Abstract
Inhaled corticosteroids can prevent acute exacerbations and emergency visits when used as part of a chronic care plan for long-term control of asthma, but low patient adherence and inadequate provider prescribing (clinical inertia) can limit these benefits. State Medicaid programs are a major source of insurance coverage for low-income children, paying for medications and preventive care, as well as bearing the cost of adverse outcomes for common chronic conditions in childhood, such as asthma. This study measured the incidence and timing of emergency department (ED) visits in the first 90 days after an initial inhaled corticosteroid prescription (ICS-Rx) among 43,156 Medicaid-enrolled children with a diagnosis of asthma in 14 southern states in 2007. One in 5 children (19.6%) with asthma had at least 1 ED visit in the first 90 days after initial ICS-Rx; 10% of these visits occurred within the first 48 hours, and 25% occurred within the first week. Continued ICS-Rx use was associated with lower risk of an ED visit. There were no racial differences in the ED visit rates. Initial ICS-Rx for Medicaid-enrolled children is a warning flag for short-term risk of asthma-related ED visits, whereas continued ICS-Rx use is protective for at least 90 days. Primary care follow-up may be needed within the first 2 days after initial ICS-Rx to prevent adverse outcomes. Medicaid programs could use claims data for surveillance of adherence to guideline-concordant therapy and for sentinel events marking windows of a higher risk for ED visits. Population Health Management 2015;18:54–60.
Introduction
Emergency department (ED) visits for asthma are largely preventable events, yet 1 in 5 children with asthma had an ED visit for asthma in 2009.1 Black and Hispanic children had a higher rate of ED visits, and those with a low income or without health insurance coverage had worse outcomes. At the health system level, costly ED visits have been a target of efforts to improve care and outcomes for asthma.
As recommended by national asthma care guidelines,2 long-term controller medications such as inhaled corticosteroids (ICS) decrease hospital admissions, ED visits, and even deaths in children with persistent asthma.3 Unfortunately, clinical inertia often leads to understaging of asthma and poor initiation of ICS long-term controller therapy (ICS prescription [Rx]) by clinicians. Real-world patient adherence to ICS-Rx can be as low as 20%.4,5 Initial ICS-Rx may be prescribed when the child is already experiencing frequent or severe exacerbations, a “window of vulnerability” for near-term exacerbation. Therefore, this study was undertaken to evaluate the incidence and timing of ED visits in the first 90 days after an initial ICS-Rx among Medicaid-enrolled children with asthma.
Methods
Study design
This was a retrospective cohort study among Medicaid-enrolled children with asthma in 14 southern states (Alabama, Arkansas, Florida, Georgia, Kentucky, Louisiana, Maryland, Missouri, Mississippi, North Carolina, South Carolina, Tennessee, Texas, and Virginia). The study was approved for human subjects research by the medical school's Institutional Review Board.
Data
The data set included 100% of the Medicaid-paid claims for calendar year 2007 in the selected states. Data were obtained from the Centers for Medicare & Medicaid Services in a standard Medicaid Analytic eXtract (MAX) file format, using the inpatient (IP), outpatient (OT=other services), prescription drug, and personal summary files.
These 2007 Medicaid claims data were derived from 20,902,393 enrollees. The study team selected 839,684 persons who had a diagnosis of asthma for at least 1 inpatient admission or at least 2 records on different dates in the outpatient file (International Classification of Diseases, Ninth Revision code: 493.xx, excluding 493.2x). The number of children age 5–12 years with asthma was 239,167. Among these children, fewer than half (122,174) had any claim for an ICS-Rx. Among these, the study team selected children who had no record of any long-term control drug claims (including ICS, oral corticosteroids, or leukotriene inhibitors) during the 90 days prior to their first ICS-Rx. In order to have an adequate window of time for looking back 90 days and forward 90 days from the first ICS-Rx, only children with an initial ICS-Rx during the period from April 1, 2007 to September 30, 2007 were included. After all inclusion and exclusion criteria were applied, there remained a cohort of 43,156 children (Fig. 1).
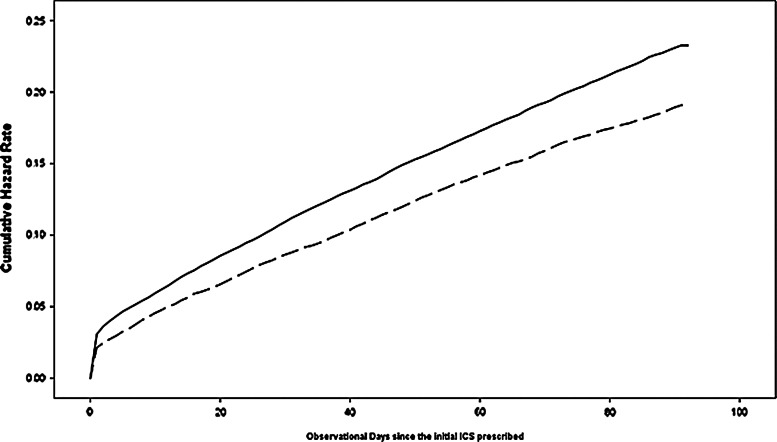
Fig. 1.
Cumulative hazard function of first emergency department visit by high vs low controller-to-total asthma drug ratio during 90-day observation period after initial inhaled corticosteroid (ICS) prescription for Medicaid enrolled children, 2007.
Variables and measures
Outcome variable—ED visits
The ED services provided were identified in both IP and OT files. For children seen in the ED but not admitted to the hospital, ED visits were identified in the OT file, whereas those who were admitted to the hospital were identified from the IP file using revenue center code values of 0450–0459 and 0981. Other charges associated with ED services were identified in the IP file by place of service. The study team deduplicated all same-day records for ED visits and added them for a cumulative count of ED visits during the 90-day observational period. The team then categorized the ED visit variable as dichotomous (yes/no) for use in multivariate models.
Independent variables
ICS-to-total asthma medication ratio
The controller-to-total asthma medication ratio expresses controller medications as a percentage of total asthma medication claims, which includes both controller and short-term reliever medications. This measure has been validated in administrative claims data, including a high correlation (0.94) between the use of 2-quarter and full-year claims.6 It also more accurately reflects the benefits of long-term controller drugs on outcomes compared with simple measures of controller adherence such as proportion of days covered (PPDC), which can increase in concert with more refills of rescue medication and show a spuriously positive association with ED visit rates.7 The study team focused on the specific subset of ICS-Rx among the controller drugs, using an ICS-to-total asthma medication ratio, which the team has shown to be predictive of risk for asthma-related ED visits.5 This variable was categorized into 2 groups (high-ICS ≥0.5, low-ICS <0.5).
Severity of asthma
Finding drug claims for 2 or more short-acting beta-agonist rescue inhalers (SABA) within the 90-day period prior to initial ICS prescription was considered an indicator of more severe asthma and is a known risk factor for asthma-related ED visits.8 Rescue drug SABAs included albuterol, levalbuterol, and pirbuterol. To identify these drugs, National Drug Codes (NDC) were linked in Medicaid pharmacy claims with the LexiComp drug database to identify the specific drug ingredients.
Other independent variables or covariates
Physician visits
Claims for physician office visit services were found in the OT file using procedure codes 99201–99205 and 99211–99215. The study team classified 90-day physician visit counts after ICS initiation for each child into 3 groups (no visit, 1 visit, and ≥2 visits).
Rural/urban
Rural/urban status was determined by merging the MAX data with county-level data from the Area Resource File (ARF) using the child's county of residence. The ARF aggregates publically available data from multiple sources providing socioeconomic and environmental characteristics. The 2003 Rural/Urban Continuum Codes9 classify counties into 3 groups: Large metro areas with at least 1 million residents; small metro areas with fewer than 1 million residents; and nonmetro (rural) areas.
Statistical analysis
Comparison of sample characteristics, demographics, socioeconomic status, and severity of asthma were examined using the chi-square test for categorical variables and analysis of variance for continuous variables. The Kaplan-Meier method was used to estimate the cumulative probability of an ED visit for children with high vs low ICS-to-total asthma drug ratios. Participants were censored on the date of the ED visit or at the end of 90 days of observation if they did not visit the ED during this time. The study team then built a Cox proportional hazard model to estimate the hazard ratio for an ED visit with high vs low ICS-Rx use, with adjustment for covariate effects. Data were analyzed using SAS 9.2 (SAS Institute Inc., Cary, NC).
Results
Table 1 shows the characteristics of the 43,156 children aged 5–12 who met inclusion criteria for having had a new ICS-Rx during the study period. Roughly one third of these children were categorized as having higher ICS-to-total asthma drug ratios. One in 5 children (19.6%) had an asthma ED visit within 90 days of ICS-Rx, including 21.1% of white, 20.2% of African American, and 15.4% of Hispanic children who had at least 1 ED visit in the first 90 days after initial ICS-Rx (Table 2). These minor racial–ethnic differences were not statistically significant on multivariate analysis.
Table 1.
Demographic Profile of Medicaid-Enrolled Children with Asthma Grouped by High vs Low ICS-to-Total Asthma Drug Ratios
| High ICS-to-total asthma drug ratio (%) | Low ICS-to-total asthma drug ratio (%) | P value | |
|---|---|---|---|
| Total | 14,391 (33.4) | 28,765 (66.6) | |
| Mean age (years) (SD) | 8.1 (2.4) | 7.8 (2.3) | <.01 |
| Race-ethnicity | |||
| White | 4483 (31.2) | 8082 (28.1) | <.01 |
| African American | 5927 (41.2) | 11,801 (41.0) | |
| Hispanic | 2832 (19.7) | 6570 (22.8) | |
| Other | 1149 (8.0) | 2312 (8.0) | |
| Sex | |||
| Female | 6029 (41.9) | 11,208 (39.0) | <.01 |
| Male | 8362 (58.1) | 17,555 (61.0) | |
| Urban/rural status | |||
| Large metro | 5501 (38.2) | 11,325 (39.4) | <.01 |
| Small metro | 5233 (36.4) | 9987 (34.7) | |
| Rural | 3657 (25.4) | 7453 (25.9) | |
| High SABA usea | |||
| No | 12,132 (84.3) | 19,379 (67.4) | <.01 |
| Yes | 2259 (15.7) | 9386 (32.6) | |
| Physician visits | |||
| None | 4486 (31.2) | 7468 (26.0) | <.01 |
| 1 | 4101 (28.5) | 7321 (25.5) | |
| ≥2 | 5804 (40.3) | 13976 (48.5) | |
| Emergency department visit | |||
| Yes | 2500 (17.4) | 5971 (20.8) | <.01 |
| No | 11,891 (82.6) | 22,794 (79.2) | |
Two or more refills of short-acting beta-agonist in 90 days.
ICS, inhaled corticosteroid; SABA, short-acting beta-agonist.
Table 2.
Relative Risk of Emergency Department Visit During the 90 Days After Initial Use of Inhaled Corticosteroid (Cox Regression Estimate)
| 90-Day ED visit incidence rate % (N) | Crude hazard ratio (95% CI) | Adjusted hazard ratioa(95%CI) | |
|---|---|---|---|
| Sex | |||
| Female | 19.6 (3386/17,237) | 1.00 | 1.00 |
| Male | 19.6 (5084/25,917) | 1.00 (0.96,1.04) | 0.99 (0.95,1.04) |
| Race-ethnicity | |||
| White | 21.1 (2645/12,565) | 1.00 | 1.00 |
| African American | 20.2 (3581/17,728) | 0.96 (0.91,1.01) | 1.04 (0.99,1.10) |
| Hispanic | 15.4 (1448/9402) | 0.71 (0.67,0.76) | 0.71 (0.66,0.76) |
| Other | 23.0 (797/3461) | 1.12 (1.03,1.21)b | 1.14 (1.05,1.23)b |
| Rural/urban status | |||
| Large metro | 18.0 (3032/16826) | 1.00 | 1.00 |
| Small metro | 20.3 (3087/15220) | 1.14 (1.08,1.19)b | 1.09 (1.04,1.15)b |
| Rural | 21.2 (2352/11110) | 1.19 (1.13,1.25)b | 1.10 (1.04,1.16 b |
| High SABA usec | |||
| No | 19.4 (6105/31511) | 1.00 | 1.00 |
| Yes | 20.3 (2366/11645) | 1.06 (1.01,1.11)b | 1.03 (0.98,1.08) |
| Physician visits | |||
| None | 16.6 (1982/11954) | 1.00 | 1.00 |
| 1 | 17.1 (1949/11422) | 1.03 (0.97,1.10) | 1.03 (0.97,1.10) |
| ≥2 | 23.0 (4540/19780) | 1.43 (1.35,1.50)b | 1.45 (1.37,1.53)b |
| ICS-Rx-to-total asthma drug ratio | |||
| High | 17.4 (2500/14,391) | 1.00 | 1.00 |
| Low | 20.8 (5971/28,765) | 1.22 (1.17,1.28)b | 1.19 (1.14,1.25)b |
Adjusted for age, sex, race, rural/urban status, physician visit count, severity of asthma, and adherence to ICS drug status.
P<.05.
Two or more refills of short-acting beta-agonist in 90 days.
CI, confidence interval; ED, emergency department; ICS-Rx, inhaled corticosteroid prescription; SABA, short-acting beta-agonist.
The timing of ED visits was not uniform throughout the 90-day follow-up period. Ten percent occurred within the first 48 hours after initial ICS-Rx and one quarter within the first week. The study team used Kaplan-Meier survival curve techniques for the whole cohort, modeling ED visits as the outcome event over the 90 days following an initial ICS-Rx (Fig. 1). The cumulative ED visit rate increased with time, and the gap between high-ICS and low-ICS groups also widened (from a 0.9% difference at the beginning to 3.4% at 90 days)(Table 3). After 90 days, the cumulative ED visit rate was 17.4% for the high-ICS group and 20.8% for the low-ICS. The Kaplan-Meier curves demonstrated a significant difference in time to the first ED visit between children with high vs. low ICS-to-total asthma drug ratios (log rank test chi-square=70.94; P≤.01).
Table 3.
Emergency Department Visit Incidence Rate (% [N]) by ICS Prescription-to-Total Asthma Drug Ratio Groups (High vs Low) through 90-day Period
| Day | ||||||||
|---|---|---|---|---|---|---|---|---|
| ICS-to-total asthma drug (N) | 1 | 2 | 3 | 7 | 14 | 28 | 56 | 90 |
| Low (28,765) | 3.0 (872) | 3.6 (1025) | 3.9 (1128) | 5.0 (1448) | 6.8 (1965) | 9.9 (2841) | 15.2 (4367) | 20.8 (5971) |
| High (14,391) | 2.1 (306) | 2.4 (349) | 2.7 (388) | 3.7 (532) | 5.3 (762) | 7.9 (1138) | 12.7 (1824) | 17.4 (2500) |
ICS, inhaled corticosteroid.
Bivariate Cox proportional hazard models generated crude hazard ratios for independent variables including sex, race, rural/urban status, ICS drug adherence (PPDC), severity of asthma, and physician visits. There were no significant racial–ethnic differences in the ED visit hazard ratios. Children in the low ICS-to-total asthma drug ratio group had a 22% higher risk of an ED visit than children in the high-ICS group. Medicaid enrollees living in small metro and rural areas had a 14%–19% higher risk of an ED visit than children living in large metro areas. Adherence to ICS-Rx, as measured by PPDC, also was a protective factor that conferred a lower risk of an ED visit. Higher asthma severity, as measured by beta-agonist use, also was associated with a higher risk of ED visits. Mutilivariate Cox proportional hazards models showed similar results. Children in the low-ICS group had a 19% higher risk of an ED visit (P<.05).
Discussion
There are 3 key findings in this study. First, the results demonstrate a window of vulnerability in the timing of ED visits for Medicaid-enrolled children with asthma after the sentinel event of an ICS-Rx, with roughly 1 in 5 children having an ED visit within 90 days, often in the first 2–7 days. Second, the study confirms and quantifies a measurable benefit of adherence to ICS-Rx with regard to preventing ED visits within the first 90 days after initial ICS-Rx. Third, no racial difference was found in 90-day ED visit rates among Medicaid enrollees with asthma after the sentinel event of an ICS-Rx.
ICS is the most effective long-term medication for asthma control across all age groups2 and is the predominant form of initial maintenance therapy for persistent asthma in children.10,11 The initial ICS-Rx may be seen as a sentinel event because it therapeutically marks a transition in disease severity, chronicity, or care that has triggered a prescription for daily anti-inflammatory therapy. An ED visit within a short time after ICS-Rx initiation could be accounted for by inadequate or delayed medication use, underestimation of asthma acuity and severity, continued exposure to environmental triggers, poor parental understanding or implementation of the asthma management plan, or some combination thereof.
Many of the children in this study might already have been experiencing an acute exacerbation at the time of their initial ICS-Rx. Timing is critical. In this cohort, one quarter of all 90-day ED visits occurred in the first week after the “sentinel event” of starting the ICS-Rx (10% within the first 2 days). The Multicenter Airway Research Collaborative found a 10% 2-week risk for unscheduled return visits to the ED or other provider after an acute exacerbation.12 Guttmann et al found a 72-hour return visit rate of approximately 6%,13 while Walsh-Kelly et al found a 7-day revisit rate of 4.1%.14 The ED visit rates for high vs. low ICS-Rx utilizers in the current study cohort began to diverge from the first day after the ICS-Rx was filled and continued to diverge over the entire 90-day follow-up period.
One key is for physicians to stage asthma patients accurately and prescribe ICS during routine chronic care rather than wait for an exacerbation. In the National Asthma Survey, more than half (52.8%) of patients with persistent asthma were not using an ICS, with African Americans being even less likely to receive an appropriate ICS-Rx (odds ratio .495).15 Moonie et al found that patients with persistent asthma in primary care practice settings were 89% less likely to receive guideline-concordant treatment than were patients with intermittent asthma, and that a structured intervention (Community Asthma Program) could improve both the accuracy of staging and guideline-concordant prescribing significantly.16
However, just prescribing an ICS-Rx is not sufficient.17 Adherence is also a challenge. Across 9 states, only 27% of adult asthma patients on Medicaid refilled a prescribed long-term controller medication prescription (LTC-Rx) at least twice.18 Among patients who did refill the LTC-Rx, only 16% adhered to therapy consistently for 6 months. Higher adherence to long-term controller therapy is associated with lower ED visit rates,19,20 but ICS-Rx adherence under “real world” conditions among Medicaid-enrolled children with asthma is only 20%.4,21
There also may be a need for closer follow-up of patients after initial ICS-Rx if it is prescribed in the context of an acute exacerbation. A follow-up telephone call the next day and a follow-up office visit within a week may be able to avert some of the preventable ED visits. Nonpersonal outreach such as an educational videotape and mailed reminders after an asthma-related ED visit is not sufficient.22 Prolonged follow-up also may be necessary to address the attrition of ICS adherence over time.23
The chronic care model is an approach that addresses both the clinician and patient sides of the asthma care equation. The conceptual heart of the model is its focus on enhancing productive interactions between a “prepared proactive practice team” and an “informed, activated patient.” For example, Fifield et al tested a chronic care practice model redesign that combined patient-focused community health worker outreach with provider-focused asthma training, structured asthma visit forms, and a Web-based asthma registry for federally qualified health center practices serving 295 low-income minority children with asthma. Appropriate prescribing and asthma control improved at all sites. 24 A second phase of the trial tested computer-based provider prompts for guideline-concordant prescribing and demonstrated an even greater impact on provider prescribing behaviors.
Are such programs cost-effective? Economic analysis of a Medicaid asthma management program for pediatricians suggested a direct cost saving of $26.44 per child per year, with a return on investment after initial implementation of $3.58 per dollar spent.25 Nurses and other members of the health care team can work together to ensure appropriate panel-based care management of children with asthma in primary care practices.26 In fact, a Cochrane Database review found nurses to be as effective as physicians in leading asthma management.27 Community pharmacy interventions also have demonstrated an impact in numerous studies.28,29
Beyond the clinical practice setting, patient and parental factors also are amenable to intervention. In a randomized trial of 305 parent–child dyads, Web-based education plus nurse case management significantly improved asthma control compared with usual care.30 Home-based asthma education improved both adherence and outcomes among 250 inner-city children.31 Flores et al showed that monthly contact with children and their families by parent mentors could reduce wheezing, asthma exacerbations, ED visits, and missed parental workdays while improving parental self-efficacy.32 Noyes and associates demonstrated a cost-effective gain of 138 symptom-free days per 100 children per month using a school-based asthma management program.33
Although this was a retrospective study, real-time monitoring of ED visits through claims data is a potential surveillance system for monitoring both process measures (eg, ICS-Rx prescribing and adherence)8 and outcomes of care (such as ED visits and hospital admissions)34 at the population health level. In Medicaid programs, these surveillance systems could provide real-time monitoring of sentinel events that flag moments of higher risk for adverse outcomes, when close follow-up, intervention, and care management might have the greatest impact.
Interestingly, no racial–ethnic disparities were found in this study's ED visit rates for patients after an initial ICS-Rx, even though previous studies have shown that minority patients have lower rates of specialist referral or follow-up visits within 5 days after an asthma-related ED visit,35 higher asthma severity, and lower rates of anti-inflammatory medication use.36 This suggests that Medicaid programs can be a significant force for health equity by providing insurance coverage and pharmacy benefits to low-income children. Even in high-prevalence and under-resourced communities, disparities are not inevitable. The Harlem Children's Zone Asthma Initiative demonstrated a 50% decline in ED visits among children whose families completed a 12-month program of home visits.37 State-led or community-based asthma coalitions have shown a positive impact in rural areas as well.38
Research using administrative claims data has known limitations. Although effective for large population surveillance of treatment adherence and adverse outcomes, claims data are not able to provide clinical data on peak flow rates or asthma staging. The study team did not differentiate between drugs within the ICS-Rx class because meta-analyses find little evidence of outcome differences based on specific ingredients.39 Using the initial ICS-Rx itself as a sentinel event is more objective than assuming it to be a proxy measure for persistent asthma.40 Although there may be some discrepancies between Medicaid claims and pharmacy records for asthma medication, one study found Medicaid claims data missed fewer of the claims than did the pharmacy records.41 One strength of claims data is that they measure real-world ICS-Rx adherence in patients who do not believe they are being observed, in contrast to studies which explicitly assess adherence by patient self-report, pill counting, or electronic means and are likely to overestimate real-world adherence. The current study approach of using the first ICS-Rx as a sentinel event would be easily reproducible in any claims-based surveillance system.
Conclusions
Asthma exacerbations requiring a visit to the ED are preventable events. Appropriate daily use of ICS could substantially decrease asthma-related ED visits, especially if prescribed before the crisis of an acute exacerbation. For now, a new ICS-Rx is a sentinel event indicating a short-term (1-week) and intermediate-term (90-day) window of greater risk for an ED visit. More fine-grained testing of interventions focused on the specific timing of follow-up contact and coordination of care after ICS-Rx will be needed in order to optimize outcomes. Finally, the absence of racial–ethnic disparities in either ICS-Rx utilization or 90-day ED visit rates suggests that Medicaid in itself is a therapeutic intervention for reducing racial–ethnic health disparities among low-income children with asthma.
Author Disclosure Statement
Drs. Rust, Zhang, Holloway, and Tyler-Hill declared no conflicts of interest with respect to the research, authorship, or publication of this article. The authors received the following financial support for the the research, authorship, or publication of this article: Initial compilation of this data set and the asthma cohort was supported by the Agency for Healthcare Research and Quality (AHRQ) grant No. R24HS019470-01; completion of the manuscript was supported by AHRQ grant No. K18 HS022444-01. Institutional research infrastructure support was provided by National Institutes of Health R-Center grant No. U54RR026137.
References
- 1.U.S. Centers for Disease Control and Prevention. Asthma's impact on the nation: data from the CDC National Asthma Control Program. Available at <http://www.cdc.gov/asthma/impacts_nation/AsthmaFactSheet.pdf>. Accessed May28, 2013
- 2.National Asthma Education and Prevention Program (NAEPP). Expert Panel Report 3 (EPR3): Guidelines for the Diagnosis and Management of Asthma; Section 4, Managing Asthma Long Term: Overview (page 278). Available at <http://www.nhlbi.nih.gov/guidelines/asthma/08_sec4_lt_ovw.pdf>. Accessed May28, 2013
- 3.Boushey HA. Effects of inhaled corticosteroids on the consequences of asthma. J Allergy Clin Immunol 1998;102:S5–S16 [DOI] [PubMed] [Google Scholar]
- 4.David C. Preventive therapy for asthmatic children under Florida Medicaid: changes during the 1990s. J Asthma 2004;41:655–661 [DOI] [PubMed] [Google Scholar]
- 5.Rust G, Zhang S, Reynolds J. Inhaled corticosteroid adherence and emergency department utilization among Medicaid-enrolled children with asthma. J Asthma 2013;50:769–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broder MS, Gutierrez B, Chang E, Meddis D, Schatz M. Ratio of controller to total asthma medications: determinants of the measure. Am J Manag Care 2010;16:170–178 [PubMed] [Google Scholar]
- 7.Yong PL, Werner RM. Process quality measures and asthma exacerbations in the Medicaid population. J Allergy Clin Immunol 2009;124:961–966 [DOI] [PubMed] [Google Scholar]
- 8.Schatz M, Zeiger RS, Yang SJ, et al. . Relationship of asthma control to asthma exacerbations using surrogate markers within a managed care database. Am J Manag Care 2010;16:327–333 [PubMed] [Google Scholar]
- 9.U.S. Department of Agriculture, Economic Research Service. Rural–urban continuum codes (2003). Available at <http://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx#.UdM3__lJvng>. Accessed July2, 2013
- 10.Guevara JP, Ducharme FM, Keren R, Nihtianova S, Zorc J. Inhaled corticosteroids versus sodium cromoglycate in children and adults with asthma. Cochrane Database Syst Rev 2006;2:CD003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng JW, Arnold RJ. Pharmacoeconomic review of medical management of persistent asthma. Allergy Asthma Proc 2008;29:109–122 [DOI] [PubMed] [Google Scholar]
- 12.Emerman CL, Cydulka RK, Crain EF, et al. . Prospective multicenter study of relapse after treatment for acute asthma among children presenting to the emergency department. J Pediatr 2001;138:318–324 [DOI] [PubMed] [Google Scholar]
- 13.Guttmann A, Zagorski B, Austin PC, et al. . Effectiveness of emergency department asthma management strategies on return visits in children: a population-based study. Pediatrics 2007;120:e1402–e1410 [DOI] [PubMed] [Google Scholar]
- 14.Walsh-Kelly CM, Kelly KJ, Drendel AL, Grabowski L, Kuhn EM. Emergency department revisits for pediatric acute asthma exacerbations: association of factors identified in an emergency department asthma tracking system. Pediatr Emerg Care 2008;25:505–510 [DOI] [PubMed] [Google Scholar]
- 15.Vaidya V, Holiday-Goodman M, Pinto S. Demographic disparities in patient-reported use of inhaled corticosteroids among patients with persistent asthma. J Asthma Allergy 2010;3:101–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moonie SA, Strunk RC, Crocker S, Curtis V, Schechtman K, Castro M. Community asthma program improves appropriate prescribing in moderate to severe asthma. J Asthma 2005;42:281–289 [DOI] [PubMed] [Google Scholar]
- 17.Small M, Vickers A, Anderson P, Kay S. The patient–physician partnership in asthma: real-world observations associated with clinical and patient-reported outcomes. Adv Ther 2010;27:591–599 [DOI] [PubMed] [Google Scholar]
- 18.Priest JL, Cantrell CR, Fincham J, Cook CL, Burch SP. Quality of care associated with common chronic diseases in a 9-state Medicaid population utilizing claims data: an evaluation of medication and health care use and costs. Popul Health Manag 2011;14:43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camargo CA, Jr, Ramachandran S, Ryskina KL, Lewis BE, Legorreta AP. Association between common asthma therapies and recurrent asthma exacerbations in children enrolled in a state Medicaid plan. Am J Health Syst Pharm 2007;64:1054–1061 [DOI] [PubMed] [Google Scholar]
- 20.Smith MJ, Rascati KL, McWilliams BC. Inhaled anti-inflammatory pharmacotherapy and subsequent hospitalizations and emergency department visits among patients with asthma in the Texas Medicaid program. Ann Allergy Asthma Immunol 2004;92:40–46 [DOI] [PubMed] [Google Scholar]
- 21.Herndon JB, Mattke S, Evans Cuellar A, Hong SY, Shenkman EA. Anti-inflammatory medication adherence, healthcare utilization and expenditures among Medicaid and Children's Health Insurance Program enrollees with asthma. Pharmacoeconomics 2012;30:397–412 [DOI] [PubMed] [Google Scholar]
- 22.Zorc JJ, Chew A, Allen JL, Shaw K. Beliefs and barriers to follow-up after an emergency department asthma visit: a randomized trial. Pediatrics 2009;124:1135–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohan J, Drotar D, McNally K, et al. . Adherence to pediatric asthma treatment in economically disadvantaged African-American children and adolescents: an application of growth curve analysis. J Pediatr Psychol 2010;35:394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fifield J, McQuillan J, Martin-Peele M, et al. . Improving pediatric asthma control among minority children participating in Medicaid: providing practice redesign support to deliver a chronic care model. J Asthma 2010;47:718–727 [DOI] [PubMed] [Google Scholar]
- 25.Cloutier MM, Grosse SD, Wakefield DB, Nurmagambetov TA, Brown CM. The economic impact of an urban asthma management program. Am J Manag Care 2009;15:345–351 [PubMed] [Google Scholar]
- 26.Kaferle JE, Wimsatt LA. A team-based approach to providing asthma action plans. J Am Board Fam Med 2012;25:247–249 [DOI] [PubMed] [Google Scholar]
- 27.Kuethe MC, Vaessen-Verberne AA, Elbers RG, Van Aalderen WM. Nurse versus physician-led care for the management of asthma. Cochrane Database Syst Rev 2013;2:CD009296. [DOI] [PubMed] [Google Scholar]
- 28.Berry TM, Prosser TR, Wilson K, Castro M. Asthma friendly pharmacies: a model to improve communication and collaboration among pharmacists, patients, and healthcare providers. J Urban Health 2011;88(suppl 1):113–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benavides S, Rodriguez JC, Maniscalco-Feichtl M. Pharmacist involvement in improving asthma outcomes in various healthcare settings: 1997 to present. Ann Pharmacother 2009;43:85–97 [DOI] [PubMed] [Google Scholar]
- 30.Gustafson D, Wise M, Bhattacharya A, et al. . The effects of combining Web-based eHealth with telephone nurse case management for pediatric asthma control: a randomized controlled trial. J Med Internet Res 2012;14:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otsuki M, Eakin MN, Rand CS, et al. . Adherence feedback to improve asthma outcomes among inner-city children: a randomized trial. Pediatrics 2009;124:1513–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flores G, Bridon C, Torres S, et al. . Improving asthma outcomes in minority children: a randomized, controlled trial of parent mentors. Pediatrics 2009;124:1522–1532 [DOI] [PubMed] [Google Scholar]
- 33.Noyes K, Bajorska A, Fisher S, Sauer J, Fagnano M, Halterman JS. Cost-effectiveness of the School-Based Asthma Therapy (SBAT) program. Pediatrics 2013;131:e709–e717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schatz M, Zeiger RS. Improving asthma outcomes in large populations. J Allergy Clin Immunol 2011;128:273–277 [DOI] [PubMed] [Google Scholar]
- 35.Shields AE, Comstock C, Weiss KB. Variations in asthma care by race/ethnicity among children enrolled in a state Medicaid program. Pediatrics 2004;113:496–504 [DOI] [PubMed] [Google Scholar]
- 36.Lieu TA, Lozano P, Finkelstein JA, et al. . Racial/ethnic variation in asthma status and management practices among children in managed Medicaid. Pediatrics 2002;109:857–865 [DOI] [PubMed] [Google Scholar]
- 37.A.I.R. Harlem. Impact. Available at <http://www.harlemasthma.org/air/impact/>. Accessed November7, 2012
- 38.Williams D, Portnoy JM, Meyerson K. Strategies for improving asthma outcomes: a case-based review of successes and pitfalls. J Manag Care Pharm 2010;16(1 suppl C):S3–S14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shepherd J, Rogers G, Anderson R, et al. . Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over. Health Technol Assess 2008;12(19):iii–iv, 1–360 [DOI] [PubMed] [Google Scholar]
- 40.Mosen DM, Macy E, Schatz M, et al. . How well do the HEDIS asthma inclusion criteria identify persistent asthma? Am J Manag Care 2005;11:650–654 [PubMed] [Google Scholar]
- 41.Mudd KE, Bollinger ME, Hsu VD, Manning A, Tsoukleris MG, Butz AM. Concordance of Medicaid and pharmacy record data in inner-city children with asthma. Contemp Clin Trials 2008;29:13–20 [DOI] [PubMed] [Google Scholar]