Abstract
Background: In healthy nonsmokers, inhaled endotoxin [lipopolysaccharide (LPS)] challenge induces airway neutrophilia and modifies innate immune responses, but the effect on mucociliary clearance (MCC), a key host defense response, is unknown. Although smokers are chronically exposed to LPS through inhaled tobacco smoke, the acute effect of inhaled LPS on both MCC and airway inflammation is also unknown. The purpose of this study was to determine the effect of inhaled LPS on MCC in nonsmokers and mild smokers with normal pulmonary function.
Methods: We performed an open-label inhalational challenge with 20,000 endotoxin units in healthy adult nonsmokers (n=18) and young adult, mild smokers (n=12). At 4 hr post LPS challenge, we measured MCC over a period of 2 hr, followed by sputum induction to assess markers of airway inflammation.
Results: No significant changes in spirometry occurred in either group following LPS challenge. Following LPS, MCC was significantly (p<0.05) slowed in nonsmokers, but not in smokers [MCC=10±9% (challenge) vs. 15±8% (baseline), MCC=14±9% (challenge) vs. 16±10% (baseline), respectively]. Both groups showed a significant (p<0.05) increase in sputum neutrophils 6 hr post LPS challenge versus baseline. Although there was no correlation between the increased neutrophilia and depressed MCC post LPS in the nonsmokers, baseline neutrophil concentration predicted the LPS-induced decrease in MCC in the nonsmokers, i.e., lower baseline neutrophil concentration was associated with greater depression in MCC with LPS challenge (p<0.05).
Conclusions: These data show that a mild exposure to endotoxin acutely slows MCC in healthy nonsmokers. MCC in mild smokers is unaffected by mild endotoxin challenge, likely due to preexisting effects of cigarette smoke on their airway epithelium.
Key words: : endotoxin, lipopolysaccharide, mucociliary clearance, airway inflammation
Introduction
Endotoxin [lipopolysaccharide (LPS)], a major component of the outer membrane of gram-negative bacteria, is a significant component of particulate pollutants in occupational settings and domestic environments,(1,2) having been identified on both PM2.5 and PM10 particles(3,4) and cigarette smoke.(5) We(6–8) and others (9–11) have shown that LPS inhalation challenge, at levels [20,000 endotoxin units (EU)] that induce airway neutrophilia but not decrements in lung function or symptoms, causes depressed phagocytic host defense function in nonsmoking, healthy adult volunteers. Like phagocyte function, mucociliary clearance (MCC) is an important primary host defense mechanism that protects the lung(12) by clearing inhaled pathogens that might otherwise persist and colonize in the airways. Although decreases in MCC can be induced by inflammation,(12) it is unknown whether MCC is affected by LPS-induced inflammation in healthy nonsmokers.
Chronic smoking has been shown to slow MCC, especially in those with a long smoking history and with development of chronic bronchitis.(13) Decreases in MCC can be induced by inflammation and almost certainly contribute to mucus plugging and decreased clearance of inhaled irritants and pathogens.(12) With increasing pack-year history, smokers are also exposed to increasing levels of LPS associated with cigarette smoke. We have shown that short-term repeated challenge of LPS in healthy nonsmokers induces tolerance to subsequent LPS-induced inflammation and innate immune activation.(14) It is unknown, however, whether smokers have acquired LPS tolerance from chronic exposure to tobacco smoke and whether the MCC response to LPS challenge is different from that of nonsmokers as a result of their preexisting chronic LPS exposure. This study examined the effect of inhaled LPS on in vivo MCC in young, otherwise healthy adult mild smokers and nonsmokers.
We performed an open-label study comparing MCC and regional particle deposition indices on a baseline study day and after inhalational challenge with 20,000 EU of Clinical Center Reference Endotoxin (CCRE) in young nonsmokers and smokers with normal baseline lung function. Four hours after inhaled endotoxin challenge, we assessed clearance of inhaled, radiolabeled particles by gamma scintigraphy over a 2-hr period. The 4-hr postchallenge time was chosen based on our and others' previous findings of increased inflammation following LPS challenge measured by lavage or induced sputum in the 3–6-hr postexposure time period.(6,15,16) Following MCC measurements, we obtained induced sputum samples for assessment of inflammatory cell counts, i.e., neutrophils and macrophages. All endpoints were compared with baseline measurements for each subject on a nonchallenge, baseline study day.
Materials and Methods
Subjects
Eighteen (5 male/13 female) healthy nonasthmatic nonsmokers (age 19–48, mean 25) and 12 (8 male/4 female) (age 21–45, mean 28) nonasthmatic mild smokers were studied. Nonsmokers and smokers had normal baseline lung function [FEV1 % pred≥80, FEV1/FVC ratio≥0.75 (FEV1, forced expiratory volume in 1 sec; FVC, forced vital capacity)] and a negative methacholine challenge (less than a 20% decrease in FEV1 at a maximum methacholine concentration of 10 mg/mL).(17) Nonsmokers had negative allergy skin tests, but smokers were only required to have no current seasonal allergy symptoms (i.e., no skin tests). The mean pack-years for these young, mild smokers was 6±3.5. Informed written consent was obtained from all subjects prior to their participation in the studies that were approved by the Biomedical Institutional Review Board of the University of North Carolina at Chapel Hill. The studies were listed on clinicaltrials.gov [NCT00839124 (nonsmokers) and NCT00753870 (smokers)].
Study design
All subjects participated in a baseline and endotoxin challenge study visit. During the subject's baseline visit, we measured, in order, spirometry, MCC of inhaled, radiolabeled particles by gamma scintigraphy(18–20) over a 2-hr period, followed by sputum induction. The subject returned the next day for a follow-up gamma camera scan (24-hr retention). At least 3 days after the baseline visit, the subject returned for the endotoxin challenge study visit. Spirometry was measured before the endotoxin challenge. Postchallenge monitoring included spirometry, vital signs, oxygen saturation, and symptom score at the following intervals post challenge: 30 and 60 min and then hourly for 3 additional hours. At 4 hr post endotoxin challenge, we measured MCC over a 2-hr period, immediately followed by induced sputum. The subject returned the following day for the 24-hr retention scan. Comparisons were made with MCC and induced sputum endpoints obtained from the same subject during the baseline visit.
Inhaled endotoxin challenge
CCRE was provided by the National Institutes of Health Clinical Center; CCRE challenge will be referred to as LPS challenge. A dose of 20,000 EU was previously shown to induce sputum neutrophilia, and was demonstrated to be safe and well tolerated in previous studies. Subjects inhaled the LPS solution (5 mL of endotoxin solution in sterile water) via a DeVilbiss Ultraneb 99 ultrasonic nebulizer. While wearing noseclips, the subject was instructed to latch his/her mouth onto the nebulizer mouthpiece and breathe normally (i.e., tidal breaths) until the solution was spent (approximately 12–14 min).
Regional particle deposition and MCC
The procedure we used for measuring and analyzing MCC in humans has been described in detail previously.(18–20) A xenon (133Xe) equilibrium lung scan was recorded for each subject on their baseline visit to allow the creation of suitable regions of interest (ROIs) for determining regional lung deposition and MCC. For each measure of MCC, the subject inhaled an aerosol (mass median aerodynamic diameter of 5 μm, geometric standard deviation=2.0) of sulfur colloid labeled with 99mTc (40 μCi; CIS-US, Inc., Bedford, MA) from a DeVilbiss 646 nebulizer. While breathing the radiolabeled aerosol, the subject matched his/her tidal flow and breathing rate at 500 mL/sec and 30/min, respectively, by following a visual flow signal while breathing in time to a metronome. Immediately following inhalation of radioaerosol (duration of less than 2 min), an initial deposition scan was recorded (sum of two 2-min images), and then continuous 2-min images were recorded for a period of 2 hr to monitor clearance of particles from the lung as the subject remained seated in front of the gamma camera. The subject returned the following day after the radiolabeled aerosol exposure to obtain a 30-min scan of 24-hr lung activity/retention.
Only the right lung was used to analyze both regional deposition and MCC because of the potential overlap of stomach and lung activity on the left side. To assess central (C) versus peripheral (P) deposition, two outline ROIs were created over the right 133Xe lung image: (1) a rectangular region around the entire right lung, and (2) a central ROI, with dimensions equal to half the whole lung ROI's width and one-half its height. The central region was positioned on the medial boundary of the lung, centered by height, 25% of the area of the whole lung ROI. The peripheral region is the area lying between the central and whole lung outline. These regions were displayed over the initial aerosol scans to determine the initial counts in each region. We then calculated the ratio of central to peripheral counts, (C/P)Tc, and normalized this ratio by dividing by the central to peripheral ratio for the 133Xe scan, (C/P)Xe:
 |
This normalization was done to account for the difference in relative lung areas and thickness between the central and peripheral regions. C/P provides an index of relative deposition between the two regions. A C/P of 1.0 reflects equal deposition in each region. However, because the central region outlines both bronchial airways and lung parenchyma surrounding these airways, a C/P of near unity reflects primarily deposition in the pulmonary airspaces distal to anatomic dead space. Increases in C/P to values greater than unity reflect an increase in central versus peripheral deposition primarily as a result of increased bronchial deposition.
Another measure of regional deposition heterogeneity is the skew of the histogram distribution (counts/pixel versus number of pixels)(21) within the right whole lung ROI, increasing with increased frequency of “hot spots” in the lung. These hot spots are presumed due to increased deposition within bronchial airways throughout the lung so that skew is independent of the specific region within the lung (e.g., central versus peripheral). To determine skew, frequency distribution histograms were constructed from the right lung deposition images, with the number of pixels with a given count value (expressed as a fraction of total pixels) on the y axis and the count values on the x axis. These histograms were analyzed for skew (a measure of histogram symmetry, the third moment about the mean of the histogram).(21) Heterogeneity of deposition increases with increasing skew (i.e., more pixels with high counts/pixel).
The whole lung ROI bordering the right lung was used to determine, by computer analysis, the whole lung retention (Rt; decay and background corrected) as a fraction of the initial counts in the right lung, over the 2-hr clearance period at 10-min intervals (two 2-min images summed for each 10-min time point, e.g., images 1 and 2 for initial time 0 and images 6 and 7 for time 10 min). Similarly, the 24-hr retention (R24) was calculated. For each retention versus time data set (e.g., mean data shown in figures), the average clearance (MCC) [or 100 * (1- Rt)] over the 2-hr period of observation (MCC) was computed (i.e., average of the 10-min clearance values from 10 to 120 min). The calculated MCC (in percent) represents the average clearance in percent at the midpoint of our 10–120-min retention versus time observations.(20)
Sputum induction and cell differentials
Sputum induction and processing were performed according to previously published methods.(22,23) In brief, subjects inhaled increasing concentrations (3%, 4%, and 5%) of hypertonic saline for 7 min each, for a total of 21 min. Manual selection of plug material was weighed and treated with 0.1% dithiothreitol (DTT) for cell dispersion. Differential cell counts were analyzed from Romanowski (Diff-Quik)–stained slides, based on 400 cells, and expressed as a percentage of total nonsquamous nucleated cells. Acceptable slides had a minimum of 50% viability (trypan blue staining) and less than 20% squamous epithelial cell content.
Statistical methods
Comparisons between baseline and postchallenge measurements were analyzed using Student's paired t test. The significance of relationships between individual variables was tested using nonparametric Spearman's rank correlation analysis (Stata for Macintosh). An overall significance level of p≤0.05 was considered to be significant. All values are expressed as the mean (±standard deviation). The number of subjects to be studied was originally based on inflammatory endpoints, specifically changes in sputum neutrophils associated with a similar acute LPS challenge in healthy nonsmokers.(22,23) But repeat measures of MCC in healthy nonsmokers in our laboratory(20) also provided paired standard deviation data that established some confidence that we could detect a significant change in MCC associated with the LPS challenge. Based on these data, an N of 15 would be required to observe a 50% change in MCC for a two-sided paired analysis with α=0.05 and power=0.80.
Results
Table 1 summarizes the baseline lung function (FEV1 % pred) of the two cohorts and the postchallenge change between pre- and post-MCC measures, i.e., 3 and 6 hr post challenge. There was no significant fall in FEV1 after LPS challenge for either of the cohorts.
Table 1.
Summary of Lung Function (FEV1) [Mean (SD)] Measures on Challenge Study Day
| FEV1 (% pred) | % base FEV1 at 30 min post challenge | % base FEV1 at 3 hr post challenge | % base FEV1 at 6 hr post challenge | |
|---|---|---|---|---|
| Healthy nonsmokers | 102 (13) | 100 (2) | 101 (2) | 100 (2) |
| Smokers | 101 (12) | 100 (2) | 100 (4) | 99 (4) |
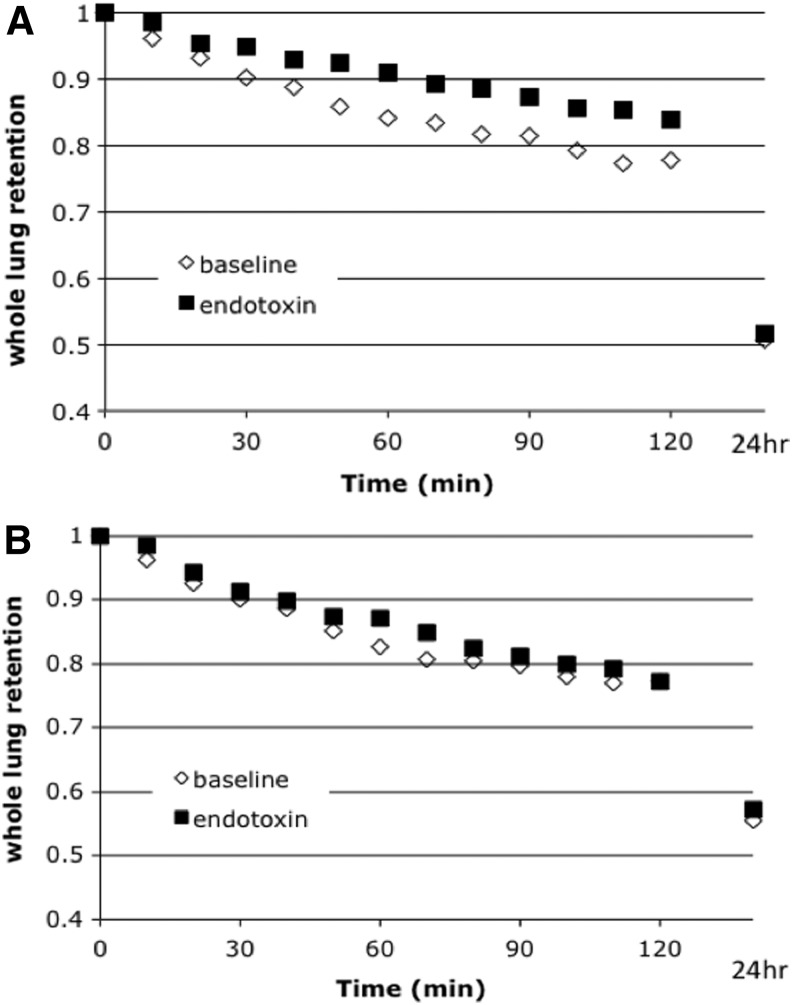
There was no difference in baseline MCC or regional deposition between the nonsmokers and smokers. In healthy nonsmoking subjects, whole lung MCC was significantly decreased after inhaled LPS challenge compared with baseline conditions (Fig. 1A and Table 2). There was no baseline versus challenge difference in regional deposition (C/P, skew, or %24hr) for the initial scan after radiolabeled aerosol inhalation (Table 1). For the smokers, there was no difference in whole lung MCC between LPS challenge and baseline study days (Fig. 1B and Table 2). There was also no difference in regional deposition for baseline versus challenge in the smokers.
FIG. 1.
(A) Whole lung MCC for baseline versus endotoxin challenge in healthy nonsmoker. (B) Whole lung MCC for baseline versus endotoxin challenge in smokers.
Table 2.
Mean (SD) of MCC and Regional Deposition Indices for Each Cohort on Baseline and LPS Challenge Study Days
| Whole-lung MCC (%) | C/P | Skew | %24hr | |
|---|---|---|---|---|
| Healthy nonsmokers | ||||
| Baseline | 15 (8) | 1.88 (0.39) | 1.83 (0.57) | 49 (14) |
| LPS challenge | 10 (9)a | 1.90 (0.51) | 1.90 (0.55) | 48 (14) |
| Smokers | ||||
| Baseline | 16 (10) | 1.75 (0.58) | 1.94 (1.12) | 44 (14) |
| LPS challenge | 14 (9) | 1.76 (0.63) | 1.75 (1.01) | 43 (15) |
p<0.05 compared with baseline by paired Student's t test.
Table 3 summarizes the results for inflammatory cell differentials obtained by sputum induction on the baseline and LPS challenge days. Only neutrophils and macrophages are shown, as they are the predominant cell types recovered from sputum. Both groups showed a significant increase in neutrophil concentrations following LPS exposure, whereas the macrophage concentrations showed no change (except as a reduced percentage of the total due to the increased percentage of neutrophils). There was a trend, although not significant, for the nonsmokers to have higher baseline neutrophil concentrations compared with the smokers. There were no significant correlations between the increase in inflammatory cells and the changes in MCC seen in the nonsmokers. However, there was a statistically significant association between baseline neutrophils (as percent total cells) and the relative decrease in MCC among the nonsmokers (p=0.026, and R=0.52 by Spearman rank correlation), i.e., the lower the baseline sputum neutrophils, the greater the relative decrease in MCC compared with baseline.
Table 3.
Mean (SD) Cell Counts From Induced Sputum (% and Cells/Mg in Sample)
| PMNs (as % (SD) and cells/mg (SD)) | Macrophages (as % (SD) and cells/mg (SD)) | |||
|---|---|---|---|---|
| Base | LPS | Base | LPS | |
| Nonsmokers (n=18) | 44.1 (27.4) | 65.9 (22.1)a | 53.6 (27) | 32.5 (22)a |
| 925 (955) | 2,706 (2958)a | 902 (665) | 945 (799) | |
| Smokers (n=12) | 50 (17) | 71 (11.3)a | 46.9 (16.3) | 23.6 (7.7)a |
| 499 (527) | 1,410 (1237)a | 453 (433)b | 472 (401) | |
PMN, polymorphonuclear neutrophils.
p<0.05 compared with baseline by paired Student's t test.
p<0.05 compared with nonsmokers' baseline by Student's t test.
Discussion
To our knowledge, this is the first study to use an LPS inhalation challenge protocol at a concentration known to induce airway inflammation in order to assess the effect of LPS on MCC (an important host defense mechanism to protect the airways from inhaled toxins and bacteria) in normal volunteers and mild smokers. The LPS challenge in this study approximates levels encountered in domestic and low-level occupational exposures, yet does not cause increased symptoms or overt changes in spirometric measures. We again found no effect on lung function for either of these groups (Table 1). However, we now report for the first time a slowing of MCC in healthy nonsmoking adults, with no effect of LPS on MCC in mild smokers.
Contrary to what we observed in nonsmokers, we found that mild smokers were resistant to the effect of LPS on MCC. Although our study was not designed to assess molecular mechanisms of response to LPS in these populations, we hypothesize that smokers may have acquired tachyphylaxis or tolerance to inhaled LPS from chronic exposure to tobacco smoke. In vitro studies of inflammatory cells have demonstrated that repeated exposure to LPS may result in tolerance to subsequent doses of LPS.(24) Such tolerance may also apply to the epithelial cells that comprise the MCC apparatus. We have recently reported that repeated short-term nasal challenge with LPS blunted inflammation (neutrophils) and decreased LPS-related innate immune responses (sCD14) when compared with a single LPS challenge in healthy volunteers.(14) Tobacco smoke is a rich source of LPS,(5) suggesting that smokers have chronic exposure to inhaled LPS. Alveolar macrophages from smokers have been found to have decreased inflammatory response to toll-like receptor 2 (TLR2) and TLR4 ligands, and smoking has been shown to decrease the antibacterial and phagocytic activities of macrophages,(25,26) suggesting that these cells from smokers have decreased LPS responsiveness.(27) Tobacco smoke preparations have been shown to modulate TLR-mediated responses in epithelial cells, although the effect of chronic smoking on epithelial cell mucin response to LPS is under-studied. However, these observations suggest that decreased inflammatory and epithelial cell response to endotoxin may account for the lack of effect of LPS on MCC we observed in the smokers.
Little is known regarding the acute effects of LPS on the various components of the mucociliary apparatus, namely, mucus secretion, epithelial cell responses, and ciliary function that might explain the slowing of MCC we observed in the healthy nonsmokers. Chronic LPS exposure stimulates production and expression of MUC5AC in animal and in vitro models.(28,29) MUC5AC is considered to be the most important mucin in the pathogenesis of mucus hypersecretion.(30) Moreover, this effect appears to be associated with inflammation, because dexamethasone, a corticosteroid, was shown to inhibit chronic LPS-induced increased MUC5AC expression in a rat study.(28) These authors also demonstrated that TLR4, found on airway epithelial and inflammatory cells, was associated with the LPS-stimulated mucus hypersecretion. However, whether a single dose of LPS in vivo acutely influences airway mucin secretions and TLR4 expression on epithelial cells is not known. On the other hand, if acute exposure to LPS acts as an irritant mucus secretagogue, MCC might acutely increase in both groups, i.e., immediately after exposure, such that after 4 hr, airway mucus might be depleted and MCC would exhibit slowing, as we observed in the nonsmokers. This phenomenon might be less apparent in smokers if they have a greater mucus-secreting capacity due to chronic exposure to irritants. In fact, the smokers may be tolerant because they can secrete more mucus, in effect maintaining a continuous mucus blanket on the airway surface over the 4-hr time period post LPS.
With regard to cilia effects, Wyatt et al.(31) exposed ciliated bovine bronchial epithelial cell cultures to hog barn-dust extract and found that ciliary beat frequency (CBF) was enhanced, but they also showed that LPS was not responsible for the increase. When LPS was added directly to the ciliated cells, there was no change in CBF. A series of earlier in vivo studies from Ohashi et al.(32–34) showed ciliary dysfunction of the middle ear and eustachian tube associated with LPS exposure in a dose-dependent fashion. Exposures to the middle ear led to otitis media with effusion in their animal model.(33,34) The mechanism of action for LPS depression of cilia function is not clear, however, from these studies.
The presence of high numbers of neutrophils seemed to be protective to slowing of MCC by LPS challenge within the nonsmokers, as evidenced by the significant correlation between baseline neutrophilia and the change in MCC in these individuals. It has been shown that polymorphonuclear neutrophils may release ATP in response to various stimuli,(35) and smokers have been shown to have elevated ATP in lavage compared with nonsmokers.(36) ATP in turn is known to enhance MCC by increasing ciliary and secretory activity.(37) This mechanism may offset the LPS-induced depression on the MCC apparatus.
Our young, mild smokers did not appear to differ in baseline MCC from the healthy nonsmokers. Given the low pack-years for our smokers and their lack of lung function impairment, such a result is not surprising and is consistent with previous findings.(12,13) What differentiates the mild smokers from the healthy nonsmokers is more likely how the MCC apparatus responds to various stimuli as we have shown here with the acute effect of endotoxin. We also studied fewer smokers than nonsmokers, although we think that unlikely to have affected either the difference in MCC (Table 2) between the two groups (p=0.81 for group comparison) or the lack of effect of LPS on MCC in smokers. With regard to the latter, an estimated N=77 would have been required to detect the absolute LPS-induced reduction of MCC=2.1±7.4% in the smokers with a power of 0.80 and one-sided α=0.05.
Finally, as suggested above, the time profile of the MCC response post LPS challenge in either of our study groups may be quite different from that of the inflammatory response. The assessment of sputum and blood inflammatory endpoints at 3–6 hr post LPS has been supported by a number of investigators and studies.(17) These studies show that, following inhaled LPS exposure, there is a rapid influx of blood neutrophils into the airways as a result of the production of chemotactic factors from activated airway macrophages. This takes place in the lung wall very shortly after exposure and peaks a few hours thereafter.(16) In our own specific case, we have been interested in comparing the response of LPS with that of ozone.(23) Early studies with ozone challenge clearly showed that airway inflammation compared at 1, 6, and 24 hr post exposure was greatest at the 6-hr time point.(38) More recent studies with LPS challenge have shown a similar time course for LPS-induced inflammation.(39,40) As discussed above, the acute response (immediately post LPS challenge) of MCC in either study group may have been quite different from what we observed at 4 hr. Nevertheless, in this initial study on the effects of LPS exposure on MCC, we wanted to maintain similar timing to our previous studies so that we could compare the MCC and inflammatory responses at or near the same time post challenge. Future studies should assess the time course of the change in MCC post LPS exposure to better understand the mechanisms by which LPS affects MCC.
Conclusion
In conclusion, we have found that an acute inhalation of LPS significantly slows whole lung MCC and increases airway neutrophils in healthy nonsmokers. The mild smokers, on the other hand, showed no effects of LPS challenge on MCC despite also showing increased neutrophilia, likely due to established tolerance from chronic LPS exposure associated with inhalation of cigarette smoke. The slowing of MCC by LPS may further enhance the toxicity of other inhaled particulate matter by increasing their residence time in the bronchial airways.
Acknowledgments
The authors are grateful to Jihong Wu and Heather Duckworth for their assistance in acquisition of MCC data, and to Heather Wells for her analysis of sputum cellularity. The project was supported by award numbers RO1HL080337 from the National Heart Lung and Blood Institute, U19AI077437 from the National Institute of Allergy and Infectious Diseases, and KL2RR025746, M01RR00046, and UL1RR025747 from the National Center of Research Resources, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the National Heart, Lung and Blood Institute, and the National Center for Research Resources of the National Institutes of Health.
Author Disclosure Statement
The authors declare that there are no conflicts of interest.
References
- 1.Mueller-Anneling , Avol E, Peters JM, and Thorne PS: Ambient endotoxin concentrations in PM10 from Southern California. Environ Health Perspect. 2004;112:583–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thorne PS, Kulhánková K, Yin M, Cohn R, Arbes SJ, and Zeldin DC: Endotoxin exposure is a risk factor for asthma: the national survey of endotoxin in United States housing. Am J Respir Crit Care Med. 2005;172:1371–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexis NE, Lay JC, Zeman K, Bennett WD, Peden DB, Soukup JM, Devlin RB, and Becker S: Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J Allergy Clin Immunol. 2006;117:1396–1403 [DOI] [PubMed] [Google Scholar]
- 4.Becker S, Fenton MJ, and Soukup JM: Involvement of microbial components and toll-like receptors 2 and 4 in cytokine responses to air pollution particles. Am J Respir Cell Mol Biol. 2002;27:611–618 [DOI] [PubMed] [Google Scholar]
- 5.Hasday JD, Bascom R, Costa JJ, Fitzgerald T, and Dubin W: Bacterial endotoxin is an active component of cigarette smoke. Chest. 1999;115:829–835 [DOI] [PubMed] [Google Scholar]
- 6.Alexis NE, and Peden DB: Blunting airway eosinophilic inflammation results in a decreased airway neutrophil response to inhaled LPS in patients with atopic asthma: a role for CD14. J Allergy Clin Immunol. 2001;108:577–580 [DOI] [PubMed] [Google Scholar]
- 7.Alexis NE, Brickey WJ, Lay JC, Wang Y, Roubey RA, Ting JP, and Peden DB: Development of an inhaled endotoxin challenge protocol for characterizing evoked cell surface phenotype and genomic responses of airway cells in allergic individuals. Ann Allergy Asthma Immunol. 2008;100:206–215 [DOI] [PubMed] [Google Scholar]
- 8.Dillon MA, Harris B, Hernandez ML, Zou B, Reed W, Bromberg PA, Devlin RB, Diaz-Sanchez D, Kleeberger S, Zhou H, Lay JC, Alexis NE, and Peden DB: Enhancement of systemic and sputum granulocyte response to inhaled endotoxin in people with the GSTM1 null genotype. Occup Environ Med. 2011;68:783–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyen V, Kassengera Z, Dinh DH, and Michel O: Time course of endotoxin-induced airways' inflammation in healthy subjects. Inflammation. 2012;35:33–38 [DOI] [PubMed] [Google Scholar]
- 10.Michel O, Nagy AM, Schroeven M, Duchateau J, Nève J, Fondu P, and Sergysels R: Dose-response relationship to inhaled endotoxin in normal subjects. Am J Respir Crit Care Med. 1997;156:1157–1164 [DOI] [PubMed] [Google Scholar]
- 11.Clapp WD, Thorne PS, Frees KL, Zhang X, Lux CR, and Schwartz DA: The effects of inhalation of grain dust extract and endotoxin on upper and lower airways. Chest. 1993;104:825–830 [DOI] [PubMed] [Google Scholar]
- 12.Wanner A, Salathe M, and O'Riordan TG: Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154:1868–1902 [DOI] [PubMed] [Google Scholar]
- 13.Goodman RM, Yergin BM, Landa JF, Golinvaux MH, and Sackner MA: Relationship of smoking history and pulmonary function tests to tracheal mucous velocity in nonsmokers, young smokers, ex-smokers and patients with chronic bronchitis. Am Rev Respir Dis. 1978;117:205–214 [DOI] [PubMed] [Google Scholar]
- 14.Doreswamy V, Alexis NE, Zhou H, and Peden DB: Nasal PMN response to repeated challenge with endotoxin in healthy volunteers. Inhal Toxicol. 2011;23:142–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nightingale JA, Rogers DF, Hart LA, Kharitonov SA, Chung KF, and Barnes PJ: Effect of inhaled endotoxin on induced sputum in normal, atopic, and atopic asthmatic subjects. Thorax. 1998;53:563–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorn J: The inflammatory response in humans after inhalation of bacterial endotoxin: a review. Inflamm Res. 2001;50:254–261 [DOI] [PubMed] [Google Scholar]
- 17.Guidelines for Methacholine and Exercise Challenge Testing—1999. Am J Respir Crit Care Med. 2000;161:309–329 [DOI] [PubMed] [Google Scholar]
- 18.Bennett WD, Almond MA, Zeman KL, Johnson JG, and Donohue JF: Effect of salmeterol on mucociliary and cough clearance in chronic bronchitis. Pulm Pharmacol Ther. 2006;19:96–100 [DOI] [PubMed] [Google Scholar]
- 19.Lay JC, Alexis NE, Zeman KL, Peden DB, and Bennett WD: In vivo uptake of inhaled particles by airway phagocytes is enhanced in mild asthmatics compared to normal volunteers. Thorax. 2009;64:313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett WD, Laube BL, Corcoran T, Zeman K, Sharpless G, Thomas K, Wu J, Mogayzel PJ Jr, Pilewski J, and Donaldson S: Multisite comparison of mucociliary and cough clearance measures using standardized methods. J Aerosol Med Pulm Drug Deliv. 2013;26:157–164 [DOI] [PubMed] [Google Scholar]
- 21.Garrard CS, Gerrity TR, Schreiner JF, and Yeates DB: The characterization of radioaerosol deposition in the healthy lung by histogram distribution analysis. Chest. 1981;80:840–842 [DOI] [PubMed] [Google Scholar]
- 22.Alexis NE, Lay JC, Almond M, Bromberg PA, Patel DD, and Peden DB: Acute LPS inhalation in healthy volunteers induces dendritic cell maturation in vivo. J Allergy Clin Immunol. 2005;115:345–350 [DOI] [PubMed] [Google Scholar]
- 23.Hernandez ML, Harris B, Lay JC, Bromberg PA, Diaz-Sanchez D, Devlin RB, Kleeberger SR, Alexis NE, and Peden DB: Comparative airway inflammatory response of normal volunteers to ozone and lipopolysaccharide challenge. Inhal Toxicol. 2010;22:648–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimada M, Tsukada H, Ishizuka O, Kon Y, Hasegawa T, Suzuki E, and Gejyo F: Lipopolysaccharide tolerance in relation to intrabronchial influx of neutrophils in the rat. Lung. 2000;178:235–248 [DOI] [PubMed] [Google Scholar]
- 25.Green GM, and Carolin D: The depressant effect of cigarette smoke on the in vitro antibacterial activity of alveolar macrophages. N Engl J Med. 1967;276:421–427 [DOI] [PubMed] [Google Scholar]
- 26.Thomas WR, Holt PG, and Keast D: Cigarette smoke and phagocyte function: effect of chronic exposure in vivo and acute exposure in vitro. Infect Immun. 1978;20:468–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H, Cowan MJ, Hasday JD, Vogel SN, and Medvedev AE: Tobacco smoking inhibits expression of proinflammatory cytokines and activation of IL-1R-associated kinase, p38, and NF-κB in alveolar macrophages stimulated with TLR2 and TLR4 agonists. J Immunol. 2007;179:6097–6106 [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Wang T, Zhang JY, Zhang SF, Liu DS, Xu D, Wang X, Chen YJ, and Wena FQ: Toll-like receptor 4 relates to lipopolysaccharide-induced mucus hypersecretion in rat airway. Arch Med Res. 2009;40:10–17 [DOI] [PubMed] [Google Scholar]
- 29.Binker MG, Binker-Cosen AA, Richards D, Oliver B, and Cosen-Binker LI: LPS-stimulated MUC5AC production involves Rac1-dependent MMP-9 secretion and activation in NCI-H292 cells. Biochem Biophys Res Commun. 2009;386:124–129 [DOI] [PubMed] [Google Scholar]
- 30.Rogers DF: Airway mucus hypersecretion in asthma: an undervalued pathology? Curr Opin Pharmacol. 2004;4:241–250 [DOI] [PubMed] [Google Scholar]
- 31.Wyatt TA, Sisson JH, Von Essen SG, Poole JA, and Romberger DJ: Exposure to hog barn dust alters airway epithelial ciliary beating. Eur Respir J. 2008;31:1249–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohashi Y, Nakai Y, Ikeoka H, Koshimo H, Esaki Y, and Kato S: Effects of bacterial endotoxin on the ciliary activity in the in vitro eustachian tube. Arch Otorhinolaryngol. 1987;244:88–90 [DOI] [PubMed] [Google Scholar]
- 33.Ohashi Y, Nakai Y, Furuya H, Esaki Y, Ikeoka H, Kato S, and Kato M: Mucociliary disease of the middle ear during experimental otitis media with effusion induced by bacterial endotoxin. Ann Otol Rhinol Laryngol. 1989;98:479–484 [DOI] [PubMed] [Google Scholar]
- 34.Ohashi Y, Nakai Y, Ikeoka H, Esaki Y, Ikeoka H, Kato S, and Kato M: Experimental otitis media with effusion induced by lipopolysaccharide from Klebsiella pneumoniae: mucociliary pathology of the middle ear. Am J Otolaryngol. 1988;9:83–89 [DOI] [PubMed] [Google Scholar]
- 35.Eltzschig HK, Eckle T, Mager A, Küper N, Karcher C, Weissmüller T, Boengler K, Schulz R, Robson SC, and Colgan SP: ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108 [DOI] [PubMed] [Google Scholar]
- 36.Lommatzsch M, Cicko S, Müller T, Lucattelli M, Bratke K, Stoll P, Grimm M, Dürk T, Zissel G, Ferrari D, Di Virgilio F, Sorichter S, Lungarella G, Virchow JC, and Idzko M: Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:928–934 [DOI] [PubMed] [Google Scholar]
- 37.Bennett WD, Noone PG, Knowles MR, and Boucher RC: Regulation of mucociliary clearance by purinergic receptors. In: Salathe M, Adler KB, Boucher RC, and Satir P, (eds). Cilia and Mucus: From Development to Respiratory Defense. Marcel Dekker, New York; pp. 347–360, 2001 [Google Scholar]
- 38.Schelegle ES, Siefkin AD, and McDonald RJ: Time course of ozone-induced neutrophilia in normal humans. Am Rev Respir Dis. 1991;143:1353–1358 [DOI] [PubMed] [Google Scholar]
- 39.Moller W, Heimbeck I, Hofer TPJ, Khadem Saba G, Neiswirth M, Frankenberger M, and Ziegler-Heitbrock L: Differential inflammatory response to inhaled lipopolysaccharide targeted either to the airways or the alveoli in man. PLoS One. 2012;7(4):e33505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janssen O, Schaumann F, Holz O, Lavae-Mokhtari B, Welker L, Winkler C, Biller H, Krug N, and Hohlfeld JM: Low-dose endotoxin inhalation in healthy volunteers—a challenge model for early clinical drug development. BMC Pulm Med. 2013;13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]