Abstract
Glaucoma is a group of diseases involving the optic nerve and associated structures, which is characterized by progressive visual field loss and typical changes of the optic nerve head (ONH). The only known treatment of the disease is reduction of intraocular pressure (IOP), which has been shown to reduce glaucoma progression in a variety of large-scale clinical trials. Nowadays, a relatively wide array of topical antiglaucoma drugs is available, including prostaglandin analogues, carbonic anhydrase inhibitors, beta-receptor antagonists, adrenergic agonists, and parasympathomimetics. In clinical routine, this allows for individualized treatment taking risk factors, efficacy, and safety into account. A major challenge is related to adherence to therapy. Sustained release devices may help minimize this problem but are not yet available for clinical routine use. Another hope arises from non-IOP-related treatment concepts. In recent years, much knowledge has been gained regarding the molecular mechanisms that underlie the disease process in glaucoma. This also strengthens the hope that glaucoma therapy beyond IOP lowering will become available. Implementing this concept with clinical trials remains, however, a challenge.
Background
Glaucoma refers to a group of multifactorial optical neuropathies associated with progressive loss of retinal ganglion cells (RGCs), leading to a characteristic pattern of visual field loss.1 Although there is general agreement that increased intraocular pressure (IOP) is the most important risk factor for onset and progression of the disease, it is, by far, not the only risk factor.2 IOP reduction remains the mainstay of glaucoma therapy. The review gives an overview of the current status of pharmacotherapy of glaucoma, a short outlook on future therapies on the horizon and discusses some of the challenges in translating such strategies into clinical application.
Physiology of aqueous humor production and outflow
IOP is regulated through the inflow and outflow of aqueous humor in the eye. The rate of aqueous humor turnover was measured to be 2.4±0.6 μL/min in the adult eye.3 The aqueous humor is produced by the epithelium of the ciliary body. It contains electrolytes, proteins, cytokines, organic solutes, and growth factors to nourish the avascular tissue of the anterior chamber.4 Most proteins are released through active secretion.5 Na+ is released by epithelial cells of the ciliary body via membrane Na+/K+ ATPases, and chloride reaches the aqueous humor via Cl− channels.6 Carbonic anhydrase forms bicarbonate (HCO3−), which is also a major component of the aqueous humor.5 The resulting osmotic gradient leads to water transport through aquaporines 1 and 4.5,7
To reach the posterior chamber, aqueous humor has to traverse the tissue components of the ciliary processes—the capillary wall, stroma, and epithelial bilayer.5 Aqueous humor then flows around the lens and through the pupil into the anterior chamber. Within the anterior chamber, a temperature gradient creates convective flow toward the cornea.8 A schematic illustration of aqueous humor dynamics is shown in Fig. 1. The major drainage pathway of aqueous humor out of the eye is via the trabecular meshwork into the Schlemm's canal (conventional pathway).9 The trabecular meshwork consists of laminar beams with a core of collagenous and elastic fibers covered by flat cells.9 It is porous and acts as a filter that drains aqueous humor passively when a certain IOP level is reached.9 Aqueous humor then reaches the Schlemm's canal, a specialized vessel that may constitute a main source of resistance to flow within its inner wall.10 Endothelial cells of the inner wall of Schlemm's canal are attached to one another through tight junctions, comparable to blood vessels. In contrast, the epithelial cells are attached to a discontinuous basement membrane, which is usually found in lymphatic vessels.10 A unique feature of the endothelium of the inner wall is the presence of so-called giant vacuoles, which have been found to be outpouchings of the endothelium into Schlemm's canal.10 These vacuoles show a large opening on the side faced toward the meshwork and in some of them also a distal opening (pore) into Schlemm's canal is present. The juxtacanalicular connective tissue consists of typical matrix components such as collagens, elastin, laminin, fibronectin, and glycosaminoglycans and is relatively loose.10 From Schlemm's canal, approximately 30 collector channels build anastomoses with the aqueous veins that finally drain aqueous humor into the systemic circulatory system.10,11
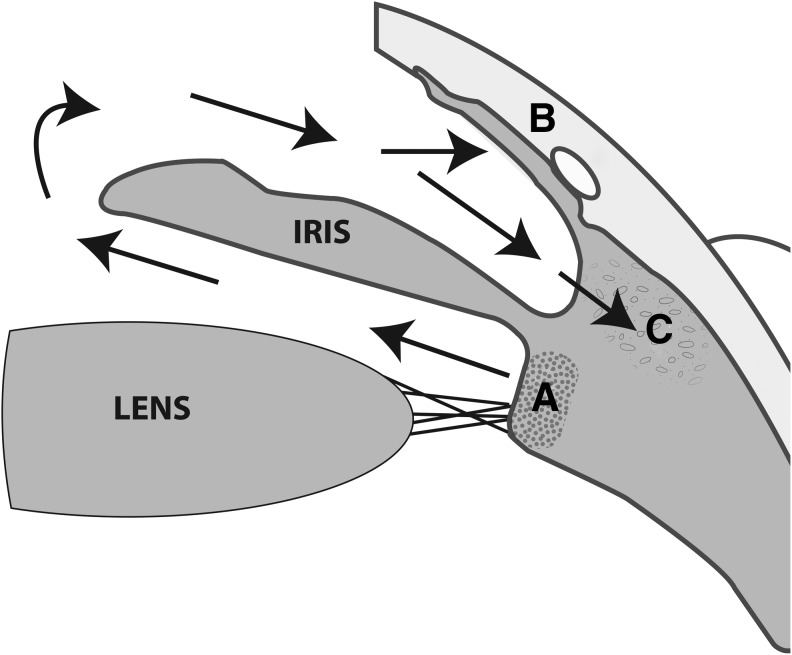
FIG. 1.
Schematic illustration of aqueous humor dynamics. Aqueous humor is produced by the epithelium of the ciliary body (A) and flows into the anterior chamber through the pupil. Most of it then leaves the anterior chamber via the trabecular meshwork into the Schlemm's canal (B). Some aqueous humor also leaves the eye through the iris root (uveoscleral outflow, C).
The main site of outflow resistance is in the juxtacanalicular tissue region.12 Whether this resistance is primarily due to trabecular meshwork cells and their extracellular matrix or the inner wall of Schlemm's canal is not fully established. Endothelial cells lining the inner wall of Schlemm's canal share some similarities with vascular and lymphatic endothelial cells, but the forces experienced by the Schlemm's canal endothelial cells through the aqueous humor flow are rather comparable to those in lymphatic endothelial cells than those in blood vessels.13 It has been hypothesized that interactions between Schlemm's canal endothelial cells and juxtacanalicular tissue are responsible for outflow resistance, but experimental evidence for this hypothesis is currently lacking.13
A small amount of aqueous humor leaves the eye via the uveoscleral route (unconventional pathway).14 In contrast to the trabecular pathway, uveoscleral outflow is relatively independent from IOP levels.14 As shown in Fig. 1, aqueous humor passes between the ciliary muscle bundles into supraciliary and suprachoroidal spaces.15 From there it is further drained into the sclera from which it is then returned to systemic circulation via lymphatic vessels outside of the eye.15 A recent study identified distinct lymphatic channels in the human ciliary body, which seem to constitute a uveolymphatic pathway, because fluid and solutes flow, at least partially, through this system.16 In a sheep model, the relative contribution of lymphatic drainage was quantified17 and a recent study has shown that this pathway of outflow is increased by latanoprost.18 In addition, it was shown that prospero homeobox protein 1, the master control gene for lymphatic development, is also expressed in Schlemm's canal.19
Pathophysiology of glaucoma
Increase in ocular pressure
The most important risk factor for glaucoma is elevated IOP. Still, not all patients with increased IOP develop glaucoma (ocular hypertensives, OHT) and not all patients with glaucoma have high IOP values (normal tension glaucoma, NTG). Nevertheless, there is evidence that increased IOP plays an important role in glaucoma pathogenesis from both clinical trials20 and basic research.9,12 The latter is related to experiments indicating trabecular meshwork dysfunction in primary open-angle glaucoma (POAG). It is nowadays generally accepted that POAG patients with increased IOP have elevated conventional outflow resistance.
In glaucoma, the trabecular meshwork shows considerable changes that are associated with the increase in outflow resistance. The meshworks from POAG patients show lower cellularity as compared with healthy controls.21 In both groups, loss of cells occurs gradually with age by which the inner tissues are more affected than the outer tissues. In untreated POAG patients, a variety of disease-related changes were observed in trabecular meshwork specimens, including thickened trabeculae, increased amounts of plaque material deposited within the cribriform layer, and abundance of long spacing collagen.22 These changes appear to be closely related to the disease process, because there is a negative correlation between the sheath-derived plaque material and axon counts.23 The mechanisms that trigger changes in the trabecular meshwork in glaucoma are not well described. A molecule that appears to play an important role in the trabecular meshwork changes in glaucoma is transforming growth factor-β2, which also may be involved in some of the glaucomatous processes at the posterior pole of the eye, including impairment of axonal transport and neurotrophic supply.24 Another mechanism that is related to loss of cells, increased accumulation of extracellular matrix proteins, changes in the cytoskeleton, cellular senescence, and the process of subclinical inflammation in the trabecular meshwork is oxidative stress.25 It has also been speculated that a hypoxic environment in glaucoma may induce aberrant epigenetic mechanisms that contribute to extracellular matrix change in the trabecular meshwork.26
Vascular aspects
Elevated IOP is the most important risk factor for glaucoma, but evidence has also accumulated that vascular factors are involved.1 The vascular theory is based on the assumption that reduced perfusion caused by either elevated IOP and low ocular perfusion pressure (OPP) or vascular dysregulation leads to glaucomatous damage.1,27 Vascular factors may contribute to the glaucomatous damage in 2 ways. On the one hand, reduced blood flow at the postlaminar and laminar structures may contribute at the primary site of damage. On the other hand, reduced retinal blood flow and abnormal retinal blood flow regulation in the retina may play a role in the processes that lead to RGC death.1 Several studies have, indeed, shown that glaucoma comes with compromised ocular blood flow. Choroidal, retinal, and ONH blood flow have been found to be reduced in patients with glaucoma compared with healthy controls.28–30 In addition, also autoregulation of ocular blood flow appears to be disturbed in this group of patients.30–32 Autoregulation is defined as the capability of a vascular bed to keep its blood flow constant despite changes in OPP.33 Large-scale studies also found low OPP, which can be estimated as the difference between mean arterial blood pressure and IOP, as a risk factor for the onset and progression of glaucoma.1,33–36 Since the interrelation between OPP, ocular blood flow, and glaucoma is beyond the scope of this review, the reader is referred to some recently published work on this topic and the involvement in glaucoma pathogenesis.1,33,37–40
Therapeutic Strategies
IOP lowering
The next chapter will summarize the most important mechanisms of action of well-established anti-glaucoma drugs. Figure 2 illustrates the influence of different substances on aqueous humor dynamics.
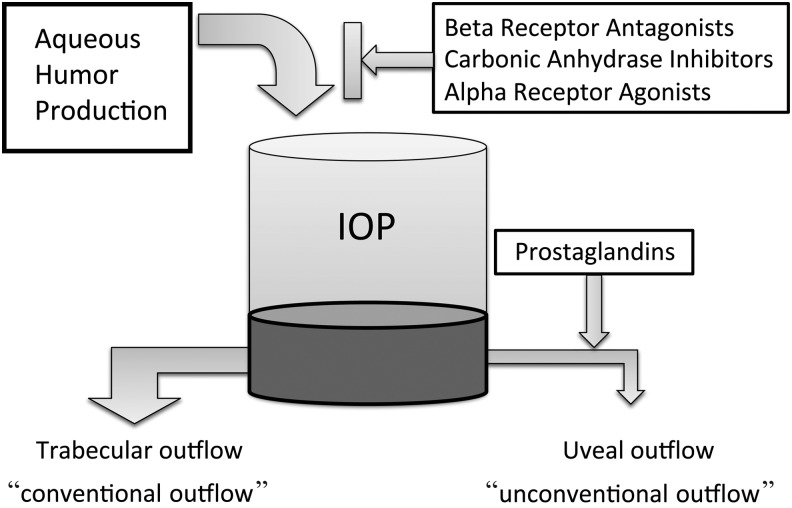
FIG. 2.
Influence of different substances on aqueous humor dynamics. Intraocular pressure (IOP) is determined by the inflow and outflow of aqueous humor in the eye. Glaucoma medications therefore either reduce aqueous humor production or increase trabecular or uveoscleral outflow.
Topical therapies
Adrenergic agonists
Adrenergic agonists stimulate alpha and/or beta receptors and provoke release of norepinephrine,41 the principal neurotransmitter of the adrenergic system. The history of adrenergic agonists is long dating back to the early 20th century when epinephrine was introduced toward dipivalyl epinephrine, clonidine, and apraclonidine.42 Nowadays, the selective alpha-2 receptor agonist brimonidine is the most important representative.43 Activation of alpha-2 receptors leads to vasoconstriction in the ciliary body associated with a decrease in aqueous humor production, but this effect is lost within a treatment period of 1 month.44 After that period, the effects of brimonidine on uveoscleral outflow predominate, increasing outflow by contraction of the ciliary muscle.44 The IOP-lowering potential of brimonidine is approximately 20%–25% from baseline.45 Although brimonidine is better tolerated than previous drugs in this class, it still exerts side effects, including contact dermatitis, ocular irritation, anterior uveitis, and hyperemia as well as fatigue, dizziness, and hypotension.43
In addition to the IOP-lowering potential, adrenergic agonists seem to have neuroprotective properties and alpha-2 receptors have been found to be present in RGCs.46 In a rat model where transient ischemia was induced, brimonidine significantly reduced RGC loss.47 In another study also carried out in rats, administration of brimonidine before inducing ischemia not only decreased RGC death but also sustained retrograde axonal transport.48 This neuroprotective effect seems to be independent of the IOP-lowering effect, as it significantly reduced RGC loss in a rat glaucoma model after intravitreal administration, even though no effect on IOP was observed in comparison to topically administered latanoprost or control.49 The mechanisms underlying the neuroprotective effects of brimonidine are not entirely clear, and several hypotheses were formulated. There is evidence from both in vitro and in vivo models that neuroprotection by alpha-2 receptor agonists is mediated via modulation of excitatory amino acid receptors to inhibit glutamate excitotoxicity.50 In addition, the neuroprotective actions of brimonidine may be caused by an upregulation of neurotrophic factors.49
One multi-center study compared the effects of brimonidine with those of timolol in NTG patients (Low-pressure Glaucoma Treatment Study, LoGTS). The primary outcome of the trial was visual field progression after a 4 year observation period. However, more than 40% of the patients did not complete the follow up with a higher drop-out rate being found in the brimonidine group compared with the timolol group. In the remaining patients, treatment with brimonidine showed less visual field progression than with timolol although both drugs reduced IOP to a comparable level.51 The study, however, has several limitations, because no information on visual field progression in the drop-outs is available and the rate of visual field loss in the timolol-treated group is higher than reported in other comparable studies.52
Beta-receptor antagonists
Beta receptors are expressed throughout the eye, and their antagonists reduce aqueous humor production in the ciliary body by decreasing intracellular cAMP concentration.53 Timolol was the first drug that came into the market and was for many years the most frequently used anti-glaucoma drug. Later, other beta-receptor antagonists with slightly different pharmacological properties such as betaxolol, carteolol, metipranolol, and levobutaxolol became available.54 The IOP-lowering potential of beta-receptor antagonists has been described to be between 20% and 25% from the initial values.45
Even though topical beta-receptor antagonists are generally well tolerated, they can induce severe systemic side effects caused by the underlying mechanism of beta-receptor blockade and are, therefore, contraindicated in some diseases. Nonspecific beta-receptor antagonists must not be prescribed to patients suffering from asthma bronchiale, decompensated chronic heart failure, symptomatic bradycardia or heart block, and history of syncope without diagnosis.55 As such, betaxolol, which pharmacologically differs from the other representatives of this class of drugs, because it exerts higher selectivity for the beta-1 receptor, was developed to be associated with the hope of equal IOP-lowering efficacy and improved pulmonary and cardiovascular risk profile.
Beta-receptor antagonists may have some neuroprotective potential, but the clinical relevance of this effect remains unclear. Timolol, metipranolol, and betaxolol were able to attenuate the effects of experimentally induced ischemia/reperfusion (I/R) injury to the rat retina, with betaxolol being more potent than the other dugs.56 Timolol also protected RGCs in an experimental model of glaucoma in rats in vivo, but in such experiments it is difficult to separate the IOP-lowering effect of the drug from direct neuroprotective effects.57 Betaxolol appears to mediate its neuroprotective effect not via a receptor-mediated mechanism but prevents excessive influx of Ca2+ and Na+ ions into neurons, in response to different injuries.58 A recent review focused on the question whether this direct neuroprotective effect of the drug along with its favorable effect on ocular hemodynamics could translate into preservation of visual fields in glaucoma.53 Indeed, the authors cite several smaller-scale clinical trials that indicate that betaxolol is less potent in reducing IOP than timolol, but at least as good as timolol in preserving visual fields. Such analysis may, however, be hampered by a publication bias and in the absence of any large-scale trials a clinically relevant neuroprotective effect of betaxolol cannot be considered as proven.
Carbonic anhydrase inhibitors
As stated earlier, carbonic anhydrase is important for aqueous humor production, as through its formation of Na+ and HCO3− ions water can enter ciliary epithelial cells. Topical carbonic anhydrase inhibitors, therefore, reduce aqueous humor formation.59 Brinzolamide and dorzolamide are currently approved for reducing IOP and provide an efficacy between 15% and 20% from the initial value.45 Topical carbonic anhydrase inhibitors are sulfonamides and special attention should be paid to possible hypersensitivities, as allergic reactions can occur manifesting as urticaria, angioedema, or pruritus.45 Other common side effects are ocular burning, stinging, bitter taste, superficial punctuate keratitis, blurred vision, tearing, headache, aesthesia, dizziness, paresthesia, and transient myopia.45 In patients with low corneal endothelial cell count, corneal edema can occur.45
Inhibition of carbonic anhydrase exerts potent vasodilator effects in the brain60–62 and, as such, hemodynamic effects of topical dorzolamide and brinzolamide have been extensively studied. Brinzolamide as well as dorzolamide have also been found to increase ONH, choroidal and retinal blood flow in patients with glaucoma and experimental animals in several studies.63–67 One study indicated that treatment with dorzolamide normalizes retinal blood flow regulation in patients with glaucoma.68 This is in agreement with data showing that a 6-month treatment with dorzolamide normalizes the ocular pressure/flow relationship.69 This effect was, however, also seen with timolol and may be related to the IOP-lowering effect of the drug.33,69
Evidence for a neuroprotective effect of topical dorzolamide has been seen in several animal experiments. A study carried out in an inherited rat glaucoma model found an upregulation in high mobility group box 1 (HMGB1) expression and a downregulation in expression of calmodulin in the glaucomatous retina.70 Both of these changes were significantly attenuated by topical administration of dorzolamide twice daily for 4 weeks.70 The authors concluded that this points toward a neuroprotective effect of this agent independent of its capacity to lower IOP, as HMGB1 seems to inhibit glial glutamate transport, leading to an increase in the extracellular glutamate concentration. Glaucoma patients treated with topical dorzolamide showed less oxidative activity, significantly higher superoxide dismutase activity, and reduced total antioxidant status in the aqueous humor compared with untreated patients.71 Still, oxidative activity was higher and antioxidative capacity was significantly lower in both glaucoma groups compared with patients suffering only from cataract.71 The antioxidative effect of dorzolamide might be dependent on the presence of mitochondria in trabecular meshwork cells, because when they are exposed to hydrogen peroxide, the antioxidant effect of dorzolamide is maximized in cell fractions containing intact mitochondria.72
To which degree these effects of topical carbonic anhydrase inhibitors transfer into clinically relevant neuroprotection is unclear. One randomized controlled study compared the effects of a 60 month treatment with either dorzolamide plus timolol versus brinzolamide plus timolol in 161 patients with POAG. Although both treatment regimens were equally effective in reducing IOP, only the dorzolamide/timolol combination group showed an increase in retrobulbar blood velocities associated with a reduced rate of visual field loss.73 On the other hand, the European Glaucoma Prevention Study (EGPS) did not find any evidence for a neuroprotective effect of dorzolamide.74 In this study, the conversion from OHT to POAG was studied. After 5 years, the mean IOP reduction was 22% in the dorzolamide group and 19% in the placebo group. The cumulative risk of converting to POAG was similar in both groups. Concerns were, however, raised about the interpretation of this trial because of a significant regression to the mean of IOP levels after 6 months of treatment and the preferential loss to follow-up in patients with higher IOPs.75
Parasympathomimetics
Parasympathomimetics induce contraction of smooth muscle cells in the ciliary body, which leads to an increase in aqueous humor outflow by widening the trabecular meshwork and Schlemm's canal.54 Pilocarpine is the best-known representative of this group of antiglaucoma drugs with an IOP-lowering capacity of 20%–25%.45 The mechanism in patients with angle-closure glaucoma differs but is not a part of the present review. Other parasympathomimetics include acetylcholine, physostigmine, or carbachol. Since parasympathomimetics induce miosis, pseudomyopia, and a wide array of systemic side effects, they are not well tolerated by patients and even contraindicated for individuals younger than 40 years.45 Other contraindications include uveitis, cataract, or neovascular glaucoma.45 Reported side effects are intestinal cramps, bronchospasm, retinal detachment, ciliary cramps, increased pupilary block, and several other topical symptoms.45
An in vitro study in retinal neurons of rats found a neuroprotective effect of pilocarpine against glutamate-induced cell death, as well as the property to maintain calcium homeostasis in these neurons.76 Pilocarpine also reduced retinal I/R damage caused by elevated IOP in vivo in experimental rats.77 This neuroprotective effect seems to be mediated via muscarinic M1 receptors, as activation of these receptors leads to upregulation of NF-E2-related factor-2 (Nrf2), which is the key transcription factor of redox homeostasis resulting in upregulation of oxidant defense genes.78 Pilocarpine significantly increased pulsatile ocular blood flow in patients with untreated OHT in one study.79 In contrast, studies conducted in patients with glaucoma as well as in healthy volunteers found no effect of pilocarpine on the pulsatile component of ocular blood flow.80,81 There is no study supporting that parasympathomimetics exert any non-IOP-related neuroprotective effects in glaucoma patients.
Prostaglandin derivatives
Prostaglandins have been found to increase uveoscleral outflow via binding to Prostaglandin F (FP) receptors, which leads to widening of the ciliary muscle and decompression of the tissue filled spaces along the ciliary muscle bundles.82 In addition, a reduction in extracellular matrix along the uveoscleral outflow pathway has been observed, which again leads to lower resistance.82 Currently available substances include bimatoprost, latanoprost, tafluprost, and travoprost. They lower IOP by 20%–35% from baseline values.45 Prostaglandin analogues are usually well tolerated with almost no systemic side effects.45,59 Common local side effects include conjunctival hyperemia, burning, stinging, eyelash changes, increased periocular skin pigmentation, and increased iris pigmentation. Therefore, unilateral use should be avoided. A few cases of cystoid macular edema in aphakic and pseudophakic patients have been reported, as well as reactivation of herpes keratitis and anterior uveitis.45 Still, prostaglandin analogues can be considered first-choice antiglaucoma medications.45
In rat RGC cultures, prostaglandin analogues showed neuroprotective effects during glutamate exposure as well as during hypoxia, as less RGCs died in comparison to untreated cultures.83 Latanoprost increased RGC survival in a rat model of glaucoma.49 In an inherited glaucoma model in rats, travoprost showed neuroprotective potential by upregulation of calmodulin and downregulation of HMGB1.70 An in vitro study found less Ca2+ influx in RGCs when exposed to glutamate as well as less apoptotic cells when co-treated with tafluprost.84 Tafluprost also reduced the number of apoptotic RGCs and increased the number of surviving RGCs after optic nerve crush in experimental rats.84 The mechanism behind the potential neuroprotective capacity of prostaglandins is not fully understood. The neuroprotective effect of latanoprost seems to be at least partially mediated by the pro-survival p44/p42 mitogen-activated protein kinase (MAPK).84,85 In contrast, bimatoprost seems to prevent from RGC loss via the Akt pathway. Stimulation of the FP receptor by bimatoprost leads to activation of the PI3K/Akt pathway, which not only has a prosurvival effect but also initiates the proapoptotic Raf/MEK/ERK pathway. Activated Akt, however, inhibits Raf phosphorilation and therefore seems to interrupt this pathway.86 Unoprostone, a prostanoid and synthetic docosanoid, seems to be neuroprotective via activation of big potassium channels (BK) which prevents Ca2+ dysregulation as induced by glutamate excitotoxicity that can result in RGC death.87
Prostaglandin analogues have been found to increase ONH and retinal blood flow in humans as well as in experimental animals.88–91 In a study in healthy volunteers, 2-week administration of topical latanoprost also improved choroidal blood flow regulation during both, an experimental increase and decrease in OPP compared with placebo, but this effect is most likely related to the ocular hypotensive effect of the drug.92
Until recently, no clinical outcome data were available for any of the prostaglandin analogues, because most of the large-scale clinical outcome trials were started before this class of antiglaucoma drugs became commercially available. A recent study, however, the United Kingdom Glaucoma Treatment Study (UKGTS), tested the hypothesis that treatment with a topical latanoprost, compared with placebo, reduces the frequency of visual field deterioration events in POAG patients over a 2-year period.93 Indeed, this study showed a significantly reduced visual field progression in the group treated with latanoprost.94 This effect can be most likely explained by the significant difference between the IOP-lowering effect in the 2 groups amounting to 4.5 mmHg in the latanoprost group and 0.6 mmHg in the placebo group.
Do topical antiglaucoma drugs offer non-IOP-lowering effects?
As mentioned in the previous sections, a wide variety of studies focused toward non-IOP-related beneficial effects of topical antiglaucoma drugs. Animal studies using topical IOP-lowering drugs have difficulties in differentiating between IOP-related and non-IOP-related effects. The same holds true for any study showing increased ocular perfusion, because it may result from either pharmacological vasodilatation or the increase in OPP associated with the decrease in IOP. Although some smaller-scale studies have indicated that this may translate into visual field preservation, there is currently no sufficient evidence for a clinically meaningful neuroprotective effect, a conclusion that has recently also been drawn by a Cochrane Review on this subject.95 Neuroprotection studies are difficult to perform for many reasons mentioned later in this review. One additional concern when comparing IOP-lowering drugs with regard to their visual field preserving effect arrives from IOP measurement itself, because lowering IOP at a certain time point does not necessarily result in a comparable 24 h IOP profile. In addition, one can never exclude that the comparator with worse visual field preservation has detrimental effects such as reducing ocular perfusion. In the absence of large-scale head-to-head clinical trials, there is currently no evidence that one drug is superior with regard to its neuroprotective potential while having the same ocular hypotensive effect, but we can also not exclude that it is the case.96
Systemic therapies
Carbonic anhydrase inhibitors
Systemic carbonic anhydrase inhibitors, such as acetazolamide, are among the most effective IOP-lowering agents currently available.97 Oral acetazolamide lowers IOP by about 30% depending on the administered dose.97 Still, considering the side effects, systemic carbonic anhydrase inhibitors are only used when topical therapy is not effective or feasible and only for a short period of time.45 Systemic side effects include renal side effects, anaphylactic reactions to sulphonamides, paresthesia, hearing dysfunction, tinnitus, loss of appetite, gastrointestinal symptoms, depression, metabolic acidosis, and electrolyte imbalance.45
Systemic administration of acetazolamide seems to increase total choroidal blood flow, although not all studies found such an effect.62,98 One study in healthy volunteers found an increase in ONH blood flow after administration of acetazolamide.99 While retinal blood flow, in general, seems to increase, blood flow in the peripapillary region of the retina appears to be reduced after treatment with acetazolamide.99,100
Osmotics
Hyperosmotics such as mannitol or glycerol are potent ocular hypotensive drugs and are used in patients with acute IOP increase. They are reserved for emergency treatment or preoperative situations, as these drugs come with serious side effects such as fluid and electrolyte imbalance, metabolic acidosis, gastrointestinal symptoms, peripheral edema, hypotension, or tachycardia. Patients must be evaluated for their kidney and heart function before administration of these agents.45,59
Impact of other systemic medications on the course of the disease
Systemic calcium channel blockers have been suggested by some authors as potential therapies for a subgroup of glaucoma patients, namely patients with NTG and a predisposition to vasospastic episodes.101,102 Usually these drugs are given in low doses that have little or no effect on blood pressure to prevent detrimental effects on perfusion pressure while inducing local vasodilatation at the eye. On the other hand, it has been reported that patients with systemic hypertension that are either treated with calcium channel blockers or angiotensin-converting enzyme inhibitors are at an increased risk for glaucoma.103 It needs, however, to be mentioned that the doses of calcium channel blockers in these reports were much higher than those usually suggested for glaucoma patients. In another report, systemic beta-receptor antagonists and nitrates were associated with a lower IOP when effects of blood pressure were factored out.104 In addition, retrospective analysis of patient data indicate that intake of statins is protective for glaucoma.105 In the absence of randomized large-scale trials, it is, however, difficult to fully explore the impact of systemic therapies on glaucoma incidence and progression, because a disease-modifying effect of the underlying disease can never be ruled out.
Adherence to medication and alternative routes of administration
Adherence to prescribed medication has been identified as a major problem in glaucoma. Although there are strategies to improve adherence to prescribed anti-glaucoma medications, including simplification of treatment regimens, reduction of costs, patient education, frequent physician visits, and pairing medication administration with specific activities,106 the rate of persistence to therapy after 1 year is considered to be less than 50%.107
Different available topical drugs differ in their frequency of dosing between once and thrice daily. When multiple drugs are prescribed, this results in complex treatment schedules that will further reduce adherence to medication. Use of fixed combinations may partly reduce this problem, because of less frequent instillation and lower exposure to toxic preservatives, thereby reducing the problem of ocular surface disease.108,109 Until recently, all fixed combinations included beta-receptor antagonists with either parasympathomimetics, prostaglandin analogues or carbonic anhydrase inhibitors. Recently, however, a fixed combination between brimonidine and brinzolamide entered the market.110
Additional problems that arise with topical antiglaucoma drugs relate to their potential toxicity at the ocular surface particularly when preservatives are added111 and the poor bioavailability. As such, there is a long-standing search for alternative sustained delivery approaches, including nanoparticle-based formulations, drug-eluting contact lenses, punctum inserts, bioadhesive matrices placed in the conjunctival sac, periocular injections, and surgically implanted drug reservoirs. A full description of these technologies is beyond the scope of this article, but the reader is referred to a recent in-depth review of this topic.112 Obviously such sustained release methods may reduce the problem of a patient's adherence to prescribed medication, improve bioavailability of the drug, and reduce side effects at the ocular surface. There are, however, still issues with sustained release systems, including safety and tolerability related partially to erratic drug release and final burst release.
IOP-lowering therapies under development
Soluble and membrane guanylate cyclases are important regulators of IOP.113 Soluble guanylate cyclases are activated by nitric oxide and generate the secondary signaling molecule cyclic guanosine monophosphate, a pathway that has been extensively studied for its potential to lower IOP.114 Nitric oxide donors reduce IOP through cell volume and contractility changes in the conventional outflow tissues. In addition, nitric oxide plays a key role in regulating ocular blood flow.115 Currently, nitric oxide donating analogs of prostaglandins are intensively studied for their ocular hypotensive effect and are under clinical development. A variety of animal studies indicate that the pharmacologically modified drugs are more efficacious than their respective prostaglandin counterparts.116–118 Recently, it was announced that BOL-303259-X, a nitric oxide-donating latanoprost analogue was superior to latanoprost in reducing IOP in patients with glaucoma or OHT.119 Prostaglandin analogues can also be modified to release hydrogen sulfide (H2S), a gas that has antioxidative properties.120 This molecule was shown to attenuate retinal ischemia and oxidative stress to RGCs in culture.121
A new way to target aqueous outflow regulation is via actin cytoskeleton-modulating signals. Among this pathway, the rho-associated kinase (ROCK) signaling pathway that is activated via secreted bioactive molecules or via integrin activation after extracellular matrix binding has attracted most interest.122 ROCK inhibitors reduce IOP by directly affecting the trabecular meshwork and Schlemm's canal. In addition, ROCK inhibitors disrupt tight junctions, result in F-actin depolymerization, and modulate intracellular calcium level.122 So far, ROCK inhibitors were tested in several clinical trials, but did not come to the market due to side effects, including conjunctival hyperemia and subconjunctival hemorrhages.123 ROCK inhibitors may also exert neuroprotective and anti-inflammatory properties124 and are currently approved in Japan for neuroprotection in subarachnoid hemorrhage. Another related approach is the use of latrunculins, which are macrolides from sponges inhibiting actin polymerization. Their ability to reduce IOP is related to an increase in trabecular meshwork outflow by disrupting the actin cytoskeleton.125,126
The adenosine pathway is another approach that is currently under investigation for glaucoma therapy. Adenosine and its agonists reduce IOP by increasing trabecular meshwork outflow127 and increase ocular blood flow.128 The problem with this type of drugs is, however, that they lose efficacy over time due to tachyphylaxis.
Other approaches
A general problem with non-IOP-related strategies to reduce glaucoma progression relates to the problem of proving efficacy in clinical trials. Reducing IOP with currently available topical antiglaucoma drugs appears to be relatively successful in reducing the rates of visual field loss over time, particularly when adherence to medication is high. As such, it is not easy to prove an additional incremental effect of another agent in a clinical trial. Indeed, the only large-scale trial that tested a non-IOP-related strategy using memantine produced negative results and the original data were never published.129 Nevertheless, it has been argued that it may be possible to perform such trials with optimized designs possibly also employing new endpoints.130 In that regard, improved outcome of retinal function131 as well as combined structural and functional outcomes may play a role in the future.132,133
Vascular approaches
Vascular targets for glaucoma management have been suggested many decades ago, but implementation of this concept has been proved to be difficult. A key problem is that no gold standard technique exists for the measurement of ocular blood flow,134–136 but recent advances in Doppler optical coherence tomography may allow for a truly noninvasive quantification as a surrogate for ocular perfusion.1,37,137 OPP appears to be unsuitable as a treatment target, because no target value can be defined. In addition, the current practice of IOP lowering also increases perfusion pressure.
Evidence has also accumulated that glaucoma is not primarily associated with reduced blood flow but rather with dysregulated perfusion.33,138 The mechanisms of autoregulation in the human eye are, however, complex and not fully explored.139–148 No strategy is currently available to normalize autoregulation in disease states associated with abnormal blood flow regulation in response to changes in perfusion pressure. In addition, the time course of loss of RGCs and decline in blood flow is not fully established. In experimental glaucoma, the onset and progression of microvessel and RGC loss are concomitant.149 The mechanism of loss of microvessels in glaucoma is unknown and requires further study.
Neuroprotection
Since the idea of neuroprotection came up, a lot of hope was associated with this approach. While a variety of experiments in animal models showed promising results, it became clearer that the concept of neuroprotection cannot easily be transferred into clinical practice, particularly when the limited knowledge on the pathophysiology of the disease is considered. This problem is shared with a variety of neurodegenerative diseases in the brain such as stroke or Alzheimer's disease. This review is not intended to give a full overview of neuroprotective strategies in glaucoma, but rather provides a short overview of some of the most interesting targets.
Mechanisms for neuroprotection
Neuroprotection is a term that originally was used in relation to neurodegenerative diseases of the brain describing direct rescue of dying neurons. Since almost 20 years, this concept has also been adapted for glaucomatous optic neuropathy. Definition of the term neuroprotection in glaucoma is not uniform in literature and has been used for strategies that directly rescue RGCs by interfering with cell death, that target glaucoma in a non-IOP-dependent matter or by strategies aimed at preventing axonal loss.150–152 In any definition of neuroprotection, one needs to consider that there is an age-related loss of nerve fibers and RGCs. The variability of this among healthy subjects in different age groups is not well described. Although significant advances have been made in our understanding of the pathways that lead to RGC death in glaucoma, no neuroprotective treatment has been shown to be clinically successful so far. Partially this may be related to our lack of understanding regarding the primary insult in glaucoma.
A complete overview of neuroprotective strategies in glaucoma is beyond the scope of this article, and the reader is referred to some extensive recent review articles.153–157 In animal models and in human glaucoma, RGCs die by apoptosis.158,159 The key elements of apoptotic cell death include chromatin condensation, shrinkage of the cell, blebbing of plasma membranes, mitochondrial disruption, as well as DNA fragmentation. Activation of apoptosis can be initiated either by extrinsic or by intrinsic mechanisms from cytochrome c release from mitochondria. This activates initiator caspases and, in turn, effector caspases that initiate apoptotic protein cleavage. This includes cytoskeletal and associated proteins such as kinases, members of the Bcl-2 family of apoptosis-related proteins, presenilins, and amyloid precursor protein.160 Inhibition of apoptosis by blocking specific caspases is one approach toward RGC survival in glaucoma. A number of inhibitors of apoptosis were identified that exert their function by binding to pro-caspases to prevent their activation or to activated caspases to block their activity.161
Excitotoxicity describes the process by which neurons are killed in response to excessive stimulation by neurotransmitters such as glutamate. This occurs when glutamate receptors such as the N-methyl-D-aspartate (NMDA) receptor and the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor are over-activated by excess release of glutamate. As a result, high levels of calcium Ca2+ enter the cell that is associated with activation of enzymes such as phospholipases, endonucleases, and proteases damaging the cytoskeleton, membrane, and DNA. Indeed, the idea of blocking the NMDA receptor has been suggested as a therapeutic target in many neurodegenerative disorders and as mentioned earlier, an NMDA receptor blocker was the only drug that was studied in a Phase III trial in glaucoma. NMDA receptor antagonists were also employed in a wide variety of clinical trials in brain disease, but were often associated with considerable side effects most likely related to the important physiological functions of NMDA receptors.162 In the brain, doubts have been raised whether so-called “single-target, single-action” agents to target neuronal cells directly and protect neurons from injurious insults can be successful.163
Neurotrophic factor deprivation has been hypothesized to play a key role in RGC death.156 Neurotrophins are diffusible molecules that promote neuronal survival by inhibiting apoptotic pathways. Their family includes nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), neurotrophin-3, and neurotrophin-4/5. Ganglion cells take up BDNF from their target neuron in the brain, and the neurotrophin is transported retrogradely along the axon to the retina. In experimental glaucoma, obstructed axonal transport of BDNF and its receptor tyrosine kinase receptor B was reported.164 As such, exogenous supplementation of BDNF has attracted much interest and was, indeed, successful in a wide variety of animal models,165–167 but the effect is temporary. Attempts to achieve prolonged action of neurotrophic factors are currently under investigation, and phase 2 trials with CNTF are ongoing.
Another strategy relates to protein misfolding describing the failure to fold into native structure associated with the production of proteins that either are inactive or have modified or toxic function. In many neurodegenerative diseases, amyloid deposits are the result of such protein misfolding. In recent years, amyloid deposits have also been implemented in the pathophysiology of glaucoma.168 Indeed, strong immunoreactivity of amyloid precursor protein and amyloid-beta was found in DBA/2J glaucomatous mouse retinas.169 Both in vitro and in vivo studies have shown that β-secretase inhibitors reduce the rate of RGC death in different models.170,171 To which degree, however, protein misfolding is implemented in human glaucoma is unclear and methods to image β-amyloidosis172 are required to elucidate how early in the disease process such changes can be seen. In a mouse model of Alzheimer's disease, impairment of endothelium-dependent regulation of neocortical microcirculation occurs before amyloid-β accumulation,173 a mechanism that has been implemented also in the pathogenesis of POAG.174
The immune system appears to play an important role in glaucoma pathophysiology175 and is associated with an extensive production of T-cells. It has been hypothesized that RGC survival is critically dependent on a balance between protective and harmful immunity in glaucoma.176 This is related to an inflammatory response and the release of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α. Hence, anti-inflammatory drugs that target TNF-α have been proposed for glaucoma treatment. Copolymer-1, a low-affinity synthetic nonencephalitogenic analogue, has been approved by the authorities for the treatment of multiple sclerosis and has been shown to be neuroprotective in a rat model of glaucoma by increasing the number of T-lymphocytes.177 Another group of proteins that is consistently upregulated in glaucoma is heat shock proteins,176 a group of highly conserved proteins that play an important role in cell survival under many conditions. What remains unclear is whether autoantibodies as observed in glaucoma are an epiphenomenon or predispose to RGC loss.178
Oxidative stress, which refers to cellular damage caused by reactive oxygen species (ROS), has been implicated in glaucoma as well as in other neurodegenerative diseases. ROS include free radicals, hydrogen peroxide, and singlet oxygen, and are often the byproducts of oxygen metabolism. Physiologically, a balance is maintained between the generation of free radicals and elimination of these free radicals. The primary source of ROS in the retina are mitochondria.179,180 Oxidative stress has numerous consequences, including cytotoxicity, alterations in signaling RGC death, oxidative protein modifications, and glial dysfunction. Oxidative stress is also implicated in the glaucomatous immune response, because ROS stimulate the antigen presenting ability of glial cells.176 Reducing oxidative stress may be achieved either via reduced production of ROS or via enhanced anti-oxidative capacity. In contrast to age-related macular degeneration,181 there is, however, little evidence that the disease process of glaucoma can be altered by supplementation with high-dose anti-oxidants.
In recent years, much effort was directed toward the maintenance of normal mitochondrial function in glaucoma.182–186 Mitochondria are membrane-bound organelles that generate most of the cell's supply of ATP used as an energy source. A decrease in mitochondrial membrane potential and an increase in membrane permeability appear to play an important role in RGC apoptosis.180 During RGC apoptosis, a number of proteins are released into the cytosol, including cytochrome c, the second mitochondria-derived activator of caspases, the apoptosis-inducing factor, endonuclease G, and the high-temperature-requirement protein A2, but the exact role of these factors in RGC death is not well defined.156 Several targets for improving mitochondrial function in glaucoma have been identified. The best characterized is Coenzyme Q10, also known as ubiquinone, which is an oil-soluble, vitamin-like substance that plays a role in the electron transport chain and participates in aerobic cellular respiration. The molecule exists in a completely oxidized form and a completely reduced form explaining its functions in the electron transport chain and as an antioxidant.
Glial cells appear to be affected early in glaucoma and their role in optic neuropathy has attracted much interest. Activated ONH astrocytes play an important role in remodeling processes.187 Reactive astrocytes in the lamina lead to cellular hypertrophy, expression of glial fibrillary acidic protein, and altered gene expression.49 Astrocytes do not only play a role in maintaining the ONH environment but also produce neurotoxic molecules such as nitric oxide, TNF-α, interleukins, or endothelins, which may promote RGC death. Endothelins are a family of potent vasoconstrictor peptides that show direct neurotoxic effects mediated via the ETB receptor.188 The vasoconstrictor actions of endothelins are primarily mediated via the ETA receptor and, indeed, administration of an ET receptor antagonist leads to an increase in ONH blood flow in glaucoma patients.189
Glial cells also play a major role in regulating ocular blood flow and the signaling between neurons and blood vessels.190,191 They appear to be involved in neurovascular coupling, and arachidonic acid metabolites produced in astrocytes have been proposed to be key mediators of neurovascular coupling.192 Indeed, neurovascular coupling in the retina appears to be disturbed early in glaucoma.190,193,194 In principle, restoration of neurovascular coupling may be an attractive approach for glaucoma, but the mechanisms that lead to its breakdown are poorly understood.
Translation of neuroprotective strategies into clinical practice
It is nowadays clear that translation of neuroprotective strategies into clinical patient care is difficult. Given that a clinical phase III trial with the NMDA receptor antagonist memantine showed negative results, it is questionable whether additional large-scale studies will be performed in near future. A large variety of barriers were identified in translating animal research into clinical setting in glaucoma neuroprotection. A key issue in this regard is the lack of adequate animal models. Currently, many animal models use IOP values that are far beyond the level of IOP that is typically seen in human POAG. In addition, despite several attempts an adequate animal model for NTG is lacking. Most animal models employ young animals only, although age is a major risk factor for the disease.130 The species used for glaucoma animal models are of critical importance. Use of primate models is expensive, but other species commonly used significantly differ in terms of optic nerve anatomy, ocular blood supply, and, most likely, biomechanical properties of the ocular tissues.
Another problem relates to the time course of disease. In humans it typically takes years to develop visual field loss, and treatment is typically initiated long after the unknown start of the disease. In animals, the damage occurs within weeks and the intervention is most often started before glaucoma is induced. Hence, any neuroprotective effect that is seen in an animal model of glaucoma may be much less pronounced when transferred into the human disease. This problem is even more severe, because in clinical trials patients are treated with IOP-lowering drugs for ethical reasons and, hence, the progression rate is slowed down. As such, it becomes more difficult to detect any additional non-IOP-related effect in clinical trials.195 Another problem relates to dosing of drugs. Animal studies often lack dose-response evaluations.130 Moreover, the bioavailability of the drug at the site of action is usually unknown.
Alternative study designs aimed at eliminating ineffective interventions from development may be an attractive goal for neuroprotection research in patients with glaucoma.130 Such futility trials for a neuroprotective agent could be potentially performed in populations of less than 100 participants with a follow-up period of 2 years.130 These futility trials may also include endpoints other than visual field testing, particularly imaging endpoints. This would also help establish imaging endpoints or combined structure/function endpoints by comparison with visual field loss.133,196 With the ongoing advances in imaging, such endpoints may become more feasible, although at the current stage imaging of single RGCs in humans is not yet possible.197
Conclusions
Since the introduction of IOP-lowering medications more than 100 years ago, our abilities to control IOP in glaucoma patients have improved significantly. Due to their excellent efficacy and safety profile, prostaglandin analogues have become the first-line therapy in patients with POAG. Several new candidates for topical IOP-lowering drugs are currently under investigation, but their future potential is difficult to estimate. A major problem with current therapy is adherence to medication. This may be improved with fixed combination therapies reducing the frequency of instillation and providing more sufficient IOP control. Ultimately, sustained release formulations may, however, be required to improve this situation. A large variety of such approaches is currently under development, but safety issues still need to be fully addressed with such approaches. The idea that glaucoma can be targeted via non-IOP-dependent strategies is supported by in vitro and animal data. Currently, however, there is insufficient evidence to claim that a specific topical antiglaucoma drug has clinically relevant neuroprotective properties in addition to lowering IOP. Moreover, no neuroprotective drug has so far been approved for glaucoma treatment and the only phase 3 study in the field has produced negative results. Major barriers in translating such strategies to clinical practice relate not only to the inadequacy of currently available animal models but also to the problems in performing clinical trials. As such, there is a need to improve our current models of glaucoma, to better characterize the potential of candidate drugs, including efficacy and safety in different animal models, drug response relationship, and pharmacokinetic data, and to validate new outcome measures for clinical trials such as combined structure-function endpoints.
Funding
A part of the experimental work mentioned in this article was supported by the following grants: Fonds zur Förderung der Wissenschaftlichen Forschung (FWF), Projects No. APP21570FW and APP21406FW, Die Österreichische Forschungsförderungsgesellschaft (FFG) project FA 607A0502, Christian Doppler Laboratories for Laser Development, and their Application in Medicine and Ocular Effects of Thiomers.
Author Disclosure Statement
The authors report no conflicts of interest.
References
- 1.Cherecheanu A.P., Garhofer G., Schmidl D., Werkmeister R., and Schmetterer L.Ocular perfusion pressure and ocular blood flow in glaucoma. Curr. Opin. Pharmacol. 13:36–42, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman A.L., and Miglior S.Risk factors for glaucoma onset and progression. Surv. Ophthalmol. 53Suppl 1:S3–S10, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Gabelt B.T., and Kaufman P.L.Aqueous humor hydrodynamics. In: Hart W.M., ed. Adler's Physiology of the Eye. 9th ed. St. Louis: MO: Mosby; 2003 [Google Scholar]
- 4.Chowdhury U.R., Madden B.J., Charlesworth M.C., and Fautsch M.P.Proteome analysis of human aqueous humor. Invest. Ophthalmol. Vis. Sci. 51:4921–4931, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goel M., Picciani R.G., Lee R.K., and Bhattacharya S.K.Aqueous humor dynamics: a review. Open Ophthalmol. J. 4:52–59, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Civan M.M., and Macknight A.D.C.The ins and outs of aqueous humour secretion. Exp. Eye Res. 78:625–631, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Schey K.L., Wang Z., Wenke J.L., and Qi Y.Aquaporins in the eye: expression, function, and roles in ocular disease. BBA-Gen. Subj. 1840:1513–1523, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heys J.J., and Barocas V.H.A boussinesq model of natural convection in the human eye and the formation of Krukenberg's spindle. Ann. Biomed. Eng. 30:392–401, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Tamm E.R.The trabecular meshwork outflow pathways: structural and functional aspects. Exp. Eye Res. 88:648–655, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Johnson M.‘What controls aqueous humour outflow resistance?’. Exp. Eye Res. 82:545–557, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bill A.Some aspects of aqueous humour drainage. Eye. 7:14–19, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Stamer W.D., and Acott T.S.Current understanding of conventional outflow dysfunction in glaucoma. Curr. Opin. Ophthalmol. 23:135–143, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramos R.F., Hoying J.B., Witte M.H., and Daniel Stamer W.Schlemm's canal endothelia, lymphatic, or blood vasculature? J. Glaucoma. 16:391–405, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Alm A., and Nilsson S.F.E.Uveoscleral outflow–a review. Exp. Eye Res. 88:760–768, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Nilsson S.F.E.The uveoscleral outflow routes. Eye. 11:149–154, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Yucel Y.H., Johnston M.G., Ly T., et al. Identification of lymphatics in the ciliary body of the human eye: a novel “uveolymphatic” outflow pathway. Exp. Eye Res. 89:810–819, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Kim M., Johnston M.G., Gupta N., Moore S., and Yucel Y.H.A model to measure lymphatic drainage from the eye. Exp. Eye Res. 93:586–591, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Tam A.L., Gupta N., Zhang Z., and Yucel Y.H.Latanoprost stimulates ocular lymphatic drainage: an in vivo nanotracer study. Transl. Vis. Sci. Technol. 2:3, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Truong T.N., Li H., Hong Y.K., and Chen L.Novel characterization and live imaging of Schlemm's canal expressing Prox-1. PLoS One. 9:e98245, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vass C., Hirn C., Sycha T., Findl O., Bauer P., and Schmetterer L. Medical interventions for primary open angle glaucoma and ocular hypertension. Cochrane Database Syst. Rev. CD003167, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarado J., Murphy C., and Juster R.Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 91:564–579, 1984 [DOI] [PubMed] [Google Scholar]
- 22.Rohen J.W., Lutjen-Drecoll E., Flugel C., Meyer M., and Grierson I.Ultrastructure of the trabecular meshwork in untreated cases of primary open-angle glaucoma (POAG). Exp. Eye Res. 56:683–692, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Gottanka J., Johnson D.H., Martus P., and Lutjen-Drecoll E.Severity of optic nerve damage in eyes with POAG is correlated with changes in the trabecular meshwork. J. Glaucoma. 6:123–132, 1997 [PubMed] [Google Scholar]
- 24.Fuchshofer R., and Tamm E.R.The role of TGF-beta in the pathogenesis of primary open-angle glaucoma. Cell. Tissue Res. 347:279–290, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Babizhayev M.A.Biomarkers and special features of oxidative stress in the anterior segment of the eye linked to lens cataract and the trabecular meshwork injury in primary open-angle glaucoma: challenges of dual combination therapy with N-acetylcarnosine lubricant eye drops and oral formulation of nonhydrolyzed carnosine. Fundam. Clin. Pharmacol. 26:86–117, 2012 [DOI] [PubMed] [Google Scholar]
- 26.McDonnell F., O'Brien C., and Wallace D.The role of epigenetics in the fibrotic processes associated with glaucoma. J. Ophthalmol. 2014:750459, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flammer J., Orgul S., Costa V.P., et al. The impact of ocular blood flow in glaucoma. Prog. Retin. Eye Res. 21:359–393, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Sehi M., Goharian I., Konduru R., et al. Retinal blood flow in glaucomatous eyes with single-hemifield damage. Ophthalmology. 121:750–758, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Resch H., Schmidl D., Hommer A., et al. Correlation of optic disc morphology and ocular perfusion parameters in patients with primary open angle glaucoma. Acta Ophthalmol. 89:e544–S549, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Portmann N., Gugleta K., Kochkorov A., Polunina A., Flammer J., and Orgul S.Choroidal blood flow response to isometric exercise in glaucoma patients and patients with ocular hypertension. Invest. Ophthalmol. Vis. Sci. 52:7068–7073, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Feke G.T., and Pasquale L.R.Retinal blood flow response to posture change in glaucoma patients compared with healthy subjects. Ophthalmology. 115:246–252, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Fuchsjager-Mayrl G., Wally B., Georgopoulos M., et al. Ocular blood flow and systemic blood pressure in patients with primary open-angle glaucoma and ocular hypertension. Invest. Ophthalmol. Vis. Sci. 45:834–839, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Schmidl D., Garhofer G., and Schmetterer L.The complex interaction between ocular perfusion pressure and ocular blood flow—relevance for glaucoma. Exp. Eye Res. 93:141–155, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Leske M.C., Heijl A., Hyman L., Bengtsson B., Dong L., and Yang Z.Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 114:1965–1972, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Leske M.C., Wu S.Y., Hennis A., Honkanen R., and Nemesure B.Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology. 115:85–93, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Bonomi L., Marchini G., Marraffa M., Bernardi P., Morbio R., and Varotto A.Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology. 107:1287–1293, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Costa V.P., Harris A., Anderson D., et al. Ocular perfusion pressure in glaucoma. Acta Ophthalmol. 92:e252–e266, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Caprioli J., and Coleman A.L.Blood pressure, perfusion pressure, and glaucoma. Am. J. Ophthalmol. 149:704–712, 2010 [DOI] [PubMed] [Google Scholar]
- 39.He Z., Vingrys A.J., Armitage J.A., and Bui B.V.The role of blood pressure in glaucoma. Clin. Exp. Optom. 94:133–149, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Leske M.C.Ocular perfusion pressure and glaucoma: clinical trial and epidemiologic findings. Curr. Opin. Ophthalmol. 20:73–78, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Philipp M., Brede M., and Hein L.Physiological significance of alpha(2)-adrenergic receptor subtype diversity: one receptor is not enough. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283:R287–R295, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Toris C.B.Pharmacotherapies for glaucoma. Curr. Mol. Med. 10:824–840, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Arthur S., and Cantor L.B.Update on the role of alpha-agonists in glaucoma management. Exp. Eye Res. 93:271–283, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Toris C.B., Camras C.B., and Yablonski M.E.Acute versus chronic effects of brimonidine on aqueous humor dynamics in ocular hypertensive patients. Am. J. Ophthalmol. 128:8–14, 1999 [DOI] [PubMed] [Google Scholar]
- 45.European Glaucoma Society. Terminology and guidelines for glaucoma. 3rd ed. Savona: Editrice DOGMA; 2008 [Google Scholar]
- 46.Kalapesi F.B., Coroneo M.T., and Hill M.A.Human ganglion cells express the alpha-2 adrenergic receptor: relevance to neuroprotection. Br. J. Ophthalmol. 89:758–763, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vidal-Sanz M., Lafuente M.P., Mayor-Torroglosa S., Aguilera M.E., Miralles de Imperial J., and Villegas-Perez M.P.Brimonidine's neuroprotective effects against transient ischaemia-induced retinal ganglion cell death. Eur. J. Ophthalmol. 11Suppl 2:S36–S40, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Lafuente López-Herrera M., amp x., et al. Transient ischemia of the retina results in altered retrograde axoplasmic transport: neuroprotection with brimonidine. Exp. Neurol. 178:243–258, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Hernandez M., Urcola J.H., and Vecino E.Retinal ganglion cell neuroprotection in a rat model of glaucoma following brimonidine, latanoprost or combined treatments. Exp. Eye Res. 86:798–806, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Dong C.-J., Guo Y., Agey P., Wheeler L., and Hare W.A.α2 adrenergic modulation of NMDA receptor function as a major mechanism of RGC protection in experimental glaucoma and retinal excitotoxicity. Invest. Ophthalmol. Vis. Sci. 49:4515–4522, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Krupin T., Liebmann J.M., Greenfield D.S., Ritch R., and Gardiner S.A randomized trial of brimonidine versus timolol in preserving visual function: results from the Low-Pressure Glaucoma Treatment Study. Am. J. Ophthalmol. 151:671–681, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Kersey T., Clement C.I., Bloom P., and Cordeiro M.F.New trends in glaucoma risk, diagnosis & management. Indian J. Med. Res. 137:659–668, 2013 [PMC free article] [PubMed] [Google Scholar]
- 53.Grieshaber M.C., and Flammer J.Is the medication used to achieve the target intraocular pressure in glaucoma therapy of relevance?—an exemplary analysis on the basis of two beta-blockers. Prog. Retin. Eye Res. 29:79–93, 2010 [DOI] [PubMed] [Google Scholar]
- 54.Pfeiffer N., Lamparter J., Gericke A., Grus F.H., Hoffmann E.M., and Wahl J.Neuroprotection of medical IOP-lowering therapy. Cell. Tissue Res. 353:245–251, 2013 [DOI] [PubMed] [Google Scholar]
- 55.Lama P.J.Systemic adverse effects of beta-adrenergic blockers: an evidence-based assessment. Am. J. Ophthalmol. 134:749–760, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Wood J.P.M., Schmidt K.G., Melena J., Chidlow G., Allmeier H., and Osborne N.N.The β-adrenoceptor antagonists metipranolol and timolol are retinal neuroprotectants: comparison with betaxolol. Exp. Eye Res. 76:505–516, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Seki M., Tanaka T., Matsuda H., et al. Topically administered timolol and dorzolamide reduce intraocular pressure and protect retinal ganglion cells in a rat experimental glaucoma model. Br. J. Ophthalmol. 89:504–507, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Osborne N.N., Wood J.P., and Chidlow G.Invited review: neuroprotective properties of certain beta-adrenoceptor antagonists used for the treatment of glaucoma. J. Ocul. Pharmacol. Ther. 21:175–181, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Sambhara D., and Aref A.A.Glaucoma management: relative value and place in therapy of available drug treatments. Ther. Adv. Chronic. Dis. 5:30–43, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kiss B., Dallinger S., Findl O., Rainer G., Eichler H.G., and Schmetterer L.Acetazolamide-induced cerebral and ocular vasodilation in humans is independent of nitric oxide. Am. J. Physiol. 276:R1661–R1667, 1999 [DOI] [PubMed] [Google Scholar]
- 61.Dahl A., Russell D., Nyberg-Hansen R., Rootwelt K., and Mowinckel P.Simultaneous assessment of vasoreactivity using transcranial Doppler ultrasound and cerebral blood flow in healthy subjects. J. Cereb. Blood Flow Metab. 14:974–981, 1994 [DOI] [PubMed] [Google Scholar]
- 62.Dallinger S., Bobr B., Findl O., Eichler H.G., and Schmetterer L.Effects of acetazolamide on choroidal blood flow. Stroke. 29:997–1001, 1998 [DOI] [PubMed] [Google Scholar]
- 63.Barnes G.E., Li B., Dean T., and Chandler M.L.Increased optic nerve head blood flow after 1 week of twice daily topical brinzolamide treatment in Dutch-belted rabbits. Surv. Ophthalmol. 44Suppl 2:S131–S140, 2000 [DOI] [PubMed] [Google Scholar]
- 64.Siesky B., Harris A., Cantor L.B., et al. A comparative study of the effects of brinzolamide and dorzolamide on retinal oxygen saturation and ocular microcirculation in patients with primary open-angle glaucoma. Br. J. Ophthalmol. 92:500–504, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Iester M., Altieri M., Michelson G., Vittone P., Traverso C.E., and Calabria G.Retinal peripapillary blood flow before and after topical brinzolamide. Ophthalmologica. 218:390–396, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Rolle T., Tofani F., Brogliatti B., and Grignolo F.M.The effects of dorzolamide 2% and dorzolamide/timolol fixed combination on retinal and optic nerve head blood flow in primary open-angle glaucoma patients. Eye (Lond). 22:1172–1179, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Fuchsjager-Mayrl G., Wally B., Rainer G., et al. Effect of dorzolamide and timolol on ocular blood flow in patients with primary open angle glaucoma and ocular hypertension. Br. J. Ophthalmol. 89:1293–1297, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nagel E., Vilser W., and Lanzl I.Dorzolamide influences the autoregulation of major retinal vessels caused by artificial intraocular pressure elevation in patients with POAG: a clinical study. Curr. Eye. Res. 30:129–137, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Fuchsjager-Mayrl G., Georgopoulos M., Hommer A., et al. Effect of dorzolamide and timolol on ocular pressure: blood flow relationship in patients with primary open-angle glaucoma and ocular hypertension. Invest. Ophthalmol. Vis. Sci. 51:1289–1296, 2010 [DOI] [PubMed] [Google Scholar]
- 70.Schallenberg M., Prokosch V., and Thanos S.Regulation of retinal proteome by topical antiglaucomatous eye drops in an inherited glaucoma rat model. PLoS One. 7:e33593, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zanon-Moreno V., Garcia-Medina J.J., Gallego-Pinazo R., Vinuesa-Silva I., Moreno-Nadal M.A., and Pinazo-Duran M.D.Antioxidant status modifications by topical administration of dorzolamide in primary open-angle glaucoma. Eur. J. Ophthalmol. 19:565–571, 2009 [DOI] [PubMed] [Google Scholar]
- 72.Saccà S., La Maestra S., Micale R.T., et al. Ability of dorzolamide hydrochloride and timolol maleate to target mitochondria in glaucoma therapy. Arch. Ophthalmol. 129:48–55, 2011 [DOI] [PubMed] [Google Scholar]
- 73.Martinez A., and Sanchez-Salorio M.Predictors for visual field progression and the effects of treatment with dorzolamide 2% or brinzolamide 1% each added to timolol 0.5% in primary open-angle glaucoma. Acta Ophthalmol. 88:541–552, 2010 [DOI] [PubMed] [Google Scholar]
- 74.Miglior S., Zeyen T., Pfeiffer N., Cunha-Vaz J., Torri V., and Adamsons I.Results of the European Glaucoma Prevention Study. Ophthalmology. 112:366–375, 2005 [DOI] [PubMed] [Google Scholar]
- 75.Quigley H.A.European Glaucoma Prevention Study. Ophthalmology. 2005;112:1642–1643; author reply 1643–1645 [DOI] [PubMed] [Google Scholar]
- 76.Zhou W., Zhu X., Zhu L., et al. Neuroprotection of muscarinic receptor agonist pilocarpine against glutamate-induced apoptosis in retinal neurons. Cell. Mol. Neurobiol. 28:263–275, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tan P.P., Yuan H.H., Zhu X., et al. Activation of muscarinic receptors protects against retinal neurons damage and optic nerve degeneration in vitro and in vivo models. CNS Neurosci. Ther. 20:227–236, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Espada S., Rojo A.I., Salinas M., and Cuadrado A.The muscarinic M1 receptor activates Nrf2 through a signaling cascade that involves protein kinase C and inhibition of GSK-3beta: connecting neurotransmission with neuroprotection. J. Neurochem. 110:1107–1119, 2009 [DOI] [PubMed] [Google Scholar]
- 79.Shaikh M.H., and Mars J.S.The acute effect of pilocarpine on pulsatile ocular blood flow in ocular hypertension. Eye (Lond). 15:63–66, 2001 [DOI] [PubMed] [Google Scholar]
- 80.Claridge K.G.The effect of topical pilocarpine on pulsatile ocular blood flow. Eye (Lond). 7:507–510, 1993 [DOI] [PubMed] [Google Scholar]
- 81.Schmetterer L., Strenn K., Findl O., et al. Effects of antiglaucoma drugs on ocular hemodynamics in healthy volunteers. Clin. Pharmacol. Ther. 61:583–595, 1997 [DOI] [PubMed] [Google Scholar]
- 82.Weinreb R.N., Toris C.B., Gabelt B.A.T., Lindsey J.D., and Kaufman P.L.Effects of prostaglandins on the aqueous humor outflow pathways. Surv. Ophthalmol. 47Suppl 1:S53–S64, 2002 [DOI] [PubMed] [Google Scholar]
- 83.Yamagishi R., Aihara M., and Araie M.Neuroprotective effects of prostaglandin analogues on retinal ganglion cell death independent of intraocular pressure reduction. Exp. Eye Res. 93:265–270, 2011 [DOI] [PubMed] [Google Scholar]
- 84.Kanamori A., Naka M., Fukuda M., Nakamura M., and Negi A.Latanoprost protects rat retinal ganglion cells from apoptosis in vitro and in vivo. Exp. Eye Res. 88:535–541, 2009 [DOI] [PubMed] [Google Scholar]
- 85.Nakanishi Y., Nakamura M., Mukuno H., Kanamori A., Seigel G.M., and Negi A.Latanoprost rescues retinal neuro-glial cells from apoptosis by inhibiting caspase-3, which is mediated by p44/p42 mitogen-activated protein kinase. Exp. Eye Res. 83:1108–1117, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Takano N., Tsuruma K., Ohno Y., Shimazawa M., and Hara H.Bimatoprost protects retinal neuronal damage via Akt pathway. Eur. J. Pharmacol. 702:56–61, 2013 [DOI] [PubMed] [Google Scholar]
- 87.Cuppoletti J., Malinowska D.H., Tewari K.P., Chakrabarti J., and Ueno R.Cellular and molecular effects of unoprostone as a BK channel activator. BBA-Biomembrans. 1768:1083–1092, 2007 [DOI] [PubMed] [Google Scholar]
- 88.Akaishi T., Kurashima H., Odani-Kawabata N., Ishida N., and Nakamura M.Effects of repeated administrations of tafluprost, latanoprost, and travoprost on optic nerve head blood flow in conscious normal rabbits. J. Ocul. Pharmacol. Ther. 26:181–186, 2010 [DOI] [PubMed] [Google Scholar]
- 89.Kimura I., Shinoda K., Tanino T., Ohtake Y., and Mashima Y.Effect of topical unoprostone isopropyl on optic nerve head circulation in controls and in normal-tension glaucoma patients. Jpn. J. Ophthalmol. 49:287–293, 2005 [DOI] [PubMed] [Google Scholar]
- 90.Ishii K., Tomidokoro A., Nagahara M., et al. Effects of topical latanoprost on optic nerve head circulation in rabbits, monkeys, and humans. Invest. Ophthalmol. Vis. Sci. 42:2957–2963, 2001 [PubMed] [Google Scholar]
- 91.Izumi N., Nagaoka T., Sato E., et al. Short-term effects of topical tafluprost on retinal blood flow in cats. J. Ocul. Pharmacol. Ther. 24:521–526, 2008 [DOI] [PubMed] [Google Scholar]
- 92.Boltz A., Schmidl D., Weigert G., et al. Effect of latanoprost on choroidal blood flow regulation in healthy subjects. Invest. Ophthalmol. Vis. Sci. 52:4410–4415, 2011 [DOI] [PubMed] [Google Scholar]
- 93.Garway-Heath D.F., Lascaratos G., Bunce C., Crabb D.P., Russell R.A., and Shah A.The United Kingdom Glaucoma Treatment Study: a multicenter, randomized, placebo-controlled clinical trial: design and methodology. Ophthalmology. 120:68–76, 2013 [DOI] [PubMed] [Google Scholar]
- 94.Garway-Heath D., Lascaratos G., Bunce C., et al. The United Kingdom Glaucoma Treatment Study (UKGTS): baseline characteristics and main outcomes. Invest. Ophthalmol. Vis. Sci. 54:2619-, 2013 [DOI] [PubMed] [Google Scholar]
- 95.Sena D.F., and Lindsley K.Neuroprotection for treatment of glaucoma in adults. Cochrane Database Syst. Rev. 2:CD006539, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schmetterer L.Are all glaucoma drugs equally effective? Acta Ophthalmol. 88:503, 2010 [DOI] [PubMed] [Google Scholar]
- 97.Kaur I.P., Smitha R., Aggarwal D., and Kapil M.Acetazolamide: future perspective in topical glaucoma therapeutics. Int. J. Pharm. 248:1–14, 2002 [DOI] [PubMed] [Google Scholar]
- 98.Zinkernagel M.S., and Ebneter A.Acetazolamide influences ocular pulse amplitude. J. Ocul. Pharmacol. Ther. 25:141–144, 2009 [DOI] [PubMed] [Google Scholar]
- 99.Haustein M., Spoerl E., and Boehm A.G.The effect of acetazolamide on different ocular vascular beds. Graefes Arch. Clin. Exp. Ophthalmol. 251:1389–1398, 2013 [DOI] [PubMed] [Google Scholar]
- 100.Rassam S.M., Patel V., and Kohner E.M.The effect of acetazolamide on the retinal circulation. Eye (Lond). 7:697–702, 1993 [DOI] [PubMed] [Google Scholar]
- 101.Flammer J., Pache M., and Resink T.Vasospasm, its role in the pathogenesis of diseases with particular reference to the eye. Prog. Retin. Eye Res. 20:319–349, 2001 [DOI] [PubMed] [Google Scholar]
- 102.Mayama C., and Araie M.Effects of antiglaucoma drugs on blood flow of optic nerve heads and related structures. Jpn. J. Ophthalmol. 57:133–149, 2013 [DOI] [PubMed] [Google Scholar]
- 103.Muskens R.P., de Voogd S., Wolfs R.C., et al. Systemic antihypertensive medication and incident open-angle glaucoma. Ophthalmology. 114:2221–2226, 2007 [DOI] [PubMed] [Google Scholar]
- 104.Khawaja A.P., Chan M.P., Broadway D.C., et al. Systemic medication and intraocular pressure in a British population: the EPIC-Norfolk Eye Study. Ophthalmology. 121:1501–1507, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McGwin G., Jr., McNeal S., Owsley C., Girkin C., Epstein D., and Lee P.P.Statins and other cholesterol-lowering medications and the presence of glaucoma. Arch. Ophthalmol. 122:822–826, 2004 [DOI] [PubMed] [Google Scholar]
- 106.Budenz D.L.A clinician's guide to the assessment and management of nonadherence in glaucoma. Ophthalmology. 116:S43–S47, 2009 [DOI] [PubMed] [Google Scholar]
- 107.Schwartz G.F., and Quigley H.A.Adherence and persistence with glaucoma therapy. Surv. Ophthalmol. 53Suppl 1:S57–S68, 2008 [DOI] [PubMed] [Google Scholar]
- 108.Hommer A.[Combination therapy in the medical treatment of glaucoma]. Klin. Monbl. Augenheilkd. 230:133–140, 2013 [DOI] [PubMed] [Google Scholar]
- 109.Hollo G., Topouzis F., and Fechtner R.D.Fixed-combination intraocular pressure-lowering therapy for glaucoma and ocular hypertension: advantages in clinical practice. Expert. Opin. Pharmacother. 15:1737–1747, 2014 [DOI] [PubMed] [Google Scholar]
- 110.Nguyen Q.H.Combination of brinzolamide and brimonidine for glaucoma and ocular hypertension: critical appraisal and patient focus. Patient Prefer. Adherence. 8:853–864, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baudouin C., Labbe A., Liang H., Pauly A., and Brignole-Baudouin F.Preservatives in eyedrops: the good, the bad and the ugly. Prog. Retin. Eye Res. 29:312–334, 2010 [DOI] [PubMed] [Google Scholar]
- 112.Knight O.J., and Lawrence S.D.Sustained drug delivery in glaucoma. Curr. Opin. Ophthalmol. 25:112–117, 2014 [DOI] [PubMed] [Google Scholar]
- 113.Buys E.S., Potter L.R., Pasquale L.R., and Ksander B.R.Regulation of intraocular pressure by soluble and membrane guanylate cyclases and their role in glaucoma. Front Mol. Neurosci. 7:38, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cavet M.E., Vittitow J.L., Impagnatiello F., Ongini E., and Bastia E.Nitric oxide (NO): an emerging target for the treatment of glaucoma. Invest. Ophthalmol. Vis. Sci. 55:5005–5015, 2014 [DOI] [PubMed] [Google Scholar]
- 115.Schmetterer L., and Polak K.Role of nitric oxide in the control of ocular blood flow. Prog. Retin. Eye Res. 20:823–847, 2001 [DOI] [PubMed] [Google Scholar]
- 116.Borghi V., Bastia E., Guzzetta M., et al. A novel nitric oxide releasing prostaglandin analog, NCX 125, reduces intraocular pressure in rabbit, dog, and primate models of glaucoma. J. Ocul. Pharmacol. Ther. 26:125–132, 2010 [DOI] [PubMed] [Google Scholar]
- 117.Impagnatiello F., Borghi V., Gale D.C., et al. A dual acting compound with latanoprost amide and nitric oxide releasing properties, shows ocular hypotensive effects in rabbits and dogs. Exp. Eye Res. 93:243–249, 2011 [DOI] [PubMed] [Google Scholar]
- 118.Krauss A.H., Impagnatiello F., Toris C.B., et al. Ocular hypotensive activity of BOL-303259-X, a nitric oxide donating prostaglandin F2alpha agonist, in preclinical models. Exp. Eye Res. 93:250–255, 2011. http://bmctoday.net/glaucomatoday/2012/04/article.asp?f=glaucoma-candidate-bol303259x-meets-primary-endpoint-in-phase-2b-study [DOI] [PubMed]
- 119.Glaucoma Candidate BOL-303259-X Meets Primary Endpoint in Phase 2b Study. Glaucoma Today: Bryn Mawr Communications LLC; 2012 [Google Scholar]
- 120.Sparatore A., Santus G., Giustarini D., Rossi R., and Del Soldato P.Therapeutic potential of new hydrogen sulfide-releasing hybrids. Expert. Rev. Clin. Pharmacol. 4:109–121, 2011 [DOI] [PubMed] [Google Scholar]
- 121.Osborne N.N., Ji D., Majid A.S., Del Soldata P., and Sparatore A.Glutamate oxidative injury to RGC-5 cells in culture is necrostatin sensitive and blunted by a hydrogen sulfide (H2S)-releasing derivative of aspirin (ACS14). Neurochem. Int. 60:365–378, 2012 [DOI] [PubMed] [Google Scholar]
- 122.Inoue T., and Tanihara H.Rho-associated kinase inhibitors: a novel glaucoma therapy. Prog. Retin. Eye Res. 37:1–12, 2013 [DOI] [PubMed] [Google Scholar]
- 123.Williams R.D., Novack G.D., van Haarlem T., and Kopczynski C.Ocular hypotensive effect of the Rho kinase inhibitor AR-12286 in patients with glaucoma and ocular hypertension. Am. J. Ophthalmol. 152:834–841 e831, 2011 [DOI] [PubMed] [Google Scholar]
- 124.Zhang K., Zhang L., and Weinreb R.N.Ophthalmic drug discovery: novel targets and mechanisms for retinal diseases and glaucoma. Nat. Rev. Drug Discov. 11:541–559, 2012 [DOI] [PubMed] [Google Scholar]
- 125.Peterson J.A., Tian B., McLaren J.W., Hubbard W.C., Geiger B., and Kaufman P.L.Latrunculins' effects on intraocular pressure, aqueous humor flow, and corneal endothelium. Invest. Ophthalmol. Vis. Sci. 41:1749–1758, 2000 [PubMed] [Google Scholar]
- 126.Peterson J.A., Tian B., Geiger B., and Kaufman P.L.Effect of latrunculin-B on outflow facility in monkeys. Exp. Eye Res. 70:307–313, 2000 [DOI] [PubMed] [Google Scholar]
- 127.Tian B., Gabelt B.T., Crosson C.E., and Kaufman P.L.Effects of adenosine agonists on intraocular pressure and aqueous humor dynamics in cynomolgus monkeys. Exp. Eye Res. 64:979–989, 1997 [DOI] [PubMed] [Google Scholar]
- 128.Polska E., Ehrlich P., Luksch A., Fuchsjager-Mayrl G., and Schmetterer L.Effects of adenosine on intraocular pressure, optic nerve head blood flow, and choroidal blood flow in healthy humans. Invest. Ophthalmol. Vis. Sci. 44:3110–3114, 2003 [DOI] [PubMed] [Google Scholar]
- 129.Osborne N.N.Recent clinical findings with memantine should not mean that the idea of neuroprotection in glaucoma is abandoned. Acta Ophthalmol. 87:450–454, 2009 [DOI] [PubMed] [Google Scholar]
- 130.Quigley H.A.Clinical trials for glaucoma neuroprotection are not impossible. Curr. Opin. Ophthalmol. 23:144–154, 2012 [DOI] [PubMed] [Google Scholar]
- 131.Zhu H., Russell R.A., Saunders L.J., Ceccon S., Garway-Heath D.F., and Crabb D.P.Detecting changes in retinal function: Analysis with Non-Stationary Weibull Error Regression and Spatial enhancement (ANSWERS). PLoS One. 9:e85654, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Harwerth R.S., Wheat J.L., Fredette M.J., and Anderson D.R.Linking structure and function in glaucoma. Prog. Retin. Eye Res. 29:249–271, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Malik R., Swanson W.H., and Garway-Heath D.F.‘Structure-function relationship’ in glaucoma: past thinking and current concepts. Clin. Exp. Ophthalmol. 40:369–380, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Riva C.E., Geiser M., and Petrig B.L.Ocular blood flow assessment using continuous laser Doppler flowmetry. Acta Ophthalmol. 88:622–629, 2010 [DOI] [PubMed] [Google Scholar]
- 135.Sugiyama T., Araie M., Riva C.E., Schmetterer L., and Orgul S.Use of laser speckle flowgraphy in ocular blood flow research. Acta Ophthalmol. 88:723–729, 2010 [DOI] [PubMed] [Google Scholar]
- 136.Stalmans I., Vandewalle E., Anderson D.R., et al. Use of colour Doppler imaging in ocular blood flow research. Acta Ophthalmol. 89:e609–S630, 2011 [DOI] [PubMed] [Google Scholar]
- 137.Grover D.S., and Budenz D.L.Ocular perfusion pressure and glaucoma. Int. Ophthalmol. Clin. 51:19–25, 2011 [DOI] [PubMed] [Google Scholar]
- 138.Mozaffarieh M., and Flammer J.Is there more to glaucoma treatment than lowering IOP? Surv. Ophthalmol. 52Suppl 2:S174–S179, 2007 [DOI] [PubMed] [Google Scholar]
- 139.Kiel J.W.Modulation of choroidal autoregulation in the rabbit. Exp. Eye Res. 69:413–429, 1999 [DOI] [PubMed] [Google Scholar]
- 140.Kiel J.W., and van Heuven W.A.Ocular perfusion pressure and choroidal blood flow in the rabbit. Invest. Ophthalmol. Vis. Sci. 36:579–585, 1995 [PubMed] [Google Scholar]
- 141.Luksch A., Polska E., Imhof A., et al. Role of NO in choroidal blood flow regulation during isometric exercise in healthy humans. Invest. Ophthalmol. Vis. Sci. 44:734–739, 2003 [DOI] [PubMed] [Google Scholar]
- 142.Fuchsjager-Mayrl G., Luksch A., Malec M., Polska E., Wolzt M., and Schmetterer L.Role of endothelin-1 in choroidal blood flow regulation during isometric exercise in healthy humans. Invest. Ophthalmol. Vis. Sci. 44:728–733, 2003 [DOI] [PubMed] [Google Scholar]
- 143.Polska E., Simader C., Weigert G., et al. Regulation of choroidal blood flow during combined changes in intraocular pressure and arterial blood pressure. Invest. Ophthalmol. Vis. Sci. 48:3768–3774, 2007 [DOI] [PubMed] [Google Scholar]
- 144.Schmidl D., Boltz A., Kaya S., et al. Role of nitric oxide in optic nerve head blood flow regulation during an experimental increase in intraocular pressure in healthy humans. Exp. Eye Res. 116:247–253, 2013 [DOI] [PubMed] [Google Scholar]
- 145.Schmidl D., Boltz A., Kaya S., et al. Role of nitric oxide in optic nerve head blood flow regulation during isometric exercise in healthy humans. Invest. Ophthalmol. Vis. Sci. 54:1964–1970, 2013 [DOI] [PubMed] [Google Scholar]
- 146.Boltz A., Schmidl D., Werkmeister R.M., et al. Role of endothelin-A receptors in optic nerve head red cell flux regulation during isometric exercise in healthy humans. Am. J. Physiol. Heart Circ. Physiol. 2013;304:H170–H174 [DOI] [PubMed] [Google Scholar]
- 147.Boltz A., Schmidl D., Werkmeister R.M., et al. Regulation of optic nerve head blood flow during combined changes in intraocular pressure and arterial blood pressure. J. Cereb. Blood Flow Metab. 33:1850–1856, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Boltz A., Told R., Napora K.J., et al. Optic nerve head blood flow autoregulation during changes in arterial blood pressure in healthy young subjects. PLoS One. 8:e82351, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Almasieh M., MacIntyre J.N., Pouliot M., et al. Acetylcholinesterase inhibition promotes retinal vasoprotection and increases ocular blood flow in experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 54:3171–3183, 2013 [DOI] [PubMed] [Google Scholar]
- 150.Schwartz M., Belkin M., Yoles E., and Solomon A.Potential treatment modalities for glaucomatous neuropathy: neuroprotection and neuroregeneration. J. Glaucoma. 5:427–432, 1996 [PubMed] [Google Scholar]
- 151.Levin L.A.Direct and indirect approaches to neuroprotective therapy of glaucomatous optic neuropathy. Surv. Ophthalmol. 43Suppl 1:S98–S101, 1999 [DOI] [PubMed] [Google Scholar]
- 152.Osborne N.N., Chidlow G., Nash M.S., and Wood J.P.The potential of neuroprotection in glaucoma treatment. Curr. Opin. Ophthalmol. 10:82–92, 1999 [DOI] [PubMed] [Google Scholar]
- 153.Baltmr A., Duggan J., Nizari S., Salt T.E., and Cordeiro M.F.Neuroprotection in glaucoma - Is there a future role? Exp. Eye Res. 91:554–566, 2010 [DOI] [PubMed] [Google Scholar]
- 154.Danesh-Meyer H.V.Neuroprotection in glaucoma: recent and future directions. Curr. Opin. Ophthalmol. 22:78–86, 2011 [DOI] [PubMed] [Google Scholar]
- 155.Kitsos E.B.G.Neuroprotective Agents in Glaucoma. In: Kubena T. ed. The Mystery of Glaucoma. INTECH; 2011. http://de.scribd.com/doc/115450822/The-Mystery-of-Glaucoma, accessed on January5, 2015 [Google Scholar]
- 156.Almasieh M., Wilson A.M., Morquette B., Cueva Vargas J.L., and Di Polo A.The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 31:152–181, 2012 [DOI] [PubMed] [Google Scholar]
- 157.Tsai J.C.Canadian Journal of Ophthalmology Lecture: translational research advances in glaucoma neuroprotection. Can. J. Ophthalmol. 48:141–145, 2013 [DOI] [PubMed] [Google Scholar]
- 158.Garcia-Valenzuela E., Shareef S., Walsh J., and Sharma S.C.Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp. Eye Res. 61:33–44, 1995 [DOI] [PubMed] [Google Scholar]
- 159.Kerrigan L.A., Zack D.J., Quigley H.A., Smith S.D., and Pease M.E.TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch. Ophthalmol. 115:1031–1035, 1997 [DOI] [PubMed] [Google Scholar]
- 160.Guerin M.B., McKernan D.P., O'Brien C.J., and Cotter T.G.Retinal ganglion cells: dying to survive. Int. J. Dev. Biol. 50:665–674, 2006 [DOI] [PubMed] [Google Scholar]
- 161.Kisiswa L., Dervan A.G., Albon J., Morgan J.E., and Wride M.A.Retinal ganglion cell death postponed: giving apoptosis a break? Ophthalmic. Res. 43:61–78, 2010 [DOI] [PubMed] [Google Scholar]
- 162.Lau A., and Tymianski M.Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. 460:525–542, 2010 [DOI] [PubMed] [Google Scholar]
- 163.Zlokovic B.V.Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat. Rev. Neurosci. 12:723–738, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pease M.E., McKinnon S.J., Quigley H.A., Kerrigan-Baumrind L.A., and Zack D.J.Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 41:764–774, 2000 [PubMed] [Google Scholar]
- 165.Di Polo A., Aigner L.J., Dunn R.J., Bray G.M., and Aguayo A.J.Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc. Natl. Acad. Sci. U S A. 95:3978–3983, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Klocker N., Kermer P., Weishaupt J.H., Labes M., Ankerhold R., and Bahr M.Brain-derived neurotrophic factor-mediated neuroprotection of adult rat retinal ganglion cells in vivo does not exclusively depend on phosphatidyl-inositol-3'-kinase/protein kinase B signaling. J. Neurosci. 20:6962–6967, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Parrilla-Reverter G., Agudo M., Sobrado-Calvo P., Salinas-Navarro M., Villegas-Perez M.P., and Vidal-Sanz M.Effects of different neurotrophic factors on the survival of retinal ganglion cells after a complete intraorbital nerve crush injury: a quantitative in vivo study. Exp. Eye Res. 89:32–41, 2009 [DOI] [PubMed] [Google Scholar]
- 168.Sivak J.M.The aging eye: common degenerative mechanisms between the Alzheimer's brain and retinal disease. Invest. Ophthalmol. Vis. Sci. 54:871–880, 2013 [DOI] [PubMed] [Google Scholar]
- 169.Goldblum D., Kipfer-Kauer A., Sarra G.M., Wolf S., and Frueh B.E.Distribution of amyloid precursor protein and amyloid-beta immunoreactivity in DBA/2J glaucomatous mouse retinas. Invest. Ophthalmol. Vis. Sci. 48:5085–5090, 2007 [DOI] [PubMed] [Google Scholar]
- 170.Yamamoto R., Yoneda S., and Hara H.Neuroprotective effects of beta-secretase inhibitors against rat retinal ganglion cell death. Neurosci. Lett. 370:61–64, 2004 [DOI] [PubMed] [Google Scholar]
- 171.Guo L., Salt T.E., Luong V., et al. Targeting amyloid-beta in glaucoma treatment. Proc. Natl. Acad. Sci. U S A. 104:13444–13449, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bolmont T., Bouwens A., Pache C., et al. Label-free imaging of cerebral beta-amyloidosis with extended-focus optical coherence microscopy. J. Neurosci. 32:14548–14556, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Iadecola C., Zhang F., Niwa K., et al. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat. Neurosci. 2:157–161, 1999 [DOI] [PubMed] [Google Scholar]
- 174.Resch H., Garhofer G., Fuchsjager-Mayrl G., Hommer A., and Schmetterer L.Endothelial dysfunction in glaucoma. Acta Ophthalmol. 87:4–12, 2009 [DOI] [PubMed] [Google Scholar]
- 175.Tezel G.The role of glia, mitochondria, and the immune system in glaucoma. Invest. Ophthalmol. Vis. Sci. 50:1001–1012, 2009 [DOI] [PubMed] [Google Scholar]
- 176.Wax M.B., and Tezel G.Immunoregulation of retinal ganglion cell fate in glaucoma. Exp. Eye Res. 88:825–830, 2009 [DOI] [PubMed] [Google Scholar]
- 177.Li X., Qian S.H., and Sun X.H.[Protection of autoimmunity induced by copolymer-1 on optic nerve: experiment with rat glaucoma models]. Zhonghua Yi Xue Za Zhi. 88:2152–2154, 2008 [PubMed] [Google Scholar]
- 178.Reichelt J., Joachim S.C., Pfeiffer N., and Grus F.H.Analysis of autoantibodies against human retinal antigens in sera of patients with glaucoma and ocular hypertension. Curr. Eye Res. 33:253–261, 2008 [DOI] [PubMed] [Google Scholar]
- 179.Tezel G.Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog. Retin. Eye Res. 25:490–513, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Osborne N.N., Alvarez C.N., and Del Olmo Aguado S. Targeting mitochondrial dysfunction as in aging and glaucoma. Drug Discov. Today, 2014 [DOI] [PubMed] [Google Scholar]
- 181.Chew E.Y.Nutrition Effects on Ocular Diseases in the Aging Eye. Invest. Ophthalmol. Vis. Sci. 2013;54:ORSF42–ORSF47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Osborne N.N.Mitochondria: their role in ganglion cell death and survival in primary open angle glaucoma. Exp. Eye Res. 90:750–757, 2010 [DOI] [PubMed] [Google Scholar]
- 183.Osborne N.N., and del Olmo-Aguado S.Maintenance of retinal ganglion cell mitochondrial functions as a neuroprotective strategy in glaucoma. Curr. Opin. Pharmacol. 13:16–22, 2013 [DOI] [PubMed] [Google Scholar]
- 184.Yu D.Y., Cringle S.J., Balaratnasingam C., Morgan W.H., Yu P.K., and Su E.N.Retinal ganglion cells: energetics, compartmentation, axonal transport, cytoskeletons and vulnerability. Prog. Retin. Eye Res. 36:217–246, 2013 [DOI] [PubMed] [Google Scholar]
- 185.Yang X.J., Ge J., and Zhuo Y.H.Role of mitochondria in the pathogenesis and treatment of glaucoma. Chin. Med. J. (Engl). 126:4358–4365, 2013 [PubMed] [Google Scholar]
- 186.Chrysostomou V., Rezania F., Trounce I.A., and Crowston J.G.Oxidative stress and mitochondrial dysfunction in glaucoma. Curr. Opin. Pharmacol. 13:12–15, 2013 [DOI] [PubMed] [Google Scholar]
- 187.Hernandez M.R.The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog. Retin. Eye Res. 19:297–321, 2000 [DOI] [PubMed] [Google Scholar]
- 188.Prasanna G., Krishnamoorthy R., and Yorio T.Endothelin, astrocytes and glaucoma. Exp. Eye Res. 93:170–177, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Resch H., Karl K., Weigert G., et al. Effect of dual endothelin receptor blockade on ocular blood flow in patients with glaucoma and healthy subjects. Invest. Ophthalmol. Vis. Sci. 50:358–363, 2009 [DOI] [PubMed] [Google Scholar]
- 190.Kur J., Newman E.A., and Chan-Ling T.Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog. Retin. Eye Res. 31:377–406, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Newman E.A.Functional hyperemia and mechanisms of neurovascular coupling in the retinal vasculature. J. Cereb. Blood Flow Metab. 33:1685–1695, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Attwell D., Buchan A.M., Charpak S., Lauritzen M., Macvicar B.A., and Newman E.A.Glial and neuronal control of brain blood flow. Nature. 468:232–243, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Garhofer G., Zawinka C., Resch H., Huemer K.H., Schmetterer L., and Dorner G.T.Response of retinal vessel diameters to flicker stimulation in patients with early open angle glaucoma. J. Glaucoma. 13:340–344, 2004 [DOI] [PubMed] [Google Scholar]
- 194.Gugleta K., Waldmann N., Polunina A., et al. Retinal neurovascular coupling in patients with glaucoma and ocular hypertension and its association with the level of glaucomatous damage. Graefes Arch. Clin. Exp. Ophthalmol. 251:1577–1585, 2013 [DOI] [PubMed] [Google Scholar]
- 195.Ergorul C., and Levin L.A.Solving the lost in translation problem: improving the effectiveness of translational research. Curr. Opin. Pharmacol. 13:108–114, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hood D.C., and Kardon R.H.A framework for comparing structural and functional measures of glaucomatous damage. Prog. Retin. Eye Res. 26:688–710, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Werkmeister R.M., Cherecheanu A.P., Garhofer G., Schmidl D., and Schmetterer L.Imaging of retinal ganglion cells in glaucoma: pitfalls and challenges. Cell. Tissue Res. 353:261–268, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]