in the hippocampal CA1 region (Escobar et al., 2011). These early postnatal changes of synaptic plasticity in hippocampus might contribute to brain dysfunctions such as learning deficits that occur later in life (Escobar et al., 2011). In our neonatal LPS treatment model, we also observed memory impairment in rats at P22 (Fig. 1A) and P50 (Fig. 1B), and less anxiety-like (anxiolytic-like) behaviors at P21 (Fig. 2A). These findings are in line with previous reports indicating that long-term hippocampal inflammation can induce anxiolytic-like behavior in rats (Fan et al., 2005, 2008; Hein et al., 2012). Additionally, Rico et al. (2010) also reported that neonatal exposure to LPS led to heightened exploratory activity in adolescent rats. Except hippocampus, several brain regions such as the amygdala and prefrontal cortex are also highly involved in the anxiety-like behavior. Thus, further studies are needed to clarify the role of LPS in anxiety-like behaviors.
Consistent with our previous studies (Fan et al., 2011b; Tien et al., 2011), neonatal LPS exposure resulted in hyperactivity in locomotion and stereotyped tasks, and other disturbances of motor behaviors, although the impaired motor functions were spontaneously recovered by P70. On the other hand, neonatal LPS-induced injury to the dopaminergic system such as the loss of dendrites and reduced tyrosine hydroxylase immunoreactivity in the substantia nigra persisted into adulthood, without discernible DA neuron loss in P70 rats (Fan et al., 2011b). When challenged with methamphetamine (METH, 0.5 mg/kg) subcutaneously, rats with neonatal LPS exposure had significantly increased locomotion and stereotypy behaviors as compared to those without LPS exposure (Tien et al., 2011). These data indicate that although neonatal LPS-induced motor neurobehavioral impairment is spontaneously recoverable, injury to the dopaminergic system and neuroinflammation are long-lasting, suggesting there might be a silent neurotoxicity (Fan et al., 2011b). In the present study, neonatal LPS exposure led to a persistent hippocampal injury as indicated by a reduced hippocampal volume and Nissl+ cell numbers in the CA1 region at P71 (Figs. 3 and 4). Therefore, such neuropathological alterations in the hippocampus were likely, responsible to learning deficits detected by the passive avoidance task (p21, P49, and P70) (Fig. 1).
Previous studies reported that male rats are more sensitive to immune challenge by bacterial infection in early life, while female rats are more sensitive to this challenge in later life (Bilbo et al., 2012; Schwarz and Bilbo, 2012; Schwarz et al., 2012). One possible contributing factor for this sex differences in immune response might be different patterns of microglial colonization of the developing rat brain between males and females, as demonstrated by studies showing that male rats have more microglia early in neonatal development (p4), whereas female rats have more micro-glia at juvenile and adult age (p30 to P60) (Bilbo et al., 2012; Schwarz and Bilbo, 2012; Schwarz et al., 2012). Our current and previous data show that a neonatal LPS exposure causes; (1) sustained inflamma-tory responses in hippocampus, (2) hippocampal injury, and (3) cognitive deficits in both male (Fig.1C) and female rats (Wang et al., 2013). However, the neonatal LPS-induced memory deficits were spontaneously recoverable by the adult stage, also in both male (Fig. 1C) and female rats (Wang et al., 2013). Our preliminary data indicate there is a sex-dependent impairment of hippocampal synaptic plasticity in rats exposure to LPS neonatally (unpublished data, Fan L.W.). As for LPS induced anxiolytic-like behavior, it was noted in both male and female rats at P21; however, this effect was only observed in adult female but not male rats (p70), when assessed by the elevated plus-maze test (Wang et al., 2013). To clarify whether neonatal LPSexposure produces different effects in different genders on hippocampal injury and hippocampus-related neurobehavioral deficits, more studies will be necessary.
Cytokine receptors are distributed throughout the brain and with high density in the hippocampus (Cunningham and De Souza,1993; Parnet et al., 2002); therefore, the hippocampus may be particularly vulnerable to infection (inflammation) and therefore important to the pathology of behavioral and cognitive disorders (Green and Nolan, 2014; Lynch et al., 2004). Our data demonstrated that IL-1ra was able to not onlysuppress sustained inflammatory responses, but also attenuated hippocampal injury as well as long-lasting cognitive deficits induced by neonatal LPS exposure, suggesting that IL-1β might play a significant role in mediating a sustained neuroinflammation, possibly by maintaining a feed-forward loop between neurons and microglia. There is evidence suggesting that an uncontrolled sustained inflammation may lead to production of neurotoxic factors contributing to the pathogenesis of neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease (Dutta et al., 2008; Glass et al., 2010; Qian etal., 2010). The hypothesis that IL-1β plays a critical role in maintaining such a feed-forward loop, is also supported by studies demonstrating that blocking IL-1 signaling pathway significantly attenuated LPS-induced inflammatory cascade and cognitive dysfunction in young adult (Terrando et al., 2010) as well as aged (Abraham and Johnson, 2009) male mice, and in addition, by studies showing that intracerebral injection of IL-1ra ameliorated LPS-induced cytokines induction in the hippocampus (Frank et al., 2012).
5. Conclusion
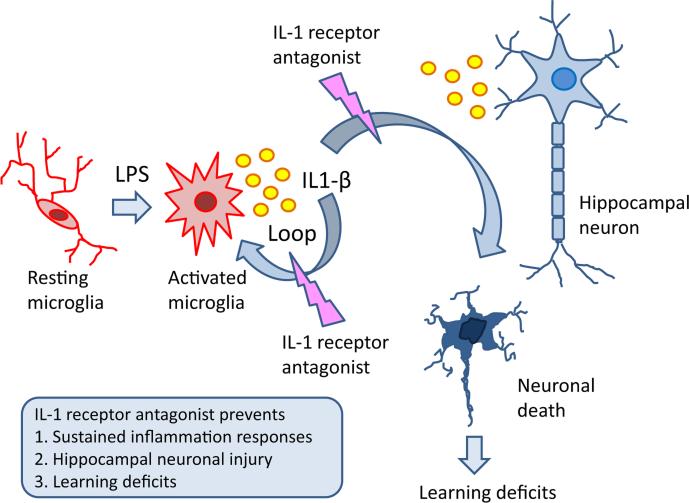
In summary, the current study demonstrated that neonatal LPS exposure led to chronic neuroinflammation, persistent neuronal injury in the hippocampus, and long-lasting cognitive deficits in adult male rats. These compromises in both structures and behaviors were attenuated by IL-1ra, suggesting that IL-1β plays a critical role in mediating these pathological changes (Fig. 6). Our findings suggest that targeting IL-1β may be a potentially novel strategy in developing therapies for neurodegenerative diseases caused by chronic neuroinflammation.
Fig. 6.
Neonatal LPS exposure leads to chronic neuroinflammation, persistent neuronal injury in the hippocampus, and long-lasting cognitive deficits in adult male rats. These compromises in both structures and behaviors were attenuated by IL-1 receptor antagonist.
Supplementary Material
HIGHLIGHTS.
IL1-ra attenuates neonatal LPS induced learning deficits in rats.
IL1-ra attenuates neonatal LPS induced hippocampal injury in rats.
IL1-ra protects against neonatal LPS induced chronic neuroinflammation in rats.
Acknowledgements
This work was supported by a NIH grant NIH/NINDS R01NS080844 (to L.W. Fan), Newborn Medicine Funds from the Department of Pediatrics, University of Mississippi Medical Center (to L.W. Fan, Y. Pang, and A.J. Bhatt), grants NSC102-2320-B-030-011 and MOST103-2320-B-030-005-MY3 from the National Science Council Taiwan (to L.T. Tien), and a grant CMFJ10006 from Chi-Mei Medical Center in Taiwan (to L.T. Tien and K.M. Lan).
Abbreviations
- LPS
lipopolysaccharide
- IL-1β
interleukin-1b
- IL-1ra
interleukin-1 receptor antagonist
- P5
postnatal day 5
- Iba1
ionized calcium binding adapter molecule 1
- IL-6
interleukin-6
- TNF
tumor necrosis factor
- ELISA
enzyme-linked immunosorbent assay
Footnotes
Conflict of interest
The authors declare that they have no competing nancial or personal interests, and that none of the author's institutions have contracts relating to this research through which it may stand to gain financially now or in the future.
Transparency document
The Transparency document associated with this article can be found in the online version.
References
- Abraham J, Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain Behav. Immun. 2009;23:396–401. doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agmo A, Belzung C. Interactions between dopamine and GABA in the control of ambulatory activity and neophobia in the mouse. Pharmacol. Biochem. Behav. 1998;59:239–247. doi: 10.1016/s0091-3057(97)00338-9. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Smith SH, Schwarz JM. A lifespan approach to neuroinflammatory and cognitive disorders: a critical role for glia. J. Neuroimmune Pharmacol. 2012;7:24–41. doi: 10.1007/s11481-011-9299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Pang Y, Lin S, Rhodes PG. Differential roles of tumor necrosis factor-alpha and interleukin-1 beta in lipopolysaccharide-induced brain injury in the neonatal rat. Brain Res. 2003;975:37–47. doi: 10.1016/s0006-8993(03)02545-9. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr., De Souza EB. Interleukin 1 receptors in the brain and endocrine tissues. Immunol. Today. 1993;14:171–176. doi: 10.1016/0167-5699(93)90281-o. [DOI] [PubMed] [Google Scholar]
- Dutta G, Zhang P, Liu B. The lipopolysaccharide Parkinson's disease animal model: mechanistic studies and drug discovery. Fundam. Clin. Pharmacol. 2008;22:453–464. doi: 10.1111/j.1472-8206.2008.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar M, Crouzin N, Cavalier M, Quentin J, Roussel J, Lante F, Batista-Novais AR, Cohen-Solal C, De Jesus Ferreira MC, Guiramand J, Barbanel G, Vignes M. Early, time-dependent disturbances of hippocampal synaptic transmission and plasticity after in utero immune challenge. Biol. Psychiatry. 2011;70:992–999. doi: 10.1016/j.biopsych.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Fan LW, Pang Y, Lin S, Tien LT, Ma T, Rhodes PG, Cai Z. Minocycline reduces lipopolysaccharide-induced neurological dysfunction and brain injury in the neonatal rat. J. Neurosci. Res. 2005;82:71–82. doi: 10.1002/jnr.20623. [DOI] [PubMed] [Google Scholar]
- Fan LW, Tien LT, Lin RC, Simpson KL, Rhodes PG, Cai Z. Neonatal exposure to lipopolysaccharide enhances vulnerability of nigrostriatal dopaminergic neurons to rotenone neurotoxicity in later life. Neurobiol. Dis. 2011a;44:304–316. doi: 10.1016/j.nbd.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan LW, Tien LT, Mitchell HJ, Rhodes PG, Cai Z. Alpha-phenyl-n-tert-butyl-nitrone ameliorates hippocampal injury and improves learning and memory in juvenile rats following neonatal exposure to lipopolysaccharide. Eur. J. Neurosci. 2008;27:1475–1484. doi: 10.1111/j.1460-9568.2008.06121.x. [DOI] [PubMed] [Google Scholar]
- Fan LW, Tien LT, Zheng B, Pang Y, Lin RC, Simpson KL, Ma T, Rhodes PG, Cai Z. Dopaminergic neuronal injury in the adult rat brain following neonatal exposure to lipopolysaccharide and the silent neurotoxicity. Brain Behav. Immun. 2011b;25:286–297. doi: 10.1016/j.bbi.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Thompson BM, Weber MD, Watkins LR, Maier SF. IL-1RA injected intra-cisterna magna confers extended prophylaxis against lipopolysaccharide-induced neuroinflammatory and sickness responses. J. Neuroimmunol. 2012;252:33–39. doi: 10.1016/j.jneuroim.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green HF, Nolan YM. Inflammation and the developing brain: consequences for hippocampal neurogenesis and behavior. Neurosci. Biobehav. Rev. 2014;40:20–34. doi: 10.1016/j.neubiorev.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann. Neurol. 2012;71:444–457. doi: 10.1002/ana.22620. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Peebles D, Mallard C. Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment. Retard. Dev. Disabil. Res. Rev. 2002;8:30–38. doi: 10.1002/mrdd.10007. [DOI] [PubMed] [Google Scholar]
- Hammond JB, Kruger NJ. The bradford method for protein quantitation. Methods Mol. Biol. 1988;3:25–32. doi: 10.1385/0-89603-126-8:25. [DOI] [PubMed] [Google Scholar]
- Hein AM, Zarcone TJ, Parfitt DB, Matousek SB, Carbonari DM, Olschowka JA, O'Banion MK. Behavioral, structural and molecular changes following long-term hippocampal IL-1beta overexpression in transgenic mice. J. Neuroimmune Pharmacol. 2012;7:145–155. doi: 10.1007/s11481-011-9294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans RH, Hunter DE, McGivern RF, Cain CD, Longo LD. Behavioral sequelae in young rats of acute intermittent antenatal hypoxia. Neurotoxicol. Teratol. 1992;14:119–129. doi: 10.1016/0892-0362(92)90060-n. [DOI] [PubMed] [Google Scholar]
- Holmin S, Mathiesen T. Intracerebral administration of interleukin-1beta and induction of inflammation, apoptosis, and vasogenic edema. J. Neurosurg. 2000;92:108–120. doi: 10.3171/jns.2000.92.1.0108. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lynch AM, Walsh C, Delaney A, Nolan Y, Campbell VA, Lynch MA. Lipopolysaccharide-induced increase in signalling in hippocampus is abrogated by IL-10–a role for IL-1 beta. J. Neurochem. 2004;88:635–646. doi: 10.1046/j.1471-4159.2003.02157.x. [DOI] [PubMed] [Google Scholar]
- Olton DS. Shock-motivated avoidance and the analysis of behavior. Psychol. Bull. 1973;79:243–251. doi: 10.1037/h0033902. [DOI] [PubMed] [Google Scholar]
- Pang Y, Cai Z, Rhodes PG. Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Brain Res. Dev. Brain Res. 2003;140:205–214. doi: 10.1016/s0165-3806(02)00606-5. [DOI] [PubMed] [Google Scholar]
- Parnet P, Kelley KW, Bluthe RM, Dantzer R. Expression and regulation of interleukin-1 receptors in the brain. Role in cytokines-induced sickness behavior. J. Neuroimmunol. 2002;125:5–14. doi: 10.1016/s0165-5728(02)00022-x. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav. Immun. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- Qian L, Flood PM, Hong JS. Neuroinflammation is a key player in Parkinson's disease and a prime target for therapy. J. Neural Transm. 2010;117:971–979. doi: 10.1007/s00702-010-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Ann. Rev. Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico JL, Ferraz DB, Ramalho-Pinto FJ, Morato S. Neonatal exposure to LPS leads to heightened exploratory activity in adolescent rats. Behav. Brain Res. 2010;215:102–109. doi: 10.1016/j.bbr.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Rubio-Perez JM, Morillas-Ruiz JM. A review: inflammatory process in Alzheimer's disease, role of cytokines. Sci. World J. 2012;2012:756357. doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt U, Waldhofer S, Weigelt T, Hiemke C. Free-choice ethanol consumption under the influence of GABAergic drugs in rats. Alcohol. Clin. Exp. Res. 2002;26:457–462. [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD. Sex glia, and development: interactions in health and disease. Horm. Behav. 2012;62:243–253. doi: 10.1016/j.yhbeh.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J. Neurochem. 2012;120:948–963. doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolidorio PC, Echeverry MB, Iyomasa M, Guimaraes FS, Del Bel EA. Anxiolytic effects induced by inhibition of the nitric oxide-cGMP pathway in the rat dorsal hippocampus. Psychopharmacology (Berlin) 2007;195:183–192. doi: 10.1007/s00213-007-0890-0. [DOI] [PubMed] [Google Scholar]
- Terrando N, Rei Fidalgo A, Vizcaychipi M, Cibelli M, Ma D, Monaco C, Feldmann M, Maze M. The impact of IL-1 modulation on the development of lipopolysaccharide-induced cognitive dysfunction. Crit. Care. 2010;14:R88. doi: 10.1186/cc9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien LT, Cai Z, Rhodes PG, Fan LW. Neonatal exposure to lipopolysaccharide enhances methamphetamine-induced reinstated behavioral sensitization in adult rats. Behav. Brain Res. 2011;224:166–173. doi: 10.1016/j.bbr.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ. Cerebral white matter injury of the premature infant-more common than you think. Pediatrics. 2003;112:176–180. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- Wang KC, Fan LW, Kaizaki A, Pang Y, Cai Z, Tien LT. Neonatal lipopolysaccharide exposure induces long-lasting learning impairment, less anxiety-like response and hippocampal injury in adult rats. Neuroscience. 2013;234:146–157. doi: 10.1016/j.neuroscience.2012.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KC, Wang SJ, Fan LW, Cai Z, Rhodes PG, Tien LT. Interleukin-1 receptor antagonist ameliorates neonatal lipopolysaccharide-induced long-lasting hyperalgesia in the adult rats. Toxicology. 2011;279:123–129. doi: 10.1016/j.tox.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson LL, Sholar PW, Mistry RS, Smith SH, Bilbo SD. Microglia and memory: modulation by early-life infection. J. Neurosci. 2011;31:15511–15521. doi: 10.1523/JNEUROSCI.3688-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast MR, Nasehi M, Pournaghshband M, Yekta BG. Dopaminergic system in CA1 modulates MK-801 induced anxiolytic-like responses. Pharmacol. Biochem. Behav. 2012;103:102–110. doi: 10.1016/j.pbb.2012.07.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.